Abstract
Lead based ammunition is a primary source of lead exposure, especially for scavenging wildlife. Lead poisoning remains the leading cause of diagnosed death for the critically endangered California condors, which are annually monitored via blood tests for lead exposure. The results of these tests are helpful in determining recent exposure in condors and in defining the potential for exposure to other species including humans. Since condors are victim to acute and chronic lead exposure, being able to measure both would lend valuable information on the rates of exposure and accumulation through time. A commercial portable x-ray fluorescence (XRF) device has been optimized to measure bone lead in vivo in humans, but this device could also be valuable for field measurements of bone lead in avian species. In this study, we performed measurements of bone Pb in excised, bare condor bones using inductively coupled plasma mass spectrometry (ICP-MS), a cadmium 109 (Cd-109) K-shell x-ray fluorescence (KXRF) system, and a portable XRF system. Both KXRF and portable XRF bone Pb measurement techniques demonstrated good correlations with ICP-MS results (r=0.93 and r=0.92 respectively), even with increasing skin thickness (r=0.86 between ICP-MS and portable XRF at 1.54 mm of soft tissue). In conclusion, our results suggest that a portable XRF could be a useful option for measurement of bone Pb in avian species in the field.
Keywords: metals, environmental monitoring, biomonitoring, avian, lead
1. Introduction
The use of lead-based ammunition in hunting has drastically increased lead (Pb) exposure to a great number of wildlife and humans in the US.(1, 2) Lead-based ammunition in the form of bullets for center-fire and rim-fire ammunition is a primary source of exposure as weight loss, or fragmentation, of the projectile results in sometimes hundreds of ingestible particles, some so small they cannot be identified with the unaided eye.(1, 3) Scavenging wildlife, who might feed upon animals killed with Pb-based ammunition are most affected.(4–7) California condors, a critically endangered species, undergo regular monitoring and testing and have demonstrated some of the ill-effects from lead exposure. Fifty-three percent of free-flying California condor deaths are attributed to Pb poisoning, the leading cause of diagnosed death in the wild-flock.(8, 9) The relationship between lead and condors is well understood because condors are monitored on an individual level, but it is highly probably that other species where calcium has a central role in bodily function are affected similarly by Pb, which primarily acts by mimicking calcium.(10–12) Currently, condors are primarily being monitored for Pb exposure by annual blood-lead analyses. The results from testing allow for a better understanding of recent exposure for the condors and of the potential exposure to other species including humans. Lead shot has been restricted nationally for use in hunting waterfowl within the United States and Canada, but lead-based ammunition is still widely used in many forms for other types of hunting and dispatch of domestic stock simply because of its mass, density, availability and cost relative to less dense metals.(2)
Since today’s free-flying condors have been found to have both acute and chronic Pb exposure, measuring lead with a biomarker more reflective of the cumulative Pb stores in the body will add to our understanding of both short and long-term rates of exposure and accumulation. In human exposures, bone Pb has a half-life of years to decades, and over 95% of the Pb in the body is finally stored in the bones.(13, 14) It would make sense for the biokinetics to be similar in other species, thus, measuring bone Pb of condors would allow us to track the chronic, life-long exposure of Pb.
Traditionally, bone Pb is measured using a cadmium-109 based K-shell x-ray fluorescence (KXRF) system, which is restricted to measurement in labs due to its size, radioisotope restrictions, and 30-minute measurement time.(15) This device uses the higher K-shell energy for Pb quantification, and therefore has less dependence on soft tissue thickness due to less attenuation of the x-rays at this energy. Modern Cd-109 KXRF system (with a cloverleaf high-purity germanium detector setup) has a detection limit of 2–3 ug/g bone mineral.
