Abstract
Objective
To investigate the predictive and concurrent validity of MRI-based cartilage thickness change between baseline (BL) and year-two (Y2) follow-up (predictive validity) and between Y2 and Y4 follow-up (concurrent validity) for symptomatic and radiographic knee osteoarthritis (OA) progression during Y2→Y4.
Methods
777 knees from 777 Osteoarthritis Initiative (OAI) participants (age: 61.3±9.0 years, BMI: 30.1±4.8 kg/m2) with Kellgren Lawrence (KL) grade 1–3 at Y2 (visit before progression interval) had cartilage thickness measurements from 3T MRI at BL, Y2 (n=777), and Y4 (n=708). Analysis of covariance and logistic regression were used to assess the association of pain progression (≥9 WOMAC units [scale 0–100], n=205/572 with/without progression) and radiographic progression (≥0.7 mm minimum joint space width loss, n=166/611 with/without progression) between Y2 and Y4 with preceding (BL→Y2) and concurrent (Y2→Y4) change in central medial femorotibial (cMFTC) compartment cartilage thickness.
Results
Symptomatic progression was associated with concurrent (Y2→Y4: −305±470μm vs. −155±346μm, OR=1.5 [1.2, 1.7]) but not with preceding cartilage thickness loss in cMFTC (−150±276μm vs. − 151±299μm, OR=0.9 95%CI: [0.8, 1.1]). Radiographic progression, in contrast, was significantly associated with both concurrent (−542±550μm vs. −98±255μm, OR=3.4 [2.6, 4.3]) and preceding cMFTC thickness loss (−229±355μm vs. −130±270μm, OR=1.3 [1.1, 1.5]).
Conclusions
These results extend previous reports that did not discern predictive vs. concurrent associations of cartilage thickness loss with OA progression. The observed predictive and concurrent validity of cartilage thickness loss for radiographic progression and observed concurrent validity for symptomatic progression provide an important step in qualifying cartilage thickness loss as a biomarker of knee OA progression.
Keywords: cartilage thickness, MRI, osteoarthritis, progression
Introduction
Knee OA substantially impacts the quality-adjusted life-years of the elderly population1. The treatment of knee OA is currently restricted to management of pain and function2, but strong efforts are ongoing to develop treatments that are ably to modify the course of the disease and to reduce both symptoms as well as the progression of structural pathology. A biomarker for clinical trials evaluating the efficacy of such disease modifying OA drugs (DMOADs) should ideally not only be sensitive to concurrent structural and symptomatic progression, but also be predictive of subsequent progression of knee OA.
In knees with radiographic OA (ROA), magnetic resonance imaging (MRI) measurements of cartilage thickness change have been shown to be significantly correlated with minimum medial radiographic joint space width (JSW)3, a structural progression measure currently accepted by the regulatory agencies for DMOAD trials. Other work has reported that increased rates of MRI cartilage thickness loss were observed prior to knee replacement surgery4,5, a “hard” clinical endpoint in knee OA closely related to symptom and function decline, and greater rates of MRI cartilage thickness loss was observed in knees with frequent pain vs. knees without pain6.
Because current diagnostic methods have been shown to be of limited value for the prediction of symptomatic and/or radiographic knee OA progression7, effort has been put into evaluating the responsiveness of imaging and biochemical biomarkers and into evaluating the association between these markers and progression of clinically relevant outcomes7. These efforts were recently undertaken under the auspices of the Foundation for the National Institutes of Health (FNIH) OA Biomarkers Consortium8–13, and we previously reported that one- and two-year (BL→Y1 and BL→Y2) loss in central medial femorotibial cartilage thickness was associated with the combination of pain and radiographic progression in knee OA from baseline (BL) to up to year four follow-up (BL→Y4)8 and this association was found to be stronger for radiographic progression than for pain progression More recently, Schaefer et al. used the FNIH design to validate a new cartilage segmentation method12 and reported odds ratios for change in cartilage volume in localized areas of the medial femur that were in the same range as that observed in the FNIH study for cartilage thickness8.
A caveat of that FNIH study design was that, whilst none of the knees met the predefined thresholds of progression at Y1, the observation period for the biomarker (BL→Y1 and BL→Y2) overlapped with that of the outcome (BL→Y2, 3 or 4)8. Therefore, it has remained unclear whether loss in cartilage thickness by MRI predicts or occurs concurrently with radiographic and symptomatic progression of knee OA.
The purpose of the present work, therefore, was to distinctly assess the predictive and concurrent validity of MRI cartilage thickness loss in the central medial femorotibial compartment, as a marker of symptomatic and radiographic knee osteoarthritis progression, using a modified study design based on the FNIH biomarker consortium8 progression criteria.
PATIENTS AND METHODS
Study Design
The current study was based on data from the Osteoarthritis Initiative (OAI), a prospective, observational cohort study (http://www.oai.ucsf.edu/, clinicaltrials.gov identifier: NCT00080171). The OAI enrolled 4,796 participants aged 45–79 years and collected clinical data, 3T MRIs, fixed-flexion radiographs, serum, and urine specimens annually at four clinical centers14. The OAI was approved by the Committee on Human Research, the Institutional Review Board (IRB) for the University of California, San Francisco (UCSF) and all four OAI clinical centers. All OAI participants provided written informed consent and this study was carried out in accordance with the IRB-approved OAI data user agreement.
