Abstract
This study aimed to investigate the consequences of maternal exposure to iodine excess (IE; 0.6 mg NaI/L) throughout pregnancy and lactation on the hypothalamus-pituitary-thyroid axis of the male offspring in adulthood. Maternal IE exposure increased hypothalamic Trh mRNA expression and pituitary Tsh expression and secretion in the adult male offspring. Moreover, the IE-exposed offspring rats presented reduced thyroid hormones levels, morphological alterations in the thyroid follicles, increased thyroid oxidative stress and decreased expression of thyroid differentiation markers (Tshr, Nis, Tg, Tpo, Mct8) and thyroid transcription factors (Nkx2.1, Pax8). Finally, the data presented here strongly suggest that epigenetic mechanisms, as increased DNA methylation, augmented DNA methyltransferases expression, hypermethylation of histone H3, hypoaceylation of histones H3 and H4, increased expression/activity of histone deacetylases and decreased expression/activity of histone acetyltransferases are involved in the repression of thyroid gene expression in the adult male offspring. In conclusion, our results indicate that rat dams’ exposure to IE during pregnancy and lactation induces primary hypothyroidism and triggers several epigenetic changes in the thyroid gland of their male offspring in adulthood.
Introduction
Thyroxin (T4) and triiodothyronine (T3) are the main hormones produced by the thyroid gland and are essential for growth, development and metabolism control in vertebrates1. Thyroid hormones (TH) are the only hormones that contain iodine, which is essential both for TH synthesis and for thyroid function control2. Indeed, iodide uptake is mediated by the activity of the sodium-iodide symporter (NIS) and is the first and limiting step for TH synthesis3.
Iodine is scarce in the environment and its consumption through natural foods as well as through iodinated salt supposedly guarantee an adequate supply of this trace element for TH synthesis4. According to the World Health Organization (WHO), the iodine consumption should be 100–150 µg per day to guarantee adequate TH production in children and adults5. Nevertheless, during specific periods as pregnancy and lactation, WHO suggests a consumption of 200–250 µg iodine per day6. Importantly, adequate iodine consumption during these critical periods of development guarantees normal maternal and foetal thyroid function.
The deleterious effects of iodine deficiency (ID) to maternal thyroid function and to foetus development are clearly reported7. However, the effects of maternal ingestion of iodine excess (IE) on foetal development are still controversial. In fact, the safety of upper limits of iodine intake is not completely defined8. Recent studies have shown that IE consumption during pregnancy and/or lactation impaired maternal thyroid function as well as promoted deleterious effects on the offspring development9–12. Additionally, previous studies have shown that hormonal/nutritional disturbances during pregnancy and lactation may interfere with the programming of gene expression through epigenetic mechanisms, as DNA methylation, histones post-translational modifications and differential miRNA expression13–15.
Although previous studies have reported the development of goitre in the foetus of mothers exposed to high iodine intake16,17, the effects of IE on the programming of thyroid dysfunction in the offspring during adulthood have never been described. Therefore, this study aimed to investigate the consequences of rat dams’ exposure to IE throughout pregnancy and lactation on the hypothalamus-pituitary-thyroid axis function of their male offspring in adult life. In addition, this study also intended to elucidate whether maternal IE exposure induces epigenetic changes in the thyroid of the male offspring.
Results
Altered morphometry parameters and serum hormonal levels in the adult male offspring of IE-exposed rat dams
As shown in Table 1, maternal IE exposure during pregnancy and lactation has not altered the body weight (BW) of the adult male rat offspring. Conversely, these animals presented a significant reduction of the dry heart weight (DHW) and the ratio between dry heart weight and body weight (DHW/BW) in comparison to the control group. Interestingly, maternal IE ingestion reduced the male offspring’s circulating levels of T3 and T4, and significantly increased the serum TSH levels in these animals in adulthood.
Table 1.
Body weight (BW), dry heart weight (DHW), dry heart to body weight ratio (DHW/BW), T3, T4 and TSH serum concentrations of adult male offspring of rat dams exposed or not to IE treatment during pregnancy and lactation periods.
| Control | 5-HI | |
|---|---|---|
| BW (g) | 354 ± 11.5 | 336 ± 6.4 |
| DHW (g) | 0.27 ± 0.01 | 0.22 ± 0.01** |
| DHW/BW (g)A | 0.76 ± 0.02 | 0.66 ± 0.01** |
| T 3 (ng/dL) | 25.0 ± 1.1 | 18.4 ± 0.7** |
| T4 (μg/dL) | 7.06 ± 0.2 | 6.31 ± 0.2* |
| TSH (ng/mL) | 1.2 ± 0.1 | 3.3 ± 0.5*** |
AValues multiplied by 1000. Results are expressed by means ± SEM, n = 6-10 per group. *P < 0.05, **P < 0.01, ***P < 0.01 vs. Control.
Altered hypothalamic and pituitary gene expression in the adult male offspring of IE-exposed rat dams
At PND90, the IE-exposed male offspring presented higher expression of the Trh and Dio2 mRNAs in the hypothalamus in comparison to the control group (Fig. 1A). Moreover, maternal IE treatment during pregnancy and lactation has decreased Gh mRNA expression and increased Trhr, Dio2, Tsha and Tshb mRNA expression in the pituitary of the male rat offspring in adulthood (Fig. 1B). Interestingly, the protein content of both subunits of TSH were significantly reduced in the pituitary of the IE-exposed male offspring (Fig. 1C).
Figure 1.
