Abstract
The MUC5B promoter polymorphism (rs35705950) has been associated with interstitial lung abnormalities (ILA) in white participants from the general population, it is not known if these findings replicate and are influenced by ILA subtype. We evaluated the associations between MUC5B genotype and ILA in cohorts with extensive imaging characterization.
We performed ILA phenotyping and MUC5B promoter genotyping in 5,308 and 9,292 participants from the AGES-Reykjavik and COPDGene cohorts.
ILA were present in 7% of participants from AGES-Reykjavik, 8% of non-Hispanic Whites (NHW) from COPDGene and 7% of African-Americans (AA) from COPDGene. While MUC5B genotype was strongly associated (after correction for multiple testing) with ILA (odds ratio [OR]=2.1, 95% confidence interval [CI] 1.8, 2.4, P=1×10−26), there was evidence of significant heterogeneity between cohorts (I2=81%). When narrowed to specific radiologic subtypes, (e.g. subpleural ILA), MUC5B genotype remains strongly associated (OR=2.6, 95% CI 2.2, 3.1, P=10×10−30) with minimal heterogeneity (I2=0%). While there was no evidence that MUC5B genotype influenced survival, there was evidence that MUC5B genotype improved risk prediction for a possible UIP or UIP pattern in NHW populations.
The MUC5B promoter polymorphism is strongly associated with ILA and specific radiologic subtypes of ILA, with varying degrees of heterogeneity in the underlying populations.
Keywords: idiopathic pulmonary fibrosis, interstitial lung disease, interstitial lung abnormalities (ILA), MUC5B, usual interstitial pneumonia (UIP), prediction
INTRODUCTION
Specific patterns of radiologic abnormalities on chest computed tomography (CT) scans (termed interstitial lung abnormalities [ILA])[1, 2], may represent an early or mild stage of pulmonary fibrosis or other interstitial lung diseases (ILD). Evidence in support of that hypothesis includes physiologic and clinical outcome data demonstrating that ILA are associated with measures of decreased pulmonary function[1–5] and exercise tolerance[6], an increased rate of respiratory symptoms[2] and death[7]. Further evidence linking ILA to pulmonary fibrosis includes the fact that the genetic polymorphism most consistently associated with idiopathic pulmonary fibrosis (IPF) (the minor allele of the single nucleotide polymorphism (SNP) rs35705950 in the promoter region of the mucin 5B [MUC5B] gene)[8] is associated with ILA in the Framingham Heart Study (FHS)[2]. Despite the latter finding, it is not known whether the association between the MUC5B promoter polymorphism and ILA replicates and whether specific radiologic patterns affect the associations.
We hypothesized that MUC5B genotype would be associated with ILA; and that, these associations would depend on specific radiologic patterns of ILA. To test these hypotheses, we evaluated the association between ILA (and radiologic subtypes of ILA) and MUC5B genotype in participants from the Age Gene/Environment Susceptibility (AGES)-Reykjavik Study and in participants from the Genetic Epidemiology of COPD Study (COPDGene). Based on the results, additional analyses were performed to determine if MUC5B genotype would influence survival and if it could help to improve risk prediction for ILA.
METHODS
Study Design
Protocols for participant enrolment in the AGES-Reykjavik study and COPDGene have been previously reported[1, 9, 10]. The AGES-Reykjavik study is a longitudinal birth cohort derived from the Reykjavik Study, which was established in 1967 and includes men and women born in Reykjavik, Iceland from 1907 to 1935 and are now followed by the Icelandic Heart Association[9]. Of the 5764 participants recruited from January 2002 to February 2006, 5308 (92%) had both chest CT and genotypic information and were included in the analysis. COPDGene is a multicentre longitudinal study of smokers designed to identify the epidemiologic and genetic risk factors for chronic obstructive pulmonary disease (COPD). Participants were excluded from COPDGene if they had a history of known lung disease other than asthma, emphysema or COPD[10]. Of the 10,364 participants recruited between November 2007 and April 2010, 9,292 (90%) had both chest CT scans and genotypic information passing quality control and were included in the analysis (this number includes 64 participants excluded from primary COPDGene analyses due to presence of bronchiectasis or ILD identified on chest CT scans after recruitment). Of the 9,292 participants included from COPDGene, 6,134 (66%) were non-Hispanic whites (NHW) and 3,158 (34%) were African-Americans (AA). Written informed consent was obtained from all participants, including consent for genetic studies. The institutional review boards of the Brigham and Women’s Hospital and participating centres approved this study.
Genotyping
All genotyping of the MUC5B promoter polymorphism (rs35705950) was done using TaqMan Genotyping Assays (Applied Biosystems)[2, 8].
Chest CT characterization
Methods for characterizing ILA in the initial 2508 participants from COPDGene and participants from AGES-Reykjavik have been previously described[1, 7], the same methods were used to characterize ILA in the remaining 6784 participants from COPDGene. Chest CT scans were evaluated by up to three readers (chest radiologists and pulmonologists) using a sequential reading method[11]. ILA were defined as specific non-dependent patterns of increased lung density including ground-glass, reticular abnormalities, diffuse centrilobular nodules, nonemphysematous cysts, honeycombing or traction bronchiectasis, affecting greater than 5% of any lung zone, (Figure 1). Chest CT scans with focal or unilateral ground-glass or reticular abnormalities, or patchy ground-glass abnormalities were considered indeterminate, (additional details in online supplementary material).
Figure 1.