We analyzed data from 853 OAI participants in the OAI core image assessment cohort15, the Pivotal OAI MRI Analyses (POMA) project5, and the OAI FNIH project8, which had at least one knee with a KL grade of 1, 2 or 3 at the Y2 follow-up visit, WOMAC pain and medial compartment minimum joint space width (mJSW) measurements at the Y2 and the Y4 follow-up visit, and cartilage thickness measurements from sagittal double-echo steady-state (DESS) sequence with water excitation at baseline (BL), year-two (Y2), and year-four (Y4) follow-up. From the 935 knees of these 853 participants, 93 knees from 76 participants were excluded because of low quality of the mJSW acquisitions (Y2 or Y4 tibia rim distance ≥6.5 mm or change in tibia rim distance between Y2 and Y4 ≥2 mm, n=89) or because of missing BMI data (n=4). To avoid the inclusion of more than one knee per participant16, knees without progression (n=31) or left knees (n=34) were excluded, if both knees met the inclusion criteria. The selection process resulted in 777 knees from 777 participants that were included in the current analysis. The MRIs for the Y4 follow-up were missing in 69 of the 777 knees (9%), mostly because the MRIs were not acquired or were of insufficient image quality. These participants were therefore only included in the analysis of the predictive validity, to maximize the sample size for this part of the analysis.
Definition of radiographic and symptomatic progression
The above knees were classified as having radiographic progression (mJSW ≥0.7mm) in the medial compartment and/or symptomatic progression (Western Ontario and McMaster Universities [WOMAC] pain progression ≥9 on scale from 0 to 100), as defined in the FNIH biomarker consortium project8. Contrary to the FNIH biomarker consortium project, knees were classified as progressor knees if the mJSW or WOMAC pain progression thresholds were exceeded between the Y2 and the Y4 follow-up visit rather than between BL and Y2, Y3 or Y4. This resulted in smaller statistical power (less progressors), but in two distinct observation periods, that is, preceding symptomatic or radiographic progression (BL→Y2) vs. one that was concurrent with progression (Y2→Y4).
Cartilage thickness measurement by MRI
Cartilage thickness measurements in the medial (MFTC) femorotibial compartment were based on a manual, quality-controlled segmentation of the cartilage surfaces from the 3T DESS MRIs acquired by the OAI8,14. All time-points of each knee (BL, Y2, and Y4 where available) were processed by the same reader using custom software (Chondrometrics GmbH, Ainring, Germany), with blinding to image acquisition order. The mean cartilage thickness (ThCtAB.Me) was computed in the MFTC, in 5 tibial subregions (central, external, internal, anterior, posterior), in 3 femoral subregions (central, external, internal)17, and in the combined central MFTC subregion (cMFTC). The BL→Y2 and the Y2→Y4 observation periods slightly differed in actual length between the participants and were hence normalized to 730-day observation periods.
Location-independent measures of subregional changes in cartilage thickness have been proposed as an alternative to location-based measures18. These measures are not designed to evaluate whether the observed changes differ significantly from zero, but they have been shown to be more sensitive to differences in change between groups than location-specific measures in observational studies19–22 as well as in interventional clinical trials23. Based on longitudinal changes in the 8 medial subregions, location-independent medial compartment cartilage thinning scores (mThinning) were computed for each observation period by summing all negative changes across the 8 medial subregions within each knee18. Similarly, location-independent medial compartment cartilage thickening scores (mThickening) were computed for each observation period by summing all positive changes across the 8 medial subregions within each knee18. Medial compartment ordered values (mOV) of subregional changes were computed by ordering the medial compartment subregional changes observed in each knee in ascending order18,19,21. mOV1 represented the subregion with the largest decrease and mOV8 the subregion with the largest increase in cartilage thickness in each knee.
Statistical analysis
The statistical analysis approach was adapted from the design of the FNIH study to allow a comparison between the results from the current study and the results from the FNIH study8. For the same reason, the longitudinal change in the cMFTC was defined as the primary analytic focus. The mean, the standard deviation (SD), and the 95% confidence intervals of the change in cartilage thickness were computed for the primary analytic focus (cMFTC) in knees with and without radiographic progression, and in knees with and without symptomatic progression. Preceding (BL→Y2) and concurrent (Y2→Y4) change in cMFTC cartilage thickness (primary analytic focus) was compared between progressor and non-progressor knees using analysis of covariance (ANCOVA), with adjustment for sex, age, WOMAC pain, medial JSN, and BMI at the respective reference visit (BL or Y2). Odds ratios (OR) per SD were computed as a measure of effect size using logistic regression, with adjustment for sex, age, WOMAC pain, medial JSN, and BMI at the respective reference visit. Because of the two progression criteria (radiographic and symptomatic) and the two observation periods (preceding and concurrent), the significance level of the primary analyses was adjusted to p=0.05/4=0.0125 (Bonferroni method) to account for 4 parallel comparisons.
The same statistical tests were applied without adjusting for multiple analyses to change in MFTC, mOV1, mOV8, mThinning, and mThickening, which were included as exploratory measures. Change in radiographic mJSW was included as an additional exploratory measure in the comparison between knees with and without symptomatic progression but not in the comparison between knees with and without radiographic progression, because the definition of radiographic progression was based on change in radiographic mJSW.
Additional sensitivity analyses were performed between knees without any progression vs. knees with isolated symptomatic progression, isolated radiographic progression, and both symptomatic and radiographic progression. The significance level for these sensitivity analyses was adjusted to p=0.05/6=0.008 (Bonferroni method) to account for the six parallel comparisons (three progression criteria and two observation periods). Again, the same statistical tests were applied without adjusting for multiple comparisons to the exploratory measures. Change in radiographic mJSW was also included as an additional exploratory measure in the comparison between knees without any progression vs. knees with isolated symptomatic progression. All analyses were performed using SPSS 23 (IBM Corporation, Armonk, NY).
Results
At the baseline visit, the 458 female and 319 male participants were on average 61.3±9.0 years old and had a body mass index of 30.1±4.8kg/m2 (Table 1). Of the 777 knees analyzed, 205 knees had symptomatic progression between the Y2 and the Y4 follow-up visit and 166 knees had radiographic progression between the Y2 and the Y4 follow-up visit (Table 1). The 69 participants with missing MRI measurements at Y4 did not differ significantly from the 708 participants with MRI measurements at Y4 with regard to age (p=0.27), BMI (p=0.07), WOMAC pain (p=0.28), mJSW (0.83), or cMFTC cartilage thickness (p=0.49) at the Y2 visit.
