Abstract
Microbial biofilms formed on biomaterials are major causes of chronic infections. Among them, Gram-positive bacteria Streptococcus mutans and Staphylococcus aureus are important pathogens causing infections associated with dental caries (tooth-decay) and other medical implants. Unfortunately, current antimicrobial approaches are ineffective in disrupting established biofilms and new methods are needed to improve the efficacy. In this study, we report that the biofilm cells of S. mutans and S. aureus can be effectively killed by low-level direct current (DC) and through synergy in concurrent treatment with DC and chlorhexidine (CHX) at low concentrations. For example, after treatment with 28 µA/cm2 DC and 50 µg/mL CHX for 1 h, the viability of biofilm cells was reduced by approximately 4 and 5 logs for S. mutans and S. aureus, respectively. These results are useful for developing more effective approaches to control pathogenic biofilms.
Electronic supplementary material
The online version of this article (10.1186/s13568-017-0505-z) contains supplementary material, which is available to authorized users.
Keywords: Biofilm, Electrochemical control, Chlorhexidine, Synergistic effects
Introduction
Biofilms are formed by microbial cells embedded in a matrix comprised of extracellular polymeric substance (EPS) containing polysaccharide, proteins, and DNA. The presence of this extracellular matrix provides protection to microbial pathogens from antimicrobials to host immune cells/factors (Liu et al. 2016; Hall and Mah 2017). Biofilms can form on both biotic and abiotic surfaces and are common causes of chronic infections including dental plaques (Smith et al. 2011; Song et al. 2015). The protection of EPS plus the dormancy of biofilm cells render these multicellular structures extremely difficult to eradicate (Kouidhi et al. 2015; Smith et al. 2011; Song et al. 2015).
Streptococcus mutans is a Gram-positive bacterium commonly found in human dental biofilms. It is a dominant species with higher biomass in dental biofilms than other Streptococcus species, including S. sanguinis, S. mitis, and S. salivarius, due to its acid tolerance and thus the capability to live in low pH environment of oral cavities (Bender et al. 1986; Harper and Loesche 1984; Kreth et al. 2005). S. mutans expresses multiple exoenzymes (glucosyltransferases) that make it the primary EPS producer in oral cavity (Falsetta et al. 2014), while it is also highly acidogenic and aciduric. S. mutans can rapidly colonize tooth surface and establish cariogenic biofilms with extracellular polysaccharides (EPS). This acidifies the local microenvironment and promotes the growth of an acidogenic microbiota, facilitating the development of dental caries (Falsetta et al. 2012, 2014).
Staphylococcus aureus is also an abundant Gram-positive bacterium, which usually harbors in the nasal passages and ears of patients (Smith et al. 2011). Previous studies have shown that S. aureus is not only a significant cause of many localized and systemic infections such as osteomyelitis (Lew and Waldvogel 2004), chronic wound infection (Hansson et al. 1995), and chronic rhinosinusitis (Stephenson et al. 2010), but also has a strong connection to dental implant infections (Salvi et al. 2008; Harris et al. 2004). The established biofilms of S. aureus, especially the methicillin-resistant S. aureus (MRSA), are highly tolerant to common antimicrobial treatments (Jones et al. 2001; O’Donnell et al. 2015; Lewis et al. 2015).
Few approaches are currently available for controlling cariogenic biofilms (Liu et al. 2016). Chlorhexidine (CHX) is considered the “gold standard” for oral antimicrobial therapy (Jones 1997). However, use of high dose CHX has adverse side effects such as tooth staining and calculus formation. Also, CHX is not recommended for long term daily therapeutic use (Flotra et al. 1971). In 1994, Costerton et al. (1994) reported bacterial killing by synergistic effects between low-level electric currents and antibiotics, a phenomenon named “bioelectric effects”. Since 1990s, direct currents (DCs) ranging from μA to mA have been reported for their bactericidal effects after a relatively long period (from several hours to days) of treatment time (Costerton et al. 1994; del Pozo et al. 2009; Schmidt-Malan et al. 2015; Spadaro et al. 1974) either by DC alone or with antibiotics together (Wattanakaroon et al. 2000; Niepa et al. 2012, 2016a). Recent studies reported that mA level DC could enhance the killing effect of 0.2% (200 µg/mL) chlorhexidine on biofilms of Gram-negative Porphyromonas gingivalis, although there was no bactericidal effect by DC alone (Lasserre et al. 2015). To explore the potential of lower levels of DC and CHX in killing dental biofilms of Gram-positive bacteria, we conducted this study using S. mutans and S. aureus as model species. We demonstrate that stainless steel electrode derived DC and CHX have strong synergy in killing S. mutans and S. aureus biofilms; and the levels of DC and CHX appear to be lower than other reported systems.