Recently a portable x-ray fluorescence (XRF) device was validated for use in measuring bone Pb in vivo in humans.(16, 17) The portable XRF has the advantage of being handheld with only a 3-minute measurement time, which would be optimal for measuring in the field. This device uses the L-shell of Pb for quantification and has an x-ray tube source. Since the energies in this device are much lower, the attenuation of soft tissue can be significant. Thus, calibration approaches have been developed to correct for soft tissue thickness in the measurement. Nonetheless, the detection limit is still dependent upon the overlying soft tissue (1.8 ug/g and 11 ug/g at 1 mm and 5 mm of soft tissue respectively).(16)
XRF technology could be used to measure bone Pb in vivo in condors to help monitor their lifetime exposure levels. Portable XRF technology, being handheld and battery powered, has significant advantages specifically for these types of field measurements. Bone Pb monitoring will help guide intervention and policy to possibly interrupt a preventable pathway of Pb exposure to wildlife and humans. In this study, we validated the use of both KXRF and portable XRF to measure bone Pb in condor bone samples, and compared the values to those obtained using inductively coupled plasma mass spectrometry (ICP-MS).
2. Materials and Methods
2.1 Condor Bone Samples
Seventeen condor bone samples were obtained from specimens held at the University of Arizona. They were bare bone samples taken from the avian equivalent of the tibia. The bones themselves were several centimeters in length and about one centimeter in width. Figure 1 below displays a sample of one of the condor tibia bones measured in the study.
Figure 1.
One of the condor tibia bone samples.
2.2 KXRF bone Pb measurement system
We used a KXRF bone Pb measurement system in this study to both further validate the portable XRF data and validate the use of XRF for condor bone measurements in comparison with ICP-MS measurements. The setup of the device is the same as used in previous studies.(16–18) The system uses four 16 mm diameter high-purified germanium (HpGe) detectors with 10 mm thickness, four feedback resistance pre-amplifiers, four digital signal-processing systems, and a computer. A 135 mCi Cd-109 source is used to irradiate condor tibia bone or bone equivalent samples to produce the Pb K x-rays. The Cd-109 source requires site-specific licensing and is not easily transportable. The bone Pb measurements (one measurement per sample) were taken for 30 minutes with the HpGe detector. The spectra were analyzed using an in-house peak-fitting program and the final Pb concentrations were calculated.(19, 20) The whole body effective dose from this system was measured to be 0.26 μSv for human adults.(21)
The condor bone samples were placed 3–5 cm from the Cd-109 source. The condor tibia bones varied slightly in size, but the center of each sample (center of the diaphysis) was aligned with the source. If bone structure is similar to humans, this should also be the area with most cortical bone and least trabecular bone, which would reflect the longest cumulative exposure.
2.3 Portable XRF bone Pb measurement system
The portable XRF used for the bone Pb measurements in this study was the Niton XL3t GOLDD+ model from Thermo Fischer Scientific Inc. (Billerica, MA). These and other similar systems typically cost between $30,000 and $50,000. Since the devices use an x-ray tube source, they typically need to be licensed for use, but the process is much simpler than with the radioisotope source used in the KXRF. This is the same device as used in previous studies of bone Pb measurements.(16, 17, 22) The commercial device allowed for customization, which we used to optimize x-ray tube settings and filtration to be used for in vivo measurements. An optimized setting of 50 kV, 40 µA, and filter combination of silver and iron were selected for bone Pb measurements as found in our previous study.(16) The device uses a thermoelectric cooled silicon drift detector with 25 mm2 area and 1 mm thickness. The device was calibrated using lead doped bone phantoms made of plaster-of-Paris ranging from 0, 5, 10, 15, 30, 50, and 100 ppm Pb and soft tissue phantoms made of Lucite, which are detailed in our previous work.(16) The spot size of the x-ray beam is <1 cm2 and the phantoms in use were large enough to completely cover the x-ray beam area. With these configurations, the skin dose to a human subject was estimated to be 31 mSv to a 1 cm2 area and the total body effective dose is 3.6 uSv for the 3-minute measurement.(22)
