Abstract
In a context of (re)emerging infectious diseases with wildlife reservoirs, understanding how animal ecology shapes epidemiology is a key issue, particularly in wild ungulates that share pathogens with domestic herbivores and have similar food requirements. For the first time in Europe, brucellosis (Brucella melitensis), a virulent zoonosis, persisted in an Alpine ibex (Capra ibex) population and was transmitted to cattle and humans. To better understand disease dynamics, we investigated the relationships between the spatial ecology of ibex and the epidemiology of brucellosis. Combining home range overlap between 37 GPS-collared individuals and visual observations of 148 visually-marked individuals monitored during the 2013–2016 period, we showed that females were spatially segregated in at least 4 units all year round, whereas males were more prone to move between female units, in particular during the rutting period. In addition to ibex age, the spatial structure in females largely contributed to variation in seroprevalence in the whole population. These results suggest that non-sexual routes are the most likely pathways of intraspecific transmission, crucial information for management. Accounting for wildlife spatial ecology was hence decisive in improving our ability to better understand this health challenge involving a wildlife reservoir.
Introduction
Wild animals have been identified as key components in the (re)emergence and worldwide spread of disease1–3. The numerical and spatial expansion of some species (for example, wild herbivores;4), along with the development of transport facilities and changes in agricultural systems (e.g. increasing size of domestic herds and decreasing human presence;5,6) have resulted in increased contact rates between wildlife and livestock7 and in the formation of disease reservoirs in wildlife2. Consequently, identifying the drivers of animal and disease distributions has become a key issue in preventing the transmission of infections between wildlife and domestic herds and the subsequent formation of reservoirs in wildlife8.
In this context, large herbivores have been recognized as particularly relevant species for studying disease processes in free-ranging populations and for understanding how wildlife-livestock interactions may affect such processes9. Indeed, wild and domestic herbivores share similar food requirements, foraging behaviours and pathogens, increasing the risks of interspecific transmission of contagious diseases. In addition, the spatial ecology (e.g. area-restricted space use, partial migration) and social organization (e.g. fission-fusion dynamics, spatial and/or sexual segregation) of wild herbivores give rise to complex host-to-host interactions (intra- or interspecific) and make disease dynamics in these species particularly tricky (see10 for a review of large herbivore ecology). Hence it is increasingly important to better understand how the ecology of large herbivores can shape disease epidemiology.
The spread of disease through a population and transmission between sympatric species are generally related to when and how individuals come into contact, either by direct interactions or by the habitat they share11,12. In social species, several characteristics such as group size, mating systems, segregation and fission-fusion dynamics have also been revealed as key factors determining intra- and interspecific epidemic dynamics13–16. For example, the social organization in badger Meles meles populations from south-west England has been shown to be of prime importance in driving bovine tuberculosis dynamics. As a result, the drastic culling that was a central part of attempts to control the disease could in practice increase movements between social units and hence the spread of disease17. The relationships between the spatial behaviour of wildlife, socio-spatial organizations and the spread of epidemics have consequently become a key research theme in disease ecology8,15.
We focused on the first report of brucellosis (Brucella melitensis) transmission from wildlife to domestic ruminants and humans, which was revealed in 2012 in the northern French Alps (Bargy massif; 46°00′N, 6°28′E, 1500–2438 m above sea level)18,19. Two human cases due to the virulent Brucella melitensis biovar3 were detected and related to the consumption of cheese made from fresh raw milk from an infected cattle herd grazing in this area20. Given that a previous outbreak involving a very similar Brucella strain had occurred in 1999 in livestock (sheep and cattle) in the same area and that all other 205 domestic herds that grazed in the massif from 1999 up to 2012 were found brucellosis-free before they were taken to alpine pastures, wildlife was suspected of being the source of disease persistence and re-emergence. Preliminary analyses confirmed the persistence of this virulent zoonotic pathogen in a wild ruminant species18,19. The local population of Alpine ibex (Capra ibex), a protected species of high patrimonial value, was heavily infected. Seroprevalence reached 38% in 2013 (95% CI [28.2; 47.8]; n = 77) and B. melitensis was isolated from several organs and lymph nodes of the first necropsied individuals suggesting high excretion levels21,22. Other large herbivores inhabiting the same area had a much lower rate of infection (chamois [Rupicapra rupicapra]; 1.8%, n = 55) or were not infected (roe deer [Capreolus capreolus], n = 44 and red deer [Cervus elaphus], n = 30). In an attempt to control the wildlife reservoir, several management actions were undertaken by the French Authorities between 2013 and 2015, including an important ibex slaughtering program: 251 individuals over 5 years old, representing > 44% of the estimated population, were culled in October 2013 and May 2014 (see Methods).
Previous brucellosis outbreaks had been reported in Iberian and Alpine wild ruminants (chamois, isard [Rupicapra pyreneica] and ibex), as self-limiting events, so that these species were generally considered dead-end hosts23–26. However, this persisting outbreak poses serious threats for public health and for the regional economy, as it occurs in a mountain agricultural area with an important production of a famous soft raw milk cheese (reblochon; agriculture revenues reaching € 20 millions in 2011). Indeed, brucellosis is a barrier to trade in animals and animal products. It causes significant losses from abortions, that generally occur during the last third of gestation27, and because the whole domestic herd has to be slaughtered when one individual is infected28. In domestic ruminants, brucellosis can be transmitted (i) from mother to young (so-called vertical transmission), (ii) by the sexual route during mating, or by the horizontal route, i.e. (iii) direct contacts between two individuals or (iv) indirect interactions between one individual and the infected secretion/abortion products of another through a contaminated environment28. This latter transmission route may be particularly important in disease spread as abortion products are heavily infected, resulting in several individuals being contaminated at once when they are aggregated and/or share the same environment. This has been confirmed in several domestic ruminants28,29 and already highlighted in wild species at the interface between bison (Bison bison) and elk (Cervus canadensis nelsoni) in the Greater Yellowstone Ecosystem (Brucella abortus 30). By contrast, little is known on how brucellosis Brucella melitensis could have persisted in that ibex population. Hence, the important public health, economical, conservation and management concerns posed by this brucellosis outbreak justified deep investigations on the relationships between the ecology of Alpine ibex and the epidemiology of brucellosis in this population.
Alpine ibex generally use distinct seasonal home ranges (migratory individuals) but can also restrict space use to a single annual home range (sedentary individuals) depending on individual and/or habitat characteristics31,32. Preliminary information on space use by ibex from the Bargy massif suggested limited seasonal migration, both in terms of the proportion of individuals concerned and of distance some individuals travelled between summer and winter ranges33. The use of a single annual home range, along with the importance of social bonds in this gregarious species, may result in the existence of spatially-separated units that use exclusive ranges most of the year. Such “spatial segregation” has been observed in several ibex populations32,34–37. Within each spatial unit, strong sexual segregation, strengthened with male age and with both social and habitat/ecological components, was reported outside the rutting period34,38,39. As a result of different foraging needs, activity patterns, social affinities and strategies to ensure reproductive success, males and females generally live in distinct groups that do not use habitats in the same way or at the same time40. The social component of this sexual segregation may predominate in spring, when groups of males and groups of females share first snow-cleared and developing pastures34. Conversely, the habitat/ecological components contribute the most to sexual segregation during summer, when females select steep areas perceived as safe to give birth and raise their offspring, while males focus on food-rich habitats40,41. Segregation based on both social and habitat components was also reported between males of different ages34,37. Sexual segregation dropped during the rutting period, however, when roving males seeking females display high movement capabilities increasing connectivity between spatially-segregated units42,43.
