Abstract
Spondyloarthritis encompasses a group of common inflammatory diseases thought to be driven by IL-17A-secreting type-17 lymphocytes. Here we show increased numbers of GM-CSF-producing CD4 and CD8 lymphocytes in the blood and joints of patients with spondyloarthritis, and increased numbers of IL-17A+GM-CSF+ double-producing CD4, CD8, γδ and NK cells. GM-CSF production in CD4 T cells occurs both independently and in combination with classical Th1 and Th17 cytokines. Type 3 innate lymphoid cells producing predominantly GM-CSF are expanded in synovial tissues from patients with spondyloarthritis. GM-CSF+CD4+ cells, isolated using a triple cytokine capture approach, have a specific transcriptional signature. Both GM-CSF+ and IL-17A+GM-CSF+ double-producing CD4 T cells express increased levels of GPR65, a proton-sensing receptor associated with spondyloarthritis in genome-wide association studies and pathogenicity in murine inflammatory disease models. Silencing GPR65 in primary CD4 T cells reduces GM-CSF production. GM-CSF and GPR65 may thus serve as targets for therapeutic intervention of spondyloarthritis.
Spondyloarthritis is an inflammatory disease with Th17 cells implicated in the pathogenesis. Here the authors show that patients with spondyloarthritis have increased numbers of GM-CSF-secreting blood and synovial lymphocytes, Th17 or not, that carry a unique transcriptional profile including enhanced GPR65 expression.
Introduction
Spondyloarthritis encompasses a group of inflammatory diseases with common pathologic and genetic features. These disorders include ankylosing spondyltis (AS), reactive arthritis (ReA), psoriatic arthritis (PsA) and enteropathic arthritis (EA)1. The prevalence of spondyloarthritis is at least 0.5% in European and US populations2,3. Only one third of patients achieve disease remission with TNF inhibitor therapies, therefore a significant unmet clinical need remains4–7.
Genetic, functional and therapeutic evidence implicates IL-17-producing cells in the pathogenesis of spondyloarthritis8–10. Within the CD4 T cell compartment, Th17 cells can be pleotropic and, in addition to the classical “type 17” cytokines IL-17A, IL-17F and IL-2211,12, can also produce IFN-γ13 and GM-CSF14. Murine models of inflammation suggest that co-expression of GM-CSF and IL-17A by CD4 T cells marks out a pathogenic subset of Th17 cells14,15, and neutralisation of GM-CSF in the Sagakuchi model of spondyloarthritis causes improvement in joint inflammation15. Noster et al.16 have provided evidence that GM-CSF and IL-17A are antagonistically regulated, but it is unclear if the CD4/GM-CSF axis is acting within the context of Th17 immunity or independently. Additional pathogenic markers of murine Th17 cells include GPR65 17, a receptor implicated in the pathogenesis of spondyloarthritis by genome-wide association studies18 .
In addition to classical CD4 Th17s, CD161-expressing CD8 T cells19, γδ T-cells20, innate lymphoid cells21 (ILC) and B cells22 have also been shown to be producers of IL-17A and/or GM-CSF. ILC are broadly divided into three groups; ILC1s express T-bet, ILC2 express GATA3 and ILC3 express RORγt and are thought to be involved in type 17 immunity23. ILCs are present in the gut24, lung25 and skin26, and have been described in the joint in psoriatic arthritis27. The contribution of ILCs to pathogenic immune responses is still being actively investigated.
Here we show that multiple lymphoid populations (including ILCs) express more GM-CSF in patients with spondyloarthritis. Increased lymphocyte GM-CSF production occurs both in the context of a polyfunctional type 17 response, but also independently of IL-17A. Primary human GM-CSF-producing CD4 cells, isolated using a multiple cytokine capture approach, show a specific transcriptional profile that includes upregulation of GPR65, and we identify a function for this receptor in CD4 lymphocyte GM-CSF production. Therapeutic targeting of GPR65 may potentially attenuate pathogenic T cells while preserving physiological type 17 immunity.
Results
GM-CSF+ lymphocytes are increased in spondyloarthritis
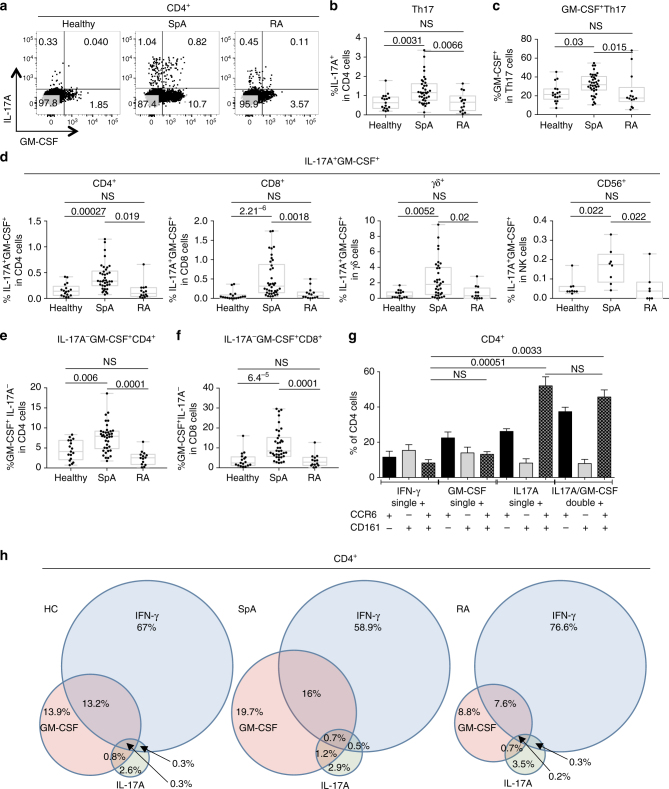
We first performed intracellular cytokine staining of ex vivo peripheral blood mononuclear cells (PBMCs) from 38 patients with axial spondyloarthritis fulfilling the assessment of spondyloarthritis (ASAS) criteria28, 14 patients with rheumatoid arthritis (RA) matched for CRP, and 17 sex and age matched healthy donors (Supplementary Table 1 shows patient characteristics, Supplementary Fig. 1A shows flow cytometry gating strategy). IL-17A+ CD4 cells (Th17 cells) were increased in spondyloarthritis compared to both control groups (Fig. 1a, b). We then looked within the Th17 compartment for the percentage of cells also expressing GM-CSF (Fig. 1c). We observed a significant increase in these GM-CSF+Th17 cells in spondyloarthritis compared to healthy donors (p = 0.0031, Kruskal–Wallis) and RA inflammatory disease controls (p = 0.0066, Kruskal–Wallis). IL-17A+GM-CSF+ cells were also increased in CD8, γδ and CD56+ lymphoid subsets in spondyloarthritis (Fig. 1d).
