ABSTRACT
Cystic fibrosis (CF) is an autosomal recessive genetic disorder characterized by dysfunction of the CFTR gene. It is a multisystem disease that most often affects White individuals. In recent decades, various advances in the diagnosis and treatment of CF have drastically changed the scenario, resulting in a significant increase in survival and quality of life. In Brazil, the current neonatal screening program for CF has broad coverage, and most of the Brazilian states have referral centers for the follow-up of individuals with the disease. Previously, CF was limited to the pediatric age group. However, an increase in the number of adult CF patients has been observed, because of the greater number of individuals being diagnosed with atypical forms (with milder phenotypic expression) and because of the increase in life expectancy provided by the new treatments. However, there is still great heterogeneity among the different regions of Brazil in terms of the access of CF patients to diagnostic and therapeutic methods. The objective of these guidelines was to aggregate the main scientific evidence to guide the management of these patients. A group of 18 CF specialists devised 82 relevant clinical questions, divided into five categories: characteristics of a referral center; diagnosis; treatment of respiratory disease; gastrointestinal and nutritional treatment; and other aspects. Various professionals working in the area of CF in Brazil were invited to answer the questions devised by the coordinators. We used the PubMed database to search the available literature based on keywords, in order to find the best answers to these questions.
Keywords: Cystic fibrosis/diagnosis, Cystic fibrosis/therapy, Cystic fibrosis/complications, Practice guideline
RESUMO
A fibrose cística (FC) é uma doença genética autossômica recessiva caracterizada pela disfunção do gene CFTR. Trata-se de uma doença multissistêmica que ocorre mais frequentemente em populações descendentes de caucasianos. Nas últimas décadas, diversos avanços no diagnóstico e tratamento da FC mudaram drasticamente o cenário dessa doença, com aumento expressivo da sobrevida e qualidade de vida. Atualmente, o Brasil dispõe de um programa de ampla cobertura para a triagem neonatal de FC e centros de referência distribuídos na maior parte desses estados para seguimento dos indivíduos. Antigamente confinada à faixa etária pediátrica, tem-se observado um aumento de pacientes adultos com FC tanto pelo maior número de diagnósticos de formas atípicas, de expressão fenotípica mais leve, assim como pelo aumento da expectativa de vida com os novos tratamentos. Entretanto, ainda se observa uma grande heterogeneidade no acesso aos métodos diagnósticos e terapêuticos para FC entre as diferentes regiões brasileiras. O objetivo dessas diretrizes foi reunir as principais evidências científicas que norteiam o manejo desses pacientes. Um grupo de 18 especialistas em FC elaborou 82 perguntas clínicas relevantes que foram divididas em cinco categorias: características de um centro de referência; diagnóstico; tratamento da doença respiratória; tratamento gastrointestinal e nutricional; e outros aspectos. Diversos profissionais brasileiros atuantes na área da FC foram convidados a responder as perguntas formuladas pelos coordenadores. A literatura disponível foi pesquisada na base de dados PubMed com palavras-chave, buscando-se as melhores respostas às perguntas dos autores.
Descritores: Fibrose cística/diagnóstico, Fibrose cística/terapia, Fibrose cística/complicações, Guia de prática clínica
INTRODUCTION
Cystic fibrosis is an autosomal recessive genetic disorder characterized by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a protein that regulates chloride transmembrane conductance. It is a multisystem disease that most often affects White individuals. In Brazil, the incidence of cystic fibrosis is estimated to be 1 in 7,576 live births; however, there are regional differences, with higher values being found in the southern states. 1
In recent decades, various advances in the diagnosis and treatment of cystic fibrosis have drastically changed the scenario of this disease, resulting in a significant increase in survival and a gain in quality of life. In Brazil, the current neonatal screening program for cystic fibrosis has broad coverage, and most of the Brazilian states have referral centers for the follow-up of individuals with the disease. Previously, cystic fibrosis was limited to the pediatric age group. However, an increase in the number of adult patients with cystic fibrosis has been observed, because of the greater number of individuals being diagnosed with atypical forms (with milder phenotypic expression) and because of the increase in life expectancy provided by the new treatments. 2 - 4 However, there is still great heterogeneity among the different regions of Brazil in terms of the access of patients with cystic fibrosis to diagnostic and therapeutic methods. The objective of this publication was to aggregate the main scientific evidence to guide the management of patients with cystic fibrosis, this body of evidence being compiled by the main health professionals involved in caring for this disease in Brazil.
METHODS
A group of 18 cystic fibrosis specialists (coordinators) devised 82 relevant clinical questions, divided into five categories: characteristics of a referral center; diagnosis; treatment of respiratory disease; gastrointestinal and nutritional treatment; and other aspects. Various professionals working in the area of cystic fibrosis in Brazil were invited to answer the questions devised by the coordinators of the guidelines.
We used the PubMed database to search the available literature based on keywords, in order to find the best answers to these questions. In addition, manual searches of references in articles or books were performed. The Oxford Centre for Evidence-Based Medicine guidelines were used to classify the level of evidence for the questions regarding the treatment chapters. The guidelines include a classification system for levels of evidence of studies, with levels of evidence ranging from “1” (highest level) to “5” (lowest level). The classification system was simplified in 2011 in order to facilitate its clinical application. Chart 1A (JBP online appendix-http://jornaldepneumologia.com.br/detalhe_anexo.asp?id=51) provides further details on the current Oxford classification system.
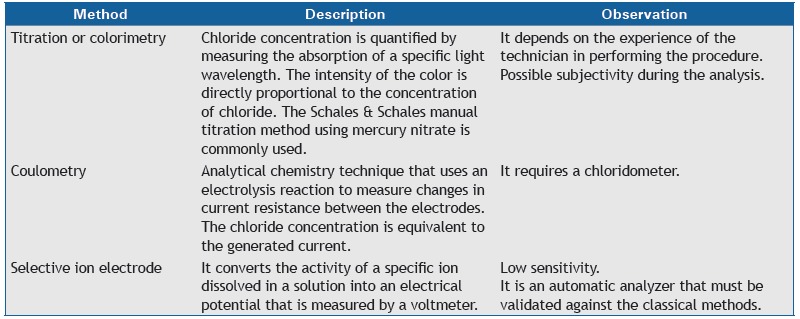
Chart 1. Methods of quantitative of sweat chloride determination.
A total of 2,352 publications were identified using the keyword search strategy, manual searches, and reference suggestions made by the authors. A total of 243 articles were selected for the present paper.
The first version of the text was written between March and August of 2016. The coordinators of each area were responsible for the validation of the level of evidence classification. In controversial cases, the questions were brought to a consensus meeting of coordinators on September 24, 2016. The final version was reviewed by the national coordinators (the first two authors) and sent to the editor of the JBP in February of 2017.
CHARACTERISTICS OF A REFERRAL CENTER
How important is a referral center in the care of patients with cystic fibrosis?
The complexity of cystic fibrosis and the peculiarities of its treatment result in the need for specialized treatment centers. 5 There is evidence that treatment at specialized referral centers, which have a multidisciplinary team, results in better clinical results, with an impact on prognosis. 6 , 7
What is a referral facility and what is a referral center?
A referral center is defined as one that treats at least 50 patients regularly. It should have a structure that meets the needs related to diagnosis, follow-up, and treatment.
A referral facility is one that treats fewer than 50 patients, and it can have a less complex structure. It should be affiliated with a referral center for the purposes of continuing education and of supplementing any needs. 5
How important is a multidisciplinary team? What would be the composition of such a team?
Given that cystic fibrosis is characterized by chronic multisystem involvement, it requires a multidisciplinary care model. 5 The care provided by a multidisciplinary team enables more comprehensive and effective treatments, resulting in an increased patient life expectancy. 5 , 8 , 9 The minimum multidisciplinary team for treating patients with cystic fibrosis should consist of the following professionals: pediatricians (when treatment is provided to children and adolescents); pulmonologists; gastroenterologists; physical therapists; nutritionists; nurses; psychologists; pharmacists; and social workers.
Are there differences between pediatric and adult centers? Are there advantages to planning for transitioning from pediatric to adult care?
Pediatric cystic fibrosis centers are quite different from adult cystic fibrosis centers. Adults have control and autonomy over their care. Pediatric centers need to meet demands that are characteristic of childhood, both in terms of structure and health professionals. Adult centers need resources to treat cases of greater complexity (comorbidities and different and more frequent complications, as well as pregnancy). 10
Transitioning an adolescent patient into an adult center is challenging, and there is evidence that transition programs optimize the process of transfer to the adult center. 11 - 15
What should the referral center infrastructure be like? What are the basic ancillary tests?
Referral centers should have multidisciplinary teams and resources so that they can provide accurate diagnoses and comprehensive care to patients with cystic fibrosis. They should be able to treat all cystic fibrosis complications, or provide referral to treatment, and should work in conjunction with facilities that are closer to the place of residence of the patients. 5 , 16 Patients should have 24-h/day access to the center or to emergency facilities affiliated with the center. 16
Each referral center should have or should ensure access to:
A laboratory for conducting tests to confirm the diagnosis of cystic fibrosis: sweat testing and/or CFTR gene mutation analysis
A pulmonary function laboratory
A microbiology laboratory with experience in and resources for identifying typical cystic fibrosis pathogens
A radiology department with CT
A clinical pathology laboratory with the capacity to perform routine tests, including hematologic tests, liver and kidney function tests, serology, and determination of proteins, vitamins, and immunoglobulins.
How important is microbiological segregation? How should it be done?
There is ample evidence that pathogen transmission can occur among individuals with cystic fibrosis, especially via droplets and contact. It can involve virulent strains, worsening disease progression. Infection control and prevention measures have been effective in decreasing pathogen transmission. Patient segregation should be instituted inside and outside the hospital setting to prevent cross infection. Cystic fibrosis centers should provide adequate structure and have a clear Infection control and prevention policy, including separate days of treatment for patients or use of different treatment spaces on the basis of patient colonization. 5 , 17 - 19
How important is commitment to care, research, and teaching?
A cystic fibrosis center should be committed to active participation in clinical and translational research, enabling patient participation in clinical trials. Education, research, and contribution to cystic fibrosis registries should be preferably performed by all centers. The various members of the multidisciplinary team should play an active role in research and education. Their work contributes to increasing and disseminating specialized knowledge, which plays a significant role in improving the quality of care. 5
What are the advantages of cooperation with cystic fibrosis patient/parent associations and with the Brazilian Cystic Fibrosis Study Group?
Cystic fibrosis patient/parent associations aim at defending the interests of this group of individuals, which includes making the disease known and improving diagnosis and treatment, in order to increase survival, improve quality of life, and integrate patients into society. 5 In North America and Europe, some of these associations still play an important role in promoting and funding scientific research and in registering patients.
In Brazil, bringing cystic fibrosis patient/parent associations closer to health professionals working in cystic fibrosis (currently represented by the Brazilian Cystic Fibrosis Study Group) would offer great advantages that could improve the current situation, such as aid in the inclusion of all Brazilian patients in the national registry (Brazilian Cystic Fibrosis Registry) and monitoring of the availability of medications in the various Brazilian states, in addition to the joining of forces to submit to the Federal Government a new (more comprehensive) directive on the care of the cystic fibrosis patient.
DIAGNOSIS
How does one confirm the diagnosis of cystic fibrosis after positive newborn screening?
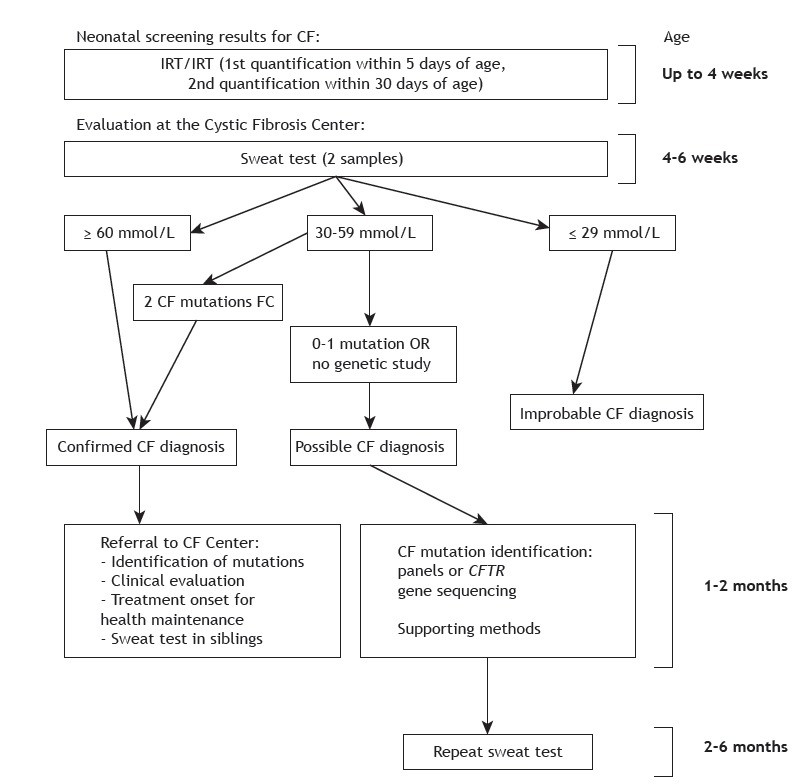
The cystic fibrosis newborn screening algorithm used in Brazil is based on two determinations of immunoreactive trypsinogen levels, the second of which is performed within 30 days of life. If screening is positive (i.e., two positive determinations), sweat testing is performed to confirm or rule out cystic fibrosis. Sweat chloride concentrations ≥ 60 mmol/L, as measured by quantitative methods, in two samples, confirm the diagnosis. Diagnostic alternatives are detection of two cystic fibrosis-related mutations and CFTR functional tests. Figure 1 shows a flowchart summarizing how infants with positive newborn screening results should be managed. 20 , 21
Figure 1. Management of cases with positive neonatal screening for cystic fibrosis. CF: cystic fibrosis; and IRT: immunoreactive trypsinogen. Adapted from Farrel et al. 21 .
Does a positive or negative newborn screening result confirm or rule out the diagnosis of cystic fibrosis?
No. Newborn screening for cystic fibrosis identifies newborns at risk for the disease, but does not confirm the diagnosis. The rate of false-positive results with the algorithm based on measurement of immunoreactive trypsinogen levels is quite high. Conversely, a negative newborn screening result does not rule out the diagnosis. 22 , 23
After confirmation of the diagnosis of cystic fibrosis in patients with positive newborn screening results, when should the patients be referred to a cystic fibrosis referral center?
Immediately after diagnosis, because cystic fibrosis requires early multidisciplinary management in order to maintain normal nutritional status and timely treat respiratory infections. 20 , 23
What are the steps involved in sweat testing? How does one ensure the quality of sweat testing?
Chart 2A (JBP online appendix) summarizes the steps that should be followed when performing sweat testing. Table 1 shows the reference values.
Chart 2. Benefits from the study of CFTR gene mutations.
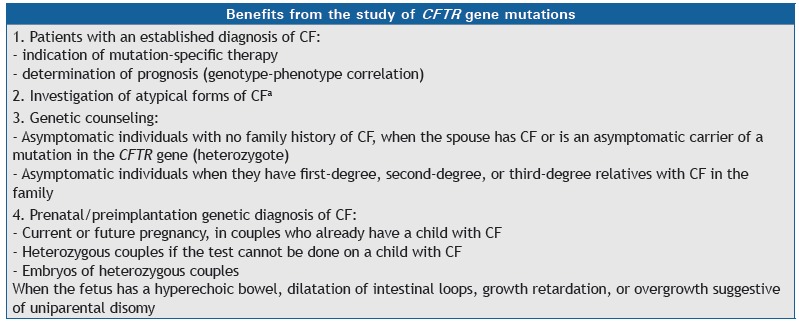
CF: cystic fibrosis. aAtypical forms of CF: symptoms consistent with CF and intermediate results in the sweat chloride test.
Table 1. Reference values for sweat test.
| Result | Chloride, mmol/L | Electrical conductivity, mmol/L |
|---|---|---|
| Normal | < 30 | < 60 |
| Intermediate | 30-59 | 60-90 |
| Positivea | ≥ 60 | > 90 |
Quantitative chloride analysis in sweat should be carried out on a different day in order to confirm the result.
It is recommended that laboratories qualified to perform sweat testing should have internal and external quality control and should perform at least 100 tests per year (at least 10 tests per year per technician). The percentage of insufficient sweat samples should not exceed 5% of the total samples collected. 24 - 26
What are the main approved methods of quantitative sweat chloride determination?
Chart 1 describes the main methods of chloride determination, all of which must be validated in each laboratory before use. 24 , 25
What is the role of sweat conductivity testing?
Despite the high level of agreement between sweat conductivity results and sweat chloride concentrations, sweat conductivity testing is still considered a screening test. 26 It is recommended that a patient with a sweat conductivity result greater than or equal to 50 mmol/L should undergo quantitative testing. Sweat conductivity testing has the advantages of being easy to use and yielding immediate results. 24 , 27 , 28
What are the minimum criteria for a laboratory to perform CFTR mutation studies?
Certification by the Brazilian National Health Oversight Agency
Capability to perform DNA extraction with different methods and from different sample types
Ability to identify the F508del mutation and other more prevalent mutations
Availability to perform CFTR mutation panel analysis and/or complete CFTR sequencing, either in its facilities, or by referral to other laboratories
Capability to interpret and report pathogenic variants
Should all patients with cystic fibrosis undergo genetic testing? How important is it to undergo genetic testing?
Yes, the identification of mutations in the CFTR gene has implications for prognosis and family planning, allowing the diagnosis of cystic fibrosis (Chart 2). In addition, there are drugs that act on specific mutations (CFTR protein correctors and potentiators), some of which have been approved in various countries, whereas others are in development. 21 , 29 - 32
What mutation panel should be investigated?
The investigation of mutations in the CFTR gene is described in Chart 3. 31 - 35
Chart 3. Stepwise molecular analysis for the identification of CFTR mutations.
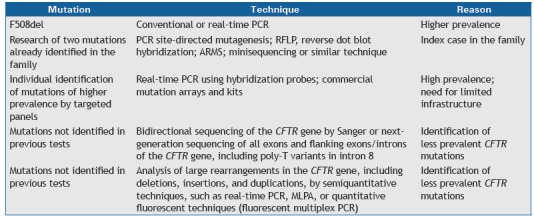
PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism; ARMS: amplification refractory mutation system; and MLPA, multiplex ligation-dependent probe amplification.
When are CFTR functional tests indicated?