As both spatial and sexual segregations have been previously reported in the Bargy massif34,35,37, we hypothesized that spatial and social organizations could shape transmission opportunities among the ibex population studied, resulting in variation in brucellosis prevalence among spatial units and among age-sex classes. To test this hypothesis, we first combined home range overlap between 37 GPS-collared individuals with visual observations of 148 marked individuals, to check the existence of several spatially-segregated units within the studied population. We then assessed the extent to which the spatial structure we identified, in addition to age (related to the duration of brucellosis exposure, and to the level of segregation between sexes and between males), was related to spatial variation in brucellosis seroprevalence. Finally, we also assessed the influence of the slaughtering program conducted in October 2013 and May 2014 on seroprevalence variation and on space use of individuals monitored during common periods before and after these operations. By coupling information on ibex space use, socio-spatial organization, and disease prevalence in the population, we were aiming for a better understanding of brucellosis dynamics and intraspecific transmission routes in this complex health and economic challenge involving wildlife.
Results
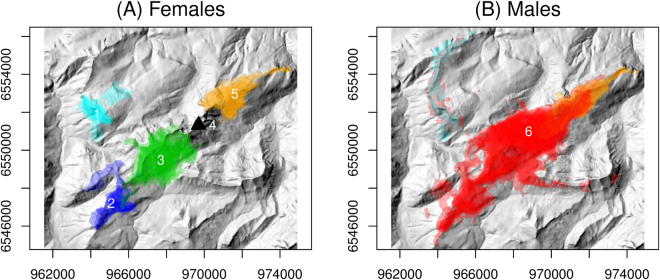
In the Bargy massif, five ibex units could be distinguished among the 37 GPS-collared ibex (Fig. 1). Four included females only or females and males (#2, #3, and #1, #5 in Fig. 1, respectively; see below for the definition of unit #4) whereas the last one consisted of males only (#6). The representation of annual home ranges of each individual on sex-specific maps revealed that females belonging to different units were indeed spatially-segregated, whereas the home ranges of females from the same unit largely overlapped in restricted areas (Fig. 2A). By contrast, males from unit #6 intensively used a large area occupied by several female units (unit #3 and #5), but also visited the areas used by units #1 and #2 and consequently overlapped most of the female ranges (Fig. 2B). The classification trees provided by the overlap between seasonal home ranges confirmed that the spatial structure in females was mostly stable all year round (Supplementary information 1). By contrast, males from unit #6 were more prone to move in the whole study area except during summer and autumn, when they used a restricted area located between the ranges of units #2 and #5, and hence largely overlapped with females from unit #3 (Supplementary information 2 and Fig. 2).
Figure 1.
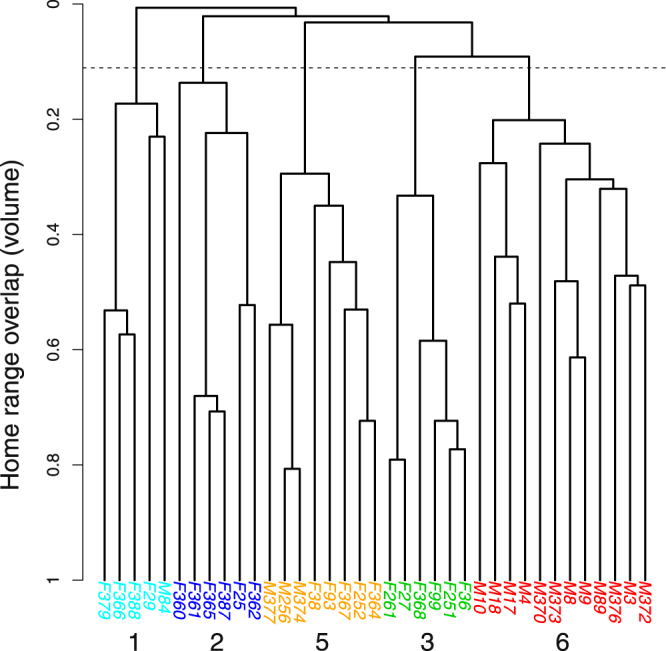
Hierarchical classification tree representing the structure in 37 GPS-collared Alpine ibex (Capra ibex) from the Bargy massif based on overlap between annual home ranges as a measure of distance between individuals.
Figure 2.
Annual home ranges of GPS-collared Alpine ibex (Capra ibex) (A) females (n=21) and (B) males (n=16) from the Bargy massif (northern French Alps). The colors correspond to the different units identified using overlap between annual home ranges as a measure of distance between individuals (see Fig. 1). The black triangle represent the area where we did not equip any female with GPS collars but where some ibex were captured or slaughtered. These maps were created using R version 3.4.180: https://cran.r-project.org.
Observations recorded each year from May to September of the 2013–2015 period confirmed that the 148 marked individuals followed the same spatial structure as that identified in the 37 GPS-collared individuals, and that capture location provided reliable information on the unit to which each individual ibex could be assigned (Supplementary information 2). This was particularly true for females: they were generally observed in the range used by GPS-collared individuals from the spatial unit to which they were assigned based on capture locations (511/530 observations, 96.4%). By contrast, visual observations confirmed males were more prone to move between these spatial units, with only 54.5% (415/762) of observations falling in the spatial range used by individuals from the spatial unit to which they were assigned.
Since some ibex were captured or slaughtered in an area that was not used by the females equipped with GPS collars (#4, black triangle in Fig. 2A), we included data on seroprevalence collected in this area as an undetermined group in the modelling procedure. In addition, given that females were spatially-structured all year round, and that a similar structure could be identified in males during some seasons, but that males from unit #6 largely overlapped with females from units #3 and #4 and other males (see Figs 1, 2A and 3), we tested for several classifications for males when investigating the influence of this spatial structure on brucellosis seroprevalence: (i) each individual male, including those from unit #6, was assigned to the female unit that used the area in which it was captured (“unitsMFall” in Supplementary information 3), (ii) males from units #3 and #4 were considered a unique group (corresponding to unit #6 in Figs 1 and 2B]) and those from the other units were assigned to the female unit that used the area in which each individual was captured (“unitsMF” in Supplementary information 3), and (iii) all the males were assigned to a unique group not spatially structured (“unitsF” in Supplementary information 3). Only the results provided by the classification that best explained the seroprevalence variation in the massif (i.e. “unitsMFall” in Supplementary information 3), are presented below.
Figure 3.
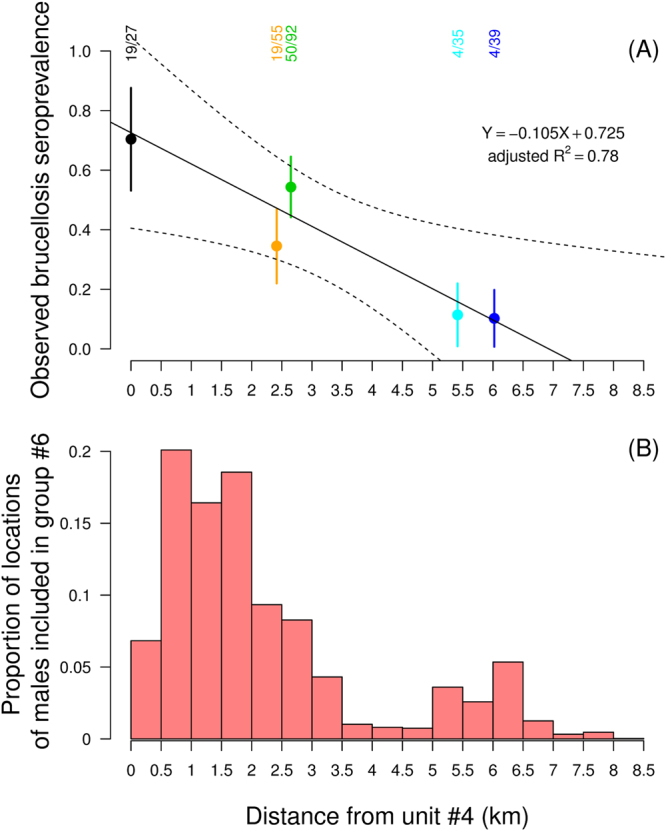
(A) Variation in the observed brucellosis seroprevalence in the identified sociospatial units of the Alpine ibex (Capra ibex) population from the Bargy massif and (B) Distribution of locations from males included in unit #6 according to distance from the highly infected unit #4. In panel (A), the solid line and the dashed lines represent predictions and the 95% confidence interval, respectively, from the weighted linear regression accounting for the number of individuals tested for brucellosis in each unit and which parameters are given on the right side. The raw numbers of seropositive versus tested Alpine ibex in each unit are given above.
