Abstract
Because cochlear implants function by stimulating the auditory nerve, it is assumed that the condition of the nerve plays an important role in the efficacy of the prosthesis. Thus, considerable research has been devoted to methods of preserving the nerve following deafness. Neurotrophins have been identified as a potential contributor to neural health, but most of the research to date has been done in young animals and for short periods (less than 3 to 6 months) after the onset of treatment. The first objective of the current experiment was to examine the effects of a neurotrophin gene therapy delivery method on spiral ganglion neuron (SGN) preservation and function in the long term (5 to 14 months) in mature guinea pigs with cochlear implants. The second objective was to examine several potential non-invasive monitors of auditory nerve health following the neurotrophin gene therapy procedure. Eighteen mature adult male guinea pigs were deafened by cochlear perfusion of neomycin and then one ear was inoculated with an adeno-associated viral vector with an Nft3-gene insert (AAV.Ntf3) and implanted with a cochlear implant electrode array. Five control animals were deafened and inoculated with an empty AAV and implanted. Data from 43 other guinea pig ears from this and previous experiments were used for comparison: 24 animals implanted in a hearing ear, nine animals deafened and implanted with no inoculation, and ten normal-hearing non-implanted ears. After 4 to 21 months of psychophysical and electrophysiological testing, the animals were prepared for histological examination of SGN densities and inner hair cell (IHC) survival. Seventy-eight percent of the ears deafened and inoculated with AAV.Ntf3 showed better SGN survival than the 14 deafened-control ears. The degree of SGN preservation following the gene therapy procedure was variable across animals and across cochlear turns. Slopes of psychophysical multipulse integration (MPI) functions were predictive of SGN density, but only in animals with preserved IHCs. MPI was not affected by the AAV.Ntf3 treatment, but there was a minor improvement in temporal integration (TI). AAV.Ntf3 treatment had significant effects on ECAP and EABR amplitude growth func-tion (AGF) slopes; the reduction in slope in deafened ears was ameliorated by the AAV.Ntf3 treatment. Slopes of the ECAP and EABR AGFs were predictive of SGN density in a broad area near and just apical to the implant. The highest ensemble spontaneous activity (ESA) values were seen in animals with surviving IHCs, but AAV.Ntf3 treatment in deafened ears resulted in slightly higher ESA values compared to deafened untreated ears. Overall, a combination of the psychophysical and electrophysiological measures can be useful for monitoring the health of the implanted cochlea in guinea pigs. These measures should be applicable for assessing cochlear health in human subjects.
Electronic supplementary material
The online version of this article (doi:10.1007/s10162-017-0633-9) contains supplementary material, which is available to authorized users.
Keywords: auditory prosthesis, neurotrophic factor, guinea pig, electrically evoked compound action potential, temporal integration
INTRODUCTION
Cochlear implant auditory prostheses improve hearing in deaf or severely hearing-impaired ears by delivering information about the acoustic environment through electrical stimulation of the auditory nerve. It is widely assumed that the condition of the auditory nerve plays a significant role in determining the quality of information transferred from the cochlear implant to the brain, although the details of the relationship between nerve status and implant function are poorly understood. Degeneration of the auditory nerve is one component thought to influence cochlear implant function. A number of studies are testing approaches to retard or prevent this degeneration and restore the nerve toward its normal state. These studies provide the opportunity to better understand the relationship between nerve status and various measures of cochlear implant function.
Previous studies have shown benefits of neurotrophin (NT) delivery for survival of auditory nerve spiral ganglion cells in deaf ears (e.g., Miller et al. 1997; Rejali et al. 2007; Wise et al. 2010; Leake et al. 2011; Atkinson et al., 2012, 2014; Budenz et al. 2015). However, these studies have mostly been done in relatively young animals and survival times after NT administration have usually been short; generally less than 3 to 6 months. Gene therapy utilizing adenoviruses has been used for NT delivery in some cases, but the duration of active secretion of the NTs with adenoviruses is limited and there are risks of immune response (Kita et al., 2004; Heilbronn and Weger, 2010; Warnock et al., 2011). Gene therapy using adeno-associated viral vectors (AAV) with various gene inserts is known from work in other systems to be safer and to have longer-lasting effects than other drug delivery systems (Lalwani et al. 1998; Dudus et al. 1999; Guy et al. 1999; Bankiewicz et al. 2006). In the study reported here, we examined the long-term effects of inoculation with the NT gene Ntf3 inserted in an AAV carrier (designated AAV.Ntf3) on auditory nerve survival and on cochlear implant function in deafened, mature guinea pigs.
Many human cochlear implant recipients have no measureable acoustic hearing and thus probably have few if any surviving inner hair cells (IHCs). However, SGN survival in deaf humans (Hinojosa and Marion 1983; Nadol et al. 2001; Fayad and Linthicum 2006) is often much better than that in the neomycin-deafened guinea pigs. The current study aimed to obtain a larger range of SGN survival in the deafened guinea pig by inoculating with AAV.Ntf3 following neomycin administration in an effort to partially protect the SGN population from the destructive results of the deafening procedure. This allowed an assessment of the functional effects of SGN survival in the absence of IHCs.
In previous reports (Kang et al. 2010; Pfingst et al. 2011; Zhou et al. 2015), we examined the effects of spiral ganglion neuron (SGN) density and IHC survival in the implanted ear on two psychophysical functions: multipulse integration (MPI) and temporal integration (TI) (described below). These studies found that good integration of pulses occurred only in animals with high SGN densities and good IHC survival. These data are reported in the current paper in more detail and with a larger number of subjects. Two electrophysiological measures are also reported in this paper: electrically evoked compound action potentials (ECAPs) recorded from the implanted electrode array and electrically evoked auditory brainstem responses (EABRs) recorded from electrodes on the surface of the head. We found that growth functions for these measures better reflect SGN densities in the lower to middle ranges (between about 4 and 49 % of normal) and in the absence of IHCs than did the psychophysical measures. Finally we report on ensemble spontaneous activity (ESA) recorded from the implanted electrodes and analyzed as previously reported by Dolan et al. (1990). The relation of this measure to SGN density and IHC survival is examined.
In summary, the objectives of the current study were (1) to assess the long-term anatomical and functional effects of AAV.Ntf3 treatment after deafening, testing the hypothesis that this gene therapy procedure facilitates long-term preservation of functional SGNs and (2) to assess several non-invasive clinically-applicable measures of implant function in animals with varying degrees of SGN survival, testing the hypothesis that these measures are predictive of the density of surviving SGNs.
METHODS
Overview
The animals used in these experiments were specific pathogen-free (SPF) pigmented guinea pigs bred and maintained by the Unit for Laboratory Animal Medicine at the University of Michigan. The animal use protocol was reviewed and approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC).
Results reported in this paper are based on data obtained from 66 ears of mature male guinea pigs. Ten ears that received no treatment or implantation were used to estimate normal histological values. Fifty six animals were used to assess effects of various deafening, gene therapy, and/or implantation procedures. These animals were first adapted to the testing environment and then trained in tasks used to measure psychophysical detection thresholds for acoustic stimuli. They were then placed in one of the four experimental, control, or comparison groups and treated as detailed below. All groups are summarized in Table 1.
TABLE 1.
Summary of guinea pig groups
| Group designation | Treatments | Number of ears |
|---|---|---|
| Normal | None | 10 |
| AAV.Ntf3—experimental | Neomycin (5 %), AAV.Ntf3, implant Implanted 30 min after inoculation Implanted 2 weeks after inoculation |
9 9 |
| AAV.empty—control | Neomycin (5 %), AAV.empty, implant Implanted 30 min after inoculation Implanted 2 weeks after inoculation |
4 1 |
| Implanted-hearing—comparison | Implant inserted into a hearing ear | 24 |
| Neomycin alone—comparison | Neomycin (5 % or 10 %), implant 5 % w/v neomycin 10 % w/v neomycin |
1 8 |
The primary experimental group (n = 18) received cochlear injection in the treatment ear with 5 % neomycin followed by cochlear inoculation with an adeno-associated virus with a NT gene insert (AAV.Ntf3). This vector is an AAV2 that transduces cells in the membranous labyrinth, both in the area of the deafened auditory epithelium and in other areas including the lateral wall marginal cells (Supplemental Fig. S1). The ear then received a cochlear implant. The implant was inserted 30 min after the inoculation in nine cases and delayed by 2 weeks in the other nine cases. The primary control group (n = 5) was treated in a similar manner except that the adeno-associated virus contained no gene insert (AAV.empty). Four of the control animals were implanted 30 min after inoculation and one was implanted 2 weeks after inoculation. For these two primary groups, the mean age and weight at the time of implantation were 8 months and 1060 g, respectively. After inoculation, testing times ranged from 5 to 14 months (mean of 8 months) before sacrifice.
There were two implanted comparison groups. One of the groups received a cochlear implant in a hearing ear (n = 24) and one group received an implant in an ear injected with neomycin but received no AAV inoculation (n = 9). Animals for these two comparison groups were implanted anywhere from 4 to 21 months (mean of 13.6 months) before sacrifice (implanted-hearing group = 5 to 21 months, mean of 15 months; neomycin alone group = 4 to 16 months, mean of 10.4 months). Some of the data for the comparison groups were drawn from previous studies (Kang et al. 2010; Pfingst et al. 2011).
Animals in all of the groups that received cochlear implants in the treatment ear received neomycin injections in the contralateral cochlea in order to deafen that ear and facilitate testing for any residual hearing in the treatment ear under free-field conditions. For the majority of the animals, the contralateral deafening procedure was performed around 4 months (range 0 to 9.1 months) before procedures began in the treatment ears. One animal was not deafened in the contralateral ear before being deafened in the test ear; this non-deafened contralateral ear was used for a normal untreated ear.
Following implantation, psychophysical and electrophysiological data were followed over time until stable results (criteria described below) were obtained. This is an important step because both psychophysical and electrophysiological measures of implant function typically reveal a loss of sensitivity to electrical stimulation over the first 2 weeks after implantation followed by a gradual recovery to relatively stable levels and then they typically remain stable for many months (Su et al. 2008; Pfingst et al. 2015). After the psychophysical and electrophysiological measures reached stable levels, the primary data sets were collected. Data for all of the functional measures were obtained for the entire primary experimental and control animals. For the two comparison groups, MPI data and ESA data were obtained from all 32 animals, but other measures were obtained for only a subset of these animals depending on the measure used.
The implanted animals were tested daily (usually 4 to 5 days/week, approximately 2 h/day) to assess psychophysical detection thresholds for electrical stimulation. Also, detection thresholds for acoustic stimulation were assessed periodically in animals that had preserved hearing. Thresholds were monitored over time until stable and then psychophysical MPI and TI data were obtained. ECAP, EABR, and ESA were also recorded periodically as detailed below. After all testing was completed, the animals were euthanized and the cochleae were harvested for histological analysis as detailed below.