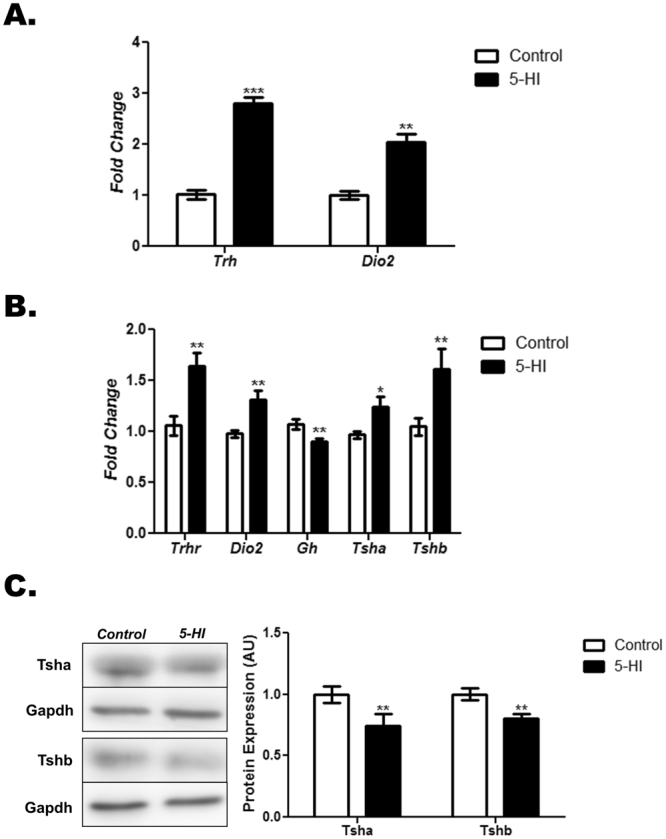
Maternal IE exposure alters gene expression in the hypothalamus and the pituitary of the adult male offspring. Hypothalamus and pituitary gene and/or protein expression were investigated in the adult male offspring of control (C) or IE-exposed rat dams (5-HI). Hypothalamic expression of Trh and Dio2 mRNAs (A) as well as pituitary Trhr, Dio2, Gh, Tsha, Tshb mRNA content (B) were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (C) Total pituitary content of Tsha and Tshb subunits were analyzed through Western blotting, using Gapdh as loading control (n = 10/group). Representative western blots are shown in the left panel. Results are expressed as means ± SEM as fold change or in arbitrary units (AU). *P < 0.05, **P < 0.01; ***P < 0.001 vs. C (Unpaired two tailed Student’s t-test).
Maternal IE exposure alters the morphology of the thyroid gland of the adult male offspring
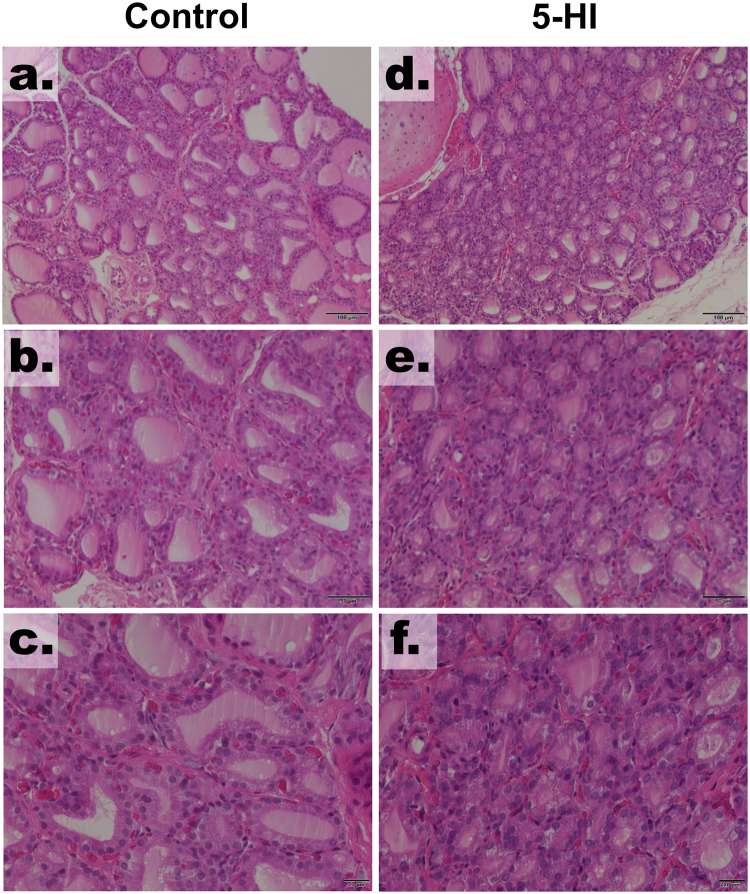
As demonstrated in Fig. 2, the male offspring of IE-exposed rat dams presented significant alterations on thyroid histology. Indeed, the offspring of the control dams presented follicles with different size and shape (Fig. 2a–c). In contrast, the adult male offspring of rat dams exposed to the IE treatment (5-HI) presented several follicles with decreased diameter and filled with lower content of thyroglobulin (Fig. 2d–f). No infiltration of immune cells was observed in any of the studied groups.
Figure 2.
Maternal IE exposure alter the thyroid morphology of the adult male offspring. Light microscope view of the thyroid follicles of the male offspring of control or IE-exposed rat dams. IE treatment decreased the diameter of the thyroid follicles and reduced the amount of colloid within the follicles. No lymphocytic infiltration was observed. Hematoxylin and eosin. Magnification 100× (a,d), 200× (b,e) and 400× (c,f).
Thyroid gene and protein expression in the adult male offspring of IE-exposed rat dams
As demonstrated in Figs 3 and 4, maternal exposure to IE during pregnancy and lactation has altered the expression of genes and proteins involved in the TH synthesis and secretion in the thyroid of male rat offspring. Indeed, the thyroid expression of Tshr, Slc5a5, Tpo, Tg and Mct8 mRNAs (Fig. 3A) and Tshr, Nis, Tpo, Tg and Mct8 proteins (Fig. 3B) were reduced in the IE-exposed male offspring. In agreement, these animals also presented significant reduction of thyroid Pax8 and Nkx2.1 mRNA (Fig. 4A) and protein (Fig. 4B) expression.
Figure 3.
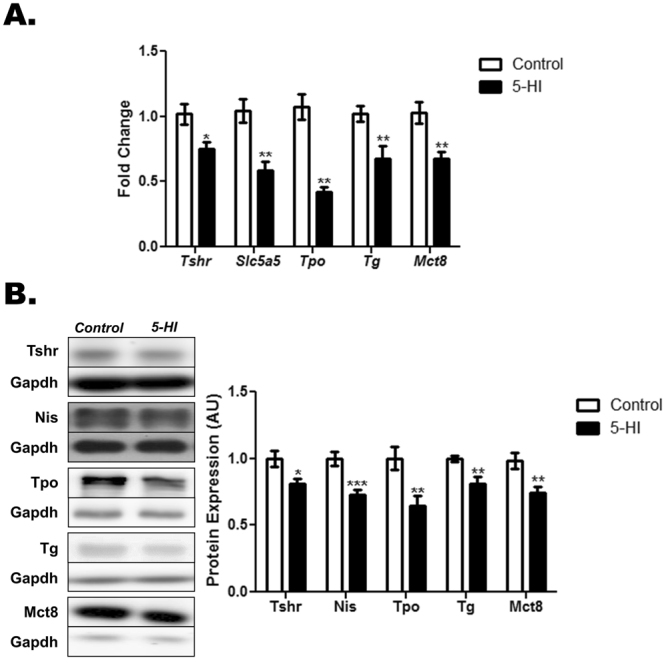
Maternal IE exposure reduces the expression of differentiation genes in the thyroid of the adult male offspring. Thyroid gene and protein expression were investigated in the adult male offspring of control (C) or IE-exposed rat dams (5-HI). (A) Tshr, Slc5a5, Tpo, Tg and Mct8 mRNA content were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (B) Total thyroid Tshr, Nis, Tpo, Tg and Mct8 protein content were analyzed through Western blotting, using Gapdh as loading control (n = 10/group). Representative western blots are shown in the left panel. Results are expressed as means ± SEM as fold change or arbitrary units (AU). *P < 0.05, **P < 0.01; ***P < 0.001 vs. C (Unpaired two tailed Student’s t-test).