Materials and methods
Bacteria strains and growth media
Staphylococcus mutans Clarke strain (ATCC 25175) was cultured in brain heart infusion (BHI) broth (BD Biosciences, San Jose, CA, U.S.) (Murchison et al. 1982). The S. aureus ALC2085 (strain RN6390 containing pALC2084) was obtained from Dr. Karin Sauer at Binghamton University (Sauer et al. 2009) and cultured in Lysogeny broth (LB) (Sambrook and Russell 2001) containing 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl, supplemented with 10 µg/mL chloramphenicol (Sigma-Aldrich, St. Louis, MO, U.S.). Both strains were routinely cultured overnight at 37 °C with shaking at 200 rpm.
Biofilm formation
Biofilms were formed on acrylic coupons (3.5 cm × 0.5 cm × 0.1 cm; McMaster-Carr, Aurora, OH, U.S.). Briefly, 25 µL of an overnight culture of S. mutans was used to inoculate a petri dish containing 25 mL of BHI medium and acrylic coupons. The culture was incubated at 37 °C for 48 h without shaking. Then the coupons with biofilms were removed from petri dish and washed three times with 0.85% NaCl solution to remove all planktonic cells and only retained the firmly attached cells for DC and CHX treatments. The S. aureus biofilm samples were prepared in the same way except that the medium was LB plus 10 µg/mL chloramphenicol and the incubation time was reduced to 24 h due to a higher growth rate of S. aureus.
Electrochemical treatment
The experimental system for DC treatment is the same as we described previously (Niepa et al. 2012, 2016b). Briefly, an electrochemical cell was constructed with two electrodes on the opposite sides of a plastic cuvette (Thermo Fisher Scientific, Pittsburg, PA, U.S.). DC was generated using a potentiostat (Potentiostat WaveNow, Pine Research Instrumentation, Raleigh, NC, U.S.) in the three electrode system with a silver wire (0.015” diameter, A-M Systems, Sequim, WA, U.S.) placed in bleach for 30 min to create an Ag/AgCl reference electrode. The DC level and voltage across the electric field were monitored and recorded using the AfterMath software (Potentiostat WaveNow, Pine Research Instrumentation, Raleigh, NC, U.S.) in the galvanostatic mode during the treatment.
DC treatment of biofilms
Each DC treatment was carried out in 3 mL 0.85% NaCl solution. First, a sterile SS304 electrode (3.5 cm × 0.95 cm × 0.05 cm) was inserted into a cuvette, followed by an acrylic coupon with S. mutans or S. aureus biofilm attached. Another sterile SS304 electrode was then inserted on the opposite side. The biofilm was treated galvanostatically with direct current (DC) for 1 h in the absence or presence of CHX (MP Biomedicals, Solon, OH, U.S.). Samples treated with DC or CHX alone and untreated samples were used as controls. After treatment, each acrylic coupon was transferred to a 10 mL tube containing 5 mL 0.85% NaCl solution. The biofilm cells were removed from the surface by gentle sonication for 1 min. The number of viable cells detached from acrylic coupons was quantified by counting colony forming units (CFUs) in the solution.
To further evaluate the effects in an environment similar to that of oral cavity, the test medium was replaced with artificial saliva medium or a mixture of 0.85% NaCl and artificial saliva medium (2:1). The recipe of artificial saliva from Pratten et al. (1998) was followed. It contains 2 g/L yeast extract, 5 g/L peptone, 2.5 g/L type III hog gastric mucin, 0.2 g/L NaCl, 0.2 g/L KCl, and 0.3 g/L CaCl2, supplemental with 1.25 mL of sterile 40% urea. The CHX was tested at 50 µg/mL to 500 µg/mL. The treatment process was the same as described above for 0.85% NaCl solution.
