Abstract
Objective
Recurrent respiratory papillomatosis (RRP) is a benign disease caused by human papillomavirus (HPV) types 6 and 11. Although a prophylactic vaccine against RRP is available, a therapeutic vaccine is needed to treat those already infected. The objective of our study was to design and test a DNA vaccine targeting HPV-11 proteins.
Study Design
Pre-clinical scientific investigation
Methods
A DNA vaccine encoding the HPV-11 E6 and E7 genes linked to calreticulin (CRT) was generated. Immunologic response to the HPV-11 CRT/E6E7 vaccine was measured by vaccinating C57BL/6 mice via electroporation and measuring CD8+ T cell responses from harvested splenocytes. A tumor cell line containing HPV11-E6E7 was created and the ability of novel DNA vaccine to control tumor growth was measured in vivo.
Results
Our vaccine generated a significant and specific CD8+ T cell response against the HPV11-E6aa41-70 peptide. The CD8+ T cell responses did not recognize E7 epitopes, indicating E6 immunodominance. CD8+ responses were augmented in the CRT-linked vaccine compared to a control non-CRT vaccine. The HPV-11 CRT/E6E7 vaccine was used to treat mice inoculated with a HPV-11 E6E7 expressing tumor cell line after temporary CD3 depletion to facilitate tumor growth. Vaccinated mice had a significantly lower tumor growth rate (p=0.029) and smaller tumor volumes compared to control mice indicating an augmented immunologic response in vaccinated mice.
Conclusions
A DNA vaccine targeting HPV-11 E6E7 generates a specific HPV-11 CD-8+ T cell response capable of reducing the growth of HPV-11 expressing tumors. DNA vaccines are a promising immunologic strategy for treating RRP.
Level of Evidence
N/A (Animal Study and basic research)
Keywords: Human Papillomavirus, HPV, DNA vaccines, HPV-11, Recurrent Respiratory Papillomatosis, RRP, Laryngeal papilloma
Introduction
Recurrent Respiratory Papillomatosis (RRP) is a viral infection of the aerodigestive tract that causes debilitating, life-long disease in pediatrics and adults. Caused by human papilloma virus (HPV) types 6 and 11, the virus induces proliferation of benign squamous papillomas, most commonly on the larynx, trachea, or other subsites such as the palate, pharyx, or lung. Although its overall incidence is low in the general population, the disease burden can be very high for affected individuals, with profound functional consequences for speech and respiration.1
Therapeutic DNA vaccine plasmids encode a disease-specific antigen and a promoter region that causes transcription of the target antigen and subsequent presentation to the immune system. This endogenous antigen expression has been shown to induce broad cellular and humoral immune responses against the encoded antigens. Since DNA is relatively stable in cells, long-term expression of the encoded antigen and maintenance of immunologic memory can be achieved.2 DNA vaccines targeting high-risk HPV types have progressed to clinical trials and have reported significant success in treating pre-neoplastic disease.3
We have previously reported the generation of a preliminary DNA vaccine targeting HPV11 oncoproteins.4 In this report, it was demonstrated that E6 is the immunodominant peptide, but no specific immunologic response was seen against E7. One strategy to enhance immunologic responses and antigen presentation is linkage of the antigen of interest to Calreticulin (CRT)5, and we then showed that linking CRT to HPV6-E7 could generate an immune response against HPV6-E7 not present in the non-CRT linked vaccine.6 However, the gold standard test for the efficacy of DNA vaccines is the ability to control growing tumors in vivo that express the target antigen. So far, this has not been demonstrated with any tumor model expressing low-risk HPV oncoproteins. As clinical trials are moving forward with DNA vaccine technology linking antigens to calreticulin (CRT) we hypothesized that we would be able to generate and control a HPV11-E6E7 tumor cell line with a novel CRT-linked HPV11 DNA vaccine.
The objectives of this study were therefore two-fold: 1) to generate a CRT-linked DNA vaccine targeting HPV11-E6E7 and compare the immune response elicited by this vaccine compared to a non-CRT control vaccine, and 2) demonstrate that our DNA vaccine is able to control HPV11-E6E7 expressing tumors in vivo. Fulfilling these objectives is an important step in the development of a clinical-grade DNA vaccine targeting low-risk HPV and would demonstrate a potential immunomodulatory strategy for the treatment of RRP.
Materials and methods
Mice
Female C57BL/6 mice, BALB/c and SKH1 mice (6–8 weeks old) were purchased from Charles River (Frederick, MD) and maintained at the Johns Hopkins University animal facility. All procedures were performed in accordance with the Johns Hopkins Animal Care and Use Committee guidelines for proper care and use of laboratory animals. All procedures were performed under an approved protocol.