The condor tibia bones were measured (one measurement for each bone with each Lucite thickness) with the device in its stand, so that the samples could be laid as flat as possible against the beam aperture and detector. Samples were measured at the same point as with the KXRF measurements, the midpoint of the length of the bone (center of the diaphysis). The bones were also measured with different Lucite thicknesses (0.54, 1, and 1.54 mm) to simulate soft-tissue covering the bones, which would increase measurement uncertainty and the corresponding detection limit for the portable XRF bone Pb measurement. We chose Lucite as a skin phantom, as Lucite was shown in a previous study using the same device to produce spectra that were identical to human skin measured over cadaver tibia.(16) The measurements were analyzed using standard peak fitting with a Gaussian and exponential background with signal related to concentration from a standard calibration curve obtained from measurements of Pb doped plaster-of-Paris and Lucite phantoms.(16)
2.4 ICP-MS Measurements
The ICP-MS measurements were done at the University of Idaho’s Analytical Sciences Laboratory. The measurements were made from the intact bone by using a 1 cm sample cut from the end of each bone (a cross-section about 3–4 cm from the center of the diaphysis), removing any soft tissue, drying, and grinding the bone. The sample was then digested in trace metal grade nitric acid (69%) using a Tecator open block digestion system. The samples were digested at 30° Celsius for 6 hours, then at 70° Celsius for 1 hour with a 1 hour ramp time, and finally at 120° Celsius for 8 hours with a 1 hour ramp time. Each sample weighed 0.25 ± 0.05 g. The digested samples were diluted to 10 mL with Type 1 water and analyzed for total lead using an Agilent 4500 ICP-MS. Standard quality control included initial and continuing calibration verification, reagent blanks, and a standard reference material.
2.5 Statistical Analysis
We used R to identify linear regressions and correlation coefficients between each of the bone Pb measurement techniques. The uncertainty values for the portable XRF measurements were determined as in previous studies using counting statistics in the fitted area for Pb.(23) The uncertainty (σ) of each measurement was calculated using the following equation,
| (1) |
, where c is the concentration, BKG is the background counts as estimated by our fitting, Gross is gross counts over the area of the fitted peak, t is measurement time, and Net is the net Pb counts from the Gaussian function in our fitting. Uncertainties for the KXRF measurements were determined similarly using counting statistics from the fitted area and normalization. Negative values for bone Pb are left as such because they represent the point estimate of bone Pb with uncertainty in the measurement. If an individual measurement is close to zero then the point estimate from that individual can be negative with the associated uncertainty. By keeping these values we can include the uncertainty of the measurements into our analysis and keep from biasing our continuous results. This is similarly done in epidemiological studies using the values as a continuous level of exposure.(24)
3. Results
3.1 Condor tibia bone samples
Table 1 shows the ICP-MS, KXRF, and portable XRF measured Pb levels of the 17 bare condor tibia bones recovered in this study. The units shown are microgram Pb per gram dry bone.
Table 1.
Pb measurements and uncertainties for ICP-MS, KXRF, and portable XRF measurements of 17 bare condor tibia bones (n=17 for each measurement technique).
| Average | Maximum | Minimum | Standard Deviation |
|
|---|---|---|---|---|
| ICP-MS (ug/g) | 14 | 45 | 0.3 | 15 |
| KXRF (ug/g) | 15 | 65 | −3.3 | 20 |
| KXRF Uncertainty (ug/g) | 2.4 | 5.8 | 1.2 | 1.0 |
| Portable XRF (ug/g) | 12 | 51 | 1.2 | 13 |
| Portable XRF Uncertainty (ug/g) | 1.3 | 2.8 | 0.8 | 0.5 |
3.2 Correlation between KXRF and ICP-MS bone Pb measurements
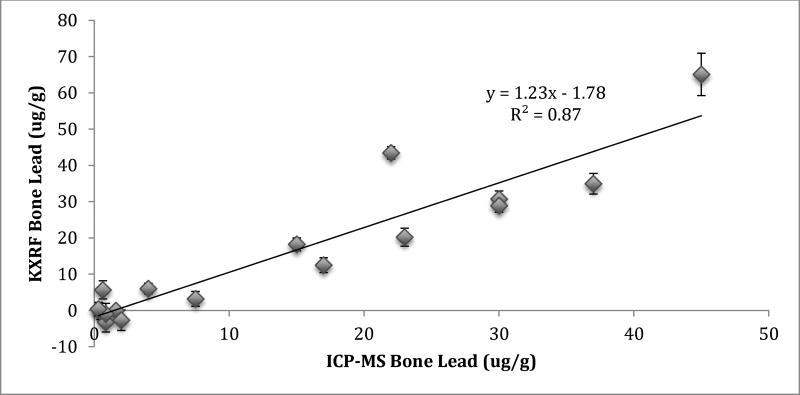
Figure 1 shows the correlation between KXRF and ICP-MS bone Pb measurements. The KXRF readings were consistently a little higher than the ICP-MS, but the correlation was quite high (r=0.93; β=1.23, Std. error=0.12; p=<0.001).