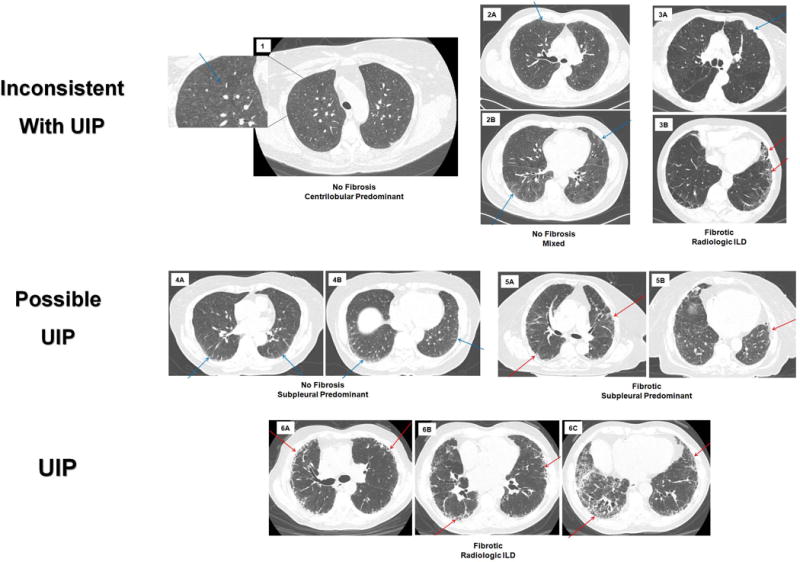
Chest computed tomographic (CT) images depicting radiologic subtypes and the overlap between subtypes of interstitial lung abnormalities (ILA). In all panels the blue arrows point to areas of ILA without fibrosis, the red arrows point to areas of ILA with fibrosis, each panel (1–6) represents one participant. Panels 1–3 demonstrate patterns of ILA that are inconsistent with usual interstitial pneumonia (UIP), panels 4–5 demonstrate patterns of ILA that are possible UIP and panel 6 is a pattern of ILA that is consistent with UIP. Panel 1 represents non-fibrotic, centrilobular predominant ILA, with an area zoomed in to highlight the centrilobular ground glass nodules. Panel 2 is non-fibrotic, mixed pattern of ILA, in 2A the blue arrow points to subpleural reticulation; in 2B the arrows demonstrate both subpleural and centrilobular ground glass. Panel 3 is fibrotic (see the red arrows in 3B), radiologic interstitial lung disease (ILD), that is inconsistent with UIP due to the pleural plaque (blue arrow) in 3A. Panel 4 is non-fibrotic, subpleural predominant ILA, blue arrows pointing to subpleural reticulation. Panel 5 is fibrotic, subpleural predominant ILA, with red arrows in both panels pointing to traction bronchiectasis. Panel 6 is fibrotic, radiologic ILD; red arrows highlight traction bronchiectasis and honeycombing.
Next, to determine if the associations between ILA and MUC5B genotype were dependent on specific radiologic patterns, further imaging-based classification was performed on all scans with ILA present. First, ILA was classified by the presence, or absence, of definite fibrosis (defined as evidence of pulmonary parenchymal architectural distortion, such as traction bronchiectasis or honeycombing)[2, 5, 7] into two groups – ILA with definite fibrosis and ILA without fibrosis. Next, the scans with ILA were classified by consistency with a usual interstitial pneumonia (UIP) pattern (inconsistent, possible and UIP) according to ATS/ERS/JRS/ALAT criteria[12]. Finally, chest CT scans with ILA were classified by the type and location of radiologic densities seen[1, 5] (online supplementary material and Figure 1). All ILA subtyping was performed by a consensus of at least three readers, who were blind to any participant specific information. Quantitative measures of emphysema (percentage of lung below 950 Hounsfield units) were measured with Airway inspector (www.airwayinspector.org)[13].
Statistical Analyses
All genetic analyses were performed using additive genetic models[8]. Logistic regression was used to assess the MUC5B SNP associations with ILA and ILA subtypes, and Cox proportional hazards models were used to analyze the time-to-mortality. In Cox models, all variables were assessed and none were found to violate the proportional hazards assumption. Multivariable models were adjusted for age, sex, and smoking behaviour (pack-years smoking). Mega analysis was performed by pooling the participant level data and P-values reported for the combined cohorts were corrected for multiple testing using a bonferroni correction. I2 values to assess heterogeneity between cohorts were calculated using the DerSimonian and Laird method[14]. To evaluate the ability of the MUC5B genotype to predict ILA (and ILA subtypes) we first evaluated clinical variables and risk factors for ILA based on prior literature[1, 2, 5, 7] and significant findings from our association analyses. Recursive partitioning using Hosmer-Lemeshow tests were used to assess goodness of fit for clinical variables (online supplementary material). Then receiver operating characteristic (ROC) curves were generated to obtain areas under the curve (AUC) and create c-statistics, and Wald tests assessed whether the addition of the MUC5B minor allele improved the ability to predict ILA. All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All p-values were two sided and a level of 0.05 was considered statistically significant.
RESULTS
ILA Prevalence and Baseline Characteristics
The prevalence of participants with ILA, indeterminate ILA status and without ILA in the AGES-Reykjavik cohort[7] has been previously reported and the percentages were similar when subset to participants with genotypic information. In AGES-Reykjavik, ILA were present in 377 (7%), 3,209 (60%) did not have ILA and 1,722 (32%) had indeterminate ILA status (Table 1). In NHW participants from COPDGene 485 (8%) had ILA, 3,667 (60%) did not have ILA and 1,982 (32%) had indeterminate ILA status. In COPDGene AA participants 223 (7%) had ILA, 1,728 (55%) did not have ILA and 1,207 (38%) had indeterminate ILA status (Table 1). In AGES-Reykjavik 4.4% (n=236) had possible UIP, 0.32% (n=17) had UIP and 2.4% (n=128) had definite fibrosis; in NHW participants from COPDGene, 3.4% (n=210) had possible UIP, 0.2% (n=12) had UIP and 1.6% (n=101) had definite fibrosis; and in AA participants from COPDGene 2.1% (n=66) had possible UIP, 0.09% (n=3) had UIP, and 0.8% (n=25) had definite fibrosis (online supplementary table 2).