Live/dead staining
To corroborate the CFU results, another set of acrylic coupons with biofilms treated with DC and CHX in the same way were stained with Live/Dead staining kit (Life Technologies Inc., Carlsbad, CA, U.S.) for 10 min. Then the biofilm samples were imaged using a fluorescence microscope (Axio Imager M1, Carl Zeiss Inc., Berlin, Germany).
Statistical analysis
All data are presented as mean ± standard deviation. Statistical significance was assessed with two-way ANOVA followed by Tukey test. Statistical significance was set as p < 0.05. All analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Effects of DC and CHX on S. mutans and S. aureus biofilms in 0.85% NaCl solution
As shown in Fig. 1, treatment with either CHX (at 5, 10, 20, 50, 100 and 200 µg/mL, Fig. 1a) or DC (at 7, 14 and 28 µA/cm2, Fig. 1b) showed moderate but statistically significant killing (p < 0.05, two-way ANOVA followed by Tukey test). For example, up to 1.2 log and 0.7 log of killing was obtained with 28 µA/cm2 DC and 50 µg/mL CHX, respectively. Furthermore, synergy was observed between DC and CHX in killing S. mutans biofilms dose dependently. Among the tested conditions, the maximum killing effect (4 logs) was observed under the condition of 28 µA/cm2 DC and 50 µg/mL CHX (p = 0.02, two-way ANOVA followed by Tukey test; Figs. 1c, 2a). The higher concentration of CHX (100 and 200 µg/mL) did not show significant increase in killing of S. mutans biofilm cells (compared to 50 µg/mL) both in the absence and presence of DC (p > 0.6, two-way ANOVA followed by Tukey test; Fig. 1a, c).
Fig. 1.

Viability of S. mutans biofilm cells after 1 h treatment. a Treatment with CHX alone. b Treatment with DC alone. c Concurrent treatment with CHX and DC. All treatments were tested in 0.85% NaCl solution
Fig. 2.

Viability of S. mutans biofilm cells after treatment with CHX, DC, or concurrent treatment. a Treatment in 0.85% NaCl. DC level: 28 µA/cm2. CHX dosage: 50 µg/mL. b Treatment in artificial saliva. DC level: 28 µA/cm2. CHX dosage: 100 or 500 µg/mL
Similar synergistic effects were also observed for S. aureus biofilms under the same treatment conditions. The number of viable S. aureus biofilm cells was reduced by more than 5 logs (p < 0.0001, two-way ANOVA with Tukey test; Fig. 3a) after treatment with 28 µA/cm2 DC and 50 µg/mL CHX for 1 h in 0.85% NaCl solution. In comparison, treatment with the same level of DC or CHX alone only reduced the number of viable biofilm cells by 60.0 ± 7.9 and 74.3 ± 2.5% (less than 1 log for both conditions), respectively.
Fig. 3.
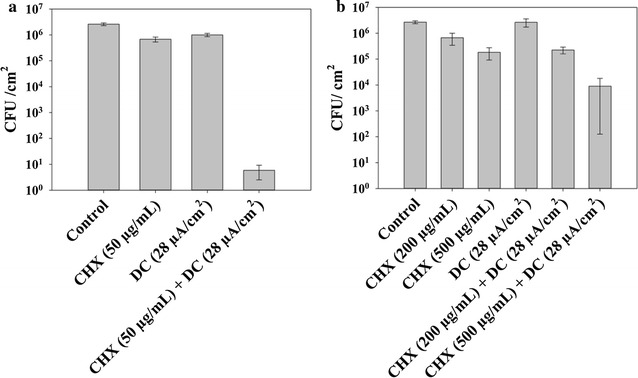
Viability of S. aureus biofilm cells after treatment with CHX, DC, or concurrent treatment. a Treatment in 0.85% NaCl. DC level: 28 µA/cm2. CHX dosage: 50 µg/mL. b Treatment in artificial saliva. DC level: 28 µA/cm2. CHX dosage: 200 or 500 µg/mL
The CFU results were corroborated with fluorescence microscopy. According to the images from Live/Dead staining of S. mutans and S. aureus biofilms, the number of live cells (green) decreased when treated with DC and CHX even at low doses (7 µA/cm2 DC and 5 µg/mL CHX for S. mutans, 28 µA/cm2 DC and 20 µg/mL CHX for S. aureus); and almost no live cells (green fluorescence only) were found on the surface of acrylic coupons after concurrent treatment with DC and CHX together (Figs. 4, 5). Compared with sample treated with DC alone, samples treated with both CHX and DC concurrently only have patches of cell debris in red, suggesting that substantial cell lysis might have occurred.
