Abstract
Core body temperature has been shown to affect vestibular end-organ and nerve afferents so that their resting discharge rate and sensitivity increase with temperature. Our aim was to determine whether these changes observed in extracellular nerve recordings of anaesthetized C57BL/6 mice corresponded to changes in the behavioural vestibulo-ocular reflex (VOR) of alert mice. The VOR drives eye rotations to keep images stable on the retina during head movements. We measured the VOR gain (eye velocity/head velocity) and phase (delay between vestibular stimulus and response) during whole-body sinusoidal rotations ranging 0.5–12 Hz with peak velocity 50 or 100 °/s in nine adult C57BL/6 mice. We also measured the VOR during whole-body transient rotations with acceleration 3000 or 6000 °/s2 reaching a plateau of 150 or 300 °/s. These measures were obtained while the mouse’s core body temperature was held at either 32 or 37 °C for at least 35 min before recording. The temperature presentation order and timing were pseudo-randomized. We found that a temperature increase from 32 to 37 °C caused a significant increase in sinusoidal VOR gain of 17 % (P < 0.001). Temperature had no other effects on the behavioural VOR. Our data suggest that temperature effects on regularly firing afferents best correspond to the changes that we observed in the VOR gain.
Keywords: mammalian vestibular system, core body temperature, vestibular primary afferents, vestibulo-ocular reflex, C57BL/6 mice
INTRODUCTION
The vestibulo-ocular reflex (VOR) is our main vision-stabilizing system during rapid head movements. The VOR receives signals from the vestibular organs in the inner ear, via primary vestibular afferents, which encode head motion. These signals are used to drive eye rotations, in the direction opposite to head rotations, so that an image remains stable on the back of the retina during head movement. The gain of the VOR, defined as the magnitude of eye velocity divided by head velocity, during typical far viewing is equal to unity. A recent mouse study has shown that core body temperature can affect the properties of the vestibular afferents so that their baseline firing rate (offset) and sensitivity (gain) to head rotations increase with rising temperature from 30–32 to 35–37 °C (Park et al. 2010). Park et al. (2010) performed extracellular recordings of vestibular afferents innervating the semicircular canals in 61 adult C57BL/6 mice. Regular and irregular vestibular primary afferents were identified based on their resting discharge firing rate and corresponding normalized coefficient of inter-spike variation (Goldberg et al. 1984; Lasker et al. 2008). Mice underwent whole-body sinusoidal rotations ranging from 0.1 to 12 Hz to determine the gain and phase of each afferent. The resting discharge rate rose by ~50 % for regular afferents and by ~90 % for irregular afferents as the temperature increased ~5 °C. Similarly, the sensitivity rose by ~20 % for regular afferents and by ~70 % for irregular afferents, as the temperature increased. The aim of our study was to determine whether the behavioural VOR was affected by these temperature-induced changes in vestibular nerve afferent properties, particularly the high-frequency increase in VOR gain, which is thought to be predominantly mediated by irregularly firing vestibular afferents (Minor et al. 1999; Clendaniel et al. 2001, 2002; Lasker et al. 1999, 2000; Migliaccio et al., 2004a, b, 2008). Given the findings by Park et al. (2010), VOR response changes due to shifts in temperature could help elucidate the contribution from regular and irregular afferents to the behavioural VOR. We measured the VOR gain, phase and latency in C57BL/6 mice during whole-body sinusoidal rotations ranging from 0.5 to 12 Hz and transient rotations with acceleration 3000 or 6000 °/s2 with velocity plateau 150 or 300 °/s. The experiments were carried out at core body temperatures of 32 and 37 °C.
METHODS
All surgical and experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales and were in strict compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Animals Preparation
A total of nine male C57BL/6 wild-type mice (aged 10 to 14 weeks) were used in this study. Each mouse underwent a single session lasting up to 4.5 h. The VOR response was measured while the core body temperature of the mouse was held at 32 or 37 °C. This is the same mouse type and temperature range as used by Park et al. (2010). To facilitate restraint during eye movement testing, a head bolt was placed with the mouse under general (sevoflurane 3–5 %) and local (1 % lidocaine with 1:100K epinephrine, ≤0.2 cm3) anaesthesia. The dorsal surface of the skull was exposed, and four stainless steel screws (M1 × l.5 mm, Small Parts Inc., Miami Lakes, FL) were implanted in the calvarium in square formation. A lightweight metal screw with a flat head was positioned in the centre of the square formation and on the skull in the midline. The screw was embedded in a dental acrylic cap engulfing the four screws. The exact implantation technique has been outlined previously (Migliaccio et al. 2011).
