Abstract
All known eukaryotes require copper for their development and survival. The essentiality of copper reflects its widespread use as a co-factor in conserved enzymes that catalyze biochemical reactions critical to energy production, free radical detoxification, collagen deposition, neurotransmitter biosynthesis and iron homeostasis. However, the prioritized use of copper poses an organism with a considerable challenge because, in its unbound form, copper can potentiate free radical production and displace iron-sulphur clusters to disrupt protein function. Protective mechanisms therefore evolved to mitigate this challenge and tightly regulate the acquisition, trafficking and storage of copper such that the metal ion is rarely found in its free form in the cell. Findings by a number of groups over the last ten years emphasize that this regulatory framework forms the foundation of a system that is capable of monitoring copper status and reprioritizing copper usage at both the cellular and systemic levels of organization. While the identification of relevant molecular mechanisms and signaling pathways has proven to be difficult and remains a barrier to our full understanding of the regulation of copper homeostasis, mounting evidence points to the mitochondrion as a pivotal hub in this regard in both healthy and diseased states. Here, we review our current understanding of copper handling pathways contained within the organelle and consider plausible mechanisms that may serve to functionally couple their activity to that of other cellular copper handling machinery to maintain copper homeostasis.
Copper in mammals
Mammals acquire copper through the diet. Dietary copper deficiency in humans is uncommon owing to the relatively low recommended daily intake of 0.9 mg/day.1 However, the true prevalence of copper deficiency in the global population may be masked by the lack of an easily assayed biomarker that is both reliable and sufficiently sensitive.2 Copper from the diet is absorbed by the intestinal epithelial cells and delivered to the liver through the portal vein, where it is released into the bloodstream to meet the demand of peripheral organs or excreted in the bile.3
The widespread use of copper largely reflects its ability to rapidly cycle between two redox states. This property has been exploited by a number of enzymes, which use the metal ion as a co-factor to catalyze biochemical reactions critical to cellular homeostasis. Cupro-proteins have important roles in oxidative metabolism, the elimination of toxic free radicals, pigment production, iron metabolism, and many others.4 Impaired function of many of these proteins disrupts these pathways and is a frequent cause of human disease.5 Despite the importance of copper to the cell, the metal ion can be toxic in excess. Copper is capable of binding and inhibiting enzyme activity,6 destabilizing iron-sulphur clusters in enzymes critical to survival,7, 8 and transferring electrons in reactions that perpetuate the production of deleterious oxidative free radicals.9, 10 Since copper is potentially dangerous, its acquisition, trafficking, storage and removal from the cell and body must be strictly controlled. The identification and characterization of mechanisms that act collectively to regulate copper homeostasis is therefore a focal point of investigation in the field.
The primary means of regulating copper homeostasis is achieved by modulating the localization and activity of transporters essential for copper import and export. In mammals, the majority of copper is transported across the plasma membrane into the cell via copper transporter 1 (CTR1), the only known high affinity copper importer.11, 12 CTR1 functions as a homotrimer, which allows it to form a pore and act as an ion channel to facilitate copper transport across the plasma membrane.13–15 Although other, lower affinity routes of copper import are known to exist they cannot compensate for the loss of CTR1 function.16 Once in the cell, copper is transferred to evolutionarily conserved, soluble chaperones that deliver the metal ion to various subcellular compartments where it is then used to mature cupro-enzymes.4 One of these chaperones, ATOX1, delivers copper to the trans-Golgi network (TGN) to ATP7A and ATP7B. These ATPases in turn translocate copper into the lumen of the TGN to facilitate the metallation of a number of secreted cupro-enzymes. In addition, ATP7A and ATP7B are both capable of regulated trafficking and redistribution to the plasma membrane where they promote copper export from the cell.17, 18
Copper in mitochondria
Mitochondria have long been recognized for their role in energy production and their active participation in a range of other processes critical to cellular homeostasis.19 However, the scope of metabolic signals originating from the organelle and the breadth of their impact on cell biology has only recently begun to be appreciated.20, 21 The vital role fulfilled by mitochondria in the homeostatic maintenance of many metal ions, including copper, is no exception in this regard.22–24 This review therefore summarizes the importance of copper to mitochondria, while placing a particular emphasis on the role of the organelle in the signaling framework that is known to regulate cellular copper homeostasis.
Mitochondria contain two known cupro-enzymes whose copper sites are matured in the intermembrane space (IMS); cytochrome c oxidase (COX) and superoxide dismutase (SOD1). COX is the terminal enzyme of the electron transport chain, and it accepts electrons from cytochrome c and subsequently transfers them to molecular oxygen to generate water. Four protons are pumped across the inner mitochondrial membrane during this reaction cycle and contribute to the electrochemical gradient that is harnessed by the ATP synthase to generate the bulk of energy that is required by the cell. COX is a multimeric protein complex composed of 14 structural subunits that are expressed by both the nuclear and mitochondrial genomes.25 The catalytic activity of the enzyme requires the insertion of multiple metal co-factors that include two heme groups (a and a3) and two copper sites (CuA and CuB). COX assembly as a whole is a complex, modular process that has been thoroughly reviewed by others.25–27 Complex biogenesis relies on more than 30 accessory proteins that function collectively to coordinate the ordered incorporation of structural subunits with the synthesis, delivery and insertion of prosthetic groups. The two copper sites of the holoenzyme are matured in the IMS by a suite of evolutionarily conserved COX assembly factors, with CuB site formation requiring at least COX17, COX19 and COX11 function and CuA site maturation depending on at least COX17, SCO1, SCO2 and COA6 function (Figure 1). Here we focus our attention on the availability of copper in the context of its insertion into the CuA site of COX2 during COX assembly, as this biochemical pathway is intrinsically linked to the regulation of cellular copper homeostasis.
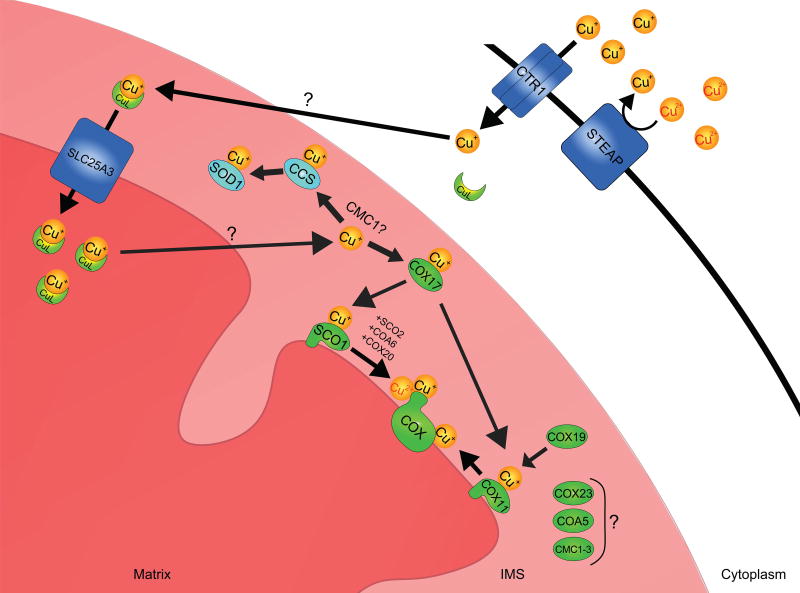
Figure 1. Mitochondrial copper acquisition and trafficking pathways in mammals.
Extracellular copper in the cupric (Cu2+) ion form is reduced by a member of the STEAP family of oxidoreductases. The cuprous (Cu+) ion is then imported into the cell by the high affinity copper transporter CTR1. How intracellular copper is trafficked to the mitochondrion is not clear; however, it is thought that copper binding to an unknown ligand triggers copper translocation from the cytosol to the mitochondrial intermembrane space (IMS). There, the copper-ligand complex is transported across the inner membrane for its storage in the matrix by SLC25A3, a member of the mitochondrial carrier family and the mammalian homolog of yeast PIC2. Matrix copper is ultimately mobilized and translocated across the inner membrane to the IMS by an unknown transporter, to metallate the cupro-enzymes SOD1 and COX during their maturation. Copper loading onto apo-SOD1 requires CCS. Copper destined for the metallation of the CuA and CuB sites of COX is initially transferred to COX17, which delivers it to the copper chaperones SCO1 and COX11. SCO1 function during the maturation of the CuA site is facilitated by at least 3 other COX assembly factors; SCO2, COA6 and COX20. Several additional COX assembly factors (COX23, COA5, CMC1–3) with unknown or poorly understood functions may also facilitate copper delivery to COX and/or regulate copper trafficking within the IMS.