Peptides and antibodies
A total of five HPV11 E7 30-mer overlapping peptides spanning the full length of the E7 protein and eight HPV11 E6 30-mer overlapping peptides spanning the full length of the E6 protein were purchased from GenScript (Piscataway, NJ). FITC-conjugated anti-mouse IFN- γ was purchased from eBioscience (San Diego, CA) and PE-conjugated anti-mouse CD8a was purchased from BioLegend (San Diego, CA). InVivoMAb anti-mouse CD3 was purchased from Bio X Cell (West Lebanon, NH).
DNA vaccination
The pcDNA3-HPV11-E6E7 vaccine was generated by cloning the codon optimized HPV11 E6E7 sequence into the pcDNA3 vector. The pcDNA3-CRT/HPV11-E6E7 vaccine was generated by cloning the HPV11-E6E7 sequence into the pcDNA3-CRT vector. Mice were vaccinated via electroporation twice a week for 3 weeks.
Intracellular cytokine staining
Splenocytes from CRT/HPV11 E6E7 vaccinated C57BL/6, SKH1 and BALB/c mice were harvested one week after the last vaccination. Splenocytes were stimulated with 2 μg overlapping HPV11 E6 or E7 peptides in the presence of 1 μl/ml GolgiPlug (BD Pharmingen, San Diego, CA). Splenocytes were stained with PE anti-mouse CD8a and fixed using a Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA). After fixation and permeabilization, cells were stained with FITC anti-mouse IFN- γ. Using a FACSCalibur, cells were analyzed for CD8 and intracellular IFN-γ.
Establishment of TC-1 HPV-11 E6E7 cell line
TC-1 is a tumor cell line that constructed from normal mice lung that was subsequently immortalized and transformed with HPV-16 E6 and E7, as well as the activated ras oncogene.7 To create the HPV-11 TC-1 variant cell line, retroviral expression plasmids containing a GFP linked to the 3′ end of the fusion genes were generated by restriction cloning (pMSCV-HPV11-E6E7-GFP). The plasmids were confirmed by direct sequencing. Subsequently TC-1 cells were transfected with these plasmids. After transfection of the TC-1, selection of clones expressing HPV-11 E6E7 was done by flow-sorting of GFP expressing cells.
Luciferase imaging
HPV11-E6E7-Luciferase plasmid (10 μg) was injected intramuscularly via electroporation. Twenty four hours after plasmid injection, mice were sedated with ketamine and injected intraperitoneally with 200 μl of 3.9 mg/ml luciferin. Ten minutes after luciferin injection, luciferase activity was measured in vivo using the IVIS Spectrum Imaging System. Luciferase imaging was repeated up to one month following plasmid injection. Bioluminescence was recorded using an image analysis software provided with the system.
In vivo Tumor Treatment with DNA Vaccine
Cells were cultured in RPMI medium supplemented with 2mM glutamine, 1mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum. Mice were depleted of CD3+ T cells by treatment with 100 μg anti-CD3 antibody for 3 consecutive days. Two days after the last depletion, mice were injected subcutaneously in the flank with 150,000 TC-1 HPV11E6E7 tumor cells. Once a palpable tumor was detected, mice were vaccinated with 2 μg CRT/HPV11 E6E7 DNA via electroporation and continued to be vaccinated twice a week. Tumor volume (length × width × height) was measured at least twice a week. As unvaccinated controls, one group received continued weekly anti-CD3 antibody treatment after tumor cell injection and the induction only group received just the first three induction anti-CD3 treatments. Tumor growth was followed for thirty days. Mice were euthanized if tumor size reached predetermined size criteria as delineated by the Johns Hopkins Animal Care and Use Committee guidelines.
Statistical Analysis
For flow cytometry experiments, a difference in means was calculated using a two-sided student’s T test. For tumor treatment experiments, the tumor growth was modeled using a linear mixed-effects model assuming random effects on the intercept and slope, and the models compared between groups using a chi squared test. Significance was attributed to p < 0.05.
Results
Comparison of non-CRT and CRT DNA vaccine
In order to directly compare the DNA vaccines, five C57BL/6 mice were vaccinated by electroporation with either pcDNA3-HPV11-E6E7 DNA vaccine or pcDNA3-CRT/HPV1-E6E7 DNA. Mice were vaccinated twice weekly, and splenocytes were harvested one week after the last vaccination. Splenocytes were stimulated with overlapping HPV11 E6 or E7 peptides to measure intracellular cytokine response. Mice vaccinated with the control pcDNA3-HPV11-E6E7 DNA vaccine did not show any detectable immunologic response (Figure 1) as splenocytes harvested from these mice and stimulated with overlapping E6 (Figure 1a and 1b) or E7 (Figure 1c and 1d) peptides did not display either E6 or E7-specific CD8+ T cell response. Mice vaccinated with pcDNA3-CRT/HPV11 E6E7 DNA and stimulated with overlapping E6 peptides (Figure 2a and 2b) generated a significantly greater number of v secreting CD8+ T-cells compared to the pcDNA3-HPV11-E6E7 DNA vaccinated group in the known immunogenic epitope of HPV11 E6 (240 vs. 6 CD8+ T cells; p = 0.01). No evident E7-specific CD8+ T cell response was observed when splenocytes were harvested with overlapping E7 peptides (Figure 2c and d).