Core Temperature Regulation
All our mice had starting core body temperatures from 30 to 32 °C, and so, a heating blanket was needed to maintain the mouse at either 32 or 37 °C. The heating blanket was shaped and positioned so that it covered the entire body including the area near the ears, while ensuring that the blanket did not cover the eyes. The core body temperature was measured and controlled using a mini rectal thermistor probe connected to an external servomechanism driving the heating blanket (FHC Inc., Bowdoinham, ME). The core body temperature and experimental session time (starting when the animal was first positioned in the VOR test platform) were recorded simultaneously with the head (motor) and eye position and velocity signals. This approach allowed us to ensure that the VOR measurements occurred within ±0.5 °C of the desired temperature and control for the possible effects of fatigue on the VOR. The animal was kept at each desired temperature for at least 35 min before measuring the VOR, thus ensuring that the vestibular organ and nerve periphery reached the core body temperature. The animal was only kept in darkness during the VOR testing. At all other times, the room lights were turned on, the eye marker arrays removed (Migliaccio et al. 2011), and the animals manually rotated for about 10 s every 2 min in order to help keep it alert. We used different temperature sequences for every mouse. Each temperature-controlled time interval lasted 45 min. We varied the time interval between the two temperatures, by pseudo-randomly including a 15–45-min rest period where the animals were not heated (dashed lines in Table 1).
TABLE 1.
The temperature presentation order and timing for each mouse was pseudo-randomized
| ID | Int. 1 (°C) | Int. 2 (°C) | Int. 3 (°C) | Int. 4 (°C) | Int. 5 (°C) |
|---|---|---|---|---|---|
| 1 | – | 32 | 37 | – | 32 |
| 2 | – | 37 | 32 | – | 37 |
| 3 | 32 | – | 37 | 32 | – |
| 4 | 32 | 37 | – | 32 | 37 |
| 5 | 37 | 32 | – | 37 | 32 |
| 6 | 37 | – | 32 | 37 | – |
| 7 | – | 32 | 37 | – | 32 |
| 8 | 32 | 37 | – | 32 | 37 |
| 9 | 37 | – | 32 | 37 | – |
Each session consisted of five intervals; each temperature-controlled interval lasted 45 min. The VOR was recorded at the end of each interval. A dashed line denotes a 15–45-min rest period during which the mouse was not heated
Data Acquisition and Analysis
The method of recording 3D binocular eye movements using high-speed video-oculography and the technique used for off-line analysis of VOR responses have been detailed in several previous studies (Migliaccio et al. 2011; Hübner et al. 2013, 2014, 2015). We measured VOR responses in darkness over most of the naturally occurring range of mouse head movement frequencies, velocities and accelerations (Beraneck et al., 2004) using horizontal, whole-body sinusoidal rotations at 0.5, 1, 2, 5, 10 and 12 Hz with peak velocities of 50 and 100 °/s and transient acceleration stimuli at 3000 and 6000 °/s2 reaching a constant velocity plateau of 150 and 300 °/s, respectively. In the following text, we refer to transient stimulus conditions using the following abbreviations: 3k150 and 6k300 (for a more detailed explanation of both rotational stimuli, see Hübner et al. 2015). The sinusoidal and transient VOR responses were measured in pseudo-randomized order during the last 10 min of each temperature-controlled (not rest) interval.
To analyse the three-dimensional VOR data, we converted eye movements acquired in eye coordinates into rotation vectors in head coordinates. Eye velocity traces with quick phases removed were inverted so that an ideal VOR would yield a gain (eye velocity/head velocity) of +1 and phase of 0 °. For sinusoidal rotations, least-square pure sine waves (frequency was a fixed variable, amplitude and phase were free variables) were fit to each head and eye velocity cycle to compute gain and phase. Positive phase lead denotes eye velocity leading head velocity. For transient steps of acceleration, we fit least-square linear regressions to the constant-acceleration and constant-velocity part of eye and head velocity traces. Using these fits, we calculated three parameters: acceleration gain (G A), constant-velocity gain (G V) and latency. G A was calculated as the average ratio of eye/head acceleration (using the slopes of the constant-acceleration fit). G V was calculated as the average ratio of eye/head velocity (using the point-by-point offset of the constant-velocity fit) during the 200 to 400 ms interval after stimulus onset. Stimulus and response onsets were calculated as the times at which the respective acceleration line-fits intercept with the zero velocity axis. The latency was defined as the time difference between stimulus (head rotation) and response (eye rotation) onset.
Statistical Analysis
Data were analysed in SPSS (Version 22; Armonk, NY) using analysis of covariance (ANCOVA) with full factorial interaction. Two ANCOVAs were used for the sinusoidal data analysis: one with gain and the other with phase as the dependent variable. The independent variables were temperature (32 or 37 °C), eye (‘left’ or ‘right’), stimulus frequency (0.5, 1, 2, 5, 10 or 12 Hz) and velocity (50 or 100 °/s). For all ANCOVAs, the covariate was the time in minutes at which the data point was collected. ANOVA was used after correcting for the effect of time. Three ANCOVAs were used for the transient data analysis: one with G A, one with G V and the other with latency as the dependent variable. The independent variables were temperature (32 or 37 °C), eye (‘left’ or ‘right’), stimulus direction (‘leftward’ or ‘rightward’) and magnitude (3k150 or 6k300). Post hoc pairwise comparisons of group means were performed using Bonferroni’s correction for two-sample t statistics. All statistical tests were performed using a significance level of P = 0.05. Unless stated otherwise, all measurement results are quoted as mean ± standard deviation.
