Abstract
In this study, the biocompatibility and in vitro degradation behaviour of a commercial zinc-based alloy (Zn-5 Al-4 Mg) were evaluated and compared with that of pure zinc for temporary orthopaedic implant applications. Biocompatibility tests were conducted using human alveolar lung epithelial cells (A549), which showed that the zinc alloy exhibits similar biocompatibility as compared to pure zinc. In vitro degradation evaluation was performed using weight loss and electrochemical methods in simulated body fluid (SBF) at 37 °C. Weight loss measurements revealed that the degradation of the zinc alloy was slightly lower during the initial immersion period (1–3 days), but marginally increased after 5 and 7 days immersion as compared to pure zinc. Potentiodynamic polarisation experiments showed that the zinc alloy exhibits higher degradation rate than pure zinc. However, electrochemical impedance spectroscopy analysis suggests that pure zinc is susceptible to localized degradation, whereas the zinc alloy exhibited passivation behaviour. Post-degradation analysis revealed localized degradation in both pure zinc and the zinc alloy.
Introduction
The emerging interest in biodegradable implants for short-term service life in orthopaedics aims to produce biomaterials with desirable biodegradability, biocompatibility and mechanical properties closer to natural bone. In recent years, a significant amount of research has been undertaken on biodegradable metals, mainly on magnesium-based materials1. Magnesium is highly biocompatible, but its undesirably high degradation rate in physiological conditions is a huge disadvantage. Hence, the recent research focus in this field has been on controlling the degradation rate of magnesium by alloying and/or surface coatings1–6.
Metallic zinc is a potential biodegradable and biocompatible material for temporary orthopaedic mini-implants such as screw, pins and plates. As an essential nutrient, zinc has many important biological functions, including development and sustenance of bones7, food intake and growth8, wound healing9, cell proliferation and division, and DNA stabilisation and replication8,10. Dietary zinc is metabolically absorbed through the small intestine as zinc ions and amnio acid complexes and it is regulated by metallothionein11. In the short-term, zinc in the body is regulated to organs such as pancreas, liver, kidneys and spleen12,13. However, in the long-term, 90% of the absorbed zinc is deposited in the muscular and skeletal system14. The biological half-life of zinc has been determined to be between 162 and 500 days15,16, and the daily recommended dose of zinc is 10–15 mg/day17. Interestingly, it has been reported that long-term administering of zinc doses ten times the daily recommended intake has produced no adverse effects in humans in relation to wound healing18, antirheumatic activity for rheumatoid arthritis19 and plasma copper levels20. In fact, high concentrations of zinc have been shown to prevent conditions like osteoporosis through promotion of osteoblastogenesis and suppression of osteoclastogenesis21,22.
Metallic zinc has physical and mechanical properties similar to those of other common biomaterials: Density = 7.14 g/cm3; Young’s Modulus = 70 GPa; Ultimate Tensile Strength (UTS) = 126–246 MPa23. The electrochemical dissolution of zinc in aqueous solutions is suggested to occur via the following reactions24,25:
| 1 |
| 2 |
where (1) and (2) represent the anodic and cathodic reactions, respectively. However, the degradation mechanism is believed to be largely reliant on even small changes in the electrolyte pH, temperature and composition, and various reaction schemes have been proposed26,27. Some principal products of dissolved zinc cations in aqueous solutions are produced via the following reactions:
| 3 |
| 4 |
| 5 |
| 6 |
Similarly, the selectivity between reactions is governed by the electrolyte conditions. These products are major constituents of the passive films formed on zinc during aqueous corrosion and are known to provide considerable degradation protection since they are thermodynamically stable at room temperature within the pH range 6–1226.
As compared to the wealth of literature on the biocompatibility and degradation of magnesium-based material1–6, the work done on zinc-based materials is limited. Although extensive research has been done over the past few decades on the corrosion behaviour of zinc and zinc-based alloys (as bulk or coated film) in chloride-containing environments for engineering applications28–34, only recently there has been a growing interest on zinc-based materials for potential biodegradable implant applications. Bowen et al.35 reported that degrading zinc has optimal biocompatibility and the degradation products supress the activities of inflammatory and smooth muscle cells. Liu et al.36 found that zinc dissolution has no significant destructive effect on erythrocyte. On the other hand, Sherier et al.37 suggested that free Zn2+ ions might hinder cell mobility and adhesion. However, Kubasek et al.38 reported that the maximum safe Zn2+ ion concentrations for U2OS and L929 cell lines are 120 µM and 80 µM, respectively. Bowen et al.39 examined the in vivo degradation behaviour of zinc for absorbable stent applications, and reported the longevity and harmless degradation of zinc metal. They observed that the degradation rate of zinc increases linearly with implantation time. Under short-term in vivo condition, zinc oxides were formed, however, after 4.5 to 6 months, calcium phosphate layers were observed. Zinc oxides seem to be inert to the immune system, but depending on the size of these oxide particles can cause cytotoxicity40. Drelich et al.41 reported that defects/cracks in the zinc oxide film increases the degradation rate.
A few binary and ternary zinc alloys (containing magnesium, aluminium, lithium, calcium, copper and/or strontium) have also been studied due to their better mechanical strength as compared to pure zinc. Muni et al.42 reported that the cell viability (normal human osteoblast cells) for Zn-3Mg was reduced by ~50% at 1 day exposure, but the cells recovered at 3 and 7 days. Dambatta et al.43 showed that homogenisation of as-cast Zn-3Mg alloy increases the degradation resistance. Vojtĕch et al.44 reported no significant difference in the degradation rate between pure zinc and zinc alloys (Zn-Mg and Zn-Al-Cu). Interestingly, they found high concentrations of calcium and phosphate in the degradation product layers. Gong et al.45 and Mostaed et al.46 reported that extruded Zn-Mg alloys are superior to their cast counterparts in terms of degradation resistance. In contrast, Shen et al.47 found that the extruded Zn-Mg alloy exhibit lower degradation resistance in comparison with the as-cast alloy. However, Shen et al.47 and Gong et al.45 agree that Zn-Mg alloys are biocompatible. Interestingly, aluminium addition to zinc has been reported to cause intergranular degradation48,49. On other hand, lithium addition to zinc has improved the degradation resistance and also exhibited excellent biocompatibility50,51. Li et al.52 reported that addition of magnesium, calcium and strontium to zinc can benefit their hemocompatibility and cytocompatibility. However, Liu et al.53 suggests that calcium or strontium addition to Zn-Mg alloy produced secondary phase particles, which increases galvanic corrosion.