CFTR functional tests are indicated when sweat testing and genetic analysis are inconclusive. In essence, these tests assess CFTR protein function by measurement of chloride transport. Currently, nasal potential difference and intestinal current measurements are internationally standardized. Other promising tests, such as assessment of CFTR function by evaporimetry and by sweat gland potential difference measurement, are being studied. 36 , 37
TREATMENT OF RESPIRATORY DISEASE
What types of respiratory samples are most appropriate, how are they obtained, and how important are they?
Respiratory secretion samples are essential for follow-up of chronic bacterial infection of the airways in patients with cystic fibrosis, as well as for identification of opportunistic infections and as a follow-up method for therapeutic interventions. Expectorated sputum is the specimen of choice. For children who cannot expectorate, collect oropharyngeal cough swabs (tonsillar region and soft palate), nasopharyngeal aspirates, secretion following inhalation of 5% hypertonic saline solution, or bronchoalveolar lavage fluid. These samples should be delivered to the laboratory immediately or kept under refrigeration for up to 3 h. 38 , 39
(Level of evidence: 4)
When should the samples be collected?
The samples should be collected at visits (with a maximum interval of 3 months), during exacerbations, and following treatment to eradicate the infection. Annual screening for mycobacteria and fungi is recommended for patients who cannot expectorate or for those with an unfavorable clinical course. 40
(Level of evidence: 5)
What are the routine culture methods and media?
Bronchoalveolar lavage fluid specimens must be quantitatively cultured. The recommended culture media for routine microbiological investigation in cystic fibrosis are as follows:
Blood agar: universal for routine microbiological investigations
Mannitol agar: selective for Staphylococcus aureus
MacConkey agar: for gram-negative bacilli (including Pseudomonas aeruginosa, Achromobacter spp., and Stenotrophomonas spp.)
Burkholderia cepacia complex-selective agar
Chocolate agar for Streptococcus pneumoniae and Haemophilus influenzae
Sabouraud agar - for fungi, including Aspergillus spp. - supplemented with chloramphenicol or gentamicin
Liquid culture media, depending on the automation available, and a solid medium, such as Lowenstein-Jensen agar. For non-tuberculosis mycobacteria, blood agar and Burkholderia cepacia selective agar can also be used provided that these media are incubated for 14 days. 39 , 41 - 45
(Level of evidence: 5)
What are the methods of bacterial identification?
Phenotypic methods: typical S. aureus, P. aeruginosa, and Stenotrophomonas maltophilia colonies are easily recognized, and few tests are needed.
Commercial, non-automated phenotypic kits: when associated with typical characteristics, they can be used for the identification of S. aureus and some glucose-nonfermenting gram-negative bacilli, such as P. aeruginosa, S. maltophilia, and Achromobacter spp., but are not suitable for the identification of B. cepacia complex, Burkholderia gladioli, Pandoraea spp., or Ralstonia spp.
Automated methods: they are not recommended for the identification of most glucose-nonfermenting gram-negative bacilli.
Molecular tests: they are recommended for the characterization of Achromobacter spp., B. cepacia complex, and the genera Ralstonia, Cupriavidus and Pandoraea.
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS): it represents a rapid alternative, but has limitations, especially in identifying glucose-nonfermenting gram-negative bacilli. 42 , 43 , 46
(Level of evidence: 5 for all methods, except MALDI-TOF MS for the identification of glucose-nonfermenting gram-negative bacilli-level of evidence: 2)
What is the role of pulmonary function testing in the management of patients with cystic fibrosis?
Spirometry should be performed starting at age 5 years at every clinical visit or at least twice a year. Testing with and without bronchodilators is recommended. Washout techniques, with determination of the lung clearance index, have increasing and promising use in identifying early lung disease.
Studies have shown that FEV1 is essential for assessing the course and progression of cystic fibrosis, as well as for early detection of acute pulmonary exacerbations, being correlated with quality of life. FEF25-75% should also be taken into consideration, since it may be altered earlier. Whole-body plethysmography and oscillometry can complement the functional assessment. 9 , 47 - 50
(Level of evidence: 5)
What imaging tests should be performed in patients with cystic fibrosis? How often?
Chest X-ray is the most widely used test in the evaluation of patients with cystic fibrosis and is correlated with pulmonary function testing in detecting disease progression. 51 , 52
Chest HRCT is more accurate in the diagnosis and follow-up of lung lesions in individuals of all ages, including children with normal pulmonary function. 53 - 55 This benefit is questionable in infants, and there are technical obstacles inherent to this age group. 56 Magnetic resonance imaging of the chest has advanced in recent years and may become a future option because it is a radiation-free method. 57
Although there is no consensus regarding the frequency of imaging tests, an annual chest X-ray is recommended. In addition, it is suggested that, in the presence of clinical, functional, or radiological deterioration, a chest HRCT should be performed. Periodic follow-up with chest HRCT every 2 to 4 years may be indicated on a case-by-case basis. In cases of pulmonary exacerbation in cystic fibrosis, chest X-ray and chest HRCT can be used, always considering the use of the lowest radiation dose possible. 58 , 59
(Level of evidence: 2 for chest HRCT in individuals of all ages, except infants) (Level of evidence: 5 for chest X-ray and magnetic resonance imaging)
How important are nebulizers in the treatment of lung disease in cystic fibrosis?
The daily treatment of lung disease in cystic fibrosis includes nebulization of various medications that are key to maintaining lung health, and an inhaler system is essential for all patients with cystic fibrosis. 60 - 62
(Level of evidence: 5)
What inhaler system should be used for each type of inhalation therapy in cystic fibrosis?
Matching a substance to be inhaled with the right type of inhaler system is essential for ensuring the efficacy of treatment. Given the great variability of devices, it is recommended that the inhaler systems tested in the clinical trials of the medications should be used. 63 , 64
The following types are often used for each therapy 64 , 65 :
Ultrasonic nebulizers: hypertonic saline
Air-jet nebulizers: tobramycin; colistimethate; dornase alfa; and hypertonic saline
Active vibrating mesh nebulizers: tobramycin; colistimethate; dornase alfa; and aztreonam
Passive vibrating mesh nebulizers that adjust to the patient’s breathing pattern: tobramycin and colistimethate
(Level of evidence: 2)
What care should be given to inhalation therapy and chest physiotherapy devices?
Devices for the treatment of lung disease in cystic fibrosis include nebulizers and equipment used in chest physiotherapy for secretion removal. Bacterial contamination of nebulizers of patients with cystic fibrosis has been described, and educational programs on cleaning and disinfection of these devices have an impact on this situation. Cleaning after each use and daily disinfection by boiling, 70-90% alcohol, isopropyl alcohol, or 3% hydrogen peroxide are recommended. 17 , 66 - 69
(Level of evidence: 3)
What chest physiotherapy techniques are indicated in the treatment of lung disease?
Chest physiotherapy techniques should be performed daily after diagnosis in all patients with cystic fibrosis. 70 Chest physiotherapy has proven clinical benefits when compared with no intervention; however, there is no evidence of the superiority of one technique over the other. Patient preference is an essential factor for adherence to treatment, but the use of devices such as positive expiratory pressure masks and oscillatory positive expiratory pressure devices such as the Flutter®, the Shaker®, and the Acapella® is of great value and gives the patient independence. 71 The use of high-frequency chest wall oscillation devices, despite also giving the patient independence, was found to be inferior to the use of positive expiratory pressure masks in a recent study. 72 Noninvasive ventilation may be used as an adjunct to airway clearance therapy and in patients with advanced disease and hypercapnic respiratory failure. 73 - 76
(Level of evidence: 2 for chest physiotherapy)
(Level of evidence: 2 for the superiority of positive expiratory pressure masks vs. high-frequency chest wall oscillation devices)
(Level of evidence: 2 for noninvasive ventilation vs. no noninvasive ventilation as an adjuvant in the treatment of patients with advanced disease and hypercapnia)
What is the role of exercise in cystic fibrosis?
Exercise (aerobic and anaerobic) can aid in functional and postural outcomes, as well as in the self-esteem of patients with cystic fibrosis. An exercise frequency of 3-5 times a week and an exercise duration of 20-30 min are recommended, with benefits being observed from 6 weeks onward. Exercise should be part of the recommendations for patients with cystic fibrosis, including during hospitalizations. Physical activity does not replace chest physiotherapy. 77 - 82
(Level of evidence: 2)
What are the indications for the use of dornase alfa and what is its dosing schedule?
Inhaled dornase alfa has proven efficacy in cystic fibrosis as demonstrated by improvement in pulmonary function and quality of life, as well as by reduction in the number of respiratory exacerbations. 83 - 89 It is recommended starting at age 6 years in patients with lung disease at any stage. 83 , 87 , 90 The recommended dose is 2.5 mg once daily, with an appropriate nebulizer. Alternate-day administration may be considered in stable patients, 91 , 92 and twice-daily administration may be considered in patients with severe disease. 102 Inhaled dornase alfa can be used at any time, at least 30 min before chest physiotherapy. 93 , 94
(Level of evidence: 1)
When should dornase alfa be used in children under 6 years of age?
The use of dornase alfa should be considered in younger patients with persistent respiratory symptoms or with evidence of early lung disease (bronchiectasis, for example). 40 , 95 - 97
(Level of evidence: 2)
What is the role of hypertonic saline and mannitol? What are their recommended concentrations?
Hypertonic saline solution and mannitol are mucokinetic substances. They function as moisturizers on the airway surface, as osmotic agents, changing the rheological properties of mucus.
Twice-daily administration of 7% hypertonic saline solution reduces the number of respiratory exacerbations and produces improvement in pulmonary function and quality of life. Long-term studies are needed to determine whether there is sustained improvement. 87 , 98 - 100
Mannitol is available as dry-powder for inhalation (400 mg twice daily). Its use is associated with reduced nebulizer treatment time, clinical improvement, and pulmonary function improvement. 101 - 103 The use of mannitol is safe and well tolerated but should be preceded by the use of inhaled bronchodilators, given that they can act as irritating substances. Both are complementary approaches to dornase alfa therapy.
(Level of evidence: 1 for hypertonic saline and for mannitol)
What should P. aeruginosa eradication therapy be like?
Eradication therapy in cases of first acquisition of P. aeruginosa or early infection with P. aeruginosa aims to eradicate the bacterium and delay chronic infection. There are various therapeutic strategies, none being superior to the other. The most widely recommended strategy is to use inhaled tobramycin (300 mg) twice daily for 28 days. 104 - 107 Sodium colistimethate (1,000,000 to 2,000,000 IU, twice daily) is an alternative with consistent results and should be associated with oral ciprofloxacin for 2-3 weeks.
Inhalation therapy may be extended for 2-3 months. Intravenous antibiotic therapy for 2 weeks may be an option in selected cases and should always be followed by inhaled antibiotic therapy. Successful eradication is defined as negative bacterial culture results over a 1-year period after treatment completion. Eradication therapy, in addition to having significant clinical benefits, may be cost-effective. 103 - 107
(Level of evidence: 1)
What should therapy for eradicating B. cepacia complex strains be like?
The B. cepacia complex consists of a group of more than 80 closely related species, 108 , 109 B. multivorans and B. cenocepacia being the predominant species infecting people with cystic fibrosis. 110
Clinical manifestations in cystic fibrosis range from no symptoms to severe conditions with rapid clinical deterioration and fulminant progression to necrotizing pneumonia, respiratory failure, and sepsis (cepacia syndrome). 110 Treatment of B. cepacia complex is difficult because of intrinsic resistance of these organisms to most antimicrobial agents available. It is therefore recommended that, whenever possible, antibiogram-guided combination therapy be used. There is no available evidence assessing the efficacy of its eradication, nor are there recommendations for inhalation therapy for chronic infection. 110 , 111
(Level of evidence: 4)
What should therapy for eradicating methicillin-resistant S. aureus be like?
Chronic infection with methicillin-resistant S. aureus is associated with worse clinical outcomes in patients with cystic fibrosis. 112 There have been reports of methicillin-resistant S. aureus eradication therapies using combinations of oral, topical, and inhaled drugs, such as sulfamethoxazole/trimethoprim, rifampin, fusidic acid, and chlorhexidine, in addition to vancomycin. Linezolid may be considered, but on the basis of less evidence. 113 Shorter treatment protocols (< 3 weeks) appear to be as effective as longer ones, as well as being less likely to result in intolerance and adverse effects. Combination therapy appears to have a greater likelihood of success than does monotherapy. 114 , 115
There is still no clear evidence of the benefits of eradication of methicillin-resistant S. aureus in patients with cystic fibrosis. 113 , 114 , 116 There is also no evidence to recommend inhaled antibiotic therapy for chronic infection with this pathogen.
(Level of evidence: 4)
What are the recommendations for chronic use of inhaled antibiotics in cystic fibrosis?
Table 2 shows the inhaled antibiotics that are used for suppression of chronic infection with P. aeruginosa. 23 , 117 , 118 The regular use of inhaled antibiotics delays deterioration of pulmonary function in patients chronically infected with P. aeruginosa. 23 , 87 , 117 - 119 Chart 4 presents the Leeds criteria, which classify respiratory infection with P. aeruginosa in patients with cystic fibrosis on the basis of respiratory secretion culture results obtained in the last 12 months. 120
Table 2. Treatment with inhaled antibiotics in accordance with a European consensus. 118 .
| Inhaled antibiotic | Dosea | Trade name |
|---|---|---|
| Aztreonam | 75 mg (3 times/day) | Cayston |
| Colistimethate sodium* | < 2 years of age: 0.5 million IU 2-10 years of age: 1 million IU > 10 years of age: 2 million IU | Colistin/Colomycin/Promixin |
| Colistimethate sodiumb (dry powder inhaler) | 1 capsule | Colobreathe |
| Tobramycin | > 6 years of age: 300 mg | Bramitob/Tobi |
| Tobramycin (dry powder inhaler) | > 6 years of age: 112 mg (4 capsules of 28 mg) | Zoteon |
Twice a day, except where otherwise indicated. bThat dose has been used in various European cystic fibrosis centers. When using I-neb® (Phillips Respironics) device, the dose should be reduced.
Chart 4. Leeds criteria for the classification of respiratory infection by Pseudomonas aeruginosa in patients with cystic fibrosis.

Pa: Pseudomonas aeruginosa. Adapted from Lee et al.(120)
Inhaled tobramycin is the most studied antibiotic, 119 , 121 , 122 and its use is recommended after age 6 years in patients with chronic infection with P. aeruginosa, regardless of disease severity, in alternating cycles of 28-days-on and 28-days-off therapy. Sodium colistimethate and aztreonam are other options. 23 , 87 , 123 , 124 Tobramycin inhalation powder has been used and shown to have equivalent efficacy to tobramycin inhalation solution, being associated with reduced treatment administration time and not requiring the use of nebulizers. 125
The recommendation of using suppression therapy in alternating months is aimed at preventing the development of bacterial resistance. In cases that are more severe, however, continued use of therapy or switching antimicrobial agents may be recommended. 124
It is advisable that the first inhalations be performed under supervision to allow for assessment of occurrence of drug-induced bronchoconstriction (wheezing, dyspnea, and chest tightness). Bronchodilator use is recommended, followed by bronchial hygiene via chest physiotherapy and, finally, antibiotic use in order to ensure greater medication deposition. 87 , 119 , 126 , 127
(Level of evidence: 1)
What are the indications for the use of azithromycin in patients with cystic fibrosis and how should azithromycin be used?
The use of oral azithromycin 3 times a week in cystic fibrosis patients over 5 years of age who are chronically colonized with P. aeruginosa results in improvement in pulmonary function and reduction in the number of exacerbations. 119 , 127 - 133
(Level of evidence: 1)
In patients who were not colonized with P. aeruginosa and had an FEV1 > 50% of the predicted value, azithromycin was found to reduce exacerbations by 50%, although with no improvement in pulmonary function. 134
(Level of evidence: 1)
The continued use of azithromycin is recommended, despite the lack of long-term assessment studies. Initial use for at least 6 months is suggested for assessment of response to therapy. 135 , 136 Side effects, such as epigastric pain, electrocardiographic changes, ototoxicity, and nontuberculous mycobacterial infection, should be monitored.
(Level of evidence: 1)
The use of azithromycin (250 mg for body weight < 40 kg and 500 mg for body weight > 40 kg; 3 times a week) is recommended in patients chronically colonized with P. aeruginosa who are over 5 years of age, as well as in those who are not colonized with P. aeruginosa and have frequent pulmonary exacerbations. Sputum sample collection for investigation of the presence of nontuberculous mycobacteria is recommended before initiation of azithromycin. 132 , 134
(Level of evidence: 2)
Given the possibility of a drug interaction between azithromycin and aminoglycosides, combined azithromycin and inhaled tobramycin use should be reassessed especially in patients with frequent exacerbations despite optimal treatment. 137
(Level of evidence: 3)
How does one recognize an acute pulmonary exacerbation?
Acute pulmonary exacerbations are characterized by clinical findings of increased cough, changes in secretion appearance, fever, abnormalities on pulmonary auscultation, decreased FEV1, decreased saturation, radiological abnormalities, and weight loss. 23
(Level of evidence: 5)
What therapy is indicated for acute pulmonary exacerbations?
For mild exacerbations (without hypoxemia or significant respiratory distress), use oral antimicrobial agents, on the basis of the last respiratory secretion culture result. For severe exacerbations or in cases of intolerance to oral medications, intravenous therapy (usually in hospital) is recommended, 139 but the choice of medications depends on previous respiratory secretion culture results and on patient history. 23
Antibiotic pharmacokinetics is different in individuals with cystic fibrosis, and dosage regimens should be adjusted 139 ) (Table 3). For P. aeruginosa, the combination of two or more antibiotics (usually a beta-lactam and an aminoglycoside) is recommended.