Deafening, Inoculation, and Implantation Procedures
Animals in the primary experimental and control groups were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg) and placed on a heating pad. A post-auricular incision was made, the temporal bone was exposed by blunt dissection, and the bulla was opened. A small cochleostomy was made in the basal turn of the cochlea approximately 0.7 mm apical to the round window using a hand drill. Through this cochleostomy, neomycin sulfate (10 μl; 5 % w/v) was slowly injected into the scala tympani (rate of 5 μl/min) using a cannula and an infusion pump. This was intended to destroy hair cells and deafen the ear. The infusion cannula was left in place for 20 min and then removed. A drop of HEALON (hyaluronic acid; 10 mg/mL; Abbott Medical Optics, Inc., Santa Ana, CA) was placed on the cochleostomy to promote penetration of administered substances through membranes, and then 5 μl of either AAV.Ntf3 or AAV.empty were infused into the scala tympani at a rate of 1 μl/min using another cannula and the infusion pump. The AAV used was AAV2 with a CBA promoter driving expression of the Ntf3 transgene. The concentration was 1 × 1012 pfu/ml. After the AAV inoculation, the cannula was removed and the cochleostomy was either temporarily covered with a piece of muscle and left alone for 30 min or the cochleostomy and the bulla were sealed with Durelon and left for 2 weeks before implantation. Thirteen animals (nine experimental and four control) were deafened, inoculated, and then implanted 30 min after AAV inoculation, and 10 animals (nine experimental and one control) were deafened and inoculated and then implanted 2 weeks later in a second surgery. The use of the 2-week delay between inoculation and implantation was based on a concern that placing a cochlear implant in the cochlea shortly after inoculation might reduce the effectiveness of the inoculation.
Anchor screws were placed at three points around bregma and used to secure a small inverted bolt (specified as the “anchor bolt”) on which the connector that interfaced with the stimulator would be mounted. Additional screws were placed for electrophysiology measurements, one for ECAP grounding placed at the midline, 1 cm caudal to bregma, (specified as the “vertex screw”) and two for EABR recordings, one 2 cm anterior to bregma, and one 1 cm from the midline and slightly behind bregma on the implant side.
For implant insertion, the muscle or Durelon was removed, the small hand drilled cochleostomy was enlarged with a diamond bur, bone dust and debris were removed from the area around the cochleostomy using a cotton pledget, and an eight-electrode scala tympani implant was inserted resulting in five to six intracochlear electrodes. The implant was secured to the bulla with a silk suture, the bulla opening was then sealed with Durelon cement and the incision was closed.
The two comparison groups of animals received no inoculation. The implantation procedure was the same for all groups. The deafening procedure for the neomycin-alone comparison group was similar to that described above; one animal received 5 % neomycin was infused with a cannula and pump and the remaining four animals received 10 % neomycin infused through the round window with a needle and syringe. In the neomycin-alone comparison group, the implant was inserted approximately 30 min after the neomycin injection.
Cochlear Implants
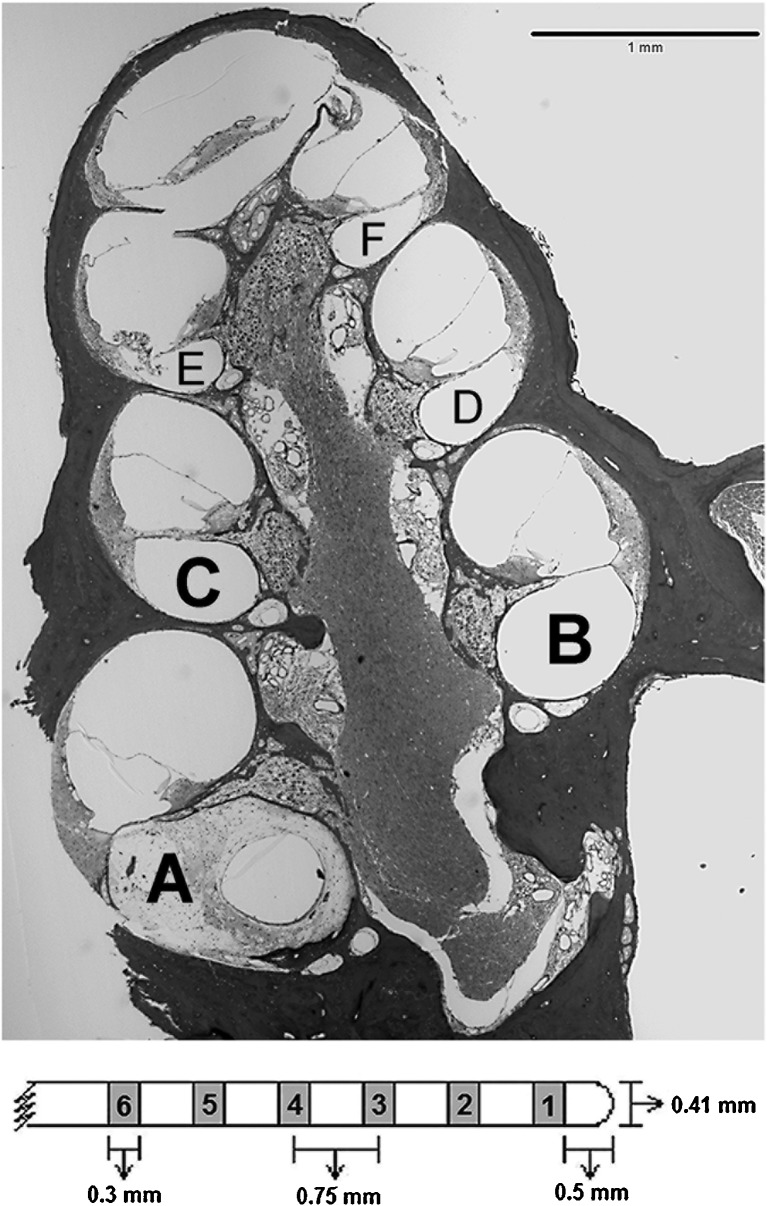
The cochlear implant electrode arrays (purchased from Cochlear Corporation, Englewood, CO) consisted of eight ring electrodes encircling a silicone rubber carrier and spaced at 0.75 mm center to center. The diameter of the implant near its apical end was 0.4 mm so that it filled the majority of the scala tympani at about 4.5 mm from the cochleostomy and could not be safely inserted further due to the rapidly decreasing diameter of the scala tympani apical to this point. The primary electrode used for stimulation in the current experiments was the second most apical electrode (electrode 2 in Fig. 1) which was located an average of 2.7 mm apical to the cochleostomy (approximately the 18-kHz region) and typically was close to the modiolar wall. Animals “a” and “m” had shallower implant insertions; in these cases the primary electrode was located around 1.7 and 2.0 mm apical to the cochleostomy, respectively. The top panel in Figure 1 shows a midmodiolar section of the guinea pig cochlea with the profiles used for data analysis labeled “A” through “F.” The implant was located in profile A.
FIG. 1.
A midmodiolar section of a guinea pig cochlea illustrating how profiles of Rosenthal’s canal were labeled for reporting histological results is shown in the top part of the figure. The profiles were labeled A through F from base to apex. The eight-electrode scala tympani implant is illustrated schematically in the lower part of the figure; only the 6 electrodes that could potentially be inserted into the cochlea are shown here; 7 and 8 were always extra-cochlear. The electrodes were numbered 1 through 8 from the apical to the basal end of the implant. Note that all six intracochlear electrodes were located in the first half of the basal turn (profile A). Electrode 2 was the default primary stimulating electrode for most of the animals tested in this experiment. If electrode 2 failed, the next closest functional electrode was used. Peri-midmodiolar sections of the cochleae analyzed in this study were centered at the location of the primary stimulating electrode in the individual animal’s implant.
Auditory Brainstem Responses
At approximately 45 days post-implantation (dpi), animals were tested for any residual acoustic hearing using auditory brainstem responses (ABRs). Animals with residual hearing were also assessed with ABRs just prior to sacrifice. To measure ABRs, animals were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg) and placed on a heating blanket with an acoustic transducer placed just inside the tragus, directed at the tympanic membrane. Needle electrodes were positioned subcutaneously at the vertex and bilaterally underneath the pinna. Tone bursts at 2, 8, and 16 kHz were tested.
Behavioral Threshold Testing Procedures
The guinea pigs were trained using positive-reinforcement-operant conditioning procedures to perform a stimulus-detection task. They were trained and tested in a sound-attenuating booth, harnessed facing a food dispenser and water spout. They were trained to initiate a trial by depressing a button on the floor of the test cage, wait through a variable period (1–6 s) for the onset of an auditory signal, and then release the button when they detected the signal. Correct detections were rewarded with a food pellet (Purina Test Diets). Failure to release the button within 1 s of stimulus onset was considered a failed trial, which was not reinforced, and the animal was required to release the button before being able to start the next trial. The method of constant stimuli was used to determine detection thresholds with stimulus levels ranging from subthreshold to suprathreshold. Psychometric functions were obtained using 10 to 20 trials per stimulus level, and threshold was defined as the level at which correct detections were indicated on 50% of the trials determined from the psychometric functions by linear interpolation. Guess rates were determined using an inaudible stimulus, and results for a given run were considered valid only if the guess rate was ≤20 %.
Animals were trained using acoustic stimuli prior to deafening. Animals with residual acoustic hearing following implantation underwent acoustic psychophysical threshold testing throughout the entire experiment using the acoustic pure tone stimuli at 50, 100, 250, 500, 1k, 2k, 8k, 16k, and 24k Hz.
After implantation, animals easily transferred their learning of detecting acoustic stimuli to detecting electrical stimuli. Psychophysical detection thresholds were measured over time from implantation until relative stability was achieved. Running samples of ten consecutive thresholds were analyzed and the data were considered stable when ten consecutive thresholds had a standard deviation of ≤2.0 dB and a linear regression of those same ten thresholds had a slope not significantly different from zero; p ≥ 0.05). The largest changes over time (typically, increases in threshold followed by gradual decreases to a stable low level) were typically observed over the first months after implantation (Su et al. 2008; Pfingst et al. 2015). Once electrical detection thresholds were considered stable, data were collected for MPI and TI functions. For animals with residual hearing, measurement of acoustic thresholds (as specified above) alternated with the electrical threshold testing.
Multipulse Integration and Temporal Integration
For MPI functions, 25 μs/phase biphasic pulses with no interphase interval were used. Thresholds for 200-ms pulse trains were measured at pulse rates ranging from 5 to 625 pps (1 to 125 pulses/stimulus) in steps of doubling. For TI functions, pulse rate was held constant at 400 pps and pulse train duration was varied from 2.5 to 320 ms (yielding 1 to 128 pulses/stimulus) in steps of doubling. Three to five thresholds for each stimulus parameter were collected in random order. Slopes of the MPI and TI functions were determined by fitting a linear function to the data.
Electrically Evoked Compound Action Potential (ECAP) Amplitude-Growth Functions (AGFs)
ECAP AGFs were recorded in awake guinea pigs while the animals were standing in the test cage. Monopolar electrode configurations were used for stimulation and recording. The default protocol for this study was to stimulate the second most apical electrode in the cochlear implant (electrode 2 in Fig. 1) and record from the adjacent, more apical, electrode (electrode 1). In cases where either of these electrodes was broken, another adjacent pair was used. The stimulating ground was at the anchor bolt and the ECAP-recording ground was at the vertex screw. The stimulus level was measured in current units (cu) where 1 cu was approximately equivalent to 1 microamp (μA). Stimulation and recording for ECAPs utilized a MED-EL “Pulsar” CI100 receiver/stimulator connected to the implant through a percutaneous electrical connector. The receiver/stimulator was connected to a standard PC via a Research Interface Box (RIB II; University of Innsbruck). Measurement parameters were controlled using custom software.