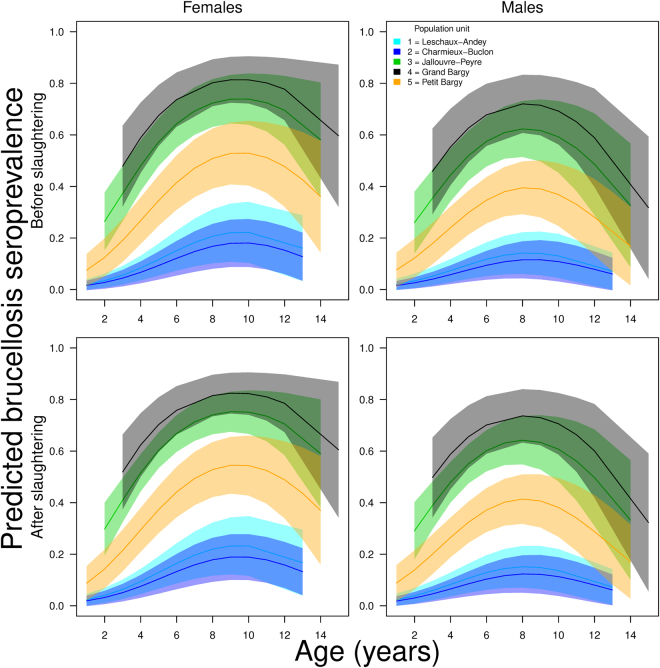
Among the multiple factors included in a logistic regression approach to explain variation in the serological status of 246 Alpine ibex individuals, the spatial structure identified in females had a strong influence on brucellosis seroprevalence in the population as a whole (i.e. including males; “unitsMFall” in Table 1), in addition to age (quadratic term, “age” in Table 1). This was revealed by the high relative importance (>0.99) and the persistent presence of both parameters in the top-ranked models (Table 1 and Supplementary information 4). Coefficients provided by the model-averaging procedure (Table 2) and the representation of the predictions from the model-averaging procedure (Fig. 4) highlighted in both sexes clear differences between units #1 and #2, where seroprevalence was <30% (raw data: 4/39 and 4/35 positive individuals, i.e. 11.4% and 10.3%), and units #3 and #4, where seroprevalence was significantly higher and could reach a maximum of 80% depending on ibex age (50/92 and 19/27, i.e. 54.3% and 70.4%). Seroprevalence values recorded in unit #5 were intermediate (<50%; raw data: 19/55, i.e. 34.5%). However, seroprevalence rose strongly during the first years of life, reached a plateau around 8–10 years in both sexes, and decreased in older individuals, in particular in males. In addition, there was a strong decrease in brucellosis seroprevalence with increasing distance between the centroid of each population unit and the centroid of the highly infected unit #4 (weighted linear model, p = 0.030, β = −10.5%.km−1, R2 = 0.78; Fig. 3A). Similarly, the proportion of locations of males included in unit #6 largely decreased with increasing distance from unit #4 (Fig. 3B).
Table 1.
Selection of models fitted to investigate variation in brucellosis seroprevalence in the Alpine ibex Capra ibex population from the Bargy massif (northern French Alps). Only the models with AICc weight ≥ 0.05 are provided here; for the full list of candidate models, see Supplementary Information 4. In model acronyms, “ + ” corresponds to additive effects and “×” to the interaction between the 2 factors. k is the number of parameters, LL is the maximum log-likelihood, ΔAICc is the difference in the Akaike information criterion between the model with the lowest AICc and the other models, and AICc weight is Akaike weight. “Age” and “sex” are ibex age (quadratic term) and sex, respectively. “UnitsMFall” are sociospatial population units (see Fig. 1 and Supplementary information 1). “Periods” opposed data collected before and after the slaughtering operations that occurred during autumn 2013 and early spring 2014.
| Model | k | LL | AICc | ΔAICc | AICc weight |
|---|---|---|---|---|---|
| unitsMFall + sex x age | 10 | −129.53 | 279.99 | 0.00 | 0.27 |
| sex + age + unitsMFall | 8 | −131.84 | 280.28 | 0.29 | 0.23 |
| age + unitsMFall | 7 | −133.17 | 280.82 | 0.83 | 0.18 |
| unitsMFall + period + sex x age | 11 | −129.17 | 281.47 | 1.47 | 0.13 |
| unitsMFall + age + period | 8 | −132.66 | 281.94 | 1.94 | 0.10 |
Table 2.
Coefficients provided by the model-averaging procedure investigating the influence of the sociospatial structure identified (i.e. unit #1 to #5), sex, age (quadratic term) and slaughtering operations on brucellosis (Brucella melitensis) seroprevalence in the Alpine ibex (Capra ibex) from the Bargy massif (northern French Alps; see Table 1 for details on the set of candidate models). In parameter acronyms, “×” corresponds to the interactive effect between both parameters. β is the estimated value, SE is the standard error of the estimated value, 95% CI is the 95% confidence interval. The reference group (intercept) corresponds to females from unit #2 before the slaughtering operations.
| Parameter | β | SE | 95% CI | p-value |
|---|---|---|---|---|
| intercept | −2.32 | 0.63 | [−3.56; −1.09] | 0.00 |
| males | −0.49 | 0.35 | [−1.11; 0.13] | 0.33 |
| age | 9.04 | 4.66 | [0.06; 18.02] | 0.06 |
| age2 | −6.19 | 3.37 | [−12.70; 0.33] | 0.07 |
| unit #4 | 3.03 | 0.75 | [1.57; 4.50] | 0.00 |
| unit #3 | 2.59 | 0.64 | [1.34; 3.83] | 0.00 |
| unit #5 | 1.65 | 0.66 | [0.36; 2.94] | 0.01 |
| unit #1 | 0.24 | 30.48 | [−59.49; 59.97] | 0.99 |
| age × males | −10.70 | 6.22 | [−21.11; −0.30] | 0.49 |
| age 2 × males | −1.64 | 3.47 | [−12.10; 8.83] | 0.85 |
| after slaughtering | 0.38 | 0.28 | [−0.40; 1.15] | 0.68 |
| age × after slaughtering | −9.05 | 2.98 | [−22.28; 4.17] | 0.82 |
| age 2 × after slaughtering | 2.08 | 1.72 | [−9.76; 13.92] | 0.93 |
| age × unit #4 | −31.14 | 1.89 | [−94.67; 32.40] | 0.98 |
| age × unit #3 | −34.73 | 1.97 | [−96.24; 26.77] | 0.97 |
| age × unit #5 | −36.78 | 2.03 | [−98.58; 25.03] | 0.97 |
| age × unit #1 | −35.23 | 2.15 | [−107.94; 37.48] | 0.98 |
| age 2 × unit #4 | 10.12 | 0.95 | [−29.60; 49.84] | 0.98 |
| age 2 × unit #3 | 14.45 | 1.01 | [−23.46; 52.37] | 0.98 |
| age 2 × unit #5 | 6.52 | 0.87 | [−32.19; 45.23] | 0.99 |
| age 2 × unit #1 | −18.41 | 1.86 | [−97.15; 60.33] | 0.99 |
| after slaughtering × unit #4 | 1.61 | 0.07 | [−1.40; 4.62] | 0.98 |
| after slaughtering × unit #3 | 1.28 | 0.06 | [−1.42; 3.97] | 0.98 |
| after slaughtering × unit #5 | −0.49 | 0.04 | [−3.19; 2.21] | 0.99 |
| after slaughtering × unit #1 | 15.02 | 30.47 | [−1914.88; 1944.92] | 1.00 |
| males × unit #4 | −0.27 | 0.02 | [−3.23; 2.69] | 1.00 |
| males × unit #3 | 1.04 | 0.03 | [−1.48; 3.56] | 0.99 |
| males × unit #5 | 0.81 | 0.02 | [−1.82; 3.43] | 0.99 |
| males × unit #1 | 1.87 | 0.04 | [−1.30; 5.04] | 0.99 |
| males × after slaughtering | −0.15 | 0.00 | [−1.44; 1.14] | 1.00 |
Figure 4.
