Abstract
A history of intermittent, limited sucrose intake (LSI) attenuates the hypothalamic-pituitary-adrenocortical (HPA) axis stress response, and neuronal activity in the basolateral amygdala (BLA) is necessary for this HPA-dampening. LSI increases the expression of plasticity-associated genes in the BLA; however, the nature of this plasticity is unknown. As BLA principal neuron activity normally promotes HPA responses, the present study tests the hypothesis that LSI decreases stress-excitatory BLA output by decreasing glutamatergic and/or increasing GABAergic inputs to BLA principal neurons. Male rats with unlimited access to chow and water were given additional access to 4 ml of sucrose (30%) or water twice-daily for 14 days, and BLA structural and functional plasticity were assessed by quantitative dual-immunolabeling and whole-cell recordings in brain slices. LSI increased vesicular glutamate transporter 1 (VGlut1)-positive (glutamatergic) appositions onto parvalbumin-positive inhibitory interneurons, and this was accompanied by increased expression of pCREB, a marker of neuronal activation that is mechanistically-linked with plasticity, within parvalbumin interneurons. LSI also increased the paired-pulse facilitation of excitatory, but not inhibitory synaptic inputs to BLA principal neurons, without affecting postsynaptic excitatory or miniature excitatory and inhibitory postsynaptic currents, suggesting a targeted decrease in the probability of evoked synaptic excitation onto these neurons. Collectively, these results suggest that LSI decreases BLA principal neuron output by increasing the excitatory drive to parvalbumin inhibitory interneurons, and decreasing the probability of evoked presynaptic glutamate release onto principal neurons. Our data further imply that palatable food consumption blunts HPA stress responses by decreasing the excitation-inhibition balance and attenuating BLA output.
Keywords: Palatable food, stress, sucrose, glutamate, GABA, parvalbumin
Introduction
The incidence of obesity in the United States is 17% in children and 34.9% in adults with an estimated annual health care cost in 2008 of $147 billion (Finkelstein et al. 2009; Ogden et al. 2014). Additionally, as developing nations gain ready access to calorically-dense food options, the effects of metabolic disorders are spreading globally (World Health Organization 2015). Daily life stressors have been identified as a major reason for snacking and overeating and in these stressful situations individuals often turn to palatable foods that are high in sugar and/or fat to cope with stress (McCann et al. 1990; Oliver and Wardle 1999; Epel et al. 2004; Ulrich-Lai and Ryan 2014; Ulrich-Lai 2016). Consistent with this idea, consumption of highly palatable food decreases emotional, behavioral and physiological responses to stress in humans (Gibson 2006; Macht and Mueller 2007; Tomiyama et al. 2011; Tryon et al. 2013) and rodents (Shide and Blass 1989; Strack et al. 1997; Pecoraro et al. 2004; la Fleur et al. 2005; Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010). However, the neural mechanisms by which palatable food intake reduces stress responses are not known.
In order to explore the mechanisms by which the consumption of highly-palatable “comfort” foods reduce stress, we developed a limited sucrose intake (LSI) paradigm in rats (Ulrich-Lai et al. 2007). In this paradigm, rats with ad libitum access to standard chow and water are also given twice-daily access to a second drink bottle containing 4 ml of either a 30% sucrose solution or plain water (as water-fed controls). During the LSI paradigm, the sucrose-fed rats typically drink the entire amount of sucrose offered (e.g., 4 ml/session, or 8 ml/day), which corresponds to ~10% of their total daily calories. The sucrose-fed rats also voluntarily reduce their food intake (approximately isocalorically to the calories obtained from sucrose), and therefore maintain similar body weight gain and adiposity as water controls (Ulrich-Lai et al. 2007). Importantly, 14 days of LSI blunts HPA axis responses to stress, including lowering plasma adrenocorticotrophic hormone (ACTH) and corticosterone responses to an acute restraint stress, as well as reducing corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Ulrich-Lai et al. 2011). Moreover, the basolateral amygdala (BLA) is ideally positioned to mediate this LSI-induced stress relief. First, naturally rewarding stimuli, like sucrose consumption, alter neuronal activity in the BLA (Muramoto et al. 1993; Yamamoto et al. 1997; Verwey et al. 2007; Fontanini et al. 2009). Second, the BLA is a key stress-regulatory brain region that promotes HPA responses to stress (Coover et al. 1973; Feldman et al. 1983; Szafarczyk et al. 1986; Goldstein et al. 1996; Bhatnagar et al. 2004). Third, restraint-induced cFos mRNA expression is attenuated in the BLA by a history of LSI, suggesting that LSI blunts BLA activation during stress (Ulrich-Lai et al. 2007). Fourth, bilateral, axon-sparing ibotenate lesions of the BLA prevent the HPA-relieving properties of LSI (while ibotenate lesions that miss the BLA do not), demonstrating that neuronal activity in the BLA is necessary for LSI-mediated blunting of the HPA response (Ulrich-Lai et al. 2010). However, the critical intra-BLA mechanisms by which LSI dampens HPA responses remain unknown.
Sucrose-induced synaptic plasticity within the BLA may play an important role in the LSI effect on the stress response. Measurement of BLA mRNA expression on the morning following the cessation of a 14-day LSI paradigm (by microarray with functional clustering analysis) indicates that the sucrose-altered genes are significantly enriched in the long-term potentiation-associated synaptic plasticity pathway (Ulrich-Lai et al. 2010). Consistent with this idea, sucrose increases the expression of multiple proteins that are strongly linked with functional and structural plasticity, including FosB/deltaFosB and phosphorylated cyclic adenosine monophosphate response element-binding protein (pCREB) (Silva et al. 1998; Nestler et al. 1999; Wallace et al. 2008; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Egan and Ulrich-Lai 2015). This suggests that BLA plasticity may underlie the HPA-blunting effects of sucrose.
The vast majority (~85%) of BLA neurons are calcium/calmodulin-dependent protein kinase II α (CaMKIIα)-positive, excitatory, glutamatergic principal neurons that are responsible for relaying information from the BLA to other brain regions (McDonald et al. 2002; Roozendaal et al. 2009). Furthermore, since principal neurons receive both excitatory, glutamatergic inputs (primarily derived from extra-BLA sources like the thalamus and prefrontal cortex) (Muller et al. 2007a; Muller et al. 2009; Roozendaal et al. 2009) and inhibitory, GABAergic inputs (primarily derived from multiple classes of intra-BLA interneurons, including those positive for paralbumin (PV), cholecystokinin (CCK) and somatostatin (SOM) (McDonald et al. 2002; McDonald and Mascagni 2002; Mascagni and McDonald 2003; Rainnie et al. 2006), the balance of these excitatory and inhibitory inputs largely determines the extent of BLA principal neuron activation. The present experiments investigated the LSI-induced changes in the glutamatergic and GABAergic innervation of BLA principal neurons using a combination of anatomical and functional assessments. We tested the hypothesis that LSI would either decrease the glutamatergic excitatory drive or increase the GABAergic inhibitory input to BLA principal neurons to cause a dampening of HPA-excitatory output from the BLA.