How copper that is imported into the cell is ultimately delivered to, and trafficked within, the mitochondrion remains an active area of investigation (Figure 1). To date, a specific copper chaperone that functions to deliver copper to the organelle has not been discovered. The copper chaperone COX17, a protein required for COX assembly,28 was previously hypothesized to shuttle copper between the cytosol and mitochondria based on its dual localization;29 however, COX17 null yeast maintain normal copper levels in their mitochondria and yeast strains in which COX17 is physically tethered to the inner membrane have wild-type levels and activity of COX.30, 31 One of the most intriguing aspects of the copper found in mitochondria is that the majority is housed in the matrix in vast excess of what is required to metallate COX and IMS-localized SOD1.30, 32 Rather than being bound by a protein, matrix copper is maintained in an anionic complex with an as yet unidentified ligand.33 The inner membrane is tightly sealed to maintain the membrane potential necessary for mitochondrial function. Recent studies in yeast have shown that the mitochondrial carrier family proteins PIC2, a phosphate transporter,34 and MRS3, an iron transporter,35 also move copper across the inner membrane.36, 37 Both proteins transport copper in a heterologous expression system, and their simultaneous deletion in yeast further decreases the amount of bioavailable copper in the matrix and leads to greater deficiencies in the activity of COX and IMS-localized SOD1.37 There are currently no known cupro-enzymes localized to the matrix. Therefore, we propose that the matrix copper store is redistributed to the IMS, where it is then used as the principal source of copper to metallate the two cupro-enzymes known to reside within the organelle (Figure 1).38 While the copper-ligand complex is exchangeable, binding of copper to the ligand may facilitate its solubility and bioavailability while preventing its non-specific interaction with the iron-sulphur cluster machinery that is also housed in the matrix.8 Matrix localization of copper may also ensure that the organelle possesses a minimal pool of copper to sustain a functional cuproproteome in the face of a copper deficiency at the whole cell level.39
Importantly, the matrix copper pool is dynamic and can expand in size,30, 40 arguing that mitochondria are candidates for the sequestration of copper from the cytosol under conditions of cellular copper overload. However, mitochondrial ultrastructure and function are severely perturbed in the livers of Wilson disease (WD) patients due to increased mitochondrial copper levels,41–43 emphasizing that there is an upper limit to the amount of copper that can be safely stored within the organelle. These findings also imply that copper uptake into mitochondria may be constitutive since it is not shut off in WD livers to protect against damage. It is also possible that the damage during copper overload is caused by transport of unbound copper since PIC2 and MRS3 are both able to transport ionic copper.36, 37 If copper accumulates in the matrix in a non-ligated form it could easily cause damage by non-specific interactions or by perturbing redox balance.8, 40 In fact, the mitochondrial damage observed in WD livers can be prevented in a rat model of the disease by exogenous treatment with methanobactin, a copper chelator which dramatically reduces the mitochondrial copper burden.44
While the matrix copper concentration appears to be independent of COX content, it is now clear that the proteins involved in maturing the CuA site of the holoenzyme are intimately linked to the generation of a mitochondrial signal that regulates cellular copper homeostasis.45–47 We, and others, have therefore conducted extensive research to further understand how copper is loaded into the CuA site of COX2 (Figure 1). Copper must be redistributed from the matrix to the IMS by a candidate transporter(s) that has yet to be identified. Once in the IMS, the copper destined for the CuA site is loaded onto COX17, a copper-binding protein with twin Cx9C motifs which functions as a chaperone to deliver the metal ion to a second metallochaperone, SCO1.48 This COX17-SCO1 interaction is essential for the delivery of copper to the CuA site as pathogenic mutations in SCO1 that inhibit this copper transfer step severely impair the assembly of the holoenzyme.49, 50 SCO1 was originally linked to copper delivery to COX through a high copy suppressor screen of a COX17 null yeast strain.28 Its copper is coordinated via a Cx3C motif and a conserved histidine residue,51–53 and is transferred directly to the CuA site of COX254 in a reaction that also requires the COX assembly factors SCO2 and COA6 (Figure 1).55–58 Interestingly, a multitude of other IMS-localized proteins containing canonical twin Cx9C motifs have been implicated in COX assembly in yeast,59 many of which have evolutionarily conserved mammalian homologues with undefined functions. We fully expect that future studies will identify members of this protein family that fulfill unique roles in delivering copper to COX, prioritizing copper utilization within the IMS, and regulating the generation and transduction of a copper-dependent, mitochondrial signal.
The second mitochondrial cupro-enzyme is SOD1. Although the majority of SOD1 is localized to the cytosol, a small but physiologically significant portion of the total protein pool is found within the IMS (Figure 1)60 and is responsible for the dismutation of superoxide anions that may otherwise oxidatively damage resident protein and lipid constituents.61 All SOD1 mRNA is translated into protein in the cytosol and then apo-SOD1 is imported into the IMS.62 SOD1 maturation in both the cytosol and the IMS requires the copper chaperone for SOD1 (CCS), which inserts copper into apo-SOD1 and catalyzes the formation of an intermolecular disulphide bond that stabilizes the homodimer and is essential for enzyme activity.63, 64 The relative distribution of CCS between the cytosol and the mitochondrion is governed by the MIA40-ERV1 disulphide relay system, which oxidizes two cysteines within its first domain to form a disulphide bond that promotes CCS retention within the IMS.65 Since both SOD1 and CCS are imported into the IMS as apo-proteins, the copper required for the maturation and catalytic activity of SOD1 must also come from the matrix copper pool.38 The mechanisms that govern copper trafficking within the IMS remain unclear, although a previous report suggests an active role for the COX assembly factor CMC1 in regulating copper distribution to COX and SOD1 (Figure 1).66 A greater understanding of these and other related regulatory inputs that govern SOD1 activation is crucial given that mutations affecting its metallation and subsequent maturation lead to increased reactive oxygen species (ROS) production,67 mitochondrial dysfunction and are a cause of the familial form of amyotrophic lateral sclerosis.68
Mitochondrial copper chaperones play a key role in the regulation of copper homeostasis