Figure 1.
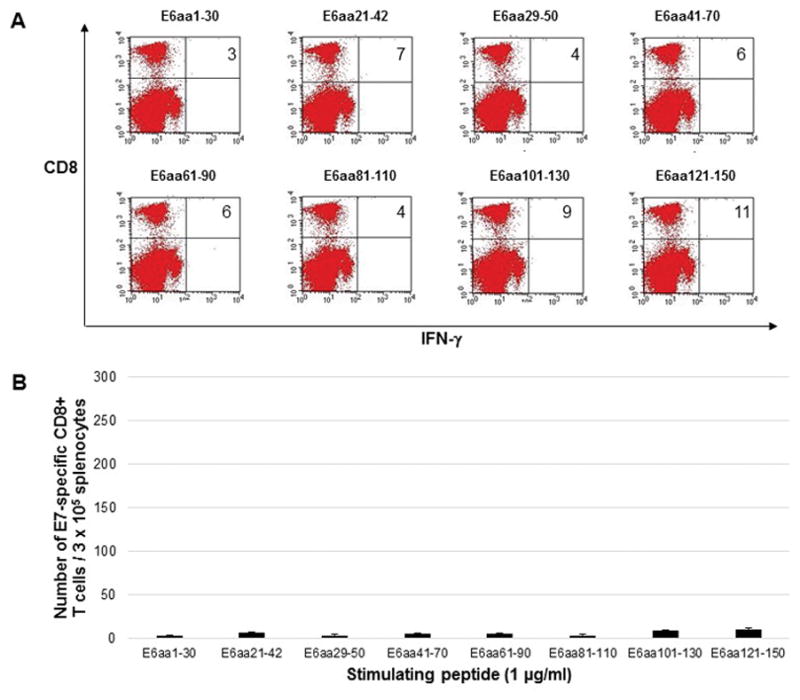
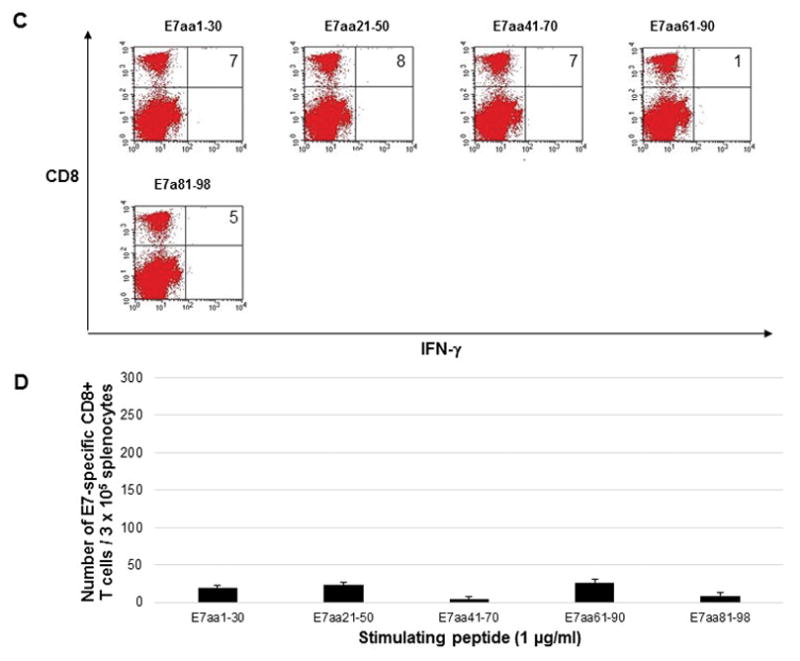
Five C57BL/6 mice were vaccinated twice weekly with 2μg HPV11-E6E7 DNA vaccine via electroporation, and splenocytes were harvested one week after the final vaccination. Splenocytes were stimulated with 1μg/ml overlapping HPV11 E6 peptides (A and B) or HPV11 E7 peptides (C and D) in the presence of 1μg/ml GolgiPlug. Cells were stained with PE anti-mouse CD8a and FITC anti-mouse IFN-γ and analyzed by flow cytometry. Sample flow cytometry from an individual mouse gated for 1 × 105 cells, with bar graphs presented for 3 × 105 cells to normalize with previously published literature.
Figure 2.