Table 1.
Demographic data, pain scores, radiographic assessments, and medial compartment cartilage thickness and minimum radiographic joint space width in participants with and without radiographic progression and participants with and without pain progression
| Symptomatic Progression | Radiographic Progression | ||||
|---|---|---|---|---|---|
| No Progression | Progression | No Progression | Progression | ||
| N preceding (BL→ Y2) | 572 | 205 | 611 | 166 | |
| N concurrent (Y2→ Y4) | 519 (90.7%) | 189 (92.2%) | 553 (90.5%) | 155 (93.4%) | |
| Sex | M | 230 (40.2%) | 89 (43.4%) | 239 (39.1%) | 80 (48.2%) |
| F | 342 (59.8%) | 116 (56.6%) | 372 (60.9%) | 86 (51.8%) | |
| KLG (BL) | 0 | 34 (5.9%) | 7 (3.4%) | 31 (5.1%) | 10 (6.0%) |
| 1 | 82 (14.3%) | 12 (5.9%) | 73 (11.9%) | 21 (12.7%) | |
| 2 | 300 (52.4%) | 109 (53.2%) | 338 (55.3%) | 71 (42.8%) | |
| 3 | 156 (27.3%) | 77 (37.6%) | 169 (27.7%) | 64 (38.6%) | |
| Med. JSN (BL) | 0 | 198 (34.6%) | 61 (29.8%) | 216 (35.4%) | 43 (25.9%) |
| 1 | 218 (38.1%) | 67 (32.7%) | 226 (37.0%) | 59 (35.5%) | |
| 2 | 156 (27.3%) | 77 (37.6%) | 169 (27.7%) | 64 (38.6%) | |
| Lat. JSN (BL) | 0 | 542 (94.8%) | 202 (98.5%) | 584 (95.6%) | 160 (96.4%) |
| 1 | 30 (5.2%) | 3 (1.5%) | 27 (4.4%) | 6 (3.6%) | |
| Pain Frequency | 0 | 86 (15.0%) | 26 (12.7%) | 82 (13.4%) | 30 (18.1%) |
| 1 | 181 (31.6%) | 59 (28.8%) | 183 (30.0%) | 57 (34.3%) | |
| 2 | 305 (53.3%) | 120 (58.5%) | 346 (56.6%) | 79 (47.6%) | |
| Age (BL) | [years] | 61.4 ± 8.9 | 61.2 ± 9.3 | 61.1 ± 9.0 | 62.1 ± 8.8 |
| BMI (BL) | [kg/m2] | 29.8 ± 4.9 | 31.0 ± 4.3 | 30.0 ± 4.8 | 30.5 ± 4.9 |
| MFTC (BL) | [mm] | 3.4 ± 0.6 | 3.4 ± 0.7 | 3.4 ± 0.6 | 3.3 ± 0.6 |
| cMFTC (BL) | [mm] | 4.1 ± 0.9 | 4.0 ± 0.9 | 4.1 ± 0.9 | 4.0 ± 0.9 |
| WOMAC (BL) | 13.6 ± 16.4 | 18.4 ± 19.0 | 15.1 ± 17.1 | 13.9 ± 17.8 | |
| WOMAC (Y2) | 16.1 ± 17.5 | 12.2 ± 13.8 | 14.6 ± 16.5 | 16.5 ± 17.5 | |
| ΔWOMAC (BL→ Y2) | 2.5 ± 17.0 | −6.2 ± 16.8 | −0.5 ± 15.5 | 2.5 ± 22.8 | |
| ΔWOMAC (Y2→ Y4) | −6.3 ± 11.7 | 20.3 ± 11.7 | −0.1 ± 15.2 | 3.8 ± 20.5 | |
| mJSW (BL) | [mm] | 4.0 ± 1.1 | 3.8 ± 1.2 | 4.0 ± 1.1 | 3.9 ± 1.3 |
| mJSW (Y2) | [mm] | 3.7 ± 1.2 | 3.6 ± 1.3 | 3.7 ± 1.2 | 3.6 ± 1.4 |
| ΔmJSW (BL→ Y2) | [μm] | −272 ± 618 | −243 ± 497 | −262 ± 580 | −272 ± 620 |
| ΔmJSW (Y2→ Y4) | [μm] | −299 ± 611 | −603 ± 831 | −120 ± 370 | −1334 ± 747 |
Symptomatic progression was defined as increase of ≥9 points on the Western Ontario and McMaster Universities (WOMAC) pain index (scale: 0 … 100) between the year 2 (Y2) and the year 4 (Y4) follow-up visit; Radiographic progression was defined as a decrease of ≥0.7mm in minimum radiographic joint space width in the medial compartment (mJSW) between the Y2 and the Y4 follow-up visit; BL: Baseline; KLG: Kellgren & Lawrence grade; Med./Lat. JSN: Medial/lateral joint space narrowing grade; Pain frequency: 0 = No pain, 1 = infrequent pain, 2 = pain on most days of a month in the previous 12 months; BMI: Body mass index; MFTC: Medial femorotibial compartment; cMFTC: central MFTC.
Primary analyses
In the period preceding the symptomatic progression (BL→Y2), no significant differences in cartilage thickness change were observed between knees with and without symptomatic progression for cMFTC (− 150±276 [−188, −112] μm vs. −151±299 [−176, −127] μm, p=0.25, OR=0.9 [0.8, 1.1], Figure 1) and the exploratory measures (Table 2). In the period concurrent with the symptomatic progression (Y2→Y4), however, knees with symptomatic progression showed a significantly greater concurrent loss in cMFTC cartilage thickness than knees without symptomatic progression (−305±470 [−373, −238] μm vs. −155±346 [−185, −125] μm, p<0.001, OR=1.5 [1.2, 1.7], Figure 1). Significant differences in concurrent change between progressor vs. non-progressor knees were also observed for the exploratory measures (Table 2).
Figure 1.