Table 1.
Baseline characteristics of participants in AGES-Reykjavik and COPDGene stratified by ILA status and race.*
| AGES-Reykjavik | P-value | COPDGene Non-Hispanic Whites | P-value | COPDGene African-Americans | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No ILA (N=3209) |
ILA (N=377) |
No ILA (N=3667) |
ILA (N=485) |
No ILA (N=1728) |
ILA (N=223) |
||||
| Age – yrs | 76 ± 5 | 78 ± 6 | <.0001 | 61 ± 9 | 64 ± 10 | <.0001 | 54 ± 7 | 55 ± 8 | 0.003 |
| Gender – no. female(%) | 1905 (59) | 171 (45) | <.0001 | 1752 (48) | 219 (45) | 0.29 | 705 (41) | 126 (57) | <.0001 |
| Body Mass Index | 27 ± 4 | 27 ± 5 | 0.50 | 29 ± 6 | 29 ± 6 | 0.25 | 29 ± 7 | 29 ± 7 | 0.33 |
| Pack Years Smoking, median (IQR)† | 2‡ (0, 25) |
20‡ (0, 50) |
<.0001 | 40 (29, 56) |
45 (34, 63) |
<.0001 | 34 (22, 46) |
35 (24, 47) |
0.49 |
| Current Smokers – no. yes (%) | 373 (12) | 69 (18) | 0.0003 | 1402 (38) | 2546 (53) | <.0001 | 1393 (81) | 183 (82) | 0.65 |
| History of COPD§ – no. (%) | – | – | – | 1474 (40) | 167 (35) | 0.02 | 381 (22) | 54 (25) | 0.44 |
| Percentage of the lung less than −950 Hounsfield Units, median (IQR) | – | – | – | 3 (0.9, 9) |
1.4 (0.5, 4.8) |
<.0001 | 1.1 (0.4, 3.3) |
0.7 (0.2, 2.4) |
<.0001 |
ILA is interstitial lung abnormality, ± values are means and standard deviations.
IQR is interquartile range
Includes cigarette and cigar smoking
COPD is chronic obstructive pulmonary disease
The baseline characteristics in AGES-Reykjavik and COPDGene, stratified by race, are presented by the presence or absence of ILA in Table 1. Baseline characteristics of participants with indeterminate ILA status, from COPDGene are presented in online supplementary table 1, and have been published previously in the AGES-Reykjavik cohort [7]. In all cohorts, participants with ILA were significantly older than those without ILA. In both AGES-Reykjavik and NHW’s from COPDGene, participants with ILA had greater pack-years of smoking and were more likely to be actively smoking, as compared to those without ILA, while in AA’s from COPDGene there were no differences associated with ILA in pack-years of smoking or current smoking status.
Interstitial Lung Abnormalities and the MUC5B promoter polymorphism
The minor allele frequency of the MUC5B promoter SNP (rs3570950) was 12.7% in AGES-Reykjavik, 10.3% in NHW participants from COPDGene and 2% in AA participants from COPDGene (consistent with reported population diversity allelic frequency in dbSNP); the SNP was found to be in Hardy-Weinberg equilibrium in all cohorts. At least one copy of the MUC5B promoter polymorphism was noted in 44% (166 of 377), in 27% (131 of 485), and in 5% (12 of 223) of those with ILA in the AGES-Reykjavik, in NHW, and in AA participants from COPDGene, respectively. After adjustment for multiple testing, MUC5B genotype was strongly associated with ILA (Odds Ratio [OR] = 2.1, 95% Confidence Interval [CI] 1.8, 2.4, P=1×10−26, despite significant heterogeneity between cohorts (I2=81%), (Table 2).
Table 2.
Association between Interstitial Lung Abnormalities (ILA) and the MUC5B promoter polymorphism*
| AGES-Reykjavik | COPDGene Non-Hispanic Whites | COPDGene African-Americans | Cohorts Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ILA Subtype | Adjusted† Odds Ratio (95% CI) |
P-Value | Adjusted† Odds Ratio (95% CI) |
P-Value | Adjusted† Odds Ratio (95% CI) |
P-Value | I2‡ | Adjusted† Odds Ratio (95% CI) |
P-Value§ (Corrected) |
| ILA | 2.7 (2.2, 3.2) |
1×10−22 | 1.6 (1.3, 2.0) |
2×10−6 | 1.5 (0.8, 2.8) |
0.19 | 81% | 2.1 (1.8, 2.4) |
1×10−26 |
| ILA without Fibrosisǁ | 2.3 (1.8, 2.9) |
5×10−13 | 1.5 (1.2, 1.9) |
0.0004 | 0.99 (0.5, 2.2) |
0.97 | 76% | 1.8 (1.5, 2.1) |
2×10−12 |
| Definite Fibrosis | 3.3 (2.4,4.4) |
5×10−15 | 2.1 (1.4, 3.1) |
0.0001 | 6.2 (2.3, 16.8) |
0.0003 | 59% | 3.0 (2.4, 3.7) |
8×10−22 |
| Centrilobular | 1.3 (0.7, 2.5) |
0.44 | 0.64 (0.4, 1.1) |
0.09 | 1.2 (0.4, 3.8) |
0.79 | 15% | 0.91 (0.63, 1.3) |
1.0 |
| Subpleural | 3.0 (2.3, 3.7) |
2×10−19 | 2.4 (1.9, 3.2) |
5×10−11 | 2.3 (0.98, 5.4) |
0.06 | 0% | 2.6 (2.2, 3.1) |
1×10−30 |
| Mixed | 2.3 (1.6, 3.3) |
2×10−5 | 1.05 (0.6, 1.7) |
0.86 | 1.06 (0.3, 3.4) |
0.93 | 66% | 1.5 (1.1, 2.0) |
0.05 |
| Radiologic ILD** | 4.4 (2.2, 9.0) |
4×10−5 | 4.8 (2.5, 8.9) |
1×10−6 | – | – | 0% | 4.4 (2.8, 6.8) |
5×10−10 |
| Inconsistent with UIP†† | 2.2 (1.7, 3.0) |
1×10−7 | 0.95 (0.7, 1.3) |
0.75 | 1.0 (0.4, 2.4) |
0.94 | 55% | 1.4 (1.1, 1.7) |
0.01 |
| Possible UIP | 2.8 (2.2, 3.6) |
9×10−17 | 2.5 (1.9, 3.3) |
1×10−11 | 2.7 (1.1, 6.3) |
0.03 | 1% | 2.7 (2.3, 3.2) |
1×10−30 |
| UIP | 4.4 (2.2, 9.0) |
4×10−5 | 4.6 (2.0, 10.4) |
0.0003 | – | – | 0% | 4.1 (2.1, 8.1) |
0.0003 |
ILA is interstitial lung abnormality. Analyses of MUC5B genotype were performed using additive genetic models, odds ratios are per copy of MUC5B minor allele.