Fig. 4.
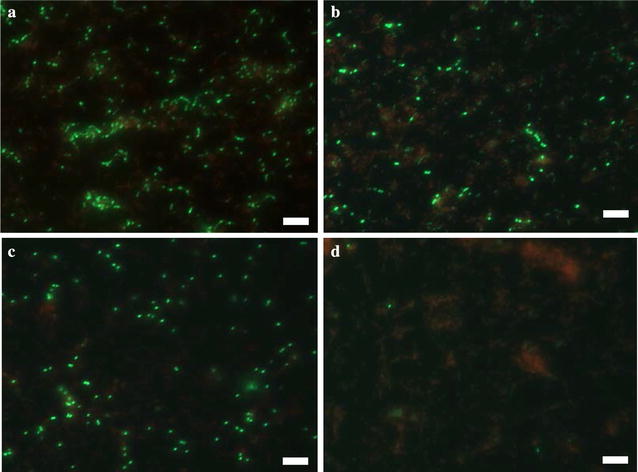
Live/dead staining of S. mutans biofilms after treatment with CHX, DC, or concurrent treatment. a Without treatment. b Treatment with 5 μg/mL CHX. c Treatment with 7 μA/cm2 DC. d Concurrent treatment with 5 μg/mL CHX plus 7 μA/cm2 DC. Bar = 20 μm
Fig. 5.
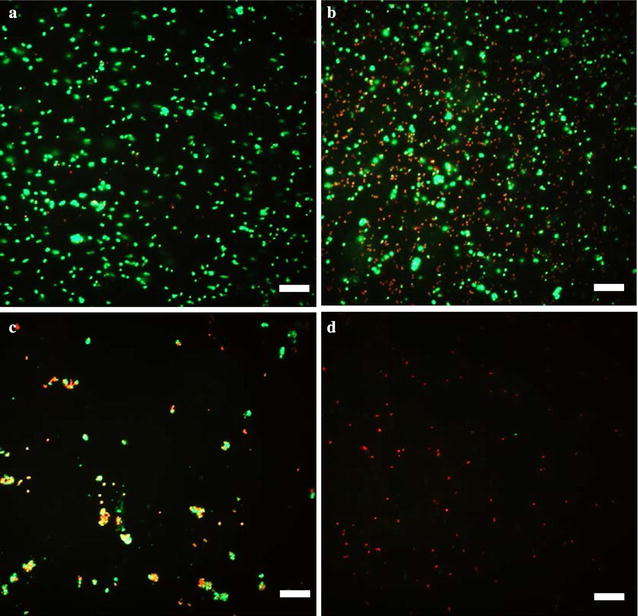
Live/dead staining of S. aureus biofilms after treatment with CHX, DC, or concurrent treatment. a Without treatment. b Treatment with 20 μg/mL CHX. c Treatment with 28 μA/cm2 DC. d Concurrent treatment with 20 μg/mL CHX and 28 μA/cm2 DC. Bar = 20 μm
During treatment, we also observed that some small particles and tiny gas bubbles were released from anode to cathode, respectively. The particles are metal oxides as we reported in our previous study on P. aeruginosa under the same experimental setup (Niepa et al. 2012, 2016a). Based on the half reaction potential of related species (Lide 2006), we speculate that hydrogen peroxide was generated by the reduction reaction of the oxygen in the solution. It will be interesting to further characterize these reactions and link the electrochemical reactions products to the killing effects. This is part of our ongoing work.