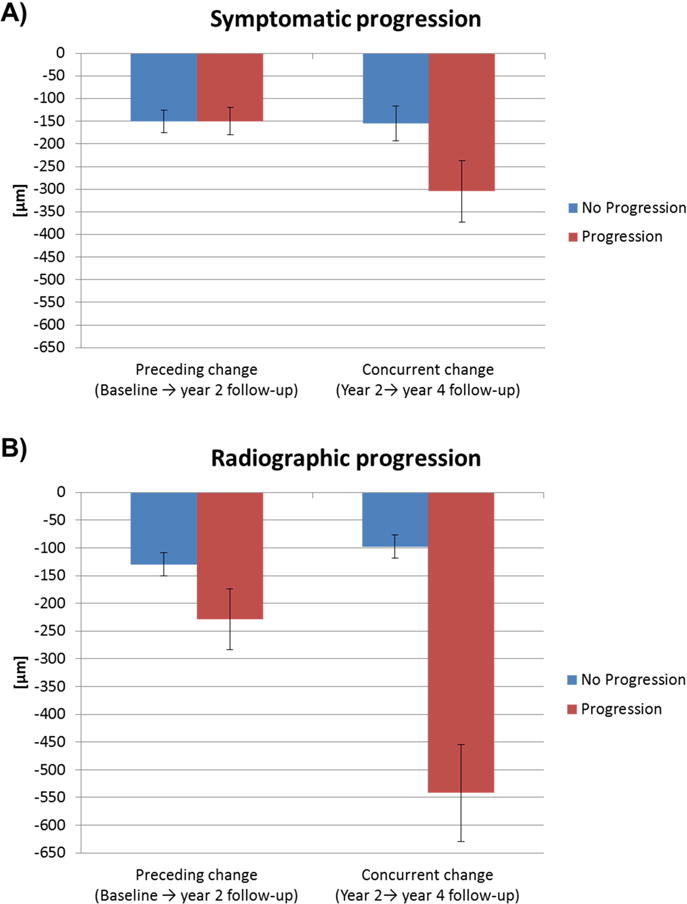
Preceding and concurrent change (±95% confidence intervals) in cMFTC cartilage thickness in A) knees with and without symptomatic progression and B) in knees with and without radiographic progression between the year 2 and the year 4 follow-up visit.
Table 2.
Preceding and concurrent longitudinal change (in μm) in exploratory measures in knees with and without radiographic progression and knees with and without symptomatic progression
| No Progression | Progression | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | SRM | Mean ± SD | 95% CI | SRM | P-Value | OR [95% CI] | |
| Symptomatic progression: | ||||||||
| Preceding change (BL→Y2): | ||||||||
| MFTC | −78 ± 188 | [−93, −62] | −0.41 | −87 ± 186 | [−113, −62] | −0.47 | 0.593 | 1.0 [0.8, 1.1] |
| mOV1 | −204 ± 192 | [−220, −189] | −207 ± 175 | [−231, −183] | 0.291 | 0.9 [0.8, 1.1] | ||
| mOV8 | 114 ± 99 | [106, 122] | 104 ± 84 | [92, 115] | 0.308 | 0.9 [0.8, 1.1] | ||
| mThinning | −546 ± 575 | [−593, −498] | −565 ± 585 | [−645, −484] | 0.399 | 0.9 [0.8, 1.1] | ||
| mThickening | 258 ± 238 | [238, 277] | 236 ± 216 | [206, 265] | 0.462 | 0.9 [0.8, 1.1] | ||
| mJSW | −272 ± 618 | [−323, −221] | −0.44 | −243 ± 497 | [−312, −175] | −0.49 | 0.234 | 0.9 [0.8, 1.1] |
| Concurrent change (Y2→Y4) | ||||||||
| MFTC | −78 ± 209 | [−96, −60] | −0.37 | −188 ± 294 | [−231, −146] | −0.64 | <0.001 | 1.6 [1.3, 1.9] |
| mOV1 | −202 ± 203 | [−220, −185] | −312 ± 299 | [−355, −270] | <0.001 | 1.6 [1.3, 1.9] | ||
| mOV8 | 110 ± 88 | [102, 118] | 93 ± 91 | [80, 106] | 0.018 | 0.8 [0.7, 1.0] | ||
| mThinning | −550 ± 676 | [−609, −492] | −909 ± 987 | [−1051, −768] | <0.001 | 1.6 [1.3, 1.9] | ||
| mThickening | 256 ± 242 | [235, 277] | 200 ± 215 | [169, 231] | 0.011 | 0.8 [0.6, 0.9] | ||
| mJSW | −300 ±620 | [−353, −246] | −0.50 | −626 ± 853 | [−748, −503] | −0.74 | <0.001 | 1.5 [1.3, 1.8] |
|
| ||||||||
| Radiographic progression: | ||||||||
| Preceding change (BL→Y2): | ||||||||
| MFTC | −65 ± 172 | [−79, −51] | −0.38 | −136 ± 228 | [−171, −101] | −0.60 | <0.001 | 1.3 [1.1, 1.6] |
| mOV1 | −190 ± 169 | [−204, −177] | −259 ± 236 | [−296, −223] | <0.001 | 1.3 [1.1, 1.5] | ||
| mOV8 | 115 ± 96 | [107, 123] | 98 ± 90 | [85, 112] | 0.136 | 0.9 [0.7, 1.0] | ||
| mThinning | −507 ± 520 | [−548, −465] | −713 ± 733 | [−825, −601] | 0.002 | 1.3 [1.1, 1.5] | ||
| mThickening | 260 ± 236 | [242, 279] | 220 ± 216 | [187, 253] | 0.166 | 0.9 [0.7, 1.1] | ||
| Concurrent change (Y2→Y4): | ||||||||
| MFTC | −52 ± 165 | [−66, −38] | −0.31 | −307 ± 339 | [−360, −253] | −0.90 | <0.001 | 3.0 [2.4, 3.9] |
| mOV1 | −172 ± 147 | [−184, −160] | −444 ± 351 | [−499, −388] | <0.001 | 3.3 [2.5, 4.2] | ||
| mOV8 | 109 ± 85 | [102, 116] | 93 ± 101 | [77, 109] | 0.093 | 0.9 [0.7, 1.0] | ||
| mThinning | −457 ± 502 | [−499, −415] | −1321 ± 1165 | [−1506, −1136] | <0.001 | 3.1 [2.4, 3.9] | ||
| mThickening | 257 ± 243 | [237, 278] | 182 ± 202 | [150, 215] | 0.011 | 0.7 [0.6, 0.9] | ||
Symptomatic progression was defined as increase of ≥9 points on the Western Ontario and McMaster Universities (WOMAC) pain index (scale: 0 … 100) between the year 2 (Y2) and the year 4 (Y4) follow-up visit; Radiographic progression was defined as a decrease of ≥0.7mm in minimum radiographic joint space width in the medial compartment (mJSW) between the Y2 and the Y4 follow-up visit; the number of knees with/without symptomatic or radiographic progression is shown in Table 1; BL: Baseline; SD: Standard deviation of the change; 95% CI: 95% confidence intervals of the change; P-Value: Computed using analysis of covariance with adjustment for sex, age, BMI, WOMAC pain, medial joint space narrowing at the respective reference visit (BL or Y2); OR: Odds ratio without adjustment or with adjustment for sex, age, BMI, WOMAC pain, medial joint space narrowing at the respective reference visit (BL or Y2); MFTC: Medial femorotibial compartment; mOV1/8: Medial compartment ordered value 1/8; mThinning/mThickening: Thinning/Thickening sum score of subregional changes in the medial compartment. Change in mJSW was only provided for the comparison between knees without and knees with symptomatic progression. Please note that the concurrent change in mJSW deviates slightly from the change shown in table 1, because the concurrent change was computed for the knees that also had MRI cartilage thickness measurements at Y4.