To date, pathogenic mutations have been described in 3 COX assembly factors crucial to the maturation of the CuA site of COX2; SCO1, SCO2 and COA6.46, 69–73 Patients with mutations in any of these genes present with fatal, tissue-specific forms of disease owing to an isolated COX deficiency. Affected tissues and fibroblasts derived from SCO1 and SCO2 patients are also profoundly copper deficient, arguing that these proteins fulfill additional roles in the regulation of cellular copper homeostasis that, when perturbed, are directly relevant to disease progression.45, 46 Whether COA6 mutations also affect cellular copper handling systems and total copper levels has yet to be reported in the literature. More than fifty SCO2 pedigrees have been identified thus far, and the overwhelming majority of affected individuals harbour at least one E140K allele and present with a fatal cardioencephalomyopathy.71, 74–76 In contrast, mutations in SCO1 are extremely rare. The three SCO1 pedigrees that have been described each harbour a unique missense allele and present with a distinct, tissue-specific form of disease. The first SCO1 patient was a compound heterozygote who carried a nonsense mutation on one allele and proline to leucine substitution on the other allele, and presented as a neonate with liver failure and encephalopathy.77 The second SCO1 patient was homozygous for a glycine to serine substitution and died at 6 months of age due to hypertrophic cardiomyopathy and encephalopathy.46 Finally, the third SCO1 patient was a compound heterozygote who harboured a nonsense mutation on one allele and a methionine to valine substitution on the other allele, and died at 5 months of age from an isolated encephalopathy.70 The underlying basis of the diverse clinical phenotypes across SCO1 pedigrees remains mysterious, particularly given that SCO1 and SCO2 are ubiquitously expressed and function collaboratively in the same biochemical pathway to promote COX assembly and regulate cellular copper homeostasis. It may be, however, that tissues rely to different extents on one or both functions of SCO1, and that the allelic variants in each SCO1 patient background are differentially compromised in this regard. Consistent with this idea, overexpression of each allelic variant in SCO1 patient fibroblasts rescued the isolated COX deficiency to varying degrees.78
How SCO1 dysfunction drives a cellular copper deficiency remains of considerable interest from both a basic biological and a clinical perspective, particularly in light of our limited understanding of the molecular mechanisms that underlie the clinical heterogeneity across SCO1 pedigrees. In SCO1 and SCO2 patient fibroblasts, the copper deficiency is caused by an increased rate of copper efflux from the cell45 that can be rescued by reducing the expression of ATP7A (Figure 2).78 The copper deficiency in SCO1, SCO2 and COX15 patient fibroblasts can also be rescued by overexpressing SCO2.45 This led us to propose a model whereby SCO2 acts upon SCO1 to modulate the generation of a mitochondrial signal that regulates the rate of copper efflux from the cell.23, 45 Subsequent studies demonstrated that SCO2 possesses a thiol-disulphide oxidoreductase activity54, 56 that may in fact allow it to regulate the generation of a redox signal by targeting the cysteines of the Cx3C motif of SCO1. The transduction of this signal at least in part requires COX19, a soluble COX assembly factor which partitions between mitochondria and the cytosol in a copper-dependent manner.78 How COX19 communicates with the efflux machinery in patient cells remains unclear. Mislocalized mitochondrial proteins are known to activate a proteostatic response in the cytosol.79, 80 Therefore, it is possible that COX19 is acting through this pathway and its presence in the cytosol somehow leads to the degradation of a target protein like the subunits of the Conserved Oligomeric Golgi (COG) complex, which was recently shown to alter the localization and abundance of both ATP7A and CTR1.81
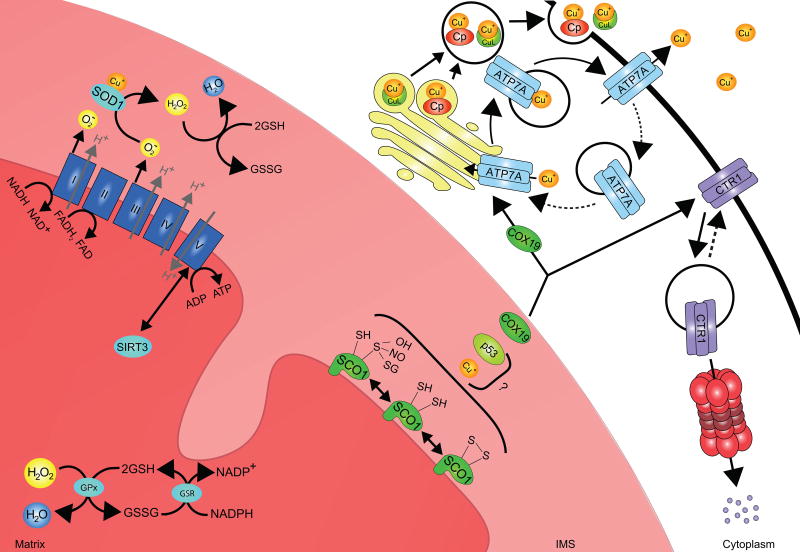
Figure 2. Potential inputs that contribute to SCO1-dependent mitochondrial signaling and the regulation of copper homeostasis.
SCO1 function is integral to a mitochondrial signaling pathway that impinges upon copper homeostasis. The redox state of its cysteinyl sulphurs, which are located within the Cx3C motif, is significantly perturbed in patients with combined COX and total copper deficiencies. Thus, signaling may be directly modulated by the proportion of the SCO1 pool with copper-loaded versus apo-Cx3C motifs, or by the balance of reversible post-translational modifications to one or both of its cysteinyl sulphurs (S-S, SH, SOH, SNO or SSG). How the functional status of SCO1 is sensed and transduced outside of the mitochondrion to effect changes in the abundance or localization of the copper import or efflux machinery remains poorly understood. COX19 partitions between the IMS and the cytosol in a copper-dependent manner, and acts alone or in concert with unknown factors to stimulate ATP7A–mediated copper export from the cell by regulating its trafficking to the plasma membrane and/or increasing the rate of secretion of copper bound to cupro-proteins (e.g Cp, ceruloplasmin) or small molecules (e.g. CuL, copper ligand). Mechanisms that couple SCO1-dependent mitochondrial signaling to the regulation of CTR1 localization or degradation are currently unknown. However, redox and/or metabolic switch mechanisms may contribute to the modulation of SCO1-dependent mitochondrial signaling. Complexes (labeled I-IV) of the electron transport chain (ETC) oxidize NADH and FADH2 while establishing a proton gradient that is harnessed by Complex V to generate ATP. Perturbations in this pathway lead to changes in the NAD+:NADH and AMP:ATP ratios and the activation of the AMPK/p53 pathway and SIRT3, both of which trigger downstream changes in metabolism that may affect cellular copper levels. Complexes I and III of the ETC also generate superoxide anions and ROS are known to promote signaling by post-translationally modifying proteins and/or altering redox balance. Changes in the mitochondrial redox state are sensed primarily by the GSH:GSSG ratio, which is buffered within the matrix by the activity of the enzymes glutathione peroxidase (GPx) and glutathione reductase (GSR).
The development and characterization of animal models of human diseases caused by mutations in SCO1 and SCO2 point to distinct, tissue-specific consequences associated with loss of SCO function, and emphasize the complex roles SCO proteins play in regulating copper homeostasis. Schon and colleagues generated a whole body Sco2 transgenic mouse that was either hemi- or homozygous for the murine equivalent of the E140K allele.82 Total tissue copper levels were unaffected in both of these transgenic lines, and the COX deficiency was unexpectedly mild, even in the heart which is profoundly COX and copper deficient in SCO2 patients.45, 46 This finding suggests that a specific threshold must be breached with respect to residual COX activity prior to eliciting a significant effect on homeostatic pathways that control cellular copper levels, which is something we indeed consistently observe in our Sco1 mouse models.47, 83 Why the mitochondrial copper pool is depleted in Sco2 mouse hearts82 when it is prioritized in SCO patient tissues and cells39 and in Sco1 null livers,47 all of which are severely copper deficient, remains unclear. Although whole body manipulation of Sco expression in the mouse is more representative of the patient condition, our initial priority was to generate and characterize mouse models in which Sco1 was specifically deleted in the liver or heart to mimic the tissue-specific defects observed in two of the SCO1 pedigrees. Deletion of Sco1 in either of these tissues produced a combined COX and copper deficiency that was ultimately lethal,47, 83 mirroring the patient condition.46, 77 Somewhat surprisingly, the copper deficiency in Sco1 null tissues differed from that observed in SCO patient fibroblasts in that it was caused by dysregulation of copper influx rather than efflux (Figure 2). CTR1 abundance was markedly reduced in Sco1 null livers and mouse embryonic fibroblasts (MEFs) and could be restored in MEFs by pharmacological inhibition of the proteasome, suggesting targeted degradation of the CTR1 protein pool.47 In contrast, CTR1 localization rather than abundance was altered in Sco1 null hearts, with the vast majority of the cellular pool of CTR1 being mislocalized to the cytosol.83 These findings collectively argue that mutations perturbing SCO1 function have tissue-specific consequences with respect to the various regulatory inputs that control the import and export machinery to regulate copper homeostasis. Consistent with this idea, we found that deletion of Sco1 in the liver or heart had distinct effects on the abundance of CCS, the only known biomarker of cytosolic copper status.