Effects in the presence of artificial saliva
Since the surface of dental implants is commonly covered with saliva, we also tested the effects of DC and CHX in the presence of artificial saliva. When artificial saliva was added to 0.85% NaCl solution as treatment medium, the killing effects were reduced but still significant (p < 0.05, two-way ANOVA followed by Tukey test; Additional file 1: Figures S1 and S2). For example, the reduction of biofilm cell viability was 98.0 ± 0.4% (~ 1.7 log) when S. aureus biofilm was treated with 50 µg/ml CHX and 28 µA/cm2 DC in a mixture of artificial saliva and 0.85% NaCl solution (1:2 v/v) (Additional file 1: Figure S1). No killing effect was observed when biofilms were treated in pure artificial saliva medium under the same dosage of CHX or DC level. However, when the concentration of CHX increased to 500 µg/mL (0.05 w/v%; the dosage used in commercial oral rising products is 0.12 w/v%) while keeping the DC level at 28 µA/cm2, the number of viable S. aureus cells in biofilm was reduced by 2.5 logs compared to untreated control (p = 0.005, two-way ANOVA followed by Tukey test; Fig. 3b). The viability of biofilm cells treated with CHX alone was reduced by approximately 1 log and no significant killing effect was observed for 28 µA/cm2 DC treatment alone (Fig. 3b). Similar results were observed for S. mutans biofilms (Fig. 2b and Additional file 1: Figure S1), although the killing of S. mutans biofilm cells in artificial saliva medium was lower than S. aureus. The number of viable cells was reduced by 0.54 log, 0.17 log, and 1.63 log when treated with CHX alone, DC alone, or concurrent treatment with CHX and DC, receptively (p = 0.02, two-way ANOVA followed by Tukey test; Fig. 2b).
Discussion
Direct currents and alternative currents (AC) are known to kill biofilm cells in the presence or absence of antibiotics, and treatment time tested to date varies from hours to days (del Pozo et al. 2009; Schmidt-Malan et al. 2015; Spadaro et al. 1974). Our group recently found synergetic effect between low level DC and the antibiotic tobramycin in killing Pseudomonas aeruginosa biofilm and persister cells (Niepa et al. 2012; 2016b). However, most of previous studies focus on biofilms formed directly on the surface of electrodes.
To mimic real applications, it is important to test biofilms that are not in direct contact with electrodes. In this study, we set a sandwich structure with biofilms formed on acrylic coupons in the middle of the electric field and about 1.5 mm from each electrode. Our results show that the viability of S. mutans and S. aureus biofilm cells (placed between two electrodes) on the surface of denture material can be reduced by low level DC and CHX through concurrent treatment in 1 h; and the effect was approximately 1–3 logs stronger than that obtained with the same level of DC or CHX alone indicating synergistic effects between DC and CHX in killing biofilm cells of these two dental bacteria. The effect was more profound in 0.85% NaCl solution than in the artificial saliva medium. The images of Live/Dead staining also confirmed that there was profound killing by concurrent treatment. We speculated that mucin and other proteins in artificial saliva medium might repress the killing effects of CHX and DC, since DC kills cells partially through the generation and movement of reactive species from electrochemical reactions (Niepa et al. 2012).
We speculated that this synergy was primarily resulted from the interaction between the products of DC treatment and CHX. In the recent studies, it showed that hydrogen peroxide was generated from electrode surface during electrical treatment of bacteria biofilms (Istanbullu et al. 2012; Sultana et al. 2015), which had been reported for its synergetic antibacterial effect with CHX against streptococcus and staphylococcus species (Steinberg et al. 1999). Furthermore, some metal ions (Zn2+, Cu2+) were shown for their capabilities to enhance the effect of CHX on different oral pathogens (Cronan et al. 2006; Drake et al. 1993). The stainless-steel electrodes used in this study have a larger surface area and can release multiple types of metal ions including Fe2+, Fe3+, Cr2+, Cr3+ and Cr6+ during DC treatment (Niepa et al. 2012). Fe2+ and Fe3+ ions were found to kill P. aeruginosa persister cells in the presence of antibiotics in an electric field (Niepa et al. 2016a). Our lab also found that Cr3+ and Cr6+ can form complex with certain antibiotic compounds, and thus increase the affinity between antibiotics and intracellular targets (Niepa et al. 2016b). It is possible that some released ions interact with CHX molecules and result in the observed synergy in killing S. mutans and S. aureus. This is part of our ongoing study.