Predictions from the model-averaging procedure investigating the influence of the sociospatial structure identified, sex, age (quadratic term) and slaughtering operations on brucellosis (Brucella melitensis) seroprevalence in the Alpine ibex (Capra ibex) from the Bargy massif (northern French Alps; see Table 1 for details on the set of candidate models). The colors correspond to the different units identified using overlap between annual home ranges as a measure of distance between individuals (see Fig. 1).
By contrast, ibex gender was less important in explaining variation in brucellosis seroprevalence (“sex”; relative importance = 0.38, i.e. 2.6 times less important than spatial struture and age) even though the interaction between age and gender was included in the model with the lowest AICc. We also detected no difference in brucellosis seroprevalence between individuals tested before (n = 54) or after (n = 192) the massive slaughtering program conducted in 2013–2014 (“periods” in Table 1, relative importance = 0.31, i.e. 3.2 times less than the spatial structure and age; Fig. 4). The average overlap between the ranges used by GPS-collared individuals monitored during common periods before and after the slaughtering operations reached 0.61 in females (SD = 0.04; range = [0.56; 0.66]; n = 4) and 0.52 in males (SD = 0.17; range = [0.26;0.74]; n = 8), i.e. values comparable with those that previously allowed including different individuals in the same spatial unit (Fig. 1 and Supplementary information 1). This result suggested that the slaughtering operations had only limited consequences on individual ibex space use in the short term (1–2 years).
Discussion
Combining space use by 37 GPS-collared individuals and visual observations of 148 marked individuals, we identified that ibex females of the Bargy massif were spatially structured, whereas males were more prone to move between the exclusive ranges used by each female unit. This spatial structure in females, in addition to age, explained the high variation in brucellosis seroprevalence in the population as a whole. By contrast, once spatial structure and age were accounted for, seroprevalence was similar in both sexes and did not change significantly after slaughtering operations. Such results provide crucial information to understanding brucellosis dynamics and identifying the major intraspecific transmission routes, key information to design management strategies for this exceptional outbreak of disease.
Our analyses confirmed that ibex females from the Bargy massif were spatially segregated in at least four units with little or no overlap all year round. Since the definition of these spatial units was based on a limited number of individuals (i.e. 21 females and 16 males) monitored with GPS collars, the existence of one or more additional unit(s) could not be excluded. However, visual observations confirmed the existence of this spatial structure in 148 marked individuals, particularly in females, and suggested it could be extrapolated to the whole population (Supplementary information 2). Besides, the existence of such a spatial structure had already been proposed in this population though it had not been accurately defined34,37. As in many other gregarious herbivore species with philopatric females (e.g.44,45, in Mediterranean mouflon,46 in chamois and isard [Rupicapra pyrenaica],47 in bighorn sheep [Ovis canadensis]), social constraints and area-restricted space use resulted in marked spatial segregation between female units36,37.
This sociospatial structure was the most determining factor influencing brucellosis seroprevalence, a result consistent with reports made in other epidemiologic contexts involving large herbivores13,14. For example, spatial segregation accounted for marked differences in the prevalence of chronic wasting disease in a mule deer (Odocoileus hemionus) population from Colorado48. It is also a key factor explaining behavioral, phenotypic, genetic and/or fitness variations in populations of large herbivores46,49–51. Previous analyses that did not consider this spatial structure found contrasting seroprevalence levels in individuals between 2 and 5 years old before/after slaughtering operations33. However, the sampling effort for these individuals was evenly distributed in spring 2012–spring 2013 (22/33 individuals tested in units #3, #4 and #5) but mostly focused on highly-infected units in spring 2014 (37/39 individuals in units #3, #4 and #5), probably explaining why this period effect was no longer detected here. These results suggest that the absolute number of animals was less determinant in disease transmission than the spatial distribution of females in the massif. However, the persistence of this spatial structure after slaughtering operations and the consequences on brucellosis seroprevalence could also be questioned. Indeed, changes in movements and space use, and social reorganizations could be expected after intense culling and removal of 40% of the population (see52 and53 for examples in perturbed populations of badgers and lions [Panthera leo]), respectively). Currently, the high overlap between the utilization distribution of GPS-collared ibex monitored before and after the slaughtering operations, and data on seroprevalence (see Results section) suggested limited consequences on space use and disease dynamics in the short term (1–2 years). Despite this apparent stability of the spatial structure and of seroprevalence levels over the study period, focusing on the dynamics of the social network structuring this population54, and of seroprevalence over the years, could in the future allow us to determine if social reorganizations had occurred, and evaluate their epidemiological consequences. It could also allow assessing the relative contribution of spatial segregation between population units, and social and habitat segregation within them, in the variation of the brucellosis seroprevalence we reported here34,37–39.
Interestingly, the structure revealed in females was also a key determinant of the seroprevalence observed in males, although males move frequently among spatial units, in particular males from unit #6 (Fig. 3, Supplementary information 1 and 2). The heterogeneous prevalence among spatial units despite frequent movements of males suggest that direct and indirect transmission due to females, in particular via the environmental contamination within each spatial unit, is probably more important for intraspecific transmission than the sexual and vertical modes28. Indeed, since males move among female spatial units, particularly during the rutting period, a purely sexual transmission would lead to spatial homogenization of brucellosis seroprevalence. However, movements of males and sexual transmission should not be excluded as a potential route of disease spread between units. Indeed, the drop in seroprevalence levels with increasing distance from the most infected unit #4, and the similar pattern in the distribution of locations of males included in unit #6 (Fig. 3), suggest the individuals using this area were of preeminent importance in the maintenance and spread of disease towards other units through source-sink dynamics55,56. As a purely speculative scenario, one could suggest that an important infectious event (e.g. one or several abortion[s]) may have occurred in this area (used by units #4, #3 and #6), infecting many individuals at the same time. During this outbreak, seroprevalence may have exceeded the level below which brucellosis disappeared naturally during previous B. melitensis outbreaks involving wild ruminants23–26. Since then, infection may have spread from the highly infected area to other units, essentially due to the movements of males from unit #6. A similar scenario, with behavioral connectivity among spatially-segregated units depending mostly on movements of males, has been recently proposed to explain pneumonia spread in bighorn sheep populations47. Nevertheless, the causes of such a hypothetic massive initial infectious event, and the mechanisms driving the exceptional persistence of this spatial pattern over time, are difficult to determine. Differences in ibex density, group size and structure between units, possibly related to landscape heterogeneity driving ibex aggregation, still have to be investigated.