Table 3. Antimicrobial agents commonly used against acute pulmonary exacerbations in cystic fibrosis patients.a .
| Bacterium | Antimicrobial agent | Dose, mg/kg/day | Intervals and route |
|---|---|---|---|
| Staphylococcus aureus | Cephalexin Cefadroxil Cefuroxime Clarithromycin Clindamycin Amoxicillin + Clavulanic acid Sulfamethoxazole/trimethoprim Oxacillin Vancomycind Teicoplanind Linezolidd Tigecyclined |
50-100 (max, 4 g/day) 30 (max, 4 g/day) 20-30 (max, 1,5 g/day) 15 (max, 1 g/day) 30-40 (max, 2,4 g/day) 50b (max. 1,5 g/day) 40c (max, 1,6 g/day) 200 (max, 8 g/day) 40-60 (max, 8 g/day) 10 (max, 400 mg/day) 20 (max, 1,2 g/day) 2 (max, 100 mg/day) |
6/6 h p.o. 12/12 h p.o. 12/12 h p.o. 12/12 h p.o. 6/6 h or 8/8 h i.v. 8/8 h or 12/12 h p.o. 12/12 h p.o. 6/6 h i.v. 6/6 h i.v. 24/24 h i.v. or i.m. 12/12 h p.o. or i.v. 12/12 h i.v. |
| Haemophilus influenzae | Amoxicillin + Clavulanic acid Cefuroxime Cefaclor |
50b (max, 1,5 g/day) 20-30 (max, 1,5 g/day) 40 (max, 1 g/day) |
8/8 h or 12/12 h p.o. 12/12 h p.o. 8/8 h p.o. |
| Pseudomonas aeruginosa | Ciprofloxacin Amikacin Tobramycin Ceftazidime Cefepimee Piperacillin + tazobactame Meropeneme Aztreonam |
30-50 (max, 1,5 g/day) 30 (max, 1,2 g/day) 20-30 (max, 1,5 g/day) 10 (max, 660 mg/day) 150 (max, 9 g/day) 150 (max, 6 g/day) 300 (max, 18 g/day) 120 (max, 6 g/day) 50 (max, 6 g/day) |
12/12 h p.o. 8/8 h i.v. 24/24 h i.v. 24/24 h i.v. 8/8 h i.v. 8/8 h i.v. 6/6 h or 8/8 h i.v. 8/8 h i.v. 8/8 h i.v. |
| Stenotrophomonas maltophilia f | Sulfamethoxazole/trimethoprim Chloramphenicol Levofloxacin |
40c (max, 1,6g/day) 60 a 80 (max, 4 g/day) 10 (max, 750 mg/day) |
12/12 h p.o. 6/6 h p.o. or i.v. < 5 years o f age: 12/12 h > 5 years of age: 24/24 h |
| Burkholderia cepacia complexf.g | Sulfamethoxazole/trimethoprim Meropenem Chloramphenicol Doxycycline |
40c (max, 1,6 g/day) 100c (max, 2,4 g/day) 120 (max, 6 g/day) 60 a 80 (max, 4 g/day) 1-2 (max, 200 mg/day) |
12/12 h p.o. 6/6 h i.v. (severe cases) 8/8 h i.v. 6/6 h p.o. or i.v. 12/12 h p.o. |
Max: maximum. aIt is recommended to control the serum level of drugs whose laboratory tests are available (e.g.: aminoglycosides and vancomycin).bAmoxicillin dose. cSulfamethoxazole dose. dThey should only be used against methicillin-resistant Staphylococcus aureus. eAlso effective against methicillin-susceptible S. aureus. fLack of standardization regarding the level of efficacy of the antimicrobial agents. gUsually resistant to many antimicrobial agents.
Treatment time for an acute pulmonary exacerbation depends on clinical response, with the recommendation being 8 to 14 days. Patients with more severe disease may benefit from longer antimicrobial therapy. 138 , 140 - 142
In addition to antibiotic therapy, the treatment of exacerbations requires the participation of a multidisciplinary team, because there is often need for oxygen supplementation, use of long-term intravenous devices, intensified chest physiotherapy, and a different nutritional approach. 138 , 143 , 144
(Level of evidence: 5)
How does one assess response to treatment?
One should observe clinical parameters, such as respiratory symptoms, fever, and weight gain, as well as improvement in pulmonary function with a view to it returning to its baseline levels. Despite intensive treatment, approximately 25% of the patients who have an acute pulmonary exacerbation requiring intravenous therapy fail to recover completely to pre-exacerbation levels of pulmonary function, 23 , 138 - 142 emphasizing the need for maintenance therapies to prevent acute pulmonary exacerbations.
(Level of evidence: 5)
When and how should oxygen therapy be used in patients with cystic fibrosis?
In hypoxemic patients, continuous oxygen supplementation is associated with increased exercise tolerance and mild improvement in sleep and school/work attendance, but does not result in increased survival.
Oxygen therapy may be indicated on a case-by-case basis when SpO2 is below 90%, for relieving dyspnea, delaying the development of cor pulmonale, and improving the aforementioned outcomes. A PaO2 < 55 mmHg or an SpO2 < 88% is an indication for oxygen therapy, regardless of symptoms. The preferred route of administration is via a nasal cannula, at the lowest flow possible to maintain SpO2 above 90%. Intermittent use may be necessary during acute pulmonary exacerbations. 145 , 146
(Level of evidence: 5)
How does one diagnose and treat pneumothorax in patients with cystic fibrosis?
Pneumothorax manifests as dyspnea and/or sudden-onset chest pain. Extensive pneumothorax requires hospitalization and chest drainage, the only indication for pleurodesis being recurrence. Noninvasive ventilation and chest physiotherapy should be performed only in patients who received drainage.
Small pneumothorax should be drained only if there is clinical instability. 23 , 147
(Level of evidence: 5 for the non-recommendation of antibiotics)
(Level of evidence: 5 for the non-discontinuation of inhaled medications)
How does one classify and treat hemoptysis in patients with cystic fibrosis?
The management of hemoptysis depends on its volume. Bleeding ≥ 5 mL requires considering treatment with antibiotics for pulmonary exacerbation. 23 , 147 Bleeding ≥ 240 mL/day or > 100 mL/day for several days requires specialized treatment, and, when there is evidence of clinical instability, bronchoscopic treatment or bronchial artery embolization is indicated. 23 , 147 Surgical intervention can be performed in the acute phase only in refractory cases.
(Level of evidence: 2)
(Level of evidence: 5 for surgical intervention)
When are invasive and noninvasive ventilation indicated in cystic fibrosis?
The use of invasive ventilation in patients with severe disease is controversial and is associated with low survival rates, especially when the indication is respiratory infection. Invasive ventilation should be considered in cases of respiratory failure due to an acute, correctable precipitating factor (massive hemoptysis, pneumothorax, and during postoperative periods).
Noninvasive ventilation may be used as an adjuvant in the treatment of exacerbations and may be indicated in patients with daytime hypercapnia and sleep disorders. The use of noninvasive ventilation has been reported to be associated with increased exercise tolerance, improved quality of life and survival, and reduced decline in pulmonary function. Its use as a resource in chest physiotherapy provides benefits regarding dyspnea, muscle fatigue, and oxygenation. 73 - 75
(Level of evidence: 2 for noninvasive ventilation)
(Level of evidence: 5 for invasive ventilation)
How does one diagnose and treat allergic bronchopulmonary aspergillosis?
Allergic bronchopulmonary aspergillosis is a common complication in cystic fibrosis. Annual measurement of total IgE is recommended as a screening strategy. The diagnostic criteria for allergic bronchopulmonary aspergillosis are presented in Chart 5. 124 , 148 Treatment is with oral prednisone with or without antifungal agents (Chart 6). 118 , 149 - 155
Chart 5. Diagnostic criteria for allergic bronchopulmonary aspergillosis.
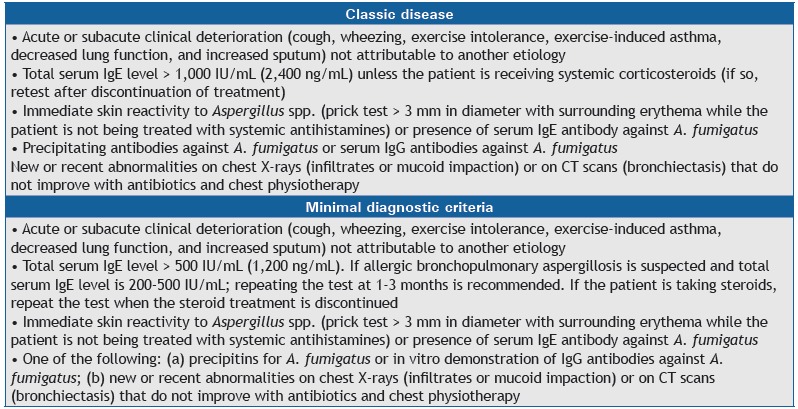
Chart 6. Allergic bronchopulmonary aspergillosis treatment.

(Level of evidence: 5 for diagnosis and treatment)
What is the approach to patients with positive sputum for Aspergillus spp.?
The presence of Aspergillus spp. in sputum usually does not mean disease. 156 If there is clinical worsening or a lack of response to antimicrobial therapy, one should investigate for allergic bronchopulmonary aspergillosis and consider fungal bronchitis. 157
(Level of evidence: 5)
What are the existing CFTR-modulating therapies, for which types of mutations have they been used, and what effects have been observed?
Potentiators increase the function of the CFTR protein that is expressed at the plasma membrane (class III, IV, and V mutations), and correctors correct defects of the protein that is not expressed at the cell membrane (class I and II mutations). 158 - 161
Ivacaftor is a potentiator that was initially studied in patients carrying the G551D mutation (a class III mutation). Its use had relevant effects resulting in a reduction in sweat chloride levels, improvement in FEV1, and weight gain, as well as in a reduction in the number of exacerbations and improvement in quality of life. Its use was subsequently approved for other class III mutations and R117H. 162 - 164
Among corrector drugs, a drug for class I mutations (ataluren) showed slight effects on pulmonary function and on the number of exacerbations in a phase 3 trial, only for patients who did not use inhaled tobramycin. 165
For the most prevalent class II mutation worldwide, F508del, the use of ivacaftor (a potentiator) in combination with lumacaftor (a corrector) was shown to produce a reduction in the number of exacerbations and a slight improvement in FEV1 and quality of life for homozygous patients, with no significant effects being observed for heterozygous patients. 166
(Level of evidence: 1 for ivacaftor in patients carrying a class III mutation [G551D])
(Level of evidence: 3 for ataluren in patients not exposed to tobramycin)
(Level of evidence: 2 for ivacaftor/lumacaftor in patients carrying a class II mutation [F508del])
Is there an indication for the use of ibuprofen in patients with cystic fibrosis?
The use of ibuprofen appears to delay the decline in pulmonary function and result in nutritional improvement, especially in children. Studies recommend doses between 20 and 30 mg/kg twice daily (maximum: 1,600 mg), in addition to monitoring of adverse events (discontinue use of ibuprofen when using aminoglycosides) and serum levels (50-100 mg/mL). Low doses are associated with a paradoxical increase in inflammation. Given the high rate of adverse events associated with ibuprofen and the difficulty in monitoring the serum level of the drug, its routine use is not recommended. The benefits of ibuprofen in patients with advanced disease are unknown. 167 - 174
(Level of evidence: 2)
What is the role of inhaled and systemic corticosteroids in cystic fibrosis?
There is no scientific evidence supporting the routine use of inhaled corticosteroids in cystic fibrosis; they can be used in patients with cystic fibrosis and asthma. It is recommended that, whenever possible, spirometry be performed to confirm the benefits of their use. 175 The chronic use of oral corticosteroids is also not recommended because of the risk of significant adverse effects, such as increased risk of diabetes and growth retardation. The effects of their short-term use and of their use during pulmonary exacerbations have yet to be elucidated. 176
(Level of evidence: 5 for not using oral corticosteroids chronically)
(Level of evidence: 5 for using inhaled corticosteroids)
What are the indications for the use of bronchodilators in cystic fibrosis?
Bronchodilators have been shown to provide benefits only in patients with confirmed bronchial hyperresponsiveness or evidence of asthma; in the latter case, bronchodilators should be used in combination with an inhaled corticosteroid. In this group of patients, an increase in pulmonary function was observed in the short and long term. Long-acting beta-agonists improved pulmonary function in the short term, with inconsistent long-lasting results, being therefore indicated only for individuals with confirmed asthma. 177 Regarding long-acting anticholinergics, tiotropium has recently been shown to be well tolerated, although the gain in pulmonary function was not statistically significantly different when compared with placebo. 178
(Level of evidence: 5 for beta-agonists in patients with cystic fibrosis and asthma)
(Level of evidence: 5 for tiotropium)
How does one diagnose and treat nontuberculous mycobacterial infections?
There has been an increase in the incidence of nontuberculous mycobacterial infections in cystic fibrosis patients, with this incidence rising with advancing age. It is associated with progressive clinical deterioration, and differentiating between colonization and infection is essential, this differentiation being based on clinical, microbiological, and radiological criteria. 179
In patients who can spontaneously expectorate, mycobacterial cultures should be performed at least annually. The most commonly identified species are M. avium-intracellulare complex, M. chelonae, and M. abscessus. 180
Treatment of the infection with at least three antibiotics, usually including a macrolide, is recommended. The antimicrobial regimen should be tailored to the nontuberculous mycobacterial species, following guideline recommendations. 179 , 180
(Level of evidence: 5)
GASTROINTESTINAL AND NUTRITIONAL TREATMENT
How to suspect, diagnose, and manage meconium ileus?
Meconium ileum is the first clinical manifestation in patients with cystic fibrosis, in 15-20% of cases. Ileal obstruction by a plug of meconium and thick mucus may arise in intrauterine life with polyhydramnios, meconial peritonitis, and ileal distension, evidenced in prenatal ultrasonography. It is manifested by the absence of stool elimination in the first 48 h of life, accompanied by abdominal distension and vomiting (acute obstructive abdomen).
Clinical treatment includes hyperosmolar enemas, use of a nasogastric tube, hydration, and control of electrolytes. Complex cases (atresia, microcolon, necrosis, or perforation) should be treated surgically using minimally invasive techniques with ileostomy and reanastomosis in a timely manner.
(Level of evidence: 5)
How should electrolyte disturbances be prevented?
Salt loss from sweat and the large body surface pose a risk for dehydration and electrolyte disturbances in infants with cystic fibrosis, even without apparent loss. Signs, such as apathy or irritability, tachypnea, and prostration, may indicate dehydration, hyponatremia, hypokalemia, and hypochloremia, which are potentially life-threatening. Newborns and infants receiving breast milk or infant formulas should be supplemented with sodium chloride at a dose of 2.5-3.0 mEq/kg/day. 23 , 182 , 183
(Level of evidence: 5)
When should exocrine pancreatic insufficiency be clinically suspected?
We should suspect it in the presence of steatorrhea, chronic diarrhea, low weight gain, and signs of hypovitaminosis. The infant may also present with edema, hypoalbuminemia, and anemia. Indirect signs are observed through the characteristics of the stool, such as oiliness, foul smell, and diarrhea. 184
(Level of evidence: 5)
How do we confirm the diagnosis of exocrine pancreatic insufficiency?
In clinical practice, the best method for confirming exocrine pancreatic insufficiency is the quantification of fecal elastase. Values < 200 μg/g of feces confirm exocrine pancreatic insufficiency (sensitivity, 86-100%). 185 Quantitative determination of fecal fat by the Van de Kamer method is considered the gold standard for the diagnosis of steatorrhea, but its use is limited by technical difficulties. 185
(Level of evidence: 4)
How should pancreatic sufficiency be followed?
The fecal elastase test is simple and reliable in patients older than two weeks of age in the absence of liquid feces. Patients with pancreatic insufficiency should be monitored annually during childhood and during periods of growth failure, weight loss, or chronic diarrhea. The semiquantitative assessment of fat in stool (steatocrit) has relative value during the follow-up of patients, and the qualitative evaluation of fecal fats (Sudan III) has only a screening value for fecal fat loss.
(Level of evidence: 5)
How should enzyme replacement therapy be performed?
The need for replacement of pancreatic enzymes varies greatly and should be evaluated individually, relating clinical symptoms to the diet of each patient. The initial doses (Table 4) and their eventual subsequent increase should be guided by the improvement/resolution of the symptoms of malabsorption.
Table 4. Recommended initial doses for pancreatic enzyme replacement therapy.a .
| Dose | Infants, per 120 mL of formula or breast milk | < 4 years of age, U/kg per meal | > 4 years of age, U/kg per meal |
|---|---|---|---|
| Initial | 2.000 U | 1.000 U | 500 U |
| Maximum per meal | 4.000 U | 2.500 U | 2.500 U |
| Snacks | --- | ½ dose | ½ dose |
Maximum daily dose: 10.000 U/kg.
Enzyme capsules should be swallowed whole and taken at the beginning of or during meals. Infants can ingest the granules mixed with milk or mashed fruit. Doses above 10,000 IU lipase/kg/day should be avoided, but they may be necessary in childhood, especially during accelerated growth phases. 186
(Level of evidence: 1)
How should the response to enzyme replacement therapy be evaluated?
The determination of the adequacy of enzyme replacement therapy is clinical: nutritional status, signs and symptoms of malabsorption, and patient’s weight gain plot should be verified. Inappropriate doses of pancreatic enzymes may result in abdominal pain, constipation, or diarrhea. Stool pattern and stool characteristics (oily, foul-smelling, floating, diarrheal, grayish, or yellowish) may indicate inadequacy of enzyme replacement therapy. 23 , 24
(Level of evidence: 5)
When should distal bowel obstruction syndrome be suspected and treated?
If there is incomplete obstruction, intermittent abdominal pain, nausea, and palpable masses in the lower right quadrant are noted, whereas in cases of complete obstruction, bilious vomiting and abdominal distension (acute obstructive abdomen) appear. The use of oral hydration and laxatives (such as polyethylene glycol) are indicated in cases of incomplete obstruction. In more severe cases, venous hydration, use of tubes, and use of enemas with polyethylene glycol or meglumine diatrizoate + sodium diatrizoate solution (Gastrografin®; Bracco Diagnostics, Canada) are indicated. Surgical treatment should be considered in cases of severe obstruction or in the presence of perforation. 187 , 188
(Level of evidence: 4)
Are there other common clinical gastrointestinal conditions in cystic fibrosis?
Approximately 30% of the patients have gastroesophageal reflux disease, and 40% have intestinal constipation. Recurrent acute pancreatitis is more common in patients with pancreatic insufficiency (10%), and rectal prolapse occurs in about 20% of the patients, especially those between 1-2 years of age. Patients with rectal prolapse and recurrent acute pancreatitis should be investigated for cystic fibrosis. The approach and treatment of all of these pathologies do not differ from those in patients without cystic fibrosis. 23
(Level of evidence: 4)
How should hepatic and biliary disease be monitored and managed?
The frequency of hepatic and biliary tract manifestations is shown in Table 5. Multilobular cirrhosis associated with hepatic insufficiency is rare, 189 but bile sludge and lithiasis in the biliary tract are common and generally asymptomatic. 190 , 191 Patient monitoring includes clinical evaluation at all visits, biochemical tests (liver enzymes and prothrombin time), and annual abdominal ultrasonography. Gastrointestinal endoscopy may be requested to investigate cases of gastrointestinal bleeding or suspected esophageal and gastric varices. Liver biopsy is rarely indicated. Patients with hepatic impairment should undergo quantification of alpha fetoprotein annually, due to the risk of hepatocellular carcinoma. 189
Table 5. Frequency of hepatic and biliary tract manifestations in patients with cystic fibrosis.
| Organ | Approximate proportion, % |
|---|---|
| Liver Increased liver enzymes Hepatic steatosis Focal biliary cirrhosis Multilobular biliary cirrhosis Neonatal cholestasis Bile duct stenosis Sclerosing cholangitis Cholangiocarcinoma |
10-35 20-60 11-70 5-15 < 2 < 2 < 1 rare |
| Gallbladder Cholelithiasis and cholecystitis Microvesicules |
10-30 24-50 |
Adapted from Debray et al.(189)
The treatment of liver disease in patients with cystic fibrosis aims to improve bile flow, viscosity, and composition. Ursodeoxycholic acid at the dose of 15-20 mg/kg/day (2-3 doses) is recommended, but its use is controversial in the medical literature. There is no indication of treatment in the case of hepatic steatosis. 190 , 192 In cases of advanced liver disease, liver transplantation may be indicated.