Methods
Overview
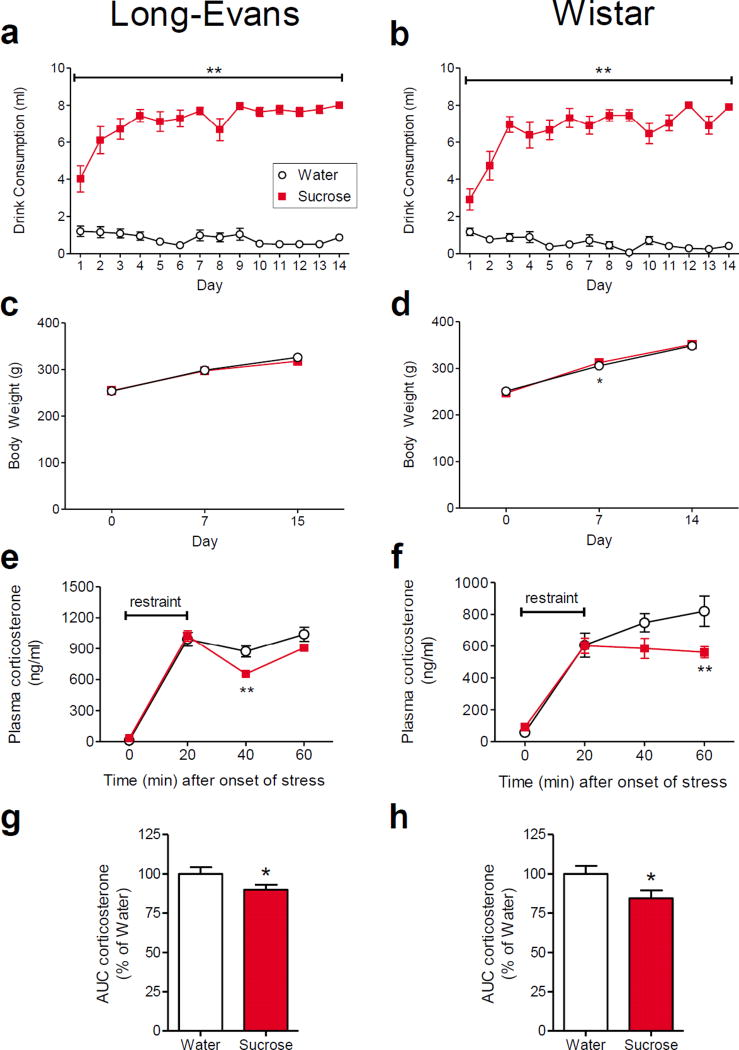
Two separate experiments were performed. Experiment 1 was performed at the University of Cincinnati and consisted of the anatomical assessments of BLA appositions; the LSI paradigm was administered to 8–10 week old, male Long-Evans rats as described in previous studies with the LSI paradigm (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011; Ulrich-Lai et al. 2015; Egan and Ulrich-Lai 2015). A follow-up anatomical assessment was performed using brain tissue sections from (Egan and Ulrich-Lai 2015) to assess the impact of repeated sucrose consumption on the expression of pCREB-immunolabeling within PV-positive interneurons. Experiment 2 was performed at Tulane University and comprised the BLA electrophysiological recordings. This experiment was performed in 4–6 week old, male Wistar rats; this age and strain of rat was used in order to increase the likelihood of obtaining viable patch clamp recordings in BLA slice preparations. The LSI model was first confirmed in the Wistar strain prior to undertaking the electrophysiological experiment. The LSI paradigm blunted the HPA response to an acute restraint stress in male Wister rats of this age to a similar extent as that seen in Long-Evans rats (Figure 1). Tulane University personnel administered the LSI paradigm and performed the restraint stress test in Experiment 2; plasma samples were then sent to the University of Cincinnati for measurement of plasma corticosterone by investigators blind to experimental group assignments.
Fig. 1. Limited sucrose intake paradigm.
Long-Evans rats (male, 8–10 weeks old; a, c, e, g) and Wistar rats (male, 4–6 weeks old; b, d, f, h) were offered a second drink bottle with a limited portion (up to 4 ml) of 30% sucrose or water for 30 minutes at scheduled times twice daily for 14 days. Sucrose-fed rats readily drank nearly the full 8 ml they were offered each day compared to water-fed controls that drank little water from the second bottle (a, b). Sucrose and water-fed rats had similar body weight gain over this 14-day period (c, d), though post-hoc analysis revealed a small but significant difference between groups at 7 days for Wistar rats. The plasma corticosterone response to a 20-minute restraint stress was significantly attenuated in sucrose-fed rats (e, f; panel e is reproduced from Figure S4 of (Ulrich-Lai et al. 2010)). The area-under-the-curve of the plasma corticosterone response to stress was significantly reduced in sucrose-fed rats (g, h). Group sizes are (a, c) 13 water and 13 sucrose rats, (e, g) 11 water and 13 sucrose rats, and (b, d, f, h) 12 water and 12 sucrose rats. *p < 0.05, **p < 0.01 vs. water-fed controls
Animals
As described above, the experiment performed at the University of Cincinnati (Experiment 1) used male (8–10 weeks old), Long-Evans rats (Harlan, Indianapolis, IN). Rats were individually housed in an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) -accredited facility on a 12 h light/dark cycle (06:00 hr lights-on and 18:00 hr lights-off) with ad libitum access to rodent chow (LM-485; Harlan Teklad, Madison, WI) and water. The rats acclimated to the facility for at least 1 week prior to starting experiments. All experiments performed at the Cincinnati site were approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC) and were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (Guide) (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011).
The experiment performed at Tulane University (Experiment 2) used male Wistar rats (4–6 weeks old, Charles River, Wilmington, MA). Rats were individually housed in an AALAC-accredited facility on a 12 h light/dark cycle (07:00 hr lights-on and 19:00 hr lights-off) with ad libitum access to rodent chow (2916; Envigo Teklad, Cambridgeshire, United Kingdom) and water. Rats were allowed to acclimate to the animal facility for 3–5 days prior to use. All experiments were approved by the Tulane University IACUC and were performed in accordance with the NIH Guide (National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011).
Limited Sucrose Intake (LSI) Paradigm
LSI was described and characterized previously (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010). In brief, all rats had ad libitum access to rodent chow and water at all times. Rats were also given limited access to a second drink bottle twice daily (at approximately 09:30 hr and 14:30 hr), which contained either 30% sucrose (MP Biomedicals, Solon, OH) dissolved in water or plain water as a control for 14 days. Rats were allowed to drink up to 4 ml per session (for a maximum of 8 ml per day) within 30 minutes. The volume of drink consumed from the second bottle was recorded, and body weight was monitored on days 1, 7, and 14 of the LSI paradigm.
Validation of LSI-induced blunting of the HPA response to stress in Wistar rats
Rats were given 14 days of LSI (n=12/group; Sucrose vs. Water). On the morning (starting ~8:30 AM) of the 15th day, rats were given a (20-minute) restraint stress by placing them in a well-ventilated rodent plastic decapitation cone (AIMS Inc., Kent Scientific Corp., Torrington, CA). Blood samples (200 µl) were collected by tail clip (as described in (Vahl et al. 2005)) into chilled tubes containing EDTA; samples were taken at 0, 20, 40, and 60 minutes after the initiation of restraint. Blood samples were then centrifuged at 3000xg for 15 minutes at room temperature and plasma was collected and stored at −20°C until measurement of corticosterone concentration by radioimmunoassay (MP Biomedicals, Orangeburg, NY).
Experiment 1: Anatomical assessment of appositions onto BLA neurons
Rats were given 14 days of LSI (n=12–13 rats/group; Sucrose vs. Water). On the morning of the following day (Day 15), rats were anesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline (Fisher Scientific, Hampton, NH), followed by 3.7% formaldehyde (Aqua Solutions, Deer Park, TX). The brains were removed and incubated in 3.7% formaldehyde overnight at room temperature, and then transferred to a solution of 30% sucrose in 0.1 M sodium phosphate-buffered saline (PBS) at 4°C. Brains were sectioned (25 µm) on a sliding microtome (Leica Microsystems; Wetzlar, Germany) in a 1-in-12 series, and sections were stored in cryoprotectant (0.1M PBS, 30% sucrose, 1% polyvinylpyrrolidone and 30% ethylene glycol) at −20°C.
Dual-immunolabeling was performed using a standard protocol that included rinsing with 50 mM potassium phosphate-buffered saline (KPBS), blocking in 0.1% bovine serum albumin (BSA; Sigma-Aldrich) with 0.2% Triton X-100 (Sigma-Aldrich) in KPBS for 1 hour at room temperature, and incubating with primary antibodies (diluted in blocking solution) overnight at 4°C. (Note that the primary antibodies that were used have been previously tested for affinity and specificity as detailed and referenced below, and in all cases immunolabeling was lost when the primary antibody was omitted from the procedure). The following day, sections were rinsed with KPBS, incubated with fluorescence-tagged secondary antibodies (diluted in 0.1% BSA in KPBS) for 1 hour at room temperature in the dark, and finally rinsed with KPBS in the dark. Note that immunolabeling performed with the CaMKIIα antibody differed from the standard protocol in that Triton X-100 was omitted from the blocking solution and the incubation period was 2 nights followed by secondary antibody application and then standard protocol conditions for the application of the second primary antibody of interest. Sections were mounted onto slides and coverslipped using a polyvinyl alcohol mounting medium with DABCO (10981; Fluka/Sigma-Aldrich).