Mother-to-offspring transmission, that may also occur in some infected but still fertile females (as in sheep and goats28), could be one of the mechanisms contributing to the exceptional persistence of the spatial pattern over time, since mother and offspring live in the same areas for a couple of years. However, infection may pass undetected before the reproductive maturity of young individuals, i.e. before 3–5 years in Alpine ibex57,58. Indeed, brucellosis can establish latent infection in sexually immature individuals and be undetectable by serological tests until the first reproductive event59–61). The increase in seroprevalence with age observed in young ibex of both sexes may reflect both their cumulative exposure throughout life and the increasing detectability of brucellosis with age. Likewise, the seroprevalence drop observed in old individuals, and particularly old males, may be related to antibodies dropping below detectable levels, or to low survival rates in old individuals infected by brucellosis62,63. However, these hypotheses are currently impossible to test in our population given that few old individuals were captured and that seropositive individuals were all euthanized.
Further research is needed to better understand the causes of persistence and the dynamics of this exceptional brucellosis outbreak in a wild ruminant species previously considered a dead-end host. These analyses should first investigate individual variation in space use of males and their relative contribution to disease spread. Whereas some males seemed to follow the same spatial structure as females, others were more prone to move between these units. Focusing on spatio-temporal variation in contact rates within and between populations units may then constitute a reliable approach to identifying risky areas and periods for intraspecific disease transmission64. Particular attention should be paid to late winter and early spring, i.e. when abortion events may occur27 and when ibex aggregation on the first snow-cleared and developing pastures is frequent, with little sexual segregation34. As the abortion products may be heavily infected28,29 and as bacteria have the ability to survive and remain infectious to other animals several months outdoors in cold and wet conditions28, even indirect contacts during this period may be particularly important in disease dynamics. In the Greater Yellowstone Ecosystem, the spatial overlap between bison and elk peaked when late-abortion and parturition events occurred for bison, increasing the risk of brucellosis Brucella abortus interspecific transmission30. In our case, this is also the period when most captures occurred, probably explaining that capture location significantly structured seroprevalence in both females and males in our population, even though both genders segregate outside of the rutting period and do not have exactly similar space use and movements. Finally, a spatially-structured epidemiological model is essential to test our hypotheses/scenario and determine the most effective management alternatives (e.g. biosafety measures, vaccination, approaches limiting transmission from highly-infected to less-infected units, culling, and combinations of these operations) promoted by the non-uniform distribution of brucellosis seroprevalence in the ibex population, i.e. differentiated management plans in the areas used by the different units, for both ibex and domestic herds8,65–67. This could offer new ethically and economically acceptable opportunities to ensure public health and preserve agricultural economy while saving this population of conservation concerns. Accounting for wildlife spatial ecology was hence decisive in improving our ability to understand this current complex health challenge involving a wildlife reservoir.
Methods
Study area, brucellosis and ibex population monitoring
Like most Alpine ibex populations, the Bargy population originates from a reintroduction, i.e., 6 males and 8 females translocated from the Mont-Pleureur population (Switzerland) between 1974 and 1976 (D. Gauthier, personal communication67). Following several bottlenecks, the genetic diversity in this population is low but comparable to levels observed in other reintroduced ibex populations (E. Quéméré, personal communication68). Some censuses were irregularly conducted on this population between the 1980’s and the late 1990’s, but no data have been collected since then. As a result, population size, spatial range and individual movements, performance (survival, reproduction, biometry) and sanitary status were unknown in 2012, when brucellosis re-emerged. Between 2012 and 2015, we captured (sometimes several times) 247 individuals by dart-gun xylazine/ketamine anesthesia (Rompun®, Bayer, Leverkusen, Germany and Imalgène®, Merial, France; 100 mg/individual). We took blood samples from all of them and tested serum samples for diagnosis of brucellosis according to the requirements of the World Organisation for Animal Health in small ruminants. Serological assays detected antibodies using the Rose Bengal test, the complement fixation test, indirect ELISA (IDEXX, Montpellier, France) and competitive ELISA (Ingenasa, Madrid, Spain), and a rapid test in the field (Anigen Rapid G.S. Brucella Ab, Bionote, Gyeonggi-do, Republic of Korea69,70; since 2014). In the present study, the serological status of animals was considered positive when at least two tests gave positive results. Seropositive individuals were shot (2013) or euthanized by embutramide intravenous injection (T61®, Intervet, Angers, France, 2014 and 2015; total = 98 positive individuals removed) whereas 149 seronegative individuals were released33. We recorded the sex and the approximate capture location (i.e. capture site, defined based on topographical landmarks) of all ibex tested, and determined age by counting horn growth annuli71.
Among the released seronegative ibex, we fitted 21 females and 16 males (between 2 and 14 years-old, 85% ≥ 4 years old, hence sexually mature) with GPS collars (GPS Plus, Vectronic Aerospace, Berlin, Germany; 530 g for females, 720 g for males, representing ≤1.5% of their body weight) programmed to record animal position every hour for at least 6 months, including at least one period when Alpine ibex generally migrate (between mid-May and mid-November31,32, see Supplementary information 5 for details on individuals equipped and on the monitoring). We screened GPS data for positional outliers based on unlikely movement characteristics72.
In addition, we marked the 149 seronegative ibex released using ear tags and/or collars so that individuals could be identified from several hundred meters using binoculars or a telescope. During the snow-free seasons (May-September) of the 2013–2015 period, we then recorded the composition (sex/age classes) of all the ibex groups observed, identity of marked individuals and reported group locations on a 100 × 100 m grid. We recorded most of these observations during the censuses we performed once a month between May and August, during which 1–2 observers travelled on 1 of the 9 tracks in the morning of a single day or 2 consecutive days (to avoid double counting). Using these mark-resight data and an immigration-emigration logit-normal model73, the population size (without newborns) estimated during summer 2013 was 567 (CI 95% [487; 660]). It dropped to 310 [275; 352] in 2014 and 277 [220; 351] in summer 2015. In 4 years, 359 individuals were removed: some because seropositivity was detected upon captures (n = 98; see above) and others (n = 261) as the result of management decisions taken by the French authorities. These decisions consisted in slaughtering individuals observed with clinical signs (autumn 2012 [n = 2] and spring 2013 [n = 8], total = 10), individuals > 5 years old (October 2013 [n = 233] and May 2014 [n = 18], total = 251).
All the ibex captured have been treated by professionals from the Office National de la Chasse et de la Faune Sauvage according to the ethical conditions detailed in the specific accreditations delivered by the Préfecture de Paris (prefectural decree 2009–014), by the French Minister of Ecology, Sustainable Development and Energy (Ministerial Orders of February 11, 2014) and by the Préfecture de la Haute-Savoie (prefectural decree 2015062-0018) in accordance with the French environmental code (Art. R421-15 to 421-31 and R422-92 to 422-94-1). Euthanasia was performed by veterinarians in accordance with the requirements of these accrediting authorities, and slaughtering operations were performed in accordance with accreditations delivered by the Préfecture de la Haute-Savoie (prefectural decrees 2013274-0001 and DDT-2015-0513). We hence confirm that we treated animals in accordance with relevant guidelines and regulations and that our protocols were approved by these institutions.
Ibex space use, overlap and spatial structure in GPS-collared ibex
Using hourly locations collected by GPS collars, we computed individual utilization distribution (UD) using the Biased Random Bridge approach (BRB74). This method is based on the biased random walk movement model. It takes into account animal’s movement path and time between locations to calculate UD, the probability density function providing likelihood of an animal occurring in each unit of a defined area, i.e. the 50 m cells of a raster grid, during the monitoring period. It supposes that animal movement is governed by a drift component (a general tendency to move in the direction of the next relocation) and a diffusion component (tendency to move in other directions than the direction of the drift). We determined the drift and diffusion components for each individual using the locations triplets collected over 3 hours and the maximum likelihood approach developed by Benhamou et al.74, respectively. We thus defined home range as the area including 95% of the space use estimated by the BRB approach.