RESULTS
Sinusoidal VOR Response
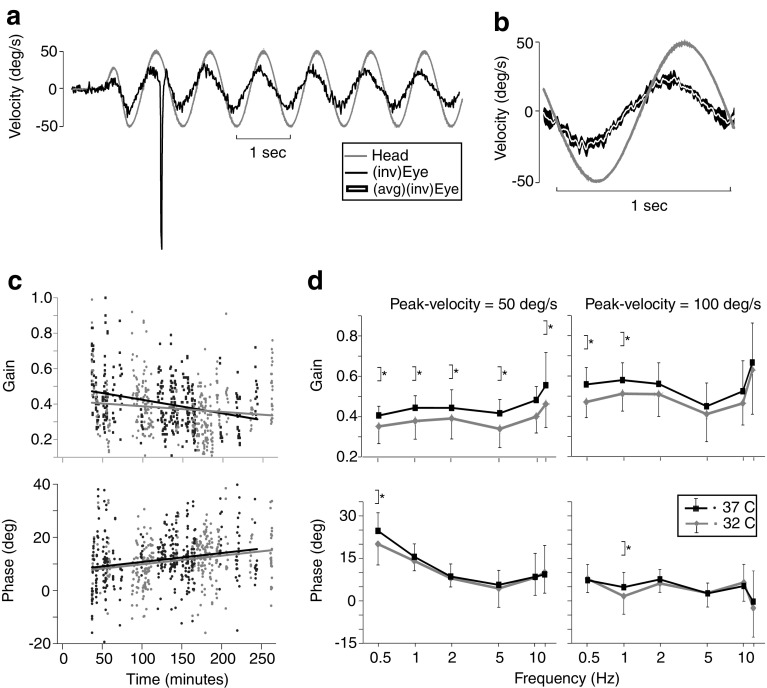
Figure 1a shows the raw data from a typical horizontal VOR response during 1 Hz sinusoids with peak velocity 50 °/s. Figure 2b shows how the single cycles were overlaid to determine the mean head and eye velocity to calculate the VOR gain and phase, which, in this example, was 0.42 ± 0.05 and 23.85 ± 3.63 °, respectively.
FIG. 1.
a Raw data from a typical horizontal VOR response during 1-Hz sinusoids with peak velocity 50 °/s. The grey trace denotes head velocity, and the black trace denotes inverted eye velocity; i.e. the eye is rotating in the opposite direction to the head. b Single full cycles were identified and overlaid to determine the mean head (grey) and eye velocity (white). A sinusoidal least-square fit was made to both head and eye velocity traces. From these fits, the VOR gain and phase were calculated. c Each data point represents the gain (top panel) and phase (bottom panel) for each trial, where each trial stimulated the VOR at one particular temperature, sinusoidal frequency and peak velocity. The time at which the trial occurred affected both the VOR gain and phase, which partially obscured the effect of temperature on VOR gain. The gain steadily decreased (~7 % per h) and the phase steadily increased (~30 % per h) with time. d VOR gain and phase across frequencies at the two different core body temperatures, 32 °C (grey) and 37 °C (black). For sinusoidal stimuli with peak velocity 50 °/s, the 5 °C rise in core temperature significantly increased the VOR gain by 21 % after pooling across frequencies. In contrast, at 100 °/s, only the VOR gains at the lower test frequencies were affected by temperature. Temperature also significantly affected the VOR phase at the two lowest test frequencies. Asterisk denotes a significant difference (P < 0.05)
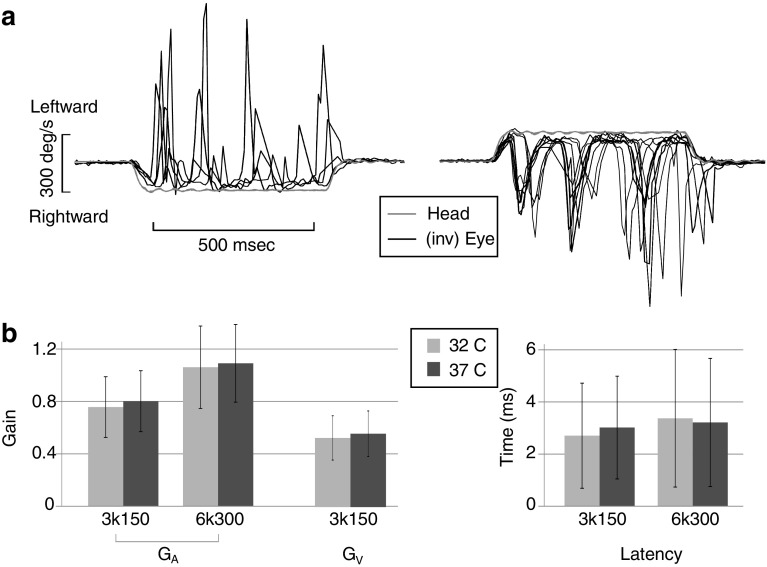
FIG. 2.