Figure 1.
Correlation between KXRF bone Pb measurements and ICP-MS bone Pb measurements in condor tibia bone samples.
3.3 Correlation between portable XRF and ICP-MS bone Pb measurements
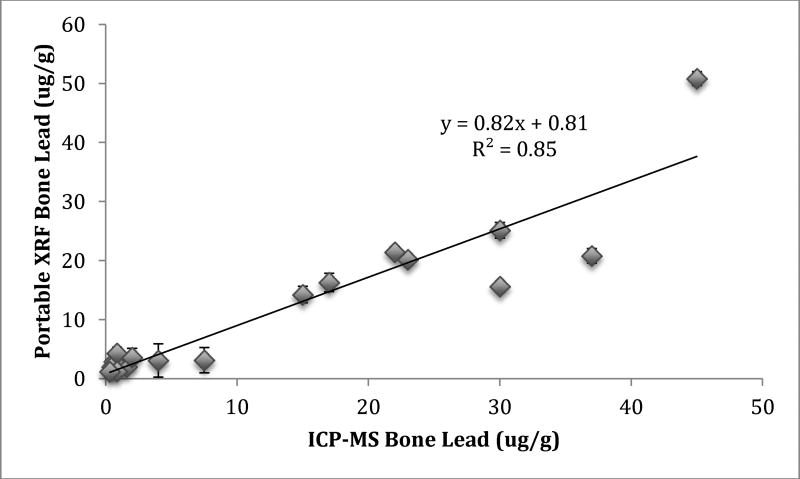
Figure 2 shows the correlation between portable XRF and ICP-MS bone Pb measurements. The portable XRF measurements were slightly lower than ICP-MS, but with almost the same correlation as KXRF and ICP-MS (r=0.92; β=0.82, Std. error=0.089; p<0.001).
Figure 2.
Correlation between portable XRF and ICP-MS bone Pb measurements in bare condor tibia bone samples.
3.4 Correlation between portable XRF and KXRF bone Pb measurements
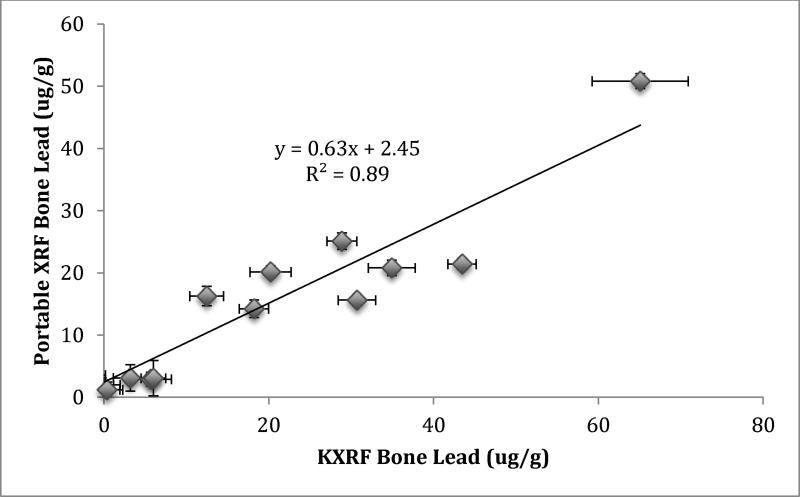
Figure 3 shows the correlation between portable XRF and KXRF bone Pb measurements. The portable XRF measured just above half the level from the KXRF, but the correlation remained quite high indicating a possible issue in quantification (r=0.94; β=0.635, Std. error=0.058; p<0.001).