For load-bearing orthopaedic applications, the mechanical integrity of the implant during service is critical. Localized degradation may affect the mechanical integrity of the implant. Unfortunately, zinc undergoes localized degradation in chloride-containing environments54–56. Hence, it is important to study the localized degradation susceptibility of zinc in physiological conditions. Literature suggests that the ternary Zn-Al-Mg alloys have superior degradation protection properties in chloride-containing solution than binary system alloys such as Zn-Mg and Zn-Al57–62. It should be noted that Zn-Al-Mg alloys are commercially available and have been widely used as galvanizing coating materials on steels due to their high degradation resistance57,59,61. This alloy system has other advantages such as better mechanical strength and relatively low density (due to lighter alloying metals such as magnesium and aluminium) as compared to pure zinc for implant applications. Therefore, it is important to understand the biocompatibility and biodegradation behaviour of a Zn-Al-Mg alloy.
In this study, the biocompatibility and biodegradation behaviour of the commercially available Zn-5 Al-4 Mg alloy were examined and compared with that of pure zinc. Weight loss and electrochemical methods were used to evaluate the biodegradation behaviour of the materials in simulated body fluid at 37 °C. Post-degradation analysis was performed using scanning electron microscope (SEM) to identify the mode of degradation.
Experimental Procedure
The chemical compositions of pure zinc and the commercial Zn-5 Al-4 Mg alloy used in this study are shown in Table 1. The hardness of the materials was measured using a Rockwell hardness tester (Model: Avery Rockwell Hardness Tester, type 6402). For the cytotoxicity testing, human alveolar lung epithelial A549 cells were used. The A549 cells utilised in this study are a human derived epithelial cell line from the lungs and respiratory tract, and is frequently used as indicator of general genotoxicity and cytotoxicity40. These cells were obtained from the American Type Culture Collection (ATCC, USA) and maintained in 25 cm2 cell culture flasks in an incubator with a humidified atmosphere at 37 °C and 5% CO2. The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, USA) supplemented with 10% FBS, 1% penicillin-streptomycin and L-glutamine (Life Technologies, Australia), designated as ‘complete medium’. The cells were cultured to a cell density of 1 × 106 cells/mL before being sub-cultured into fresh media 2–3 times a week. The metal samples were ground with SiC paper up to 2500 grit and later polished with 1 μm alumina powder solution, washed with distilled water and then ultrasonically cleaned in ethanol. Subsequently, the samples were pre-incubated in the complete medium until 96 h at 37 °C in a humidified atmosphere with 5% CO2 to obtain the extraction medium, which was used for the cytotoxicity analysis. Metabolic activity of A549 cells exposed to the samples was assessed using the MTS assay which measures the absorbance (490 nm) of the purple dye formazan generated by live cells when exposed to the MTS reagent. (Promega MTS CellTiter 96® aqueous kit, Promega, USA). Briefly, 10,000 cells in 100 µL were seeded into 96-well tissue culture plates (Sarstedt, Germany). After allowing for overnight attachment, the cells were exposed to 100 µL of the extraction medium obtained at 1, 2, 3 and 4-day exposure period. Wells containing cells exposed to the “complete medium” served as positive control. Data were obtained from three independent experiments, each performed in triplicate.
Table 1.
Composition of pure zinc and zinc alloy (Zn-5 Al-4 Mg), all wt.%.
| Mg | Al | Ca | Fe | Pb | Zn | |
|---|---|---|---|---|---|---|
| Pure Zn | <0.001 | 0.001 | <0.001 | 0.001 | 0.002 | Bal. |
| Zn Alloy | 4.35 | 4.46 | 0.035 | 0.002 | 0.002 | Bal. |
In addition, DAPI (4′,6-diamidino-2-phenylindole) staining was carried out to study the changes in nuclear morphology of A549 cells after exposure to the extraction media. A549 cells were allowed to attach overnight on chambered slides (Lab-Tek, Proscitech) at a density of 106 cells per mL and subsequently incubated with the extraction media for up to 4 days. At the end of the incubation period, all cells were collected and washed with Dulbeccos’ phosphate buffered saline (Life Technologies, USA), subjected to fixation and were mounted on Superfrost slides (Proscitech, Australia) using ProLong® Gold Antifade Reagent with DAPI (Molecular Probes, Life Technologies, USA). The slides were subsequently incubated at room temperature for 24 h in the dark before visualization using a Zeiss LSM710 confocal laser scanning microscope (Carl Zeiss, Germany).
In vitro degradation behaviour of pure zinc and Zn-5 Al-4 Mg alloy was evaluated by weight loss analysis and electrochemical methods, i.e., potentiodynamic polarisation and electrochemical impedance spectroscopy (EIS), in simulated body fluid (SBF) maintained at a body temperature of 37.5 ± 0.5 °C and pH of 7.4–7.6. The chemical composition of the SBF is given in Table 2 63. Prior to the in vitro degradation testing, the samples were ground with SiC paper up to 2500 grit and later polished with 1 μm alumina powder solution, and washed with distilled water and then ultrasonically cleaned in ethanol. In the weight loss testing, the samples were immersed in SBF at a static condition and the weight losses were recorded after 1 to 7 days immersion. Electrochemical experiments were conducted using a potentiostat/galvanostat and a frequency response analyser (Model: ACM Gill AC, ACM Instruments). A typical three-electrode system consisting of graphite as a counter electrode, Ag/AgCl electrode as a reference electrode and the sample as a working electrode was used in this study. The potentiodynamic polarisation experiments were conducted at a scan rate of 0.5 mV/sec. The EIS experiments were performed over the frequency range of 1 × 105 Hz to 1 × 10−2 Hz and at an AC amplitude of 5 mV. The EIS data were analysed using equivalent circuit modelling (Software: ZSimpWin v3.21, Princeton Applied Research). All the in vitro degradation tests were conducted in triplicate. Scanning electron microscope (SEM) was used to analyse the post-degradation samples.