The appositional anatomical studies were performed with two primary objectives. First, appositions of glutamate and GABA synaptic terminals on principal neuron somata and neurites were assessed. Dual-immunolabeling was performed using a primary antibody directed against CaMKIIα (mouse monoclonal; 1:200; Clone 6G9, ab22609; abcam , Cambridge, United Kingdom; or 05–532; EMD Millipore, Billerica, MA) (Erondu and Kennedy 1985; Rostkowski et al. 2009; Giassi et al. 2012) to label BLA principal neurons, combined with a primary antibody directed against either the vesicular glutamate transporter 1 (VGlut1; rabbit polyclonal; 1:1000; 135303; Synaptic Systems, Gottingen, Germany) (Garbelli et al. 2008) to label glutamatergic terminals (Kaneko and Fujiyama 2002) or glutamic acid decarboxylase (GAD; mouse monoclonal; 1:100; Hybridoma Product GAD-6 deposited by Gottlieb, D.I., Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA) (Chang and Gottlieb 1988; Kultas-Ilinsky et al. 2011) to label GABAergic terminals. Second, since GABAergic inputs to principal neurons of the BLA derive from multiple subtypes of inhibitory interneurons (Mascagni and McDonald 2003; Rainnie et al. 2006) that could undergo changes in excitatory drive in response to LSI, dual-immunolabeling was performed to quantify glutamatergic appositions onto each of the 3 primary BLA interneuron subtypes: SOM (D-20) (goat polyclonal; 1:250; sc-7819; Santa Cruz Biotechnology, Dallas, TX) (Cox et al. 2008); CCK (mouse monoclonal; 1:1000; ab37274; CCK8; abcam) (McDonald and Mascagni 2001); and PV (mouse monoclonal; 1:1000; P3088, Sigma-Aldrich) (Rainnie et al. 2006). The fluorescence-labeled secondary antibodies that were used include: Alexa Fluor 488 Donkey Anti-Goat IgG (488 DAG) (1:500; A-11055, Life Technologies, Carlsbad, CA), Alexa Fluor 488 Donkey Anti-Rabbit IgG (488 DAR) (1:500; A21206; Life Technologies), Alexa Fluor 488 Donkey Anti-mouse IgG (488 DAM) (1:500; A21202, Life Technologies), Alexa Fluor 488 Goat Anti-Mouse IgG (488 GAM) (1:500; A11001; Life Technologies), Cy-3 AffiniPure Donkey Anti-Rabbit IgG (Cy-3 DAR) (1:500; 711-165-152, Jackson Immuno Research, West Grove, PA), and Cy-3 AffiniPure Donkey Anti-Mouse IgG (Cy-3 DAM) (1:500; 715-165-150; Jackson Immuno Research).
Imaging and anatomical analysis of appositions
Immunolabeling was analyzed by first capturing z-stack images (630 × magnification, 0.5 µm optical sections) of coronal sections of the BLA (preferred level of −2.3 to −2.56 mm from Bregma) using an Axio Imager.M2 with Apotome.2 fluorescence microscope and Zen 2 Pro software (Zeiss, Oberkochen, Germany). Four z-stack images of the BLA (generally bilateral images from 2 BLA sections) were obtained for each rat, when tissue quality permitted. Apposition counts were then performed for all of the labeled BLA neuronal cell bodies that were completely contained within their respective z-stack images (i.e., the cell did not extend beyond the rostral-caudal or lateral margins of the section); on average this yielded 4 CamKIIα-, 5 PV-, 3 SOM-, and 4 CCK-positive cells per rat. If no cells, or only 1 cell, meeting these criteria was available for a given animal, the rat was excluded from the analysis due to insufficient replicates. This occurred most frequently for the analysis of SOM-positive cells, in keeping with the observation that they are less common than either PV- or CCK-positive cells, comprising just 17% of all BLA interneurons (McDonald and Mascagni 2002; Muller et al. 2007b).
The total numbers of appositions for each neurotransmitter type (VGlut1 or GAD) were counted onto each identified neuronal soma, as well as on all of the neuron’s visible neurites, throughout all of the optical sections of the z-stack. Appositions were defined as areas where there was no visible space between the immunoreactive bouton and the postsynaptic cell membrane, as described in (Flak et al. 2009). Care was taken to mark the location of each apposition on a particular optical section for comparison with those in the adjacent optical sections to prevent double-counting (i.e., in cases where an individual apposition appeared in two adjacent optical sections). The postsynaptic immunolabel filled each of the BLA neuron types (CamKIIα, SOM, CCK, PV) to allow clear visualization of appositions onto the somata or neurites. The immunolabeling and imaging protocol likely did not capture the entire dendritic tree, so neurite apposition counts are primarily reflective of proximal dendrites. In order to calculate the density of appositions onto the somata, soma size was approximated by using the cell radius on the optical section in which the soma was largest to calculate the volume of the soma assuming a spheroid shape, as described in (Flak et al. 2009). The number of appositions onto each soma was then divided by the soma volume. In order to calculate the density of appositions onto the neurites, the length of each neurite was measured, and the number of appositions onto each neurite was divided by the neurite length (Flak et al. 2009). All anatomical analyses were performed by investigators blind to treatment group assignments.
Anatomical assessment of pCREB and PV co-expression
Brain tissue sections from (Egan and Ulrich-Lai 2015) were used to assess the impact of LSI on pCREB-immunolabeling within PV-positive interneurons. pCREB expression was selected because it is strongly linked with both neuronal activation and synaptic plasticity (Silva et al. 1998; Huang et al. 2000; Miyamoto 2006), and our prior work indicates that its expression is increased in the BLA of rats following repeated sucrose drink (Ulrich-Lai et al. 2010; Egan and Ulrich-Lai 2015). Moreover, unstressed rats have little pCREB-positive labeling throughout brain (Kovács and Sawchenko 1996; Ahmed et al. 2006; Kwon et al. 2006; Yang et al. 2016), suggesting that the pCREB expression that is observed in the present tissue primarily results from the prior drink exposure, and can be used to determine whether the consumption of sucrose drink vs. water induces pCREB-immunolabeling in BLA PV-interneurons.
In brief, rats were given 14 days of LSI (n=12 rats/group; Sucrose vs. Water). On the morning of day 14, at 2 hours after presentation of the second drink bottle, rats were anesthetized, perfused with paraformaldehyde, and brains were collected and sectioned as described above. Dual-immunolabeling for pCREB and PV was performed on a 1-in-4 series of BLA sections. Sections were rinsed in KPBS, incubated in 2% hydrogen peroxide (in KPBS) for 20 min, rinsed, and blocked with KPBS with 0.1% BSA and 0.2% Triton-X 100 for 1 hour. Sections were incubated in primary antibody directed against pCREB (rabbit polyclonal; 1:500; 06–19; Millipore, Billerica, MA) overnight at 4°C. Sections were then rinsed, incubated in Biotinylated Goat Anti-rabbit IgG (1:500; BA-1000; Vector Laboratories, Burlingame, CA) for 1 hour, rinsed, incubated with avidin-biotin-peroxidase (1:1000; Vectastain ABC solution; Vector Laboratories, Burlingame, CA) for 1 hour, rinsed, and incubated in Cy-3 Streptavidin (1:500; 016-160-084; Jackson Immunoresearch, West Grove, PA) for 1 hour. Sections were then rinsed and incubated in primary antibody directed against parvalbumin, and 488 DAM secondary antibody, as described above. Dual immunolabeling was visualized using conventional fluorescent microscopy with the same microscope and imaging system as described above. The total number of BLA cells that were positive for PV, either alone or in combination with pCREB, were counted by an observer unaware of group assignment, in order to determine the percentage of PV-positive cells that co-labeled for pCREB. The cell counts were performed for all sections that contained the BLA, and generally resulted in bilateral counts from 3–5 BLA sections per rat, depending on tissue availability.
Experiment 2: Electrophysiological recordings from BLA principal neurons following LSI
Rats were given 14 days of LSI (Sucrose vs. Water). On the morning of the following day (Day 15), rats were anesthetized with a mixture of ketamine/xylazine (80/5 mg/kg body weight, I.P.) and transcardially perfused with ice-cold modified artificial cerebrospinal fluid (aCSF) containing (in mM):124 NaCl, 5 KCl, 6 MgSO4, 1.2 NaH2PO4, 26 NaHCO3, 0.25 CaCl2, 10 glucose, and bubbled with a 95% O2- 5% CO2 gas mixture. In some experiments, NaCl was replaced by an equimolar concentration of sucrose to improve neuronal viability. The brain was then rapidly collected and 3–4 coronal slices, 300 µm in thickness and containing the BLA, were sectioned on a vibratome (Vibratome 3000) and bisected along the third ventricle in the same ice-cold, modified aCSF. Slices were placed in oxygenated, modified aCSF at 32–34°C for recovery (20–30 min) and then transferred to a holding chamber in standard aCSF at room temperature (25°C), where they were allowed to equilibrate for at least 1 hour before being transferred to the recording chamber, one slice at a time. The standard aCSF contained (in mM): 124 NaCl, 5 KCl, 2 MgSO4, 1.2 NaH2PO4, 26 NaHCO3, 2 CaCl2, 10 glucose, and bubbled with a 95% O2 −5% CO2 gas mixture; pH was maintained at 7.25–7.35 and the osmolarity was 300–310 mOsmol.