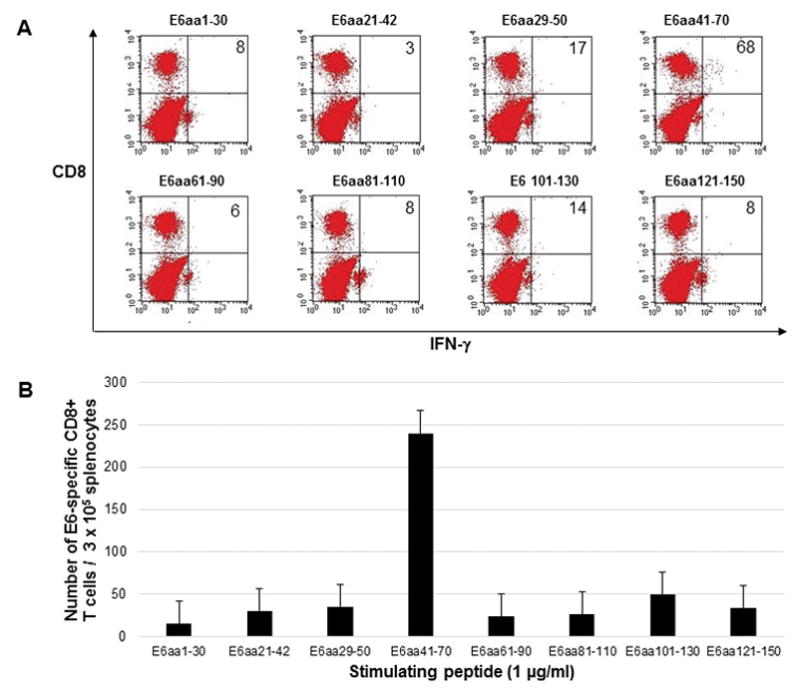
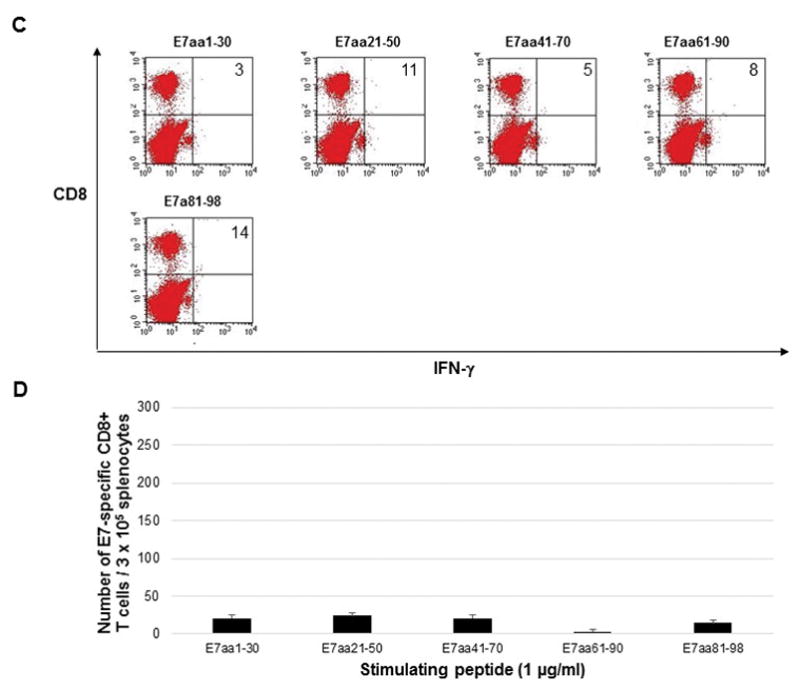
Five C57BL/6 mice were vaccinated twice weekly with 2μg CRT/HPV11-E6E7 DNA vaccine via electroporation, and splenocytes were harvested one week after the final vaccination. Splenocytes were stimulated with 1μg/ml overlapping HPV11 E6 peptides (A and B) or HPV11 E7 peptides (C and D) in the presence of 1μg/ml GolgiPlug. Cells were stained with PE anti-mouse CD8a and FITC anti-mouse IFN-γ and analyzed by flow cytometry. Sample flow cytometry data from an individual mouse gated for 0.5 × 105 cells, bar graph presented for 3 × 105 cells to normalize with previously published literature.
As our future experimental aims depended on generating a robust T cell response, the vaccination regimen was then modified to observe the effects of vaccination frequency on immunologic response of the pcDNA3-CRT/HPV11 E6E7 DNA vaccine in C57BL/6 mice. Five mice in each group were vaccinated either twice weekly for 3 weeks or clustered at 3 consecutive days a week for 3 weeks. Control mice received no vaccination. As shown in Figure 3, no significant difference was observed in CD8+ T cell response between the clustered and weekly vaccinated mice (294 vs. 249 CD8+ T cells; p = 0.13). No detectable IFN-γ secreting CD8+ T cells was observed in the control group.
Figure 3.