Fig. 1.
GM-CSF+ cells are expanded in multiple lymphoid populations in spondyloarthritis. a Representative flow cytometry plots comparing intracellular staining for IL-17A and GM-CSF in healthy, spondyloarthritis (SpA) and rheumatoid arthritis (RA) PBMCs (gated on live CD3+CD4+ lymphocytes). b Total IL-17A+CD4+ cells (Th17) and c percentage of Th17 cells also expressing GM-CSF for healthy (n = 17) SpA (n = 38) and RA (n = 14). d Percentage of IL-17A+GM-CSF+ cells within the CD4+, CD8+, γδ+ and CD56+ gates in the same cohort of patients and controls. Percentage of GM-CSF+ CD4 e and CD8 f independently of IL-17A in the cohort of patients and controls. g CD161 and CCR6 expression was measured by flow cytometry on single positive IL-17A+, IFN-γ+, GM-CSF+ and IL-17A+GM-CSF+ double-positive CD4 cells (n = 8; mean + SEM shown, Friedman’s test). h Venn diagram of mean PBMC cytokine data from healthy donors, SpA and RA patients derived by Boolean gating, the percentage of each cytokine is represented in relation to the total number of cytokine-producing CD4 T cells. All statistical analysis (except g, Friedman’s) determined by Kruskal–Wallis test with p-values calculated using Dunn’s multiple comparisons test
Next we looked at the percentage of cells producing GM-CSF independently of IL-17A. We observed a significant expansion of GM-CSF+IL-17A− cells in both CD4 and CD8 cells in spondyloarthritis compared to the two control populations (Fig. 1e, f), but saw no difference in the CD56 and γδ compartments (Supplementary Fig. 1D, E), and no difference in the percentage of IFN-γ+ cells was seen in the CD4, γδ and CD56 compartments (Supplementary Fig. 1B–E). GM-CSF+ CD4 frequency was not affected by co-existent psoriasis or IBD (Supplementary Fig. 2A). We did however note increased GM-CSF+ CD4 T cells in patients on anti-TNF therapy, but even with the exclusion of these patients from the analysis, GM-CSF+ CD4 cells were still elevated in spondyloarthritis compared to controls (Supplementary Fig. 2B).
Phenotypic analysis showed that single-positive GM-CSF+ CD4+ cells expressed CCR6 and CD161 at similar frequencies to single-positive IFN-γ+CD4+ cells, whilst double-positive IL-17A+GM-CSF+ CD4+ cells more commonly expressed CCR6 and CD161 (similar to single-positive IL-17A+CD4+ cells) (Fig. 1g). We used Boolean gating to delineate the functional overlap of IL-17A, IFN-γ and GM-CSF production by CD4 T cells in healthy controls, spondyloarthritis and RA (Fig. 1h). In all three cohorts, GM-CSF+ CD4 T cells overlapped with both Th1 and Th17 cells but also existed independently of these two effector subsets. In spondyloarthritis the overall GM-CSF pool was expanded both independently and in combination with of Th1 and Th17.
Spondyloarthritis synovial T cells are enriched for GM-CSF
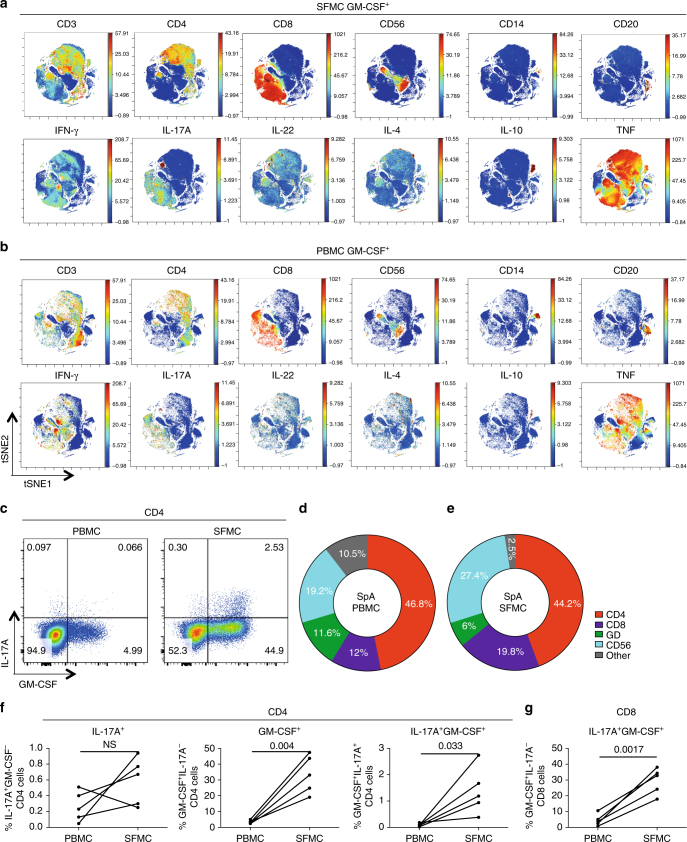
Since spondyloarthritis is characterised by joint inflammation, we next studied synovial fluid mononuclear cells (SFMC) from the inflamed joints of 5 patients with peripheral spondyloarthritis (Supplementary Table 2 shows patient characteristics). We compared the cellular sources of GM-CSF in spondyloarthritis PBMCs and SFMCs using time-of-flight mass cytometry (CyTOF) using a panel of 20 surface and 11 intracellular markers. We gated on all live GM-CSF+ events and performed an unbiased clustering analysis of all parameters using viSNE29 (Supplementary Fig. 3A, C shows the CyTOF gating and validation of CyTOF using flow cytometry). We observed CD4 cells to be the largest population of GM-CSF-producing cell in both spondyloarthritis peripheral blood and synovial fluid, with significant contributions from CD8 and CD56 cells (Supplementary Fig. 2A, B). Both SFMCs and (matched) PBMCs showed co-production of GM-CSF with IL-17A, IFN-γ, IL-4, IL-10 and TNF (Fig. 2a, b lower sets of panels) confirming and extending our observations in Fig. 1h. We confirmed these data by flow cytometry for 5 additional matched PBMC/SFMC spondyloarthritis samples (Fig. 2c–g). Figure 2c shows not only a marked increase in the size of the CD4 GM-CSF production (>9-fold) in the joint relative to the blood, but also that in this patient 89% of IL-17A+ synovial CD4 T cells co-expressed GM-CSF. In this additional cohort of 5 patients, no significant expansion of IL-17A+ single-positive CD4 cells in the synovial fluid relative to the blood was seen. However, both IL-17A−GM-CSF+ CD4 cells and double-positive IL-17A+GM-CSF+ CD4 and CD8 cells were markedly increased in spondyloarthritis synovial fluid relative to blood (Fig. 2f, g). Additionally, synovial tissue cultures of CD4 T cells from 4 spondyloarthritis patients showed comparable high levels (>30%) of GM-CSF production (Supplementary Fig. 4).