Models are adjusted for age, sex and tobacco exposure
I2 calculated using DerSimonian and Laird Method
P-value corrected for multiple testing using a bonferroni correction
Fibrosis is evidence of pulmonary parenchymal architectural distortion
ILD is interstitial lung disease. Analysis was not done in African-Americans; no participants with radiologic ILD had the MUC5B genotype
UIP is usual interstitial pneumonia. Analysis was not done in African-Americans; no participants with UIP had the MUC5B genotype
The MUC5B Promoter Polymorphism and Radiologic Patterns of ILA
While there was some variability in the associations between the MUC5B promoter polymorphism and radiologic subtypes of ILA across cohorts; consistent patterns emerged. For example, after adjustment for covariates, despite moderate heterogeneity between cohorts (I2=59%), MUC5B genotype was consistently associated with definite fibrosis (OR=3.0, 95% CI 2.4, 3.7, P=8×10−22) compared to those without ILA, Table 2, Figure 1. There was also evidence that in addition to consistent association (or lack of) with MUC5B genotype, that when narrowed to specific radiologic phenotypes; there was minimal heterogeneity between cohorts. After adjustment for covariates, the MUC5B promoter polymorphism was consistently associated with a possible UIP pattern (OR=2.7, 95% CI 2.3, 3.2, P=1×10−30), with essentially no between cohort heterogeneity (I2=1%), (Table 2, Figure 1). While, there was no evidence for an association with the MUC5B promoter polymorphism when ILA was limited to those with a centrilobular pattern (OR=0.91, 95% CI 0.63, 1.3, P=1.0, I2=15%), (Table 2, Figure 1). Additional results, subset to participants by age are presented in online supplementary tables 3 and 4.
The MUC5B Promoter Polymorphism and ILA Prediction
Based on the consistent associations between the MUC5B promoter polymorphism and ILA subtypes we sought to determine if knowledge of MUC5B genotype alone could predict definite fibrosis, and a possible UIP or a UIP pattern, on chest CT. In all cohorts, MUC5B genotype improved risk prediction for definite fibrosis (c-statistic 0.64, 95% CI 0.60–0.69, P<0.0001, c-statistic 0.57, 95% CI 0.52–0.62, P=0.0007, c-statistic 0.58, 95% CI 0.50–0.65, P=0.0005 in the AGES-Reykjavik, NHW, and in AA participants from COPDGene, respectively). In the NHW populations, MUC5B genotype improved risk prediction for having a possible UIP or UIP pattern (c-statistic 0.66, 95% CI 0.61–0.71, P<0.0001, c-statistic 0.60, 95% CI 0.57–0.63, P<0.0001 in the AGES-Reykjavik and in NHW participants from COPDGene, respectively), risk prediction was not improved in African-Americans (c-statistic 0.52, 0.49–0.56, P=0.06 in AA participants from COPDGene), see Table 3, Figure 2.
Table 3.