In the period preceding the radiographic progression (BL→Y2), a significantly greater loss in cMFTC cartilage thickness was observed in knees with radiographic progression than in knees without radiographic progression (−229±355 [−284, −175] μm vs. −130±270 [−151, −108] μm, p=0.002, OR=1.3 [1.1, 1.5], Figure 1). Exploratory analyses also revealed significant differences between knees with and without radiographic progression in the MFTC, the mOV1, and the mThinning score (Table 2). In the period concurrent with the radiographic progression (Y2→Y4), knees with progression were found to display a significantly greater concurrent loss in cMFTC cartilage thickness than knees without progression (− 542±550 [−629, −455] μm vs. −98±255 [−119, −76] μm, p<0.001, OR=3.4 [2.6, 4.3], Figure 1). Significant differences between knees with and without progression were also observed for most of the exploratory measures (Table 2).
Sensitivity analysis
Sensitivity analyses of the changes in knees without any progression, with isolated symptomatic progression, with isolated radiographic progression, and in knees with both symptomatic and radiographic progression (Supplemental Table 1) showed significantly greater loss in cMFTC cartilage thickness in knees with subsequent isolated radiographic progression as compared to knees without any progression (− 268±367μm vs. −125±275 μm, p<0.001, OR=1.5 [1.2, 1.9], Table 3). The cMFTC change in knees with subsequent isolated symptomatic progression and in knees with combined symptomatic and radiographic progression did not differ significantly from the cMFTC change observed in knees without any progression (Table 3). During the period concurrent with progression, the difference in cMFTC change between knees with symptomatic progression vs. knees without any progression (−145±254 μm vs. −86±244 μm, p=0.03, OR=1.4 [1.0, 1.9]) failed to reach the adjusted significance level. Knees with isolated radiographic progression (−456±521 μm, p<0.001, OR=3.1 [2.3, 4.3]) and knees with combined symptomatic and radiographic progression (−687±571 μm, p<0.001, OR=5.3 [3.5, 8.1]) showed a significantly greater cMFTC loss than knees without any progression (Table 3).
Table 3.
Sensitivity analysis of preceding and concurrent change (in μm) in cartilage thickness and minimum radiographic joint space width (mJSW) in knees without any progression vs. knees with isolated symptomatic progression, with isolated radiographic progression, and with both symptomatic and radiographic progression
| No progression | Isolated symptomatic progression | Isolated radiographic progression | Combined symptomatic & radiographic progression | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD [95% CI] |
Mean ± SD [95% CI] |
OR [95% CI] | P-Value | Mean ± SD [95% CI] |
OR [95% CI] | P-Value | Mean ± SD [95% CI] |
OR [95% CI] | P-Value | |
| Preceding change (BL→Y2): | ||||||||||
| cMFTC | −125 ± 275 [−150, −100] |
−145 ± 254 [−187, −104] |
1.0 [0.8, 1.3] | 0.823 | −268 ± 367 [−338, −197] |
1.5 [1.2, 1.9] | <0.001 | −161 ± 327 [−245, −77] |
0.9 [0.7, 1.3] | 0.682 |
| MFTC | −61 ± 173 [−77, −45] |
−79 ± 170 [−107, −51] |
1.1 [0.9, 1.3] | 0.632 | −151 ± 232 [−196, −107] |
1.5 [1.2, 1.8] | <0.001 | −108 ± 220 [−165, −51] |
1.1 [0.8, 1.4] | 0.601 |
| mOV 1 | −189 ± 171 [−204, −173] |
−196 ± 162 [−223, −170] |
1.0 [0.8, 1.2] | 0.842 |
−274 ± 254 [−323, −225] |
1.4 [1.2, 1.7] | <0.001 | −233 ± 201 [−285, −181] |
1.1 [0.8, 1.4] | 0.611 |
| mOV 16 | 119 ± 101 [110, 129] |
102 ± 77 [89, 114] |
0.8 [0.7, 1.0] | 0.070 | 92 ± 84 [76, 108] |
0.8 [0.6, 1.0] | 0.028 | 110 ± 99 [84, 135] |
1.0 [0.8, 1.3] | 0.962 |
| mThinning | −501 ± 516 [−548, −454] |
−525 ± 532 [−612, −438] |
1.0 [0.8, 1.2] | 0.861 | −743 ± 756 [−888, −597] |
1.4 [1.1, 1.7] | <0.001 | −660 ± 694 [−840, −481] |
1.1 [0.8, 1.4] | 0.505 |
| mThickening | 271 ± 245 [249, 294] |
225 ± 203 [192, 258] |
0.8 [0.7, 1.0] | 0.068 | 197 ± 195 [160, 235] |
0.7 [0.6, 0.9] | 0.012 | 261 ± 246 [198, 324] |
1.1 [0.8, 1.4] | 0.688 |