The stimulus for ECAP recordings was a biphasic pulse, with 45-μs phase duration and 2.1-μs interphase interval. Pulses were delivered at 50 pps for 20 iterations. The recording amplifier was blanked for 165 μs following electrical pulse onset to avoid saturation artifacts. Prior to recording the ECAP, the maximum stimulus level (MSL) that could be used for each stimulation site was determined. The MSL was based on behavior (observed facial twitch or pinna movement) or by compliance limit of the stimulator/electrode combination, whichever was lower. The MSL for the ECAP experiments was typically set 10 to 20 cu below the current level eliciting a facial nerve response. To obtain an ECAP AGF, the program selected stimulus levels at 15 amplitudes evenly spaced from zero to the MSL, while the order of stimulus levels was permuted (8 1 9 2 10 15 5 6 14 13 12 7 3 4 11).
For every amplitude step, responses to an anodic leading biphasic pulse and a cathodic leading biphasic pulse were averaged to reduce stimulation artifacts and the response to zero-amplitude stimulation was recorded and subtracted from this average to reduce non-stimulus related effects like switch-on artifacts. The resulting waveforms obtained for each of the 15 amplitude steps were plotted and analyzed using a custom-made Matlab program. This program picked all negative peaks (Ns) and positive peaks (Ps), which were then verified by visual inspection of the waveforms. Given that stimulus artifacts could obscure P1 under our testing conditions, the N1 to P2 response amplitudes were used. The N1 to P2 amplitudes in μV were plotted against stimulus current in μA to obtain input-output AGFs. The measurements in awake animals required a more limited range of stimulus levels compared to studies in anesthetized animals (for example Ramekers et al. 2014) so that mostly only the linear part of the sigmoidal population response was observed. To fit the data and to account for measurement error at low-stimulus levels, only ECAP amplitude response values above 100 μV were used to calculate slopes of the AGFs. Linear regression was applied to fit all data points from 100 μV to the MSL, providing the ECAP amplitudes continued to increase as a function of level. Typically, input-output functions were monotonic with an increase in amplitude for every increased current step. However, in the rare cases (~4 %) where the ECAP amplitudes started to decline as the stimulus approached the MSL, these declining points were excluded from the fit.
ECAPs were recorded frequently throughout the first 30 days starting on the day of surgery and then monthly until the animal completed all psychophysical experiments and then one final ECAP was recorded on or close to the day of animal sacrifice. Slopes of ECAP AGFs fluctuated over the first 90 dpi, typically decreasing during the first few days after implantation and then increasing to a steeper slope which was relatively stable after about 90 dpi. Means of the growth-function slopes obtained from 150 dpi to the day of animal sacrifice were used for comparison to the histological data so that an estimate of the growth functions was obtained during a stable period close to the time of histological assessment.
Electrically Evoked Auditory Brainstem Response (EABR) AGFs
EABR AGFs were recorded under ketamine (40 mg/kg) and xylazine (10 mg/kg) anesthesia at 30-day intervals following implantation. EABR responses were recorded either from recording screws in the animal’s skull or, in a few cases, from needles placed through the skin at the return and/or ground locations. The active electrode was the anchor bolt placed at bregma (or the screw 1 cm posterior to bregma on the midline if the anchor bolt was not making good contact). The return electrode was on the midline 2 cm anterior to bregma and the ground electrode was 1 cm lateral to bregma on the implanted side. Neural activity was amplified with a bioamplifier (P55 A.C. Pre-Amplifier; Grass Instrument Co.), converted to a digital signal using a TDT AD1 and recorded using the BioSig32 program (Tucker Davis Technologies). The bioamplifier was set to a gain of 10,000 and a bandwidth from 0.1 to 3 kHz.
Animals were stimulated with 25-μs duration monophasic alternating polarity pulses at 50 pps, 2048 pulses per trial. To obtain an input-output function, the beginning amplitude tested was set to a level (300 μA) known to be below the level for detectable facial nerve stimulation. Threshold was determined by decreasing stimulus levels in 50 μA steps above 200 μA and 10–20 μA steps below 200 μA until neural responses became small and could not be detected reliably. Then steps of 5 μA were tested around this level to determine threshold. The EABR threshold was defined as the lowest level at which there was a repeatable P wave with a P-N difference of 0.25 μA. A 0-μA level was also tested to determine the resting noise level and help discriminate evoked responses from noise. Once threshold was determined, the MSL was determined by increasing the level in 50-μA steps until facial movement was seen or the ABR started to decrease as a function of level. If facial movement was evoked, the level was decreased in 25-μA steps until no facial movement was seen; this level or the level at which the EABR amplitude started to decrease was considered the MSL.
To generate the EABR AGFs, peak to peak amplitudes were plotted against current level. As peak shape and characteristics varied across animals and stimulation levels, peak amplitude and latency were used to determine which peak to measure and follow over time. The largest P peak occurring within 1.2–2.0 ms was designated the P peak. The N peak was defined as the N peak (latency of about 0.7–1.0 ms) that preceded the P peak. Peak to peak amplitude was defined as the difference between these two peaks. EABR input-output function slopes were calculated by fitting a linear regression to all points from a P-N difference of 0.25 μV to the MSL except that points where the peak amplitude decreased as a function of level were not used.
EABRs were recorded monthly starting on day 30 after implantation and continuing throughout the experiment with the final EABR being obtained on the day that the animal was euthanized. As with behavioral and ECAP measures, slopes of EABR AGFs typically fluctuated over the first 90 dpi, but then became stable. Means of slopes obtained from 150 dpi to the sacrifice date were used for comparison to the histological data.
Ensemble Spontaneous Activity (ESA)
ESA recordings (Dolan et al. 1990; Searchfield et al. 2004) were used to measure the amount of spontaneous activity in the auditory nerve following implantation, deafening and/or NT treatment. Animals were tested at approximately 10, 30, and 90 dpi and just before sacrifice. Spontaneous electrical noise was recorded from the cochlear implant electrodes under ketamine (40 mg/kg) and xylazine (10 mg/kg) anesthesia while the animals were in a sound-attenuating chamber (Acoustic Systems model AS02893). Electrical potentials were recorded from each electrode in the cochlear implant electrode array using a monopolar recording configuration with the anchor bolt as ground, but only the data from the primary test electrode (usually Electrode 2) are reported in this paper. The recorded electrical potentials were filtered from 300 Hz to 3.0 kHz, amplified with a gain of 10,000, and transmitted to a spectrum analyzer (SR760, Stanford Research Systems, Sunnyvale, CA, USA). The SR760 had a sampling rate of 256 kHz. The frequency span of the SR760 was set from zero to 12.5 kHz, which resulted in analysis of 32-ms time domain records. A fast Fourier transform (FFT) was performed on each record, using Blackman-Harris windowing. The frequency resolution of the resulting FFT was 31.25 Hz. One hundred fifty FFTs were acquired and averaged (linear, RMS averaging). A Matlab program, written in-house, was used to retrieve the waveforms from the SR760 and store the data. To compare results across animals, response levels (dB re 1 V) were averaged across the frequency range from 593.75 to 1312.5 Hz; a range within which a peak in the spectrum would occur if spontaneous neural activity was present.
Histology
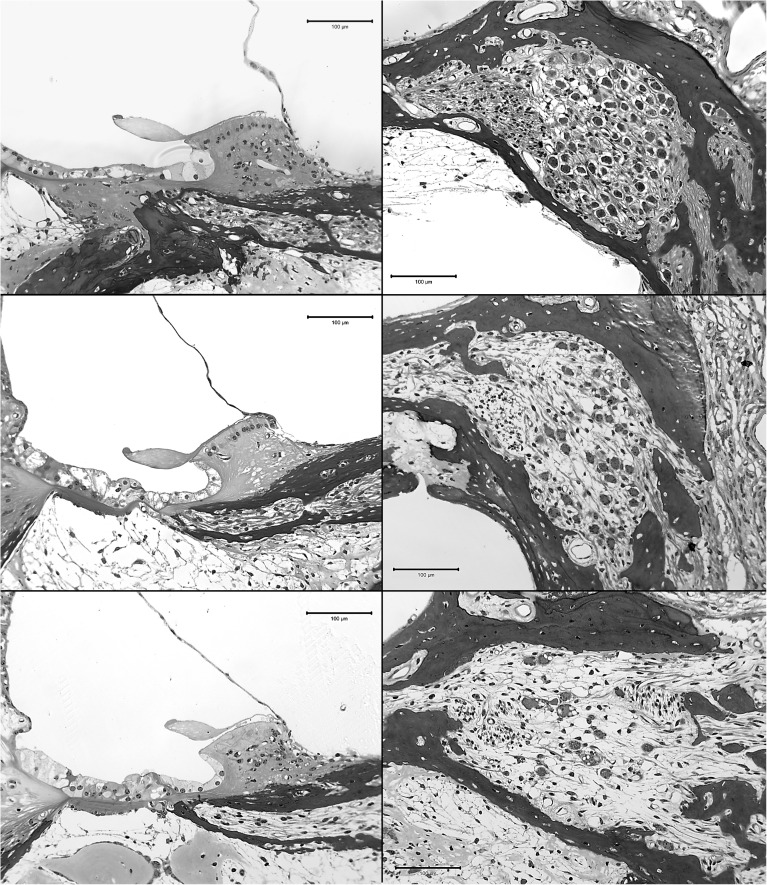
After psychophysical and electrophysiological data collection was complete, animals were anesthetized and perfused intravascularly with 4 % paraformaldehyde, with the exception of a few of the comparison animals that were perfused with 2 % glutaraldehyde in 3 % cacodylate buffer. Temporal bones were extracted with the implant remaining in place in the implanted ear. The tissues were decalcified in 3 % EDTA for 3 to 6 months until the bone was soft. When decalcification was complete and the cochlear implant electrodes were visible through the bone, the cochleae were marked in the lateral wall at the location of the primary electrode that was used for stimulation in this study (usually electrode 2). The implant was then gently removed. Tissues were embedded in JB-4 (Electron Microscopy Sciences, Hatfield, PA, USA) and sectioned with glass knives to obtain 3-μm thick sections in a near-midmodiolar plane centered at the location of the previously made mark and thus centered at the location of the primary test electrode. This provided views of six profiles that were labeled A through F as illustrated in Figure 1. Figure 2 shows examples of peri-midmodiolar sections illustrating the basilar membrane area (left column) and Rosenthal’s canal (right column) in deafened cochleae from three of the animals from this study.
FIG. 2.
Examples of the basilar membrane area (left column) and SGN cell bodies in Rosenthal’s canal (right column) from profile A; examples for three cases are shown. Peri-midmodiolar sections were made at the location of the primary stimulating electrode in each case. Animal “g” (top row; survival time 183 dpi) had a neomycin injection into the scala tympani followed 30 min later by AAV.Ntf3 inoculation, 30 min before implantation. In this case, IHCs remained apical to profile A despite the neomycin injection. Animal “c” (middle row; survival time 377 dpi) received a similar treatment to animal “g” but no surviving IHCs were found in any of the profiles. A deafened-control animal (bottom row; survival time 176 dpi) had a neomycin injection followed by AAV.empty inoculation 30 min before implantation; no IHCs were found in any of the turns. SGN densities and other details for these three cases are plotted along with those for all other cases in the following figures.