Figure 4.
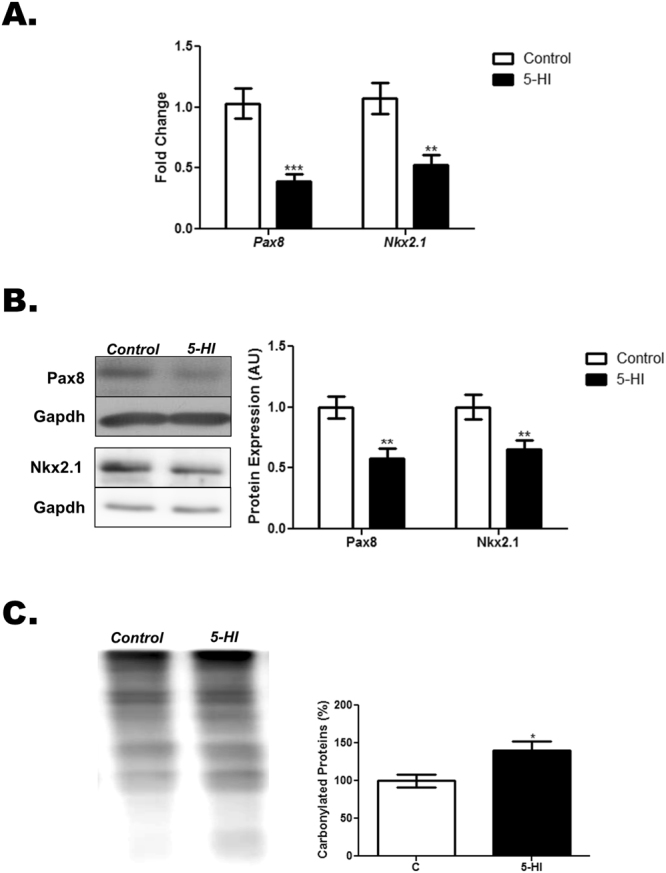
Maternal IE exposure reduces thyroid transcription factors expression and increases thyroid oxidative stress in the adult male offspring. Thyroid gene expression and protein carbonylation were investigated in the adult male offspring of control (C) or IE-exposed rat dams (5-HI). (A) Pax8 and Nkx2.1 mRNA content were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (B) Total thyroid Pax8 and Nkx2.1 protein content were analyzed through Western blotting, using Gapdh as loading control (n = 10/group). Representative western blots are shown in the left panel. (C) Protein carbonylation was analyzed through Western blotting by using specific primary and secondary antibodies to detect carbonylated proteins. Ponceau S staining was used as loading control (n = 6/group). Representative western blots are shown in the left panel. Results are expressed as means ± SEM as fold change, arbitrary units (AU) or percentage (%), respectively. *P < 0.05, **P < 0.01; ***P < 0.001 vs. C (Unpaired two tailed Student’s t-test).
Increased thyroid oxidative stress status in the adult male offspring of IE-exposed rat dams
Maternal exposure to IE treatment during pregnancy and lactation has significantly increased the amount of carbonylated proteins in the thyroid of the adult male offspring in comparison to the control animals (Fig. 4C).
Altered peripheral conversion of TH in the adult male offspring of IE-exposed rat dams
As presented in Fig. 5, maternal IE exposure significantly reduced Dio1 mRNA expression in the liver and the kidney of the adult male offspring (Fig. 5A). In accordance, the D1 activity was reduced in both tissues of IE-exposed offspring in comparison to control group (Fig. 5B).
Figure 5.
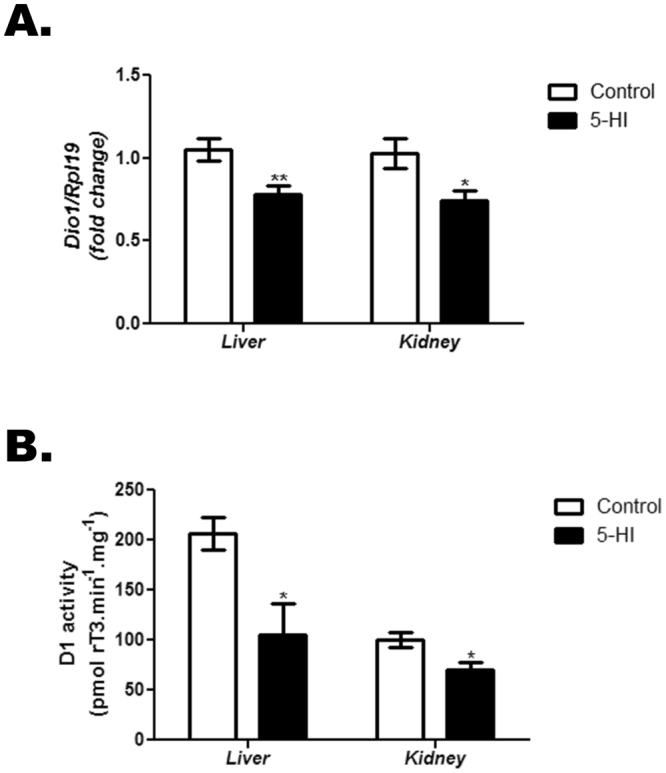
Maternal IE exposure reduces liver and kidney Dio1 mRNA expression and D1 activity in the adult male offspring. Dio1 expression and D1 activity were analyzed in the liver and kidney of the adult male offspring of control (C) or IE-exposed rat dams (5-HI). (A) Dio1 mRNA expression was analyzed in the liver and the kidney by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (B) D1 activity was evaluated in the microsomes from liver and kidney of the offspring rats (n = 3–5/group). Results are expressed as means ± SEM as fold change or as picomols rT3.min−1.mg−1, respectively. *P < 0.05; ** P < 0.01 vs. C (Unpaired two tailed Student’s t-test).