Neurons in the BLA were selected for recordings by their location and their roughly pyramidal-shaped soma under infrared-differential interference contrast optics. Whole-cell patch clamp recordings were performed on a fixed-stage upright microscope (Olympus BXW51WI) at 30–32°C. Patch pipettes were pulled on a horizontal electrode puller (P97, Sutter Intr.) with a tip resistance of 4–5 MΩ. The internal patch solution used for recordings of inhibitory postsynaptic currents (IPSCs) contained (in mM): 120 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 4 Mg-ATP, and 30 HEPES. The patch solution used for recordings of excitatory postsynaptic currents (EPSCs) contained (in mM): 120 K-gluconate, 10 KCl, 1 NaCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 2 Mg-ATP, 0.3 Na-GTP, and 10 HEPES. The pH of the patch solutions was adjusted to 7.25–7.35 with CsOH or KOH respectively, and the osmolarity was adjusted to 295–300 mOsmol with D-sorbitol.
All recordings were performed in voltage-clamp mode using a Multiclamp 700A amplifier and pCLAMP 9 software (Molecular Devices, Sunnyvale, CA). Recordings with an unstable input resistance or series resistance (i.e., that changed > 20%) were not included in our analyses. All spontaneous and evoked postsynaptic currents were recorded at a holding potential of −60 mV; IPSCs were recorded in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 µM; Sigma-Aldrich, St. Louis, MO) and D-(−)-2-amino-5-phosphonopentanoic acid (AP-5, 40 µM; Sigma-Aldrich) to isolate GABAergic synaptic currents and EPSCs were recorded in the presence of bicuculline methiodide (10 µM; Sigma-Aldrich) to isolate glutamatergic synaptic currents. In a subset of neurons, tetrodotoxin (TTX, 1 µM; Sigma-Aldrich) was applied to block action potential-dependent neurotransmitter release, and miniature EPSCs (mEPSCs) and miniature IPSCs (mIPSCs) were recorded. Electrical recordings were low-pass filtered at 2 kHz, digitized at 10 kHz, and stored on a computer hard drive for off-line analysis. Synaptic currents were analyzed for changes in mean frequency, amplitude and decay time (defined as the elapsed time from the peak to 63% decay to baseline) using the Minianalysis 6.0 program (Synaptosoft Inc., Decatur, GA). The frequency of miniature synaptic currents primarily reflects the probability of spontaneous (unstimulated) presynaptic neurotransmitter release and/or the number of presynaptic release sites (Queenan et al. 2012); the amplitude primarily reflects the postsynaptic response (Queenan et al. 2012); and the decay time primarily reflects the amount of neurotransmitter clearance/reuptake and receptor desensitization (Takahashi et al. 1995).
A paired-pulse paradigm was used to study the probability of evoked presynaptic neurotransmitter release. A concentric stimulation electrode (FHC, Bowdoin, ME) was placed on the external capsule (EC) neighboring the BLA. Two synaptic responses (S1 and S2) were evoked by a pair of stimuli (~0.2 ms, 0.2–0.4 mA) delivered every 10 s at interstimulus intervals of 45 ms for EPSCs and 55 ms for IPSCs. Paired-pulse facilitation was expressed as the ratio of the amplitude of the second synaptic response to the amplitude of the first synaptic response (S2/S1), with an increase or decrease in the paired-pulse ratio indicating a reduced or enhanced probability of evoked neurotransmitter release, respectively (Dobrunz and Stevens 1997; Saviane et al. 2002).
Statistical analyses
All data are expressed as means ± standard error. Plasma corticosterone, drink consumption, and body weight were analyzed by two-way ANOVA (comparing DRINK and TIME, with TIME as a repeated measures factor) followed by protected Fisher’s LSD post-hoc tests. In instances where variance was not homogenous, data underwent a square root transformation prior to ANOVA. For the integrated plasma corticosterone response to stress (area-under-the-curve), sucrose and water were compared by one-tailed Student’s unpaired t-test based on the a priori directional hypothesis that sucrose reduces the corticosterone response to acute stress, as observed in previous work (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011; Egan and Ulrich-Lai 2015). For all other measures, sucrose and water groups were compared by two-tailed Student’s unpaired t-test; in instances where the variance was not homogenous, a two-tailed nonparametric Mann-Whitney U test was used instead. p < 0.05 was considered statistically significant.
Results
Blunting of the HPA response to stress by LSI
The effect of the LSI paradigm on metabolic and HPA end points was assessed in 8–10 week old male Long-Evans rats and 4–6 week old male Wistar rats. When rats of both strains, with free access to chow and water, were given additional twice-daily access to a second drink bottle containing 30% sucrose drink (LSI), they drank significantly more from the second drink bottle than control rats that were offered water (Long-Evans: Drink F1,24 = 148.01, p < 0.0001; Time F13,24 = 4.85, p < 0.0001; and Drink × Time interaction F13,24 = 9.23, p < 0.0001; Sucrose > Water at all time points on post-hoc analysis, p < 0.01; Wistar: Drink F1,22 = 255, p < 0.0001; Time F13,22 = 5.89, p < 0.0001; and Drink × Time interaction F13,22 = 10.85, p < 0.0001; Sucrose > Water at all time points on post-hoc analysis, p < 0.01; Figure 1a,b). Sucrose intakes quickly reached the maximum allowed (i.e., 4 ml/session, or 8 ml/day). Moreover, the LSI paradigm did not alter body weight in the Long-Evans rats (Drink F1,24 = 0.25, p = 0.620; Time F2,24 = 326.42, p < 0.0001; Drink × Time interaction F2,24 = 0.71, p = 0.496; Figure 1c) and had minimal effects on body weight in the Wistar rats (Drink F1,22 = 0.09, p = 0.768; Time F2,22 = 0.14, p < 0.0001; Drink × Time interaction F2,22 = 3.24, p = 0.048; Sucrose > Water only at the 7 day time point on post-hoc analysis, p < 0.05; Figure 1d).
On the morning following completion of the 14-day LSI paradigm, the HPA axis response to an acute, 20-minute restraint stress was reduced in the LSI groups compared to the control groups for both Long-Evans and Wistar rats. Plasma corticosterone levels were lower at 40 or 60 min (p < 0.01) after the initiation of stress in the LSI Long-Evans and Wistar rats, respectively (Long-Evans (This panel is reproduced from a cohort that was previously published and is shown for comparison to Wistar rats (Ulrich-Lai et al. 2010)): Drink F1,22 = 1.86, p = 0.186; Time F3,22 = 614.71, p < 0.0001; and Drink × Time interaction F3,22 = 6.15, p = 0.0009; Sucrose < Water at the 40 min time point on post-hoc analysis, p < 0.01; Wistar: Drink F1,22 = 2.90, p = 0.103; Time F3,22 = 107.89, p < 0.0001; and Drink × Time interaction F3,22 = 3.48, p = 0.021; Sucrose < Water at the 60 min time point on post-hoc analysis, p < 0.01; Figure 1e,f). In both strains, sucrose-fed rats had a reduced integrated plasma corticosterone response relative to water controls (Long-Evans: t22 = 1.94, p =0.032; Wistar: t22 = 2.19, p = 0.019; Figure 1g,h). These results mirror those observed previously for this paradigm (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011; Ulrich-Lai et al. 2015; Egan and Ulrich-Lai 2015) and indicate that the LSI paradigm effectively reduces HPA axis responses to acute stress in rats of both ages and strains.
Experiment 1: Anatomical assessment of appositions onto BLA neurons
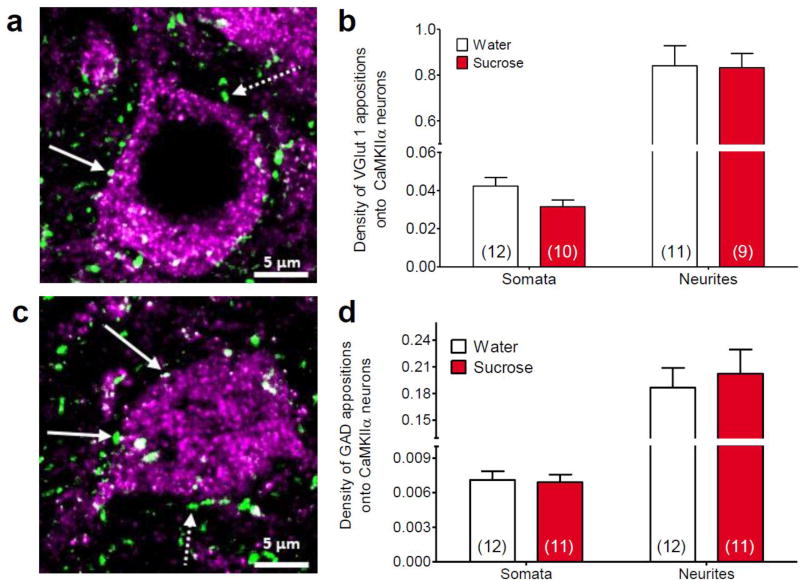
Analysis of appositions onto the somata and neurites of CamKIIα-positive BLA principal neurons revealed that the number of VGlut1-positive appositions was not altered by LSI (somata t18 = 1.84, p = 0.082; neurites t15 = 0.23, p = 0.818; Figure 2a,b). Similarly, the number of GAD-positive appositions onto the somata and neurites of CamKIIa-positive principal neurons was not altered by LSI (somata t21 = 0.18, p = 0.852; neurites t21 = 0.45, p = 0.657), Figure 2c,d). The volume of the CamKIIα-positive principal neurons was not altered by sucrose intake (water = 2362 ± 204.3 µm3 vs. sucrose = 2421 ± 183.2 µm3; t46 = 0.21, p = 0.832).