a Raw data from typical horizontal VOR responses during transient rotations with acceleration 3000 °/s2 and plateau velocity 150 °/s. The grey traces denote head velocity, and the black traces denote inverted eye velocity responses; i.e. the eye is rotating in the opposite direction to the head. The overlaid responses are shown for both leftward and rightward transients. b The mean acceleration gain G A, velocity gain G V and latency of the transient VOR at the two core body temperatures (32 °C grey; 37 °C black) were not significantly different. Only the stimulus magnitude affected the transient VOR response
The VOR gain was significantly affected by temperature (ANCOVA: F 1,731 = 5.35, P < 0.05). The time at which the VOR gain was recorded seemed to affect the gain between trials, where each trial stimulated the VOR at one particular temperature, sinusoidal frequency and peak velocity. For each animal, we fit regression lines to the individual VOR gains for each trial versus session time and observed a decrease in gain with time trend (i.e. a negative slope coefficient) in 7/9 animals. Analysis after pooling the individual trial gains of all animals showed that the time at which the VOR gain was recorded significantly affected the gain (ANCOVA: F 1,731 = 49.26, P < 0.001), such that it decreased with time (with time in min; gain = −0.00034 × time + 0.42 at 32 °C; gain = −0.0008 × time + 0.50 at 37 °C) (see Fig. 1c, top panel). We corrected for the time effect by adding 0.00034 × time to the 32 °C gains and adding 0.0008 × time to the 37 °C gains. After this correction, temperature effects on VOR gain became highly significant (ANOVA: F 1,731 = 57.82, P < 0.001). ANOVA revealed no significant interactions between temperature and stimulus frequency (ANOVA: F 5,731 = 0.13, P = 0.99), between temperature and stimulus velocity (ANOVA: F 1,731 = 0.71, P = 0.4) or between all three factors (ANOVA: F 5,731 = 0.60, P = 0.70). However, at 50 °/s, the difference in VOR gain between the two temperatures was significant at all test frequencies, whereas at 100 °/s, the difference was significant only at the two lower test frequencies of 0.5 and 1 Hz (see Fig. 1d, top row). At 50 °/s and pooled across frequencies, the VOR gain increased from 0.33 ± 0.1 at 32 °C to 0.40 ± 0.1 at 37 °C, i.e. a 0.07 ± 0.01 or 21 ± 3 % increase. The temperature-induced VOR gain increase was smaller at 100 °/s compared to 50 °/s, where the VOR gain went from 0.50 ± 0.15 at 32 °C to 0.55 ± 0.14 at 37 °C, only a 10 ± 3 % increase. When pooled across all conditions, the VOR gain increased from 0.41 ± 0.15 at 32 °C to 0.48 ± 0.15 at 37 °C, a difference of 0.07 ± 0.01, representing a 17 ± 3 % increase from 32 to 37 °C. Similar to our prior findings, the VOR gain was affected by stimulus peak velocity (ANOVA: F 1,731 = 377.58, P < 0.001), which increased from 0.36 ± 0.11 at 50 °/s to 0.53 ± 0.15 at 100 °/s (see Fig. 1d, top row). Frequency also affected the VOR gain (ANOVA: F 5,731 = 31.45, P < 0.001), which increased from 0.42 ± 0.13 at 0.5 Hz to 0.55 ± 0.20 at 12 Hz. There was a significant interaction between peak velocity and frequency (ANCOVA: F 5,731 = 4.1, P < 0.002), so that the VOR gain increased from 0.32 ± 0.07 at 0.5 Hz and 50 °/s to 0.65 ± 0.21 at 12 Hz and 100 °/s.