Perturbations in cellular copper homeostasis are also observed in patients with pathogenic mutations in other ancillary factors that are critical to the maturation and insertion of the heme groups of COX1 during COX assembly. Heart from SURF1 patients,46 fibroblasts from COX10 and COX15 patients,45 and Cox10 null mouse livers47 all have significantly lower levels of cellular copper than control samples. The ability of SCO2 overexpression to rescue the copper deficiency in COX15 patient fibroblasts45 and the fact that the redox state of the Cx3C motif of SCO1 is altered in COX10 and COX15 patient fibroblasts78 both suggest that SCO1-dependent, mitochondrial signaling can be perturbed indirectly by mutations in proteins that impair other facets of COX assembly.
The combined COX and copper deficiency in SCO1, SCO2 and COA6 patient backgrounds can be rescued to varying degrees by copper supplementation. The original studies focused solely on the effect of copper salts on COX in SCO2 patient myoblasts84 and fibroblasts,85 and showed a full recovery of enzyme activity that was both time- and dose-dependent. This success motivated a further evaluation of the potential therapeutic benefit of copper supplementation in a SCO2 patient. Although it was found that the brain remained refractory to treatment, subcutaneous administration of copper reversed the cardiac hypertrophy and normalized heart function.86 Similarly, exogenous copper treatment rescued the loss of COA6 function in patient fibroblasts73 and in relevant yeast and zebrafish models.57 In contrast, copper supplementation, even at high doses, was only able to partially complement the COX deficiency in SCO1 patient fibroblasts.55 In retrospect, these findings are not that surprising given the relative severity of the copper deficiency in various SCO1 cell types,45 and the fact that SCO1 catalyzes the transfer of copper to the CuA site of COX2 in a reaction that is facilitated by SCO2 and COA6.54, 56, 58, 87 These observations in fact collectively support the idea that SCO1 sits at the head of a mitochondrial pathway, and that its functional status directly impinges upon COX assembly and the generation of a signal that regulates copper homeostasis (Figure 2).23, 24 The exact identity of this mitochondrial signal and how it is transduced to copper handling machinery localized elsewhere in the cell is not yet known. Copper is an endogenous modulator of neuronal cell biology,15 and it may therefore be that the metal ion is playing an active role in SCO1-dependent mitochondrial signaling. However, we restrict our discussion below to the possibility that the organelle instead uses a redox and/or metabolic switch mechanism to sense copper and dynamically regulate copper homeostasis.
Mitochondria, redox regulation and control of copper homeostasis
Although the endoplasmic reticulum (ER) was once thought to be the only oxidizing compartment in the cell, it is now appreciated that limiting glutaredoxin amounts enables thiol oxidation within the IMS.88 Pioneering work from the Herrmann group established that the import and folding of many proteins within this mitochondrial compartment, including a suite of COX assembly factors with canonical twin Cx9C motifs, requires a disulphide relay system89, 90 that bares remarkable similarity to the one operating in the ER.91 Mitochondria are also major sources of superoxide anion and hydrogen peroxide, and a number of resident enzymes are dedicated to the conversion and consumption of these ROS.92 However, it is well established that these ROS are more than simply by-products of metabolism and that they offer an essential means of regulating mitochondrial protein function via the post-translational modification (PTM) of reactive residues.93 Elegant studies of other, non-mitochondrial proteins like OxyR further emphasize that direct modulation of a redox active cysteine via its sulfenylation, glutathionylation or nitrosylation allows for exquisite tuning of protein function.94 Therefore, one of the more obvious possibilities is that mitochondrial signaling through SCO1 is directly controlled by redox regulation of its Cx3C motif,95 which is contained within an evolutionarily conserved thioredoxin fold (Figure 2).96, 97 A large number of other COX assembly factors exist with reactive cysteine residues that also localize to the IMS, and a subset of these proteins have already been shown to physically interact with SCO1.49, 50, 98 As such, it is tempting to speculate that redox-dependent PTM of one or more of these proteins may significantly influence their physical association with SCO1, and provide an additional means of modulating the activity of this mitochondrial signaling pathway (Figure 2).
Glutathione (GSH) is another critical player in cellular redox balance, and recent findings are expanding upon its established role in the regulation of copper homeostasis. GSH donates an electron to reduce oxidized targets, and its millimolar concentration in healthy cells makes GSH the principal redox buffer against oxidative damage.99 GSH also binds copper, a property that further adds to its capacity to buffer against toxicity100 but also appears to directly support CTR1-mediated copper import into the cell.101 Copper bound to GSH within the cytosol is ultimately transferred down an affinity gradient to soluble metallochaperones,102 which then traffic the metal ion to various subcellular compartments. GSH can also modulate copper delivery and flux through individual trafficking pathways via PTM of protein function. Thus far, the secretory pathway has received the most attention in this regard with glutathionylation impairing the activity of the soluble chaperone ATOX1 and the TGN ATPases ATP7A and ATP7B,103–105 and deglutathionylation of these proteins rendering their cysteines accessible to copper and stimulating copper delivery to the secretory pathway.
The importance of GSH and its oxidized form, GSSG, to copper homeostasis in mitochondria and to SCO1-dependent signaling has not been well studied and warrants further investigation. GSH freely diffuses across the outer mitochondrial membrane, and the organelle contains roughly 10–15% of the cellular GSH pool in its matrix.99 A portion of the cellular glutathione reductase pool is also found in the mitochondrial matrix,106 providing it with a regenerative system for this redox couple (Figure 2). Matrix redox balance is nonetheless perturbed by mutations in ATP7A or ATP7B, which lead to mitochondrial hyper accumulation of copper and organelle dysfunction.40, 43 However, whether GSH participates in copper delivery to mitochondria, whether the GSH:GSSG ratio affects copper trafficking within the organelle and whether any of these activities are impaired in copper handling disorders remain important, open questions.
Changes in the GSH:GSSG ratio in the cytosol may also affect copper homeostasis by altering mitochondrial morphology. Mitochondria are dynamic organelles that constantly fuse and divide to mix their contents, which is essential to preserve their function and maintain cellular health.107 There is an emerging literature supporting the idea that signaling through ROS and reactive nitrogen species leads to PTM of the fusion and fission machinery to regulate mitochondrial dynamics.108 GSSG has also been shown to be a potent inducer of organelle fusion.109 Fusion of individual organelles into a reticular network is a common stress response in cells to protect the overall health of their mitochondrial population,107 and a GSH deficiency has been reported in skeletal muscle of mitochondrial disease patients with electron transport chain defects.110 While gross mitochondrial morphology does not appear to differ between control, SCO1, SCO2 and ATP7A patient fibroblasts,39, 40 a potential relationship between redox-dependent changes in mitochondrial dynamics and copper homeostasis has yet to be explored in mechanistic detail in more applicable disease models and contexts. It is imperative that future studies like these also consider the possible interplay between redox and the release of mitochondria-derived vesicles, which were originally identified by the McBride group and are now known to deliver specific, selective and physiologically relevant cargo to peroxisomes and endolysosomes.111–113
Metabolic control of copper homeostasis
SCO1-dependent mitochondrial signaling may also be influenced by changes in energy homeostasis. Mitochondria play a key role in regulating various aspects of metabolism because they produce the bulk of ATP consumed by the cell, and synthesize essential macromolecules like lipids and iron-sulphur clusters that are then used in the biogenesis of membranes and maturation of proteins. Some of the intermediates produced by these metabolic pathways also function as signaling molecules. Acetate and citrate are both released from mitochondria and converted in the cytosol to acetyl CoA, which is subsequently used in acetylation reactions to control gene expression in the nucleus.93 Reversible acetylation also occurs within the mitochondrion and is regulated in part by members of the sirtuin family of NAD+-dependent deacetylases, including SIRT3 (Figure 2).114 SIRT3 is normally sequestered via a physical interaction with the ATP synthase; however, an increase in NAD+ levels and a loss of mitochondrial membrane potential results in its release from this complex.115 SIRT3 then deacetylates a suite of matrix proteins, which is essential for the recovery of membrane potential and, by extension, mitochondrial function and cell survival. Intriguingly, SIRT3 also modulates the activity of iron regulatory protein 1 and is required for cellular iron metabolism,116 opening up the possibility that the mitochondrial acetylome may also contribute to the regulation of copper homeostasis by virtue of the intimate link between the metabolism of these two metal ions.