Fig. 2. Limited sucrose intake did not change appositions on basolateral amygdala principal neurons.
(a) Maximum intensity projection image of calcium/calmodulin-dependent protein kinase II α (CaMKIIα)-labeled neuron (violet) with vesicular glutamate transporter 1 (VGlut1)-positive appositions (green). (b) Analysis of basolateral amygdala tissue collected after 14 days of limited sucrose intake (Sucrose) revealed no significant differences in the number of VGlut1-labeled appositions on either the somata or neurites of CaMKIIα-labeled neurons. (c) Maximum intensity projection image of CaMKIIα-labeled neuron (violet) with glutamic acid decarboxylase (GAD)-positive appositions (green). (d) There were no significant differences in the number of GAD-labeled appositions on either the somata or neurites of CaMKIIα-labeled neurons. Numbers in parentheses on bar graphs indicate the number of rats that had sufficient replicates to meet inclusion criteria. Solid arrows indicate examples of appositions and dotted arrows indicate immunoreactive spots that did not meet apposition criteria
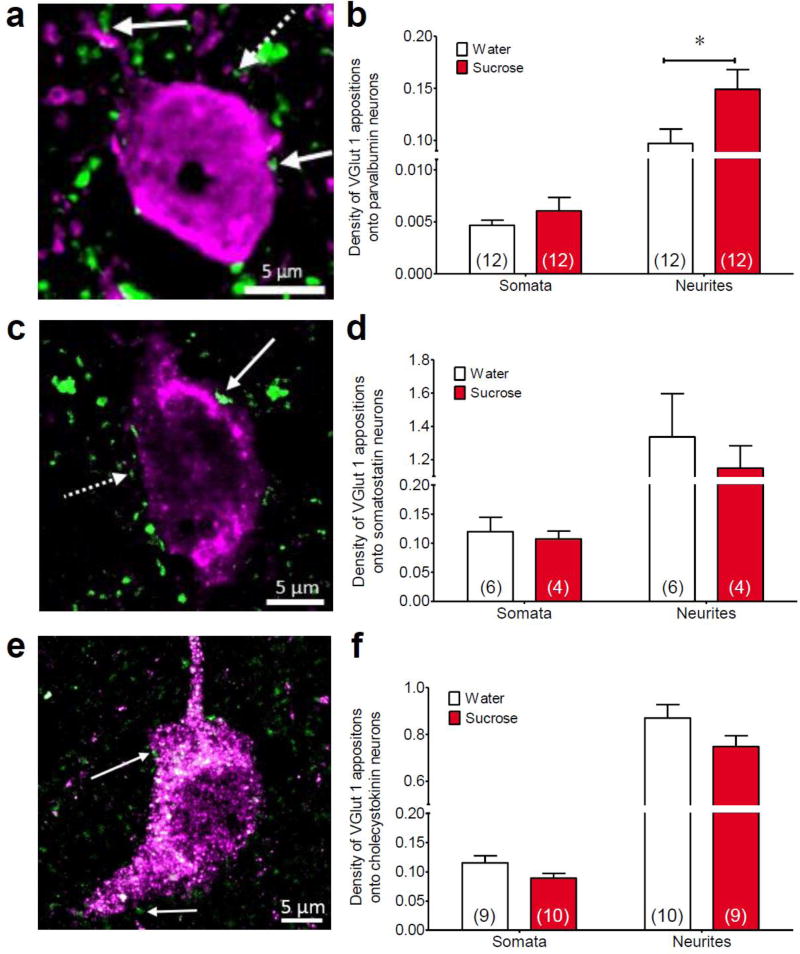
While the above data suggested that total GABAergic appositions derived from all sources was not altered by sucrose drink, this does not preclude the possibility that sucrose may specifically impact a small subset of the BLA interneurons. For example, LSI may increase glutamatergic tone onto a particular interneuron subtype, which could then provide greater inhibition of the principal neurons, and this would not be detectable using measures of GABAergic appositions on BLA principal neurons. In order to test for this possibility, VGlut1-positive appositions were quantified on each of the three main subpopulations of BLA interneurons expressing PV, SOM, and CCK (Mascagni and McDonald 2003; Rainnie et al. 2006). LSI increased the number of VGlut1-positive appositions onto the neurites, but not the somata, of PV-positive neurons in the BLA (somata t22 = 1.00, p = 0.327; neurites t22 = 2.23, p = 0.036, Figure 3a,b). In contrast, sucrose did not affect VGlut1-positive appositions onto the somata or the neurites of SOM-positive neurons (somata t8 = 0.39, p = 0.707, neurites t8 = 0.55, p = 0.599; Figure 3c,d) or CCK-positive neurons (somata t17 = 1.83, p = 0.086, neurites t17 = 1.61, p = 0.125; Figure 3e,f). Interneuron cell volumes were not altered by sucrose intake (PV: water = 3888 ± 432.9 µm3 vs. sucrose = 3826 ± 297.3 µm3, t22 = −0.12, p = 0.455; SOM: water = 949.0 ± 108.4 µm3 vs. sucrose = 1153 ± 130.4 µm3, t8 = 1.20, p = 0.265; CCK: water = 354.8 ± 51.4 µm3 vs. sucrose = 396.9 ± 28.1 µm3, t17 = 0.74, p = 0.470).
Fig. 3. Limited sucrose intake increased appositions on parvalbumin-labeled basolateral amygdala inhibitory interneurons.
Immunohistochemical analysis of glutamatergic synaptic innervation of interneuron subtypes in the basolateral amygdala was performed following limited sucrose intake (Sucrose). Maximum intensity projection images of vesicular glutamate transporter 1 (VGlut1) appositions (green) on (a) parvalbumin-, (c) somatostatin- and (e) cholecystokinin-positive neurons in the basolateral amygdala (interneurons are labeled purple). (b) There was a significant increase in the number of VGlut1-labeled appositions on parvalbumin neurons, specifically on neurites, following sucrose treatment. However, there was no difference in the number of VGlut1-labeled appositions on either the somata or neurites of somatostatin (d) or cholecystokinin neurons (f). Numbers in parentheses on bar graphs indicate the number of rats that had sufficient replicates to meet inclusion criteria. Solid arrows indicate examples of appositions and dotted arrows indicate immunoreactive spots that did not meet apposition criteria. *p < 0.05 vs. water-fed controls
Anatomical assessment of pCREB expression within PV-positive interneurons
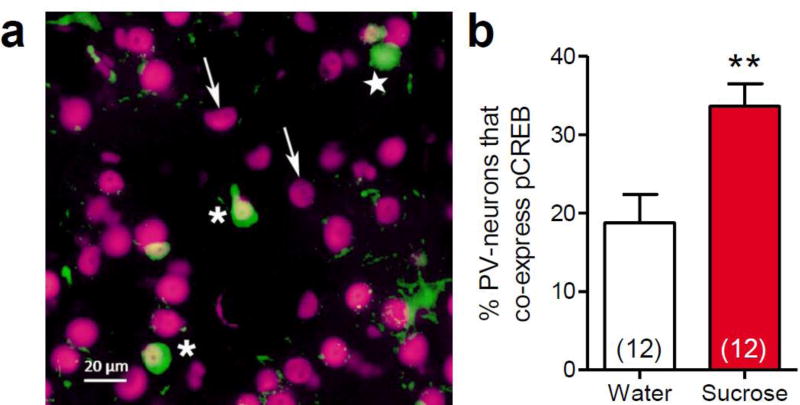
The LSI-induced increase in VGlut1-appositions onto PV-positive neurons suggests that repeated sucrose consumption may activate PV-interneurons, leading to activity-dependent plasticity that increases their glutamatergic appositions. The phosphorylation and activation of CREB, to form pCREB, is a useful marker for this process, as pCREB expression occurs as a consequence of neuronal activation, and pCREB mediates activity-dependent plasticity at glutamatergic synapses (Silva et al. 1998; Huang et al. 2000; Miyamoto 2006). We therefore performed dual immunolabeling for pCREB and PV in order to determine the impact of repeated sucrose consumption in the LSI paradigm on pCREB expression in PV-positive BLA neurons. The total number of PV-positive neurons that were present in the BLA sections was not altered by sucrose intake (water = 34.4 ± 5.3 cells vs. sucrose = 43.4 ± 3.7 cells, t22 = 1.40, p = 0.177). However, LSI increased the proportion of these PV-positive neurons that co-expressed pCREB compared to water controls (t22 = 3.22, p = 0.004, Figure 4a,b). These data suggest that repeated sucrose intake activates a subset of BLA PV-interneurons, increasing their expression of a known mediator of glutamatergic synaptic plasticity.