Fig. 2.
Spondyloarthritis synovial fluid T cells are highly enriched for GM-CSF production ex vivo. CyTOF viSNE clustering analysis of all live GM-CSF+ events from paired ex vivo spondyloarthritis (SpA) synovial fluid mononuclear cells (SFMC) a and PBMC b. In the top panels of a and b CD3, CD4, CD8, CD56, CD14 and CD20 are shown as the third parameter. The bottom panels of a and b show IFN-γ, IL-17A, IL-22, IL-4, IL-10 and TNF as third parameters. c Representative flow cytometry plots, gated on CD4 cells, from matched SpA patient PBMCs and SFMC showing IL-17A and GM-CSF production. d–e Flow cytometry data of 5 matched ex vivo SpA PBMC d and SFMC e samples showing the mean cellular components of the GM-CSF pool. f Paired PBMC and SFMC flow cytometry data gated on CD4 showing the percentage of IL-17A+GM-CSF−, IL-17A−GM-CSF+ and IL-17A+GM-CSF+ in SpA patients (n = 5, paired t-test). g Paired PBMC and SFMC flow cytometry data showing the percentage of IL-17A+GM-CSF+ CD8 cells in SpA (n = 5, paired t-test)
ILC3 are enriched in the inflamed joint and produce GM-CSF
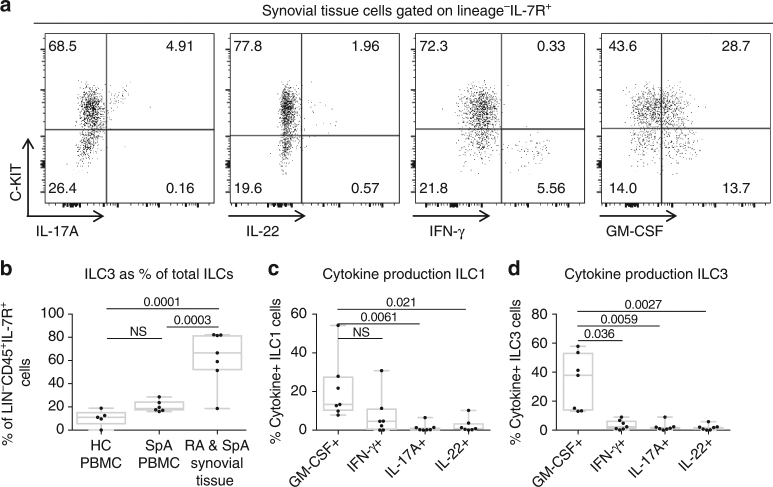
The presence of innate lymphoid cells (ILC) in human joints has recently been described in psoriatic arthritis, a form of spondyloarthritis27, and we next asked if GM-CSF is produced by these cells. We obtained synovial tissue from 4 spondyloarthritis and 3 RA patients undergoing arthroplasty (Supplementary Table 2 shows patient characteristics) and derived synovial tissue mononuclear cells by in vitro explant culture. The flow cytometry gating strategy used to identify ILCs in blood and synovial tissue cultures is shown in Supplementary Fig. 5. Lineage-negative, IL-7R+ cells were indeed present in the synovial tissue and produced cytokines upon stimulation (Fig. 3a). The predominant ILC population in spondyloarthritis tissue was C-KIT+ ILC3s, whilst in the blood of healthy donors and spondyloarthritis patients ILC1 cells were the predominant subset (Fig. 3b). Figure 3c, d shows that GM-CSF was the most commonly produced cytokine for both ILC1 and ILC3 subsets in the synovial tissue-derived cells. In the synovial cultures, ILC1 showed reduced IFN-γ, while ILC3 showed increased GM-CSF compared to peripheral blood from spondyloarthritis and healthy donors (Supplementary Fig. 5B–E).
Fig. 3.
Type 3 innate lymphoid cells (ILC3) are enriched in inflammatory arthritis synovial tissue and produce GM-CSF. a Representative flow cytometry plots showing cytokine production by synovial ILCs. Lineage negative (Gating strategy shown in Supplementary Fig. 5. Lineage cocktail: CD3, CD5, CD8, CD11b, CD11c, CD19, CD20, CD34, TCR-γδ, CRTH2) CD45+ cells were then gated on IL-7R expression. ILC1 subset defined as Lin−CD45+IL-7R+C-KIT− and ILC3 subset defined as Lin−CD45+IL-7R+C-KIT+. b Relative enrichment of ILC3s as percentage of total ILCs in inflammatory arthritis synovial tissue explant cultures (n = 7), compared to ex vivo PBMCs from axial spondyloarthritis (SpA) (n = 6) and healthy control (n = 5) (ANOVA). c, d. Intracellular expression of GM-CSF IFN-γ, IL-17A and IL-22 in stimulated ILCs plotted against C-KIT expression to identify ILC1 and ILC3 cytokine production (n = 7 patients with inflammatory arthritis, 4 SpA/3 RA) (Friedman’s multiple comparison test)
GM-CSF CD4 cells have a distinct transcriptional profile
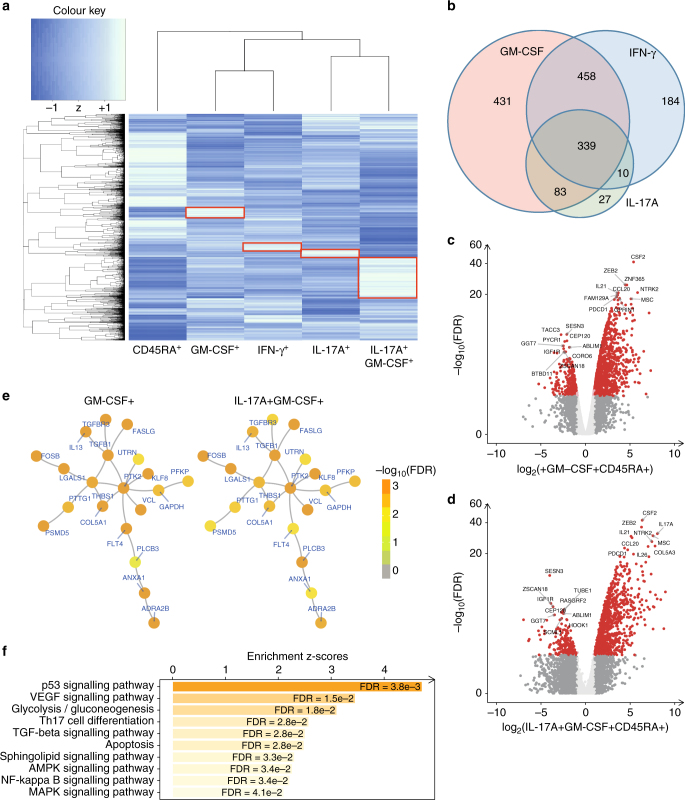
Given that our patient and control data suggest that GM-CSF+CD4+ may constitute a distinct functional subset, we next asked if GM-CSF+ CD4 T cells have a distinct transcription signature compared to classical IFN-γ+ Th1 cells and IL-17A+ Th17 cells. In the absence of known surface markers that define these cells we developed a triple-cytokine capture assay (Supplementary Fig. 6A, B shows cytokine capture-stained cells and FACS sorting strategy). This allowed us to purify CD4 T cells producing GM-CSF, IL-17A and IFN-γ both in isolation and in combination. We FACS sorted to high purity (>95%) GM-CSF+, IL-17A+ and IFN-γ+ single-positive cells, IL-17A+GM-CSF+ double-positive cells and cytokine-negative CD45RA+ naive CD4 T cells, from an initial cohort of 4 healthy donors. Normalised RNAseq gene counts for IL17A, IFNG and CSF2 and for the transcription factors RORC, TBX21 and PTPRC are shown in Supplementary Fig. 6C. Unbiased hierarchical gene clustering analysis of the 5 sorted subsets showed GM-CSF+ single