MUC5B Genotype and Interstitial Lung Abnormality Prediction*
| MUC5B Minor Allele | Clinical Data† | Clinical Data + MUC5B Minor Allele | P-value for the comparison of Clinical to Clinical + MUC5B | ||||
|---|---|---|---|---|---|---|---|
| C-Statistic (95% CI) |
P-Value | C-Statistic (95% CI) |
P-Value | C-Statistic (95% CI) |
P-Value | ||
| AGES-Reykjavik | |||||||
| ILA | 0.61 (0.59, 0.64) |
<.0001 | 0.64 (0.61, 0.66) |
<.0001 | 0.72 (0.69, 0.74) |
<.0001 | <.0001 |
| Subpleural & Radiologic ILD‡ | 0.63 (0.60, 0.67) |
<.0001 | 0.66 (0.63, 0.70) |
<.0001 | 0.72 (0.69, 0.76) |
<.0001 | <.0001 |
| Definite Fibrosis§ | 0.64 (0.60, 0.69) |
<.0001 | 0.70 (0.65, 0.75) |
<.0001 | 0.75 (0.70, 0.79) |
<.0001 | 0.004 |
| Possible UIP & UIPǁ | 0.64 (0.61, 0.67) |
<.0001 | 0.64 (0.61, 0.68) |
<.0001 | 0.72 (0.69, 0.75) |
<.0001 | 0.001 |
| Non-Hispanic Whites – COPDGene | |||||||
| ILA | 0.54 (0.52, 0.62) |
<.0001 | 0.57 (0.55, 0.60) |
<.0001 | 0.58 (0.56, 0.61) |
<.0001 | 0.20 |
| Subpleural & Radiologic ILD | 0.60 (0.57, 0.63) |
<.0001 | 0.72 (0.69, 0.75) |
<.0001 | 0.75 (0.72, 0.78) |
<.0001 | 0.0006 |
| Definite Fibrosis | 0.57 (0.52, 0.62) |
<.0001 | 0.76 (0.71, 0.80) |
<.0001 | 0.76 (0.72, 0.81) |
<.0001 | 0.22 |
| Possible UIP & UIP | 0.60 (0.57, 0.63) |
<.0001 | 0.71 (0.68, 0.75) |
<.0001 | 0.75 (0.72, 0.78) |
<.0001 | 0.0008 |
| African-Americans – COPDGene | |||||||
| ILA | 0.51 (0.49, 0.52) |
0.3 | 0.58 (0.54, 0.62) |
0.0006 | 0.59 (0.55, 0.62) |
0.0001 | 0.52 |
| Subpleural & Radiologic ILD | 0.52 (0.49, 0.55) |
0.1 | 0.67 (0.62, 0.74) |
<.0001 | 0.59 (0.55, 0.62) |
<.0001 | 0.47 |
| Definite Fibrosis | 0.58 (0.50, 0.65) |
0.0005 | 0.67 (0.62, 0.74) |
<.0001 | 0.73 (0.62, 0.85) |
0.005 | 0.34 |
| Possible UIP & UIP | 0.52 (0.49, 0.56) |
0.06 | 0.69 (0.63, 0.77) |
<.0001 | 0.70 (0.63, 0.76) |
<.0001 | 0.50 |
ILA is interstitial lung abnormality; analyses using the MUC5B genotype were performed using additive genetic models.
Clinical Data includes age, sex and pack-years of tobacco exposure
ILD is interstitial lung disease
Definite Fibrosis is evidence pulmonary parenchymal architectural distortion
UIP is usual interstitial pneumonia
Figure 2.
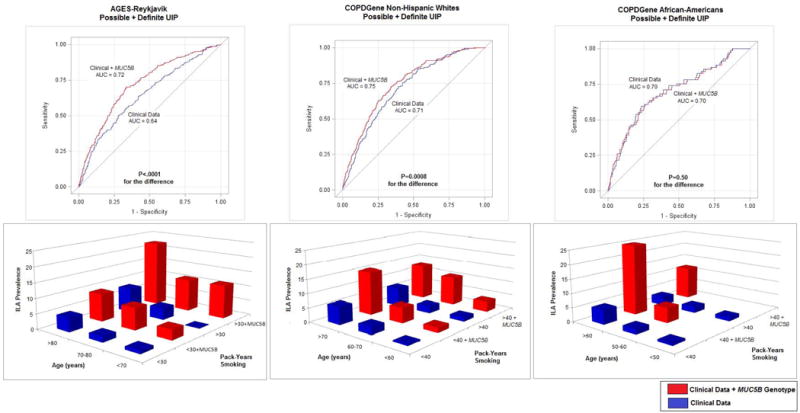
Receiver operator curves and bar graphs depicting interstitial lung abnormality (ILA), specifically possible and definite usual interstitial pneumonia (UIP) prediction, using baseline clinical information (age, sex and pack-years of smoking) and then with adding the MUC5B promoter polymorphism in each cohort. In the bar graphs the addition of MUC5B minor allele is for at least one copy of the minor allele.
Next, we sought to determine if carrying the MUC5B genotype would add to clinical characteristics and increase risk prediction for ILA subtypes. When added to models of best fitting clinical characteristics (age, sex, and pack-years of smoking), the MUC5B genotype improved risk prediction for definite fibrosis in AGES-Reykjavik (c-statistic 0.70 to 0.75, P=0.004 for comparison) but not in populations from COPDGene (c-statistic 0.76 to 0.76, P=0.22 for comparison, and c-statistic 0.70 to 0.73, P=0.34 for comparison, NHW, and in AA participants from COPDGene, respectively), (Table 3). When added to models of best fitting clinical characteristics, the MUC5B genotype improved risk prediction for having a possible UIP or UIP pattern in white populations (c-statistic 0.70 to 0.76, P=0.001 for comparison, and c-statistic 0.71 to 0.75, P=0.0008 for comparison in AGES-Reykjavik and in NHW’s from COPDGene, respectively) but not in AA participants from COPDGene (c-statistic 0.70 to 0.70, P=0.50 for comparison), (Table 3 and Figure 2). Additional models for risk prediction are presented in Table 3 and online supplementary table 5.
Survival and the MUC5B Promoter Polymorphism
Finally, we sought to determine if the MUC5B promoter polymorphism influenced survival amongst participants with ILA. Over a median follow up time of 8.3 years [Interquartile Range (IQR) 4.8, 9.6], in AGES-Reykjavik, of the 378 participants with ILA, 210 (56%) had died. Of the participants with ILA from COPDGene with both mortality and genetic information available, over a median follow up time of 5.4 years (IQR 4.6, 6.1), 59 (15%) of the 399 non-Hispanic whites with ILA had died and 13 (8%) of the 165 African-Americans with ILA had died (none of the deaths in AA participants occurred in those with the MUC5B promoter polymorphism). There was no association between the MUC5B minor allele and survival (HR=1.0, 95% CI 0.8, 1.3, P=0.95, and HR=1.2, 95% CI 0.75, 2.0, P=0.41, in the AGES-Reykjavik and NHW participants from COPDGene, respectively). Similar results were seen when ILA was subset to include only those with various ILA subtypes.