Fig. 4. Limited sucrose intake increased pCREB expression within parvalbumin-labeled basolateral amygdala neurons.
Immunohistochemical analysis of pCREB and parvalbumin co-expression in the basolateral amygdala was performed following limited sucrose intake (Sucrose). (a) Image of dual-immunolabeling for pCREB (magenta) and parvalbumin (green) in the basolateral amygdala. Asterisks denote neurons that expresses both pCREB and parvalbumin; a star denotes a parvalbumin neuron that does not express pCREB; and arrows indicate examples of pCREB-positive cells that do not express parvalbumin. (b) There was a significant increase in the proportion of PV-positive neurons in the basolateral amygdala that co-expressed pCREB-labeling. Numbers in parentheses on bar graphs indicate the number of rats per group. *p<0.01 vs. water-fed controls
Experiment 2: Electrophysiological recordings from BLA principal neurons following LSI
Amygdala slices were prepared from control and LSI rats, and whole-cell patch clamp recordings were performed in principal neurons of the BLA to test for LSI-induced changes in passive electrical properties and GABAergic and glutamatergic synaptic inputs. No change in the input resistance or resting potential of the BLA principal neurons was found following LSI. The mean input resistance was 343.5 ± 21.3 MΩ in the water controls and 341.6 ± 57 MΩ in the LSI group (t58 = 0.07, p = 0.94). The holding current required to voltage clamp the cells at −60 mV was −63.8 ± 7.3 pA in the controls and −57 ± 5.9 pA in the LSI group (t58 = 0.73, p = 0.47; n = 30 cells in each group for both membrane resistance and holding current).
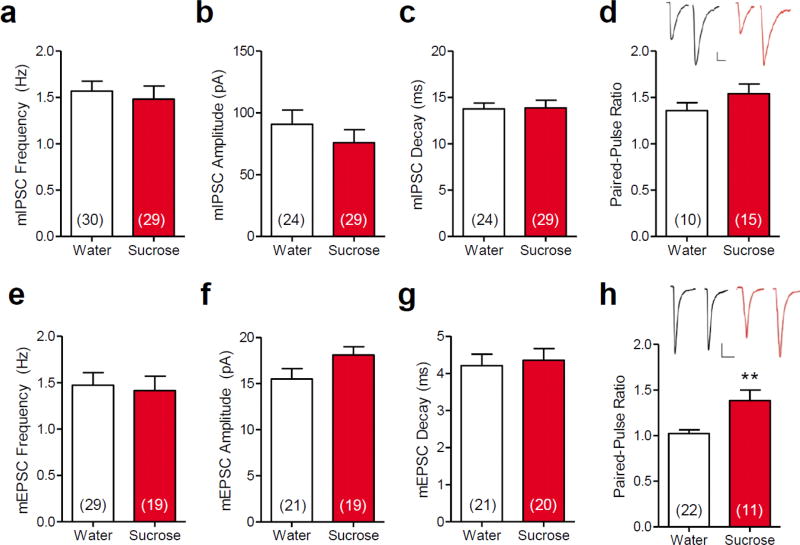
When GABAergic inhibitory synaptic inputs to BLA neurons were examined, no change in mIPSCs was detected following LSI. There was no difference in the frequency (t57 = 0.50, p = 0.621, Figure 5a), amplitude (t51 = 0.96, p = 0.343, Figure 5b) or decay (t51 = 0.11, p = 0.916, Figure 5c) of mIPSCs between sucrose and water-fed rats. Similarly, there was no change in the evoked IPSC paired-pulse ratio (t23 = 1.25, p = 0.222, Figure 5d) following sucrose treatment. Collectively, these results indicate that LSI does not alter GABAergic synaptic inputs to the BLA principal neurons.
Fig. 5. Limited sucrose intake increased the probability of evoked excitatory input to basolateral amygdala principal neurons.
Patch clamp recordings revealed that limited sucrose intake (Sucrose) had no effect on the (a) frequency, (b) amplitude, or (c) decay of mIPSCs, or on (d) the paired-pulse ratio of evoked IPSCs. Limited sucrose intake had no effect on mEPSC (e) frequency, (f) amplitude or (g) decay, but (h) increased the evoked EPSC paired-pulse ratio, suggesting a decrease in the probability of evoked glutamate release at excitatory synapses following sucrose exposure. Numbers in parentheses on bar graphs indicate the number of principal neurons that were recorded from rats that received prior LSI vs. water controls. Insets in panels (d) and (h) show representative paired eIPSCs and eEPSCs, respectively, recorded in neurons from control (Water) and sucrose-treated rats. Scale bars: 25 pA, 20 ms; **p < 0.01 vs. water-fed controls
Sucrose treatment also had no effect on mEPSCs, though it did alter evoked excitatory synaptic responses. Thus, there was no difference in the mEPSC frequency (t45 = 0.28, p = 0.776, Figure 5e), amplitude (t38 = 1.77, p = 0.085, Figure 5f) or decay (t38 = 0.34, p = 0.736, Figure 5g) between LSI-treated and control rats. However, sucrose increased the EPSC paired-pulse ratio (U = 53, n1 = 22, n2 = 11, p = 0.009, Figure 5h). Therefore, sucrose consumption did not alter the probability of spontaneous glutamate release, the postsynaptic glutamate sensitivity, or the glutamate clearance, but it decreased the probability of evoked glutamate release onto BLA principal neurons.
Discussion
Sucrose consumption prior to exposure to an acute stress reduces the HPA response to the stress (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010), and activation of the BLA is required for the HPA-dampening effect of sucrose (Ulrich-Lai et al. 2010). Output from BLA principal neurons is primarily thought to increase stress responses (Coover et al. 1973; Feldman et al. 1983; Szafarczyk et al. 1986; Goldstein et al. 1996; Bhatnagar et al. 2004), so we postulated that sucrose acts by reducing stress-excitatory BLA output. Moreover, analyses of BLA mRNA and protein expression suggest that this reduced stress-excitatory output may be mediated by sucrose-induced neural plasticity in the BLA (Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2015; Egan and Ulrich-Lai 2015). Therefore, we measured indices of structural and functional plasticity in the BLA following LSI. Our results suggest that LSI decreases BLA output by shifting the synaptic excitation/inhibition balance in BLA principal neurons towards inhibition (via an enhanced excitatory drive to local PV inhibitory interneurons) and away from excitation (via a reduced probability of evoked glutamate release).
Effect of LSI on principal neurons
BLA output is determined by the activation state of the principal neurons in the BLA, which in turn is a function of the intrinsic excitability of the principal neurons and the balance between excitatory and inhibitory synaptic inputs to those neurons. We found with paired-pulse analysis that LSI reduced the probability of stimulation-evoked glutamate release onto BLA principal neurons. We found no effect of LSI on the intrinsic excitability; the probability of unstimulated glutamate release or the number of glutamatergic release sites; or on the postsynaptic sensitivity to glutamate release in our recordings of mEPSCs. The LSI paradigm also had no effect on spontaneous or evoked inhibitory inputs to BLA principal neurons. Quantification of VGlut-1- and GAD-immunoreactive profiles showed no change in the glutamatergic and GABAergic appositions on the BLA principal neurons following LSI. Together, these findings indicate that LSI may act primarily via an upstream, pre-synaptic mechanism as opposed to a post-synaptic mechanism. For example, it is possible that the reduced probability of evoked, but not spontaneous, glutamate release by sucrose treatment is due to differential modulation of different pools of synaptic vesicles. Recent studies suggest that spontaneous and evoked/synchronous vesicle release events are mediated by different pools of synaptic vesicles that are subject to distinct regulatory processes (reviewed in (Ramirez and Kavalali 2011; Kavalali 2015)). We recently reported that stress-induced glucocorticoids stimulate retrograde endocannabinoid release at inhibitory synapses in the BLA that differentially modulates evoked and spontaneous GABA release (Di et al. 2016), supporting the possibility that evoked and spontaneous glutamate release in the BLA may also be regulated by distinct processes. Alternatively, it has previously been shown that GABAB receptor-mediated inhibition of glutamatergic terminals can occur in multiple brain regions, including lateral amygdala (Pan et al. 2009; Bosch and Ehrlich 2015). It is intriguing to speculate that LSI may reduce the probability of evoked glutamate release onto BLA principal neurons through a similar GABAB-mediated process, and further that this may be a consequence of increased activation of PV interneurons (as discussed below). Future work will be directed towards identifying the mechanism(s) by which LSI impacts evoked glutamatergic transmission.