positive cells to cluster more closely with IFN-γ+ single positive cells while IL-17A+GM-CSF+ double-positive cells clustered with IL-17A+ single positives (Fig. 4a shows mean data, Supplementary Fig. 6E shows all donors). Differential gene expression analysis (compared to CD45RA+ T cells) showed GM-CSF single positive cells to have a gene expression profile with both unique (Fig. 4a, highlighted in red boxes) and shared features compared to IFN-γ+ and IL-17A+ single-positive cells (Fig. 4b). Volcano plots with the top 10 upregulated and downregulated genes in the GM-CSF+ (Fig. 4c) and IL-17A+GM-CSF+ (Fig. 4d) are shown in addition to network analysis for these two subsets (Fig. 4e). Pathway analysis for the genes common to GM-CSF+ CD4 cells in Fig. 4f showed altered p53 signalling, glycolysis, Th17 and apoptosis pathways (individual genes used to define each pathway are listed in Supplementary Table 3).
Fig. 4.
GM-CSF positive CD4 cells have a distinct transcriptional profile. RNA was extracted from 5 FACS sorted CD3+CD4+ T-cell populations: CD45RA+ (IFN-γ−IL-17A−GM-CSF−), GM-CSF+, IFN-γ+, IL-17A+ and IL-17A+GM-CSF+ double-positive, of triple cytokine capture-labelled activated PBMCs from four healthy donors, pooled and sequenced on the Illumina HiSeq 4000 platform. a Unbiased hierarchical gene clustering analysis of the 5 sorted subsets from the combined data set of 4 donors. b Differential gene expression profiles of GM-CSF+, IL-17A+ and IFN-γ+ single-positive cell subsets (compared to CD45RA+ population) showing unique and shared expressed genes. False discovery rate (FDR) for this analysis was set at <0.05%. c, d Volcano plots of differential gene expression. Fold changes in the x-axis versus FDR in the y-axis are plotted for genes identified in GM-CSF+ cells c and IL-17A+GM- CSF+ cells d, Coloured in red are genes with FDR <5% and at least twofold changes (upregulated or downregulated). Highlighted in text are the most significant genes (upregulated or downregulated). e Visualisation of the gene network identified by integrative analysis of RNA sequencing data with gene interaction data. This network contains 20 genes that were commonly regulated in both IL-17A+GM-CSF+ cells and GM-CSF+ cells. Illustrations are using the same network layout with nodes colour-coded according to cell-specific FDR in the GM-CSF+ population (left) and the IL-17A+GM-CSF+ population (right). f Bar plots of enriched pathways for 391 genes commonly regulated in IL-17A+GM-CSF+ and GM-CSF+ cells. Displayed in the x-axis are enrichment z-scores with FDR labelled inside. Supplementary Table 3 shows the listing of gene members per pathway
GPR65 expression is associated with GM-CSF
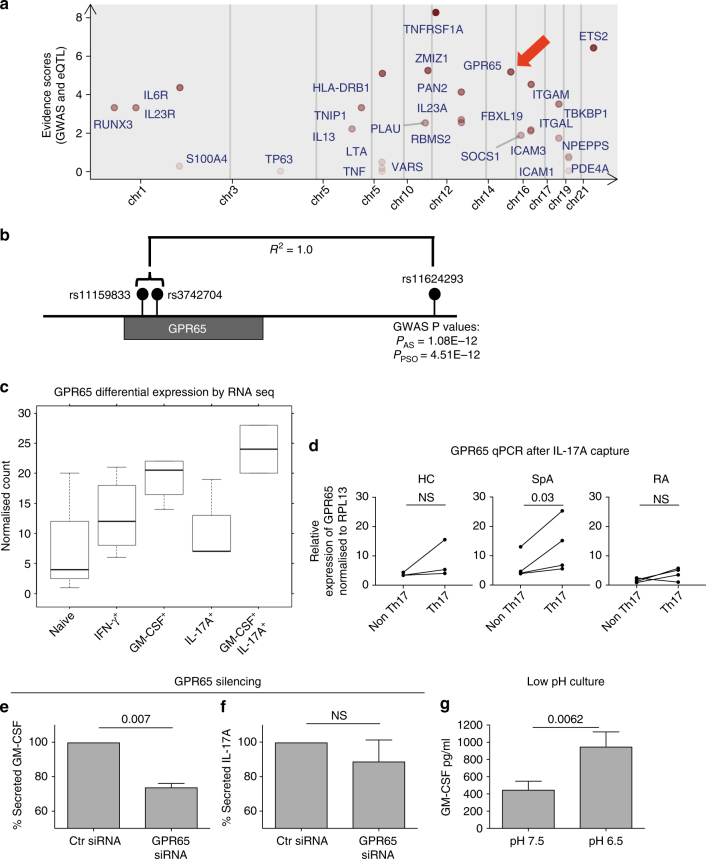
Next we asked if any genes selectively expressed in GM-CSF+ cells (both independently and in combination with IL-17A) might have be potentially pathogenic, integrating our RNA sequencing data with eQTL and GWAS data sets (Fig. 5a). GPR65, a proton-sensing G-protein coupled receptor was the fourth highest ranked gene identified from our bioinformatics ranking analysis and was upregulated in the GM-CSF+ and IL-17A+GM-CSF+ but not in IL-17A+ cells. (Fig. 5c). GPR65 is in complete linkage disequilibrium with a SNP associated with ankylosing spondylitis through GWAS studies18 (Fig. 5b). Increased expression of GPR65 in GM-CSF+ and IL-17A+GM-CSF+ was confirmed by qPCR in a second independent cohort of capture-sorted cells from 3 healthy donors (Supplementary Fig. 6D). Next we studied expression of GPR65 in pooled Th17 cells from additional spondyloarthritis patients, healthy donors and RA disease controls. We purified ex vivo CD4 IL-17A+ cells by single cytokine capture from 4 spondyloarthritis, 4 RA and 3 healthy controls. Since we showed that in spondyloarthritis nearly 40% of Th17 cells co-expressed GM-CSF (Fig. 1c), we hypothesised that ex vivo sorted Th17 cells from spondyloarthritis would have higher expression of GPR65 compared to the control populations, and this was confirmed in Fig. 5d. We silenced GPR65 in primary human T cells (confirmed by qPCR, Supplementary Fig. 7A) and observed a significant downregulation of GM-CSF but not IL-17A (Fig. 5e, f). Since GPR65 is a known extracellular pH sensor30, we cultured isolated primary human CD4 cells in media with a pH of 6.5 and observed a significant increase in GM-CSF production (Fig. 5g).