(Level of evidence: 5)
How should the nutritional status of patients with cystic fibrosis be monitored?
There is a strong association between pulmonary function and nutritional status. Periodic monitoring is necessary, especially regarding anthropometry, pulmonary function, gastrointestinal function, quality and quantity of food ingested, body composition, and biochemical evaluation.
Patients are more vulnerable to malnutrition during periods of rapid growth, and special attention should be paid to the first 12 months after the diagnosis, the first year of life, and the peripuberal period. 193 The parameters and periodicity for monitoring are shown in Chart 7. 194
Chart 7. Nutritional parameters and suggested monitoring frequency in patients with cystic fibrosis.
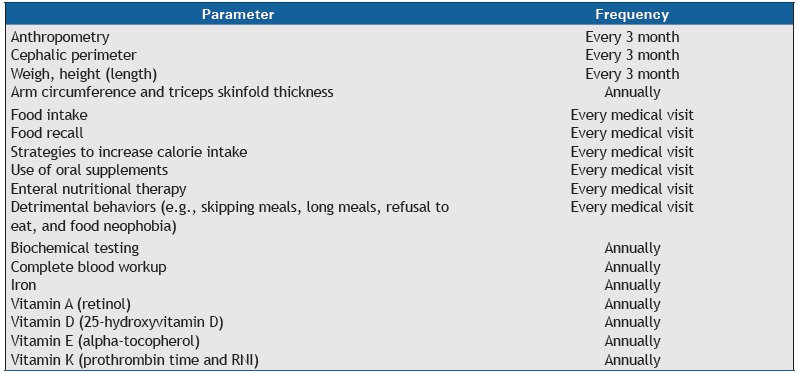
RNI: recommended nutrient intake. Adapted from Stallings et al.(194)
(Level of evidence: 5)
What reference data should be used?
The growth curves used in Brazil are those by the World Health Organization (2006-2007), 195 , 196 and anthropometric data must be obtained and recorded in every medical visit (Chart 8).
Chart 8. Anthropometric indices recommended by the World Health Organization and adopted by the Brazilian Ministry of Health for the evaluation of the nutritional status of children and adolescents.

BMI: body mass index.
(Level of evidence: 5)
How to define nutritional deficiencies?
In children and adolescents, height-for-age, weight-for-age, weight-for-height and body mass index (BMI)-for-age Z-scores with results < −2 reveal anthropometric deficiencies. It is suggested to associate the anthropometric evaluation with the growth rate and the target height. For adults, the recommended BMI is ≥ 22 kg/m2 for women and ≥ 23 kg/m2 for men. Additional body composition assessments should include skinfold thickness and arm circumference measurements, as well as bioimpedance, to help determine optimal nutritional status. 197
(Level of evidence: 5)
What are the main strategies for the prevention and treatment of nutritional disorders in cystic fibrosis patients?
The prevention of nutritional disorders presupposes the ingestion of a hypercaloric and high protein diet, vitamin supplementation, enzyme replacement therapy, and control of cystic fibrosis-related infections/exacerbations/other comorbidities. Treatment involves behavioral therapy, use of nutritional supplements, and the use of enteral diet via a nasoenteral tube in an acute phase and via gastrostomy for prolonged use. 198 - 201
(Level of evidence: 5)
What factors should be evaluated in the growth failure?
It is recommended to evaluate the intake of macronutrients and micronutrients, as well as to control malabsorption and infections/exacerbations. In addition, comorbidities, such as electrolyte disturbances, gastroesophageal reflux disease, bacterial overgrowth, diabetes, or behavioral appetite disorders should be evaluated. Less frequent causes of growth failure include lactose intolerance, celiac disease, food allergy, and inflammatory bowel disease. 193 , 202
(Level of evidence: 5)
What are the options for nutritional therapy?
All of the patients should receive dietary advice. Behavioral therapy may be helpful for children between 3-12 years of age. Energy intake of 110-200% of recommended values for age and sex, with 35-40% of the energy supplied by lipids is recommended, as well as is vitamin supplementation, according to the needs of the patients. The use of dietary supplements and/or increased caloric density of the diet are indicated for patients showing weight loss or weight gain failure for 2-6 months, according to the age bracket. Enteral nutritional support by means of tubes is reserved for more severe cases and for short periods of time. Gastrostomy is indicated for long-term nutritional therapy. Parenteral nutrition is an exceptional measure, indicated for patients whose digestive tract cannot be used (during postoperative period or in the presence of critical illness) or in cases of short bowel syndrome. 193 , 202 Figure 2 shows the algorithm for the management of patients with low weight or inadequate weight gain.
Figure 2. Algorithm for patients with low weight or insufficient weight gain. CF: cystic fibrosis; PBI: proton pump inhibitor; GI: gastrointestinal; ENT: enteral nutrition therapy; CFRD: cystic fibrosis-related diabetes; and GERD: gastroesophageal reflux disease. Adapted from Sinaasappel et al. 202 .
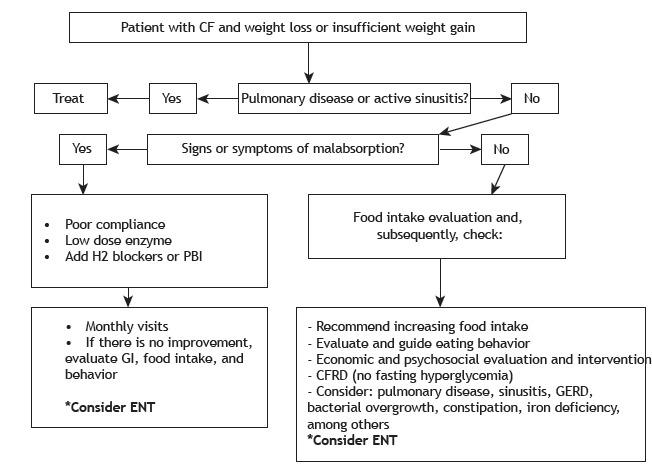
(Level of evidence: 5)
What are the requirements for vitamin supplementation in cystic fibrosis patients?
Patients with cystic fibrosis are at high risk of developing fat-soluble vitamin deficiency due to exocrine pancreatic insufficiency. The recommended doses vary widely (Table 6 and Table 1A-online appendix). 193 , 202 Fat-soluble vitamins are better absorbed when given in combination with a meal and pancreatic enzymes.
Table 6. Doses for fat-soluble vitamin supplementation in patients with cystic fibrosis.
| Age | Individual daily vitamin supplementation | |||
|---|---|---|---|---|
| Vitamin A, IU (µg) | Vitamin E, mg | Vitamin K, mg | Vitamin D, IU | |
| 0-12 months | 1,500 (510) | 40-50 | 0,3-0,5 | 400-500 |
| 1-3 years | 5,000 (1,700) | 80-150 | 0,3-0,5 | 800-1,000 |
| 4-8 years | 5,000-10,000 (1,700-3,400) | 100-200 | 0,3-0,5 | 800-1,000 |
| > 8 years | 10,000 (3,400) | 200-400 | 0,3-1,0 | 800-2,000 |
| Adults | 10,000 (3,400) | 200-400 | 2,5-5,0a | 800-2,000 |
| amg/week. | ||||
(Level of evidence: 2 for vitamin E)
(Level of evidence: 5 for vitamin A)
(Level of evidence: 3 for vitamin D)
(Level of evidence: 2 for vitamin K)
OTHER ASPECTS
When and how should diabetes be investigated in patients with cystic fibrosis?
Approximately 20% of the adolescents and 40% of the adults develop cystic fibrosis-related diabetes, resulting in worsening of nutrition, worsening of pulmonary function, and increase in morbidity and mortality rates, even in its asymptomatic phase. 208 Every cystic fibrosis patient older than 10 years of age should undergo the oral glucose tolerance test for the determination of blood glucose levels after fasting for 8 h and 2 h after the ingestion of 1.75 g/kg of glucose (maximum, 75 g). 209 - 213 The oral glucose tolerance test should be performed preferably when the patient is clinically stable.
The test is also recommended for patients with unexplained clinical worsening, prior to transplantation, in use of systemic corticosteroids, and before and during gestation, as well as in patients receiving enteral nutrition as nutritional support.
Blood glucose levels for the diagnosis of cystic fibrosis-related diabetes are similar to those for non-cystic fibrosis-related diabetes (Table 7).
Table 7. Screening and diagnostic criteria for cystic fibrosis-related diabetes and interpretation of blood serum glucose results by means of the oral glucose tolerance test.
| OGTT, mg/dl | Interpretation | Fasting BSG | BSG-2 h | BSG-1 h |
|---|---|---|---|---|
| • Healthy patients > 10 years of age • Prior to transplantation • Pregnancy scheduling • During pregnancy |
Glucose tolerance Glucose intolerance CFRD Indeterminate |
< 126 < 126 ≥ 126 < 126 |
< 140 140-199 ≥ 200 < 140 |
≥ 200 |
| Monitorização de glicemia de jejum e GPP de 2 h | ||||
| • During hospitalization • Outpatient setting 1. Pulmonary exacerbation, treated with i.v. antibiotic therapy or systemic glucocorticosteroids 2. Monthly, during and after nocturnal enteral feeding Glycated hemoglobin Random blood glucose testing |
DRFC DRFC DRFC |
GP de jejum ≥ 126 mg/dl GPP ≥ 200 mg/dl (persistente por 48 h) |
≥ 200 mg/dl durante ou após a dieta |
≥ 6,5% (< 6,5% não exclui) Glicemia ao acaso ≥ 200 mg/dl + poliúria e polidipsia |
OGTT: oral glucose tolerance test; BSG: blood serum glucose; BSG-2h: blood serum glucose 2 h after glucose intake during OGTT, GP-1h: blood serum glucose 2 h after glucose intake during OGTT; CFRD: cystic fibrosis-related diabetes; and PBG: postprandial blood glucose.
Adapted from Sermet-Gaudelus et al.(213)
(Level of evidence: 2)
What is the current recommended treatment for cystic fibrosis-related diabetes?
Cystic fibrosis-related diabetes should be treated with insulin. 208 - 210 Mean insulin doses range from 0.38 to 0.58 IU/kg/day, 214 distributed between slow-acting basal insulin and long-acting or ultralong-acting basal insulin at meals. Calories and carbohydrates should not be restricted, but complex carbohydrates and foods with low glycemic index should be favored and distributed in smaller portions and shorter intervals (2-3 h). Patients with changes in glucose levels during exacerbations might benefit from intermittent insulin administration. There is no definite consensus regarding the treatment of glucose intolerance.
(Level of evidence: 2 for insulin treatment)
(Level of evidence: 3 for recommendations regarding calories and carbohydrates)
When and how should osteopenia/osteoporosis be investigated in cystic fibrosis patients?
Low bone mineral density is common in cystic fibrosis patients, and it might occur since childhood. Bone densitometry is the gold standard test for diagnosing osteopenia/osteoporosis and should be performed in patients between 8-10 years of age. In patients younger than 20 years of age, the sites for that assessment are the total body and lumbar spine, whereas, in those aged 20 years or more, the sites are the hip and lumbar spine. The results should be adjusted for gender, age, and ethnicity, and they should be expressed as a Z-score for patients < 50 years of age and as a T-score for patients > 50 years of age and menopausal women. Bone densitometry should be repeated every 1-5 years, depending on the clinical classification of the findings. 23 , 215
(Level of evidence: 4 for body sites for bone densitometry)
(Level of evidence: 5 for confirming the need for bone densitometry)
What is the recommended treatment for osteoporosis/osteopenia in cystic fibrosis patients?
In order to prevent bone mass loss, it is recommended to maintain an adequate nutritional status, perform low-impact physical exercises, and avoid the use of inhaled or oral corticosteroids. 23 , 215
(Level of evidence: 5)
If the diagnosis of osteopenia is confirmed (Z-score between −1.0 and −2.5), vitamin D, vitamin K, and calcium supplementation should be initiated. 216
(Level of evidence: 2)
If the diagnosis of osteoporosis is confirmed (Z-score < −2.5), prescribe bisphosphonates: alendronate v.o. (70 mg/week or 10 mg/day); risedronate v.o. (35 mg/week or 5 mg/day); pamidronate i.v. (0.5-1.0 mg/kg/day for 3 days every 3 months); or zoledronic acid i.v. (0.025-0.05 mg/kg/day in a single dose every 6 months). 213 , 217
(Level of evidence: 1 for bisphosphonates)
When should the use of long-term venous access be considered and what are the options?
The evaluation of the need for using a long-stay intravenous device is based on the expected length of therapy, characteristics of the medications, such as pH (<5 or > 9), osmolarity (> 600 mOsmol/L), and the irritant capacity of such medications, as well as the durability of the intravenous device, the clinical condition of the patient, and the possibility of associated complications (Figure 3). 218
Figure 3. Selection of long-term endovenous device. PICC: peripherally inserted central catheter. Adapted from Santolim et al. 218 .
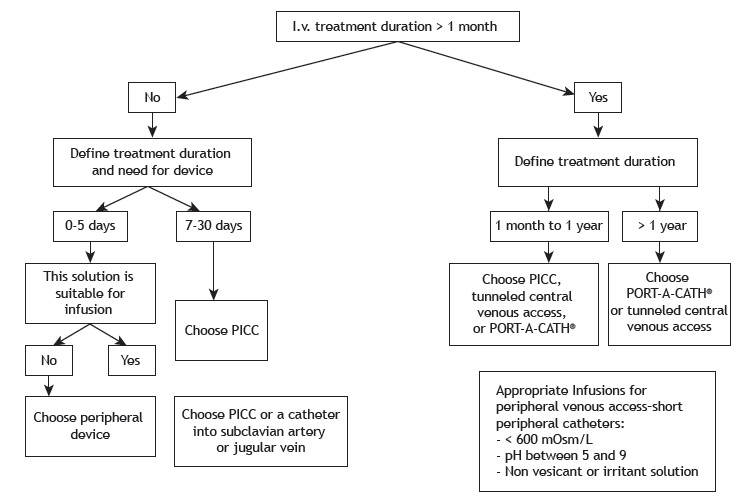
Options for long-term venous access devices include central peripherally inserted central catheters, central venous catheters (Intracath®, Becton Dickinson, Sandy, UT, USA), tunneled catheters (e.g., Hickman type), and totally implantable catheters (PORT-A-CATH®, Smiths Medical, Minneapolis, MN, USA). In a preserved venous network, the first option is the central peripherally inserted central catheter valve technology, which allows its intermittent use. 218
(Level of evidence: 4 for venous access)
When and how should sinus disease be approached in cystic fibrosis patients?
Sinonasal manifestations are common in patients with cystic fibrosis, especially nasal obstruction due to nasal polyposis and chronic rhinosinusitis. The extent of sinonasal disease may not correlate with the symptoms.
The patient should have routine otorhinolaryngological evaluation, since sinus disease may be related to pulmonary exacerbations. Imaging examinations are indicated only for surgical planning or investigation of complicated cases. 219
Treatment of sinonasal disease in cystic fibrosis patients consists of anti-inflammatory drugs, antibiotics, topical medications, and surgery. 219 , 220
A study with the use of nasal dornase alfa in post-nasal endoscopic surgery patients showed benefits. However, the effectiveness depends on the surgical dilation of the paranasal sinus ostia to allow the medication to reach the sinus mucosa. 219 , 221 - 223
In relation to nasal topical corticosteroids, studies have shown positive effects on both the improvement of nasal symptoms and the reduction of polyps. 224 , 225
Surgical treatment should be considered in the persistence of nasal obstruction even after clinical treatment, in cases of anatomical obstruction, when there is a relationship with pulmonary exacerbations, in cases of lung transplantation, or in patients whose symptoms affect their quality of life. 219
No studies on the use of mucokinetic drugs are available, and the recommendations are extrapolated from patients without cystic fibrosis. It is advocated that 7% hypertonic saline solution would be more adequate due to its mucokinetic effect in cystic fibrosis patients, but the use of 3% saline solution is more widespread.
(Level of evidence: 2 for dornase alfa)
(Level of evidence: 3 for surgical treatment)
(Level of evidence: 5 for further recommendations)
When should lung transplantation be indicated and when should the patient be referred for it?
Lung transplantation should be considered in patients with cystic fibrosis whose predicted life expectancy < 50% in 2 years and who have functional class III or IV according to the New York Heart Association. Although there is no clear indicator of a 2-year survival, a decrease in FEV1 < 30% is related to a 2-year mortality of approximately 40% in males and 55% in females. Because the mean time on the waiting list for lung transplantation is approximately 2 years, adult patients with cystic fibrosis should be referred for lung transplantation under the following conditions: FEV1 < 30%; six-minute walk test distance < 400 m; clinical or functional worsening, especially in females; hypoxemia or hypercapnia (PaO2 < 60 mmHg and PaCO2 > 50 mmHg); and pulmonary artery systolic pressure > 35 mmHg. Patients with episodes of pneumothorax or hemoptysis should be referred early. Regarding pediatric patients, long-term results are less consistent, and, although the referral criteria are similar to those abovementioned, the indication should be individualized, taking into account the availability and expertise of the transplant team. 23 , 226 - 228
(Level of evidence: 5)
How should palliative care be in patients with cystic fibrosis and advanced lung disease?
Open and frank dialogue about the progression of the disease should be promoted early on, and palliative care should be provided by the staff responsible for the patient. The team must be trained and qualified regarding the basic principles of analgesia and sedation and be able to treat symptoms, such as pain, nausea, anxiety, and dyspnea.
Oftentimes, palliative care is instituted with the remainder of the active treatment. The desire of the patient and his/her family members, not only in general terms, but also in terms of investment in situations of emergency and end-of-life, should be known by the whole team.
(Level of evidence: 5)
What are the recommendations regarding the use of contraceptive methods in patients with cystic fibrosis?