We observed an increase in phase with time trend (i.e. a positive slope coefficient) in the same 7/9 animals that had a gain decrease with time trend. Analysis after pooling the individual trial phases of all animals showed that the time at which the VOR phase was recorded significantly affected the phase (ANCOVA: F 1,731 = 51.54, P < 0.001), such that the phase increased in lead with time (with time in min; phase = [0.032 × time + 6.22]° at 32 °C; phase = [0.032 × time + 6.98]° at 37 °C) (see Fig. 1c, bottom panel). After correcting for the time effect by subtracting 0.032 × time from all phase values, temperature effects on VOR phase became significant (ANOVA: F 1,731 = 4.03, P < 0.05), only at the low test frequencies of 0.5 Hz (50 °/s) and 1 Hz (100 °/s) (see Fig. 1d, bottom row). There were no significant interactions between temperature and stimulus frequency (ANOVA: F 5,731 = 0.65, P = 0.66), between temperature and stimulus velocity (ANOVA: F 1,731 = 0.16, P = 0.69) or between all three factors (ANOVA: F 5,731 = 1.28, P = 0.27). Similar to our prior findings, VOR phase was affected by stimulus peak velocity (ANOVA: F 1,731 = 172.87, P < 0.001), which decreased from 11.02 ± 8.60 ° at 50 °/s to 4.14 ± 8.2 ° at 100 °/s (see Fig. 1d bottom row). Frequency also affected the VOR phase (ANOVA: F 5,731 = 40.0, P < 0.001), which decreased from 14.64 ± 9.61 ° at 0.5 Hz to 3.89 ± 13.13 ° at 12 Hz. There was a significant interaction between peak velocity and frequency (ANOVA: F 5,731 = 20.97, P < 0.001), so that the VOR phase decreased from 21.99 ± 7.26 ° at 0.5 Hz and 50 °/s to −1.42 ± 14.5 ° at 12 Hz and 100 °/s.
Transient VOR Response
Figure 2a shows the raw data for typical horizontal VOR responses during transient rotations with acceleration 3000 °/s2 and plateau velocity 150 °/s. The overlaid responses are shown for both leftward and rightward transients. Line fits during the constant acceleration and velocity portions of the stimulus were used to calculate the acceleration (G A) and velocity gains (G V) and latency. For this example, the leftward G A, G V and latency were 0.53 ± 0.15, 0.7 ± 0.05 and 6.88 ± 2.38 ms, respectively.
The acceleration gain G A was affected by the stimulus magnitude (ANCOVA: F 1,288 = 86.54, P < 0.001) and increased when pooled across conditions from 0.78 ± 0.23 at 3k150 to 1.08 ± 0.30 at 6k300, a difference of 0.3 ± 0.03. G A was not affected by temperature (ANCOVA: F 1,288 = 1.56, P = 0.21) or time (ANCOVA: F 1,288 = 1.74, P = 0.19) (see Fig. 2b, left panel). Similarly, the velocity gain G V was not affected by temperature (ANCOVA: F 1,288 = 0.29, P = 0.59) or time (ANCOVA: F 1,288 = 0.14, P = 0.71). The mean G V at 3k150 was 0.54 ± 0.17 (see Fig. 2b, left panel). G V was not calculated for 6k300 transient rotations because there was no evident eye velocity plateau. The VOR latency was not affected by temperature (ANCOVA: F 1,288 = 0.17, P = 0.68), time (ANCOVA: F 1,288 = 3.81, P = 0.05) or stimulus magnitude (ANCOVA: F 1,288 = 2.61, P = 0.11). The mean latency was 3.08 ± 2.29 ms (see Fig. 2b, right panel).
DISCUSSION
In this study, we measured the mouse behavioural VOR at core body temperatures of 32 and 37 °C. Summed across all the sinusoidal stimuli, we found a significant increase of 17 % in VOR gain from the lower to higher temperature. The temperature effect was greater during sinusoidal stimuli with peak velocity 50 °/s (21 % gain increase) compared to 100 °/s (10 %). The VOR phase at the two lowest test frequencies was also affected by temperature. The time at which each data point was recorded in the session significantly affected the sinusoidal VOR gain and phase; i.e. the gain steadily decreased by ~7 % per hour and the phase steadily increased by ~30 % per hour. During transient stimuli, we found no effect of temperature on the VOR. The acceleration gain was only affected by the magnitude of the transient stimulus, whereas the velocity gain and latency were not affected by any of the stimulus parameters.
Limitations in Data Interpretation and Comparison
Before interpretation and comparison of our data with that from by Park et al. (2010) can be performed, it is important to note major differences and potential confounding factors between the two studies. First, the ages of the mice tested were different between studies. Park et al. (2010) tested mice 6–8 weeks old so that a craniotomy could be performed with minimal trauma. In our study, we tested mice 10–14 weeks old to ensure that their cranium had hardened sufficiently for head bolt implantation, which was needed to keep the (unanaesthetized mouse) head fixed during rotational testing. However, in rodents, the vestibular canals and afferent responses have been shown to be fully mature at 3 weeks old (rat: Curthoys 1979). Studies have shown that 4-week-old mice hair cell currents are functionally mature (Rusch et al. 1998), and type I hair cells, which form after type IIs, are fully formed (Anniko 1990; Nordemar 1983), suggesting minimal differences in VOR system function between 6–8- and 10–14-week age groups. Second, Park et al. (2010) tested both sexes, whereas in our study, we only tested male mice. Estrous cycle has been shown to affect mouse behaviour and other physiological parameters including core body temperature (Sanchez-Alavez et al. 2011). The Park et al. (2010) study used a total of 62 mixed gender mice, so the effects of estrous cycle on the results were likely negated, whereas in our behavioural study of nine mice , it would likely have been significant, which is why we only tested males. Third, Park et al. (2010) performed a craniotomy to expose the vestibular nerve. Although we heated our animals using the same technique as Park et al. (2010), i.e. a heating jacket/pad connected to a servomechanism with input from a rectal probe to control core body temperature, it is possible that head temperature in our mice with intact cranium was closer to core temperature than mice with craniotomy, which should result in more consistent temperature effects. Fourth, unlike Park et al. (2010), our mice were unanaesthetized, which introduces several confounding factors, such as fatigue leading to attention deficit, VOR suppression and the contribution of other systems and changes in physiological parameters that could affect the behavioural VOR. We examine these limitations further below.