Table 2.
Chemical composition of the simulated body fluid (SBF).
| Chemical | Amount (/L) |
|---|---|
| NaCl | 8.036 (g) |
| NaHCO3 | 0.352 (g) |
| KCl | 0.225 (g) |
| K2HPO4∙3H2O | 0.23 (g) |
| MgCl2∙6H2O | 0.311 (g) |
| 1 M HCl | 40 (mL) |
| CaCl2 | 0.293 (g) |
| Na2SO4 | 0.072 (g) |
| TRIS buffera | 6.063 (g) |
aTRIS buffer = tris(hydroxylmethylaminomethane).
Results and Discussion
Biocompatibility
The cell viability (cytotoxicity) of pure zinc and the zinc alloy on A549 cells is shown in (Fig. 1) as compared to cells exposed to the complete medium. A549 cells exposed to the extraction media obtained from the zinc or zinc alloy samples did not demonstrate cytotoxicity at the end of the 4 days testing period. Figure 1b–d show the nuclear morphology of the treated cells after DAPI staining. Normally, cells undergoing apoptosis exhibit characteristic condensation of the nuclear material. In the present study, cells exposed to zinc or the zinc alloy demonstrated nuclear morphology similar to the control cells (exposed to cell culture medium alone) even after the 96 h exposure period, further confirming the non-toxic nature of the samples. These results serve as a preliminary indication of the biocompatibility of pure zinc and the zinc alloy.
Figure 1.
(a) Cell viability of pure zinc and zinc alloy exposed to A549 human lung alveolar epithelial cells. Cytotoxic response of human lung epithelial cells exposed to extraction medium was assessed using the MTS assay. Specific absorbance from untreated cells was used as the reference for normalising the test-well data to calculate “% of control cell viability. Values are expressed as mean ±SEM (n = 3 separate experiments each performed in triplicate). CLSM images of DAPI staining of (b) cells exposed to complete medium, (c) zinc alloy and (d) pure zinc, after 4 days exposure to A549 human lung alveolar epithelial cells.
Potentiodynamic polarisation
The potentiodynamic polarisation curves of pure zinc and the zinc alloy are shown in Fig. 2, and the electrochemical data obtained from the curves are presented in Table 3. The corrosion potential (Ecorr) of the zinc alloy was slightly (~10 mV) more noble as compared to pure zinc. The cathodic polarisation curves suggest that the cathodic activity was higher for the zinc alloy in comparison with pure zinc. This difference in cathodic activity can be attributed to the alloying elements. In the case of the anodic side of the polarisation curves, the dissolution behaviour of the zinc alloy was higher than pure zinc. However, both pure zinc and the zinc alloy did not show any active passive region or breakdown potential. The corrosion current density (icorr) calculated from the cathodic curves suggested that the icorr value of the zinc alloy is ~85% higher than pure zinc i.e., 17.7 µA/cm2 and 9.55 µA/cm2, respectively. The calculated degradation rate for the zinc alloy was 0.32 mm/y and for pure zinc 0.14 mm/y. As expected, the degradation rate of pure zinc and the zinc alloy was significantly lower than that of pure magnesium (degradation rate = 0.54 mm/y; icorr = 23.5 µA/cm2)64. Post-polarisation SEM micrographs of pure zinc and the zinc alloy are shown in Fig. 3. The morphology of pure zinc revealed localized attack (Fig. 3a and b). In the case of the zinc alloy, the localized attack increased, as demonstrated by the relative larger areas of evident damage (Fig. 3c and d).
Figure 2.

Potentiodynamic polarisation curves of pure zinc and zinc alloy in SBF.
Table 3.
Electrochemical data obtained from the potentiodynamic polarisation curves of pure zinc and zinc alloy (Zn-5 Al-4 Mg).
| Ecorr (mVAg/AgCl) | βa (mV/decade) | βc (mV/decade) | icorr (μA/cm2) | Corrosion Rate (mm/y) | |
|---|---|---|---|---|---|
| Pure Zn | −1032 ± 5 | 318 | −417 | 9.55 ± 1.1 | 0.14 |
| Zn Alloy | −1020 ± 5 | 295 | −202 | 17.7 ± 1.2 | 0.32 |
Values represent means of triplicate samples ± absolute standard deviations.
Figure 3.
SEM micrographs of: (a) & (b) pure zinc and (c) & (d) zinc alloy, after potentiodynamic polarisation in SBF.
EIS
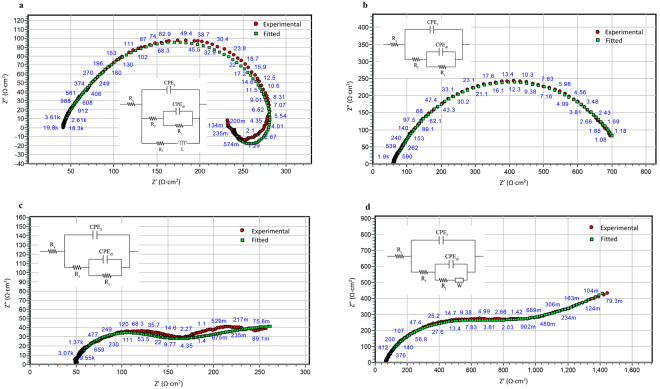
The EIS spectra for pure zinc and the zinc alloy over 72 h immersion in SBF is shown in Fig. 4. The equivalent circuits (EC) models used and the fitting for pure zinc and the zinc alloy after 2 h and 72 h immersion are shown in Fig. 5. The data obtained from EIS modelling are presented in Table 4. After 2 h immersion, pure zinc showed a capacitive loop and an inductive loop. The low frequency inductive loop is a general indication of localized degradation65,66 or adsorption of intermediate corrosion products or ions onto the surface67,68. The zinc alloy showed two capacitive loops, but no inductive loop. The high frequency capacitive loop can be attributed to charge transfer resistance and the mid-frequency capacitive loop is related to the film resistance. The EC model used for zinc alloy consisted of the following elements: Rs (solution resistance), Rct (charge transfer resistance), CPEdl (double layer capacitance) and Rf (film resistance). For the pure zinc, which exhibited an inductive loop, L (inductance) and CPEf (capacitance due to film effect) elements were added. The polarisation resistance (Rp) of the samples was calculated by adding the Rct and Rf. The Rp of the zinc alloy after 2 h exposure to SBF was 63% higher than that of pure zinc (pure zinc = 250.56 Ω∙cm2; zinc alloy = 408.39 Ω∙cm2).