DISCUSSION
This study adds important contributions to our understanding of the extent of the associations of ILA with the MUC5B promoter polymorphism (rs35705950), and to the origins of pulmonary fibrosis in general. First, this study replicates the association between MUC5B genotype and ILA[2]. Moreover, we found that the MUC5B promoter variant is associated with fibrotic ILA in African-Americans, a population with low allelic frequency of the rs35705950. Second, this study provides important information on the consistency of ILA subtypes associated with MUC5B genotype; specifically the evidence for consistent, and minimal heterogeneity, of associations between some overlapping ILA subtypes (e.g. subpleural ILA and possible UIP). Finally, although our study provides evidence that MUC5B genotype, in addition to clinical characteristics, may help to improve risk prediction for important ILA subtypes (e.g. possible UIP or UIP patterns) in NHW populations, our study also demonstrates that MUC5B genotype will not likely be helpful in differentiating those with ILA who have better and worse survival.
The consistency of replication between the MUC5B genotype and ILA subtypes, provides further support to the growing evidence[2] that some chest CT imaging patterns can reliably identify a common phenotype that shares a genetic background noted in patients with IPF[8, 15–17]. These findings, coupled with evidence that undiagnosed research participants with ILA are more likely to have physiologic decrements[1–4, 18], elevated fibrosis biomarkers[19, 20], when followed over time can experience imaging progression[5], accelerated lung function decline,[5] and an increased rate of mortality,[5, 7] all bolster the case that some subtypes of ILA likely represent an early and/or mild case of undiagnosed pulmonary fibrosis.
Although the pathogenic processes leading to pulmonary fibrosis that result from the MUC5B promoter polymorphism are not entirely understood, some steps in this process have been elucidated. Increasing copies of the minor allele of the MUC5B promoter polymorphism are associated with increased promoter activity[21] leading to increased expression of MUC5B in the lung in general[8, 22], and specifically in the bronchiolar epithelium[21]. In IPF patients, increased expression of cilium-associated genes (including MUC5B) is associated with increased amounts of honeycombing[23]. Although it remains unclear how increased expression of MUC5B results in pulmonary fibrosis, our findings add to those noted in IPF patients which demonstrate that increased MUC5B expression in the lung tends to result in a radiologic appearance dominated by subpleural reticular infiltrates and fibrosis both in patients with IPF[24] and in those with undiagnosed interstitial abnormalities.
To properly interpret these findings it is important to consider the characteristics of the study populations. We previously demonstrated an association between the MUC5B genotype and ILA in a white population from the FHS[2]. The AGES-Reykjavik cohort, although similar to the FHS in that it is also a general population sample of NHWs, is unique in that it is entirely comprised of older adults from a geographically and genetically isolated population from Iceland[25, 26]. In contrast, COPDGene includes a population of smokers with and without COPD, and excluded those known to have significant interstitial lung disease. Consistent replication in these populations, and the minimal between cohort heterogeneity seen with specific radiologic patterns, provides further evidence that the MUC5B promoter polymorphism confers a strong risk to develop a subpleural fibrotic process that can be detected in adults regardless of mitigating factors such as differences in geography and smoking prevalence. The MUC5B promoter polymorphism, although relatively common in European and American populations (with at least one copy occurring in ~20% of Europeans and in ~11% of Americans), is rare in African populations (~ 0.6%)[27]. The prevalence of having at least one copy of the minor allele of the MUC5B promoter polymorphism in AAs (which has not previously been reported) from COPDGene was 4%. Additional studies will be needed to understand the unique factors that contribute to an ILA prevalence of 7% in this population.
Additionally, our findings demonstrate that MUC5B genotype improves risk prediction, particularly for detecting the presence of a possible UIP or UIP pattern among NHW populations. This finding is remarkable given the more modest improvements in risk that have been noted in multimarker genetic prediction models for established clinical disease entities such as breast cancer[28] and cardiovascular events,[29] and the lack of evidence that multimarker genetic profiles can improve risk prediction for subclinical atherosclerosis[30]. Our findings suggest that MUC5B genotype, in addition to important clinical variables, could be helpful in determining those most likely to develop an early stage of pulmonary fibrosis. In contrast, our findings do not suggest that MUC5B genotype will help to identify those with ILA who have an improved survival as has been noted in patients with IPF[31]. Instead our findings demonstrate that MUC5B genotype is important, but not the only, factor that can increase the risk for ILA (and ILA progression)[5] which when present, can lead to an increased rate of mortality[7].
This study has several limitations. First, although we were able to demonstrate similar associations between the MUC5B promoter polymorphism and specific radiologic subtypes of ILA in AAs from COPDGene, the smaller sample size and lower prevalence of the minor allele, may have limited the statistical power to demonstrate an improvement in risk prediction. Second, while MUC5B genotype is associated with a possible UIP pattern across all populations, the magnitude of this association is less than that observed in patients with clinically identified IPF[8, 16]. Finally, we cannot rule out the possibility that small sample size, within some ILA subtypes specifically, could have limited our statistical power to detect an association between MUC5B genotype and survival in subgroup analyses.