Each experiment lasted around 4 h. During this time, we saw a consistent decrease in gain and corresponding increase in phase with time. We think that the major reason for this change was fatigue. At 32 °C, mice required minimal heating; i.e. the baseline temperature of mice was 30–32 °C, yet, there was clear a drop in gain and an increase in phase lead with time. At 37 °C, heating was required and the effect of time on gain and phase increased. Taken together, the data suggest that the time effect is in part, but not entirely, due to changes in core temperature. We think that fatigue is a reasonable explanation because it occurs at baseline (temperature) and increases with core temperature; i.e. increased temperature likely increases alertness initially but leads to greater fatigue. Although there are no studies examining fatigue and changes in vestibular neurons, studies show that attention deficits, which can be related to fatigue, can significantly decrease behavioural VOR responses (Weissman et al. 1989; Matta and Enticott 2004).
Our data were collected using the UV fluorescent marker array tracking technique previously outlined in Migliaccio et al. (2011). VOR gains can be lower using this technique because the head-fixed diffused-UV (non-point) light source leaks into the visible spectrum. Although the constricted pupils are covered by the marker array, some of this light might enter the eye resulting in some VOR suppression. In studies where we used an infrared light source instead, which presumably leaked less into the visible spectrum, the gains were ~10 % higher (Hübner et al. 2014, 2015, 2017; Khan et al. 2017). This lighting problem affects all video-oculography techniques to varying extent because most light sources leak into the visible spectrum. Even if some VOR suppression were driving VOR gain changes (i.e. adaptation) during the ten or so minutes of testing, then exposure to ten or more minutes of normal viewing conditions (our interval of normal viewing was at least 35 min) should have been sufficient to wash out any adaptation.
Anaesthesia could affect the normal response of other systems that compensate for changes in temperature. For example, a preliminary study by Han et al. (2007) showed that the efferent vestibular system (EVS) affects primary vestibular afferents. This same single-unit afferent study in anaesthetized mice, however, showed a large difference in afferent characteristics between alpha9 knockout (compromised EVS) and wild-type (normal EVS) mice, suggesting that the EVS was minimally affected by anaesthesia; i.e. assuming an anaesthetized EVS behaves similarly to a compromised EVS. But temperature may have affected other systems that could influence vestibular afferent responses. Conversely, core temperature changes in an unanaesthetized animal likely trigger complex neural system responses, all working at different timescales, and physiological parameter changes that could affect the behavioural VOR.
Primary Vestibular Afferents and the Behavioural VOR
VOR signal processing is thought to occur along tonic (velocity-sensitive) and phasic (acceleration-sensitive) signal pathways (Minor et al. 1999; Lasker et al. 2000). Morphologically, these tonic and phasic pathways resemble the response dynamics (latency and sensitivity) of regularly and irregularly discharging vestibular primary afferents and second-order vestibular neurons, respectively (Hullar and Minor, 1999; Hullar et al. 2005). The tonic pathway is primarily active during stimuli with low-acceleration components and can encode bilateral head rotations over a large range, whereas the phasic pathway has high sensitivity and predominantly encodes short-latency, high-acceleration, unilateral head rotations. As a result, the rapid acceleration response G A at the beginning of a transient step stimulus, or high-acceleration (high frequency and high peak velocity) sinusoid, preferentially activates the phasic pathway. In contrast, the constant-velocity response G V of a transient step stimulus, or low-acceleration (low frequency and low peak velocity) sinusoid, predominantly relies on the velocity-sensitive tonic pathway (Lasker et al. 2000).