Female patients with cystic fibrosis should be advised of the contraceptive methods available (hormonal methods, intrauterine devices, barrier methods, and sterilization). 230 The efficacy of these methods is similar to that for the general population, except for the lower activity of hormonal contraceptives with the use of the new drugs (ivacaftor and lumacaftor). 231 Male patients, despite the fact that almost all are infertile, should be advised of the risks of sexually transmitted diseases. Although there is evidence of greater severity in the use of oral contraceptives in females, supposedly related to hormonal issues, their use does not seem to influence the evolution of cystic fibrosis. 232 Genetic counseling should be offered to all patients and their families, facilitating the prevention of cystic fibrosis in the affected families.
(Level of evidence: 5 for indication of contraceptives)
(Level of evidence: 5 for genetic counseling)
How to approach pregnancy in cystic fibrosis patients?
The pregnant woman with cystic fibrosis must be closely followed by the multidisciplinary team and by an obstetrician specializing in high-risk pregnancy. Oral glucose tolerance test and ultrasonography should be performed quarterly, as well as nutritional and pulmonary function monitoring.
Respiratory exacerbations should be treated aggressively. Most drugs used to treat cystic fibrosis do not compromise the fetus. Whenever possible, vaginal delivery should be performed with epidural anesthesia. 233 - 236 )
(Level of evidence: 5 for vaginal delivery)
What is the best way to approach infertility in cystic fibrosis patients?
Infertility or subfertility in both sexes usually accompanies cystic fibrosis. Female infertility appears to be related to the thickening of cervical mucus, whereas male infertility is related to congenital and bilateral absence of the vas deferens. 237 Sperm counts should be offered to every patient who wants to know his fertility level. Referral centers should provide access to various specialists, including gynecologists, urologists, geneticists, and human reproduction specialists, to guide couples and patients on investigation strategies and infertility treatments.
(Level of evidence: 5)
What is the importance of treatment compliance in cystic fibrosis?
The therapies recommended for the treatment of cystic fibrosis, despite their proven efficacy in survival, cause burden on patients, interfere with their quality of life, and impair their compliance with the treatment due to the complexity of the therapeutic regimens.
Strategies to overcome barriers and appropriate psychosocial interventions to improve compliance should be implemented by professionals from specialized centers. Open communication and discussion might help identify the key barriers, addressing the problems inherent to each family unit. These actions are essential, since adequate compliance with the actions inherent to the disease is related to relevant clinical benefits. 239 - 242
(Level of evidence: 5)
What is the relevance of anxiety and depression in the management of cystic fibrosis patients?
The prevalence of anxiety and depression among patients with cystic fibrosis is extremely high, especially in women. High rates are also found in the parents of such patients. The referral center must be prepared to identify, support, and treat the patients and their family members. The multidisciplinary team should be attentive in order to identify these comorbidities, and an annual screening using specific questionnaires or structured conversations is suggested. In the face of suspected anxiety or depression, a trained professional can confirm the diagnosis and allow psychological or medication interventions. 243
(Level of evidence: 5)
FINAL CONSIDERATIONS
The scenario of cystic fibrosis in Brazil and worldwide has undergone drastic changes, due to the incorporation of new technologies for the diagnosis and treatment of the disease. In this context, the life expectancy of the patients has increased significantly, which will bring about the need for changes in the performance of health care professionals and the incorporation of new therapeutic resources.
Patients with cystic fibrosis have complex needs for the management of their disease, requiring specialized care that involves a multidisciplinary team, as well as an adequate health care structure and access to advanced medical resources.
The present Brazilian guidelines were prepared in partnership with several Brazilian medical societies and received contributions from several Brazilian professionals involved in the care of patients with cystic fibrosis, aiming at the homogenization of diagnosis and treatment of the disease nationwide.
ACKNOWLEDGMENTS
The names listed here (in alphabetical order) answered the questions related to the categories of the present guidelines and take part in the Brazilian Guidelines for the Diagnosis and Treatment of Cystic Fibrosis Work Group:
Characteristics of a referral center: Leonardo Araújo Pinto, Luciana Freitas Velloso Monte, Laurinda Yoko Shinzato Higa, and Tania Wrobel Folescu.
Diagnosis: Fernando Augusto de Lima Marson, Isabela Sad, Maria de Fátima Correa Pimenta Servidoni, Paulo Kussek, and Salmo Raskin.
Treatment of respiratory disease: Adriana Della Zuana, Albin Augustin, Anneliese Hoffmann, Beatriz Barbisan, Bruno Hochhegger, Carlos Emilio Levy, Claudine Sarmento da Veiga, Claudio Ricachinevsky, Concetta Esposito, Dante Escuissato, Diego Brandemburgo, Elisabeth Marques, Evanirso de Aquino, Gilberto Bueno Fischer, Joaquim Carlos Rodrigues, Leonardo Araújo Pinto, Leticia Machado, Lucia Muramato, Luciana Freitas Velloso Monte, Lusmaia Damasceno Camargo Costa, Marcio Donadio, Marcos César Santos de Castro, Maria Angela Ribeiro, Maria Angélica Santana, Mariane Canan, Marina Buarque de Almeida, Murilo Britto, Paulo Roth Tarso Dalcin, Regina Terse Trindade Ramos, Sonia Chiba, and Valéria de Carvalho Martins.
Gastrointestinal and nutritional treatment: Claudine Lacerda, Eliana Barbosa, Elizabet Vilar Guimarães, Gabriel Hessel, Jocemara Gurmini, Lenycia Neri, Marcelo Coelho Nogueira, Mônica Chang Wayhs, and Miriam Isabel Santos Simon.
Other aspects: Arlene Gonçalves dos Santos Fernandes, Claudia de Castro de Silva, Concetta Esposito, Cristiano Túlio Maciel Albuquerque, Edna Lúcia Souza, Fernando Antonio de Abreu e Silva, Paulo de Tarso Dalcin, Renata Maria de Noronha, Ricardo Teixeira, Sandra Helena Machado, Spencer Marcantonio Camargo, Tatiana Rozov, Ticiana da Costa Rodrigues, and Valéria de Carvalho Martins.
*Study organized by the Grupo Brasileiro de Estudos de Fibrose Cística, with the support of the Sociedade Brasileira de Pneumologia e Tisiologia, Brasília (DF) and the Sociedade Brasileira de Pediatria, Rio de Janeiro (RJ) Brasil.
Financial support: This study received assistance in editorial matters and medical writing from Springer Healthcare, with financial support from Roche Brasil, Teva Brasil, Zambon Laboratórios Farmacêuticos, and Vertex Pharmaceuticals. The authors take full responsibility for the content of this publication. The sponsors had no influence on data collection or analysis or on the decision to publish the information presented here.
REFERENCES
- 1.Raskin S, Pereira-Ferrari L, Reis FC, Abreu F, Marostica P, Rozov T. Incidence of cystic fibrosis in five different states of Brazil as determined by screening of p F508del, mutation at the CFTR gene in newborns and patients. J Cyst Fibros. 2008;7(1):15–22. doi: 10.1016/j.jcf.2007.03.006. https://doi.org/10.1016/j.jcf.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 2.Ong T, Ramsey BW. Update in Cystic Fibrosis 2014. Am J Respir Crit Care Med. 2015;192(6):669–675. doi: 10.1164/rccm.201504-0656UP. https://doi.org/10.1164/rccm.201504-0656UP [DOI] [PubMed] [Google Scholar]
- 3.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372(4):351–362. doi: 10.1056/NEJMra1300109. https://doi.org/10.1056/NEJMra1300109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle MP. Nonclassic cystic fibrosis and CFTR-related diseases. Curr Opin Pulm Med. 2003;9(6):498–503. doi: 10.1097/00063198-200311000-00009. https://doi.org/10.1097/00063198-200311000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Conway S, Balfour-Lynn IM, De Rijcke K, Drevinek P, Foweraker J, Havermans T. European Cystic Fibrosis Society Standards of Care Framework for the Cystic Fibrosis Centre. J Cyst Fibros. 2014;13(1):S3–22. doi: 10.1016/j.jcf.2014.03.009. https://doi.org/10.1016/j.jcf.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahadeva R, Webb K, Westerbeek RC, Carroll NR, Dodd ME, Bilton D. Clinical outcome in relation to care in centres specialising in cystic fibrosis cross sectional study. BMJ. 1998;316(7147):1771–1775. doi: 10.1136/bmj.316.7147.1771. https://doi.org/10.1136/bmj.316.7147.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl MEB. Factors influencing outcomes in cystic fibrosis a center-based analysis. Chest. 2003;123(1):20–27. doi: 10.1378/chest.123.1.20. https://doi.org/10.1378/chest.123.1.20 [DOI] [PubMed] [Google Scholar]
- 8.Bell SC, Robinson PJ, Fitzgerald DS. Cystic fibrosis standards of care Australia. Sidney: Cystic Fibrosis Australia; 2008. [Google Scholar]
- 9.Cystic Fibrosis Trust . Standards for the Clinical Care of Children and Adults with cystic fibrosis in the UK. Second . London: Cystic Fibrosis Trust; 2011. https://www.cysticfibrosis.org.uk/the-work-we-do/clinical-care/consensus-documents [Google Scholar]
- 10.Elborn JS, Bell SC, Madge SL, Burgel PR, Castellani C, Conway S. Report of the European Respiratory Society/European Cystic Fibrosis Society task force on the care of adults with cystic fibrosis. Eur Respir J. 2016;47(2):420–428. doi: 10.1183/13993003.00592-2015. https://doi.org/10.1183/13993003.00592-2015 [DOI] [PubMed] [Google Scholar]
- 11.Okumura MJ, Kleinhenz ME. Cystic Fibrosis Transitions of Care Lessons Learned and Future Directions for Cystic Fibrosis. Clin Chest Med. 2016;37(1):119–126. doi: 10.1016/j.ccm.2015.11.007. https://doi.org/10.1016/j.ccm.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 12.Okumura MJ, Ong T, Dawson D, Nielson D, Lewis N, Richards M. Improving transition from paediatric to adult cystic fibrosis care programme implementation and evaluation. BMJ Qual Saf. 2014;23(1):i64–i72. doi: 10.1136/bmjqs-2013-002364. https://doi.org/10.1136/bmjqs-2013-002364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duguépéroux I, Tamalet A, Sermet-Gaudelus I, Le Bourgeois M, Gérardin M, Desmazes-Dufeu N. Clinical changes of patients with cystic fibrosis during transition from pediatric to adult care ?J Adolesc. Health. 2008;43(5):459–465. doi: 10.1016/j.jadohealth.2008.03.005. https://doi.org/10.1016/j.jadohealth.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 14.Reid GJ, Irvine MJ, McCrindle BW, Sananes R, Ritvo PG, Siu SC. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pt 1Pediatrics. 2004;113(3):e197–e205. doi: 10.1542/peds.113.3.e197. https://doi.org/10.1542/peds.113.3.e197 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry SR, Keaton M, Nasr SZ. Evaluation of a cystic fibrosis transition program from pediatric to adult care. Pediatr Pulmonol. 2013;48(7):658–665. doi: 10.1002/ppul.22647. https://doi.org/10.1002/ppul.22647 [DOI] [PubMed] [Google Scholar]
- 16.Machado CD, Matos MA. Rede de Atenção à Saúde para Pessoas com Fibrose Cística--Padronização dos Cuidados na Fibrose Cística. Condições de oferta dos centros de referência, cuidados compartilhados, cuidados de transição e internação. Belo Horizonte: Secretaria de Estado de Saúde de Minas Gerais; 2017. pp. [1 p.]–[1 p.].http://www.saude.mg.gov.br/images/documentos/Livro%20REDE%20DE%20ATENCaO%20A%20SAUDE%20PARA%20PESSOAS%20COM%20FIBROSE%20CISTICA.pdf [Google Scholar]
- 17.Saiman L, Siegel JD, LiPuma JJ, Brown RF, Bryson EA, Chambers MJ. Infection prevention and control guideline for cystic fibrosis 2013 update. Infect Control Hosp Epidemiol. 2014;35(1):S1–S67. doi: 10.1086/676882. https://doi.org/10.1086/676882 [DOI] [PubMed] [Google Scholar]
- 18.Jain M, Saiman LM, Sabadosa K, LiPuma JJ. Point does the risk of cross infection warrant exclusion of adults with cystic fibrosis from cystic fibrosis foundation events? Yes. Chest. 2014;145(4):678–680. doi: 10.1378/chest.13-2404. https://doi.org/10.1378/chest.13-2404 [DOI] [PubMed] [Google Scholar]
- 19.Conway S. Segregation is good for patients with cystic fibrosis. J R Soc Med. 2008;101(1):S31–S35. doi: 10.1258/jrsm.2008.s18007. https://doi.org/10.1258/jrsm.2008.s18007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya K, Wotton T, Wiley V. The evolution of blood-spot newborn screening. Transl Pediatr. 2014;3(2):63–70. doi: 10.3978/j.issn.2224-4336.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR. Guidelines for diagnosis of cystic fibrosis in newborns through older adults Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. https://doi.org/10.1016/j.jpeds.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos GP, Domingos MT, Wittig EO, Riedi CA, Rosario NA. Neonatal cystic fibrosis screening program in the state of Paraná evaluation 30 months after implementation [Article in Portuguese]. J Pediatr (Rio J) 2005;81(3):240–244. https://doi.org/10.2223/JPED.1345 [PubMed] [Google Scholar]
- 23.Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P. European Cystic Fibrosis Society Standards of Care Best Practice guidelines. J Cyst Fibros. 2014;13(1):S23–S42. doi: 10.1016/j.jcf.2014.03.010. https://doi.org/10.1016/j.jcf.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 24.Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181S:S4–S15.e1. doi: 10.1016/j.jpeds.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 25.Heap S, Griffiths P, Elborn S, Harris B, Wayte A, Wallis CE. Guidelines for the Performance of the Sweat Test for the Investigation of Cystic Fibrosis in the UK, 2nd version. London: Royal College of Paedriatics and Child Health; 2014. pp. [1 p.]–[1 p.].http://www.rcpch.ac.uk/system/files/protected/page/Sweat%20Guideline%20v3%20reformat_2.pdf [Google Scholar]
- 26.LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ., Jr Cystic Fibrosis Foundation Diagnostic sweat testing the Cystic Fibrosis Foundation guidelines. J Pediatr. 2007;151(1):85–89. doi: 10.1016/j.jpeds.2007.03.002. https://doi.org/10.1016/j.jpeds.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Lezana JL, Vargas MH, Karam-Bechara J, Aldana RS, Furuya ME. Sweat conductivity and chloride titration for cystic fibrosis diagnosis in 3834 subjects. J Cyst Fibros. 2003;2(1):1–7. doi: 10.1016/S1569-1993(02)00146-7. https://doi.org/10.1016/S1569-1993(02)00146-7 [DOI] [PubMed] [Google Scholar]
- 28.Mattar AC, Leone C, Rodrigues JC, Adde FV. Sweat conductivity an accurate diagnostic test for cystic fibrosis? J Cyst Fibros. 2014;13(5):528–533. doi: 10.1016/j.jcf.2014.01.002. https://doi.org/10.1016/j.jcf.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 29.Brodlie M, Haq IJ, Roberts K, Elborn JS. Targeted therapies to improve CFTR function in cystic fibrosis. Genome Med. 2015;7:101–101. doi: 10.1186/s13073-015-0223-6. https://doi.org/10.1186/s13073-015-0223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettit RS, Fellner C. CFTR Modulators for the Treatment of Cystic Fibrosis. P T. 2014;39(7):500–511. [PMC free article] [PubMed] [Google Scholar]
- 31.Dequeker E, Stuhrmann M, Morris MA, Casals T, Castellani C, Claustres M. Best practice guidelines for molecular genetic diagnosis of cystic fibrosis and CFTR-related disorders--updated European recommendations. Eur J Hum Genet. 2009;17(1):51–65. doi: 10.1038/ejhg.2008.136. https://doi.org/10.1038/ejhg.2008.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellani C, Cuppens H, Macek M, Jr, Cassiman JJ, Kerem E, Durie P. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7(3):179–196. doi: 10.1016/j.jcf.2008.03.009. https://doi.org/10.1016/j.jcf.2008.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J. Cystic fibrosis terminology and diagnostic algorithms. Thorax. 2006;61(7):627–635. doi: 10.1136/thx.2005.043539. https://doi.org/10.1136/thx.2005.043539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferec C, Cutting GR. Assessing the Disease-Liability of Mutations in CFTR. Cold Spring Harb Perspect Med. 2012;2(12):a009480–a009480. doi: 10.1101/cshperspect.a009480. https://doi.org/10.1101/cshperspect.a009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskowitz SM, Chmiel JF, Sternen DL, Cheng E, Gibson RL, Marshall SG. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet Med. 2008;10(12):851–868. doi: 10.1097/GIM.0b013e31818e55a2. https://doi.org/10.1097/GIM.0b013e31818e55a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beekman JM, Sermet-Gaudelus I, de Boeck K, Gonska T, Derichs N, Mall MA. CFTR functional measurements in human models for diagnosis, prognosis and personalized therapy Report on the pre-conference meeting to the 11th ECFS Basic Science Conference, Malta, 26-29 March 2014. J Cyst Fibros. 2014;13(4):363–372. doi: 10.1016/j.jcf.2014.05.007. https://doi.org/10.1016/j.jcf.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 37.De Boeck K, Kent L, Davies J, Derichs N, Amaral M, Rowe SM. CFTR biomarkers time for promotion to surrogate end-point. Eur Respir J. 2013;41(1):203–216. doi: 10.1183/09031936.00057512. https://doi.org/10.1183/09031936.00057512 [DOI] [PubMed] [Google Scholar]