Approximately 45 sections were collected per cochlea. The first section chosen for analysis was a section close to the mark that indicated the location of the primary stimulating electrode. If that section was damaged, the next closest section was used. Four other sections were then selected at intervals separated by a minimum of six sections (~18 μm) in order to prevent counting a cell more than once. The peri-midmodiolar sections used for SGN counts were stained with toluidine blue. The specimens were observed with a Leica DMRB microscope (Leica, Eaton, PA, USA) and photographed with a CCD Cooled SPOT-RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA). The cross-sectional area within Rosenthal’s canal containing SGN cell bodies was determined using ImageJ software, and the cell bodies in each of the five selected sections were counted. SGN density was calculated by dividing the number of cells counted by this cross-sectional area. Only cells with soma diameters of 12–25 μm and a well-defined nuclear envelope of 5–9 μm in diameter were considered. Shrunken, irregularly shaped cells were not counted. In the basilar membrane area, IHCs in each profile, if present, were counted. To avoid counting the same hair cell in two sections, IHCs were only counted if both the nucleus and stereocilia were present.
SGN densities presented below are for profiles A, B, and C. They are designated SGN-A density, SGN-B density, and SGN-C density. These profiles were chosen for comparison to the functional data because they represented the region closest to the implant (profile A) and profiles just apical to the implant (profiles B and C) which would be most likely to reflect pathology affecting responses to the cochlear implant stimulation.
Statistical Analysis
Effects of treatment on SGN densities were analyzed using ANOVA with densities in profiles A–C treated as three response variables. Non-independence of profiles was characterized by regressing the more apical profiles (B and C) on the focal profile (A). Variation in functional measures (e.g., MPI, ECAP) was analyzed by a model comparison strategy to test for independent effects of potentially interacting factors (treatment and SGN density). In this approach, ANOVA is used to compare the variances explained by two models differing in the number of explanatory variables. The model with more explanatory variables is considered to be a significant improvement on the simpler model if it reduces the residual (unexplained) variation more than would be expected by chance given the larger number of terms, as evaluated by an F test. Subsequently, significant relationships between the measure and SGN density were described by univariate regressions. In analyses with multiple response variables, F was estimated from Pillai’s trace. To test for outliers, all cases included in a regression analysis were evaluated to determine whether they deviated from the general trend more than expected under a model of normally distributed deviations. These analyses were performed using base R 3.3.0 (R Core Team, 2016), except for the outlier test, which used the outlier test function in the car package (Fox and Weisberg, 2011).
RESULTS
Histological Effects of Treatments
Cochlear Implants in Hearing Ears Usually Did Not Induce Appreciable SGN Loss
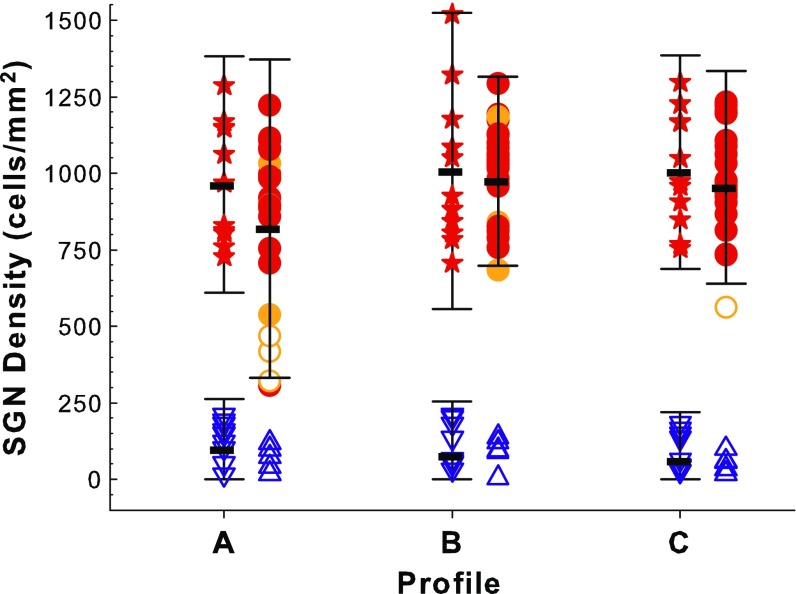
The animals implanted in a hearing ear with no neomycin deafening (circles in Fig. 3) showed a similar range of SGN densities, with a few exceptions, to normal (non-implanted animals (stars in Fig. 3). Five of the animals had SGN densities in profile 1 that were more than two standard deviations below the mean for the normal, non-implanted group. All but one of these five had less than 80 % SGN survival in profile A. As a group, the animals implanted in a hearing ear had SGN densities that were not significantly different from normal animals that received no treatment of any kind (F 3,29 = 1.03, p = 0.393). The difference between the means of these two groups was greatest in profile A, but the post hoc test of only this profile was not significant (F 1,32 = 2.46, p = 0.127). Differences between these groups in the other profiles were even smaller and farther from significance (p > 0.45). These results indicated that insertion trauma and other surgical side effects did not usually lead to SGN loss.
FIG. 3.
SGN densities for four comparison and control groups: normal animals (stars); animals implanted in a hearing ear (circles); animals deafened with neomycin prior to implantation (inverted triangles) and animals deafened with neomycin and treated with AAV.empty prior to implantation (upright triangles). SGN densities in profiles A–C are shown for each individual. Ears with greater than 80 % inner hair cell survival in the specified profile are indicated by filled red symbols. Symbols with orange fill indicate IHC survival between 1 and 79 %, and open symbols indicate that no IHC were found in the specified profile. For each group, bars indicate the mean for the specified profile and error bars extended to ±2 SD of that mean. Data for the two deafened groups were not significantly different, so these two groups were combined to form a “deafened-control” group for further analysis.
Ganglion Cell Survival After Neomycin Was Not Improved by AAV.empty
In contrast to animals implanted in a hearing ear, SGN densities in animals implanted after neomycin deafening with no NT treatment were uniformly very low (blue symbols in Fig. 3). In these neomycin deafened animals, SGN survival in profiles A, B, and C was <25 % of the mean for the normal animals. These ranges were nearly identical to those seen in many previously tested animals that were treated with neomycin injections alone prior to implantation. SGN survival in five animals given both neomycin and AAV.empty (blue triangles in Fig. 3) was not significantly different from that in animals given only neomycin (inverted triangles) (F 3,10 = 1.74, p = 0.222). These two groups overlapped broadly in all three profiles and no individual animal given neomycin plus AAV.empty had an SGN density greater than one standard deviation above the mean of the neomycin alone group in any profile. These results demonstrate that AAV.empty did not enhance SGN survival after neomycin deafening. Therefore; these groups were combined as a single “deafened-control” group in subsequent analyses.
SGN Survival Was Enhanced by AAV.Ntf3 Treatment
SGN survival in the 18 neomycin injected, AAV.Ntf3 inoculated, implanted ears ranged from near-normal to poor across animals and across profiles of Rosenthal’s canal (black squares labeled a through r in Fig. 4). SGN densities were not significantly different between animals receiving implants and AAV.Ntf3 in the same surgery as the neomycin treatment and those receiving implants and AAV.Ntf3 2 weeks after neomycin (F 3,14 = 0.218, p = 0.882). Post hoc analyses comparing these two treatment groups showed that SGN survival was not significantly different in any individual profile (p > 0.64); therefore, these two groups were combined in subsequent analyses.
FIG. 4.
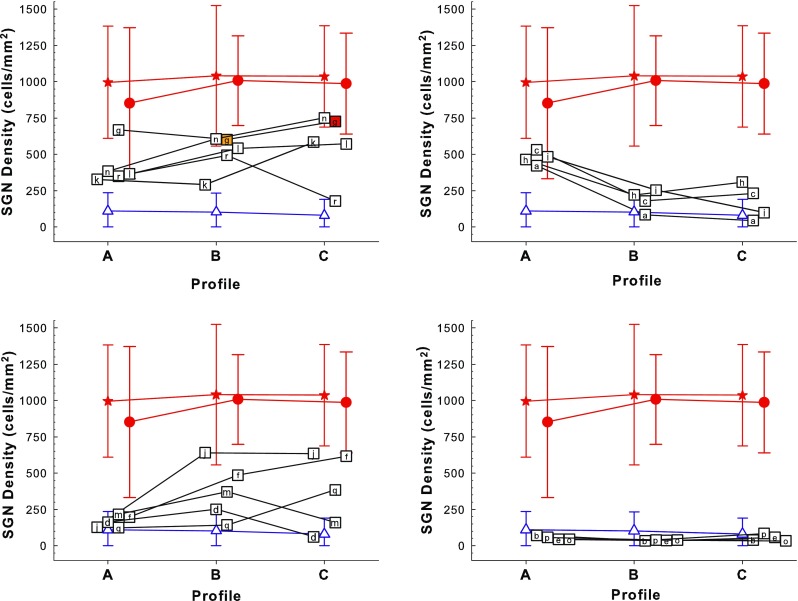
Density of SGN cell bodies in Rosenthal’s canal in the first three half-turns (profiles A, B, and C) of the cochlea. Symbols are jittered along the x axis to avoid overlap. To make a clearer presentation, the data are divided among four panels based on post hoc qualitative assessment of the patterns across profiles, as detailed in Table 2. Each symbol containing a letter (“a” through “r”) represents an individual animal from the experimental group, which received neomycin followed by AAV.Ntf3 and a cochlear implant. Note that animal “g” had remaining hair cells in profiles B (40 %) and C (100 %) as indicated by the orange and red fill in the symbols. Animal “a” had some remaining hair cells apical to profile D. Animal “p” had measurable behavioral responses to acoustic stimulation at 250 Hz to 2 kHz, but no IHCs were found on histological analysis. For reference, each panel also shows the means plus and minus two standard deviations of SGN densities for the comparison and control groups: Red filled stars are for the normal animals, red filled circles are for the implanted-hearing group, and blue triangles are for the 14 deafened-controls. Figure 3 gives the details for these three groups.
A three-way analysis comparing SGN survival in the AAV.Ntf3-treated group, the deafened-control group, and implanted-hearing group indicated that there were significant differences (F 6,106 = 13.7, p < 0.0001). Post hoc pairwise tests confirmed that SGN density in the AAV.Ntf3-treated group was significantly different from both the implanted-hearing group (F 3,37 = 52.7, p < 0.0001) and the deafened-control group (F 3,28 = 4.64, p = 0.009).
In this study, 14 of the 18 ears (78 %) that were inoculated with AAV.Ntf3 following neomycin injection had SGN survival in at least one profile (A–C) that was better than that for any of the deafened-control animals; i.e., the SGN count was more than two standard deviations above the mean of that for the deafened-control group (Fig. 4). The four remaining animals inoculated with AAV.Ntf3 were indistinguishable from the deafened-control group (Fig. 4, bottom right panel).