| mJSW | −265 ± 603 [−320, −211] |
−252 ± 500 [−334, −170] |
0.9 [0.8, 1.1] | 0.497 | — | — | ||||
|
| ||||||||||
| Concurrent change (Y2→Y4): | ||||||||||
| cMFTC | −86 ± 244 [−109, −62] |
−136 ± 287 [−186, −87] |
1.4 [1.0, 1.9] | 0.031 | −456 ± 521 [−560, −351] |
3.1 [2.3, 4.3] | <0.001 | −687 ± 571 [−837, −537] |
5.3 [3.5, 8.1] | <0.001 |
| MFTC | −41 ± 157 [−56, −26] |
−85 ± 184 [−117, −54] |
1.5 [1.2, 2.1] | 0.003 | −238 ± 308 [−300, −176] |
2.7 [1.9, 3.6] | <0.001 | −421 ± 360 [−516, −327] |
6.3 [3.8, 10.2] | <0.001 |
| mOV 1 | −161 ± 134 [−174, −148] |
−208 ± 177 [−239, −178] |
1.7 [1.3, 2.4] | <0.001 | −381 ± 321 [−446, −317] |
3.2 [2.3, 4.4] | <0.001 | −548 ± 377 [−647, −449] |
6.4 [4.0, 10.2] | <0.001 |
| mOV 16 | 111 ± 87 [103, 120] |
101 ± 80 [87, 115] |
0.9 [0.7, 1.1] | 0.186 | 104 ± 95 [85, 123] |
1.0 [0.8, 1.2] | 0.718 | 75 ± 109 [46, 104] |
0.6 [0.4, 0.9] | 0.002 |
| mThinning | −425 ± 478 [−471, −379] |
−560 ± 560 [−657, −463] |
1.6 [1.2, 2.2] | 0.005 | −1096 ± 1047 [−1307, −885] |
2.8 [2.0, 3.9] | <0.001 | −1698 ± 1260 [−2029, −1366] |
5.4 [3.5, 8.4] | <0.001 |
| mThickening | 264 ± 246 [241, 288] |
234 ± 232 [194, 274] |
0.9 [0.7, 1.1] | 0.192 | 217 ± 223 [172, 262] |
0.9 [0.7, 1.1] | 0.298 | 124 ± 146 [86, 163] |
0.3 [0.2, 0.5] | <0.001 |
| mJSW | −88 ± 389 [−125, −51] |
−212 ± 328 [−268, −155] |
2.1 [1.4, 3.3] | 0.001 | — | — | ||||
Symptomatic progression was defined as increase of ≥9 points on the Western Ontario and McMaster Universities (WOMAC) pain index (scale: 0 … 100) between the year 2 (Y2) and the year 4 (Y4) follow-up visit; Radiographic progression was defined as a decrease of ≥0.7mm in minimum radiographic joint space width in the medial compartment (mJSW) between the Y2 and the Y4 follow-up visit; the number of knees without progression, with isolated symptomatic, with isolated radiographic, and with both symptomatic and radiographic progression is shown in Supplemental Table 1 BL: Baseline; SD: Standard deviation of the change; 95% CI: 95% confidence intervals of the change; P-Value: Computed using analysis of covariance with adjustment for sex, age, BMI, WOMAC pain, medial joint space narrowing at the respective reference visit (BL or Y2); OR: Odds ratio without adjustment or with adjustment for sex, age, BMI, WOMAC pain, medial joint space narrowing at the respective reference visit (BL or Y2); MFTC: Medial femorotibial compartment; cMFTC: central MFTC; mOV1/8: Medial compartment ordered value 1/8; mThinning/mThickening: Thinning/Thickening sum score of subregional changes in the medial compartment. Change in mJSW was only provided for the comparison between knees without any progression and knees with isolated symptomatic progression. Please note that the concurrent change in mJSW deviates slightly from the change shown in table 1, because the concurrent change was computed for the knees that also had MRI cartilage thickness measurements at Y4.
Discussion
The current study is the first to dissect the predictive vs. concurrent validity of MRI cartilage thickness loss versus symptomatic and radiographic (structural) progression of knee OA. The results revealed a significant association of cMFTC cartilage thickness loss with both concurrent symptomatic (WOMAC pain) and concurrent radiographic (mJSW) progression. More importantly, cMFTC cartilage thickness loss was found to be predictive of subsequent radiographic progression, whereas no significant association could be demonstrated with subsequent symptomatic progression. Exploratory analyses showed that the association between concurrent cMFTC cartilage thickness loss and OA progression was strongest for knees with both symptomatic and radiographic progression.
As part of the OAI FNIH biomarker consortium project, we previously reported that two-year cMFTC cartilage thickness loss was significantly associated with combined radiographic and symptomatic progression8. The current study significantly extends these findings, in that the study design clearly separated concurrent and predictive observation periods for MRI cartilage thickness, with the predictive observation period (BL→Y2) not overlapping with the period during which progression was detected (Y2→Y4).