- 38.Cuthbertson L, Rogers GB, Walker AW, Oliver A, Hafiz T, Hoffman LR. Time between collection and storage significantly influences bacterial sequence composition in sputum samples from cystic fibrosis respiratory infections. J Clin Microbiol. 2014;52(8):3011–3016. doi: 10.1128/JCM.00764-14. https://doi.org/10.1128/JCM.00764-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cystic Fibrosis Trust . Laboratory Standards for Processing Microbiological Samples from People with Cystic Fibrosis. 1st . London: Cystic Fibrosis Trust; 2010. https://www.cysticfibrosis.org.uk/~/media/documents/the-work-we-do/care/consensus-docs-with-new-address/laboratory-standards.ashx?la=en [Google Scholar]
- 40.Lahiri T, Hempstead SE, Brady C, Cannon CL, Clark K, Condren ME, et al. Clinical Practice Guidelines From the Cystic Fibrosis Foundation for Preschoolers With Cystic Fibrosis. Pediatrics. 2016;137(4):e20151784. doi: 10.1542/peds.2015-1784. https://doi.org/10.1542/peds.2015-1784 [DOI] [PubMed] [Google Scholar]
- 41.Kiska DL, Riddell SW. Practical Laboratory Aspects of Cystic Fibrosis Microbiology an Update, Part II. Clin Microbiol Newsl. 2012;34(5):35–41. https://doi.org/10.1016/j.clinmicnews.2012.02.001 [Google Scholar]
- 42.Gilligan PH. Infections in patients with cystic fibrosis diagnostic microbiology update. Clin Lab Med. 2014;34(2):197–217. doi: 10.1016/j.cll.2014.02.001. https://doi.org/10.1016/j.cll.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilligan PH, Kiska Dl, Appleman MD. Cystic fibrosis microbiology. Cumitech 43. Washington, DC: ASM Press; 2006. [Google Scholar]
- 44.Burns JL, Rolain JM. Culture-based diagnostic microbiology in cystic fibrosis can we simplify the complexity? J Cyst Fibros. 2014;13(1):1–9. doi: 10.1016/j.jcf.2013.09.004. https://doi.org/10.1016/j.jcf.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Caballero Jde D, del Campo R, Tato M, Gómez G de la Pedrosa E, Cobo M, López-Causapé C. Microbiological diagnostic procedures for respiratory cystic fibrosis samples in Spain towards standard of care practices. BMC Microbiol. 2014;14:335–335. doi: 10.1186/s12866-014-0335-y. https://doi.org/10.1186/s12866-014-0335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marko DC, Saffert RT, Cunningham SA, Hyman J, Walsh J, Arbefeville S. Evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of nonfermenting gram-negative bacilli isolated from cultures from cystic fibrosis patients. J Clin Microbiol. 2012;50(6):2034–2039. doi: 10.1128/JCM.00330-12. https://doi.org/10.1128/JCM.00330-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habib AR, Manji J, Wilcox PG, Javer AR, Buxton JA, Quon BS. A systematic review of factors associated with health-related quality of life in adolescents and adults with cystic fibrosis. Ann Am Thorac Soc. 2015;12(3):420–428. doi: 10.1513/AnnalsATS.201408-393OC. https://doi.org/10.1513/AnnalsATS.201408-393OC [DOI] [PubMed] [Google Scholar]
- 48.Rosenfeld M, Allen J, Arets BH, Aurora P, Beydon N, Calogero C. An official American Thoracic Society workshop report optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc. 2013;10(2):S1–S11. doi: 10.1513/AnnalsATS.201301-017ST. https://doi.org/10.1513/AnnalsATS.201301-017ST [DOI] [PubMed] [Google Scholar]
- 49.Subbarao P, Milla C, Aurora P, Davies JC, Davis SD, Hall GL. Multiple-Breath Washout as a Lung Function Test in Cystic Fibrosis A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc. 2015;12(6):932–939. doi: 10.1513/AnnalsATS.201501-021FR. https://doi.org/10.1513/AnnalsATS.201501-021FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tridello G, Volpi S, Assael BM, Meneghelli I, Passiu M, Circelli M. Lung function comparison between two decades in cystic fibrosis children A single centre study. Pediatr Pulmonol. 2015;50(12):1237–1243. doi: 10.1002/ppul.23314. https://doi.org/10.1002/ppul.23314 [DOI] [PubMed] [Google Scholar]
- 51.Cleveland RH, Zurakowski D, Slattery DM, Colin AA. Chest radiographs for outcome assessment in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):302–305. doi: 10.1513/pats.200611-179HT. https://doi.org/10.1513/pats.200611-179HT [DOI] [PubMed] [Google Scholar]
- 52.de Jong PA, Lindblad A, Rubin L, Hop WC, de Jongste JC, Brink M. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2006;61(1):80–85. doi: 10.1136/thx.2005.045146. https://doi.org/10.1136/thx.2005.045146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ernst CW, Basten IA, Ilsen B, Buls N, Van Gompel G, De Wachter E. Pulmonary disease in cystic fibrosis assessment with chest CT at chest radiography dose levels. Radiology. 2014;273(2):597–605. doi: 10.1148/radiol.14132201. https://doi.org/10.1148/radiol.14132201 [DOI] [PubMed] [Google Scholar]
- 54.Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;184(7):816–821. doi: 10.1164/rccm.201105-0816OC. https://doi.org/10.1164/rccm.201105-0816OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang EY, Miller RR, Müller NL. Bronchiectasis comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology. 1995;195(3):649–654. doi: 10.1148/radiology.195.3.7753989. https://doi.org/10.1148/radiology.195.3.7753989 [DOI] [PubMed] [Google Scholar]
- 56.Thia LP, Calder A, Stocks J, Bush A, Owens CM, Wallis C. Is chest CT useful in newborn screened infants with cystic fibrosis at 1 year of age. Thorax. 2014;69(4):320–327. doi: 10.1136/thoraxjnl-2013-204176. https://doi.org/10.1136/thoraxjnl-2013-204176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sileo C, Corvol H, Boelle PY, Blondiaux E, Clement A, Ducou Le Pointe H. HRCT and MRI of the lung in children with cystic fibrosis comparison of different scoring systems. J Cyst Fibros. 2014;13(2):198–204. doi: 10.1016/j.jcf.2013.09.003. https://doi.org/10.1016/j.jcf.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 58.Sanders DB, Li Z, Brody AS. Chest computed tomography predicts the frequency of pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc. 2015;12(1):64–69. doi: 10.1513/AnnalsATS.201407-338OC. https://doi.org/10.1513/AnnalsATS.201407-338OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson TE, Leung AN, Chen X, Moss RB, Emond MJ. Cystic fibrosis HRCT scores correlate strongly with Pseudomonas infection. Pediatr Pulmonol. 2009;44(11):1107–1117. doi: 10.1002/ppul.21107. https://doi.org/10.1002/ppul.21107 [DOI] [PubMed] [Google Scholar]
- 60.Agent P, Parrott H. Inhaled therapy in cystic fibrosis agents, devices and regimens. Breathe (Sheff) 2015;11(2):110–118. doi: 10.1183/20734735.021014. https://doi.org/10.1183/20734735.021014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins N. Nebulizer therapy in cystic fibrosis an overview. J R Soc Med. 2009;102(1):11–17. doi: 10.1258/jrsm.2009.s19003. https://doi.org/10.1258/jrsm.2009.s19003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cystic Fibrosis Trust . Nebuliser therapy in cystic fibrosis. London: Cystic Fibrosis Trust; Factsheet; 2013. https://www.cysticfibrosis.org.uk/the-work-we-do [Google Scholar]
- 63.Geller DE. The science of aerosol delivery in cystic fibrosis. Pediatr Pulmonol. 2008;43(S9):S5–S17. https://doi.org/10.1002/ppul.20860 [Google Scholar]
- 64.Daniels T, Mills N, Whitaker P. Nebuliser systems for drug delivery in cystic fibrosis. Cochrane Database Syst Rev. 2013;(4):CD007639–CD007639. doi: 10.1002/14651858.CD007639.pub2. [DOI] [PubMed] [Google Scholar]
- 65.Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi: 10.1183/09031936.00166410. https://doi.org/10.1183/09031936.00166410 [DOI] [PubMed] [Google Scholar]
- 66.Della Zuana A, Garcia Dde O, Juliani RC, Silva LV., Filho Effect that an educational program for cystic fibrosis patients and caregivers has on the contamination of home nebulizers. J Bras Pneumol. 2014;40(2):119–127. doi: 10.1590/S1806-37132014000200004. https://doi.org/10.1590/S1806-37132014000200004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Towle D, Callan DA, Farrel PA, Egan ME, Murray TS. Baby bottle steam sterilizers disinfect home nebulizers inoculated with bacterial respiratory pathogens. J Cyst Fibros. 2013;12(5):512–516. doi: 10.1016/j.jcf.2012.11.013. https://doi.org/10.1016/j.jcf.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 68.Reychler G, Leonard A, Van Ossel C, Godding V, Gigi J, Simon A. Impact of hypochlorite-based disinfection on bacterial contamination of cystic fibrosis patients' home-nebulisers. J Hosp Infect. 2009;72(4):351–357. doi: 10.1016/j.jhin.2009.05.011. https://doi.org/10.1016/j.jhin.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 69.Merritt K, Hitchins VM, Brown SA. Safety and cleaning of medical materials and devices ?J Biomed Mater. Res. 2000;53(2):131–136. doi: 10.1002/(sici)1097-4636(2000)53:2<131::aid-jbm1>3.0.co;2-i. https://doi.org/10.1002/(SICI)1097-4636(2000)53:2<131::AID-JBM1>3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- 70.Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2015;(12):CD001401–CD001401. doi: 10.1002/14651858.CD001401.pub3. https://doi.org/10.1002/14651858.cd001401.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McIlwaine M, Button B, Dwan K. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;(6):CD003147–CD003147. doi: 10.1002/14651858.CD003147.pub4. https://doi.org/10.1002/14651858.cd003147.pub4 [DOI] [PubMed] [Google Scholar]
- 72.McIlwaine MP, Alarie N, Davidson GF, Lands LC, Ratjen F, Milner R. Long-term multicentre randomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax. 2013;68(8):746–751. doi: 10.1136/thoraxjnl-2012-202915. https://doi.org/10.1136/thoraxjnl-2012-202915 [DOI] [PubMed] [Google Scholar]
- 73.Moran F, Bradley JM, Piper AJ. Non-invasive ventilation for cystic fibrosis. Cochrane Database Syst Rev. 2013;(4):CD002769–CD002769. doi: 10.1002/14651858.CD002769.pub4. https://doi.org/10.1002/14651858.cd002769.pub4 [DOI] [PubMed] [Google Scholar]
- 74.Young AC, Wilson JW, Kotsimbos TC, Naughton MT. Randomised placebo controlled trial of non-invasive ventilation for hypercapnia in cystic fibrosis. Thorax. 2008;63(1):72–77. doi: 10.1136/thx.2007.082602. https://doi.org/10.1136/thx.2007.082602 [DOI] [PubMed] [Google Scholar]
- 75.Holland AE, Denehy L, Ntoumenopoulos G, Naughton MT, Wilson JW. Non-invasive ventilation assists chest physiotherapy in adults with acute exacerbations of cystic fibrosis. Thorax. 2003;58(10):880–884. doi: 10.1136/thorax.58.10.880. https://doi.org/10.1136/thorax.58.10.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lester MK, Flume PA. Airway-clearance therapy guidelines and implementation. Respir Care. 2009;54(6):733–750. doi: 10.4187/002013209790983205. https://doi.org/10.4187/002013209790983205 [DOI] [PubMed] [Google Scholar]
- 77.Radtke T, Nolan SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev. 2015;(6):CD002768–CD002768. doi: 10.1002/14651858.CD002768.pub3. https://doi.org/10.1002/14651858.cd002768.pub3 [DOI] [PubMed] [Google Scholar]
- 78.Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter CR, Kelsey S. Strength vs aerobic training in children with cystic fibrosis a randomized controlled trial. Chest. 2004;126(4):1204–1214. doi: 10.1378/chest.126.4.1204. https://doi.org/10.1378/chest.126.4.1204 [DOI] [PubMed] [Google Scholar]
- 79.Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol. 2002;33(3):194–200. doi: 10.1002/ppul.10015. https://doi.org/10.1002/ppul.10015 [DOI] [PubMed] [Google Scholar]
- 80.Dwyer TJ, Elkins MR, Bye PT. The role of exercise in maintaining health in cystic fibrosis. Curr Opin Pulm Med. 2011;17(6):455–460. doi: 10.1097/MCP.0b013e32834b6af4. https://doi.org/10.1097/mcp.0b013e32834b6af4 [DOI] [PubMed] [Google Scholar]
- 81.Schindel CS, Hommerding PX, Melo DA, Baptista RR, Marostica PJ, Donadio MV. Physical exercise recommendations improve postural changes found in children and adolescents with cystic fibrosis a randomized controlled trial. J Pediatr. 2015;166(3):710–716. doi: 10.1016/j.jpeds.2014.12.001. https://doi.org/10.1016/j.jpeds.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 82.Rovedder PM, Flores J, Ziegler B, Casarotto F, Jaques P, Barreto SS. Exercise programme in patients with cystic fibrosis a randomized controlled trial. Respir Med. 2014;108(8):1134–1140. doi: 10.1016/j.rmed.2014.04.022. https://doi.org/10.1016/j.rmed.2014.04.022 [DOI] [PubMed] [Google Scholar]
- 83.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB. Cystic fibrosis pulmonary guidelines chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–969. doi: 10.1164/rccm.200705-664OC. https://doi.org/10.1164/rccm.200705-664OC [DOI] [PubMed] [Google Scholar]
- 84.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–642. doi: 10.1056/NEJM199409083311003. https://doi.org/10.1056/NEJM199409083311003 [DOI] [PubMed] [Google Scholar]
- 85.Jones AP, Wallis C. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2010;(3):CD001127–CD001127. doi: 10.1002/14651858.CD001127.pub2. https://doi.org/10.1002/14651858.cd001127.pub2 [DOI] [PubMed] [Google Scholar]
- 86.Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Jacobs JR, Yegin A. Clinical use of dornase alpha is associated with a slower rate of FEV1 decline in cystic fibrosis. Pediatr Pulmonol. 2011;46(6):545–553. doi: 10.1002/ppul.21388. https://doi.org/10.1002/ppul.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB. Cystic fibrosis pulmonary guidelines Chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187(7):680–689. doi: 10.1164/rccm.201207-1160oe. https://doi.org/10.1164/rccm.201207-1160OE [DOI] [PubMed] [Google Scholar]
- 88.Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139(6):813–820. doi: 10.1067/mpd.2001.118570. https://doi.org/10.1067/mpd.2001.118570 [DOI] [PubMed] [Google Scholar]
- 89.Rozov T, de Oliveira VZ, Santana MA, Adde FV, Mendes RH, Paschoal IA. Dornase alfa improves the health-related quality of life among Brazilian patients with cystic fibrosis--a one-year prospective study. Pediatr Pulmonol. 2010;45(9):874–882. doi: 10.1002/ppul.21267. https://doi.org/10.1002/ppul.21267 [DOI] [PubMed] [Google Scholar]
- 90.McCoy K, Hamilton S, Johnson C. Effects of 12-week administration of dornase alfa in patients with advanced cystic fibrosis lung disease Pulmozyme Study Group. Chest. 1996;110(4):889–895. doi: 10.1378/chest.110.4.889. https://doi.org/10.1378/chest.110.4.889 [DOI] [PubMed] [Google Scholar]
- 91.Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax. 2002;57(10):841–846. doi: 10.1136/thorax.57.10.841. https://doi.org/10.1136/thorax.57.10.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suri R, Metcalfe C, Lees B, Grieve R, Flather M, Normand C. Comparison of hypertonic saline and alternate-day or daily recombinant human deoxyribonuclease in children with cystic fibrosis a randomised trial. Lancet. 2001;358(9290):1316–1321. doi: 10.1016/S0140-6736(01)06412-1. https://doi.org/10.1016/S0140-6736(01)06412-1 [DOI] [PubMed] [Google Scholar]
- 93.Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics. 2005;116(4):e549–e554. doi: 10.1542/peds.2005-0308. https://doi.org/10.1542/peds.2005-0308 [DOI] [PubMed] [Google Scholar]
- 94.Yang C, Chilvers M, Montgomery M, Nolan SJ. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2016;4:CD001127–CD001127. doi: 10.1002/14651858.CD001127.pub3. https://doi.org/10.1002/14651858.cd001127.pub3 [DOI] [PubMed] [Google Scholar]
- 95.Berge MT, Wiel E, Tiddens HA, Merkus PJ, Hop WC, de Jongste JC. DNase in stable cystic fibrosis infants a pilot study. J Cyst Fibros. 2003;2(4):183–188. doi: 10.1016/S1569-1993(03)00090-0. https://doi.org/10.1016/S1569-1993(03)00090-0 [DOI] [PubMed] [Google Scholar]
- 96.Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest x-rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than age 5 years a preliminary study. Pediatr Pulmonol. 2001;31(5):377–382. doi: 10.1002/ppul.1061. https://doi.org/10.1002/ppul.1061 [DOI] [PubMed] [Google Scholar]
- 97.McKenzie SG, Chowdhury S, Strandvik B, Hodson ME. Investigators of the Epidemiologic Registry of Cystic Fibrosis Dornase alfa is well tolerated data from the epidemiologic registry of cystic fibrosis. Pediatr Pulmonol. 2007;42(10):928–937. doi: 10.1002/ppul.20685. https://doi.org/10.1002/ppul.20685 [DOI] [PubMed] [Google Scholar]
- 98.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. https://doi.org/10.1056/NEJMoa043900 [DOI] [PubMed] [Google Scholar]
- 99.Wark PA, McDonald V. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2000;(2):CD001506–CD001506. doi: 10.1002/14651858.CD001506. [DOI] [PubMed] [Google Scholar]
- 100.Dentice RL, Elkins MR, Middleton PG, Bishop JR, Wark PA, Dorahy DJ. A randomised trial of hypertonic saline during hospitalisation for exacerbation of cystic fibrosis. Thorax. 2016;71(2):141–147. doi: 10.1136/thoraxjnl-2014-206716. https://doi.org/10.1136/thoraxjnl-2014-206716 [DOI] [PubMed] [Google Scholar]
- 101.Aitken ML, Bellon G, De Boeck K, Flume PA, Fox HG, Geller DE. Long-term inhaled dry powder mannitol in cystic fibrosis an international randomized study. Am J Respir Crit Care Med. 2012;185(6):645–652. doi: 10.1164/rccm.201109-1666OC. https://doi.org/10.1164/rccm.201109-1666OC [DOI] [PubMed] [Google Scholar]
- 102.Bilton D, Robinson P, Cooper P, Gallagher CG, Kolbe J, Fox H. Inhaled dry powder mannitol in cystic fibrosis an efficacy and safety study. Eur Respir J. 2011;38(5):1071–1080. doi: 10.1183/09031936.00187510. https://doi.org/10.1183/09031936.00187510 [DOI] [PubMed] [Google Scholar]
- 103.Bilton D, Bellon G, Charlton B, Cooper P, De Boeck K, Flume PA. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. J Cyst Fibros. 2013;12(4):367–376. doi: 10.1016/j.jcf.2012.11.002. https://doi.org/10.1016/j.jcf.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 104.Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev. 2014;(11):CD004197–CD004197. doi: 10.1002/14651858.CD004197.pub4. https://doi.org/10.1002/14651858.cd004197.pub4 [DOI] [PubMed] [Google Scholar]