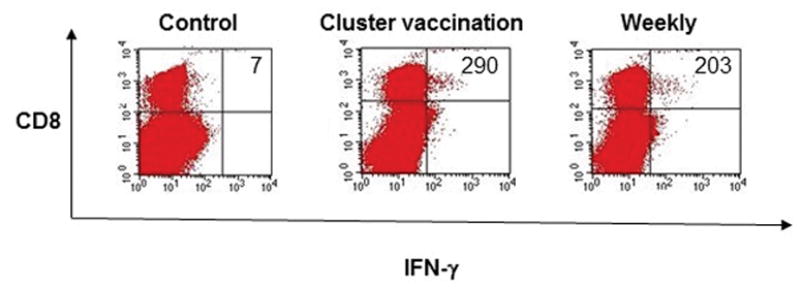
C57BL/6 mice were vaccinated either once a week or 3 consecutive days a week for 3 weeks with 2μg CRT/HPV11 E6E7 DNA via electroporation. Unvaccinated mice were used as a control. Splenocytes were harvested one week after the final vaccination. Splenocytes were stimulated with 1μg/ml HPV11-E6aa41-70 peptide in the presence of 1μg/ml GolgiPlug. Cells were stained with PE anti-mouse CD8a and FITC anti-mouse IFN-γ and analyzed by flow cytometry. Sample flow cytometry data from an individual mouse gated for 3 × 105 cells.
Immunologic responses to vaccination in different mouse strains
To pursue our aim of treating in vivo tumors expressing the low-risk HPV oncoproteins, a TC-1 HPV-11 E6E7 tumor cell line was created. However, when injected into C57BL/6 mice, no tumor growth was seen, in contrast to the control TC-1 cell line, which developed rapidly growing tumors. This result suggested that the low-risk HPV oncoproteins were sufficiently immunogenic in the genetic background of C57BL/6 mice that the tumors were controlled by the native immune system.
In order to test this hypothesis, DNA plasmids were designed and created that expressed HPV11-E6E7 linked to luciferase as reporter gene to allow for in vivo imaging of E6E7 expression. Two C57BL/6, BALB/c, and immunodeficient athymic nude mice were injected by electroporation with HPV11-E6E7-luciferase, and bioluminescent imaging performed serially. All three strains of mice showed an uptake of the HPV11 E6E7 luciferase plasmid 24 hours after plasmid injection (Figure 4). However, Luciferase activity was no longer present in the BALB/c mice at three days and in the C57BL/6 mice after one week. Bioluminescence was still present in the athymic nude mice after one month (Figure 4b). These results demonstrate that the rapid reduction of low-risk HPV oncoprotein expression was due immunologic clearance in the immunocompetent mice strains.
Figure 4.
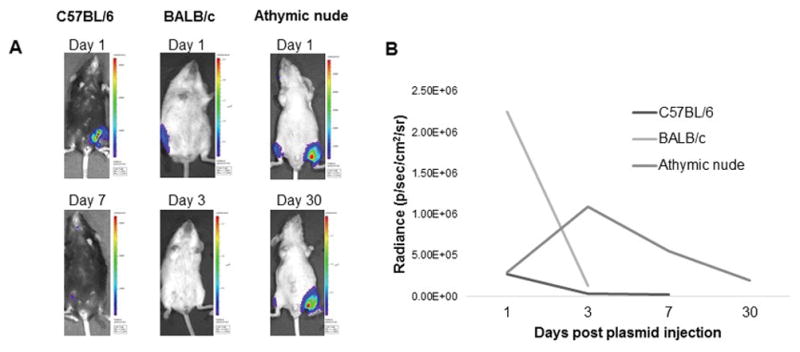
C57BL/6, BALB/c, and athymic nude and mice were injected with 10 μg HPV11 E6E7 luciferase plasmid in the hind leg, and measured serially for luciferase activity. At Day 1, all mice showed uptake of the plasmid, but the signal was cleared by Day 3 in BALB/c and Day 7 in C57BL/6 mice, but persisted at Day 30 in nude mice. A) Representative images of luciferase activity in C57BL/6, athymic nude and BALB/c mice showing plasmid activity. B) Quantification of the change in luciferase signal over time.
To further explore the immunologic response to HPV11-E6E7 in different strains of mice, BALB/c and SKH-1 (an outbred mouse strain) mice were vaccinated with the same vaccination regimen as previously performed in C57BL/6 mice. Splenocytes were harvested and stimulated with E6 and E7 overlapping peptides. Robust CD8+ T cell response was observed from splenocytes harvested from SKH1 mice, similar to results observed in C57BL/6 mice (Figure 5a). The immunogenic epitope for HPV11 E6 was found to be within the E6aa41-70, the same epitope as in C57BL/6 mice. E6 peptide stimulation of splenocytes harvested from vaccinated BALB/c mice did not elicit any E6-specific CD8+ T cell response. As shown in Figure 5b, vaccination with pcDNA3-CRT/HPV11-E6E7 DNA vaccine did not induce E7-specific CD8+ T cell response in either the vaccinated SKH1 or BALB/c mice. These experiments verified the immunodominance of the E6 antigen in our DNA vaccine model showed that other mouse strains did not respond more favorably to our DNA vaccine compared to C57BL/6 mice. Therefore we concluded that C57BL/6 mouse strain was the most promising genetic background in which to pursue our aim of in vivo tumor treatment.