A potential limitation of the current study is that the analyses were based on MRI readings available from 3 different studies5,8,15. To ensure that the participants included were suitable for the analysis, a strict set of eligibility criteria was applied that was very similar to the original FNIH biomarker consortium study8. Hence the current analyses were restricted to knees with predominantly mild to moderate (KLG 1–3) medial tibiofemoral knee OA and excluded knees with fixed-flexion radiography positioning errors. All analyses were adjusted for WOMAC pain and medial JSN grade at the respective reference visit to account for potential differences between the strata studied. A further limitation of the current study is that the Y4 visit cartilage thickness measurements were missing in about 9% of the knees, albeit these participants had mJSW and WOMAC pain assessments at Y4 for detecting the presence or absence of progression; these were thus included in the analysis of predictive validity (BL→Y2). Please also note that the ORs reported in this study were reported as measures of effect size and should not be interpreted as relative risk, because parts of the sample (FNIH participants) were selected based on the presence or absence of the outcome (progression), and because the relatively large number of progressor knees would most likely cause the ORs to overestimate the relative risk. Besides the primary analytic focus, i.e. the longitudinal change in the cMFTC, the study included other exploratory structural measures in order to facilitate a comparison between these measures as potential biomarkers. The adjustment for multiple statistical comparisons, however, only took the four primary analyses into account (preceding and concurrent change in cMFTC cartilage thickness in knees with vs. knees without symptomatic / radiographic progression). Further, the significance level for the primary analyses was also not adjusted for multiple comparisons between changes in knees without any progression versus that in knees with isolated symptomatic, isolated radiographic, or combined symptomatic and radiographic progression, as these were regarded as sensitivity analyses. The statistical significance reported for the four primary analyses must thus be interpreted in light of the adjustment made for 4 parallel primary analyses vs. other exploratory and sensitivity analyses, for which no adjustment was made.
Given the focus on knees with medial femorotibial OA, lateral compartment measures were not included in the current study. For the same reason, location-independent measures were computed from cartilage thickness changes in medial compartment subregions only8. The strength of the observed associations was similar for medial compartment location-independent measures of cartilage thickness loss (mOV1 and mThinning) and for location-based measures, although a recent study has suggested that location-independent measures may be more sensitive and informative in demonstrating DMOAD efficacy23. Hence, it is useful to ascertain whether location-independent measures of MRI-based cartilage loss demonstrate similar predictive and concurrent validity of symptomatic and radiographic progression to more traditional measures of region-specific cartilage loss. The results obtained for change in mJSW were similar to those observed for cMFTC and other exploratory measures. The association for concurrent change with isolated symptomatic progression was, however, even somewhat stronger for mJSW than for cMFTC.
The two main comparisons used different stratifications (±symptomatic progression, ±radiographic progression) of the same cohort to study their predictive and concurrent association with MRI-based cartilage thickness loss. Additional analyses explored the association between preceding and concurrent cartilage loss in knees with both symptomatic and radiographic progression, in those with isolated symptomatic progression, in those with isolated radiographic progression, vs. knees without any progression. These sensitivity analyses suggest that when actively ruling out radiographic progression, isolated symptomatic progression is not significantly associated with concurrent cMFTC cartilage thickness change. Isolated symptomatic progression, however, appeared to be associated with concurrent change in exploratory measures such as MFTC, mOV1, mThinning and radiographic mJSW. Interestingly, the combination of both symptomatic progression and radiographic progression displayed a notably stronger association with concurrent cartilage thickness loss than radiographic progression alone. Knees with both symptomatic and structural worsening are of particular clinical interest because they are more likely to approach end-stage disease in the foreseeable future and, thus, display a greater likelihood of undergoing knee replacement surgery5,24.
The association between radiographic progression and cartilage thickness change was found to be stronger for the concurrent than for the predictive period. Previous studies have reported that change in MRI-based cartilage measures are significantly correlated with concurrent change in radiographic JSW3,25. Still, an important finding from the current study is that cartilage thickness change is not only associated with concurrent but also with subsequent radiographic progression. These findings suggest that longitudinal cartilage morphometry by MRI has predictive validity for subsequent structural disease progression. Modestly greater cartilage thickness loss was observed concurrent with but not prior to symptomatic progression.
In conclusion, medial compartment cartilage thickness loss, as measured by MRI, was found to be associated with both concurrent and subsequent radiographic progression and with concurrent symptomatic progression. Interestingly, the sensitivity analyses indicated that MRI-based cartilage thickness loss was more strongly associated with concurrent combined symptomatic and radiographic progression than with concurrent isolated radiographic or isolated symptomatic progression. These results demonstrate concurrent and predictive validity of MRI cartilage thickness loss and, hence, provide an important step in qualifying cartilage thickness loss as a biomarker of clinically relevant progression of knee OA.
Supplementary Material
Acknowledgments
Financial support
The authors thank the following operators at Chondrometrics GmbH: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Jana Matthes, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr for the segmentation of the MRI data. We thank Susanne Maschek for quality control readings of the segmentations. Further, the authors would like to thank the readers of the fixed flexion radiographs at Boston University for the central KL grading, the OAI investigators, clinic staff and OAI participants at each of the OAI clinical centers and the team at the OAI coordinating center for their contributions in acquiring the publicly available clinical and imaging data.
This work is based on data from the Osteoarthritis Initiative (OAI): The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health. Funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation; GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the Consortium and OAI is managed by the FNIH.
The image analysis in this study was partly funded by the FNIH OA Biomarkers Consortium, with grants, direct and in-kind contributions, provided by: AbbVie; Amgen Inc.; Arthritis Foundation; Bioiberica S.A.; DePuy Mitek, Inc.; Flexion Therapeutics, Inc.; GlaxoSmithKline; Merck KGaA; Rottapharm | Madaus; Sanofi; and Stryker. Other parts of funding were provided by a direct grant from Merck KGaA, by a contract with the University of Pittsburgh (Pivotal OAI MRI Analyses [POMA]: NIH/NHLBI Contract No. HHSN2682010000 21C), by a vendor contract from the OAI coordinating center at University of California, San Francisco (N01-AR-2-2258), and by an ancillary study to the OAI held by the Division of Rheumatology, Feinberg School of Medicine, Northwestern University (R01 AR52918).