Thyroid DNA methylation status in the adult male offspring of IE-exposed rat dams
Maternal exposure to IE has increased both the mRNA (Fig. 6A) and protein (Fig. 6B) expression of the DNA methyltransferases 1 and 3 in the thyroid of the adult male offspring. Accordingly, global DNA methylation was also significantly increased in the thyroid of these animals in comparison to the control group (Fig. 6C).
Figure 6.
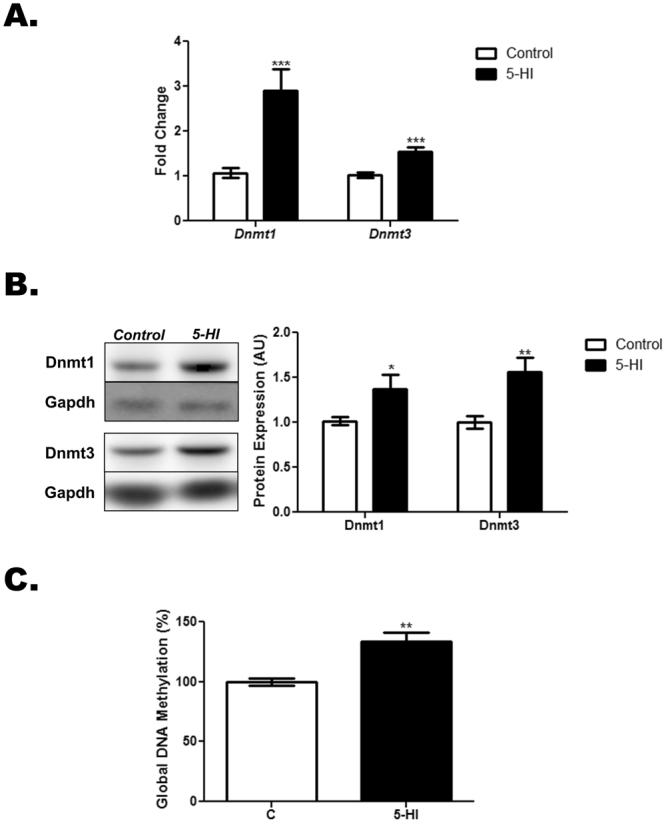
Maternal IE exposure increases Dnmts expression and DNA methylation in the thyroid of the adult male offspring. Thyroid lobes were obtained from the adult male offspring of control (C) or IE-exposed rat dams (5-HI). (A) Dnmt1 and Dnmt3 mRNA content were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (B) Total Dnmt1 and Dnmt3 protein content were analyzed in the thyroid through Western blotting, using Gapdh as loading control (n = 10/group). Representative western blots are shown in the left panel. (C) Thyroid DNA methylation was assessed through Imprint® Methylated DNA Quantification Kit. Results are expressed as means ± SEM as fold change, arbitrary units (AU) or percentage (%), respectively. *P < 0.05, **P < 0.01; ***P < 0.001 vs. C (Unpaired two tailed Student’s t-test).
Histones post-translational modifications in the thyroid of IE-exposed adult male offspring
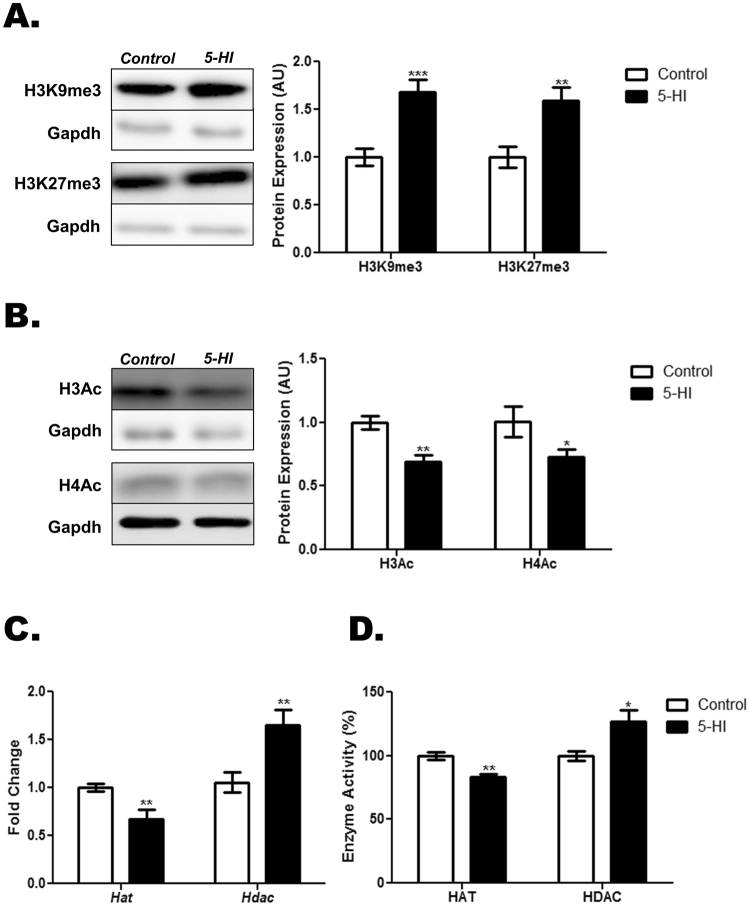
As shown in Fig. 7A, the exposure of rat dams to IE during pregnancy and lactation has increased the lysine-9 and lysine-27 methylation of the histone H3 in the thyroid of adult male offspring. Conversely, IE treatment has reduced the acetylation of the histones H3 and H4 in the thyroid of these animals in comparison to the control ones (Fig. 7B). Moreover, IE-exposed adult male offspring presented a significant reduction of the Hat mRNA content and an increased expression of the Hdac mRNA in the thyroid gland (Fig. 7C). Furthermore, the HAT activity was reduced, whereas the HDAC activity was increased in the thyroid of the IE-exposed male offspring in comparison to the control animals (Fig. 7D).
Figure 7.