- 105.Proesmans M, Vermeulen F, Boulanger L, Verhaegen J, De Boeck K. Comparison of two treatment regimens for eradication of Pseudomonas aeruginosa infection in children with cystic fibrosis. J Cyst Fibros. 2013;12(1):29–34. doi: 10.1016/j.jcf.2012.06.001. https://doi.org/10.1016/j.jcf.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 106.Ratjen F, Munck A, Kho P, Angyalosi G. ELITE Study Group Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis the ELITE trial. Thorax. 2010;65(4):286–291. doi: 10.1136/thx.2009.121657. https://doi.org/10.1136/thx.2009.121657 [DOI] [PubMed] [Google Scholar]
- 107.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165(9):847–856. doi: 10.1001/archpediatrics.2011.136. https://doi.org/10.1001/archpediatrics.2011.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vandamme P, Dawyndt P. Classification and identification of the Burkholderia cepacia complex Past, present and future. Syst Appl Microbiol. 2011;34(2):87–95. doi: 10.1016/j.syapm.2010.10.002. https://doi.org/10.1016/j.syapm.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 109.Depoorter E, Bull MJ, Peeters C, Coenye T, Vandamme P, Mahenthiralingam E. Burkholderia an update on taxonomy and biotechnological potential as antibiotic producers. Appl Microbiol Biotechnol. 2016;100(12):5215–5229. doi: 10.1007/s00253-016-7520-x. https://doi.org/10.1007/s00253-016-7520-x [DOI] [PubMed] [Google Scholar]
- 110.Zlosnik JE, Zhou G, Brant R, Henry DA, Hird TJ, Mahenthiralingam E. Burkholderia species infections in patients with cystic fibrosis in British Columbia, Canada 30 years' experience. Ann Am Thorac Soc. 2015;12(1):70–78. doi: 10.1513/AnnalsATS.201408-395OC. https://doi.org/10.1513/AnnalsATS.201408-395OC [DOI] [PubMed] [Google Scholar]
- 111.Horsley A, Webb K, Bright-Thomas R, Govan J, Jones A. Can early Burkholderia cepacia complex infection in cystic fibrosis be eradicated with antibiotic therapy. Front Cell Infect Microbiol. 2011;1:18–18. doi: 10.3389/fcimb.2011.00018. https://doi.org/10.3389/fcimb.2011.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303(23):2386–2392. doi: 10.1001/jama.2010.791. https://doi.org/10.1001/jama.2010.791 [DOI] [PubMed] [Google Scholar]
- 113.Vanderhelst E, De Wachter E, Willekens J, Pierard D, Vincken W, Malfroot A. Eradication of chronic methicillin-resistant Staphylococcus aureus infection in cystic fibrosis patients An observational prospective cohort study of 11 patients. J Cyst Fibros. 2013;12(6):662–666. doi: 10.1016/j.jcf.2013.04.009. https://doi.org/10.1016/j.jcf.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 114.Hall H, Gadhok R, Alshafi K, Bilton D, Simmonds NJ. Eradication of respiratory tract MRSA at a large adult cystic fibrosis centre. Respir Med. 2015;109(3):357–363. doi: 10.1016/j.rmed.2015.01.013. https://doi.org/10.1016/j.rmed.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 115.Vallières E, Rendall JC, Moore JE, McCaughan J, Hoeritzauer AI, Tunney MM, et al. MRSA eradication of newly acquired lower respiratory tract infection in cystic fibrosis. ERJ Open Res. 2016;2(1):00064–02015. doi: 10.1183/23120541.00064-2015. https://doi.org/10.1183/23120541.00064-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Macfarlane M, Leavy A, McCaughan J, Fair R, Reid AJ. Successful decolonization of methicillin-resistant Staphylococcus aureus in paediatric patients with cystic fibrosis (CF) using a three-step protocol. J Hosp Infect. 2007;65(3):231–236. doi: 10.1016/j.jhin.2006.10.011. https://doi.org/10.1016/j.jhin.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 117.Ryan G, Singh M, Dwan K. Inhaled antibiotics for long-term therapy in cystic fibrosis. Cochrane Database Syst Rev. 2011;(3):CD001021–CD001021. doi: 10.1002/14651858.CD001021.pub2. https://doi.org/10.1002/14651858.cd001021.pub2 [DOI] [PubMed] [Google Scholar]
- 118.Cystic Fibrosis Trust . Antibiotic Treatment for Cystic Fibrosis. Third . London: Cystic Fibrosis Trust; 2009. https://www.cysticfibrosis.org.uk/the-work-we-do [Google Scholar]
- 119.Silva LV, Filho, Ferreira Fde A, Reis FJ, Britto MC, Levy CE, Clark O. Pseudomonas aeruginosa infection in patients with cystic fibrosis scientific evidence regarding clinical impact, diagnosis, and treatment. J Bras Pneumol. 2013;39(4):495–512. doi: 10.1590/S1806-37132013000400015. https://doi.org/10.1590/S1806-37132013000400015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. https://doi.org/10.1016/S1569-1993(02)00141-8 [DOI] [PubMed] [Google Scholar]
- 121.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328(24):1740–1746. doi: 10.1056/NEJM199306173282403. https://doi.org/10.1056/NEJM199306173282403 [DOI] [PubMed] [Google Scholar]
- 122.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. https://doi.org/10.1056/NEJM199901073400104 [DOI] [PubMed] [Google Scholar]
- 123.Oermann CM, Retsch-Bogart GZ, Quittner AL, Gibson RL, McCoy KS, Montgomery AB. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol. 2010;45(11):1121–1134. doi: 10.1002/ppul.21301. https://doi.org/10.1002/ppul.21301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balfour-Lynn IM. Clinical Guidelines: Care of Children with Cystic Fibrosis. Royal Brompton Hospital. 6th edition. London: Royal Brompton Hospital; 2014. [Google Scholar]
- 125.Konstan MW, Flume PA, Kappler M, Chiron R, Higgins M, Brockhaus F. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients The EAGER trial. J Cyst Fibros. 2011;10(1):54–61. doi: 10.1016/j.jcf.2010.10.003. https://doi.org/10.1016/j.jcf.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Littlewood KJ, Higashi K, Jansen JP, Capkun-Niggli G, Balp MM, Doering G. A network meta-analysis of the efficacy of inhaled antibiotics for chronic Pseudomonas infections in cystic fibrosis. J Cyst Fibros. 2012;11(5):419–426. doi: 10.1016/j.jcf.2012.03.010. https://doi.org/10.1016/j.jcf.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 127.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T. Cystic Fibrosis Foundation pulmonary guideline pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc. 2014;11(10):1640–1650. doi: 10.1513/AnnalsATS.201404-166OC. https://doi.org/10.1513/AnnalsATS.201404-166OC [DOI] [PubMed] [Google Scholar]
- 128.Clement A, Tamalet A, Leroux E, Ravilly S, Fauroux B, Jais JP. Long term effects of azithromycin in patients with cystic fibrosis A double blind, placebo controlled trial. Thorax. 2006;61(10):895–902. doi: 10.1136/thx.2005.057950. https://doi.org/10.1136/thx.2005.057950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis a randomised, placebo-controlled crossover trial. Lancet. 2002;360(9338):978–984. doi: 10.1016/s0140-6736(02)11081-6. https://doi.org/10.1016/S0140-6736(02)11081-6 [DOI] [PubMed] [Google Scholar]
- 130.Florescu DF, Murphy PJ, Kalil AC. Effects of prolonged use of azithromycin in patients with cystic fibrosis A meta-analysis. Pulm Pharmacol Ther. 2009;22(6):467–472. doi: 10.1016/j.pupt.2009.03.002. https://doi.org/10.1016/j.pupt.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 131.Kabra SK, Pawaiya R, Lodha R, Kapil A, Kabra M, Vani AS. Long-term daily high and low doses of azithromycin in children with cystic fibrosis a randomized controlled trial. J Cyst Fibros. 2010;9(1):17–23. doi: 10.1016/j.jcf.2009.09.001. https://doi.org/10.1016/j.jcf.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 132.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa a randomized controlled trial. JAMA. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. https://doi.org/10.1001/jama.290.13.1749 [DOI] [PubMed] [Google Scholar]
- 133.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203–CD002203. doi: 10.1002/14651858.CD002203.pub4. https://doi.org/10.1002/14651858.cd002203.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa a randomized controlled trial. JAMA. 2010;303(17):1707–1715. doi: 10.1001/jama.2010.563. https://doi.org/10.1001/jama.2010.563 [DOI] [PubMed] [Google Scholar]
- 135.Tramper-Stranders GA, Wolfs TF, Fleer A, Kimpen JL, van der Ent CK. Maintenance azithromycin treatment in pediatric patients with cystic fibrosis long-term outcomes related to macrolide resistance and pulmonary function. Pediatr Infect Dis J. 2007;26(1):8–12. doi: 10.1097/01.inf.0000247109.44249.ac. https://doi.org/10.1097/01.inf.0000247109.44249.ac [DOI] [PubMed] [Google Scholar]
- 136.Willekens J, Eyns H, Malfroot A. How long should we maintain long-term azithromycin treatment in cystic fibrosis patients. Pediatr Pulmonol. 2015;50(1):103–104. doi: 10.1002/ppul.22981. https://doi.org/10.1002/ppul.22981 [DOI] [PubMed] [Google Scholar]
- 137.Nick JA, Moskowitz SM, Chmiel JF, Forssén AV, Kim SH, Saavedra MT. Azithromycin may antagonize inhaled tobramycin when targeting Pseudomonas aeruginosa in cystic fibrosis. Ann Am Thorac Soc. 2014;11(3):342–350. doi: 10.1513/AnnalsATS.201310-352OC. https://doi.org/10.1513/AnnalsATS.201310-352OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ. Cystic fibrosis pulmonary guidelines treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–808. doi: 10.1164/rccm.200812-1845PP. https://doi.org/10.1164/rccm.200812-1845PP [DOI] [PubMed] [Google Scholar]
- 139.de Groot R, Smith AL. Antibiotic pharmacokinetics in cystic fibrosis Differences and clinical significance. Clin Pharmacokinet. 1987;13(4):228–253. doi: 10.2165/00003088-198713040-00002. https://doi.org/10.2165/00003088-198713040-00002 [DOI] [PubMed] [Google Scholar]
- 140.Plummer A, Wildman M. Duration of intravenous antibiotic therapy in people with cystic fibrosis. Cochrane Database Syst Rev. 2011;(1):CD006682–CD006682. doi: 10.1002/14651858.CD006682.pub3. https://doi.org/10.1002/14651858.cd006682.pub3 [DOI] [PubMed] [Google Scholar]
- 141.Collaco JM, Green DM, Cutting GR, Naughton KM, Mogayzel PJ., Jr Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med. 2010;182(9):1137–1143. doi: 10.1164/rccm.201001-0057OC. https://doi.org/10.1164/rccm.201001-0057OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.VanDevanter DR, O'Riordan MA, Blumer JL, Konstan MW. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010;11:137–137. doi: 10.1186/1465-9921-11-137. https://doi.org/10.1186/1465-9921-11-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hurley MN, Prayle AP, Flume P. Intravenous antibiotics for pulmonary exacerbations in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;(7):CD009730–CD009730. doi: 10.1002/14651858.CD009730.pub2. https://doi.org/10.1002/14651858.cd009730.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Döring G, Conway SP, Heijerman HG, Hodson ME, Høiby N, Smyth A. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis a European consensus. Eur Respir J. 2000;16(4):749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. https://doi.org/10.1034/j.1399-3003.2000.16d30.x [DOI] [PubMed] [Google Scholar]
- 145.Balfour-Lynn IM, Field DJ, Gringras P, Hicks B, Jardine E, Jones RC. BTS guidelines for home oxygen in children. Thorax. 2009;64(2):ii1–i26. doi: 10.1136/thx.2009.116020. https://doi.org/10.1136/thx.2009.116020 [DOI] [PubMed] [Google Scholar]
- 146.Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database Syst Rev. 2013;(7):CD003884–CD003884. doi: 10.1002/14651858.CD003884.pub4. https://doi.org/10.1002/14651858.cd003884.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC. Cystic fibrosis pulmonary guidelines pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182(3):298–306. doi: 10.1164/rccm.201002-0157OC. https://doi.org/10.1164/rccm.201002-0157OC [DOI] [PubMed] [Google Scholar]
- 148.Stevens DA, Moss RB, Kurup VP, Knutsen AP, Greenberger P, Judson MA. Allergic bronchopulmonary aspergillosis in cystic fibrosis--state of the art Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis. 2003;37(3):S225–S264. doi: 10.1086/376525. https://doi.org/10.1086/376525 [DOI] [PubMed] [Google Scholar]
- 149.Elphick HE, Southern KW. Antifungal therapies for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2014;(11):CD002204–CD002204. doi: 10.1002/14651858.CD002204.pub3. https://doi.org/10.1002/14651858.cd002204.pub3 [DOI] [PubMed] [Google Scholar]
- 150.Dhooria S, Agarwal R. Diagnosis of allergic bronchopulmonary aspergillosis a case-based approach. Future Microbiol. 2014;9(10):1195–1208. doi: 10.2217/fmb.14.74. https://doi.org/10.2217/fmb.14.74 [DOI] [PubMed] [Google Scholar]
- 151.Agarwal R, Chakrabarti A, Shah A, Gupta D, Meis JF, Guleria R. Allergic bronchopulmonary aspergillosis review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy. 2013;43(8):850–873. doi: 10.1111/cea.12141. https://doi.org/10.1111/cea.12141 [DOI] [PubMed] [Google Scholar]
- 152.Mahdavinia M, Grammer LC. Management of allergic bronchopulmonary aspergillosis a review and update. Ther Adv Respir Dis. 2012;6(3):173–187. doi: 10.1177/1753465812443094. https://doi.org/10.1177/1753465812443094 [DOI] [PubMed] [Google Scholar]
- 153.Cohen-Cymberknoh M, Blau H, Shoseyov D, Mei-Zahav M, Efrati O, Armoni S. Intravenous monthly pulse methylprednisolone treatment for ABPA in patients with cystic fibrosis. J Cyst Fibros. 2009;8(4):253–257. doi: 10.1016/j.jcf.2009.04.008. https://doi.org/10.1016/j.jcf.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 154.Wong R, Wong M, Robinson PD, Fitzgerald DA. Omalizumab in the management of steroid dependent allergic bronchopulmonary aspergillosis (ABPA) complicating cystic fibrosis. Paediatr Respir Rev. 2013;14(1):22–24. doi: 10.1016/j.prrv.2012.11.004. https://doi.org/10.1016/j.prrv.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 155.Casciaro R, Naselli A, Cresta F, Ros M, Castagnola E, Minicucci L. Role of nebulized amphotericin B in the management of allergic bronchopulmonary aspergillosis in cystic fibrosis Case report and review of literature. J Chemother. 2015;27(5):307–311. doi: 10.1179/1973947814Y.0000000194. [DOI] [PubMed] [Google Scholar]
- 156.Aaron SD, Vandemheen KL, Freitag A, Pedder L, Cameron W, Lavoie A. Treatment of Aspergillus fumigatus in patients with cystic fibrosis a randomized, placebo-controlled pilot study. PLoS One. 2012;7(4):e36077. doi: 10.1371/journal.pone.0036077. https://doi.org/10.1371/journal.pone.0036077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest. 2006;130(1):222–226. doi: 10.1378/chest.130.1.222. https://doi.org/10.1378/chest.130.1.222 [DOI] [PubMed] [Google Scholar]
- 158.Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis correction of the underlying CFTR defect. Lancet Respir Med. 2013;1(2):158–163. doi: 10.1016/S2213-2600(12)70057-7. https://doi.org/10.1016/S2213-2600(12)70057-7 [DOI] [PubMed] [Google Scholar]
- 159.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13(6):674–680. doi: 10.1016/j.jcf.2014.09.005. https://doi.org/10.1016/j.jcf.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 160.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190(2):175–184. doi: 10.1164/rccm.201404-0703OC. https://doi.org/10.1164/rccm.201404-0703OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Taylor-Cousar J, Niknian M, Gilmartin G, Pilewski JM. VX11-770-901 investigators Effect of ivacaftor in patients with advanced cystic fibrosis and a G551D-CFTR mutation Safety and efficacy in an expanded access program in the United States. J Cyst Fibros. 2016;15(1):116–122. doi: 10.1016/j.jcf.2015.01.008. https://doi.org/10.1016/j.jcf.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 162.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Resp Crit Care Med. 2013;187(11):1219–1225. doi: 10.1164/rccm.201301-0153OC. https://doi.org/10.1164/rccm.201301-0153OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevínek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. https://doi.org/10.1056/NEJMoa1105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363(21):1991–2003. doi: 10.1056/NEJMoa0909825. https://doi.org/10.1056/NEJMoa0909825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kerem E, Konstan MW, De Boeck K, Accurso FJ, Sermet-Gaudelus I, Wilschanski M. Ataluren for the treatment of nonsense-mutation cystic fibrosis a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med. 2014;2(7):539–547. doi: 10.1016/S2213-2600(14)70100-6. https://doi.org/10.1016/S2213-2600(14)70100-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2(7):527–538. doi: 10.1016/S2213-2600(14)70132-8. https://doi.org/10.1016/S2213-2600(14)70132-8 [DOI] [PubMed] [Google Scholar]
- 167.Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;28(2):331–346. doi: 10.1016/j.ccm.2007.02.002. https://doi.org/10.1016/j.ccm.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 168.Fennell PB, Quante J, Wilson K, Boyle M, Strunk R, Ferkol T. Use of high-dose ibuprofen in a pediatric cystic fibrosis center. J Cyst Fibros. 2007;6(2):153–158. doi: 10.1016/j.jcf.2006.06.003. https://doi.org/10.1016/j.jcf.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 169.Flume PA, Van Devanter DR. State of progress in treating cystic fibrosis respiratory disease. BMC Med. 2012;10:88–88. doi: 10.1186/1741-7015-10-88. https://doi.org/10.1186/1741-7015-10-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–854. doi: 10.1056/NEJM199503303321303. https://doi.org/10.1056/NEJM199503303321303 [DOI] [PubMed] [Google Scholar]
- 171.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–1089. doi: 10.1164/rccm.200702-181OC. https://doi.org/10.1164/rccm.200702-181OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lands LC, Stanojevic S. Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;(6):CD001505–CD001505. doi: 10.1002/14651858.CD001505.pub3. https://doi.org/10.1002/14651858.cd001505.pub3 [DOI] [PubMed] [Google Scholar]
- 173.Chmiel JF, Konstan MW, Accurso FJ, Lymp J, Mayer-Hamblett N, VanDevanter DR. Use of ibuprofen to assess inflammatory biomarkers in induced sputum Implications for clinical trials in cystic fibrosis. J Cyst Fibros. 2015;14(6):720–726. doi: 10.1016/j.jcf.2015.03.007. https://doi.org/10.1016/j.jcf.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 174.Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis Canadian safety and effectiveness trial. J Pediatr. 2007;151(3):249–254. doi: 10.1016/j.jpeds.2007.04.009. https://doi.org/10.1016/j.jpeds.2007.04.009 [DOI] [PubMed] [Google Scholar]