Changes in ATP levels may also be relevant to the transduction of a SCO1-dependent mitochondrial signal that regulates copper homeostasis. When ATP levels are low, two ADP molecules are brought together to produce ATP and AMP, and AMP accumulation leads to the activation of the AMP kinase (AMPK) (Figure 2). AMPK in turn phosphorylates the tumour suppressor protein p53,117 which is known to transactivate SCO2 expression to modulate the balance between the utilization of respiratory and glycolytic pathways.118 Interestingly, a portion of the total p53 pool is found within the IMS and is imported into the organelle by the MIA40-ERV1 disulphide relay system.119 Attenuated MIA40 function reduces the abundance of IMS-localized p53 and results in its nuclear enrichment.119 It is therefore conceivable that p53 senses the functional status of SCO1, via direct or indirect mechanisms, and its ability to do so is perturbed in affected tissues of COX deficient patients. Consistent with this idea, deletion of the single SCO gene in Drosophila heart produced a dilated cardiomyopathy that was rescued by disrupting p53 function.120
Concluding remarks
Roughly 25 years ago, it was discovered that mitochondria generate signals that alter nuclear gene expression and that the organelle releases cytochrome c from the IMS to stimulate apoptosis.19 The implication of these original discoveries was that the functional status, or fitness, of the organelle population was constantly being communicated throughout the cell to regulate various biological processes and preserve homeostasis. Seminal discoveries by many other groups have subsequently reinforced this idea and expanded upon the breadth and nature of signals mitochondria employ towards this end.93 The release of copper may in fact allow mitochondria to communicate with the rest of the cell given that an active role for transition metals in signal transduction is now appreciated.121 However, our understanding of the role of the organelle in the regulation of copper homeostasis is only in its infancy. The mitochondrion itself remains a “black box” of sorts, with most mechanistic elements of copper handling aside from COX assembly having yet to be described. It is clear that the relationship between copper, redox and metabolism is intertwined, and it may be further complicated by functional contacts formed between mitochondria and the plasma membrane122 or mitochondria and the ER. Physical association with the plasma membrane may provide a direct conduit for CTR1-mediated delivery of copper to the organelle or for mitochondrial regulation of CTR1 function. Dynamic associations between mitochondria and the ER are essential for lipid and calcium exchange, and it has been proposed that the ER possesses a labile pool of copper123 that may serve as an additional source of the metal ion for the organelle.124 Studies that further identify the mitochondrial machinery that handles copper, characterize how it is regulated, and clarify how its activity is in turn communicated outside of the organelle to modulate cellular copper uptake and efflux will be invaluable to advancing our understanding of relevant human diseases and basic biology alike.
Acknowledgments
Our programs are supported by grants-in-aid of research from the Canadian Institutes of Health Research (SCL) and National Institutes of Health (PAC, SCL). SCL is also a CIHR New Investigator awardee.
References
- 1.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US); Washington (DC): 2001. pp. 224–257. [PubMed] [Google Scholar]
- 2.Prohaska JR. Impact of copper deficiency in humans. Ann. N.Y. Acad. Sci. 2014;1314:1–5. doi: 10.1111/nyas.12354. [DOI] [PubMed] [Google Scholar]
- 3.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta. 2012;1823:1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 5.Tisato F, Marzano C, Porchia M, Pellei M, Santini C. Copper in diseases treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010;30:708–749. doi: 10.1002/med.20174. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamoorthy L, Cotruvo JA, Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Wal MN, Guan T, Smaga LP, Farhi SL, New EJ, Lutsenko S, Chang CJ. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 2016;12:586–592. doi: 10.1038/nchembio.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brancaccio D, Gallo A, Piccioli M, Novellino E, Ciofi-Baffoni S, Banci L. [4Fe-4S] Cluster Assembly in Mitochondria and Its Impairment by Copper. J. Am. Chem. Soc. 2017;139:719–730. doi: 10.1021/jacs.6b09567. [DOI] [PubMed] [Google Scholar]
- 9.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 10.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Dancis A, Yuan DS, Haile D, Askwith C, Eide D, Moehle C, Kaplan J, Klausner RD. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Peña MM, Nose Y, Thiele DJ. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 13.Aller SG, Unger VM. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Feo CJ, Aller SG, Siluvai GS, Blackburn NJ, Unger VM. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodani SC, Firl A, Chan J, Nam CI, Aron AT, Onak CS, Ramos-Torres KM, Paek J, Webster CM, Feller MB, Chang CJ. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl. Acad. Sci. U.S.A. 2014;111:16280–16285. doi: 10.1073/pnas.1409796111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, Strausak D, Camakaris J, Mercer JF. Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J. Biol. Chem. 2002;277:46736–46742. doi: 10.1074/jbc.M208864200. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am. J. Physiol. 1999;276:G639–G646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- 19.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Mehta MM, Weinberg SE, Chandel NS. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 2017 doi: 10.1038/nri.2017.66. [DOI] [PubMed] [Google Scholar]
- 22.Pierrel F, Cobine PA, Winge DR. Metal Ion availability in mitochondria. Biometals. 2007;20:675–682. doi: 10.1007/s10534-006-9052-9. [DOI] [PubMed] [Google Scholar]
- 23.Leary SC, Winge DR, Cobine PA. “Pulling the plug” on cellular copper: the role of mitochondria in copper export. Biochim. Biophys. Acta. 2009;1793:146–153. doi: 10.1016/j.bbamcr.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leary SC. Redox regulation of SCO protein function: controlling copper at a mitochondrial crossroad. Antioxid. Redox Signal. 2010;13:1403–1416. doi: 10.1089/ars.2010.3116. [DOI] [PubMed] [Google Scholar]
- 25.Soto IC, Fontanesi F, Liu J, Barrientos A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta. 2012;1817:883–897. doi: 10.1016/j.bbabio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiburek L, Hansikova H, Tesarova M, Cerna L, Zeman J. Biogenesis of eukaryotic cytochrome c oxidase. Physiol. Res. 2006;55:S27–S41. doi: 10.33549/physiolres.930000.55.S2.27. [DOI] [PubMed] [Google Scholar]
- 27.Dennerlein S, Rehling P. Human mitochondrial COX1 assembly into cytochrome c oxidase at a glance. J. Cell Sci. 2015;128:833–837. doi: 10.1242/jcs.161729. [DOI] [PubMed] [Google Scholar]
- 28.Glerum DM, Shtanko A, Tzagoloff A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J. Biol. Chem. 1996;271:14504–14509. doi: 10.1074/jbc.271.24.14504. [DOI] [PubMed] [Google Scholar]
- 29.Beers J, Glerum DM, Tzagoloff A. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J. Biol. Chem. 1997;272:33191–33196. doi: 10.1074/jbc.272.52.33191. [DOI] [PubMed] [Google Scholar]