Figure 4.
Nyquist plots of pure zinc and zinc alloy after: (a) 2 hours, (b) 1 day, (c) 2 days and (d) 3 days immersion in SBF.
Figure 5.
EIS fitting of experimental data for: (a) pure zinc after 2 hours, (b) pure zinc after 72 hours, (c) zinc alloy after 2 hours and (d) zinc alloy after 72 hours immersion in SBF. (Insets: Equivalent circuits).
Table 4.
EIS spectra equivalent circuit modelling data of pure zinc and zinc alloy (Zn-5 Al-4 Mg).
| Immersion Time | Rf (Ω∙cm2) (x 102) | CPEf (Ω−1∙cm−2∙s−n) (x 10−5) | n | Rt (Ω∙cm2) (x 102) | CPEdl (Ω−1∙cm−2∙s−n) (x 10−5) | n | RL (Ω∙cm2) (x 102) | L (Ω∙cm2∙s) | W (Ω−1∙cm−2∙s−0.5) (x 102) | Rp (Ω∙cm2) (x 102) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pure Zn | 2 h | 0.88 ± 0.09 | 1.40 ± 0.18 | 0.87 | 1.62 ± 0.1 | 2.09 ± 0.14 | 0.85 | 9.58 ± 0.47 | 109 ± 7.317 | — | 2.5 ± 0.17 |
| 1d | 0.91 ± 0.06 | 0.79 ± 0.29 | 0.8 | 2.27 ± 0.35 | 4.29 ± 0.32 | 0.86 | — | — | — | 3.19 ± 0.43 | |
| 2d | 0.97 ± 0.02 | 0.75 ± 0.2 | 0.99 | 2.9 ± 0.2 | 4.73 ± 0.87 | 0.81 | — | — | — | 3.87 ± 0.29 | |
| 3d | 0.96. ± 0.01 | 0.71 ± 0.1 | 1 | 5.93 ± 0.46 | 6.65 ± 1.06 | 0.69 | — | — | — | 6.9 ± 0.5 | |
| Zn Alloy | 2 h | 0.83 ± 0.06.45 | 5.86 ± 2.11 | 0.74 | 3.25 ± 0.55 | 607.12 ± 69.61 | 0.32 | — | — | — | 4.08 ± 0.5 |
| 1d | 2.26 ± 0.76 | 13.27 ± 1.07 | 0.69 | 3.36 ± 0.42 | 899.67 ± 234.09 | 0.63 | — | — | — | 5.63 ± 1.26 | |
| 2d | 2.89 ± 1 | 8.11 ± 1.09 | 0.79 | 11.68 ± 0.78 | 283.10 ± 38.15 | 0.36 | — | — | 3.14 ± 0.49 | 14.57 ± 1.75 | |
| 3d | 2.17 ± 0.3 | 51.26 ± 17.33 | 0.18 | 26.82 ± 1.83 | 21.41 ± 3.61 | 0.79 | — | — | 0.08 ± 0.01 | 28.99 ± 2.04 |
Values represent means of triplicate samples ± absolute standard deviation.
After 24 h exposure, the Rp of the zinc alloy increased by 38%. In the case of pure zinc, the inductive loop disappeared and the Rp increased by 27% (Rp = 319.61 Ω∙cm2). However, after 48 h exposure, the zinc alloy continued to display passivation effect, but the mid-frequency capacitive loop has transformed to Warburg impedance. This type of behaviour has been reported in the literature for zinc metal69. The significance of a Warburg impedance is the presence of a porous passive film facilitating diffusion controlled processes69,70. A Warburg diffusion element was used to model the EIS spectra for the zinc alloy. The Rp of the zinc alloy was ~4 times higher than pure zinc (pure zinc = 387.36 Ω∙cm2, zinc alloy = 1457.2 Ω∙cm2) after 48 h exposure. The trend continued even after 72 h exposure, the Rp values for pure zinc and zinc alloy were 690.13 Ω∙cm2 and 2899.66 Ω∙cm2, respectively.
Weight Loss
The weight loss measurements for pure zinc and the zinc alloy are shown in Fig. 6. As expected, the weight loss increased with increasing exposure. Interestingly, the weight loss data for the zinc alloy were not remarkably different to the pure zinc during 1 to 7 days immersion in SBF. After 1 and 3 days immersion, the weight loss of the alloy was marginally lower than pure zinc, but after 5 and 7 days the trend reversed. The macrographs of pure zinc and the zinc alloy after each interval of immersion are shown in Fig. 7. Both pure zinc and the zinc alloy have undergone localized degradation and the intensity has increased with increasing immersion time. It was interesting to note that the pitting nucleation was not high, but the growth of pits was very rapid. After 7 days immersion, the localized degradation attack was remarkably high in both pure zinc and the zinc alloy. The overall degradation rates derived from the weight loss test were 0.31 mm/y for pure zinc and 0.35 mm/y for the zinc alloy.
Figure 6.
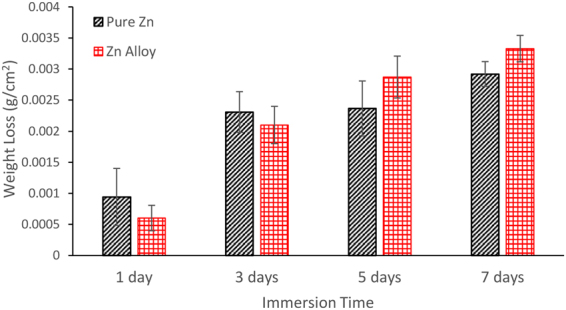
Weight loss results for pure zinc and zinc alloy over 7 days immersion in SBF. (Each bar represents the mean of a triplicate sample, and error bars represent standard deviations).