SGN densities in the AAV.Ntf3-treated group usually were intermediate between the implanted-hearing group and the deafened-control group, but they also exhibited relatively large differences between profiles (Fig. 4). For clarity of presentation, the 18 AAV.Ntf3-treated ears were divided into four groups based on the pattern of SGN density across the three profiles. These four post hoc groups are described in Table 2 and shown in the four panels of Figure 4. For comparison, each panel also shows the mean and 95 % confidence intervals for SGN density in the ten normal ears (stars), the 24 implanted-hearing ears (circles), and the 14 deafened-control ears (triangles). Five cases showed relatively even distributions of SGN density across the three profiles (Fig. 4, upper-left panel). Four other cases showed the highest SGN densities in profile A, near the cochlear implant, and relatively low densities apical to the implant (Fig. 4, upper-right panel). A third pattern (five cases) is shown in the lower-left panel of Figure 4: relatively poor SGN survival in profile A but much better survival apical to profile A. The fourth panel in Figure 4 shows the four NT-treated cases that did not show better survival than the 14 deafened-controls. As suggested by the presence of these four patterns within the AAV.Ntf3-treated group, SGN-B and -C densities were not significantly correlated with SGN-A density in this group (F 2,15 = 1.62, p = 0.232); regression on SGN-A density accounted for only 17.5 % of the variation in SGN density in profile B and only 13.6 % in profile C.
TABLE 2.
Range of SGN preservation results for the 18 ears in the experimental group, which received neomycin injection, AAV.Ntf3 inoculation, and cochlear implantation. Individual ears are designated by the lower case letters “a” through “r” in column 1. Survival times in days post-inoculation (dpi) are shown in column 2
| SGN survival | Survival time (dpi) |
Comments and exceptions |
|---|---|---|
| Fair to good in all profiles | ||
| g | 183 | IHCs in profiles apical to profile A |
| n | 209 | |
| l | 217 | |
| r | 227 | SGN density poor only in profile C |
| k | 184 | |
| Fair to good in profile A, poor in profiles B–C | ||
| c | 377 | |
| i | 189 | |
| h | 149 | |
| a | 361 | IHCs in profiles apical to profile D |
| Poor in profile A, fair to good in profiles B and/or C | ||
| j | 198 | |
| f | 238 | |
| m | 323 | SGN density good only in profile B |
| q | 168 | SGN density good only in profile C |
| d | 426 | SGN density good only in profile B |
| Poor in all profiles | ||
| b | 386 | |
| p | 273 | |
| e | 183 | |
| o | 195 | |
AAV.Ntf3 Inoculation Had Long-lasting Effects in the Absence of Hair Cells
In the neomycin-injected animals, no surviving hair cells were found in profile A. For most of these animals, no hair cells were found in any of the other profiles and there was no measurable acoustic hearing, with the following three exceptions. Animal “g” had surviving hair cells in all profiles apical to A in spite of the neomycin injection. Animal “a” had no hair cells found in profiles A through D but did have a few remaining IHCs apical to profile D. Both “g” and “a” had some preserved acoustic hearing at the tested frequencies (50 Hz to 24 kHz) with psychophysical detection thresholds for animal “g” ranging from 23 to 76 dB SPL and thresholds for animal “a” ranging from 76 dB SPL to inaudible. Animal “p” had behavioral detection responses to low-frequency acoustic stimulation (250 Hz to 2 kHz, with thresholds ranging from 76 to 108 dB SPL), but no hair cells were found in either cochlea at the time of sacrifice. The remaining neomycin-treated animals depicted in Figure 4 (open squares) had no measurable acoustic hearing and no remaining hair cells were found in profiles A through C, or apical to profile C in cases where this could be examined. These animals all had a flat epithelial layer, or in a few cases, a small cluster of non-descript cells, replacing the organ of Corti (examples in Fig. 2 right panel).
Survival times from inoculation to euthanasia (dpi) for the NT-inoculated group are shown in Table 2. These times varied considerably across animals depending on the time required to complete the psychophysical and electrophysiological experiments. The 12 AAV.Ntf3-treated ears that had fair to good SGN survival despite the absence of IHCs had inoculation-to-euthanasia durations ranging from 149 (approximately 5 months) to 426 days (approximately 14 months), suggesting that the effects of the successful inoculations were long-lasting in implanted ears that received some electrical stimulation. Animals with poor SGN survival span a similar range of survival times. Overall, the correlation between SGN density and dpi was not statistically significant (F 3,14 = 1.62, p = 0.229). Including the presence or absence of IHC did not significantly improve the explanation for variation in SGN density (F 3,13 = 1.83, p = 0.192). The interaction between IHC and dpi also was not significant (F 3,13 = 0.598, p = 0.628), indicating that IHC survival did not differ with duration. Separate regressions of SGN densities on dpi were not significant; none pass the Bonferroni criterion for a table of three tests (0.0167) and correlations with dpi accounted for <10 % of the variation in SGN density in profiles A and B and 23 % of the variation in profile C. Thus, differences in SGN density among animals were not related to differences in survival time.
Functional Effects of Treatments
The experimental and control treatments administered in this experiment resulted in a large range of SGN survival and a variety of SGN survival patterns across profiles A through C near and apical to the implanted electrode array. Below, we explore the relationships between the experimental and control treatments and various psychophysical and electrophysiological measures of cochlear implant function.
Multipulse Integration Was Not Affected by the AAV.Ntf3 Treatment
Previously, we reported that slopes of psychophysical threshold versus pulse rate functions were steep (good MPI) only in animals with excellent cochlear health, which included high levels of SGN survival, presence of IHCs, and evidence of ESA (Kang et al., 2010; Pfingst et al. 2011; Zhou et al. 2015). In Figure 5, we combine the results from these previous experiments with results for additional animals and report SGN densities and IHC counts in profiles A, B, and C. The top-left panel shows examples of the MPI functions for electrical stimulation in profile A. In the top-right and lower panels, the slopes of the MPI functions are plotted as a function of SGN density in profiles A, B, and C as indicated on the abscissa. None of the animals in the AAV.Ntf3-treated group (squares) and deafened-control group (triangles) showed good MPI. Slopes of the MPI functions in all of these cases were shallower than −1 dB/doubling of pulse rate. In contrast, 58% of the animals in the implanted-hearing group had MPI slopes steeper than −1 dB/doubling.
FIG. 5.
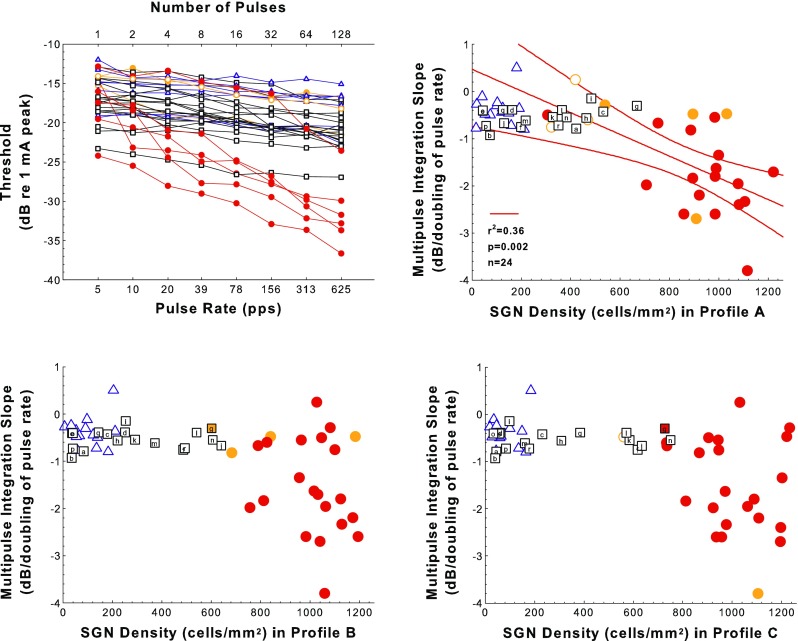
Multipulse integration (MPI) results. Examples of MPI functions (psychophysical detection threshold vs. pulse rate for 200-ms pulse trains) from animals from the various treatment groups are shown in the top-left panel. Slopes of the MPI functions plotted as a function of SGN density in Rosenthal’s canal for profiles A, B, and C for experimental, control, and comparison animals are shown in the top-right (SGN-A) and the two bottom panels (SGN-B and SGN-C). The 18 animals deafened and then treated with AAV.Ntf3 are represented by squares; letters indicate individual animals as in Figure 4. The 14 deafened-control cases are represented by triangles. The 24 implanted-hearing cases are represented by circles. In all panels, red and orange symbols indicate the presence of inner hair cells (IHCs): red fill ≥80 % IHC survival, orange fill 1 to 79 % IHCs; and open symbols indicate the absence of IHCs in that profile. The open orange symbols indicate that IHCs were not present in the indicated profile but were present in some other profiles. The red regression line and corresponding statistics show the significant correlation between MPI slope and SGN-A density for the implanted-hearing group; red curving lines are 95 % confidence ranges. This correlation was not significant for SGN-B or SGN-C.
The implanted-hearing and deafened-control groups differed greatly in both MPI slope and SGN-A density, but the AAV.Ntf3-treated group was not intermediate in MPI slope despite being intermediate in SGN-A density (Fig. 5). Multiple group analysis indicated that the differences among treatment groups were significant (F 2,52 = 14.5, p < 0.0001). ANOVA found that SGN-A density combined with treatment accounted for significantly more of the variation in MPI slope than did treatment alone (F 3,49 = 8.83, p < 0.0001). In addition, there was a significant interaction between SGN-A and treatment (F 2,49 = 5.50, p = 0.0070). These latter results reflect the different relationships between SGN-A variation and MPI slope within the three groups. The deafened-control and AAV.Ntf3-treated groups had shallow MPI slopes that were independent of SGN-A density (deafened-control group, F 1,12 = 0.0552, p = 0.818; AAV.Ntf3-treated, F 1,16 = 2.14, p = 0.163). Outlier tests indicated that the treated ears with surviving IHC did not have exceptional MPI slopes for their SGN-A densities (p > 0.10). Some implanted-hearing ears had shallow MPI slopes, but less than half of the animals in this group had SGN-A densities in the same range as those for the other two groups. Because 5 of 24 implanted-hearing ears had both shallow MPI slopes and relatively low SGN-A density, there was a significant correlation between slope and density in this group (F 1,22 = 12.4, p = 0.0019); but when those five ears were excluded from the analysis, the correlation was not significant (F 1,17 = 1.24, p = 0.281). The results indicate that shallowing of MPI slopes was not a specific effect of the deafening (it occurred also in non-deafened animals) and was not ameliorated by SGN-A preservation to the extent that preservation was achieved in the AAV.Ntf3-treated group. Although SGN density was not perfectly correlated between profiles, especially in the AAV.Ntf3-treated group, including the SGN-B and SGN-C densities did not explain more variation in MPI slope than did the analysis using only SGN-A and treatment (F 2,49 = 0.024, p = 0.976). Thus, variation of SGN density in the more apical profiles did not contribute significantly to variation in MPI slope.
There Was a Small Effect of AAV.Ntf3 Treatment on Temporal Integration (TI)
Like MPI, there was a big difference between implanted-hearing and deafened-control groups, but unlike MPI, AAV.Ntf3-treated were intermediate in TI slopes as well as in SGN-A density (Fig. 6). For TI, there was a significant effect of treatment (F 2,31 = 26.0, p < 0.0001), but including SGN-A density did not explain more variation than treatment alone (F 1,30 = 1.95, p = 0.173). Each group was significantly different from the others in TI (F > 12.4, p < 0.002) and TI was not correlated with SGN-A density in any of the groups (F < 1.5, p > 0.27). Including the SGN-B and SGN-C densities did not explain more variation in TI slope than did the analysis using only SGN-A and treatment (F 2,28 = 0.578, p = 0.568). The effect of the treatment was not due to changes in SGN-A numbers. Variation of SGN density in the more apical profiles did not contribute significantly to variation in TI slope.