We then used the overlap in volume between individual annual UD as a proxy of the distance and contact rates between individuals75,76. This dimensionless overlap index not only accounts for the area shared by two individuals but also for the relative use of this area by both individuals. It ranges from 0 (i.e. no spatial overlap) to 1 (i.e. 100% spatial overlap and identical use of this area by two individuals, or by one individual during two periods). We relied on a hierarchical classification procedure to investigate the existence of a spatial structure77 and on multiple methods to determine the best number of spatially-segregated groups in ibex monitored by GPS collars (see78 for details). We defined this best number of groups as the number provided by the majority of the methods tested. In order to evaluate the potential seasonal variation in this spatial structure, we also performed the same classification procedure using overlap between seasonal UD distinguishing spring (April-June), summer (July-August), autumn (September-09 November), the rutting period (10 November–14 January) and winter (15 January–March; Supplementary information 1).
In addition, we also checked whether visual observations of seronegative individuals marked with ear tags or collars confirmed the existence of the spatial structure we identified in the 37 GPS-collared individuals. Each individual was assigned to the closest unit based on the location where it was captured (possible for n = 148 individuals). We then computed the proportion of locations of individual assigned to the focal unit that actually fall within the range used by GPS-collared individuals from the same unit.
Finally, we also relied on the overlap in volume between the UD of GPS-collared individuals monitored during common periods before and after the slaughtering program to assess the influence of these operations on Alpine ibex space use.
Relationships between spatial structure in GPS-collared ibex and brucellosis seroprevalence at the population scale
To test for the effect of the spatial structure we identified on seroprevalence variation in the whole ibex population, we first performed the same assignment to the different spatial units as for marked individuals. We assigned each individual tested for brucellosis for the first time (recaptures were removed from analyses) to the closest unit based on the location where it was captured or slaughtered. This assignment was possible for 246 individuals ranging from 1 to 15 years old, after removing ibex having obvious clinical signs, to avoid bias in prevalence estimation.
We then included the spatial structure identified in GPS-collared ibex in a modeling procedure aimed at investigating the drivers of brucellosis seroprevalence in the massif between 2012 and 2015. The serological status of each individual was coded as 1 (positive) or 0 (negative) and this response variable was analyzed using logistic regression (generalized linear models with a binomial error distribution and a logit link function). We tested for the influence of ibex sex, age as a continuous variable in years (linear and quadratic terms; for clarity, only results including quadratic terms, providing the best results, are presented). We also tested for a “period” effect, opposing data collected before and after the first slaughtering operations that occurred during autumn 2013 and spring 2014; this was done to test the hypothesis proposed earlier for potential consequences of slaughtering operations on brucellosis prevalence (by potentially modifying ibex space use and involving social reorganizations33). We compared all the models including the additive effects of these variables. We also tested for sex-specific variation in seroprevalence with age to account for the gradual age-specific segregation between males and females and between males of different ages34,37,38. In addition, given that a large majority of the individuals slaughtered were > 5 years old, we also tested for age-specific consequences of these operations by including an interactive effect between age and period. We based our model ordering on Akaike’s Information Criterion with second-order adjustment (AICc). We considered the set of models for which the difference in AICc with the lowest AICc value (ΔAICc) was <2 the best models79. In addition, we assessed the relative importance of each explanatory variable by summing the Akaike weights across all the models where each variable occurs79. Akaike weights can be interpreted as the probability that a model is the best model, given the data and the set of candidate models. Finally, we represented the sex- and age-specific variation in seroprevalence in the different spatial units predicted by a model-averaging procedure to account for uncertainty in model selection79.
We performed all analyses using R version 3.4.180, and “adehabitatHR”, “adehabitatLT”81 packages for the computation of Utilization Distribution and overlap, and “AICcmodavg” for the model-averaging approach82.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request to anyone who wishes to repeat our analyses or collaborate with us.
Electronic supplementary material
Acknowledgements
We warmly thank all the professionals from the Office National de la Chasse et de la Faune Sauvage (SD74, USF, UFM, Amélie Vaniscotte, Elodie Petit), the Laboratoire Départemental d’Analyses Vétérinaires de la Savoie, the Direction Départementale de la Protection des Populations de la Haute-Savoie, the Fédération Départementale des Chasseurs de la Haute-Savoie and Asters–Conservatoire d’espaces naturels for their technical support in capturing ibex, analyzing samples and monitoring GPS-collared individuals and population abundance. We gratefully acknowledge Denise Mirat for correcting the grammar and two anonymous reviewers for their very helpful comments on previous drafts of this paper.
Author Contributions
J.H., J.-P.H., Y.G. and C.T. were key contributors to study creation and design. J.H., J.-P.H., P.F., Y.G., and P.M. participated in data acquisition. All the authors contributed equally to the analysis and interpretation of data, and to drafting and critically reviewing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15803-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife–threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 2.Kruse H, Kirkemo A-M, Handeland K. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 2004;10:2067–2072. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostfeld RS. Interactions between mammals and pathogens: an introduction. J. Mammal. 2015;96:2–3. doi: 10.1093/jmammal/gyu009. [DOI] [Google Scholar]
- 4.Putman, R., Appollonio, M. & Andersen, R. Ungulate Management in Europe: Problems and Practices (Cambridge University Press, New York, USA, 2011).
- 5.Tatem AJ, Rogers DJ, Hay S. Global transport networks and infectious disease spread. Adv. Parasit. 2006;62:293–343. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Martínez A, Olaizola A, Bernués A. Trajectories of evolution and drivers of change in european mountain cattle farming systems. Animal. 2009;3:152–165. doi: 10.1017/S1751731108003297. [DOI] [PubMed] [Google Scholar]
- 7.Richomme C, Gauthier D, Fromont E. Contact rates and exposure to inter-species disease transmission in mountain ungulates. Epidemiol. Infect. 2006;134:21–30. doi: 10.1017/S0950268805004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostfeld RS, Glass GE, Keesing F. Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol. Evol. 2005;20:328–336. doi: 10.1016/j.tree.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Jolles AE, Ezenwa VO. Ungulates as model systems for the study of disease processes in natural populations. J. Mammal. 2015;96:4–15. doi: 10.1093/jmammal/gyu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz, H. & Loison, A. Large herbivores across biomes. In Dannell, K., Duncan, O., Bergström, R. & Pastor, R. (eds.) Large herbivores ecology, ecosystem dynamics and conservation, 19–49 (Cambridge Unversity Press, Cambridge, UK., 2006).
- 11.Barbour, A. & Mollison, D. Epidemics and random graphs. In Stochastic processes in epidemic theory, 86–89 (Springer Verlag, Berlin Heidelberg, Germany, 1990).
- 12.White PCL, Brown JA, Harris S. Badgers (Meles meles), cattle and bovine tuberculosis (Mycobacterium bovis): a hypothesis to explain the influence of habitat on the risk of disease transmission in southwest England. P. Roy. Soc. Lond. B Biol. 1993;253:277–284. doi: 10.1098/rspb.1993.0114. [DOI] [PubMed] [Google Scholar]
- 13.Altizer, S. et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol., Evol. Syst. 517–547 (2003).