Maternal IE exposure induces post-translational modifications in the thyroid of the adult male offspring. Thyroid lobes were obtained from the adult male offspring of control (C) or IE-exposed rat dams (5-HI). Thereafter, the methylation status of the lysines 9 and 27 of histone H3 (A) and acetylation status of the histones H3 and H4 (B) were evaluated through Western blotting analysis, using Gapdh as loading control (n = 8/group). Representative western blots are shown in the left panels. (C) Thyroid Hat and Hdac mRNA expression were analyzed by Real-Time PCR and normalized to Rpl19 mRNA content (n = 10/group). (D) Thyroid HAT activity was measured with a colorimetric assay kit, while HDAC activity was assessed with a fluorimetric assay kit. Results are expressed as means ± SEM as arbitrary units (AU) (A-B), fold change (C) or percentage (%) (D),. *P < 0.05, **P < 0.01; ***P < 0.001 vs. C (Unpaired two tailed Student’s t-test).
Discussion
The results presented here demonstrate that the exposure of rat dams to IE during pregnancy and lactation induces primary hypothyroidism in their male offspring in adulthood. Additionally, the data strongly suggest that several epigenetic mechanisms are involved in the repression of thyroid gene expression observed in the IE-exposed animals.
Epidemiological data and animal studies suggest that maternal environment during intrauterine and/or lactation periods has a critical role in the programming of gene expression of the offspring18,19. Moreover, disturbances during these critical periods of development are commonly related to increased susceptibility to develop neuronal, cardiovascular, endocrine or metabolic diseases in the adult life20,21. In accordance, our data indicated that the maternal ingestion of high concentrations of iodine significantly altered the function of the hypothalamus-pituitary-thyroid axis of the male rat offspring in adulthood. This finding was in agreement with earlier epidemiological observations that reported the occurrence of hypothyroidism and goitre in the newborns of IE-exposed mothers12,22,23. However, our study was the first to evaluate, using an animal model, the impact of maternal IE exposure in the function of hypothalamus-pituitary-thyroid axis of the male offspring during adult life.
The hypothyroid condition was confirmed by several molecular and biochemical parameters. Firstly, there was a significant increase of serum TSH levels in the offspring of IE-exposed rat dams. This result seems to be associated with the reduction of serum T3 and T4 concentrations also observed in these animals. Indeed, since Tsh mRNA expression is down regulated by TH24, the increased mRNA expression of both subunits of Tsh in the pituitary reinforce the hypothyroid condition in the IE-exposed offspring. Additionally, the expression of other genes that are known to be regulated by TH, as Dio2 and Gh 25–27 were also altered in the pituitary of the offspring derived from IE-treated rat dams. Importantly, the reduced action of TH in the male offspring’s hypothalamus-pituitary axis was also confirmed by the increased expression of Trh and Trhr mRNAs that are known to be down regulated by T3 28. In fact, the latter result also suggests an increased action of Trh in the thyrotropes of IE-exposed animals and could justify the increased secretion rate of TSH in the bloodstream of these animals.
The reduced TH action in peripheral tissues was strengthened by the decreased DHW and DHW/BW ratio in the offspring of IE-exposed rat dams. As described before, TH regulate the expression of several structural and functional proteins in the heart29. Therefore, the reduction of DHW is commonly observed in hypothyroid animals30.
The repression of thyroid gene expression and, consequently, the reduced serum TH levels in IE-exposed animals may be related to the significant reduction of the mRNA/protein expression of Pax8 and Nkx2.1. In fact, these transcription factors are responsible for controlling the expression of several differentiation genes in the thyroid cells31. In addition, it has been reported that the oxidative stress reduces the transcriptional activity of these transcription factors and their binding to the promoters of thyroid genes32. Moreover, previous studies demonstrated that IE acutely increases the production of reactive oxygen species (ROS) in the thyroid33. Indeed, as demonstrated here, maternal exposure to IE during pregnancy and lactation augmented the content of carbonylated proteins in the thyroid of the adult male offspring, suggesting an increased oxidative stress in this gland. Although the protein carbonylation is an important hallmark of oxidative damage34, the absence of additional analyses about other oxidative stress parameters is a limitation of this study.
Taken together, our data suggest that the IE-induced reduction of Pax8/Nkx2.1 content associated with their reduced transcriptional activity might contribute to the decreased expression of genes/proteins involved in the TH synthesis and secretion (Tshr, Nis, Tpo, Tg and Mct8). Interestingly, the reduction of the thyroid follicles’ diameter and the decreased colloid content within the follicles in the IE-exposed animals concur with the observed reduction of Tg protein content in these animals. It is worth noting that the physiological mechanism that protects the thyroid gland from the deleterious effects of IE, known as the Wolff-Chaikoff effect, is not mature until the 36th week of pregnancy in humans 35. Therefore, the impact of a direct effect of IE on the regulation of thyroid gene expression during the intrauterine period is probably more intense in the developing foetus than in the adult individuals.
Besides the impairment of the thyroid function in the IE-exposed offspring, the decreased serum TH levels, especially T3, might also be a consequence of the reduced activity of D1 in the liver and the kidney. In fact, it is well known that D1 activity is the main responsible for the peripheral conversion of T4 to T3 36. Furthermore, the impaired D1 activity is in accordance with the observed reduction of Dio1 mRNA in both tissues of IE-exposed animals.
The adult male offspring of the rat dams exposed to IE during pregnancy and lactation presented an increased expression of Dnmt1 and Dnmt3 in the thyroid. Actually, both enzymes are involved in the establishment and maintaining of cytosine methylation in the DNA37. Therefore, this result is in accordance with the increased global DNA methylation status that was observed in the thyroid of the IE-exposed offspring rats. Moreover, these data concur with the repression of thyroid gene expression in these animals, since DNA methylation is intimately related to the inactivation of gene transcription38. The trigger event responsible for the increased DNA methylation in IE-exposed animals need to be further explored. Indeed, previous studies reported that the hypothyroid condition increased the DNA methylation and the expression of Dnmts in the hippocampus and muscle of adult rats39,40. Therefore, the decreased TH circulating levels in the IE-exposed animals could be involved in the increased DNA methylation of the thyroid gland. However, a direct role of IE in this parameter cannot be ruled out. Finally, the increased oxidative stress in the thyroid gland that was reported here might also be involved in the increased DNA methylation, since ROS have been previously associated with several molecular responses that affect the cellular epigenome41.