- 175.Balfour-Lynn IM, Welch K. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev. 2014;(10):CD001915–CD001915. doi: 10.1002/14651858.CD001915.pub4. https://doi.org/10.1002/14651858.cd001915.pub4 [DOI] [PubMed] [Google Scholar]
- 176.Cheng K, Ashby D, Smyth RL. Oral steroids for long-term use in cystic fibrosis. Cochrane Database Syst Rev. 2013;(6):CD000407–CD000407. doi: 10.1002/14651858.CD000407.pub3. https://doi.org/10.1002/14651858.cd000407.pub3 [DOI] [PubMed] [Google Scholar]
- 177.Halfhide C, Evans HJ, Couriel J. WITHDRAWN Inhaled bronchodilators for cystic fibrosis. Cochrane Database Syst Rev. 2016;2:CD003428–CD003428. doi: 10.1002/14651858.CD003428.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ratjen F, Koker P, Geller DE, Langellier-Cocteaux B, Le Maulf F, Kattenbeck S. Tiotropium Respimat in cystic fibrosis Phase 3 and Pooled phase 2/3 randomized trials. J Cyst Fibros. 2015;14(5):608–614. doi: 10.1016/j.jcf.2015.03.004. https://doi.org/10.1016/j.jcf.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 179.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F. An official ATS/IDSA statement diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. https://doi.org/10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 180.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(1):i1–22. doi: 10.1136/thoraxjnl-2015-207360. https://doi.org/10.1136/thoraxjnl-2015-207360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Carlyle BE, Borowitz DS, Glick PL. A review of pathophysiology and management of fetuses and neonates with meconium ileus for the pediatric surgeon. J Pediatr Surg. 2012;47(4):772–781. doi: 10.1016/j.jpedsurg.2012.02.019. https://doi.org/10.1016/j.jpedsurg.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 182.Guimarães EV, Schettino GC, Camargos PA, Penna FJ. Prevalence of hyponatremia at diagnosis and factors associated with the longitudinal variation in serum sodium levels in infants with cystic fibrosis. J Pediatr. 2012;161(2):285–289. doi: 10.1016/j.jpeds.2012.01.052. https://doi.org/10.1016/j.jpeds.2012.01.052 [DOI] [PubMed] [Google Scholar]
- 183.Coates AJ, Crofton PM, Marshall T. Evaluation of salt supplementation in CF infants. J Cyst Fibros. 2009;8(6):382–385. doi: 10.1016/j.jcf.2009.08.006. https://doi.org/10.1016/j.jcf.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 184.Cystic Fibrosis Foundation Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 Suppl):S73–S93. doi: 10.1016/j.jpeds.2009.09.001. https://doi.org/10.1016/j.jpeds.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O'Sullivan BP, Baker D, Leung KG, Reed G, Baker SS, Borowitz D. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013;162(4):808–812. doi: 10.1016/j.jpeds.2012.10.008. https://doi.org/10.1016/j.jpeds.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 186.Stern RC, Eisenberg JD, Wagener JS, Ahrens R, Rock M, doPico G. A comparison of the efficacy and tolerance of pancrelipase and placebo in the treatment of steatorrhea in cystic fibrosis patients with clinical exocrine pancreatic insufficiency. Am J Gastroenterol. 2000;95(8):1932–1938. doi: 10.1111/j.1572-0241.2000.02244.x. https://doi.org/10.1111/j.1572-0241.2000.02244.x [DOI] [PubMed] [Google Scholar]
- 187.Colombo C, Ellemunter H, Houwen R, Munck A, Taylor C, Wilschanski M. Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. J Cyst Fibros. 2011;10(2):S24–S28. doi: 10.1016/S1569-1993(11)60005-2. https://doi.org/10.1016/S1569-1993(11)60005-2 [DOI] [PubMed] [Google Scholar]
- 188.Subhi R, Ooi R, Finlayson F, Kotsimbos T, Wilson J, Lee WR. Distal intestinal obstruction syndrome in cystic fibrosis presentation, outcome and management in a tertiary hospital (2007-2012) ANZ J Surg. 2014;84(10):740–744. doi: 10.1111/ans.12397. https://doi.org/10.1111/ans.12397 [DOI] [PubMed] [Google Scholar]
- 189.Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10(2):S29–S36. doi: 10.1016/S1569-1993(11)60006-4. https://doi.org/10.1016/S1569-1993(11)60006-4 [DOI] [PubMed] [Google Scholar]
- 190.Sokol RJ, Durie PR. Recommendations for management of liver and biliary tract disease in cystic fibrosis Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. J Pediatr Gastroenterol Nutr. 1999;28(1):S1–13. doi: 10.1097/00005176-199900001-00001. https://doi.org/10.1097/00005176-199900001-00001 [DOI] [PubMed] [Google Scholar]
- 191.Williams SG, Westaby D, Tanner MS, Mowat AP. Liver and biliary problems in cystic fibrosis. Br Med Bull. 1992;48(4):877–892. doi: 10.1093/oxfordjournals.bmb.a072583. https://doi.org/10.1093/oxfordjournals.bmb.a072583 [DOI] [PubMed] [Google Scholar]
- 192.Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13(6):529–536. doi: 10.1097/MCP.0b013e3282f10a16. https://doi.org/10.1097/MCP.0b013e3282f10a16 [DOI] [PubMed] [Google Scholar]
- 193.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–259. doi: 10.1097/00005176-200209000-00004. https://doi.org/10.1097/00005176-200209000-00004 [DOI] [PubMed] [Google Scholar]
- 194.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Clinical Practice Guidelines on Growth and Nutrition Subcommittee Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. https://doi.org/10.1016/j.jada.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 195.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. https://doi.org/10.2471/BLT.07.043497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children's Fund. Geneva: WHO; 2009. [PubMed] [Google Scholar]
- 197.Alicandro G, Battezzati A, Bianchi ML, Loi S, Speziali C, Bisogno A. Estimating body composition from skinfold thicknesses and bioelectrical impedance analysis in cystic fibrosis patients. J Cyst Fibros. 2015;14(6):784–791. doi: 10.1016/j.jcf.2015.07.011. https://doi.org/10.1016/j.jcf.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 198.Francis DK, Smith J, Saljuqi T, Watling RM. Oral protein calorie supplementation for children with chronic disease. Cochrane Database Syst Rev. 2015;(5):CD001914–CD001914. doi: 10.1002/14651858.CD001914.pub2. https://doi.org/10.1002/14651858.cd001914.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smyth RL, Rayner O. Oral calorie supplements for cystic fibrosis. Cochrane Database Syst Rev. 2014;(11):CD000406–CD000406. doi: 10.1002/14651858.CD000406.pub4. https://doi.org/10.1002/14651858.cd000406.pub4 [DOI] [PubMed] [Google Scholar]
- 200.Engelen MP, Com G, Deutz NE. Protein is an important but undervalued macronutrient in the nutritional care of patients with cystic fibrosis. Curr Opin Clin Nutr Metab Care. 2014;17(6):515–520. doi: 10.1097/MCO.0000000000000100. https://doi.org/10.1097/MCO.0000000000000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Trabulsi J, Schall JI, Ittenbach RF, Olsen IE, Yudkoff M, Daikhin Y. Energy balance and the accuracy of reported energy intake in preadolescent children with cystic fibrosis. Am J Clin Nutr. 2006;84(3):523–530. doi: 10.1093/ajcn/84.3.523. [DOI] [PubMed] [Google Scholar]
- 202.Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG. Nutrition in patients with cystic fibrosis a European Consensus. J Cyst Fibros. 2002;1(2):51–75. doi: 10.1016/s1569-1993(02)00032-2. https://doi.org/10.1016/S1569-1993(02)00032-2 [DOI] [PubMed] [Google Scholar]
- 203.Maqbool A, Stallings VA. Update on fat-soluble vitamins in cystic fibrosis. Curr Opin Pulm Med. 2008;14(6):574–581. doi: 10.1097/MCP.0b013e3283136787. https://doi.org/10.1097/MCP.0b013e3283136787 [DOI] [PubMed] [Google Scholar]
- 204.Okebukola PO, Kansra S, Barrett J. Vitamin E supplementation in people with cystic fibrosis. Cochrane Database Syst Rev. 2014;(12):CD009422–CD009422. doi: 10.1002/14651858.CD009422.pub2. https://doi.org/10.1002/14651858.cd009422.pub2 [DOI] [PubMed] [Google Scholar]
- 205.Bonifant CM, Shevill E, Chang AB. Vitamin A supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2014;(5):CD006751–CD006751. doi: 10.1002/14651858.CD006751.pub4. https://doi.org/10.1002/14651858.cd006751.pub4 [DOI] [PubMed] [Google Scholar]
- 206.Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85(5):1307–1311. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 207.Jagannath VA, Fedorowicz Z, Thaker V, Chang AB. Vitamin K supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2015;1:CD008482–CD008482. doi: 10.1002/14651858.CD008482.pub4. https://doi.org/10.1002/14651858.cd008482.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J. Diabetes-related mortality in adults with cystic fibrosis Role of genotype and sex. Am J Respir Crit Care Med. 2015;191(2):194–200. doi: 10.1164/rccm.201403-0576OC. https://doi.org/10.1164/rccm.201403-0576OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G. Clinical care guidelines for cystic fibrosis-related diabetes a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. https://doi.org/10.2337/dc10-1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moran A, Pillay K, Becker DJ, Acerini CL. International Society for Pediatric and Adolescent Diabetes ISPAD Clinical Practice Consensus Guidelines 2014 Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2014;15(20):65–76. doi: 10.1111/pedi.12178. https://doi.org/10.1111/pedi.12178 [DOI] [PubMed] [Google Scholar]
- 211.Middleton PG, Wagenaar M, Matson AG, Craig ME, Holmes-Walker DJ, Katz T. Australian standards of care for cystic fibrosis-related diabetes. Respirology. 2014;19(2):185–192. doi: 10.1111/resp.12227. https://doi.org/10.1111/resp.12227 [DOI] [PubMed] [Google Scholar]
- 212.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis five year prospective study. BMJ. 1995;311(7006):655–659. doi: 10.1136/bmj.311.7006.655. https://doi.org/10.1136/bmj.311.7006.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sermet-Gaudelus I, Castanet M, Retsch-Bogart G, Aris RM. Update on cystic fibrosis-related bone disease a special focus on children. Paediatr Respir Rev. 2009;10(3):134–142. doi: 10.1016/j.prrv.2009.05.001. https://doi.org/10.1016/j.prrv.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 214.Sunni M, Bellin MD, Moran A. Exogenous insulin requirements do not differ between youth and adults with cystic fibrosis related diabetes. Pediatr Diabetes. 2013;14(4):295–298. doi: 10.1111/pedi.12014. https://doi.org/10.1111/pedi.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–1896. doi: 10.1210/jc.2004-1629. https://doi.org/10.1210/jc.2004-1629 [DOI] [PubMed] [Google Scholar]
- 216.Haworth CS, Jones AM, Adams JE, Selby PL, Webb AK. Randomised double blind placebo controlled trial investigating the effect of calcium and vitamin D supplementation on bone mineral density and bone metabolism in adult patients with cystic fibrosis. J Cyst Fibros. 2004;3(4):233–236. doi: 10.1016/j.jcf.2004.08.002. https://doi.org/10.1016/j.jcf.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 217.Conwell LS, Chang AB. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2014;(3):CD002010–CD002010. doi: 10.1002/14651858.CD002010.pub4. https://doi.org/10.1002/14651858.cd002010.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Santolim TQ, Santos LA, Giovani AM, Dias VC. The strategic role of the nurse in the selection of IV devices. Br J Nurs. 2012;21(21):S28, S30-2. doi: 10.12968/bjon.2012.21.Sup21.S28. [DOI] [PubMed] [Google Scholar]
- 219.Chaaban MR, Kejner A, Rowe SM, Woodworth BA. Cystic fibrosis chronic rhinosinusitis a comprehensive review. Am J Rhinol Allergy. 2013;27(5):387–395. doi: 10.2500/ajra.2013.27.3919. https://doi.org/10.2500/ajra.2013.27.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Robertson JM, Friedman EM, Rubin BK. Nasal and sinus disease in cystic fibrosis. Paediatr Respir Rev. 2008;9(3):213–219. doi: 10.1016/j.prrv.2008.04.003. https://doi.org/10.1016/j.prrv.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 221.Cimmino M, Nardone M, Cavaliere M, Plantulli A, Sepe A, Esposito V. Dornase alfa as postoperative therapy in cystic fibrosis sinonasal disease. Arch Otolaryngol Head Neck Surg. 2005;131(12):1097–1101. doi: 10.1001/archotol.131.12.1097. https://doi.org/10.1001/archotol.131.12.1097 [DOI] [PubMed] [Google Scholar]
- 222.Mainz JG, Schien C, Schiller I, Schädlich K, Koitschev A, Koitschev C. Sinonasal inhalation of dornase alfa administered by vibrating aerosol to cystic fibrosis patients a double-blind placebo-controlled cross-over trial. J Cyst Fibros. 2014;13(4):461–470. doi: 10.1016/j.jcf.2014.02.005. https://doi.org/10.1016/j.jcf.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 223.Mainz JG, Schiller I, Ritschel C, Mentzel HJ, Riethmüller J, Koitschev A. Sinonasal inhalation of dornase alfa in CF A double-blind placebo-controlled cross-over pilot trial. Auris Nasus Larynx. 2011;38(2):220–227. doi: 10.1016/j.anl.2010.09.001. https://doi.org/10.1016/j.anl.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 224.Hadfield PJ, Rowe-Jones JM, Mackay IS. A prospective treatment trial of nasal polyps in adults with cystic fibrosis. Rhinology. 2000;38(2):63–65. [PubMed] [Google Scholar]
- 225.Beer H, Southern KW, Swift AC. Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis. Cochrane Database Syst Rev. 2015;(6):CD008253–CD008253. doi: 10.1002/14651858.CD008253.pub4. https://doi.org/10.1002/14651858.cd008253.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rampolla R. Lung transplantation an overview of candidacy and outcomes. Ochsner J. 2014;14(4):641–648. [PMC free article] [PubMed] [Google Scholar]
- 227.Hirche TO, Knoop C, Hebestreit H, Shimmin D, Solé A, Elborn JS. Practical guidelines lung transplantation in patients with cystic fibrosis. Pulm Med. 2014;2014:621342–621342. doi: 10.1155/2014/621342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S. A consensus document for the selection of lung transplant candidates 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. https://doi.org/10.1016/j.healun.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 229.Sands D, Repetto T, Dupont LJ, Korzeniewska-Eksterowicz A, Catastini P, Madge S. End of life care for patients with cystic fibrosis. J Cyst Fibros. 2011;10(2):S37–S44. doi: 10.1016/S1569-1993(11)60007-6. https://doi.org/10.1016/S1569-1993(11)60007-6 [DOI] [PubMed] [Google Scholar]
- 230.Plant BJ, Goss CH, Tonelli MR, McDonald G, Black RA, Aitken ML. Contraceptive practices in women with cystic fibrosis. J Cyst Fibros. 2008;7(5):412–414. doi: 10.1016/j.jcf.2008.03.001. https://doi.org/10.1016/j.jcf.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 231.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. doi: 10.1056/NEJMoa1409547. https://doi.org/10.1056/NEJMoa1409547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kernan NG, Alton EW, Cullinan P, Griesenbach U, Bilton D. Oral contraceptives do not appear to affect cystic fibrosis disease severity. Eur Respir J. 2013;41(1):67–73. doi: 10.1183/09031936.00018712. https://doi.org/10.1183/09031936.00018712 [DOI] [PubMed] [Google Scholar]
- 233.Government of South Australia . Department for Health and Ageing. SA Maternal & Neonatal Clinical Network. Clinical practice guideline on cystic fibrosis in pregnancy. 3. Adelaide: Department for Health and Ageing; 2015. [Google Scholar]
- 234.Edenborough FP, Borgo G, Knoop C, Lannefors L, Mackenzie WE, Madge S. Guidelines for the management of pregnancy in women with cystic fibrosis. J Cyst Fibros. 2008;7(1):S2–32. doi: 10.1016/j.jcf.2007.10.001. https://doi.org/10.1016/j.jcf.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 235.Goddard J, Bourke SJ. Cystic fibrosis and pregnancy. TOG. 2009;11(1):19–24. https://doi.org/10.1576/toag.11.1.19.27464 [Google Scholar]
- 236.McMullen AH, Pasta DJ, Frederick PD, Konstan MW, Morgan WJ, Schechter MS. Impact of pregnancy on women with cystic fibrosis. Chest. 2006;129(3):706–711. doi: 10.1378/chest.129.3.706. https://doi.org/10.1378/chest.129.3.706 [DOI] [PubMed] [Google Scholar]
- 237.Chan HC, Ruan YC, He Q, Chen MH, Chen H, Xu WM. The cystic fibrosis transmembrane conductance regulator in reproductive health and disease. J Physiol. 2009;587(10):2187–2195. doi: 10.1113/jphysiol.2008.164970. https://doi.org/10.1113/jphysiol.2008.164970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kerem E, Conway S, Elborn S, Heijerman H, Consensus Committee Standards of care for patients with cystic fibrosis a European consensus. J Cyst Fibros. 2005;4(1):7–26. doi: 10.1016/j.jcf.2004.12.002. https://doi.org/10.1016/j.jcf.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 239.Duff AJ, Latchford GJ. Motivational interviewing for adherence problems in cystic fibrosis. Pediatr Pulmonol. 2010;45(3):211–220. doi: 10.1002/ppul.21103. https://doi.org/10.1002/ppul.21103 [DOI] [PubMed] [Google Scholar]
- 240.Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest. 2014;146(1):142–151. doi: 10.1378/chest.13-1926. https://doi.org/10.1378/chest.13-1926 [DOI] [PubMed] [Google Scholar]
- 241.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis challenges to disease self-management. J Cyst Fibros. 2009;8(2):91–96. doi: 10.1016/j.jcf.2008.09.007. https://doi.org/10.1016/j.jcf.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ziaian T, Sawyer MG, Reynolds KE, Carbone JA, Clark JJ, Baghurst PA. Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. J Paediatr Child Health. 2006;42(10):596–600. doi: 10.1111/j.1440-1754.2006.00943.x. https://doi.org/10.1111/j.1440-1754.2006.00943.x [DOI] [PubMed] [Google Scholar]
- 243.Quittner AL, Abbott J, Georgiopoulos AM, Goldbeck L, Smith B, Hempstead SE. International Committee on Mental Health in Cystic Fibrosis Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax. 2016;71(1):26–34. doi: 10.1136/thoraxjnl-2015-207488. https://doi.org/10.1136/thoraxjnl-2015-207488 [DOI] [PMC free article] [PubMed] [Google Scholar]