- 14.Ezenwa VO. Host social behavior and parasitic infection: a multifactorial approach. Behav. Ecol. 2004;15:446–454. doi: 10.1093/beheco/arh028. [DOI] [Google Scholar]
- 15.Bansal S, Grenfell BT, Meyers LA. When individual behaviour matters: homogeneous and network models in epidemiology. J. Roy. Soc. Interface. 2007;4:879–891. doi: 10.1098/rsif.2007.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smieszek T, Fiebig L, Scholz RW. Models of epidemics: when contact repetition and clustering should be included. Theor. Biol. Med. Model. 2009;6:1–15. doi: 10.1186/1742-4682-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicente J, Delahay RJ, Walker NJ, Cheeseman C. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. J. Anim. Ecol. 2007;76:348–360. doi: 10.1111/j.1365-2656.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 18.Garin-Bastuji B, et al. Reemergence of Brucella melitensis infection in wildlife, France. Emerg. Infect. Dis. 2014;20:1570. doi: 10.3201/eid2009.131517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mick V, et al. Brucella melitensis in France: persistence in wildlife and probable spillover from Alpine ibex to domestic animals. Plos One. 2014;9:1–9. doi: 10.1371/journal.pone.0094168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mailles A, et al. Re-emergence of brucellosis in cattle in France and risk for human health. Euro Surveillance. 2012;17:30. [PubMed] [Google Scholar]
- 21.Freycon, P. Rôle du bouquetin Capra ibex dans l’épidémiologie de la brucellose à Brucella melitensis en Haute-Savoie. Vet. Med. thesis, VetAgroSup Lyon–Université Claude Bernard Lyon 1 (2015).
- 22.Gilot-Fromont, E. et al. Receptivity, sensitivity or sociality: what makes ibex a good host for Brucella melitensis? In European College of Zoological Medicine, Brussels (2016).
- 23.Ferroglio E, Tolari F, Bollo E, Bassano B. Isolation of Brucella melitensis from Alpine ibex. J. Wild. Dis. 1998;34:400–402. doi: 10.7589/0090-3558-34.2.400. [DOI] [PubMed] [Google Scholar]
- 24.Gauthier, D., Hars, J. & Rossi, L. Brucellosis in free ranging chamois (Rupicapra rupicapra) and its relationships with domestic breeding. In Third Conference of European Wildlife Disease Association, Edinburg, Scotland (1998).
- 25.Muñoz PM, et al. Spatial distribution and risk factors of brucellosis in iberian wild ungulates. BMC Infect. Dis. 2010;10:46. doi: 10.1186/1471-2334-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hars J, et al. Un foyer de brucellose chez les ongulés sauvages du massif du Bargy en Haute-Savoie. Bul. épidémiologique, santé animale et alimentation. 2013;60:2–7. [Google Scholar]
- 27.Alton, G. G. Brucella melitensis. In Animal brucellosis, 383–409 (CRC Press, Boca Raton, USA, 1990).
- 28.Díaz AE. Epidemiology of brucellosis in domestic animals caused by Brucella melitensis, Brucella suis and Brucella abortus. Rev. Sci. Tech. OIE. 2013;32:43–51. doi: 10.20506/rst.32.1.2188. [DOI] [PubMed] [Google Scholar]
- 29.Morgan WJB. Brucellosis in animals: diagnosis and control. P. Roy. Soc. Med. 1969;62:56–58. [PMC free article] [PubMed] [Google Scholar]
- 30.Proffitt KM, White PJ, Garrott RA. Spatio-temporal overlap between Yellowstone bison and elk–implications of wolf restoration and other factors for brucellosis transmission risk. J. Appl. Ecol. 2010;47:281–289. doi: 10.1111/j.1365-2664.2010.01770.x. [DOI] [Google Scholar]
- 31.Girard I, Adrados C, Bassano B, Janeau G. Application de la technologie GPS au suivi du déplacement de bouquetins des Alpes (Capra ibex Ibex l.) dans les parcs nationaux de la Vanoise et du Grand Paradis (Italie) Travaux scientifiques du Parc National de la Vanoise. 2009;24:105–126. [Google Scholar]
- 32.Marchand, P. Mouvements, occupation de l’espace, sélection de l’habitat et interactions chez les bouquetins des Alpes Capra ibex suivis par colliers GPS dans le Parc National des Ecrins. Tech. Rep., Parc National des Ecrins (2015).
- 33.Hars J, et al. Surveillance et gestion d’un foyer de brucellose chez le bouquetin dans le massif du Bargy (Haute-Savoie) Faune Sauvage. 2015;306:11–20. [Google Scholar]
- 34.Villaret J-C, Bon R. Social and spatial segregation in Alpine ibex (Capra ibex) in Bargy, French Alps. Ethology. 1995;101:291–300. doi: 10.1111/j.1439-0310.1995.tb00366.x. [DOI] [Google Scholar]
- 35.Bon R, Campan R. Unexplained sexual segregation in polygamous ungulates: a defense of an ontogenetic approach. Behav. Process. 1996;38:131–154. doi: 10.1016/S0376-6357(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 36.Conradt L. Social segregation is not a consequence of habitat segregation in red deer and feral soay sheep. Anim. Behav. 1999;57:1151–1157. doi: 10.1006/anbe.1999.1067. [DOI] [PubMed] [Google Scholar]
- 37.Bon R, Rideau C, Villaret JC, Joachim J. Segregation is not only a matter of sex in alpine ibex Capra ibex ibex. Animal Behav. 2001;62:495–504. doi: 10.1006/anbe.2001.1776. [DOI] [Google Scholar]
- 38.Villaret JC, Bon R, Rivet A. Sexual segregation of habitat by the Alpine ibex in the French Alps. J. Mammal. 1997;78:1273–1281. doi: 10.2307/1383070. [DOI] [Google Scholar]
- 39.Grignolio S, Rossi I, Bassano B, Apollonio M. Predation risk as a factor affecting sexual segregation in Alpine ibex. J. Mammal. 2007;88:1488–1497. doi: 10.1644/06-MAMM-A-351R.1. [DOI] [Google Scholar]
- 40.Ruckstuhl, K. E. & Neuhaus, P. (eds.) Sexual segregation in vertebrates - Ecology of the two sexes (Cambridge University Press, Cambridge, UK., 2006).