Additionally, post-translational modifications in the N-terminal tails of histones are also involved in the activation or repression of gene transcription42. In accordance, our data indicated that the thyroid of IE-exposed rats presented hypermethylation in the lysines 9 and 27 of the histone H3 as well as reduced acetylation of the histones H3 and H4. The latter results are in agreement with the reduced expression/activity of HAT and increased expression/activity of HDAC in the thyroid of IE-exposed animals. It is noteworthy that the methylation of histones is commonly associated with transcriptional repression, whereas the acetylation of the N-terminal tail of these proteins is usually associated with transcriptional activation43. As discussed above for DNA methylation, hypothyroidism has also been previously associated with reduced acetylation of histones in the hippocampus as well as with increased activity of histones deacetylases in the liver39,44. Moreover, a previous study indicated that TH regulate the myosin expression in the cardiac cells through post-translational modifications in the histones45. Therefore, the hypothyroid condition observed in the IE-exposed animals could also be associated with the histones post-translational modifications that were described herein. Even so, a direct role of IE on this molecular event cannot be excluded.
It is worth mentioning that the gene expression programming during the intrauterine life has been described as a “predictive adaptive response” that promotes a physiological adjustment in the offspring to guarantee their survival in the same environment of their mother46. Therefore, the IE-induced reduction of thyroid gene/protein expression could be an adaptive mechanism to protect the offspring’s thyroid from the deleterious effects triggered by IE and to guarantee the TH production if these animals were maintained in an IE environment. However, since the IE-exposed animals were supplied with a normal iodine diet after the end of the weaning until adulthood, the reduced thyroid gene expression could not be sufficient to guarantee an adequate production of TH, inducing the primary hypothyroid condition that was observed in these animals in the adult life. Importantly, future studies about the thyroid function of the IE-exposed offspring in the end of the weaning (PND21) will add valuable information about the predictive adaptive response hypothesis.
In summary, our results demonstrated that the exposure of rat dams to IE altered the hypothalamus-pituitary-thyroid axis function of their male offspring in adult life. Additionally, our data also suggest that increased DNA methylation and histones post-translational modifications are involved in the repression of thyroid gene expression in the IE-exposed animals. Therefore, by using an animal model, our data reinforce the pivotal role of maternal diet in the foetal development and the early programming of adult diseases.
Methods
Animals and Treatments
Virgin male and female Wistar rats were obtained from the Animal Breeding Centre at the Institute of Biomedical Sciences, University of Sao Paulo. The animals were housed at constant temperature (23 ± 1 °C), 12:12-h light-dark cycle schedule, with free access to food and water. At eight weeks of age, female Wistar rats were mated with male rats at a proportion of 2:1. After confirming the pregnancy through the presence of spermatozoa in the vaginal smear, the pregnant rats were housed in individual cages and randomly divided into the following groups: Control (C): Rat dams supplied with distilled water during pregnancy and lactation periods. 5-HI: Rat dams supplied with distilled water supplemented with 0.6 mg/L NaI during pregnancy and lactation periods. The dose of treatment was chosen based on previous studies and represents five times the normal daily consumption of iodine by rats9,47.
At postnatal day (PND) 1, the litters were culled to eight pups per dam and kept at this proportion until the end of the weaning. At PND21 the male rats were housed in cages (five animals per cage) and maintained with water and food ad libitum. At PND90, the rats were anesthetized and euthanized by decapitation (Fig. 8). The blood, hypothalamus, pituitary, thyroid gland, heart, kidney and liver were collected for performing the molecular and/or biochemical analysis described below.
Figure 8.
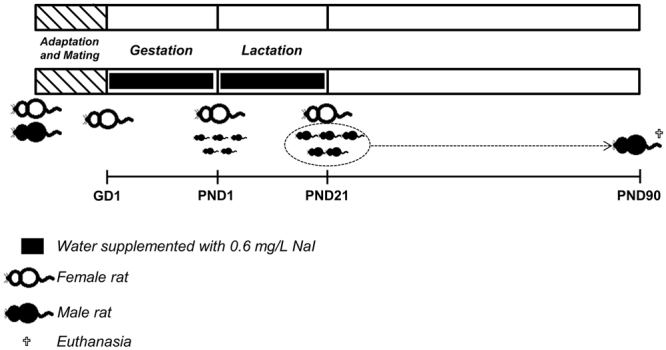
Schematic representation of experimental protocol. Male and female Wistar rats were mated and after confirming the pregnancy through the presence of spermatozoa in the vaginal smear, the pregnant rats (GD1) were treated or not with water supplemented with 0.6 mg/L NaI during pregnancy and lactation periods. At postnatal day (PND) 1, the litters were culled to eight pups per dam and kept at this proportion until the end of the weaning. At PND21 the male rats were housed in cages and maintained with water and food ad libitum. At PND90, the adult male rats were anesthetized and euthanized by decapitation and tissues were collected for performing several molecular and biochemical analyses.
The experimental protocol was approved by the Institute of Biomedical Sciences/University of São Paulo-Ethical Committee for Animal Research (no. 155/2012) and the protocols are in accordance with the ethics principles in animal research adopted by the National Council for the Control of Animal Experimentation.
Gene expression analysis
Total RNA was extracted using Trizol following the manufacturer’s protocol (LifeTechnologies, Carlsbad, CA, USA). Then, the mRNA expression of thyrotropin releasing hormone (Trh), type 2 iodothyronine deiodinase (Dio2), thyrotropin releasing hormone receptor (Trhr), alpha subunit of thyrotropin (Tsha), beta subunit of thyrotropin (Tshb), growth hormone (Gh), paired box 8 (Pax8), thyroid transcription factor 1 (Nkx2.1), thyrotropin receptor (Tshr), sodium-iodide symporter (Slc5a5), thyroperoxidase (Tpo), thyroglobulin (Tg), monocarboxilate transporter 8 (Mct8), type 1 iodothyronine deiodinase (Dio1), DNA methyltransferase 1 (Dnmt1), DNA methyltransferase 3 (Dnmt3), histone acetyltransferase (Hat) histone deacetylase (Hdac) were evaluated by RT-qPCR according to the manufacturer’s recommendations (Invitrogen, Carlsbad, CA, USA). Primer sequences are described in Supplementary Table 1. The 2−ΔΔCt method was used to measure gene expression variations using Rpl19 as a housekeeping gene. Melting curves were analysed to confim the amplification of a single PCR product.