Fig. 5.
GPR65 expression is associated with GM-CSF+ and GM-CSF+IL-17A+ CD4 T cells. a Evidence score analysis showing top ranking genes identified in GM-CSF+ and IL-17A+GM-CSF+ cells. Red arrow shows GPR65 b Location of AS and psoriasis-associated GPR65 single-nucleotide polymorphism rs11624293 in relation the two SNPs identified in eQTL data sets. c Expression of GPR65 in RNA sequencing analysis of the 5 sorted primary T cell populations isolated by multiple cytokine capture (p = 0.012, ANOVA). d Expression of GPR65 by qPCR in ex vivo IL-17A− and IL-17A+ cytokine captured CD4 T cells from patients and controls (paired t-tests). GM-CSF e and IL-17A f measured by ELISA in culture supernatants of primary human CD4 cells in the presence GPR65 siRNA or control siRNA (mean + SEM, paired t-tests). g GM-CSF was measured by ELISA in culture supernatants of primary human CD4 cells cultured at a pH of 7.5 or in media acidified to a pH of 6.5 for 72 h (mean + SEM, paired t-tests)
IL-7 promotes expansion of GM-CSF in CD4 T cells
Since IL-7 has been previously been shown to be a driver of GM-CSF in a STAT5 dependent manner in murine models, we sought to investigate the effects of IL-7 on human CD4 cells. Supplementary Fig. 8A, B show that IL-7 enhances the GM-CSF production by CD4 cells, with a doubling of the number of cells expressing this cytokine. IL-7 had no effect on IL-17A, IL-22, IFN-γ, TNF or IL-17A/GM-CSF double-positive cells. Adding recombinant human IL-7 to explant synovial tissue cultures from patients with inflammatory arthritis (Supplementary Fig. 8C) increased both the percentage of GM-CSF+ CD4 cells and GM-CSF production by ELISA. There was also a statistically significant increase (p = 0.03) in the percentage of IL-22+ cells but no difference in IFN-γ+, IL-17A+ and IL-17A+GM-CSF+ cells. However, culture in the presence of IL-7 did not increase expression of GPR65 (Supplementary Fig. 7B). Finally, we sought to investigate the downstream target of GM-CSF in ex vivo PBMCs and SFMCs from spondyloarthritis patients by looking at pSTAT5 induction in response to recombinant human GM-CSF. We observed a clear induction of pSTAT5 in CD14 monocytes in the blood and synovial fluid but no effect on CD4+, CD8+, CD56+ and CD20+ cells (Supplementary Fig. 8D).
Discussion
We here show that human spondyloarthritis is characterised by a program of lymphoid GM-CSF production in multiple compartments, including CD4 and CD8 T cells, γδ and innate lymphoid cells (ILC). This overlaps with an expansion of “type 17” cytokine production previously reported for CD49 and γδ20 cells and confirmed by our data. Notably in spondyloarthritis, GM-CSF production is increased both independently of, and in combination with, IL-17A and IFN-γ. This, together with our data showing that CD4 T cells from synovial fluid are further enriched for GM-CSF production, suggests that the GM-CSF programme may be a primary pathogenic process.
We also show increased numbers of lineage-negative IL-7R-positive type 3 innate lymphoid cells (ILC3) in inflamed joints, and further show that ILC3 cells derived from the joint abundantly produce GM-CSF upon in vitro stimulation, albeit after in vitro culture. C-Kit-expressing ILC3 are thought to contribute to the cytokine environment through the production of IL-17A and IL-22 in response to tissue stress23,31, and have recently been shown to produce GM-CSF in the gut24. We show that ILC1s are also present in the joint and show a downregulation of IFN-γ relative to blood. Thus, our data suggest that GM-CSF may be an important mediator in both innate and adaptive immune responses in spondyloarthritis.
Several lines of evidence have suggested that T cell-derived GM-CSF may be pathogenic in murine and human inflammatory diseases. Neutralization of GM-CSF has been shown to be effective in the Sagakuchi mouse model of spondyloarthritis15 and in EAE models of neuro-inflamation16, with a T cell specific-GM-CSF knockout shown to be protected from disease32. GM-CSF-mediated immunity has been implicated in several human diseases. Elevated GM-CSF-producing T cells have been shown in the joints of patients with Juvenile Inflammatory Arthritis33. Moreover an increased percentage of GM-CSF+ CD4 T cells have been found in the cerebrospinal fluid of patients with multiple sclerosis (MS) compared to non-MS controls16, and CD4 and CD8 T cells expressing GM-CSF have been shown to be elevated in the peripheral blood of patients with MS34.
In this study, we observed GM-CSF production both independently and in combination with Th17 subsets. Importantly, we here show that the percentage of IL-17A+GM-CSF+ double-positive cells is greatly increased in the peripheral blood of patients with spondyloarthritis, with further increases in the ex vivo synovial fluid. Th17 cells co-expressing GM-CSF are thought to be particularly pathogenic in animal models of inflammatory disease17,35,36. Lee et al.35 showed pathogenic Th17 cells to be dependent on IL-23 and downstream TGF-β3 signalling, with Csf2 (GM-CSF) one of the key upregulated genes in murine pathogenic Th17 cells. Csf2 was also shown recently to be upregulated in pathogenic Th17 cells isolated from the central nervous system of diseased experimental autoimmune encephalitis (EAE) mice17.