Figure 3.
Correlation between portable XRF and KXRF bone Pb measurements in bare condor tibia bone samples.
3.5 Portable XRF bone Pb measurements with added soft tissue
Soft tissue was simulated using Lucite slabs of 0.54, 1.08, and 1.54 mm thicknesses. The correlation values and uncertainties for portable XRF (pXRF) measurements at each Lucite thickness compared to both KXRF and ICP-MS bone Pb results are shown in Table 2. The mean bone lead did not change with the addition of Lucite, and the uncertainty in the measurements only went up slightly. We also did a repeated measure of the condor bones with 1 mm of Lucite thickness and found the differences to be non-significant with a p-value of 0.97 using a Mann-Whitney-Wilcoxon test.
Table 2.
Linear regression results from portable XRF bone lead at 0.54, 1.08, and 1.54 mm compared with KXRF and ICP-MS bone lead.
| Regressed against pXRF |
KXRF Bone Lead Coefficient (p-value, Std. Error) |
ICP-MS Bone Lead Coefficient (p-value, Std. Error) |
pXRF Bone Lead Mean±SD |
pXRF Uncertainty Mean±SD |
|---|---|---|---|---|
| 0.54 mm Lucite | 17±21 | 2.8±0.8 | ||
| Slope | 1.00 (<0.001, 0.10) | 1.17 (<0.001, 0.21) | ||
| Intercept | 1.38 (0.59, 2.48) | 0.6 (0.89, 4.24) | ||
| Rho | 0.93 | 0.82 | ||
|
|
||||
| 1.08 mm Lucite | 17±21 | 2.4±0.9 | ||
| Slope | 0.98 (<0.001, 0.11) | 1.25 (<0.001, 0.18) | ||
| Intercept | 2.05 (0.47, 2.78) | −0.23 (0.95, 3.51) | ||
| Rho | 0.91 | 0.88 | ||
|
|
||||
| 1.54 mm Lucite | 14±17 | 3.0±1.1 | ||
| Slope | 0.73 (<0.001, 0.12) | 0.98 (<0.001, 0.15) | ||
| Intercept | 3.08 (0.89, 2.89) | 0.65 (0.83, 2.98) | ||
| Rho | 0.85 | 0.86 | ||
|
|
||||
4. Discussion
Monitoring bone Pb levels of wildlife should allow for easier identification of lifetime exposure levels to further understand the environmental hazards of metals. Bone Pb, when measured alongside other possible covariates such as age and location, give us a better idea of the risks and average exposures of wildlife to Pb. From our results, there is excellent agreement between the different measurement approaches. These results also support the viability of monitoring condor bone Pb levels in vivo using XRF. The average uncertainty of 2.4 ± 1.0 ug/g for KXRF measurements and 1.3 ± 0.5 ug/g for portable XRF measurements demonstrate that each of these techniques could be used to effectively quantify bone Pb level for wildlife, and distinguish highly exposed individuals from normal environmental exposures.