Temperature Effects on the Behavioural VOR
If irregular afferents mediate the phasic pathway and the sensitivity of these increased with temperature, then one would predict the following. First, the VOR gain should increase more with increasing frequency and velocity at higher temperatures because both affect the stimulus acceleration, which is predominantly encoded by the phasic component of the VOR response. Second, the phase should lead more with increasing sinusoidal frequency and velocity at higher temperatures, or the latency should decrease for high-acceleration transients, because irregular afferents are most sensitive to head movements and will be recruited first, before the regulars, during the early portion of the VOR response. This prediction contrasts with our data showing that temperature affects the low-acceleration (low velocity and low frequency) sinusoidal VOR gain and phase and does not affect the high-acceleration transient VOR gain and latency. Therefore, our findings suggest that the contribution of regular afferents is most affected by temperature.
Park et al. (2010) showed that the resting rate and sensitivity of irregular afferents were most affected by temperature, but perhaps because irregular afferents are only a small part (~30 %; Baird et al. 1988) of the total peripheral vestibular afferent signal, moderate changes in these do not have a large effect on the VOR. For example, Park et al. (2010) showed that the resting rate of regular and irregular afferents increased by 50 and 90 %, respectively, when the temperature increased by 5 °C. However, the resting rate of the vestibular nerve signal due to the contribution of regular and irregular afferents increased by 35 % (70 % of 50 %) and 27 % (30 % of 90 %), respectively, when taking into account the ratio of regular to irregular afferents. So, the change in contribution from regular afferents, due to temperature-induced changes in resting rate, was ~30 % larger than that from irregular afferents. This line of reasoning, however, cannot be repeated when considering changes in afferent sensitivity due to temperature, which was ~4 times larger in irregular afferents. Our data suggest that temperature effects on the afferent resting rate, not sensitivity, are mediating the changes that we measured in sinusoidal VOR gain. Perhaps, central adaptation mechanisms are better at compensating for temperature-induced changes in sensitivity rather than resting firing rate. It is also possible that compensation is closer to ideal for the highly modifiable phasic pathway compared to the relatively less adaptable tonic pathway mediated mostly by regular firing afferents, so that postcompensation temperature effects would seem most apparent in the tonic pathway (Minor et al. 1999; Clendaniel et al. 2001, 2002; Migliaccio et al. 2004a, b, 2008). This hypothesis is consistent with the finding that during caloric testing, a common clinical test that examines the function of the labyrinth using warm and cold water or air administered to the external auditory canal, there is a small component of the low-frequency (note the head is kept stationary) vestibular-evoked eye movement that is due to the direct effect of temperature on vestibular-nerve afferents and/or hair cells (Young and Anderson 1974; Suzuki et al. 1998; Minor and Goldberg 1990). Our findings are also consistent with a primate study by Minor and Goldberg (1991) which showed that modifying the response of irregular afferents via galvanic stimulation had minimal effect on the behavioural VOR. These authors confirmed that their galvanic stimulus had minimal effect on regular afferents, but ablated the irregular afferent response. Similarly, several human transmastoid galvanic studies, which presumably also preferentially reduce the response of irregular afferents, showed minimal effects on the behavioural VOR (Karlberg et al. 2000; Migliaccio et al. 2013).
Acknowledgements
A.A. Migliaccio was supported by The Garnett Passe and Rodney Williams Memorial Foundation Senior/Principal Research Fellowship in Otorhinolaryngology and NHMRC Project Grant APP1061752. P. P. Hübner was supported by a University of New South Wales (UNSW) International Research Scholarship and a Neuroscience Research Australia (NeuRA) supplementary scholarship.
Compliance with Ethical Standards
All surgical and experimental procedures were approved by the Animal Care and Ethics Committee of the University of New South Wales and were in strict compliance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
References
- Anniko M. Development of the vestibular system. JR. Coleman (Ed.), Development of sensory systems in mammals, Wiley, New York, 341–440, 1990
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E, Uno A. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. 2004;92:1668–1684. doi: 10.1152/jn.00158.2004. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. IV Responses after spectacle-induced adaptation. J Neurophysiol. 2001;86:1594–1611. doi: 10.1152/jn.2001.86.4.1594. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Differential adaptation of the linear and nonlinear components of the horizontal vestibuloocular reflex in squirrel monkeys. J Neurophysiol. 2002;88:3534–3540. doi: 10.1152/jn.00404.2002. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. The development of function of horizontal semicircular canal primary neurons in the rat. Brain Res. 1979;167:41–52. doi: 10.1016/0006-8993(79)90261-0. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Han GC, Lasker DM, Vetter DE, Minor LB. (2007) Extracellular recordings from semicircular canal afferents in mice that lack the alpha 9 nicotinic acetylcholine receptor subunit in ARO midwinter meeting, pp. 1–2