Figure 7.
Macrographs of pure zinc and zinc alloy after 1, 3, 5 and 7-day immersion in SBF.
Mechanism
The EIS spectra suggests that the zinc alloy exhibits passivation behaviour. This can be attributed to the alloying elements in the zinc alloy, especially aluminium. Literature on the corrosion behaviour of aluminium-containing zinc alloy coatings, e.g., Zn-Al71 and Zn-Al-Mg61,72, in chloride-containing solution suggests that aluminium forms a thick and complex layers. Volovitch, et al.62 reported that aluminium formed basic aluminium-oxides in the initial stages of corrosion of a Zn-Al-Mg alloy. Studies have also suggested that aluminium has a lower dissolution tendency as compared to zinc and magnesium in Zn-Al-Mg alloy system60,62. On the other hand, magnesium forms magnesium hydroxide in aqueous solutions, which is a protective film, but in chloride-containing solution, the protective film converts to soluble magnesium chloride, as shown below73.
| 7 |
| 8 |
In the current study, the Warburg impedance observed under long-term EIS suggests that the film formed on the alloy is porous in nature and introduces diffusion characteristics; hence, the stability of the film in physiological condition was only temporary.
To further understand the passivation behaviour of pure zinc and the zinc alloy in the physiological environment that contains chloride ions, the Pourbaix diagrams of zinc, aluminium and magnesium were used74. Figure 8 (a–d) shows the Ecorr values of pure zinc and the zinc alloy embedded on the Pourbaix diagrams. Although (Fig. 8a) suggests that the potentials of pure zinc and the zinc alloy are in the passive region, the presence of chloride shifts the passivity region towards the higher pH regions and hence undergo dissolution. It can be noted that the experimental conditions confined pure zinc and the zinc alloy to the active ZnCl+ region throughout the immersion period (Fig. 8b). Hence, zinc did not show any strong passivation. The passivation observed in the EIS experiments (Fig. 4) of the zinc alloy could be attributed to aluminium, which is stable in the physiological pH range (Fig. 8c). Magnesium is however not stable in that pH range (Fig. 8d).
Figure 8.
Pourbaix diagram for Zn-C-Cl-H2O system at 25 °C with Ecorr (EH) values for pure zinc and zinc alloy and the physiological pH range overlayed (adapted from [74]).
The potentiodynamic polarisation curves suggest that the cathodic activity of the zinc was higher as compared to pure zinc (Fig. 2). This can be attributed to the alloying elements in the zinc alloy. Although oxygen reduction reaction is the predominant cathodic reaction for zinc metal, hydrogen evolution reaction is feasible in the zinc alloy due to the presence of the alloying elements such as magnesium and aluminium. It should be noted that the hydrogen-evolution exchange current densities of the alloying elements magnesium and aluminium are higher than that of zinc (~10−8 −10−9, ~10−10 and ~10−11 A/cm2 respectively75,76). Therefore, the cathodic current of the alloy was higher than that of pure zinc. The anodic reaction of the zinc alloy was also higher than pure zinc, which could be due to selective leaching of elements under accelerated conditions. Magnesium being more reactive than aluminium and zinc, selective leaching of magnesium could have caused the increase in anodic current during polarisation. However, the EIS experiments, which is non-destructive, revealed passivation behaviour in the alloy. The weight loss method also showed improved degradation resistance of the zinc alloy as compared to pure zinc during the initial immersion period, but exhibited localized degradation with increasing exposure, probably due to galvanic effect.
The study suggests that the zinc alloy exhibited similar biocompatibility to pure zinc. It was interesting to see that the biodegradation resistance of the zinc alloy, which has been used for galvanization for its excellent corrosion resistance, was not superior to that of pure zinc in physiological conditions. During the initial immersion period, the zinc alloy exhibited passivation behaviour, but the passivity became less stable with exposure time and ultimately gave rise to localized degradation similar to pure zinc. However, this zinc alloy has some attractive properties (density and hardness) as compared to pure zinc, which are essential for load-bearing implant applications. Due to the presence of light metals, the density of the zinc alloy is approximately 17% lower than pure zinc (pure zinc = 7.14 g/cm3 and zinc alloy = 5.908 g/cm3). The hardness of the zinc alloy was approximately 14% higher than pure zinc (pure zinc = 79.2 HRB and zinc alloy = 89.9 HRB). The biocompatibility and the attractive physical and mechanical properties make the commercial zinc alloy a potential material for temporary mini-implant applications. However, surface engineering is essential to delay the localized degradation of the commercial zinc alloy.
Conclusions
The biocompatibility and in vitro degradation behaviour of a commercial zinc alloy (Zn-5 Al-4 Mg) were evaluated and compared with that of pure zinc. The zinc alloy showed similar biocompatibility to pure zinc in the cytotoxicity assay conducted using human alveolar lung epithelial cells (A549). The aluminium content in the alloy improved the passivation behaviour, but was only temporary in the physiological conditions. The potentiodynamic polarisation results suggested that the zinc alloy degradation rate is marginally higher than pure zinc owing to the higher hydrogen exchange current density of the alloying elements (magnesium and aluminium) as compared to zinc. The localized degradation susceptibility of the zinc alloy was similar to pure zinc. In addition to the comparable biocompatibility and biodegradability of the zinc alloy as compared to pure zinc, the alloy exhibits lower density and higher hardness, which make it more attractive for load-bearing orthopaedic applications.
Acknowledgements
The authors would like to thank Sun Metals Corporation Pty. Ltd. (Queensland, Australia) for providing the zinc and zinc alloy samples. A.L received funding from the Australian Research Council and the National Health and Medical Research.