Our flow cytometry, CyTOF and gene expression data suggest that GM-CSF+ CD4 T cells have a distinct phenotypic and transcriptional profile. Several reports have shown GM-CSF production to be more aligned with a STAT5 rather than a STAT3 programme16, with IL-7 implicated as a driver of STAT5-mediated GM-CSF-driven inflammation in the mouse EAE model37. Moreover, it has been shown that GM-CSF production by CD4 T cells is influenced by IL-1β38,39, with GM-CSF in turn acting via monocytes to boost IL-1β production40. We recently showed that RORγt inhibition of human Th17 cells in vitro led to suppression of IL-17A+ but not GM-CSF+ CD4 T cells in vitro, suggesting GM-CSF can act independently of RORγt41. In the absence of clear surface phenotypic markers to identify GM-CSF+ CD4 T cells and IL-17A+GM-CSF+ double-positive CD4 T cells, our RNA sequencing data represent an important insight into the transcriptional regulation of these cells. We show GM-CSF+ cells to have a transcriptional profile that is distinct from IFN-γ+ and IL-17A+ cells, supporting the concept that GM-CSF+ T cells may be a distinct T-cell phenotype. Gene clustering analysis identified distinct, as well as overlapping groups of differentially expressed genes for both GM-CSF+ cells and IL-17A+GM-CSF+ double-positive cells.
In this study, we observe the percentage of GM-CSF+ CD4 and CD8 T cells in spondyloarthritis to be significantly higher than rheumatoid arthritis (RA), as well as healthy controls. Interestingly, the percentage of GM-CSF+ CD4 cells was especially high in patients receiving anti-TNF therapy, suggesting that this pathway is downstream of TNF. This increase in GM-CSF may be due to the fact that anti-TNF treated patients represent a subset with more aggressive disease requiring the need for biological therapies. Alternatively, the effects of TNF blockade may lead to a loss of negative feedback leading to an increase of GM-CSF. An increase in Th17 numbers after anti-TNF has been previously reported in RA42 and the two mechanisms may be similar.
Our data would support development of clinical trials of anti-GM-CSF in spondyloarthritis, particularly in view of the safety and efficacy already reported in RA43,44. Even though we did not observe an increase in circulating GM-CSF+ lymphocytes in RA, the efficacy of the anti-GM-CSF antibody in this disease may be predominantly due to the local effects of GM-CSF in synovial tissue, where increased numbers of cells expressing the GM-CSF receptor have been described in both RA and psoriatic arthritis45. Additionally, in RA, GM-CSF has been shown to play a function in inflammatory dendritic cell differentiation46.
We here show that both human GM-CSF-producing CD4 T cells, and GM-CSF+ Th17 cells are characterized by high levels of GPR65 expression. GPR65 is G-protein coupled receptor with an extracellular proton sensing domain47 associated with ankylosing spondyloarthritis by GWAS18. Its function in immune cells remains unclear. We show culture of primary human CD4 cells in an acidic environment significantly upregulates GM-CSF while silencing of GPR65 reduced GM-CSF. Notably, a low pH environment has been reported in the inflamed synovium48 . Of interest, Gpr65 expression was upregulated in pathogenic murine Th17 cells, with knockout protecting against EAE17. The upregulation of GPR65 in ex vivo sorted spondyloarthritis Th17 cells further validates a function for this receptor in driving pathogenic T cell responses in human disease. Since silencing of GPR65 in human CD4 cells leads to a significant decrease in GM-CSF, inhibition of GPR65 using small molecules might constitute a promising therapeutic approach, in combination with or as an alternative to current therapies, although the potential human in vivo side effect profile of such agents remains to be determined. Such an approach may potentially preserve the protective function of non GM-CSF secreting Th17 cells49.
Taken together, our data suggest an important function in human spondyloarthritis for GM-CSF production by multiple lymphocyte populations, including CD4, CD8 and γδ T cells and ILCs, within the context of globally enhanced type 17 immunity. GM-CSF-producing CD4 cells have a distinct transcriptional profile, which includes expression of GPR65. Our data provide a strong scientific rationale for clinical trials targeting GM-CSF in spondyloarthritis and additionally identify further targets including GPR65 involved in this likely ‘pathogenic’ module.
Methods
Patient and control recruitment
Peripheral blood samples were recruited, following informed written consent, from patients attending the Oxford University Hospitals National Health Service Foundation Trust (Ethics reference number 06/Q1606/139, National Health Service, Health Research Authority, South Central—Oxford C Research Ethics Committee). All spondyloarthritis patients met assessment of spondyloarthritis international society (ASAS) criteria for axial spondyloarthritis28. Age and sex matched healthy donors and rheumatoid arthritis (RA) patients meeting American college of rheumatology/European league against rheumatism 2010 criteria50 matched for C-reactive protein were recruited. Patient and control characteristics are shown in Supplementary Table 1. Power calculation was based on 3 pairwise comparisons and observed differences in frequency of Th17 cells from a previous cohort of patients51. A minimum sample size of 11 was needed for each group for power of 0.8 and type 1 error rate of 5%.
Synovial fluid from patients with peripheral spondyloarthritis as defined by ASAS criteria28 or psoriatic arthritis as defined by classification criteria for psoriatic arthritis (CASPAR) criteria52 were also recruited under the same ethics as the peripheral blood cohort with informed written consent. Additional synovial tissue samples from spondyloarthritis28 and RA50 patients were studied (ethics reference number 09/H0606/11, National Health Service, Health Research Authority, South Central—Oxford C Research Ethics Committee). Patient characteristics are shown in Supplementary Table 2.
Flow cytometry
The cells were stimulated with phorbol myristate acetate (125 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Sigma-Aldrich), in the presence of Golgiplug (Brefeldin A) and Golgistop (Monensin) (both from BD Biosciences diluted according to manufacturer’s instructions) and incubated for four hours at 37 °C in 5% CO2. Staining antibodies and dilutions shown in Supplementary Table 4. The cells were stained on ice and in the dark for 20 min, then permeabilised using Cytofix/Cytoperm fixation and permeabilization solution (BD Biosciences) for 30 min and stained for intracellular cytokines. Flow cytometry was performed on a BD Fortessa calibrated daily with calibration and tracking beads from BD Biosciences. The data were analysed using FlowJo software (Treestar).
CyTOF staining
A volume of 8 μl of 103Rh intercalator (Fluidigm) was added to cells and incubated for a 15 min at 37 °C. Cells were resuspended in 50 μl staining buffer containing surface antibody master mix and incubated for 20 min at room temperature, washed, then re-suspended in 500 μl of fix buffer (Fluidigm-Maxpar) and incubated at room temperature for 20 min. Cells were permeabilised using 2 ml of perm-S-buffer (Fluidigm-Maxpar) and intracellular staining was performed for 30 min at room temperature. All antibodies used are listed in Supplementary Table 5. Finally, cells were suspended in 500 μl of the 191Ir/193Ir intercalator and incubated overnight. Equalisation beads (Fluidigm-Maxpar) were added at a concentration of 1:10 and the sample was acquired on a CyTOF-1 machine. Analysis of data was carried out using FlowJo software (Treestar) and Cytobank software.