Recently, Lasserre et al. (2015) reported that the viability of P. gingivalis biofilm could be reduced by 81.1 and 98.9% in 10 min when treated with 2000 µg/mL (0.2 w/v%) CHX alone and concurrent treatment with same dosage of CHX and 5882 µA/cm2 DC, receptively; while the treatment with DC itself did not kill P. gingivalis cells. The biofilms were cultured on the discs of a Modified Robbins Device (MRD), which were placed between two electrodes of platinum wires in the MRD’s chamber. This is an exciting discovery, but the DC level appears high and may not be suitable for in vivo therapy, especially for the implants close to nervous systems that do not tolerate more than a maximum current density of 30 µA/cm2 (McCreery et al. 1990; Shannon 1992; Clark 2003). Hence, it is necessary to reduce DC to µA level for future in vivo applications. In this study, we treated S. aureus and S. mutans biofilm without direct contact to electrodes by placing an acrylic coupon in the middle of a low-level electric field and parallel to the electrode surfaces. By using stainless steel as electrode material, the level of DC and CHX in our study are much lower (28 µA/cm2 DC and 50 µg/mL CHX), and strong killing effects (3–4 logs) were obtained.
CHX is bacteriostatic at low concentrations by affecting the integrity of bacterial cell wall and bactericidal at high concentrations by disrupting the cell (McDonnell and Russell 1999). S. mutans and S. aureus appear to be quite susceptible to CHX according to MIC data (< 8 μg/mL) (Chung et al. 2006). However, the maximum killing of preformed biofilms by CHX alone in our experimental system was only less than 1.5 logs even with a dosage up to 500 μg/mL.
Through synergy with DC, CHX was found to be more effective in killing biofilm cells. The effective doses of CHX we used were only 50 μg/mL (0.005 w/v%) in 0.85% NaCl solution and 500 μg/mL (0.05 w/v%) in artificial saliva medium. This CHX level is expected to be safe because the commercial products for oral wash have approximately 1200 µg/mL (0.12 w/v%)–2000 µg/mL (0.2 w/v%) of CHX. The exact mechanism for such synergistic killing is unknown and is part of our ongoing research.
In summary, we demonstrated that the biofilm cells of two Gram-positive pathogenic bacteria, S. mutans and S. aureus, could be efficiently killed by concurrent treatment with low level DC and CHX in 1 h. This electrochemical control is effective against the biofilms formed on the acrylic materials. The synergistic effect between DC and CHX can help design new devices and strategies for controlling pathogenic biofilms. The interaction between electrochemical products and CHX may play a significant role in the observed synergy in biofilm killing, which deserves further study.
Authors’ contributions
HW and DR designed the research. DR supervised the research. HW performed all experiments and analyzed data with DR. HW and DR wrote the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
We thank Dr. Hyun Koo at University of Pennsylvania for helpful comments on the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its supplement materials.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The authors are grateful for the support of the Milton and Ann Stevenson Endowment.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AC
alternative currents
- ANOVA
analysis of variance
- BHI
brain heart infusion
- CHX
chlorhexidine
- DC
direct current
- EPS
extracellular polysaccharides
- MRD
modified Robbins device
- LB
lysogeny broth
- S. aureus
Staphylococcus aureus
- S. mutans
Streptococcus mutans
Additional file
Additional file 1: Figure S1. Viability of S. aureus biofilm cells after 1 h treatment with 50 μg/mL CHX alone, 28 μA/cm2 DC alone or concurrent treatment with CHX and DC. The treatments were tested in a mixture of 0.85% NaCl and artificial saliva medium (2:1, v/v). Figure S2. Viability of S. mutans biofilm cells after 1 h treatment with 50 μg/mL CHX alone, 28 μA/cm2 DC alone or concurrent treatment with CHX and DC. The treatments were tested in a mixture of 0.85% NaCl and artificial saliva medium (2:1, v/v).
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13568-017-0505-z) contains supplementary material, which is available to authorized users.
Contributor Information
Hao Wang, Email: hwang50@syr.edu.
Dacheng Ren, Phone: +1-315-443-4409, Email: dren@syr.edu.