FIG. 6.
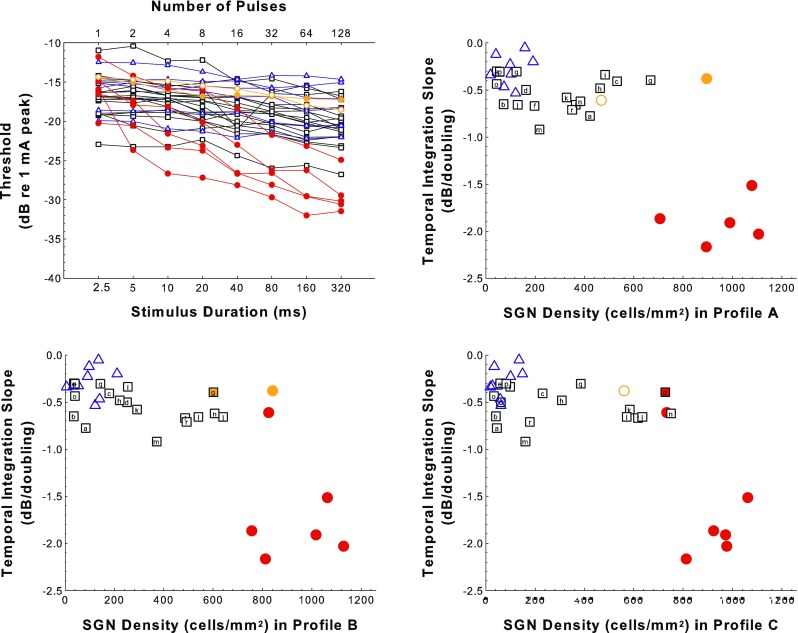
Temporal integration (TI) results. Examples of TI functions (psychophysical detection threshold vs. pulse-train duration for 400 pps pulse trains) for animals from the various treatment groups are shown in the top-left panel. Slopes of the TI functions are plotted as a function of SGN density for profiles A, B, and C for experimental, control, and comparison animals in the top-right panel (SGN-A) and the two bottom panels (SGN-B and SGN-C). Symbols and colors follow the same rules as described in the caption of Figure 5. However, note that for the deafened-control and implanted-hearing comparison groups TI functions were measured for fewer animals than were used for the MPI data shown in Figure 5.
Note that TI was tested only in a subset (n = 33) of the 56 animals that underwent MPI testing; fewer animals from the implanted-hearing and deaf-control groups underwent TI testing.
ECAP Amplitude Growth Function (AGF) Slopes Were Affected by Nft3 Treatment
For ECAP AGFs, many AAV.Ntf3-treated ears that were intermediate between deafened-control and implanted-hearing in SGN-A density were also intermediate in ECAP AGF slopes (Fig. 7). There was a significant effect of treatment group (F 2,25 = 8.31, p = 0.0017), but including SGN-A density did not explain more variation than treatment alone (F 2,24 = 0.368, p = 0.550). Pairwise tests showed that the deafened-control and AAV.Ntf3-treated groups had ECAP AGF slopes that were not significantly different (3.05 vs. 5.67 μV/μA; F 1,20 = 3.69, p = 0.069), whereas the difference between the AAV.Ntf3-treated and implanted-hearing groups mean slopes was statistically significant (5.67 vs. 9.70 μV/μA;, F 1,21 = 8.96, p = 0.0098). The slopes of the ECAP AGFs were not correlated with SGN-A density within any of the treatment groups (F < 0.85, p > 0.37). The outlier test rejected the hypothesis that IHC preservation in some AAV.Ntf3-treated ears influenced the correlation between ECAP growth function slope and SGN-A density (p > 0.24). Unlike the psychophysical functions, variation of the ECAP AGF slope was better explained by including SGN-B and SGN-C densities in the analysis (F 2,22 = 4.53, p = 0.0226), reflecting the significant correlations in the AAV.Ntf3-treated group between ECAP AGF slopes and SGN densities in profiles B (F 1,15 = 13.2, p = 0.0025) and C (F 1,15 = 14.3, p = 0.0018). SGN density in the more apical profiles was better correlated with the slope because some individuals with steep slopes had relatively low SGN-A densities (f, j, and l) and some with moderate to shallow slopes had relatively high SGN-A densities (a, c, h, and i). These results suggest lower ECAP AGF slopes were an effect of deafening that could be ameliorated by SGN preservation, and that preservation of SGN density distant from the active electrode contributed to maintenance of steep ECAP AGFs.
FIG. 7.
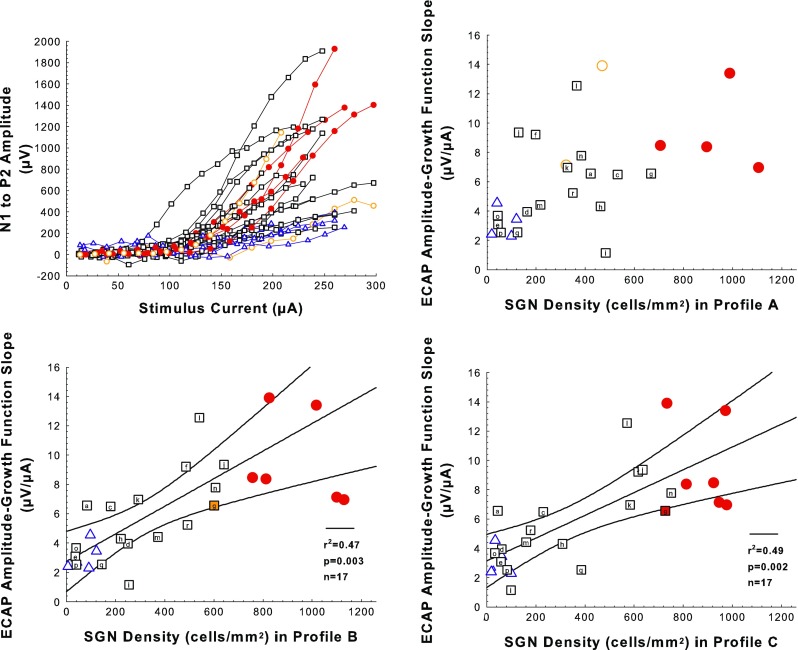
Electrically evoked compound action potential (ECAP) amplitude growth function (AGF) results. Examples of ECAP AGFs (ECAP level versus stimulus level) are shown in the top-left panel. Slopes of the ECAP AGFs plotted as a function of SGN density for profiles A, B, and C for experimental, control, and comparison animals are shown in the top-right panel (SGN-A) and the two bottom panels (SGN-B and SGN-C). Symbols and colors follow the same rules as described for Figure 5 but the number of comparison cases for which ECAP data were obtained is smaller. Slopes for individual cases represent mean slopes of multiple growth functions obtained during a period when slopes were relatively stable over time (day 150 post-implantation to sacrifice). The number of slopes per animal averaged 8.0 and the standard deviations averaged 1.1 μV/μA. The black regression lines and corresponding statistics show the statistical relationship between ECAP AGF slopes and SGN density for the AAV.Ntf3 group; black curving lines are 95 % confidence ranges.
Results for EABR AGFs Were Similar to Those for ECAP AGFs
The relationship of EABR AGFs to deafening and SGN density were very similar to those for ECAP AGFs because here, too, many AAV.Ntf3-treated ears that were intermediate between deafened-control and implanted-hearing in SGN-A density were also intermediate in EABR AGF slope (Fig. 8). There was a significant effect of treatment (F 2,30 = 6.65, p = 0.0041), but including SGN-A density did not explain more variation than treatment, alone (F 1,29 = 3.88, p = 0.0586). EABR slopes of the AAV.Ntf3-treated group were significantly different from the deafened-control group (F 1,25 = 4.58, p = 0.0424) but not from the implanted-hearing group (F 1,22 = 3.86, p = 0.0623). EABR AGF slope was not correlated with SGN-A density within any group (F < 3.7, p > 0.07). The hypothesis that IHC preservation in some AAV.Ntf3-treated ears influenced the correlation between EABR AGF slope and SGN-A density was rejected by the outlier test; the sole specimen that deviated from the predicted value enough to be considered an outlier was animal “l” (p = 0.041), which did not have residual IHCs. Variation in EABR slope was better explained by including SGN-B and SGN-C densities in the analysis (F 2,27 = 4.44, p = 0.0215), reflecting the significant correlations in the AAV.Ntf3-treated group between EABR and SGN densities in profiles B (F 1,16 = 8.57, p = 0.0099) and C (F 1,16 = 11.0, p = 0.0043). As with ECAP, several ears had EABR AGF slopes that were better explained by SGN densities in the more apical profiles than by the density in profile A. Thus, similar to the results for ECAP AFGs, reduction in EABR AGF slope also was an effect of deafening that could be ameliorated by SGN preservation, including preservation of SGN density apical to the active electrode.
FIG. 8.
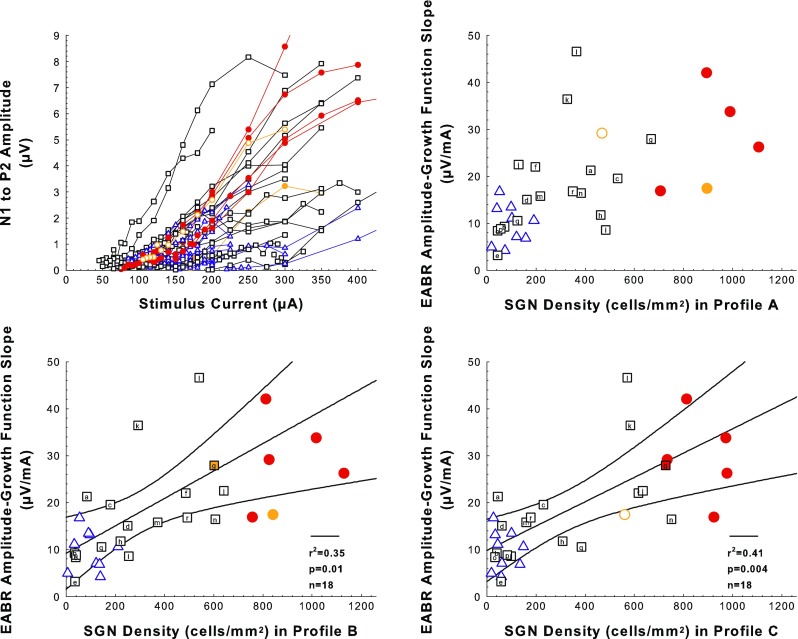
Electrically evoked auditory brainstem (EABR) amplitude growth function (AGF) results. Examples of EABR AGFs (EABR level versus stimulus level) are shown in the top-left panel. Slopes of EABR AGFs plotted as a function of SGN density for profiles A, B, and C for experimental, control, and comparison animals are shown in the top-right (SGN-A) and the two bottom panels (SGN-B and SGN-C). Symbols and colors follow the same rules as described for Figure 5 but the number of comparison cases for which EABR data were obtained is smaller. Slopes for individual cases represent mean slopes of multiple growth functions obtained during a period when slopes were relatively stable over time (day 150 post-implantation to sacrifice). The number of slopes per animal averaged 4.1 and the standard deviations averaged 3.8 μV/mA. The black regression lines and corresponding statistics show the relationship between EABR AGF slopes and SGN density for the AAV.Ntf3 group; black curving lines are 95 % confidence ranges.