Author Contributions
C.M., S.S.b and M.R. performed the experiments. B.K.M. and A.L. supervised the study. B.K.M., S.S.b, S.S.a and C.M. wrote the manuscript. All authors commented on and approved the manuscript before submission.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng YF, Gu XN, Witte F. Biodegradable metals. Materials Science and Engineering: R: Reports. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. [DOI] [Google Scholar]
- 2.Koç E, Kannan MB, Ünal M, Candan E. Influence of zinc on the microstructure, mechanical properties and in vitro corrosion behavior of magnesium–zinc binary alloys. Journal of Alloys and Compounds. 2015;648:291–296. doi: 10.1016/j.jallcom.2015.06.227. [DOI] [Google Scholar]
- 3.Kannan MB, Raman RKS. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials. 2008;29:2306–2314. doi: 10.1016/j.biomaterials.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Alabbasi A, Mehjabeen A, Kannan MB, Ye Q, Blawert C. Biodegradable polymer for sealing porous PEO layer on pure magnesium: An in vitro degradation study. Applied Surface Science. 2014;301:463–467. doi: 10.1016/j.apsusc.2014.02.100. [DOI] [Google Scholar]
- 5.Alabbasi A, Liyanaarachchi S, Kannan MB. Polylactic acid coating on a biodegradable magnesium alloy: An in vitro degradation study by electrochemical impedance spectroscopy. Thin Solid Films. 2012;520:6841–6844. doi: 10.1016/j.tsf.2012.07.090. [DOI] [Google Scholar]
- 6.Kannan MB. Enhancing the performance of calcium phosphate coating on a magnesium alloy for bioimplant applications. Materials Letters. 2012;76:109–112. doi: 10.1016/j.matlet.2012.02.050. [DOI] [Google Scholar]
- 7.Yamaguchi M. Role of zinc in bone formation and bone resorption. Journal of Trace Elements in Experimental Medicine. 1998;11:119–135. doi: 10.1002/(SICI)1520-670X(1998)11:2/3<119::AID-JTRA5>3.0.CO;2-3. [DOI] [Google Scholar]
- 8.MacDonald RS. The Role of Zinc in Growth and Cell Proliferation. The Journal of Nutrition. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 9.Andrews M, Gallagher-Allred C. The role of zinc in wound healing. Advances in Skin & Wound Care. 1999;12:137. [PubMed] [Google Scholar]
- 10.Wu FYH, Wu CW. Zinc in DNA Replication and Transcription. Annual Review of Nutrition. 1987;7:251–272. doi: 10.1146/annurev.nu.07.070187.001343. [DOI] [PubMed] [Google Scholar]
- 11.Plum LM, Runk L, Haase H. The Essential Toxin: Impact of Zinc on Human Health. Int J Environ Res Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eggleton WGE. The zinc and copper contents of the organs and tissues of Chinese subjects. Biochemical Journal. 1940;34:991–997. doi: 10.1042/bj0340991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J, et al. Toxicity of zinc oxide nanoparticles in rats treated by two different routes: single intravenous injection and single oral administration. Journal of Toxicology and Environmental Health, Part A. 2015;78:226–243. doi: 10.1080/15287394.2014.949949. [DOI] [PubMed] [Google Scholar]
- 14.Wastney M, Aamodt R, Rumble W, Henkin R. Kinetic analysis of zinc metabolism and its regulation in normal humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1986;251:R398–R408. doi: 10.1152/ajpregu.1986.251.2.R398. [DOI] [PubMed] [Google Scholar]
- 15.Elinder, C. G. & Piscator, M. In Handbook on the Toxicology of Metals (eds L. Friberg, G. F. Nordberg, & V. B. Vouk) (Elsevier/North-Holland, 1980).
- 16.Sigel, H. Metal Ions in Biological Systems: Volume 16: Methods Involving Metal Ions and Complexes in Clinical Chemistry. (Taylor & Francis, 1983).
- 17.Medicine, I. O. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. (The National Academies Press, 2001). [PubMed]
- 18.Pories WJ, Henzel JH, Rob CG, Strain WH. Acceleration of healing with zinc sulfate. Annals of Surgery. 1967;165:432–436. doi: 10.1097/00000658-196703000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simkin P. Oral zinc sulphate in rheumatoid arthritis. The Lancet. 1976;308:539–542. doi: 10.1016/S0140-6736(76)91793-1. [DOI] [PubMed] [Google Scholar]
- 20.Samman S, Roberts D. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. The Medical Journal of Australia. 1987;146:246–249. doi: 10.5694/j.1326-5377.1987.tb120232.x. [DOI] [PubMed] [Google Scholar]
- 21.Brzóska MM, Rogalska J. Protective effect of zinc supplementation against cadmium-induced oxidative stress and the RANK/RANKL/OPG system imbalance in the bone tissue of rats. Toxicology and Applied Pharmacology. 2013;272:208–220. doi: 10.1016/j.taap.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Luo X, et al. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomaterialia. 2014;10:477–485. doi: 10.1016/j.actbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Porter, F. C. Zinc Handbook: Properties, Processing, and Use In Design. (Taylor & Francis, 1991).
- 24.Qu Q, Li L, Bai W, Yan C, Cao C-N. Effects of NaCl and NH4Cl on the initial atmospheric corrosion of zinc. Corrosion Science. 2005;47:2832–2840. doi: 10.1016/j.corsci.2004.11.010. [DOI] [Google Scholar]
- 25.Mouanga M, Berçot P, Rauch JY. Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: Corrosion layer characterization. Corrosion Science. 2010;52:3984–3992. doi: 10.1016/j.corsci.2010.08.003. [DOI] [Google Scholar]
- 26.Zhang, X. G. Corrosion and Electrochemistry of Zinc. (Springer US, 2013).