Figure 5.
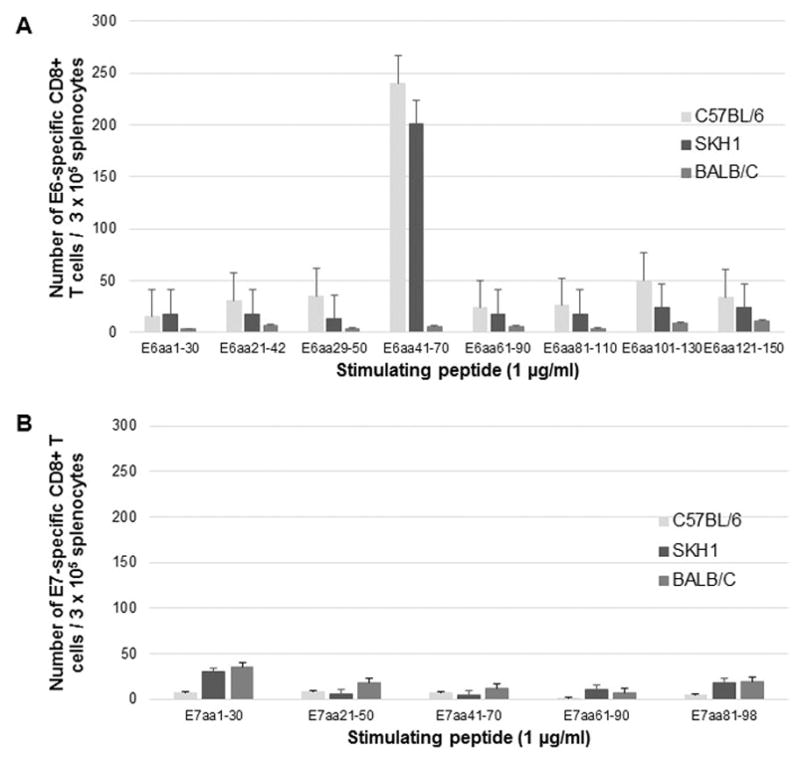
C57BL/6, BALB/c and SKH1 mice were vaccinated twice weekly with 2μg CRT/HPV11-E6E7 DNA vaccine via electroporation, and splenocytes were harvested one week after the final vaccination. Splenocytes were stimulated with 1μg/ml overlapping A) HPV11 E6 peptides or B) HPV11 E7 peptides in the presence of 1μg/ml GolgiPlug. 3 × 105 cells were stained with PE anti-mouse CD8a and FITC anti-mouse IFN-γ and analyzed by flow cytometry.
In vivo tumor treatment
As we had previously found that the TC-1 HPV11-E6E7 cell line did not grow in fully immunocompetent mice, C57BL/6 mice were immunocompromised by CD3 depletion followed by tumor injection two days after the last depletion. With continued CD3 depletion on a weekly basis, the TC-1 HPV11-E6E7 tumors grew exponentially, displaying similar growth kinetics to the control TC-1 cell line. If CD3 depletion was stopped after the initial induction, we found that tumors would grow more slowly as the immune system reconstituted.
We therefore designed a tumor treatment experiment using three experimental arms: continued CD3 depletion, CD3 induction only, and CD3 induction followed by pcDNA3-CRT/HPV11 E6E7 DNA vaccination. At the initial development of a palpable tumor, mice were assigned in a 1:2:2 ratio to these three groups by a predetermined randomization sequence, and the result of this experiment is presented in Table 1. There was no significant difference in tumor size at first measurable day between the continued CD3, induction only and vaccinated groups (p=0.22). As expected, the continued CD3 depletion group experienced rapid tumor growth (86 mm3/day), with large tumors (mean size = 1442 mm3) that required euthanasia prior to the predetermined experimental endpoint (10/10 mice). Vaccinated mice had smaller tumors than CD3 induction only mice (384 mm3 vs. 645 mm3; p = 0.072) and a significantly lower growth rate (20 mm3/day vs. 34 mm3/day; p = 0.029) demonstrating that we were able to elicit an effective T cell response against the HPV11-E6E7 proteins capable of augmenting the native immune response.