- Hübner PP, Lim R, Brichta AM, Migliaccio AA. Glycine receptor deficiency and its effect on the horizontal vestibulo-ocular reflex: a study on the SPD1J mouse. J Assoc Res Otolaryngol. 2013;14:249–259. doi: 10.1007/s10162-012-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. Velocity-selective adaptation of the horizontal and cross-axis vestibulo-ocular reflex in the mouse. Exp Brain Res. 2014;232:3035–3046. doi: 10.1007/s00221-014-3988-8. [DOI] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in the high-frequency response and short-term adaptation of the vestibulo-ocular reflex. J Neurophysiol. 2015;114:3154–3165. doi: 10.1152/jn.00307.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner PP, Khan SI, Migliaccio AA. The mammalian efferent vestibular system plays a crucial role in vestibulo-ocular reflex compensation after unilateral labyrinthectomy. J Neurophysiol. 2017;117:1553–1568. doi: 10.1152/jn.01049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibulo-ocular reflexes. J Neurophysiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- Karlberg M, McGarvie L, Magnusson M, Aw ST, Halmagyi GM. The effects of galvanic stimulation on the human vestibulo-ocular reflex. Neuroreport. 2000;11:3897–3901. doi: 10.1097/00001756-200011270-00058. [DOI] [PubMed] [Google Scholar]
- Khan SI, Hübner PP, Brichta AM, Smith DW, Migliaccio AA. Aging reduces the high-frequency and short-term adaptation of the vestibulo-ocular reflex in mice. Neurobiol Aging. 2017;51:122–131. doi: 10.1016/j.neurobiolaging.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Backous DD, Lysakowski A, Davis GL, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. II. Responses after canal plugging. J Neurophysiol. 1999;82:1271–1285. doi: 10.1152/jn.1999.82.3.1271. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III Responses after labyrinthectomy. J Neurophysiol. 2000;83:2482–2496. doi: 10.1152/jn.2000.83.5.2482. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Han GC, Park HJ, Minor LB. Rotational responses of vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. J Assoc Res Otolaryngol. 2008;9:334–348. doi: 10.1007/s10162-008-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta FV, Enticott JC. The effects of state of alertness on the vestibulo-ocular reflex in normal subjects using the vestibular rotational chair. J Vestib Res. 2004;14:387–391. [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC, Jiradejvong P, Lasker DM, Clendaniel RA, Minor LB. The 3-dimensional vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. Exp Brain Res. 2004;159:433–446. doi: 10.1007/s00221-004-1974-2. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human horizontal vestibulo-ocular reflex is eliminated by a partial peripheral gentamicin lesion. Exp Brain Res. 2004;159:92–98. doi: 10.1007/s00221-004-1974-2. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Minor LB, Carey JP. Vergence-mediated modulation of the human angular vestibulo-ocular reflex is unaffected by canal plugging. Exp Brain Res. 2008;186:581–587. doi: 10.1007/s00221-007-1262-z. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Meierhofer R, Della Santina CC. Characterization of the 3D angular vestibulo-ocular reflex in C57BL6 mice. Exp Brain Res. 2011;210:489–501. doi: 10.1007/s00221-010-2521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio AA, Della Santina CC, Carey JP. Transmastoid galvanic stimulation does not affect the vergence-mediated gain increase of the human angular vestibulo-ocular reflex. Exp Brain Res. 2013;224:489–499. doi: 10.1007/s00221-012-3330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. 1991;11:1636–1648. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Influence of static head position on the horizontal nystagmus evoked by caloric, rotational and optokinetic stimulation in the squirrel monkey. Exp Brain Res. 1990;82:1–13. doi: 10.1007/BF00230832. [DOI] [PubMed] [Google Scholar]
- Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I Normal Responses J Neurophysiol. 1999;82:1254–1270. doi: 10.1152/jn.1999.82.3.1254. [DOI] [PubMed] [Google Scholar]
- Nordemar H. Postnatal development of the vestibular sensory epithelium in the mouse. Acta Otolaryngol. 1983;96:447–456. doi: 10.3109/00016488309132731. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lasker DM, Minor LB. Static and dynamic discharge properties of vestibular-nerve afferents in the mouse are affected by core body temperature. Exp Brain Res. 2010;200:269–275. doi: 10.1007/s00221-009-2015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci. 1998;18:7487–7501. doi: 10.1523/JNEUROSCI.18-18-07487.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr) 2011;33:89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kadir A, Hayashi N, Takamoto M. Direct influence of temperature on the semicircular canal receptor. J Vestib Res. 1998;8:169–173. doi: 10.1016/S0957-4271(97)00003-7. [DOI] [PubMed] [Google Scholar]
- Weissman BM, DiScenna AO, Ekelman BL, Leigh RJ. Effect of eyelid closure and vocalization upon the vestibulo-ocular reflex during rotational testing. Ann Otol Rhinol Laryngol. 1989;98:548–550. doi: 10.1177/000348948909800710. [DOI] [PubMed] [Google Scholar]
- Young JH, Anderson DJ. Response patterns of primary vestibular neurons to thermal and rotational stimuli. Brain Res. 1974;79:199–212. doi: 10.1016/0006-8993(74)90411-9. [DOI] [PubMed] [Google Scholar]