The condor tibia bones were all free of soft tissue for our measurements. This would lower the uncertainties for KXRF and portable XRF measurements. The KXRF is much less sensitive to changes in soft tissue thickness, so we primarily focused on the associated changes with portable XRF measurements.(25) We used Lucite as a proxy for soft tissue in our measurements, which was shown previously to produce almost identical spectra to human skin measured in cadaver tibia bones.(16) There was an overall slight increase in measurement uncertainty with the addition of 1.5 mm of Lucite (1.3 to 3.0 ug/g), which we expected. Even with this increase in soft tissue the uncertainty remains quite low, and the correlation value did not change by a significant margin nor did the quantification change significantly. The detection limit for portable XRF bone Pb measurements was shown to be about 10 ug/g for skin thicknesses of 5 mm(16), which would be sufficient for measurement of any avian species. Avian species will likely have much thinner soft tissue thicknesses, and, thus, an even lower detection limit. This shows great promise for using the portable XRF for in-field measurements of live caught birds, which would allow for much greater ease of monitoring chronic Pb exposures.
The uncertainties for the KXRF bone Pb measurements are higher than we would have predicted. This is likely because the samples themselves were much smaller than we would typically have in human bone measurements. The KXRF measurement typically has a cone beam shape due to collimation of the cadmium-109 source, which will produce signal throughout the bone due to the high energy. Thus, the KXRF measurements sample over a large portion of the bone and, for smaller bone samples, give smaller sampling area and lower signal, which increases uncertainty in the counting statistics and quantified results.(25) For live specimens, the bone will be longer and have a larger sampling area than seen with the samples measured here. Thus, true in vivo measurements may have a larger sampling area and signal during measurements, which would give a lower uncertainty than what we found in this study.
The quantification of the XRF measurements should be equivalent in the units presented. The KXRF bone Pb results had an average of 15.4 ug/g and the ICP-MS bone Pb results average was 14.0 ug/g. KXRF data is normalized to the coherent scattering peak in the spectra, which gives results in ug/g bone mineral as a consequence. The KXRF was converted from ug/g bone mineral to ug/g dry bone to be comparable to ICP-MS results using the factor of 1.5 that represents the difference between bone mineral and dry bone. All results shown use these same units for comparison between the analytical methods. The portable XRF result should be close to ug/g dry bone from the calibration using plaster-of-Paris phantoms, if we assume that differences in the bone matrix for the portable XRF would have little impact over the quantification results. In our previous study, we compared quantification results when using a plaster-of-Paris calibration standards and ground bone calibration standards, which showed little to no difference between the two methods.(16)
The average of the portable XRF bone Pb results of 12.2 ug/g was slightly lower than predicted, which could be a result of poor geometry during measurements with bare bone. Slight grooves in the bone or unaccounted for air space would cause a lower signal and, therefore, a lower quantified result. In vivo measurements would not have these same issues, since these grooves in the bone would be covered by soft tissue, which would also prevent unaccounted for air space. We can account for the soft tissue in our calibration to correct the signal for the changes in geometry. In addition, the bone section measured by ICP-MS was taken from a section not measured by either portable XRF or KXRF, which would introduce some variability between measurements (which also means our correlation estimates between XRF and ICP-MS are likely underestimated compared to what would be found if the same exact bone portion was measured with each). As the measurement location changes from the center of the diaphysis to the epiphysis, the composition of the bone changes from mostly cortical (~95% at the center of diaphysis in humans) to become higher percentages of trabecular bone.(26) This would result in different accumulation and resorption rates with the differences in bone type. Previous work in human tibia has shown that the results do change along (−0.11 ug/g/cm proximal distal location from mid-tibia) and within (between 5–10 ug/g greater at the surface of the bone).(27) However, since the bias is in the opposite direction of the core and surface, and the proximal distal location variability is quite small, this does not seem to explain our current results. More study is needed to identify whether this is a conversion issue, effect of the difference between measurement methods, or effect from the sample geometry.
In summary, both KXRF and portable XRF bone Pb measurement techniques demonstrated good correlations with ICP-MS results. The uncertainties of each technique were low enough to feasibly measure bone Pb in vivo. At higher levels of soft tissue thickness the portable XRF should have higher uncertainty, but this is unlikely to be much of an issue for avian species. Portable XRF would be the optimal device for measurement of bone Pb in vivo on avian species in the field given the impracticality of Cd-109 KXRF measurements in the field.