Table 1.
| Continued CD3 depletion (n=10) | Induction only (n=19) | Induction + Vaccination (n=19) | p-value | |
|---|---|---|---|---|
| Tumor size at first measurable day (mm3 (SD)) | 23 (8) | 31 (12) | 28 (2) | 0.22 |
| Mean tumor size at last measured day (mm3) | 1442 (965) | 645 (549) | 384 (274) | 0.072 |
| Growth rate (mm3/day (95% CI)) | 86 (51 – 122) | 34 (23 – 46) | 20 (15 – 25) | 0.029 |
Discussion
Prophylactic vaccines against low and high-risk HPV types are remarkably effective at preventing HPV infection and the subsequent development of HPV-associated diseases.8 However, prophylactic vaccines are not effects for individuals that are already infected with HPV, or those who have developed HPV-associated diseases.9 In contrast, therapeutic vaccines are designed to treat those with established HPV-associated diseases by priming the immune system to the foreign viral antigen. In the present study, we evaluated the immunologic response of a DNA vaccine targeting HPV-11 E6E7. Linked to CRT, the HPV-11 E6E7 DNA vaccine developed in this study was able to induce T-cell mediated immunity and enhance the kinetics of the immunologic response to a HPV11 E6E7 tumor cell line.
Our data show that vaccination with CRT/HPV11-E6E7 DNA vaccine generated a strong E6-specific and weak E7-specific CD8+ T-cell immune response in vaccinated C57BL/6 mice. These data confirm previous findings using a DNA vaccine targeting HPV11 E6E7 that was not linked to CRT.4 While it is possible to induce an E7 response to vaccination by designing a vaccine containing only E76, it appears that in the C57BL/6 background, E6 is the dominant immunologic antigen when both E6 and E7 protein are expressed together. Vaccines designed for human use often include both antigens, to maximize the chances for antigen presentation, and there are many trials currently underway in HPV-associated diseases.10 CRT linkage with our antigen enhanced the immunologic response as expected, a strategy we chose since CRT-linked DNA vaccines are now in clinical trials.11
Unlike the parent TC-1 cell line, TC-1 HPV11 E6E7 tumors were not able to be established in fully immunocompetent C57BL/6 mice. To confirm that is was our target antigens that were responsible for this effect, injection of luciferase-conjugated HPV11 E6E7 was also rapidly cleared by BALB/c mice after three days and C57BL/6 mice in seven days. In contrast, athymic nude mice, maintained signal as long as they were followed. Interestingly, vaccination with CRT/HPV11-E6E7 DNA vaccine elicited a CD8+ immunologic response in C57BL/6 and SKH1 but not in BALB/c mice, suggesting that CD4+ mediated immunologic responses may have a role in tumor clearance. Similar to our results, Shepherd et al and Khammanivong et al also found no detectable responses to E7 peptides in BALB/c mice but identified an antigenic epitope in C57BL/6 after mice were immunized with HPV16 E7 proteins.12,13 Wang et al., using CRT/mE6 and CRT/mE7 vaccine strategies, were also unable to detect CD8+ T cell responses in vaccinated BALB/c mice.14 Analogous to these previous findings, we also demonstrate a lack of immunologic response in BALB/c mice after vaccination with CRT/HPV11 E6E7. The BALB/c mice were unable to generate CD8+ T cell response against any of the overlapping E6 or E7 peptides, including the E6aa41-70 peptide, which is a previously identified epitope in C57BL/6 and SKH1 mice. We conclude that the E6 and E7 antigens may lack an immunodominant epitope that is able to interact with the MHC class I haplotype found in the BALB/c strain.
With these findings in mind, we therefore established a tumor model using CD3 depletion in C57BL/6 mice, to severely reduce both the CD4+ and CD8+ T cell populations and to permit tumor growth. When mice were fully CD3 depleted, the tumor grew exponentially, similar in growth kinetics to the parent cell line TC-1. Other models of murine papilloma also require CD3 depletion in order to establish papilloma in immunocompetent mice.14,15 These results support the conclusion that HPV infections can be controlled by T-cell immunity, and we then sought to show that this immunity could be augmented by DNA vaccination. By an initial burst of immunosuppression, and then allowing some tumor growth while the immune system recovered, we were able demonstrate that the growth rate of the tumor was significantly impacted by our DNA vaccination. Therefore, the antigen-specific T-cell response generated by vaccination is a functional response capable of recognizing and eliminating cells carrying the target antigen.
For HPV-associated disease like RRP, this targeted immunologic strategy is ideal, as the underlying defect in RRP appears to be an inherent or induced deficiency in the cytotoxic T cell response needed to eliminate virally infected cells.16 This immune polarization away from a CD8+ T cell response could potentially be addressed by priming the immune system with DNA vaccination. The HPV E6 and E7 proteins in RRP are ideal tumor-specific antigens for therapeutic vaccination because the proteins are encoded only by the viral genome and are uniquely expressed by all virus-infected cells. Since the E6 and E7 oncoproteins are required for the induction and maintenance of the papilloma phenotype17, these proteins are constitutively expressed within the papilloma cells, including infected basal epithelia, which cannot evade a directed immune response through antigen loss of E6 or E7.18 We therefore believe that DNA vaccination has a promising future for the treatment of RRP and other HPV-associated diseases.