ROLE OF THE STUDY SPONSOR
The statistical analysis and writing of this article was independent from and not contingent upon approval from the study sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
W. Wirth is a part time employee and co-owner of Chondrometrics GmbH.
D.J. Hunter is a consultant for Flexion and Merck KGaA.
C.K. Kwoh has received research support from Merck Serono and Abbvie.
C. Ladel is an employee of Merck KGaA.
F. Eckstein is CEO/CMO and co-owner of Chondrometrics GmbH, which has received funding from the FNIH OA Biomarker Consortium for the quantitative analysis of cartilage data in this study. He has received consulting fees from Merck KGaA as well as honoraria from Medtronic (less than $10,000 each). M. C. Nevitt and L. Sharma have no conflicts of interest.
- Study conception and design: WW, DH, FE
- Acquisition of data: WW, MN, CK, LS, FE
- Analysis & interpretation of data: All authors
- Writing of first manuscript draft: WW and FE
- Critical manuscript revision and approval of final manuscript: All authors
Wolfgang Wirth had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinicaltrials.gov identification: NCT00080171
REFERENCE LIST
- 1.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154(4):217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Wirth W, Duryea J, Hellio Le Graverand M-PP, John MR, Nevitt M, Buck RJ, et al. Direct comparison of fixed flexion, radiography and MRI in knee osteoarthritis: responsiveness data from the Osteoarthritis Initiative. Osteoarthr Cartil. 2013;21(1):117–125. doi: 10.1016/j.joca.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein F, Kwoh CK, Boudreau R, Wang Z, Hannon M, Cotofana S, et al. Quantitative magnetic resonance imaging measures of cartilage predict knee replacement – a case-control study from the Osteoarthritis Initiative. Ann Rheum Dis. 2013;(72):707–714. doi: 10.1136/annrheumdis-2011-201164. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein F, Boudreau RM, Wang Z, Hannon MJ, Wirth W, Cotofana S, et al. Trajectory of cartilage loss within 4 years of knee replacement – a nested case-control study from the Osteoarthritis Initiative. Osteoarthr Cartil. 2014;22(10):1542–1549. doi: 10.1016/j.joca.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63(8):2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. 2014;28(1):61–71. doi: 10.1016/j.berh.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus V, Katz JN, et al. Cartilage thickness change as an imaging biomarker of knee osteoarthritis progression – data from the fnih OA biomarkers consortium. Arthritis Rheumatol (Hoboken, NJ) 2015;67(12):3184–3189. doi: 10.1002/art.39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semi-Quantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data from the FNIH OA Biomarkers Consortium. Arthritis Rheumatol (Hoboken, NJ) 2016;68(10):2422–2431. doi: 10.1002/art.39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter D, Nevitt M, Lynch J, Kraus VB, Katz JN, Collins JE, et al. Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2016;75(9):1607–1614. doi: 10.1136/annrheumdis-2015-207602. [DOI] [PubMed] [Google Scholar]
- 11.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis. 2017;76(1):186–195. doi: 10.1136/annrheumdis-2016-209252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer LF, Sury M, Yin M, Jamieson S, Smith SE, Lynch J, et al. Quantitative measurement of medial femoral knee cartilage volume – analysis of the OA Biomarkers Consortium FNIH Study cohort. Osteoarthr Cartil. 2017;25(7):1107–1113. doi: 10.1016/j.joca.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort – Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17(1):466. doi: 10.1186/s12891-016-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8:622–630. doi: 10.1038/nrrheum.2012.113. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckstein F, Mc Culloch CE, Lynch JA, Nevitt M, Kwoh CK, Maschek S, et al. How do short-term rates of femorotibial cartilage change compare to long-term changes? Four year follow-up data from the osteoarthritis initiative. Osteoarthr Cartil. 2012;20(11):1250–1257. doi: 10.1016/j.joca.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranstam J. Repeated measurements, bilateral observations and pseudoreplicates, why does it matter? Osteoarthr Cartil. 2012;20(6):473–475. doi: 10.1016/j.joca.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein F, Buck R, Wirth W. Location-independent analysis of structural progression of osteoarthritis – taking it all apart, and putting the puzzle back together makes the difference. Semin Arthritis Rheum. 2017;46(4):404–410. doi: 10.1016/j.semarthrit.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61(7):917–924. doi: 10.1002/art.24613. [DOI] [PubMed] [Google Scholar]
- 20.Buck RJ, Wyman BT, Hellio Le Graverand MP, Hunter D, Vignon E, Wirth W, et al. Using ordered values of subregional cartilage thickness change increases sensitivity in detecting risk factors for osteoarthritis progression. Osteoarthr Cartil. 2011;19(3):302–308. doi: 10.1016/j.joca.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Wirth W, Buck R, Nevitt M, Le Graverand MPH, Benichou O, Dreher D, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography–data from the OA initiative. Osteoarthr Cartil. 2011;19(6):689–699. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of Change and Sensitivity to Change in Cartilage Morphology in Healthy Knees and in Knees With Mild, Moderate, and End-Stage Radiographic Osteoarthritis : Results From 831 Participants From the Osteoarthritis Initiative. Arthritis Care Res(Hoboken) 2011;63(3):311–319. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckstein F, Wirth W, Guermazi A, Maschek S, Aydemir A. Intra-articular sprifermin not only increases cartilage thickness, but also reduces cartilage loss – location-independent post hoc analysis using MR imaging. Arthritis Rheumatol. 2015;67(11):2916–2922. doi: 10.1002/art.39265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddle DL, Jiranek WA. Knee osteoarthritis radiographic progression and associations with pain and function prior to knee arthroplasty: a multicenter comparative cohort study. Osteoarthr Cartil. 2015;23(3):391–396. doi: 10.1016/j.joca.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res(Hoboken) 2010;62(7):932–937. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