Western blotting analysis
Pituitary and thyroid total protein content were extracted using RIPA buffer [50 mM Tris (pH 8), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS] supplemented with protease inhibitor cocktail. Therefore, the total proteins extracts were subjected to electrophoresis in 6, 10, or 15% polyacrylamide gels and transferred to nitrocellulose membranes, which were blocked and incubated with specific primary and secondary antibodies diluted in 3% BSA, 0.1% Tween 20, Tris-buffered saline overnight at 4 C. After chemiluminescent reaction detection, blots densitometries were analysed by using the ImageJ Software (National Institutes of Health, Bethesda, MD). Primary antibodies are described in Supplementary Table 2.
Thyroid histological analysis
Thyroid histology was performed as previously described48. Hematoxylin-eosin-stained sections were examined in a Nikon Eclipse E600 microscope and photographs were captured with a digital camera (Roper Scientific, Trenton, NJ, USA).
Thyroid oxidative stress analysis
Protein carbonylation status in the thyroid gland was analysed by using the OxyBlot™ Protein Oxidation Detection Kit, following the instructions of the manufacturer (EMD Millipore Headquarters, Billerica, MA, USA). Briefly, the extracted proteins were subjected to Western Blotting, as described above. Therefore, the membranes were subsequently incubated with a primary antibody to detect the carbonyl groups and then with a peroxidase-conjugated secondary antibody. Blots were developed and analysed as described above. Ponceau S staining was used as a loading control to normalize the analysis of the protein carbonylation content in the thyroid gland. Results are presented as percentage in comparison to the control group.
D1 activity analysis
Liver and kidney D1 activity were determined as previously described49. D1 activity was expressed as picomol rT3.min−1.mg−1.
DNA extration and DNA global methylation analysis
Total DNA was extracted with the PureLink® Genomic DNA Kit, following the manufacturer’s intructions (LifeTechnologies, Carlsbad, CA, USA). Thereafter, global DNA methylation status was assessed by the Imprint® Methylated DNA Quantification Kit (Sigma Aldrich, St. Louis, MO, USA).
Histone post-translational modifications assessment
The analysis of the methylation or acetylation status of histones H3 and H4 N-terminal tails was performed by Western blotting, following the protocol described above. The post-translational modifications were assessed by using specific antibodies to detect histone methylation (H3K27me3 and H3K9me3) or histone acetylation (H3Ac e H4Ac). In addition, the activity of thyroid HAT and HDAC enzymes were evaluated by using a HAT Activity Colorimetric Assay Kit and a HDAC Activity Assay Kit, respectively, following protocol indicated by the manufacturer (Sigma Aldrich, St. Louis, MO, USA).
Determination of TSH, T3, T4 serum concentrations
Rat TSH, T4, and T3 serum levels were determined by the Milliplex Luminex® kit #RTHY-30K (EMD Millipore Headquarters, Billerica, MA, USA), following the manufacturer’s instructions.
Data analysis
All data was reported as means ± SEM. Comparisons between two groups were made using the Unpaired two-tailed Student’s t test. Statistical analysis was performed by using GraphPad Prism (GraphPad Software, San Diego, CA). Differences were considered statistically significant at p-value < 0.05.
Electronic supplementary material
Acknowledgements
The authors are grateful to Leonice Lourenço Poyares for excellent technical support. This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) to CSN (2012/24391-6) and to MTN (2013/05629-4). This study was also supported by a grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to CSN (461419/2014-9).
Author Contributions
The animals’ treatments and experiments were performed at the Institute of Biomedical Sciences by C.S.N. Gene and protein expression were evaluated by C.S.N. and R.B.S. Protein carbonylation analysis was performed by C.S.N. D1 activity assays were performed by C.S.N., T.P. and V.M.C.C. Epigenetic analysis was assessed by C.S.N. The study was designed by C.S.N. and M.T.N. The manuscript was written by C.S.N. The final version was approved by all authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15529-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carvalho, D. P. & Dupuy, C. Thyroid hormone biosynthesis and release. Mol Cell Endocrinol, 10.1016/j.mce.2017.01.038 (2017). [DOI] [PubMed]
- 2.Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I- symporter (NIS): mechanism and medical impact. Endocr Rev. 2014;35:106–149. doi: 10.1210/er.2012-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen, L. B., Andersen, S., Ovesen, L. & Laurberg, P. In Comprehensive Handbook of Iodine (eds Gerard, N. Burrow & Ronald Watson) 332–337 (Academic Press, 2009).
- 5.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. 2012;70:553–570. doi: 10.1111/j.1753-4887.2012.00528.x. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):108–117. doi: 10.1111/j.1365-3016.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 7.Stinca S, et al. Moderate-to-Severe Iodine Deficiency in the “First 1000 Days” Causes More Thyroid Hypofunction in Infants Than in Pregnant or Lactating Women. J Nutr. 2017;147:589–595. doi: 10.3945/jn.116.244665. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Pearce EN. Reproductive endocrinology: Iodine intake in pregnancy–even a little excess is too much. Nat Rev Endocrinol. 2015;11:260–261. doi: 10.1038/nrendo.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano-Nascimento C, et al. Iodine excess exposure during pregnancy and lactation impairs maternal thyroid function in rats. Endocrine connections. 2017 doi: 10.1530/EC-17-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. 2015;100:1630–1638. doi: 10.1210/jc.2014-3704. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, et al. Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC neuroscience. 2012;13:121. doi: 10.1186/1471-2202-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connelly KJ, et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. The Journal of pediatrics. 2012;161:760–762. doi: 10.1016/j.jpeds.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riddihough G, Zahn LM. Epigenetics. What is epigenetics? Introduction. Science (New York, N.Y.) 2010;330:611. doi: 10.1126/science.330.6004.611. [DOI] [PubMed] [Google Scholar]
- 14.Loh KM, Lim B. Epigenetics: Actors in the cell reprogramming drama. Nature. 2012;488:599–600. doi: 10.1038/488599a. [DOI] [PubMed] [Google Scholar]