References
- Bender GR, Sutton SV, Marquis RE. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53:331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Choo JH, Lee MH, Hwang JK. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006;13:261–266. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Clark G. Cochlear Implants: Fundamentals and applications. New York: Springer Verlag; 2003. p. 171. [Google Scholar]
- Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother. 1994;38:2803–2809. doi: 10.1128/AAC.38.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan CA, Potempa J, Travis J, Mayo JA. Inhibition of Porphyromonas gingivalis proteinases (gingipains) by chlorhexidine: synergistic effect of Zn(II) Oral Microbiol Immunol. 2006;21:212–217. doi: 10.1111/j.1399-302X.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother. 2009;53:41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake DR, Grigsby W, Cardenzana A, Dunkerson D. Synergistic, growth-inhibitory effects of chlorhexidine and copper combinations on Streptococcus mutans, Actinomyces viscosus, and Actinomyces naeslundii. J Dent Res. 1993;72:524–528. doi: 10.1177/00220345930720020901. [DOI] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Lemos JA, Silva BB, Agidi S, Scott-Anne KK, Koo H. Novel antibiofilm chemotherapy targets exopolysaccharide synthesis and stress tolerance in Streptococcus mutans to modulate virulence expression in vivo. Antimicrob Agents Chemother. 2012;56:6201–6211. doi: 10.1128/AAC.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, Gonzalez-Begne M, Watson G, Krysan DJ, Bowen WH, Koo H. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–1981. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotra L, Gjermo P, Rolla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971;79:119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- Hansson C, Hoborn J, Moller A, Swanbeck G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm Venereol. 1995;75:24–30. doi: 10.2340/00015555752430. [DOI] [PubMed] [Google Scholar]
- Harper DS, Loesche WJ. Growth and acid tolerance of human dental plaque bacteria. Arch Oral Biol. 1984;29:843–848. doi: 10.1016/0003-9969(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Harris LG, Richards PG. Staphylococcus aureus adhesion to different treated titanium surfaces. J Mat Sci Mat Med. 2004;15:311–314. doi: 10.1023/B:JMSM.0000021093.84680.bb. [DOI] [PubMed] [Google Scholar]
- Istanbullu O, Babauta J, Duc Nguyen H, Beyenal H. Electrochemical biofilm control: mechanism of action. Biofouling. 2012;28(8):769–778. doi: 10.1080/08927014.2012.707651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- Jones SM, Morgan M, Humphrey TJ, Lappin-Scott H. Effect of vancomycin and rifampicin on methicillin-resistant Staphylococcus aureus biofilms. Lancet. 2001;357:40–41. doi: 10.1016/S0140-6736(00)03572-8. [DOI] [PubMed] [Google Scholar]
- Kouidhi B, Al Qurashi YM, Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb Pathog. 2015;80:39–49. doi: 10.1016/j.micpath.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasserre JF, Leprince JG, Toma S, Brecx MC. Electrical enhancement of chlorhexidine efficacy against the periodontal pathogen Porphyromonas gingivalis within a biofilm. New Microbiol. 2015;38:511–519. [PubMed] [Google Scholar]
- Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- Lewis N, Parmar N, Hussain Z, Baker G, Green I, Howlett J, Kearns A, Cookson B, McDonald A, Wilson M, Ready D. Colonization of dentures by Staphylococcus aureus and MRSA in out-patient and in-patient populations. Eur J Clin Microbiol Infect Dis. 2015;34:1823–1826. doi: 10.1007/s10096-015-2418-6. [DOI] [PubMed] [Google Scholar]
- Lide DR. CRC handbook of chemistry and physics. 87. Boca Raton: CRC Press; 2006. [Google Scholar]
- Liu Y, Kamesh AC, Xiao Y, Sun V, Hayes M, Daniell H, Koo H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 2016;105:156–166. doi: 10.1016/j.biomaterials.2016.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, Yuen TG, Bullara L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans Biomed Eng. 1990;7:996–1001. doi: 10.1109/10.102812. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H, Larrimore S, Hull S, Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants with altered cellular morphology or chain length. Infect Immun. 1982;38(1):282–291. doi: 10.1128/iai.38.1.282-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepa TH, Gilbert JL, Ren D. Controlling Pseudomonas aeruginosa persister cells by weak electrochemical currents and synergistic effects with tobramycin. Biomaterials. 2012;33:7356–7365. doi: 10.1016/j.biomaterials.2012.06.092. [DOI] [PubMed] [Google Scholar]