- 27.Farmer ED, Webb AH. Zinc passivation and the effect of mass transfer in flowing electrolyte. Journal of Applied Electrochemistry. 1972;2:123–136. doi: 10.1007/BF00609128. [DOI] [Google Scholar]
- 28.Baugh LM. Corrosion and polarization characteristics of zinc in neutral—acid media — I. Pure zinc in solutions of various sodium salts. Electrochimica Acta. 1979;24:657–667. doi: 10.1016/0013-4686(79)87048-6. [DOI] [Google Scholar]
- 29.Cachet C, Wiart R. The kinetics of zinc dissolution in chloride electrolytes: Impedance measurements and electrode morphology. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 1980;111:235–246. doi: 10.1016/S0022-0728(80)80043-X. [DOI] [Google Scholar]
- 30.McKubre MCH, Macdonald DD. The Dissolution and Passivation of Zinc in Concentrated Aqueous Hydroxide. Journal of The Electrochemical Society. 1981;128:524–530. doi: 10.1149/1.2127450. [DOI] [Google Scholar]
- 31.Wasekar NP, Jyothirmayi A, Hebalkar N, Sundararajan G. Influence of pulsed current on the aqueous corrosion resistance of electrodeposited zinc. Surface and Coatings Technology. 2015;272:373–379. doi: 10.1016/j.surfcoat.2015.03.038. [DOI] [Google Scholar]
- 32.Prosek T, Nazarov A, Bexell U, Thierry D, Serak J. Corrosion mechanism of model zinc–magnesium alloys in atmospheric conditions. Corrosion Science. 2008;50:2216–2231. doi: 10.1016/j.corsci.2008.06.008. [DOI] [Google Scholar]
- 33.Ramanauskas R, Quintana P, Maldonado L, Pomés R, Pech-Canul MA. Corrosion resistance and microstructure of electrodeposited Zn and Zn alloy coatings. Surface and Coatings Technology. 1997;92:16–21. doi: 10.1016/S0257-8972(96)03125-8. [DOI] [Google Scholar]
- 34.Ramanauskas R, Juškėnas R, Kaliničenko A, Garfias-Mesias LF. Microstructure and corrosion resistance of electrodeposited zinc alloy coatings. Journal of Solid State Electrochemistry. 2004;8:416–421. doi: 10.1007/s10008-003-0444-2. [DOI] [Google Scholar]
- 35.Bowen PK, et al. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Materials Science and Engineering C – Materials for Biological Applications. 2015;56:467–72. doi: 10.1016/j.msec.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Sun J, Yang Y, Pu Z, Zheng Y. In vitro investigation of ultra-pure Zn and its mini-tube as potential bioabsorbable stent material. Materials Letters. 2015;161:53–6. doi: 10.1016/j.matlet.2015.06.107. [DOI] [Google Scholar]
- 37.Shearier ER, et al. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc. ACS Biomaterials Science & Engineering. 2016;2:634–42. doi: 10.1021/acsbiomaterials.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubásek J, et al. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Materials Science and Engineering: C. 2016;58:24–35. doi: 10.1016/j.msec.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Bowen PK, Drelich J, Goldman J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. (Weinheim, Ger.) 2013;25:2577–2582. doi: 10.1002/adma.201300226. [DOI] [PubMed] [Google Scholar]
- 40.Saptarshi SR, Duschl A, Lopata AL. Biological reactivity of zinc oxide nanoparticles with mamalian test systems: an overview. Nanomedicine. 2015;10:2075–2092. doi: 10.2217/nnm.15.44. [DOI] [PubMed] [Google Scholar]
- 41.Drelich AJ, Bowen PK, Lalonde L, Goldman J, Drelich JW. Importance of oxide film in endovascular biodegradable zinc stents. Surface Innovations. 2016;4:133–40. doi: 10.1680/jsuin.16.00014. [DOI] [Google Scholar]
- 42.Murni NS, Dambatta MS, Yeap SK, Froemming GR, Hermawan H. Cytotoxicity evaluation of biodegradable Zn-3 Mg alloy toward normal human osteoblast cells. Materials Science and Engineering: C. 2015;49:560–6. doi: 10.1016/j.msec.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 43.Dambatta MS, et al. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3 Mg alloy as potential biodegradable implant material. Materials & Design. 2015;85:431–7. doi: 10.1016/j.matdes.2015.06.181. [DOI] [Google Scholar]
- 44.Vojtěch D, Kubásek J, Šerák J, Novák P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomaterialia. 2011;7:3515–3522. doi: 10.1016/j.actbio.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Gong H, Wang K, Strich R, Zhou JG. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015;103:1632–40. doi: 10.1002/jbm.b.33341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mostaed E, et al. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. Journal of the Mechanical Behavior of Biomedical Materials. 2016;60:581–602. doi: 10.1016/j.jmbbm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Shen C, et al. Mechanical properties, in vitro degradation behavior, hemocompatibility and cytotoxicity evaluation of Zn-1.2 Mg alloy for biodegradable implants. RSC Advances. 2016;6:86410–9. doi: 10.1039/C6RA14300H. [DOI] [Google Scholar]
- 48.Bowen, P. K. et al. Evaluation of Wrought Zn-Al alloys (1, 3, and 5 wt% Al) Through Mechanical and In Vivo Corrosion Testing for Stent Applications. Journal of Biomedical Materials Research: Part B - Applied Biomaterials, 10.1002/jbm.b.33850 (2017). [DOI] [PubMed]
- 49.Guillory RJ, et al. Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants. ACS Biomaterials Science & Engineering. 2016;2:2355–64. doi: 10.1021/acsbiomaterials.6b00591. [DOI] [PubMed] [Google Scholar]
- 50.Zhao S, et al. Structural characteristics and in vitro biodegradation of a novel Zn-Li alloy prepared by induction melting and hot rolling. Metallurgical and Materials Transactions A. 2017;48:1204–15. doi: 10.1007/s11661-016-3901-0. [DOI] [Google Scholar]
- 51.Zhao S, et al. Zn-Li alloy after extrusion and drawing: structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Materials Science and Engineering C – Materials for Biological Applications. 2017;76:301–12. doi: 10.1016/j.msec.2017.02.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, et al. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Materials & Design. 2015;83:95–102. doi: 10.1016/j.matdes.2015.05.089. [DOI] [Google Scholar]
- 53.Liu X, et al. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn–1.5 Mg alloy. Journal of Alloys and Compounds. 2016;664:444–52. doi: 10.1016/j.jallcom.2015.10.116. [DOI] [Google Scholar]
- 54.Abd El Aal EE. On the pitting corrosion currents of zinc by chloride anions. Corrosion Science. 2004;46:37–49. doi: 10.1016/S0010-938X(03)00110-0. [DOI] [Google Scholar]
- 55.Chen Y, et al. Comparative corrosion behavior of Zn with Fe and Mg in the course of immersion degradation in phosphate buffered saline. Corrosion Science. 2016;111:541–555. doi: 10.1016/j.corsci.2016.05.039. [DOI] [Google Scholar]
- 56.Haleem SMAE. Dissolution Current and Pitting Potential of Zinc in KOH Solutions in Relation to the Concentration of Aggressive Ions. British Corrosion Journal. 1976;11:215–218. doi: 10.1179/000705976798319900. [DOI] [Google Scholar]
- 57.Uranaka, M. & Shimizu, T. Corrosion resistance of hot-dip Zn-6% Al-3% Mg alloy coated steel sheet used in automotive parts. Metallurgical Science and Tecnology30 (2013).