- 30.Cobine PA, Ojeda LD, Rigby KM, Winge DR. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004;279:14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 31.Maxfield AB, Heaton DN, Winge DR. Cox17 is functional when tethered to the mitochondrial inner membrane. J. Biol. Chem. 2004;279:5072–5080. doi: 10.1074/jbc.M311772200. [DOI] [PubMed] [Google Scholar]
- 32.Garber Morales J, Holmes-Hampton GP, Miao R, Guo Y, Münck E, Lindahl PA. Biophysical characterization of iron in mitochondria isolated from respiring and fermenting yeast. Biochemistry. 2010;49:5436–5444. doi: 10.1021/bi100558z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobine PA, Pierrel F, Winge DR. Copper trafficking to the mitochondrion and assembly of copper metalloenzymes. Biochim. Biophys. Acta. 2006;1763:759–772. doi: 10.1016/j.bbamcr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Hamel P, Saint-Georges Y, de Pinto B, Lachacinski N, Altamura N, Dujardin G. Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol. Microbiol. 2004;51:307–317. doi: 10.1046/j.1365-2958.2003.03810.x. [DOI] [PubMed] [Google Scholar]
- 35.Foury F, Roganti T. Deletion of the mitochondrial carrier genes MRS3 and MRS4 suppresses mitochondrial iron accumulation in a yeast frataxin-deficient strain. J. Biol. Chem. 2002;277:24475–24483. doi: 10.1074/jbc.M111789200. [DOI] [PubMed] [Google Scholar]
- 36.Vest KE, Leary SC, Winge DR, Cobine PA. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem. 2013;288:23884–23892. doi: 10.1074/jbc.M113.470674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vest KE, Wang J, Gammon MG, Maynard MK, White OL, Cobine JA, Mahone WK, Cobine PA. Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae. Open Biol. 2016;6:150223. doi: 10.1098/rsob.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobine PA, Pierrel F, Bestwick ML, Winge DR. Mitochondrial matrix copper complex used in metallation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 2006;281:36552–36559. doi: 10.1074/jbc.M606839200. [DOI] [PubMed] [Google Scholar]
- 39.Dodani SC, Leary SC, Cobine PA, Winge DR, Chang CJ. A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J. Am. Chem. Soc. 2011;133:8606–8616. doi: 10.1021/ja2004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharjee A, Yang H, Duffy M, Robinson E, Conrad-Antoville A, Lu YW, Capps T, Braiterman L, Wolfgang M, Murphy MP, Yi L, Kaler SG, Lutsenko S, Ralle M. The Activity of Menkes Disease Protein ATP7A Is Essential for Redox Balance in Mitochondria. J. Biol. Chem. 2016;291:16644–16658. doi: 10.1074/jbc.M116.727248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sternlieb I. Mitochondrial and fatty changes in hepatocytes of patients with Wilson’s disease. Gastroenterology. 1968;55:354–367. [PubMed] [Google Scholar]
- 42.Roberts EA, Robinson BH, Yang S. Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease. Mol. Genet. Metab. 2008;93:54–65. doi: 10.1016/j.ymgme.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 43.Zischka H, Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann. N.Y. Acad. Sci. 2014;1315:6–15. doi: 10.1111/nyas.12347. [DOI] [PubMed] [Google Scholar]
- 44.Lichtmannegger J, Leitzinger C, Wimmer R, Schmitt S, Schulz S, Kabiri Y, Eberhagen C, Rieder T, Janik D, Neff F, Straub BK, Schirmacher P, DiSpirito AA, Bandow N, Baral BS, Flatley A, Kremmer E, Denk G, Reiter FP, Hohenester S, Eckardt-Schupp F, Dencher NA, Adamski J, Sauer V, Niemietz C, Schmidt HH, Merle U, Gotthardt DN, Kroemer G, Weiss KH, Zischka H. Methanobactin reverses acute liver failure in a rat model of Wilson disease. J. Clin. Invest. 2016;126:2721–2735. doi: 10.1172/JCI85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leary SC, Cobine PA, Kaufman BA, Guercin GH, Mattman A, Palaty J, Lockitch G, Winge DR, Rustin P, Horvath R, Shoubridge EA. The human cytochrome c oxidase assembly factors SCO1 and SCO2 have regulatory roles in the maintenance of cellular copper homeostasis. Cell Metab. 2007;5:9–20. doi: 10.1016/j.cmet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Stiburek L, Vesela K, Hansikova H, Hulkova H, Zeman J. Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am. J. Physiol. Cell Physiol. 2009;296:1218–1226. doi: 10.1152/ajpcell.00564.2008. [DOI] [PubMed] [Google Scholar]
- 47.Hlynialuk CJ, Ling B, Baker ZN, Cobine PA, Yu LD, Boulet A, Wai T, Hossain A, El Zawily AM, McFie PJ, Stone SJ, Diaz F, Moraes CT, Viswanathan D, Petris MJ, Leary SC. The Mitochondrial Metallochaperone SCO1 Is Required to Sustain Expression of the High-Affinity Copper Transporter CTR1 and Preserve Copper Homeostasis. Cell Rep. 2015;10:933–943. doi: 10.1016/j.celrep.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR. Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J. Biol. Chem. 2004;279:35334–35340. doi: 10.1074/jbc.M404747200. [DOI] [PubMed] [Google Scholar]
- 49.Cobine PA, Pierrel F, Leary SC, Sasarman F, Horng YC, Shoubridge EA, Winge DR. The P174L mutation in human Sco1 severely compromises Cox17-dependent metallation but does not impair copper binding. J. Biol. Chem. 2006;281:12270–12276. doi: 10.1074/jbc.M600496200. [DOI] [PubMed] [Google Scholar]
- 50.Banci L, Bertini I, Ciofi-Baffoni S, Leontari I, Martinelli M, Palumaa P, Sillard R, Wang S. Human Sco1 functional studies and pathological implications of the P174L mutant. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15–20. doi: 10.1073/pnas.0606189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lode A, Kuschel M, Paret C, Rödel G. Mitochondrial copper metabolism in yeast: interaction between Sco1p and Cox2p. FEBS Lett. 2000;485:19–24. doi: 10.1016/s0014-5793(00)02176-1. [DOI] [PubMed] [Google Scholar]
- 52.Nittis T, George GN, Winge DR. Yeast Sco1, a protein essential for cytochrome c oxidase function is a Cu(I)-binding protein. J. Biol. Chem. 2001;276:42520–42526. doi: 10.1074/jbc.M107077200. [DOI] [PubMed] [Google Scholar]
- 53.Horng YC, Leary SC, Cobine PA, Young FB, George GN, Shoubridge EA, Winge DR. Human Sco1 and Sco2 function as copper-binding proteins. J. Biol. Chem. 2005;280:34113–34122. doi: 10.1074/jbc.M506801200. [DOI] [PubMed] [Google Scholar]
- 54.Morgada MN, Abriata LA, Cefaro C, Gajda K, Banci L, Vila AJ. Loop recognition and copper-mediated disulfide reduction underpin metal site assembly of CuA in human cytochrome oxidase. Proc. Natl. Acad. Sci. U.S.A. 2015;112:11771–11776. doi: 10.1073/pnas.1505056112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leary SC, Kaufman BA, Pellecchia G, Guercin GH, Mattman A, Jaksch M, Shoubridge EA. Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum. Mol. Genet. 2004;13:1839–1848. doi: 10.1093/hmg/ddh197. [DOI] [PubMed] [Google Scholar]
- 56.Leary SC, Sasarman F, Nishimura T, Shoubridge EA. Human SCO2 is required for the synthesis of CO II and as a thiol-disulphide oxidoreductase for SCO1. Hum. Mol. Genet. 2009;18:2230–2240. doi: 10.1093/hmg/ddp158. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh A, Trivedi PP, Timbalia SA, Griffin AT, Rahn JJ, Chan SS, Gohil VM. Copper supplementation restores cytochrome c oxidase assembly defect in a mitochondrial disease model of COA6 deficiency. Hum. Mol. Genet. 2014;23:3596–3696. doi: 10.1093/hmg/ddu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh A, Pratt AT, Soma S, Theriault SG, Griffin AT, Trivedi PP, Gohil VM. Mitochondrial disease genes COA6, COX6B and SCO2 have overlapping roles in COX2 biogenesis. Hum. Mol. Genet. 2016;25:660–671. doi: 10.1093/hmg/ddv503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longen S, Bien M, Bihlmaier K, Kloeppel C, Kauff F, Hammermeister M, Westermann B, Herrmann JM, Riemer J. Systematic analysis of the twin cx(9)c protein family. J. Mol. Biol. 2009;393:356–368. doi: 10.1016/j.jmb.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 60.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta V. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001;276:38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 61.Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Jaronen M, Arens E, Akerman K, Chan PH, Koistinaho J. Deleterious role of superoxide dismutase in the mitochondrial intermembrane space. J. Biol. Chem. 2008;283:8446–8452. doi: 10.1074/jbc.M706111200. [DOI] [PubMed] [Google Scholar]