In conclusion, our study demonstrates that the MUC5B promoter polymorphism is associated with undiagnosed chest CT findings consistent with an early stage of pulmonary fibrosis. Our study also provides some specificity for the associations by demonstrating that MUC5B genotype is associated with subpleural ILA and a possible UIP pattern, but not with centrilobular predominant abnormalities. Additionally, the MUC5B genotype may help to predict the presence of specific subtypes of ILA on chest CT. Although it is not known if treating early stages of pulmonary fibrosis will help to prevent the accelerated pulmonary function decline[5] and early mortality[7] with which they are associated, the fact that MUC5B genotype may improve risk detection for a possible UIP pattern suggests a path forward.
Supplementary Material
Acknowledgments
COPDGene is supported by NIH Award Number R01 HL089897 and R01 HL089856.
Funding
Dr. Putman is supported by NIH Grant Number T32 HL007633. Dr. G. Gudmundsson is supported by Oddur Olafsson Fund, project grant 141513-051 from the Icelandic Research Fund and Landspitali Scientific Fund A-2015-030 and A-2016-023. Dr. Nishino is supported by NIH Grant Number K23 CA157631. Dr. Ross is supported by NIH Grant Number K25 HL130637. Dr. San José Estépar is supported by NIH Grant Numbers: K25 HL104085 and R01 HL116473. Dr. Miller is supported by NIH Grant Number T32 HL007633. Dr. Yamada is supported by project grant 141513-051 from the Icelandic Research Fund and Landspitali Scientific Fund A-2015-030. Dr. El-Chemaly is supported by NIH Grant Number R01 HL130275. Dr. Raby is supported by NIH Grant Numbers: U01 AI095219, R01 HL0866601, R01 HL118455, R01 HL123546, R01 HL130974, and P01 HL132825. Dr. Cho is supported by NIH Grant Number R01 HL113264. Dr. Rosas is supported by NIH Grant Numbers: R01 HL130974, R01 HL129920, and P01 HL114501. Dr. Washko is supported by NIH Grant Number: R01 HL122464 and R01 HL116473. Dr. Schwartz is supported by NIH Grant Numbers: R25 ES025476, P01 HL092870, R01 HL097163, R33 HL120770, UH2 HL123442, and the Veterans Administration Grant Number I01 BX001534. Dr. Silverman is supported by NIH Grants: R01 HL089856, R01 HL113264, R33 HL120794, P01 HL114501. The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by NIA grant: 27120120022C, NIH contracts N01-AG-1-2100 and HHSN27120120022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). Dr. Gudnason is supported by NIA grant: 27120120022C and project grant 141513-051 from the Icelandic Research Fund. Dr. Hunninghake and this work were supported by NIH Grant Numbers: R01 HL111024, R01 HL130974 and project grant 141513-051 from the Icelandic Research Fund.
Obtained Funding: G. Gudmundsson, Gudnason, Hunninghake, Silverman
Footnotes
Author Contributions:
Study Design: G. Gudmundsson, Gudnason, Hatabu, Hunninghake, Rosas, Schwartz, Silverman, Washko
Acquisition, analysis or interpretation of the data: Araki, Aspelund, Cho, El-Chemaly, Eiríksdottír, E. Gudmundsson, G. Gudmundsson, Gudnason, Hatabu, Hunninghake, Nishino, Putman, Ross, San Jose Estepar, Schwartz, Sigurdsson, Silverman, Washko, Yamada, Yanagawa
Critical Revision of the manuscript for important intellectual content: Araki, Aspelund, Cho, El-Chemaly, Eiríksdottír, E. Gudmundsson, G. Gudmundsson, Gudnason, Harris, Hatabu, Hunninghake, Launer, Miller, Nishino, Putman, Raby, Rosas, Ross, San Jose Estepar, Schwartz, Sigurdsson, Silverman, Tomiyama, Washko, Yamada, Yamagawa
Statistical Analysis: Hunninghake and Putman
This paper is subject to the NIH public access policy: http://www.nih.gov/about/publicaccess/Finalpublicaccessimplementation031505.htm.
Take Home Message: MUC5B genotype is associated with specific subtypes of ILA, with varying heterogeneity in the underlying populations.
References
- 1.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D’Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Investigators CO Lung volumes and emphysema in smokers with interstitial lung abnormalities. The New England journal of medicine. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Murphy E, Steele MP, Loyd JE, Schwarz MI, Fingerlin TE, Rosas IO, Washko GR, O’Connor GT, Schwartz DA. MUC5B promoter polymorphism and interstitial lung abnormalities. The New England journal of medicine. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respiratory medicine. 2010;104(11):1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Schwartz DA, Rosas IO, Washko GR, O’Connor GT, Hunninghake GM. Development and Progression of Interstitial Lung Abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Hunninghake GM, Investigators CO Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185(7):756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O’Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O’Connor GT, Washko GR, Rosas IO, Hunninghake GM, Evaluation of CLtIPSEI, Investigators CO Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA. 2016;315(7):672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. The New England journal of medicine. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American journal of epidemiology. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. Copd. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San Jose Estepar R, Silverman EK, Rosas IO, Hatabu H. Identification of early interstitial lung disease in smokers from the COPDGene Study. Academic radiology. 2010;17(1):48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ, Fibrosis AEJACoIP An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JC, Estepar RS, Diaz A, Westin CF, Kikinis R, Silverman EK, Washko GR. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Medical image computing and computer-assisted intervention: MICCAI International Conference on Medical Image Computing and Computer-Assisted Intervention. 