Cytokine capture
Fresh lymphocyte cones were obtained from the NHS blood and transplant service and PBMCs obtained by density centrifugation. CD4 T cells were isolated by negative selection (Miltenyi). The next day cells were stimulated with phorbol myristate acetate (125 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Sigma-Aldrich) for 2 h. Cells were stained on ice for 5 min with IL-17A, IFN-γ and GM-CSF capture antibodies (all Miltenyi biotech) and incubated at 37 °C for 45 min. At 5 min intervals the mixture was resuspended. Cells were then placed on ice to stop cytokine secretion and stained with MACS detection antibodies (IL-17A-PE, IFN- γ -FITC, GM-CSF-Biotin) all at a concentration of 1:25 μl. In addition, cells were also stained with CD45RA-PerCpCy5.5, CD3 BV605, CD4 BV421 and ef780 viability dye followed by with anti-biotin APC secondary. Sorting was carried out using an automated cell sorter BD Aria. Cells were sorted at 4 °C into tubes containing sterile PBS.
Synovial tissue cultures
Freshly obtained surgical material was re-suspended in 25 ml of sterile RPRMI immediately. The sample was placed in a large petri dish and cut into 3–4 mm pieces by hand. 20–30 pieces of the sample were placed in a 6-well plate containing 5 ml of warm of RPMI supplemented with 10% human serum containing 100 IU/ml IL-2 (Peprotech) + IL-7 10 ng/ml (Peprotech). Volume of 2.5 ml of media was aspirated every 72 h and replaced with the same volume containing IL-2 100 IU/ml + IL-7 10 ng/ml. After 14 days non-adherent cells were washed off the plate and filtered through a 70 μm filter. Cells were counted, rested overnight and stimulated in the morning for intracellular cytokine staining.
RNA extraction
The AllPrep DNA/RNA/miRNA kit (Qiagen, catalogue number 80224) was used for RNA extraction. FACS sorted cells were spun down and re-suspended in 350 μl of RLTplus buffer and transferred to 2 ml tubes. Samples were then stored at −80 °C for batched RNA extraction. Homogenisation of the sample was carried out using the QIAshredder (Qiagen). DNase I was used during the extraction protocol to minimise DNA contamination. RNA was eluted into 35 μl of RNase-free water. The RNA amount was quantified by nano-drop and the RNA samples stored at −80 °C for storage until ready for sequencing.
RNA sequencing
RNA sequencing was carried out at the Wellcome Trust Centre for Human Genetics core facility. RNA underwent quality control testing using a bioanalyser followed by cDNA library preparation. Paired end sequencing was performed at 100 base pairs on each side of the DNA fragment on the HiSeq 4000 platform. Total of 20–80 million fragments were sequenced per sample.
Analytical methods
Prism version 6 was used for statistical analysis. Bioinformatic analysis of RNA sequencing samples was carried out using validated packages in R. Reads were mapped to human genome reference sequence GRCh37 with tophat253, deduplicated using samtools v.1.254. Gene counts were retrieved using htseq-cout55 and the Ensembl gene annotation. DESeq256, an R package, was used for differential gene expression analysis.
Pathway and network analysis
Genes common to IL-17A+GM-CSF+ and GM-CSF+ were defined if they, as compared to naive CD45RA+ cells, were differentially expressed (FDR <5%) in both IL-17A+GM-CSF+ cells and GM-CSF+ cells but not in IL-17A+ cells. Pathway analysis was conducted using the XGR software to identify enriched pathways (compiled from KEGG; June 2017). Network analysis was performed using the dnet package to identify a gene network that contains groups of interconnected genes. In brief, the dNetPipeline function in the package took as inputs a list of common genes with the significance level (i.e., the per-gene maximum FDR in both cells) and output a gene network (FDR <1%) from a human gene interaction data (compiled from the STRING database; May 2017).
Prioritisation of key genes
We applied an in-house prioritisation system using random forest plot modelling57 to generate evidence scores prioritising key genes involved in spondyloarthritis. We utilised GWAS summary data (risk variants and their detected p-values) for susceptibility in spondyloarthritis58,59 and eQTL summary data60,61 to prioritise the 1643 genes that were differentially expressed in GM-CSF+ CD4 cells from our data set. We prioritised genes based upon the relative weight of evidence of GWAS association and the eQTL significance level linking a SNP to a gene.
Statistical analysis
Prism version 6 was used for statistical analysis. When comparing two unpaired groups with normal distribution of data a two-tailed unpaired t-test was used for statistical analysis. When comparing two unpaired groups with data that was not normally distributed a Mann–Whitney test was used. For paired data, a paired t-test and Wilcoxon matched-pairs signed-rank tests were used for parametric and non-parametric data, respectively. When comparing more than two unpaired groups, a one-way analysis of variance (ANOVA) with Bonferroni’s correction was used for parametric data. Where the data were not normally distributed a Kruskal–Wallis test with Dunn’s multiple comparison analysis was used for statistical analysis. For matched data with more than two groups, a one-way ANOVA with Greenhouse-Geisser correction was performed in parametric data sets. In non-parametric data a Friedman test, a Dunn’s multiple comparison analysis was performed. Significance was defined as p ≤ 0.05.
Data availability
RNA sequencing data have been deposited in the National Centre for Biotechnology Information gene expression omnibus under the accession number GSE103930. The remainder of the data that support the findings of this study are available on request from the corresponding author. The patient data are not publicly available due to them containing information that could compromise research participant privacy.
Electronic supplementary material
Acknowledgements
This research was funded by the Wellcome Trust through a fellowship award to M.H.A.-M., Arthritis Research UK (programme grant 20773, J.C.K., P.B. and I.P.), the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (P.B. and A.R.). We would like to acknowledge funding by the Structural Genomics Consortium, a registered charity (number 1097737), that receives funds from AbbVie, Bayer Pharma AG, Boehringer Ingelheim, the Canada Foundation for Innovation, Eshelman Institute for Innovation, Genome Canada, Innovative Medicines Initiative (EU/EFPIA) [ULTRA-DD grant no. 115766], Janssen, Merck KGaA Darmstadt Germany, MSD, Novartis Pharma AG, the Ontario Ministry of Economic Development and Innovation, Pfizer, Sao Paulo Research Foundation-FAPESP, Takeda, and the Wellcome Trust [106169/ZZ14/Z]. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Author contributions
M.H.A.-M. Designed and performed experiments, analysed data and wrote the manuscript. L.C. contributed to designing the study and performed experiments. H.F. analysed data. A.R., J.d.W., N.Y. and A.H. performed experiments. I.P. analysed data. B.P.F. contributed to designing the study. D.S., Y.Y. and S.B. performed experiments. K.D., R.G. and B.K. contributed with clinical samples. F.P. and J.C.K. contributed to designing of the study. P.B. contributed to designing of the study and writing the manuscript.