- 62.Kawamata H, Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 2010;13:1375–1384. doi: 10.1089/ars.2010.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Field LS, Furukawa Y, O’Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J. Biol. Chem. 2003;278:28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 64.Fetherolf MM, Boyd SD, Winkler DD, Winge DR. Oxygen-dependent activation of Cu, Zn-superoxide dismutase-1. Metallomics. 2017;9:1047–1059. doi: 10.1039/c6mt00298f. [DOI] [PubMed] [Google Scholar]
- 65.Klöppel C, Suzuki Y, Kojer K, Petrungaro C, Longen S, Fiedler S, Keller S, Riemer J. Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol. Mol. Biol. Cell. 2011;22:3749–3757. doi: 10.1091/mbc.E11-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horn D, Al-Ali H, Barrientos A. Cmc1p is a conserved mitochondrial twin CX9C protein involved in cytochrome c oxidase biogenesis. Mol. Cell Biol. 2008;28:4354–4364. doi: 10.1128/MCB.01920-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Said Ahmed M, Hung WY, Zu JS, Hockberger P, Siddique T. Increased reactive oxygen species in familial amyotrophic lateral sclerosis with mutations in SOD1. J. Neurol. Sci. 2000;176:88–94. doi: 10.1016/s0022-510x(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 68.Tokuda E, Okawa E, Ono S. Dysregulation of intracellular copper trafficking pathway in a mouse model of mutant copper/zinc superoxide dismutase-linked familial amyotrophic lateral sclerosis. J. Neurochem. 2009;111:181–191. doi: 10.1111/j.1471-4159.2009.06310.x. [DOI] [PubMed] [Google Scholar]
- 69.Valnot I, von Kleist-Retzow JC, Barrientos A, Gorbatyuk M, Taanman JW, Mehaye B, Rustin P, Tzagoloff A, Munnich A, Rötig A. A mutation in the human heme A:farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- 70.Leary SC, Antonicka H, Sasarman F, Weraarpachai W, Cobine PA, Pan M, Brown GK, Brown R, Majewski J, Ha KC, Rahman S, Shoubridge EA. Novel mutations in SCO1 as a cause of fatal infantile encephalopathy and lactic acidosis. Hum. Mutat. 2013;34:1366–1370. doi: 10.1002/humu.22385. [DOI] [PubMed] [Google Scholar]
- 71.Papadopoulou LC, Sue C, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, Coster RV, Lyon G, Scalais E, Lebel R, Kaplan P, Shanske S, De Vivo DC, Bonilla E, Hirano M, DiMauro S, Schon EA. Fatal infantile cardioencephalomyopathy with COX deficiency mutations in SCO2, a COX assembly gene. Nat. Genet. 1999;23:333–337. doi: 10.1038/15513. [DOI] [PubMed] [Google Scholar]
- 72.Jaksch M, Ogilvie I, Yao J, Kortenhaus G, Bresser HG, Gerbitz KD, Shoubridge EA. Mutations in SCO2 are associated with a distinct form of hypertrophic cardiomyopathy and cytochrome c oxidase deficiency. Hum. Mol. Genet. 2000;9:795–801. doi: 10.1093/hmg/9.5.795. [DOI] [PubMed] [Google Scholar]
- 73.Baertling F, van den Brand M, Hertecant JL, Al-Shamsi A, van den Heuvel L, Distelmaier F, Mayatepek E, Smeitink JA, Nijtmans LG, Rodenburg RJ. Mutations in COA6 cause cytochrome c oxidase deficiency and neonatal hypertrophic cardiomyopathy. Hum. Mutat. 2015;36:34–38. doi: 10.1002/humu.22715. [DOI] [PubMed] [Google Scholar]
- 74.Jaksch M, Horvath R, Horn N, Auer DP, Macmillan C, Peters J, Gerbitz KD, Kraegeloh-Mann I, Muntau A, Karcagi V, Kalmanchey R, Lochmuller H, Shoubridge EA, Freisinger P. Homozygosity (E140K) in SCO2 causes delayed infantile onset of cardiomyopathy and neuropathy. Neurology. 2001;57:1440–1446. doi: 10.1212/wnl.57.8.1440. [DOI] [PubMed] [Google Scholar]
- 75.Vesela K, Hansikova H, Tesarova M, Martasek P, Elleder M, Houstek J, Zeman J. Clinical, biochemical and molecular analyses of six patients with isolated cytochrome c oxidase deficiency due to mutations in the SCO2 gene. Acta. Paediatr. 2004;93:1312–1317. doi: 10.1080/08035250410008761. [DOI] [PubMed] [Google Scholar]
- 76.Pronicka E, Piekutowska-Abramczuk D, Szymańska-Dębińska T, Bielecka L, Kowalski P, Luczak S, Karkucińska-Więckowska A, Migdał M, Kubalska J, Zimowski J, Jamroz E, Wierzba J, Sykut-Cegielska J, Pronicki M, Zaremba J, Krajewska-Walasek M. The natural history of SCO2 deficiency in 36 Polish children confirmed the genotype-phenotype correlation. Mitochondrion. 2013;13:801–816. doi: 10.1016/j.mito.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Valnot I, Osmond S, Gigarel N, Mehaye B, Amiel J, Cormier-Daire V, Munnich A, Bonnefont J, Rustin P, Rötig A. Mutations of the SCO1 gene in mitochondrial cytochrome c oxidase deficiency with neonatal-onset hepatic failure and encephalopathy. Am. J. Hum. Genet. 2000;67:1104–1109. doi: 10.1016/s0002-9297(07)62940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leary SC, Cobine PA, Nishimura T, Verdijk RM, de Krijger R, de Coo R, Tarnopolsky MA, Winge DR, Shoubridge EA. COX19 mediates the transduction of a mitochondrial redox signal from SCO1 that regulates ATP7A–mediated cellular copper efflux. Mol. Biol. Cell. 2013;24:683–691. doi: 10.1091/mbc.E12-09-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A. Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature. 2015;524:485–488. doi: 10.1038/nature14951. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Chen XJ. A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature. 2015;527:481–484. doi: 10.1038/nature14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Comstra HS, McArthy J, Rudin-Rush S, Hartwig C, Gokhale A, Zlatic SA, Blackburn JB, Werner E, Petris M, D’Souza P, Panuwet P, Barr DB, Lupashin V, Vrailas-Mortimer A, Faundez V. The interactome of the copper transporter ATP7A belongs to a network of neurodevelopmental and neurodegeneration factors. Elife. 2017;29:e24722. doi: 10.7554/eLife.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang H, Brosel S, Acin-Perez R, Slavkovich V, Nishino I, Khan R, Goldberg IJ, Graziano J, Manfredi G, Schon EA. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum. Mol. Genet. 2010;19:170–180. doi: 10.1093/hmg/ddp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker ZN, Jett K, Boulet A, Hossain A, Cobine PA, Kim BE, El Zawily AM, Lee L, Tibbits GF, Petris MJ, Leary SC. The Mitochondrial Metallochaperone SCO1 Maintains CTR1 at the Plasma Membrane to Preserve Copper Homeostasis in the Murine Heart. Hum. Mol. Genet. 2017 doi: 10.1093/hmg/ddx344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaksch M, Paret C, Stucka R, Horn N, Müller-Höcker J, Horvath R, Trepesch N, Stecker G, Freisinger P, Thirion C, Müller J, Lunkwitz R, Rödel G, Shoubridge EA, Lochmüller H. Cytochrome c oxidase deficiency due to mutations in SCO2, encoding a mitochondrial copper-binding protein, is rescued by copper in human myoblasts. Hum. Mol. Genet. 2001;10:3025–3035. doi: 10.1093/hmg/10.26.3025. [DOI] [PubMed] [Google Scholar]
- 85.Salviati L, Hernandez-Rosa E, Walker WF, Sacconi S, DiMauro S, Schon EA, Davidson MM. Copper supplementation restores cytochrome c oxidase activity in cultured cells from patients with SCO2 mutations. Biochem. J. 2002;363:321–327. doi: 10.1042/0264-6021:3630321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Freisinger P, Horvath R, Macmillan C, Peters J, Jaksch M. Reversion of hypertrophic cardiomyopathy in a patient with deficiency of the mitochondrial copper binding protein Sco2: is there a potential effect of copper? J. Inherit. Metab. Dis. 2004;27:67–79. doi: 10.1023/B:BOLI.0000016614.47380.2f. [DOI] [PubMed] [Google Scholar]