2009;12(Pt 2):690–698. doi: 10.1007/978-3-642-04271-3_84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, Richards TJ, Juan-Guardela BM, Vij R, Han MK, Martinez FJ, Kossen K, Seiwert SD, Christie JD, Nicolae D, Kaminski N, Garcia JG. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. The lancet Respiratory medicine. 2013;1(4):309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189(7):770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, Collard HR, Wolters PJ, Bradford WZ, Kossen K, Seiwert SD, du Bois RM, Garcia CK, Devine MS, Gudmundsson G, Isaksson HJ, Kaminski N, Zhang Y, Gibson KF, Lancaster LH, Cogan JD, Mason WR, Maher TM, Molyneaux PL, Wells AU, Moffatt MF, Selman M, Pardo A, Kim DS, Crapo JD, Make BJ, Regan EA, Walek DS, Daniel JJ, Kamatani Y, Zelenika D, Smith K, McKean D, Pedersen BS, Talbert J, Kidd RN, Markin CR, Beckman KB, Lathrop M, Schwarz MI, Schwartz DA. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle TW, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, Hatabu H, Rosas IO, Hunninghake GM. Interstitial Lung Abnormalities and Reduced Exercise Capacity. Am J Respir Crit Care Med. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, Gochuico BR, Hinckley Stukovsky K, Kaufman JD, Hrudaya Nath P, Newell JD, Jr, Palmer SM, Rabinowitz D, Raghu G, Sell JL, Sieren J, Sonavane SK, Tracy RP, Watts JR, Williams K, Kawut SM, Lederer DJ. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016 doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho JE, Gao W, Levy D, Santhanakrishnan R, Araki T, Rosas IO, Hatabu H, Latourelle JC, Nishino M, Dupuis J, Washko GR, O’Connor GT, Hunninghake GM. Galectin-3 is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201509-1753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, Lara AR, Schwarz MI, Evans CM, Schwartz DA. MUC5B Promoter Variant rs35705950 Affects MUC5B Expression in the Distal Airways in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;193(4):464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kropski JA, Pritchett JM, Zoz DF, Crossno PF, Markin C, Garnett ET, Degryse AL, Mitchell DB, Polosukhin VV, Rickman OB, Choi L, Cheng DS, McConaha ME, Jones BR, Gleaves LA, McMahon FB, Worrell JA, Solus JF, Ware LB, Lee JW, Massion PP, Zaynagetdinov R, White ES, Kurtis JD, Johnson JE, Groshong SD, Lancaster LH, Young LR, Steele MP, Phillips JA, III, Cogan JD, Loyd JE, Lawson WE, Blackwell TS. Extensive phenotyping of individuals at risk for familial interstitial pneumonia reveals clues to the pathogenesis of interstitial lung disease. Am J Respir Crit Care Med. 2015;191(4):417–426. doi: 10.1164/rccm.201406-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, Rosen R, Neidermyer AJ, McKean DF, Groshong SD, Cool C, Cosgrove GP, Lynch DA, Brown KK, Schwarz MI, Fingerlin TE, Schwartz DA. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax. 2013;68(12):1114–1121. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung JH, Peljto AL, Chawla A, Talbert JL, McKean DF, Rho BH, Fingerlin TE, Schwarz MI, Schwartz DA, Lynch DA. CT Imaging Phenotypes of Pulmonary Fibrosis in the MUC5B Promoter Site Polymorphism. Chest. 2016;149(5):1215–1222. doi: 10.1016/j.chest.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor R, Bryant J, Gudnason V, Sigurdsson G, Humphries S. A study of familial hypercholesterolaemia in Iceland using RFLPs. J Med Genet. 1989;26(8):494–498. doi: 10.1136/jmg.26.8.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heutink P, Oostra BA. Gene finding in genetically isolated populations. Human molecular genetics. 2002;11(20):2507–2515. doi: 10.1093/hmg/11.20.2507. [DOI] [PubMed] [Google Scholar]
- 27.Online Medelian Inheritance in Man. Mucin 5 sB, tracheobronchial: MUC5B. OMIM no. 600770. (http://useastensemblorg/Homo_sapiens/Variation/Population?db=core;r=11:1219491-1220491;v=rs35705950;vdb=variation;vf=10314659).
- 28.Wacholder S, Hartge P, Prentice R, Garcia-Closas M, Feigelson HS, Diver WR, Thun MJ, Cox DG, Hankinson SE, Kraft P, Rosner B, Berg CD, Brinton LA, Lissowska J, Sherman ME, Chlebowski R, Kooperberg C, Jackson RD, Buckman DW, Hui P, Pfeiffer R, Jacobs KB, Thomas GD, Hoover RN, Gail MH, Chanock SJ, Hunter DJ. Performance of common genetic variants in breast-cancer risk models. N Engl J Med. 2010;362(11):986–993. doi: 10.1056/NEJMoa0907727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kathiresan S, Melander O, Anevski D, Guiducci C, Burtt NP, Roos C, Hirschhorn JN, Berglund G, Hedblad B, Groop L, Altshuler DM, Newton-Cheh C, Orho-Melander M. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358(12):1240–1249. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 30.Hernesniemi JA, Seppala I, Lyytikainen LP, Mononen N, Oksala N, Hutri-Kahonen N, Juonala M, Taittonen L, Smith EN, Schork NJ, Chen W, Srinivasan SR, Berenson GS, Murray SS, Laitinen T, Jula A, Kettunen J, Ripatti S, Laaksonen R, Viikari J, Kahonen M, Raitakari OT, Lehtimaki T. Genetic profiling using genome-wide significant coronary artery disease risk variants does not improve the prediction of subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study and the Health 2000 Survey–a meta-analysis of three independent studies. PLoS One. 2012;7(1):e28931. doi: 10.1371/journal.pone.0028931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JG, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, Gibson KF, Seibold MA, Brown KK, Talbert JL, Markin C, Kossen K, Seiwert SD, Murphy E, Noth I, Schwarz MI, Kaminski N, Schwartz DA. Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA. 2013;309(21):2232–2239. doi: 10.1001/jama.2013.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