Highlights.
California condors have extensive chronic exposure to lead reflected in their bone.
KXRF, portable XRF, and ICP-MS bone lead measurements shared strong correlations.
Portable XRF is well-suited for field measurement of bone lead in avian species.
Acknowledgments
This work was supported by the Purdue University Nuclear Regulatory Commission (NRC) Faculty Development Grant [NRC-HQ-11-G-38-0006], the National Institute for Occupational Safety and Health (NIOSH) R21 grant [1R21OH010044], and Purdue Ross Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest with this work.
References
- 1.Hunt WG, Watson RT, Oaks JL, Parish CN, Burnham KK, Tucker RL, et al. Lead bullet fragments in venison from rifle-killed deer: potential for human dietary exposure. PLoS One. 2009;4(4):e5330. doi: 10.1371/journal.pone.0005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen P, Asmund G, Riget F. High human exposure to lead through consumption of birds hunted with lead shot. Environ Pollut. 2004;127(1):125–9. doi: 10.1016/s0269-7491(03)00255-0. [DOI] [PubMed] [Google Scholar]
- 3.Kollander B, Widemo F, Agren E, Larsen EH, Loeschner K. Detection of lead nanoparticles in game meat by single particle ICP-MS following use of lead-containing bullets. Analytical and bioanalytical chemistry. 2017;409(7):1877–85. doi: 10.1007/s00216-016-0132-6. [DOI] [PubMed] [Google Scholar]
- 4.Hunt WG, Burnham W, Parish CN, Burnham KK, Mutch B, Oaks JL. Bullet Fragments in Deer Remains: Implications for Lead Exposure in Avian Scavengers. Wildlife Society Bulletin (1973–2006) 2006;34(1):167–70. [Google Scholar]
- 5.Stauber E, Finch N, Talcott PA, Gay JM. Lead poisoning of bald (Haliaeetus leucocephalus) and golden (Aquila chrysaetos) eagles in the U.S. inland Pacific northwest region--an 18-year retrospective study: 1991–2008. J Avian Med Surg. 2010;24(4):279–87. doi: 10.1647/2009-006.1. [DOI] [PubMed] [Google Scholar]
- 6.Herring G, Eagles-Smith CA, Wagner MT. Ground Squirrel Shooting and Potential Lead Exposure in Breeding Avian Scavengers. PLoS One. 2016;11(12):e0167926. doi: 10.1371/journal.pone.0167926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naidoo V, Wolter K, Botha CJ. Lead ingestion as a potential contributing factor to the decline in vulture populations in southern Africa. Environ Res. 2017;152:150–6. doi: 10.1016/j.envres.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein ME, Doak DF, George D, Burnett J, Brandt J, Church M, et al. Lead poisoning and the deceptive recovery of the critically endangered California condor. Proc Natl Acad Sci U S A. 2012;109(28):11449–54. doi: 10.1073/pnas.1203141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rideout BA, Stalis I, Papendick R, Pessier A, Puschner B, Finkelstein ME, et al. Patterns of mortality in free-ranging California Condors (Gymnogyps californianus) Journal of wildlife diseases. 2012;48(1):95–112. doi: 10.7589/0090-3558-48.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Church ME, Gwiazda R, Risebrough RW, Sorenson K, Chamberlain CP, Farry S, et al. Ammunition is the principal source of lead accumulated by California condors re-introduced to the wild. Environ Sci Technol. 2006;40(19):6143–50. doi: 10.1021/es060765s. [DOI] [PubMed] [Google Scholar]
- 11.Ecke F, Singh NJ, Arnemo JM, Bignert A, Helander B, Berglund AMM, et al. Sublethal Lead Exposure Alters Movement Behavior in Free-Ranging Golden Eagles. Environ Sci Technol. 2017;51(10):5729–36. doi: 10.1021/acs.est.6b06024. [DOI] [PubMed] [Google Scholar]