- 41.Ruckstuhl KE, Neuhaus P. Behavioral synchrony in ibex groups: effects of age, sex and habitat. Behaviour. 2001;138:1033–1046. doi: 10.1163/156853901753286551. [DOI] [Google Scholar]
- 42.Willisch C, Neuhaus P. Alternative mating tactis and their impact on survival in adult male Alpine ibex (Capra ibex) J. Mammal. 2009;90:1421–1430. doi: 10.1644/08-MAMM-A-316R1.1. [DOI] [Google Scholar]
- 43.Willisch CS, Neuhaus P. Social dominance and conflict reduction in rutting male Alpine ibex. Capra ibex.Behav. Ecol. 2009;21:372–380. doi: 10.1093/beheco/arp200. [DOI] [Google Scholar]
- 44.Martins AG, et al. Population subdivision among mouflon sheep (Ovis gmelini) ewes and ranging behaviour of rams during the rut. J. Zool. 2002;258:27–37. doi: 10.1017/S0952836902001176. [DOI] [Google Scholar]
- 45.Marchand P, et al. Coupling scale-specific habitat selection and activity reveals sex-specific food/cover trade-offs in a large herbivore. Anim. Behav. 2015;102:169–187. doi: 10.1016/j.anbehav.2015.01.011. [DOI] [Google Scholar]
- 46.Loison A, Jullien J-M, Menaut P. Subpopulation structure and dispersal in two populations of chamois. J. Mammal. 1999;80:620–632. doi: 10.2307/1383306. [DOI] [Google Scholar]
- 47.Borg NJ, et al. Behavioral connectivity among bighorn sheep suggests potential for disease spread. J.Wildl. Manage. 2017;81:38–45. doi: 10.1002/jwmg.21169. [DOI] [Google Scholar]
- 48.Conner MM, Miller MW. Movement patterns and spatial epidemiology of a prion disease in mule deer population units. Ecol. Appl. 2004;14:1870–1881. doi: 10.1890/03-5309. [DOI] [Google Scholar]
- 49.Petit E, Aulagnier S, Bon R, Dubois M, Crouau-Roy B. Genetic structure of populations of the Mediterranean mouflon (Ovis gmelini) J. Mammal. 1997;78:459–467. doi: 10.2307/1382898. [DOI] [Google Scholar]
- 50.Crampe J-P, et al. Patrons de reproduction des femelles d’isard (Rupicapra pyrenaica pyrenaica) dans une population non chassée et conséquences démographiques. Can. J. Zool. 2006;84:1263–1268. doi: 10.1139/z06-123. [DOI] [Google Scholar]
- 51.Garel M, et al. Selective harvesting and habitat loss produce long-term life history changes in a mouflon population. Ecol. Appl. 2007;17:1607–1618. doi: 10.1890/06-0898.1. [DOI] [PubMed] [Google Scholar]
- 52.Carter S, et al. Culling-induced social perturbation in Eurasian badgers Meles meles and the management of TB in cattle: an analysis of a critical problem in applied ecology. P. Roy. Soc. Lond. B Biol. 2007;274:2769–2777. doi: 10.1098/rspb.2007.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson Z, Valeix M, Loveridge AJ, Madzikanda H, Macdonald DW. Socio-spatial behaviour of an African lion population following perturbation by sport hunting. Biol. Conserv. 2011;144:114–121. doi: 10.1016/j.biocon.2010.08.005. [DOI] [Google Scholar]
- 54.Farine DR, Whitehead H. Constructing, conducting and interpreting animal social network analysis. J.Anim. Ecol. 2015;84:1144–1163. doi: 10.1111/1365-2656.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pulliam HR. Sources, sinks, and population regulation. Am. Nat. 1988;132:652–661. doi: 10.1086/284880. [DOI] [Google Scholar]
- 56.Grenfell B, Harwood J. Meta) population dynamics of infectious diseases. Trends Ecol. Evol. 1997;12:395–399. doi: 10.1016/S0169-5347(97)01174-9. [DOI] [PubMed] [Google Scholar]
- 57.Toïgo, C. et al. Female reproductive success and costs in an alpine capital breeder under contrasting environments. Ecoscience 427–433 (2002).
- 58.Rughetti M, Dematteis A, Meneguz PG, Festa-Bianchet M. Age-specific reproductive success and cost in female Alpine ibex. Oecologia. 2015;178:197–205. doi: 10.1007/s00442-014-3192-3. [DOI] [PubMed] [Google Scholar]
- 59.Thorne, E. T. Brucellosis. In Williams, E. S. & Barker, I. K. (eds.) Infectious Diseases of Wild Mammals, 372–395 (Iowa State University Press, Ames, USA, 2000).
- 60.Rhyan JC, et al. Pathogenesis and epidemiology of brucellosis in Yellowstone bison: serologic and culture results from adult females and their progeny. J. Wildl. Dis. 2009;45:729–739. doi: 10.7589/0090-3558-45.3.729. [DOI] [PubMed] [Google Scholar]
- 61.Treanor JJ, et al. Estimating probabilities of active brucellosis infection in Yellowstone bison through quantitative serology and tissue culture. J. Appl.Ecol. 2011;48:1324–1332. doi: 10.1111/j.1365-2664.2011.02058.x. [DOI] [Google Scholar]
- 62.Joly DO, Messier F. The effect of bovine tuberculosis and brucellosis on reproduction and survival of wood bison in Wood Buffalo National Park. J. Anim. Ecol. 2005;74:543–551. doi: 10.1111/j.1365-2656.2005.00953.x. [DOI] [Google Scholar]
- 63.Benavides JA, et al. Estimating loss of Brucella abortus antibodies from age-specific serologic data in elk. EcoHealth. 2017;14:234–243. doi: 10.1007/s10393-017-1235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Long JA, Nelson TA. Measuring dynamic interaction in movement data. Transactions GIS. 2013;17:62–77. doi: 10.1111/j.1467-9671.2012.01353.x. [DOI] [Google Scholar]
- 65.Haydon DT, et al. Low-coverage vaccination strategies for the conservation of endangered species. Nature. 2006;443:692–695. doi: 10.1038/nature05177. [DOI] [PubMed] [Google Scholar]
- 66.Lawson, A. B. Statistical methods in spatial epidemiology (John Wiley & Sons, West Sussex, England, 2006).
- 67.Anses. Mesures de maîtrise de la brucellose chez les bouquetins du Bargy. Avis de l’Anses et Rapport d’expertise collective, Juillet 2015 (2015).
- 68.Biebach I, Keller LF. Inbreeding in reintroduced populations: the effects of early reintroduction history and contemporary processes. Conserv. Genet. 2010;11:527–538. doi: 10.1007/s10592-009-0019-6. [DOI] [Google Scholar]
- 69.Anses. Note d’appui scientifique et technique de l’Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail relatif au “dépistage des bouquetins infectés de brucellose sur le terrain”. Tech. Rep. (2014).
- 70.Corde, Y. et al. A rapid test for identifying B. melitensis infection in an Alpine ibex (Capra ibex) reservoir in the French Alps. Brucell. 2014 Int. Res. Conf., including the 67th Annu. Brucell. Res. Meet., Berlin, Ger. (2014).
- 71.Michallet, J., Grand, B. & Bonardi, J. La population de bouquetins des Alpes du massif de Belledonne-Sept Laux (département de l’Isère). Bulletin Mensuel de l’Office National de la Chasse 19–24 (1988).
- 72.Bjørneraas K, Van Moorter B, Rolandsen CM, Herfindal I. Screening global positioning system location data for errors using animal movement characteristics. J. Wildl. Manage. 2010;74:1361–1366. doi: 10.1111/j.1937-2817.2010.tb01258.x. [DOI] [Google Scholar]
- 73.McClintock, B. T., White, G. C., Burnham, K. P. & Pryde, M. A. A generalized mixed effects model of abundance for mark-resight data when sampling is without replacement. In Thomson, D. L., Cooch, E. G. & Conroy, M. J. (eds.) Modeling demographic processes in marked populations, 271–289 (Springer, 2009).
- 74.Benhamou S. Dynamic approach to space and habitat use based on biased random bridges. PloS One. 2011;6:e14592. doi: 10.1371/journal.pone.0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fieberg J, Kochanny CO. Quantifying home-range overlap: the importance of the utilization distribution. J. Wildl. Manage. 2005;69:1346–1359. doi: 10.2193/0022-541X(2005)69[1346:QHOTIO]2.0.CO;2. [DOI] [Google Scholar]
- 76.Robert K, Garant D, Pelletier F. Keep in touch: does spatial overlap correlate with contact rate frequency? J. Wildl. Manage. 2012;76:1670–1675. doi: 10.1002/jwmg.435. [DOI] [Google Scholar]
- 77.Gordon AD. A review of hierarchical classification. J. Roy. Stat. Soc. A (General) 1987;150:119–137. doi: 10.2307/2981629. [DOI] [Google Scholar]
- 78.Charrad M, Ghazzali N, Boiteau V, Niknafs A. nbclust: an R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014;61:1–36. doi: 10.18637/jss.v061.i06. [DOI] [Google Scholar]
- 79.Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference - A practical information-theoretic approach - Second edition (Springer Verlag, New York, USA, 2002).
- 80.R Development Core Team. R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria (2017).
- 81.Calenge C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006;197:516–519. doi: 10.1016/j.ecolmodel.2006.03.017. [DOI] [Google Scholar]
- 82.Mazerolle, M. J. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c) (2016). R package version 2.0–4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on request to anyone who wishes to repeat our analyses or collaborate with us.