Ensemble Spontaneous Activity Was Affected by AAV.Ntf3 Treatment
As expected, clear peaks in the ESA spectra near 900 Hz were seen for animals with high numbers of surviving IHCs (≥80 % of normal in profile A; red traces in the top-left panel of Fig. 9), and these animals had the highest levels of ESA activity in that region (average ESA levels in the spectrum between 593.75 and 1312.5 Hz). Animals with moderate IHC survival (<80 % of normal in profile A) showed a peak of activity in the 900-Hz region but with lower average ESA levels (orange traces). Animals with no hair cells in the sections examined showed no clear peak in this frequency region and had the lowest average ESA levels in this region (blue and most of the black traces). It is worth noting that among animals with no IHCs in the examined slides, seven of the animals that received AAV.Ntf3 inoculations (43.8 % of the AAV.Ntf3-group) had higher average ESA levels than any of the deafened-control animals. ESA values for these seven AAV.Ntf3 inoculated cases without IHCs ranged from −43.0 to −42.6 dB re 1 V while the range for the deafened-control animals was −43.1 to −47.9 dB re 1 V.
FIG. 9.
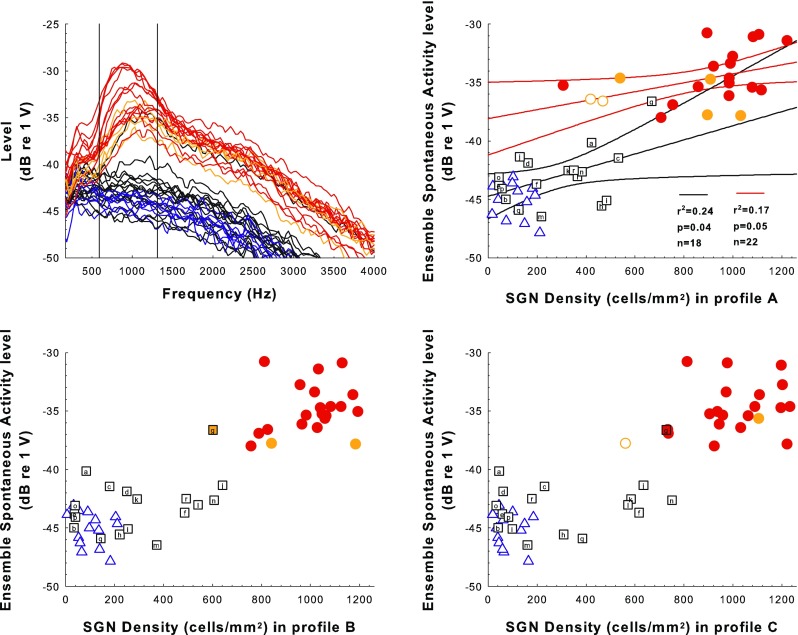
Ensemble spontaneous activity (ESA) results. Examples of ESA spectra for animals from the various treatment groups obtained within a few days of sacrifice are shown in the top left panel. ESA levels averaged over the range between 593.75 to 1312.5 Hz that are plotted as a function of SGN density for profiles A, B, and C for experimental, control, and comparison animals are shown in the top-right (SGN-A) and the two bottom panels (SGN-B and SGN-C). Symbols and colors follow the same rules as described for Figure 5. Note that the black ESA spectrum trace in the upper-left panel that is similar to the orange traces is from animal “g” where there were no hair cells found in profile A but hair cells were present in profile B and more apical profiles. In the top-right panel, the black and red regression lines and corresponding statistics show the relationship between ESA levels and SGN density for the AAV.Ntf3 (black traces) and implanted-hearing group (red traces), respectively; curving lines are 95 % confidence ranges.
Results for ESA levels (averaged between 593.75 and 1312.5 Hz) relative to SGN density showed that AAV.Ntf3-treated ears with greater SGN preservation were closer in value to the implanted-hearing ears than those with poorer SGN preservation. However, a regression line fit to the AAV.Ntf3-group data (black regression line in the top-right panel of Fig. 9) was too shallow to predict the peak values observed in the implanted-hearing group which had near-normal SGN densities (circles in top-right panel of Fig. 9). There was a significant effect of AAV.Ntf3 treatment (F 2,50 = 123, p < 0.0001), and including SGN-A density explained more variation than treatment alone (F 1,49 = 7.04, p = 0.0107). However, the interaction between SGN-A density and treatment was not significant (F 2,47 = 1.12, p = 0.334), reflecting the nearly parallel regression slopes for the two groups that had significant correlations (AAV.Ntf3-treated black and implanted-hearing red regression lines in the top-right panel of Fig. 9). AAV.Ntf3-treated ears that had surviving hair cells did not deviate from expected peak levels sufficiently to be considered outliers (p < 0.27). Including the SGN-B and SGN-C densities did not explain more variation in ESA levels than did the analysis using only SGN-A and treatment (F 2,47 = 1.18, p = 0.315) and there was not a significant correlation between ESA levels and SGN density in profile B or C in any of the three treatment groups (F < 2.6, p > 0.13), indicating ESA recorded at the active electrode was not significantly influenced by SGN preservation apical to profile A. The results suggest that ESA levels in deafened ears can be enhanced by SGN preservation, but that there are probably effects of deafening not measured by SGN density and not fully prevented or reversed by the Ntf3 treatment in this experiment.
DISCUSSION
Inoculation with AAV.Ntf3 after deafening was effective in preserving higher SGN densities compared to cases inoculated with an empty virus and cases with no inoculation. These results were seen in mature animals with electrically stimulated cochlear implants and at sacrifice times ranging from 5 to 14 months after inoculation. While this result is promising, there is certainly room for improvement. In the AAV.Ntf3-treated animals in which no surviving IHCs were found and there was no measurable residual hearing, the percent SGN preservation in the region closest to the stimulating electrode (profile A) ranged from 44 cells/mm2 (about 4 % of normal) to 484 cells/mm2 (about 49 % of normal). If the two animals in which surviving IHCs were found are included, the range is about 4 to 67 % of normal. Higher percentages and more consistent results are desirable.
The primary purpose of this experiment was to use AAV.Ntf3 treatment paradigms that were designed based on the existing literature on AAV gene expression in other systems and to determine the long-term effects of that treatment on SGN density and on measures of cochlear implant function. Currently, we do not have a detailed picture of the expression of NT-3 in this paradigm but several points are evident from the work done to date: Following injection of the viral vector into the perilymph (as done here), the gene expression was mostly found in the mesothelial cells that line the scala tympani (Supplemental Fig. S1). In a previous study (Budenz et al., 2015), we found that NT-3 levels, sampled 3 months after AAV.Ntf3 inoculation, were variable across animals ranging from well above threshold (66.6 and 111.4 pg/ml) to undetectable. Possible reasons for this variability include the technical difficulty of measuring NT-3 levels in small perilymph samples and/or variability in turnover in the mesothelial cells that were transduced by the viral vector. From the current experiment, it is evident that the histological and functional effects of the AAV.Ntf3 treatment lasted well beyond 3 months in many cases. Additional work is needed to determine the location and time course of NT-3 expression, its consistency across animals, and its relationship to anatomical and functional effects such as those observed in this study.
It is possible that electrical stimulation of the implant contributed to the survival of SGNs. Some previous studies have shown that chronic electrical stimulation of the implant can enhance or extend but not replace the effects of NTs on SGN survival (e.g., Shepherd et al. 2005, 2008), while others have shown protective effects of electrical stimulation alone (e.g., Miller and Altschuler, 1995; Leake et al., 1999). It is important to note that the amount of electrical stimulation received by animals in the present study was far less than that received in studies using chronic stimulation. Psychophysical testing lasted for a maximum of 2 hours per day and involved mostly short bursts of pulsatile stimuli at levels near the detection thresholds. Electrophysiological testing covered the whole dynamic range but lasted only about 20 min per day.
This study was not designed to evaluate the interaction of electrical and acoustic stimulation; none of the animals received AAV.Ntf3 inoculation without electrical stimulation. Furthermore, the variability in effects of inoculation was high, so a large number of cases would be required to determine if electrical stimulation had an additional effect. However, it is clear from the deafened-control cases that electrical stimulation alone, at the levels used for psychophysical and electrophysiological testing in the current experiment, was not sufficient to enhance SGN survival in these neomycin-deafened ears.
Five of the AAV.Ntf3 inoculated ears in the current study had low SGN densities in profile A, where the implant was located, but good survival apical to the implant (Fig. 4, lower-left panel). It might be that in some cases, the implant or the tissue response that typically develops near the implant was interfering with the gene therapy procedure. This might be due to death of the transduced cells, blocking the diffusion of the NTs from the transduced cells to the SGNs, or absorption of the NTs by fibrous tissue. Possible solutions are use of “soft surgery” techniques or other procedures to limit or prevent the growth of fibrous tissue. Delaying the implantation for 2 weeks after inoculation was of little or no benefit, suggesting that poor results of inoculation were not due to the insertion of the implant shortly after inoculation interfering with the inoculation.
In contrast to the above cases, four of the AAV.Ntf3 inoculated ears had good survival in profile A but poor survival in profiles B and C (Fig. 4, upper-right panel). We saw no systematic difference in the extent of fibrous tissue in these cases compared to those with relatively poor survival in profile A. This outcome may be related to poor spread of the viral vector from the site of inoculation to other areas in the cochlea.
While it is desirable for therapeutic purposes to decrease the variability across animals in the histological response to the gene therapy procedures, the variability proved useful for the purpose of assessing the relationship between nerve survival and our measures of implant function. Three types of functional measures were tested in these experiments: psychophysical pulse-integration measures (MPI and TI), electrophysiological growth functions (ECAP and EABR), and electrophysiological measures of spontaneous activity (ESA).
The psychophysical MPI and TI measures showed good integration (slopes larger than −1 dB/doubling) only when SGN densities were greater than 706 cells/mm2 (greater than about 71 % of normal) in profile A. These cases were all implanted in hearing ears and all had surviving IHCs (red and orange circles in Figs. 5 and 6). It is not possible from our current data to tell if the IHCs, the high SGN densities, or some other variable was necessary to achieve good multipulse and TI. In our guinea pig studies, the high SGN densities were correlated with, and probably supported by, the high IHC survival. However, we have seen good multipulse and TI at some stimulation sites in human subjects who had no measurable hearing and thus probably had few if any IHCs (Zhou et al., 2012; Zhou and Pfingst 2014; Zhou et al., 2015). Human subjects with cochlear implants can have a high percentage of SGNs in some regions despite the absence of IHCs (e.g., Fayad and Linthicum 2006), so it is possible that the steep slopes of the integration functions seen in our human studies were due to high SGN densities. It is also noteworthy that a few guinea pigs that had high IHC and SGN survival had shallow MPI and TI slopes, suggesting that some other variable that is normally correlated with IHC and SGN survival was responsible for the steep slopes. Further research is needed to determine if near-normal populations of SGNs can be preserved in the region of the implant and if this variable alone contributes to good MPI or if the presence of a population of healthy hair cells is required.