- 58.Salgueiro Azevedo M, Allély C, Ogle K, Volovitch P. Corrosion mechanisms of Zn(Mg,Al) coated steel in accelerated tests and natural exposure: 1. The role of electrolyte composition in the nature of corrosion products and relative corrosion rate. Corrosion Science. 2015;90:472–481. doi: 10.1016/j.corsci.2014.05.014. [DOI] [Google Scholar]
- 59.Salgueiro Azevedo M, Allély C, Ogle K, Volovitch P. Corrosion mechanisms of Zn(Mg,Al) coated steel: 2. The effect of Mg and Al alloying on the formation and properties of corrosion products in different electrolytes. Corrosion Science. 2015;90:482–490. doi: 10.1016/j.corsci.2014.07.042. [DOI] [Google Scholar]
- 60.Prosek T, Persson D, Stoulil J, Thierry D. Composition of corrosion products formed on Zn–Mg, Zn–Al and Zn–Al–Mg coatings in model atmospheric conditions. Corrosion Science. 2014;86:231–238. doi: 10.1016/j.corsci.2014.05.016. [DOI] [Google Scholar]
- 61.Schürz S, et al. Chemistry of corrosion products on Zn–Al–Mg alloy coated steel. Corrosion Science. 2010;52:3271–3279. doi: 10.1016/j.corsci.2010.05.044. [DOI] [Google Scholar]
- 62.Volovitch P, Vu TN, Allély C, Abdel Aal A, Ogle K. Understanding corrosion via corrosion product characterization: II. Role of alloying elements in improving the corrosion resistance of Zn–Al–Mg coatings on steel. Corrosion Science. 2011;53:2437–2445. doi: 10.1016/j.corsci.2011.03.016. [DOI] [Google Scholar]
- 63.Kannan MB. Influence of microstructure on the in-vitro degradation behaviour of magnesium alloys. Materials Letters. 2010;64:739–742. doi: 10.1016/j.matlet.2010.01.022. [DOI] [Google Scholar]
- 64.Alabbasi A, Kannan MB, Walter R, Stormer M, Blawert C. Performance of pulsed constant current silicate-based PEO coating on pure magnesium in simulated body fluid. Materials Letters. 2013;106:18–21. doi: 10.1016/j.matlet.2013.04.047. [DOI] [Google Scholar]
- 65.Kannan MB, Koc E, Unal M. Biodegradability of β-Mg17Al12 phase in simulated body fluid. Materials letters. 2012;82:54–56. doi: 10.1016/j.matlet.2012.05.047. [DOI] [Google Scholar]
- 66.Jin S, Amira S, Ghali E. Electrochemical Impedance Spectroscopy Evaluation of the Corrosion Behavior of Die Cast and Thixocast AXJ530 Magnesium Alloy in Chloride Solution. Advanced Engineering Materials. 2007;9:75–83. doi: 10.1002/adem.200600199. [DOI] [Google Scholar]
- 67.Maleeva MA, Rybkina AA, Marshakov AI, Elkin VV. The effect of atomic hydrogen on the anodic dissolution of iron in a sulfate electrolyte studied with impedance spectroscopy. Protection of Metals. 2008;44:548–556. doi: 10.1134/S0033173208060039. [DOI] [Google Scholar]
- 68.Keddam M, Mottos OR, Takenouti H. Reaction Model for Iron Dissolution Studied by Electrode Impedance: I. Experimental Results and Reaction Model. Journal of The Electrochemical Society. 1981;128:257–266. doi: 10.1149/1.2127401. [DOI] [Google Scholar]
- 69.Song S, Chen Z. Initial Corrosion of Pure Zinc Under NaCl Electrolyte Droplet Using a Zn-Pt-Pt Three-Electrode System. Int. J. Electrochem. Sci. 2013;8:6852–6863. [Google Scholar]
- 70.Emran, K. M. Effect of Concentration and temperature on the corrosion properties of the Fe-Ni-Mn alloy in HCL solutions. Res Chem Intermed, doi:1007/s11164-013-1473-9 (2013).
- 71.Zhang X, Leygraf C, Odnevall Wallinder I. Atmospheric corrosion of Galfan coatings on steel in chloride-rich environments. Corrosion Science. 2013;73:62–71. doi: 10.1016/j.corsci.2013.03.025. [DOI] [Google Scholar]
- 72.Persson D, Thierry D, LeBozec N, Prosek T. In situ infrared reflection spectroscopy studies of the initial atmospheric corrosion of Zn–Al–Mg coated steel. Corrosion Science. 2013;72:54–63. doi: 10.1016/j.corsci.2013.03.005. [DOI] [Google Scholar]
- 73.Song G, Atrens A, St John D, Wu X, Nairn J. The anodic dissolution of magnesium in chloride and sulphate solutions. Corrosion Science. 1997;39:1981–2004. doi: 10.1016/S0010-938X(97)00090-5. [DOI] [Google Scholar]
- 74.Møller, P. In National Association for Surface Finishing Annual Conference and Trade Show (SUR/FIN 2013) 583–591 (National Association for Surface Finishing, 2013).
- 75.Mathaudhu, S. N., Sillekens, W. H., Neelameggham, N. R. & Hort, N. Magnesium Technology 2012. (Wiley, 2012).
- 76.Roberge, P. Corrosion Engineering: Principles and Practice. (McGraw-Hill Education, 2008).