Reducing the probability of evoked excitatory synaptic input to the BLA principal neurons should reduce BLA output under conditions that stimulate BLA activity. For example, BLA principal neurons are activated by glutamatergic inputs during acute stress exposure (Davis et al. 1994; Hubert et al. 2014; Masneuf et al. 2014; Ito et al. 2015), and dampening by prior LSI of stress-induced glutamate release would be expected to blunt principal neuron activation during stress, consistent with the reduction in stress-induced cFos mRNA expression in BLA principal neurons seen following LSI (Ulrich-Lai 2007). Furthermore, as BLA principal neuron output promotes HPA axis activation during stress (Coover et al. 1973; Feldman et al. 1983; Szafarczyk et al. 1986; Goldstein et al. 1996; Bhatnagar et al. 2004), the reduction in principal neuron activation following LSI may contribute to the LSI-induced attenuation of stress-evoked HPA activation.
Effect of LSI on PV interneurons
While the above data suggest that the total GABAergic tone onto BLA principal neurons (i.e., derived from all interneuron sources) was not altered by sucrose drink, this does not preclude the possibility that sucrose may specifically impact a small subset of the BLA interneurons. For example, LSI may activate a particular interneuron subtype, which could then provide greater inhibition of principal neurons in a manner that is not readily detectable when global measures of GABAergic tone are employed. In support of this idea, sucrose increased the expression of pCREB, a marker of neuronal activation that is linked with synaptic plasticity (Silva et al. 1998; Huang et al. 2000; Miyamoto 2006), within PV-positive interneurons, and increased glutamatergic appositions onto BLA PV-positive interneurons. Together, these data imply that sucrose consumption may specifically activate PV interneurons, promoting plastic changes that drive greater glutamatergic stimulation following LSI. By extension, this recruitment of PV interneurons by sucrose could promote principal neuron inhibition, as PV-positive interneurons innervate the perisomatic region of principal neurons (Kemppainen and Pitkänen 2000; McDonald and Betette 2001; Rainnie et al. 2006; Muller et al. 2006) and inhibit principal neuron activity (Woodruff and Sah 2007b; Woodruff and Sah 2007a). Moreover, the principal neurons are the main source of the glutamatergic input to the PV interneurons (Smith et al. 2000; McDonald et al. 2005; Woodruff and Sah 2007b), providing an opportunity for reciprocal PV/principal neuron interactions. In fact, reciprocal PV/principal neuron interactions have been linked with mediating principal neuron feed-forward inhibition, which regulates the synchronized firing of principal neurons and may contribute to spike-timing-dependent synaptic plasticity in the BLA (Woodruff and Sah 2007b; Ryan et al. 2012). Thus, if sucrose promotes these PV/principal neuron reciprocal interactions by increasing principal neuron glutamatergic inputs onto PV-positive interneurons, then this has the potential to impact multiple aspects of principal neuron activity.
Importantly, recent studies have reported that there is considerable heterogeneity within the population of BLA PV interneurons in terms of their intra-BLA connectivity and electrophysiological properties (Woodruff and Sah 2007b; Bienvenu et al. 2012). For instance, some subtypes are activated by stimuli that are noxious, anxiogenic or stressful, whereas others are either unaffected or inhibited by these inputs (Rainnie et al. 2006; Reznikov et al. 2008; Hale et al. 2010; Bienvenu et al. 2012; Wolff et al. 2014). In the present work, repeated sucrose consumption induced pCREB-labeling within ~36% of PV-interneurons (compared to in ~18% co-expression in water controls), supporting the idea that PV interneurons are a heterogeneous population, with a subset that are activated by sucrose. As the role of these diverse subtypes of PV-positive interneurons in stress regulation by the BLA is completely unexplored, further work is needed to identify if/how they each contribute to HPA axis activation during stress.
Potential impact of age and strain of rats
Prior work with the LSI paradigm used young-adult Long-Evans rats, and as in the present study, we invariably observe that these rats quickly begin to drink the sucrose in amounts approaching the daily maximum, and decrease their chow intake isocalorically, resulting in no effect on body weight gain when compared to water controls (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011). When these same rats receive a novel restraint stress, there is a robust increase in circulating corticosterone that typically peaks at ~800–1000 ng/ml in the water controls, though the temporal dynamics vary slightly between cohorts, such that the peak corticosterone appears between 20–60 min after stress onset (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011). Moreover, prior sucrose intake in the LSI paradigm blunts this corticosterone response to restraint, consistently reducing the integrated (area-under-the-curve) corticosterone response by 10–20%; and again, the exact timing of the sucrose effect fluctuates somewhat among cohorts, occurring at one or more time points between 20–60 min after stress onset (Ulrich-Lai et al. 2007; Ulrich-Lai et al. 2010; Christiansen et al. 2011; Ulrich-Lai et al. 2011). The present work tested the LSI paradigm for the first time in younger (e.g., early adolescent) male Wistar rats, providing the opportunity to compare the impact of LSI across the two strains and ages. The younger Wistar rats also quickly began to drink sucrose in near-maximal amounts, and sucrose caused a subtle increase in body weight after 7 days of exposure, that was no longer different from water-controls by 14 days of drink intake. The peak plasma corticosterone response to restraint occurred at 60 min after stress onset and was ~800 ng/ml, a profile that falls within the historical range of several prior cohorts of adult Long-Evans rats (as described above). Importantly, the younger Wistar rats with a history of sucrose intake had a lower corticosterone response to stress that appeared at the 60 min post-stress time point, and the integrated corticosterone response was decreased by ~16%, placing the temporal dynamics and magnitude of the sucrose-induced corticosterone-blunting within the historical range of the adult Long-Evans rats. Prior work has shown that pre-adolescent rats typically have larger and/or prolonged HPA axis responses to stress when compared to adults, with the corticosterone response becoming adult-like at ~30–40 days of age (Gomez et al. 2004; McCormick and Mathews 2007; Romeo et al. 2016). This may indicate that in the present study, the corticosterone response of the younger Wistar rats reached the adult-like stage prior to restraint testing. However, HPA axis tone can also vary between rat strains, and some evidence suggests that adult Long-Evans rats generally have greater HPA activation than adult Wistar rats (Tannahill et al. 1988; Tohei et al. 2003). By extension, it is possible that the younger Wistar rats had not yet reached their adult-like corticosterone phenotype, as this might be expected to lower their HPA activation compared to Long-Evans rats. Given the potential contributions of strain and age to HPA reactivity, it is noteworthy that the LSI paradigm produced similar corticosterone-blunting in the young-adult Long-Evans and early-adolescent Wistar rats, demonstrating that the stress-blunting effects of LSI generalize across these ages and strains of rats.
Perspectives
The present work identified indices of functional plasticity and structural remodeling in the BLA following repeated sucrose consumption. Collectively, these observations are suggestive of overall blunted principal neuron activation. This result is intriguing as these data were obtained from BLA slices taken from otherwise normal rats. That is, a history of repeatedly ingesting small amounts of highly-palatable food is itself sufficient to alter basal BLA structure/function. Future work will be directed towards understanding how these changes in BLA local synaptic circuitry lead to altered BLA responsivity during acute stress, for example by assessing the impact of LSI on acute stress-induced plasticity in the BLA. These studies provide critical insight into the neural and molecular mechanisms by which the consumption of highly-palatable food confers stress protection, and suggest a basis for the consumption of so-called ‘comfort’ foods for the relief of stress symptoms.
Acknowledgments
This work was supported by National Institutes of Health grants R01 DK091425 (YMU), K01 DK078906 (YMU), F32 DK102334 (AEBP), T32 DK059803 (AEBP, AEE), and an Albert J. Ryan Foundation Fellowship (AEE). We would like to thank Sriparna Ghosal for her excellent technical assistance.