- 15.Marx V. Epigenetics: Reading the second genomic code. Nature. 2012;491:143–147. doi: 10.1038/491143a. [DOI] [PubMed] [Google Scholar]
- 16.Thomas Jde V, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res. 2009;72:344–347. doi: 10.1159/000249162. [DOI] [PubMed] [Google Scholar]
- 17.De Wolf D, et al. Congenital hypothyroid goiter and amiodarone. Acta Paediatr Scand. 1988;77:616–618. doi: 10.1111/j.1651-2227.1988.tb10717.x. [DOI] [PubMed] [Google Scholar]
- 18.Mathers JC. Early nutrition: impact on epigenetics. Forum Nutr. 2007;60:42–48. doi: 10.1159/000107066. [DOI] [PubMed] [Google Scholar]
- 19.Gicquel C, El-Osta A, Le Bouc Y. Epigenetic regulation and fetal programming. Best Pract Res Clin Endocrinol Metab. 2008;22:1–16. doi: 10.1016/j.beem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Sinclair KD, Lea RG, Rees WD, Young LE. The developmental origins of health and disease: current theories and epigenetic mechanisms. Soc Reprod Fertil Suppl. 2007;64:425–443. doi: 10.5661/rdr-vi-425. [DOI] [PubMed] [Google Scholar]
- 21.Joss-Moore LA, Lane RH. The developmental origins of adult disease. Curr Opin Pediatr. 2009;21:230–234. doi: 10.1097/MOP.0b013e328326773b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama S, et al. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004;14:1077–1083. doi: 10.1089/thy.2004.14.1077. [DOI] [PubMed] [Google Scholar]
- 23.Carswell F, Kerr MM, Hutchison JH. Congenital goitre and hypothyroidism produced by maternal ingestion of iodides. Lancet. 1970;1:1241–1243. doi: 10.1016/S0140-6736(70)91736-8. [DOI] [PubMed] [Google Scholar]
- 24.Shupnik MA, Chin WW, Habener JF, Ridgway EC. Transcriptional regulation of the thyrotropin subunit genes by thyroid hormone. J Biol Chem. 1985;260:2900–2903. [PubMed] [Google Scholar]
- 25.Arrojo EDR, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta. 2013;1830:3956–3964. doi: 10.1016/j.bbagen.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guadano-Ferraz A, Escamez MJ, Rausell E, Bernal J. Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci. 1999;19:3430–3439. doi: 10.1523/JNEUROSCI.19-09-03430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva FG, Giannocco G, Santos MF, Nunes MT. Thyroid hormone induction of actin polymerization in somatotrophs of hypothyroid rats: potential repercussions in growth hormone synthesis and secretion. Endocrinology. 2006;147:5777–5785. doi: 10.1210/en.2006-0110. [DOI] [PubMed] [Google Scholar]
- 28.Chiamolera MI, Wondisford FE. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology. 2009;150:1091–1096. doi: 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- 29.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 30.Hu LW, Benvenuti LA, Liberti EA, Carneiro-Ramos MS, Barreto-Chaves ML. Thyroxine-induced cardiac hypertrophy: influence of adrenergic nervous system versus renin-angiotensin system on myocyte remodeling. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1473–1480. doi: 10.1152/ajpregu.00269.2003. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez LP, Lopez-Marquez A, Santisteban P. Thyroid transcription factors in development, differentiation and disease. Nat Rev Endocrinol. 2015;11:29–42. doi: 10.1038/nrendo.2014.186. [DOI] [PubMed] [Google Scholar]
- 32.Kambe F, Nomura Y, Okamoto T, Seo H. Redox regulation of thyroid-transcription factors, Pax-8 and TTF-1, is involved in their increased DNA-binding activities by thyrotropin in rat thyroid FRTL-5 cells. Mol Endocrinol. 1996;10:801–812. doi: 10.1210/mend.10.7.8813721. [DOI] [PubMed] [Google Scholar]
- 33.Serrano-Nascimento C, et al. Excess iodide downregulates Na(+)/I(−) symporter gene transcription through activation of PI3K/Akt pathway. Mol Cell Endocrinol. 2016;426:73–90. doi: 10.1016/j.mce.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Fedorova M, Bollineni RC, Hoffmann R. Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass spectrometry reviews. 2014;33:79–97. doi: 10.1002/mas.21381. [DOI] [PubMed] [Google Scholar]
- 35.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10:136–142. doi: 10.1038/nrendo.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 37.Siedlecki P, Zielenkiewicz P. Mammalian DNA methyltransferases. Acta Biochim Pol. 2006;53:245–256. [PubMed] [Google Scholar]
- 38.Tate PH, Bird AP. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3:226–231. doi: 10.1016/0959-437X(93)90027-M. [DOI] [PubMed] [Google Scholar]
- 39.Sui L, Li BM. Effects of perinatal hypothyroidism on regulation of reelin and brain-derived neurotrophic factor gene expression in rat hippocampus: Role of DNA methylation and histone acetylation. Steroids. 2010;75:988–997. doi: 10.1016/j.steroids.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Peng S, et al. The gene expressions of DNA methylation/demethylation enzymes and cytochrome c oxidase subunit 4 in skeletal muscle of thyroidectomized rats. Afr. J. Biotechnol. 2011;10:730–733. doi: 10.5897/AJB11.2099. [DOI] [Google Scholar]
- 41.Cyr AR, Domann FE. The redox basis of epigenetic modifications: from mechanisms to functional consequences. Antioxid Redox Signal. 2011;15:551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 43.Kimura H. Histone modifications for human epigenome analysis. J Hum Genet. 2013;58:439–445. doi: 10.1038/jhg.2013.66. [DOI] [PubMed] [Google Scholar]
- 44.Cordeiro A, et al. Thyroid hormone regulation of Sirtuin 1 expression and implications to integrated responses in fasted mice. J Endocrinol. 2013;216:181–193. doi: 10.1530/JOE-12-0420. [DOI] [PubMed] [Google Scholar]
- 45.Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–1980. doi: 10.1152/ajpheart.00644.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth defects research. Part C, Embryo today: reviews. 2011;93:12–18. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- 47.Li N, Jiang Y, Shan Z, Teng W. Prolonged high iodine intake is associated with inhibition of type 2 deiodinase activity in pituitary and elevation of serum thyrotropin levels. Br J Nutr. 2012;107:674–682. doi: 10.1017/S0007114511003552. [DOI] [PubMed] [Google Scholar]
- 48.Calil-Silveira J, et al. Underlying Mechanisms of Pituitary-Thyroid Axis Function Disruption by Chronic Iodine Excess in Rats. Thyroid. 2016;26:1488–1498. doi: 10.1089/thy.2015.0338. [DOI] [PubMed] [Google Scholar]
- 49.Marassi MP, et al. Sexual dimorphism in thyroid function and type 1 iodothyronine deiodinase activity in pre-pubertal and adult rats. The Journal of endocrinology. 2007;192:121–130. doi: 10.1677/joe.1.06901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.