Conclusion
A DNA vaccine targeting HPV-11 E6E7 generates a specific HPV-11 CD-8+ T cell response capable of reducing the growth of HPV-11 expressing tumors. DNA vaccines are a promising immunologic strategy for treating RRP.
Acknowledgments
Financial Support for this study was provided by the Triological Society Career Development Award (S. Best) and the NIDCD Mentored Patient-Oriented Research Career Development Award 1K23DC014758 (S. Best). We thank Nae-Yuh Wang, PhD for his statistical support.
Footnotes
Conflict of Interest: T.-C. Wu and Richard B.S. Roden are co-founders of, and have an equity ownership interest in Papivax LLC. They also own Papivax Biotech Inc. stock options and are members of Papivax Biotech Inc.’s Scientific Advisory Board. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. The other authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1.Venkatesan NN, Pine HS, Underbrink MP. Recurrent respiratory papillomatosis. Otolaryngol Clin North Am. 2012;45(3):671–694. viii–ix. doi: 10.1016/j.otc.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. Cancer vaccines. Nat Med. 1998;4(5 Suppl):525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 3.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng S, Best SR, Hung CF, et al. Characterization of human papillomavirus type 11-specific immune responses in a preclinical model. Laryngoscope. 2010;120(3):504–510. doi: 10.1002/lary.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng WF, Hung CF, Chen CA, et al. Characterization of DNA vaccines encoding the domains of calreticulin for their ability to elicit tumor-specific immunity and antiangiogenesis. Vaccine. 2005;23(29):3864–3874. doi: 10.1016/j.vaccine.2004.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng S, Mattox A, Best SR, et al. Identification of the murine H-2D(b) and human HLA-A*0201 MHC class I-restricted HPV6 E7-specific cytotoxic T lymphocyte epitopes. Cancer Immunol Immunother. 2016;65(3):261–271. doi: 10.1007/s00262-016-1793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–26. [PubMed] [Google Scholar]
- 8.Group FIIS, Dillner J, Kjaer SK, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coskuner ER, Ozkan TA, Karakose A, Dillioglugil O, Cevik I. Impact of the quadrivalent HPV vaccine on disease recurrence in men exposed to HPV Infection: a randomized study. J Sex Med. 2014;11(11):2785–2791. doi: 10.1111/jsm.12670. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Farmer E, Wu TC, Hung CF. Perspectives for therapeutic HPV vaccine development. J Biomed Sci. 2016;23(1):75. doi: 10.1186/s12929-016-0293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez RD, Huh WK, Bae S, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecol Oncol. 2016;140(2):245–252. doi: 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepherd PS, Tran TT, Rowe AJ, et al. T cell responses to the human papillomavirus type 16 E7 protein in mice of different haplotypes. J Gen Virol. 1992;73( Pt 5):1269–1274. doi: 10.1099/0022-1317-73-5-1269. [DOI] [PubMed] [Google Scholar]
- 13.Khammanivong V, Liu XS, Liu WJ, et al. Paucity of functional CTL epitopes in the E7 oncoprotein of cervical cancer associated human papillomavirus type 16. Immunol Cell Biol. 2003;81(1):1–7. doi: 10.1046/j.1440-1711.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang JW, Jiang R, Peng S, Chang YN, Hung CF, Roden RB. Immunologic Control of Mus musculus Papillomavirus Type 1. PLoS Pathog. 2015;11(10):e1005243. doi: 10.1371/journal.ppat.1005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handisurya A, Day PM, Thompson CD, Bonelli M, Lowy DR, Schiller JT. Strain-specific properties and T cells regulate the susceptibility to papilloma induction by Mus musculus papillomavirus 1. PLoS Pathog. 2014;10(8):e1004314. doi: 10.1371/journal.ppat.1004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1/T(H)2 cytokine imbalance. Clin Immunol. 1999;93(3):302–311. doi: 10.1006/clim.1999.4784. [DOI] [PubMed] [Google Scholar]
- 17.Genovese NJ, Broker TR, Chow LT. Nonconserved lysine residues attenuate the biological function of the low-risk human papillomavirus E7 protein. J Virol. 2011;85(11):5546–5554. doi: 10.1128/JVI.02166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pim D, Banks L. Interaction of viral oncoproteins with cellular target molecules: infection with high-risk vs low-risk human papillomaviruses. APMIS. 2010;118(6–7):471–493. doi: 10.1111/j.1600-0463.2010.02618.x. [DOI] [PubMed] [Google Scholar]