Footnotes
Compliance with Ethical Standards
The authors declare that they have no conflicts of interest. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
References
- Ahmed T, Frey JU, Korz V. Long-term effects of brief acute stress on cellular signaling and hippocampal LTP. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:3951–3958. doi: 10.1523/JNEUROSCI.4901-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Annals of the New York Academy of Sciences. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- Bienvenu TCM, Busti D, Magill PJ, et al. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron. 2012;74:1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D, Ehrlich I. Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. The Journal of Physiology. 2015;593:4387–4409. doi: 10.1113/JP270645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. Journal of Neuroscience. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AM, DeKloet AD, Ulrich-Lai YM, Herman JP. “Snacking” causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiology & Behavior. 2011;103:111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coover G, Ursin H, Levine S. Corticosterone and avoidance in rats with basolateral amygdala lesions. Journal of Comparative and Physiological Psychology. 1973;85:111–122. doi: 10.1037/h0034858. [DOI] [PubMed] [Google Scholar]
- Cox DJ, Racca C, Lebeau FEN. β-adrenergic receptors are differentially expressed in distinct interneuron subtypes in the rat hippocampus. The Journal of Comparative Neurology. 2008;509:551–565. doi: 10.1002/cne.21758. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends in Neurosciences. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Di S, itoga CA, Fisher MO, et al. Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. Journal of Neuroscience. 2016;36:8461–8470. doi: 10.1523/JNEUROSCI.2279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/S0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Egan AE, Ulrich-Lai YM. Activation of physiological stress responses by a natural reward: Novel vs. repeated sucrose intake. Physiology & Behavior. 2015;150:43–52. doi: 10.1016/j.physbeh.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, et al. Are stress eaters at risk for the metabolic syndrome? Annals of the New York Academy of Sciences. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. Journal of Neuroscience. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Siegel RA, Conforti N. Differential effects of medial forebrain bundle lesions on adrenocortical responses following limbic stimulation. Neuroscience. 1983;9:157–163. doi: 10.1016/0306-4522(83)90053-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs. 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. Journal of Comparative Neurology. 2009;517:156–165. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. The Journal of Neuroscience. 2009;29:2486–2495. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbelli R, Inverardi F, Medici V, et al. Heterogeneous expression of SNAP-25 in rat and human brain. The Journal of Comparative Neurology. 2008;506:373–386. doi: 10.1002/cne.21505. [DOI] [PubMed] [Google Scholar]
- Giassi ACC, Harvey-Girard E, Valsamis B, Maler L. Organization of the gymnotiform fish pallium in relation to learning and memory: I. Cytoarchitectonics and cellular morphology. The Journal of Comparative Neurology. 2012;520:3314–3337. doi: 10.1002/cne.23097. [DOI] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiology & Behavior. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. Journal of Neuroscience. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 2004;145:59–70. doi: 10.1210/en.2003-0565. [DOI] [PubMed] [Google Scholar]
- Hale MW, Johnson PL, Westerman AM, et al. Multiple anxiogenic drugs recruit a parvalbumin-containing subpopulation of GABAergic interneurons in the basolateral amygdala. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1285–1293. doi: 10.1016/j.pnpbp.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:6317–25. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Li C, Rainnie DG, Muly EC. Effects of stress on AMPA receptor distribution and function in the basolateral amygdala. Brain Structure and Function. 2014;219:1169–1179. doi: 10.1007/s00429-013-0557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A. Observation of distressed conspecific as a model of emotional trauma generates silent synapses in the prefrontal-amygdala pathway and enhances fear learning, but ketamine abolishes those effects. Neuropsychopharmacology. 2015;40:2536–2545. doi: 10.1038/npp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neuroscience Research. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kavalali ET. The mechanisms and functions of spontaneous neurotransmitter release. Nature Reviews Neuroscience. 2015;16:5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkänen A. Distribution of parvalbumin, calretinin, and calbindin- D28k immunoreactivity in the rat amygdaloid complex and colocalization with γ- aminobutyric acid. Journal of Comparative Neurology. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. Journal of Molecular Neuroscience. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Ilinsky IA, Verney C. Glutamic acid decarboxylase isoform 65 immunoreactivity in the motor thalamus of humans and monkeys: γ-aminobutyric acidergic connections and nuclear delineations. The Journal of Comparative Neurology. 2011;519:2811–2837. doi: 10.1002/cne.22653. [DOI] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, et al. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neurosci. 2006;142:1281–1292. doi: 10.1016/j.neuroscience.2006.07.027. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49:667–674. doi: 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Research. 2003;976:171–184. doi: 10.1016/S0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- Masneuf S, Lowery-Gionta E, Colacicco G, et al. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosomatic Medicine. 1990;52:97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology Biochemistry and Behavior. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Research. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Research. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. The Journal of Comparative Neurology. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology. 2007a;505:314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Dopaminergic innervation of pyramidal cells in the rat basolateral amygdala. Brain Structure and Function. 2009;213:275–288. doi: 10.1007/s00429-008-0196-y. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. The Journal of Comparative Neurology. 2007b;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. The Journal of Comparative Neurology. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 2011 doi: 10.17226/12910. [DOI] [Google Scholar]
- Nestler EJ, Kelz MB, Chen J. ΔFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Research. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014;311:806–9. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Wardle J. Perceived effects of stress on food choice. Physiology & Behavior. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Pan B-X, Dong Y, Ito W, et al. Selective gating of glutamatergic inputs to excitatory neurons of amygdala by presynaptic GABAb receptor. Neuron. 2009;61:917–929. doi: 10.1016/j.neuron.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, et al. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Queenan BN, Lee KJ, Pak DTS. Wherefore Art Thou, Homeo(stasis)? Functional Diversity in Homeostatic Synaptic Plasticity. Neural Plasticity. 2012;2012:1–12. doi: 10.1155/2012/718203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. The Journal of Comparative Neurology. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Current Opinion in Neurobiology. 2011;21:275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. Journal of Comparative Neurology. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neuroscience & Biobehavioral Reviews. 2016;70:206–216. doi: 10.1016/j.neubiorev.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rostkowski AB, Teppen TL, Peterson DA, Urban JH. Cell-specific expression of neuropeptide Y Y1 receptor immunoreactivity in the rat basolateral amygdala. The Journal of Comparative Neurology. 2009;517:166–176. doi: 10.1002/cne.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Ehrlich DE, Jasnow AM, et al. Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS ONE. 2012;7:e35320. doi: 10.1371/journal.pone.0035320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Savtchenko LP, Raffaelli G, et al. Frequency- dependent shift from paired- pulse facilitation to paired- pulse depression at unitary CA3- CA3 synapses in the rat hippocampus. The Journal of Physiology. 2002;544:469–476. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behavioral Neuroscience. 1989;103:1168–1175. doi: 10.1037//0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annual Review of Neuroscience. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Smith Y, Paré JF, Paré D. Differential innervation of parvalbumin- immunoreactive interneurons of the basolateral amygdaloid complex by cortical and intrinsic inputs. Journal of Comparative Neurology. 2000;416:496–508. doi: 10.1002/(SICI)1096-9861(20000124)416:4<496::AID-CNE6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. The American Journal of Physiology. 1997;272:R840–8. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- Szafarczyk A, Caracchini M, Rondouin G, et al. Plasma ACTH and corticosterone responses to limbic kindling in the rat. Experimental Neurology. 1986;92:583–590. doi: 10.1016/0014-4886(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kovalchuk Y, Attwell D. Pre- and postsynaptic determinants of EPSC waveform at cerebellar climbing fiber and parallel fiber to Purkinje cell synapses. Journal of Neuroscience. 1995;15:5693–5702. doi: 10.1523/JNEUROSCI.15-08-05693.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill LA, Dow RC, Fairhall KM, et al. Comparison of adrenocorticotropin control in Brattleboro, Long-Evans, and Wistar rats. Measurement of corticotropin-releasing factor, arginine vasopressin, and oxytocin in hypophysial portal blood. Neuroendocrinology. 1988;48:650–657. doi: 10.1159/000125077. [DOI] [PubMed] [Google Scholar]
- Tohei A, Mogi Y, Kon H, et al. Strain difference in pituitary-adrenal axis between Wistar-Imamichi and Long Evans adult male rats. Exp Anim. 2003;52:437–439. doi: 10.1538/expanim.52.437. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36:1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon MS, Carter CS, DeCant R, Laugero KD. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiology & Behavior. 2013;120:233–242. doi: 10.1016/j.physbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM. Self-medication with sucrose. Curr Opin Behav Sci. 2016;9:78–83. doi: 10.1016/j.cobeha.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, et al. Pleasurable behaviors reduce stress via brain reward pathways. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Wang X, et al. Statistical modeling implicates neuroanatomical circuit mediating stress relief by “comfort” food. Brain Structure and Function. 2015:1–16. doi: 10.1007/s00429-015-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiology & Behavior. 2011;103:104–110. doi: 10.1016/j.physbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, et al. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metabolism. 2014;19:910–925. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. American Journal of Physiology Endocrinology and Metabolism. 2005;289:E823–8. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neurosci. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, et al. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. Journal of Neuroscience. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SBE, Gründemann J, Tovote P, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. Journal of Neurophysiology. 2007a;98:2956–2961. doi: 10.1152/jn.00739.2007. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. The Journal of Neuroscience. 2007b;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. [Accessed 20 May 2016];Obesity and overweight. 2015 httpwww.who.intmediacentrefactsheetsfsen.
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neuroscience Letters. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- Yang L, Shi L-J, Yu J, Zhang Y-Q. Activation of protein kinase A in the amygdala modulates anxiety-like behaviors in social defeat exposed mice. Mol Brain. 2016;9:3. doi: 10.1186/s13041-015-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]