- Niepa TH, Snepenger LM, Wang H, Sivan S, Gilbert JL, Jones MB, Ren D. Sensitizing Pseudomonas aeruginosa to antibiotics by electrochemical disruption of membrane functions. Biomaterials. 2016;74:267–279. doi: 10.1016/j.biomaterials.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Niepa TH, Wang H, Gilbert JL, Dabrowiak JC, Ren D. Synergy between tobramycin and trivalent chromium ion in electrochemical control of Pseudomonas aeruginosa. Acta Biomater. 2016;36:286–295. doi: 10.1016/j.actbio.2016.03.028. [DOI] [PubMed] [Google Scholar]
- O’Donnell LE, Smith K, Williams C, Nile CJ, Lappin DF, Bradshaw D, Lambert M, Robertson DP, Bagg J, Hannah V, Ramage G. Dentures are a reservoir for respiratory pathogens. J Prosthodont. 2015;25(2):99–104. doi: 10.1111/jopr.12342. [DOI] [PubMed] [Google Scholar]
- Pratten J, Wills K, Barnett P, Wilson M. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J Appl Microbiol. 1998;84:1149–1155. doi: 10.1046/j.1365-2672.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Furst MM, Lang NP, Persson GR. One-year bacterial colonization patterns of Staphylococcus aureus and other bacteria at implants and adjacent teeth. Clin Oral Implants Res. 2008;19:242–248. doi: 10.1111/j.1600-0501.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- Sambrook, J, Russell, DW (2001) Molecular cloning: a laboratory manual. Cold spring harbor laboratory press
- Sauer K, Steczko J, Ash SR. Effect of a solution containing citrate/methylene blue/parabens on Staphylococcus aureus bacteria and biofilm, and comparison with various heparin solutions. J Antimicrob Chemother. 2009;63:937–945. doi: 10.1093/jac/dkp060. [DOI] [PubMed] [Google Scholar]
- Schmidt-Malan SM, Karau MJ, Cede J, Greenwood-Quaintance KE, Brinkman CL, Mandrekar JN, Patel R. Antibiofilm activity of low-amperage continuous and intermittent direct electrical current. Antimicrob Agents Chemother. 2015;59:4610–4615. doi: 10.1128/AAC.00483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV. A model of safe levels for electrical stimulation. IEEE Trans Biomed Eng. 1992;39:424–426. doi: 10.1109/10.126616. [DOI] [PubMed] [Google Scholar]
- Smith A, Buchinsky FJ, Post JC. Eradicating chronic ear, nose, and throat infections: a systematically conducted literature review of advances in biofilm treatment. Otolaryngol Head Neck Surg. 2011;144:338–347. doi: 10.1177/0194599810391620. [DOI] [PubMed] [Google Scholar]
- Song F, Koo H, Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 2015;94:1027–1034. doi: 10.1177/0022034515587690. [DOI] [PubMed] [Google Scholar]
- Spadaro JA, Berger TJ, Barranco SD, Chapin SE, Becker RO. Antibacterial effects of silver electrodes with weak direct current. Antimicrob Agents Chemother. 1974;6:637–642. doi: 10.1128/AAC.6.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D, Heling I, Daniel I, Ginsburg I. Antibacterial synergistic effect of chlorhexidine and hydrogen peroxide against Streptococcus sobrinus, Streptococcus faecalis and Staphylococcus aureus. J Oral Rehabil. 1999;26:151–156. doi: 10.1046/j.1365-2842.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- Stephenson MF, Mfuna L, Dowd SE, Wolcott RD, Barbeau J, Poisson M, James G, Desrosiers M. Molecular characterization of the polymicrobial flora in chronic rhinosinusitis. J Otolaryngol Head Neck Surg. 2010;39:182–187. [PubMed] [Google Scholar]
- Sultana ST, Atci E, Babauta JT, Falghoush AM, Snekvik KR, Call DR, Beyenal H. Electrochemical scaffold generates localized, low concentration of hydrogen peroxide that inhibits bacterial pathogens and biofilms. Sci Rep. 2015;5:14908. doi: 10.1038/srep14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanakaroon W, Stewart PS. Electrical enhancement of Streptococcus gordonii biofilm killing by gentamicin. Arch Oral Biol. 2000;45:167–171. doi: 10.1016/S0003-9969(99)00132-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its supplement materials.