- 87.Pacheu-Grau D, Bareth B, Dudek J, Juris L, Vögtle FN, Wissel M, Leary SC, Dennerlein S, Rehling P, Deckers M. Cooperation between COA6 and SCO2 in COX2 maturation during cytochrome c oxidase assembly links two mitochondrial cardiomyopathies. Cell Metab. 2015;21:823–833. doi: 10.1016/j.cmet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 88.Kojer K, Peleh V, Calabrese G, Herrmann JM, Riemer J. Kinetic control by limiting glutaredoxin amounts enables thiol oxidation in the reducing mitochondrial intermembrane space. Mol. Biol. Cell. 2015;26:195–204. doi: 10.1091/mbc.E14-10-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005;121:1059–1069. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Herrmann JM, Riemer J. Mitochondrial disulfide relay: redox-regulated protein import into the intermembrane space. J. Biol. Chem. 2012;287:4426–4433. doi: 10.1074/jbc.R111.270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324:1284–1287. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 92.Riemer J, Schwarzländer M, Conrad M, Herrmann JM. Thiol switches in mitochondria: operation and physiological relevance. Biol. Chem. 2015;396:465–482. doi: 10.1515/hsz-2014-0293. [DOI] [PubMed] [Google Scholar]
- 93.Chandel NS. Evolution of Mitochondria as Signaling Organelles. Cell Metab. 2015;22:204–206. doi: 10.1016/j.cmet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Kim SO, Merchant K, Nudelman R, Beyer WFJ, Keng T, DeAngelo J, Hausladen A, Stamler JS. OxyR: a molecular code for redox-related signaling. Cell. 2002;109:383–396. doi: 10.1016/s0092-8674(02)00723-7. [DOI] [PubMed] [Google Scholar]
- 95.Williams JC, Sue C, Banting GS, Yang H, Glerum DM, Hendrickson WA, Schon EA. Crystal structure of human SCO1: implications for redox signaling by a mitochondrial cytochrome c oxidase “assembly” protein. J. Biol. Chem. 2005;280:15202–15211. doi: 10.1074/jbc.M410705200. [DOI] [PubMed] [Google Scholar]
- 96.Chinenov YV. Cytochrome c oxidase assembly factors with a thioredoxin fold are conserved among prokaryotes and eukaryotes. J. Mol. Med. (Berl) 2000;78:239–242. doi: 10.1007/s001090000110. [DOI] [PubMed] [Google Scholar]
- 97.Balatri E, Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Solution structure of Sco1: a thioredoxin-like protein Involved in cytochrome c oxidase assembly. Structure. 2003;11:1431–1443. doi: 10.1016/j.str.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Stroud DA, Maher MJ, Lindau C, Vögtle FN, Frazier AE, Surgenor E, Mountford H, Singh AP, Bonas M, Oeljeklaus S, Warscheid B, Meisinger C, Thorburn DR, Ryan MT. COA6 is a mitochondrial complex IV assembly factor critical for biogenesis of mtDNA-encoded COX2. Hum. Mol. Genet. 2015;24:5404–5415. doi: 10.1093/hmg/ddv265. [DOI] [PubMed] [Google Scholar]
- 99.Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Freedman JH, Ciriolo MR, Peisach J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- 101.Maryon EB, Molloy SA, Kaplan JH. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol. Cell Physiol. 2013;304:768–779. doi: 10.1152/ajpcell.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 103.Singleton WC, McInnes KT, Cater MA, Winnall WR, McKirdy R, Yu Y, Taylor PE, Ke BX, Richardson DR, Mercer JF, La Fontaine S. Role of glutaredoxin1 and glutathione in regulating the activity of the copper-transporting P-type ATPases, ATP7A and ATP7B. J. Biol. Chem. 2010;285:27111–27121. doi: 10.1074/jbc.M110.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hatori Y, Clasen S, Hasan NM, Barry AN, Lutsenko S. Functional partnership of the copper export machinery and glutathione balance in human cells. J. Biol. Chem. 2012;287:26678–26687. doi: 10.1074/jbc.M112.381178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatori Y, Yan Y, Schmidt K, Furukawa E, Hasan NM, Yang N, Liu CN, Sockanathan S, Lutsenko S. Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat. Commun. 2016;16:10640. doi: 10.1038/ncomms10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–D1257. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 108.Willems PH, Rossignol R, Dieteren CE, Murphy MP, Koopman WJ. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015;22:207–218. doi: 10.1016/j.cmet.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep. 2012;13:909–915. doi: 10.1038/embor.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hargreaves IP, Sheena Y, Land JM, Heales SJ. Glutathione deficiency in patients with mitochondrial disease: implications for pathogenesis and treatment. J. Inherit. Metab. Dis. 2005;28:81–88. doi: 10.1007/s10545-005-4160-1. [DOI] [PubMed] [Google Scholar]
- 111.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr. Biol. 2008;18:102–108. doi: 10.1016/j.cub.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 112.Cadete VJ, Deschênes S, Cuillerier A, Brisebois F, Sugiura A, Vincent A, Turnbull D, Picard M, McBride HM, Burelle Y. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 2016;594:5343–5362. doi: 10.1113/JP272703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McLelland GL, Lee SA, McBride HM, Fon EA. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J. Cell Biol. 2016;214:275–291. doi: 10.1083/jcb.201603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13653–13658. doi: 10.1073/pnas.222538099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang W, Nagasawa K, Münch C, Xu Y, Satterstrom K, Jeong S, Hayes SD, Jedrychowski MP, Vyas FS, Zaganjor E, Guarani V, Ringel AE, Gygi SP, Harper JW, Haigis MC. Mitochondrial Sirtuin Network Reveals Dynamic SIRT3-Dependent Deacetylation in Response to Membrane Depolarization. Cell. 2016;167:985–1000. doi: 10.1016/j.cell.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeong SM, Lee J, Finley LW, Schmidt PJ, Fleming MD, Haigis MC. SIRT3 regulates cellular iron metabolism and cancer growth by repressing iron regulatory protein 1. Oncogene. 2015;34:2115–2124. doi: 10.1038/onc.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 118.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 119.Zhuang J, Wang PY, Huang X, Chen X, Kang JG, Hwang PM. Mitochondrial disulfide relay mediates translocation of p53 and partitions its subcellular activity. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17356–17361. doi: 10.1073/pnas.1310908110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martínez-Morentin L, Martínez L, Piloto S, Yang H, Schon EA, Garesse R, Bodmer R, Ocorr K, Cervera M, Arredondo JJ. Cardiac deficiency of single cytochrome oxidase assembly factor scox induces p53-dependent apoptosis in a Drosophila cardiomyopathy model. Hum. Mol. Genet. 2015;24:3608–3622. doi: 10.1093/hmg/ddv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chang CJ. Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 2015;11:744–747. doi: 10.1038/nchembio.1913. [DOI] [PubMed] [Google Scholar]
- 122.Westermann B. The mitochondria-plasma membrane contact site. Curr. Opin. Cell Biol. 2015;35:1–6. doi: 10.1016/j.ceb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Park SY, Kim W, Park SH, Han J, Lee J, Kang C, Lee MH. An endoplasmic reticulum-selective ratiometric fluorescent probe for imaging a copper pool. Chem. Commun. 2017;53:4457–4460. doi: 10.1039/c7cc01430a. [DOI] [PubMed] [Google Scholar]
- 124.Giacomello M, Pellegrini L. The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ. 2016;23:1417–1427. doi: 10.1038/cdd.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]