The ECAP and EABR AGFs were shallow in deafened-control animals that had low SGN densities but steeper in AAV.NTF3-treated animals that had higher SGN densities as well as in implanted-hearing animals. Thus, they could be useful measures for following the efficacy of NT gene therapy and hearing preservation procedures over time after implantation. These two measures involved stimulation at suprathreshold levels, so current spread would be greater than for threshold measures and probably reached areas apical to the implants where SGN survival was often better than in profile A. This might explain why the correlations of the electrophysiological growth function slopes were better when SGN densities in profiles B and C were included in the analysis.
Slopes of the ECAP and EABR AGFs for implanted-hearing ears with IHC survival were generally not higher than those for AAV.Ntf3-treated ears with intermediate SGN densities and no identified IHCs (Figs. 7 and 8). This suggests that the presence of IHCs might have counteracted the benefit of the higher SGN densities in the implanted-hearing ears. A possible reason for this might be that the IHCs created spontaneous activity in the auditory nerve so that some of the surviving SGNs were in a refractory state and thus unresponsive to the electrical stimulus on any given trial.
ESA levels in these experiments were highest in animals with surviving IHCs. However, seven of the 18 AAV.Ntf3-treated animals with no identified IHCs had higher ESA levels than any of the deafened-control animals, suggesting that the NT treatment might have had a slight effect on the ESA. In theory, the ESA level should depend on some non-linear combination of the density of neurons near the recording electrodes, the proximity of the neurons to the recording site, and the amount of spontaneous activity in each neuron. The lack of correlation of ESA with SGN density in the absence of IHCs suggests that the slightly higher ESA levels found in some deaf NT-treated ears were not due to the effect of AAV.Ntf3 on nerve density. The fact that the AAV.Ntf3 treatment was associated with a small amount of spontaneous activity in some SGNs might be related to regrowth of peripheral processes which can occur with NT treatment (Shibata et al. 2011). However, it is unlikely that this small amount of spontaneous activity could serve as a reliable indicator of the condition of the auditory nerve.
The correlations of SGN density with the psychophysical and electrophysiological measures tested in the current study do not imply causation. Furthermore, at best, SGN density measures accounted for less than 50 % of the variance in our functional measures. Many other aspects of cochlear biology are likely to be affected by NT treatment and they are likely to be correlated with SGN density to some extent. As noted above, the highest SGN densities were found only in cases with high IHC survival, so we cannot say if it is the IHCs or the SGN densities or some other biological variable that is responsible for the slopes of the MPI and TI functions. For example, as noted above, NT treatment can lead to regrowth of peripheral neurites into the basilar membrane area (Shibata et al. 2011). The histological procedures used in our current study did not allow us to assess all possible biological variables, any one or combination of which could be responsible for the variance in the functional measures. Rather, we refer to the more general theoretical construct that we call “cochlear health,” which represents a suite of traits that include or are correlated with survival of auditory nerve fibers.
In combination, the psychophysical and electrophysiological measures examined in this study can be useful for estimating the health of the implanted cochlea to some extent. In humans with cochlear implants, these measures could be useful for non-invasively estimating cochlear health and monitoring the effects of treatments designed to maintain or improve the health of the implanted cochlea. Recently, we have shown that the MPI slopes can predict ear differences in speech recognition in subjects with bilateral implants (Zhou and Pfingst 2014) and ECAP slopes have also proved to be correlated with speech recognition under limited conditions (Kim et al. 2010). Thus, the combination of these measures might be useful for selecting the best sites for use in processing strategies and for identifying weaker sites that require special rehabilitation using adjustment of stimulation parameters or ultimately, tissue engineering. These potential uses warrant further examination.
Electronic supplementary material
In a set of animals not formally included in this study, we tested the pattern of transduction of an AAV2 vector with a reporter gene (AAV.GFP) and determined that 20 days after the vector was injected into the perilymph of mature deafened guinea pigs, GFP-labeled cells (arrows) were sparsely distributed in the mesothelium lining the scala tympani in both the apical and basal areas of the cochlear partition. GFP was seen in the basal turn (a and b) and as far apically as the third turn (c). In every whole-mount inspected, a small number of cells was transduced, indirectly suggesting that transduction with an AAV2 carrying the Ntf3 gene leads to low levels of NT-3 secreted into cochlear fluids. In a control ear that was not injected with the virus, GFP positive cells were absent (d). These whole-mount preparations include the bone surrounding the osseous spiral lamina which introduces a low level of auto-fluorescence seen in all images. All panels are the same scale (bar = 20 μm). (GIF 101 kb).
Acknowledgements
This work was supported by NIH grants R01 DC007634, R01 DC010412, R56 DC010786, R01 DC015809, and P30 DC 005188, the University of Michigan Center for Organogenesis, and a contract from MED-EL. We express appreciation to Cameron Budenz and James Wiler for assistance with AAV inoculations, Lisa Kabara and Melissa Watts for assistance with the electrophysiology, and Lisa Beyer for histological preparation.
Compliance with Ethical Standards
Conflict of Interest Disclosure Statement
One of the authors, Stefan B. Strahl, is an employee of MED-EL GmbH, which was one of the sponsors of this research. None of the other authors have any financial relationship with any of the sponsors.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10162-017-0633-9) contains supplementary material, which is available to authorized users.
References
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Hume CR, O'Leary SJ, Shepherd RK, Richardson RT. Neurotrophin gene therapy for sustained neural preservation after deafness. PLoS One. 2012;7:e52338. doi: 10.1371/journal.pone.0052338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT. Viability of long-term gene therapy in the cochlea. Sci Rep. 2014;4:4733. doi: 10.1038/srep04733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Budenz CL, Wong HT, Swiderski DL, Shibata SB, Pfingst BE, Raphael Y (2015) Differential effects of AAV.BDNF and AAV.Ntf3 in the deafened adult guinea pig ear. Sci Rep 5:8619 [DOI] [PMC free article] [PubMed]
- Dolan DF, Nuttall AL, Avinash G. Asynchronous neural activity recorded from the round window. J Acoust Soc Am. 1990;87:2621–2627. doi: 10.1121/1.399054. [DOI] [PubMed] [Google Scholar]
- Dudus L, Anand V, Acland GM, Chen SJ, Wilson JM, Fisher KJ, Maguire AM, Bennett J. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vis Res. 1999;39:2545–2553. doi: 10.1016/S0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} companion to applied regression. Second. Thousand Oaks CA: Sage; 2011. [Google Scholar]
- Guy J, Qi X, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999;117:929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010;197:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Marion M. Histopathology of profound sensorineural deafness. Ann N Y Acad Sci. 1983;405:459–484. doi: 10.1111/j.1749-6632.1983.tb31662.x. [DOI] [PubMed] [Google Scholar]
- Kang SY, Colesa DJ, Swiderski DL, Su GL, Raphael Y, Pfingst BE. Effects of hearing preservation on psychophysical responses to cochlear implant stimulation. J Assoc Res Otolaryngol. 2010;11:245–265. doi: 10.1007/s10162-009-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JR, Abbas PJ, Brown CJ, Etler CP, O'Brien S, Kim LS. The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol. 2010;31:1041–1048. doi: 10.1097/MAO.0b013e3181ec1d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Y, Nogimura H, Ida M, Ozawa Y, Kageyama Y, Ohi S, Ito Y, Matsushita K, Takahashi T, Suzuki K, Kazui T, Hayashi S, Enosawa S, Li XK, Suzuki S. Time course of gene expression after the injection of adenoviral vectors containing CTLA4IG gene. Transplant Proc. 2004;36:2443–2445. doi: 10.1016/j.transproceed.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Lalwani AK, Walsh BJ, Carvalho GJ, Muzyczka N, Mhatre AN. Expression of adeno-associated virus integrated transgene within the mammalian vestibular organs. Am J Otol. 1998;19:390–395. [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Hetherington AM, Stakhovskaya O. Brain-derived neurotrophic factor promotes cochlear spiral ganglion cell survival and function in deafened, developing cats. J Comp Neurol. 2011;519:1526–1545. doi: 10.1002/cne.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–562. doi: 10.1002/(SICI)1096-9861(19991004)412:4<543::AID-CNE1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Miller JM, Altschuler RA. Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion cells following deafness. Ann Otol Rhinol Laryngol Suppl. 1995;166:57–60. [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Internat J Devel Neurosci. 1997;15:631–643. doi: 10.1016/S0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Colesa DJ, Hembrador S, Kang SY, Middlebrooks JC, Raphael Y, Su GL. Detection of pulse trains in the electrically stimulated cochlea: effects of cochlear health. J Acoust Soc Am. 2011;130:3954–3968. doi: 10.1121/1.3651820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Hughes AP, Colesa DJ, Watts MM, Strahl SB, Raphael Y. Insertion trauma and recovery of function after cochlear implantation: evidence from objective functional measures. Hear Res. 2015;330:98–105. doi: 10.1016/j.heares.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SF, Grolman W. Auditory nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol. 2014;15:187–202. doi: 10.1007/s10162-013-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searchfield GD, Munoz DJ, Thorne PR. Ensemble spontaneous activity in the guinea-pig cochlear nerve. Hear Res. 2004;192:23–35. doi: 10.1016/j.heares.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242:100–109. doi: 10.1016/j.heares.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Budenz CL, Bowling SA, Pfingst BE, Raphael Y. Nerve maintenance and regeneration in the damaged cochlea. Hear Res. 2011;281:56–64. doi: 10.1016/j.heares.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su GL, Colesa DJ, Pfingst BE. Effects of deafening and cochlear implantation procedures on post-implantation psychophysical electrical detection thresholds. Hear Res. 2008;241:64–72. doi: 10.1016/j.heares.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Method Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- Wise AK, Hume CR, Flynn BO, Jeelall YS, Suhr CL, Sgro BE, O'Leary SJ, Shepherd RK, Richardson RT. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Kraft CT, Colesa DJ, Pfingst BE. Integration of pulse trains in humans and guinea pigs with cochlear implants. J Assoc Res Otolaryngol. 2015;16:523–534. doi: 10.1007/s10162-015-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Relationship between multipulse integration and speech recognition with cochlear implants. J Acoust Soc Am. 2014;136:1257–1268. doi: 10.1121/1.4890640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Xu L, Pfingst BE. Characteristics of detection thresholds and maximum comfortable loudness levels as a function of pulse rate in human cochlear implant users. Hear Res. 2012;284:25–32. doi: 10.1016/j.heares.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In a set of animals not formally included in this study, we tested the pattern of transduction of an AAV2 vector with a reporter gene (AAV.GFP) and determined that 20 days after the vector was injected into the perilymph of mature deafened guinea pigs, GFP-labeled cells (arrows) were sparsely distributed in the mesothelium lining the scala tympani in both the apical and basal areas of the cochlear partition. GFP was seen in the basal turn (a and b) and as far apically as the third turn (c). In every whole-mount inspected, a small number of cells was transduced, indirectly suggesting that transduction with an AAV2 carrying the Ntf3 gene leads to low levels of NT-3 secreted into cochlear fluids. In a control ear that was not injected with the virus, GFP positive cells were absent (d). These whole-mount preparations include the bone surrounding the osseous spiral lamina which introduces a low level of auto-fluorescence seen in all images. All panels are the same scale (bar = 20 μm). (GIF 101 kb).