- 12.Kerper LE, Hinkle PM. Lead uptake in brain capillary endothelial cells: activation by calcium store depletion. Toxicology and applied pharmacology. 1997;146(1):127–33. doi: 10.1006/taap.1997.8234. [DOI] [PubMed] [Google Scholar]
- 13.Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect. 1991;91:4. doi: 10.1289/ehp.919133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry PS. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32(2):119–39. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chettle DR, Scott MC, Somervaille LJ. Lead in bone: sampling and quantitation using K X-rays excited by 109Cd. Environ Health Perspect. 1991;91:49–55. doi: 10.1289/ehp.919149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Specht AJ, Weisskopf M, Nie LH. Portable XRF Technology to Quantify Pb in Bone In Vivo. J Biomark. 2014;2014:398032. doi: 10.1155/2014/398032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Specht AJ, Lin Y, Weisskopf M, Yan C, Hu H, Xu J, et al. XRF-measured bone lead (Pb) as a biomarker for Pb exposure and toxicity among children diagnosed with Pb poisoning. Biomarkers. 2016:1–6. doi: 10.3109/1354750X.2016.1139183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie H, Chettle D, Luo L, O'Meara J. In vivo investigation of a new 109Cd gamma-ray induced K-XRF bone lead measurement system. Phys Med Biol. 2006;51(2):351–60. doi: 10.1088/0031-9155/51/2/011. [DOI] [PubMed] [Google Scholar]
- 19.Somervaille LJ, Nilsson U, Chettle DR, Tell I, Scott MC, Schutz A, et al. In vivo measurements of bone lead-a comparison of two x-ray fluorescence techniques used at three different bone sites. Phys Med Biol. 1989;34(12) doi: 10.1088/0031-9155/34/12/007. [DOI] [PubMed] [Google Scholar]
- 20.Bevington P, Robinson D. Data reduction and error analysis for the physical sciences. New York, NY: McGraw-Hill; 2003. [Google Scholar]
- 21.Nie H, Chettle D, Luo L, O'Meara J. Dosimetry study for a new in vivo X-ray fluorescence (XRF) bone lead measurement system. Nuclear Instruments and Methods in Physics Research B. 2007:225–30. [Google Scholar]
- 22.Nie H, Sanchez S, Newton K, Grodzins L, Cleveland RO, Weisskopf MG. In vivo quantification of lead in bone with a portable x-ray fluorescence system--methodology and feasibility. Phys Med Biol. 2011;56(3):N39–51. doi: 10.1088/0031-9155/56/3/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Specht AJ, Mostafaei F, Lin Y, Xu J, Nie LH. Measurements of Strontium Levels in Human Bone In Vivo Using Portable X-ray Fluorescence (XRF) Applied Spectroscopy. 2017 doi: 10.1177/0003702817694383. 0003702817694383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim R, Aro A, Rotnitzky A, Amarasiriwardena C, Hu H. K x-ray fluorescence measurements of bone lead concentration: the analysis of low-level data. Phys Med Biol. 1995;40(9):1475–85. doi: 10.1088/0031-9155/40/9/007. [DOI] [PubMed] [Google Scholar]
- 25.Behinaein S, Chettle DR, Marro L, Malowany M, Fisher M, Fleming DE, et al. Factors influencing uncertainties of in vivo bone lead measurement using a (109)Cd K X-ray fluorescence clover leaf geometry detector system. Environmental science Processes & impacts. 2014;16(12):2742–51. doi: 10.1039/c4em00446a. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Milder FL, Burger DE. X-ray fluorescence: issues surrounding the application of a new tool for measuring burden of lead. Environ Res. 1989;49(2):295–317. doi: 10.1016/s0013-9351(89)80074-x. [DOI] [PubMed] [Google Scholar]
- 27.Todd AC, Parsons PJ, Tang S, Moshier EL. Individual variability in human tibia lead concentration. Environ Health Perspect. 2001;109(11):1139–43. doi: 10.1289/ehp.011091139. [DOI] [PMC free article] [PubMed] [Google Scholar]