Competing interests
Paul Bowness has received research grant funding from the Merck Research Laboratories and Celgene. Mohammad Hussein Al-Mossawi has received unrestricted research grant funding from UCB Pharma. The remaining authors declare no competing financial interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01771-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 2.Reveille JD. Epidemiology of spondyloarthritis in North America. Am. J. Med. Sci. 2011;341:284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun J, et al. Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 1998;41:58–67. doi: 10.1002/1529-0131(199801)41:1<58::AID-ART8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Brandt J, et al. Long-term efficacy and safety of etanercept after readministration in patients with active ankylosing spondylitis. Rheumatology. 2005;44:342–348. doi: 10.1093/rheumatology/keh475. [DOI] [PubMed] [Google Scholar]
- 5.van der Heijde D, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 6.Braun J, van der Heijde D. Novel approaches in the treatment of ankylosing spondylitis and other spondyloarthritides. Expert Opin. Investig. Drugs. 2003;12:1097–1109. doi: 10.1517/13543784.12.7.1097. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijde D, et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann. Rheum. Dis. 2009;68:922–929. doi: 10.1136/ard.2007.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reveille JD, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 2010;42:123–127. doi: 10.1038/ng.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 10.Baeten D, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 11.Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melton AC, et al. Regulation of IL-17A production is distinct from IL-17F in a primary human cell co-culture model of T cell-mediated B cell activation. PLoS ONE. 2013;8:e58966. doi: 10.1371/journal.pone.0058966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoreschi K, et al. Generation of pathogenic TH17 cells in the absence of TGF-[bgr] signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Behi M, et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiomi A, et al. GM-CSF but Not IL-17 is critical for the development of severe interstitial lung disease in SKG mice. J. Immunol. 2014;193:849–859. doi: 10.4049/jimmunol.1303255. [DOI] [PubMed] [Google Scholar]
- 16.Noster R, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci. Transl. Med. 2014;6:241ra80. doi: 10.1126/scitranslmed.3008706. [DOI] [PubMed] [Google Scholar]
- 17.Gaublomme JT, et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 2015;163:1400–1412. doi: 10.1016/j.cell.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortes A, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 2013;45:730–738. doi: 10.1038/ng.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LJ, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenna TJ, et al. Enrichment of circulating IL-17-secreting IL-23 receptor-positive γδ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64(5):1420–1429. doi: 10.1002/art.33507. [DOI] [PubMed] [Google Scholar]
- 21.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 22.Li R, et al. Proinflammatory GM-CSF–producing B cells in multiple sclerosis and B cell depletion therapy. Sci. Transl. Med. 2015;7:310ra166–310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 23.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 24.Pearson C, et al. ILC3 GM-CSF production and mobilisation orchestrate acute intestinal inflammation. eLife. 2016;5:e10066. doi: 10.7554/eLife.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011;12:1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salimi M, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leijten EFA, et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol. 2015;67:2673–2678. doi: 10.1002/art.39261. [DOI] [PubMed] [Google Scholar]
- 28.Rudwaleit M, et al. The assessment of SpondyloArthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2010;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 29.Amir ED, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JQ, et al. TDAG8 Is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004;279:45626–45633. doi: 10.1074/jbc.M406966200. [DOI] [PubMed] [Google Scholar]
- 31.Geremia A, et al. IL-23–responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 33.Piper C, et al. Brief report: T cell expression of granulocyte–macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014;66:1955–1960. doi: 10.1002/art.38647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasouli J, et al. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β therapy. J. Immunol. 2015;194:5085–5093. doi: 10.4049/jimmunol.1403243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain R, et al. Interleukin-23-induced transcription factor blimp-1 promotes pathogenicity of T helper 17 cells. Immunity. 2016;44:131–142. doi: 10.1016/j.immuni.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng W, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24:1387–1402. doi: 10.1038/cr.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD. Inflammasome-derived IL-1β regulates the production of GM-CSF by CD4+ T Cells and γδ T Cells. J. Immunol. 2012;188:3107–3115. doi: 10.4049/jimmunol.1103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 40.Khameneh HJ, Isa SABM, Min L, Wee Nih F, Ruedl C. GM-CSF signalling boosts dramatically IL-1Production. PLoS ONE. 2011;6:e23025. doi: 10.1371/journal.pone.0023025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit J, et al. RORγt inhibitors suppress TH17 responses in inflammatory arthritis and inflammatory bowel disease. J. Allergy Clin. Immunol. 2016;137:960–963. doi: 10.1016/j.jaci.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 42.Alzabin S, et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann. Rheum. Dis. 2012;71:1741–1748. doi: 10.1136/annrheumdis-2011-201024. [DOI] [PubMed] [Google Scholar]
- 43.Behrens F, et al. MOR103, a human monoclonal antibody to granulocyte–macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 2015;74:1058–1064. doi: 10.1136/annrheumdis-2013-204816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burmester GR, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 2013;72:1445–1452. doi: 10.1136/annrheumdis-2012-202450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greven DEA, et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015;74:1924–1930. doi: 10.1136/annrheumdis-2014-205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds G, et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann. Rheum. Dis. 2015;75:899–907. doi: 10.1136/annrheumdis-2014-206578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi JW, Lee SY, Choi Y. Identification of a putative G protein-coupled receptor induced during activation-induced apoptosis of T cells. Cell Immunol. 1996;168:78–84. doi: 10.1006/cimm.1996.0051. [DOI] [PubMed] [Google Scholar]
- 48.Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop. Scand. 1969;40:634–641. doi: 10.3109/17453676908989529. [DOI] [PubMed] [Google Scholar]
- 49.Huang X-P, et al. Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature. 2015;527:477–483. doi: 10.1038/nature15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010;69:1580–1588. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 51.Bowness P, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in Ankylosing Spondylitis. J. Immunol. 2011;186:2672–2680. doi: 10.4049/jimmunol.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor W, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 53.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, et al. The sequence alignment/map format and SAMtools. Bioinforma. Oxf. Engl. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinforma. Oxf. Engl. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein BA, Polley EC, Briggs FBS. Random forests for genetic association studies. Stat. Appl. Genet. Mol. Biol. 2011;10:32. doi: 10.2202/1544-6115.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellinghaus D, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 2016;48:510–518. doi: 10.1038/ng.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fairfax BP, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343:1246949. doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raj T, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344:519–523. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data have been deposited in the National Centre for Biotechnology Information gene expression omnibus under the accession number GSE103930. The remainder of the data that support the findings of this study are available on request from the corresponding author. The patient data are not publicly available due to them containing information that could compromise research participant privacy.