Abstract
Estrogen is a potent steroid with pleiotropic effects, which have yet to be fully elucidated. Estrogen has both nuclear and non-nuclear effects. The rapid response to estrogen, which involves a membrane associated estrogen receptor(ER) and is protective, involves signaling through PI3K, Akt, and ERK 1/2. The nuclear response is much slower, as the ER-estrogen complex moves to the nucleus, where it functions as a transcription factor, both activating and repressing gene expression. Several different ERs regulate the specificity of response to estrogen, and appear to have specific effects in cardiac remodeling and the response to injury. However, much remains to be understood about the selectivity of these receptors and their specific effects on gene expression. Basic studies have demonstrated that estrogen treatment prevents apoptosis and necrosis of cardiac and endothelial cells. Estrogen also attenuates pathologic cardiac hypertrophy. Estrogen may have great benefit in aging as an anti-inflammatory agent. However, clinical investigations of estrogen have had mixed results, and not shown the clear-cut benefit of more basic investigations. This can be explained in part by differences in study design: in basic studies estrogen treatment was used immediately or shortly after ovariectomy, while in some key clinical trials, estrogen was given years after menopause. Further basic research into the underlying molecular mechanisms of estrogen’s actions is essential to provide a better comprehension of the many properties of this powerful hormone.
Keywords: estrogen, aging, cardiovascular, inflammation, cardiac hypertrophy, endothelial, cardiac myocyte, heart failure, HRT, menopause
1. Introduction
Estrogen is a potent steroid hormone present in high levels in females from adolescence to menopause and in low levels in men. Much anecdotal evidence accumulated over many years had supported the idea that estrogen post-menopause reduced cardiovascular disease. In the 1990’s several double blind, controlled trials of estrogen replacement post-menopause were conducted including the Women’s Health Initiative(WHI) and the Heart and Estrogen/progestin Replacement Study (HERS).(Hulley et al., 1998; Grady et al., 2002; Writing Group for the Women’s Health Initiative Investigators, 2002) These studies showed an increased risk of both cancer and cardiovascular disease in those taking estrogen replacement. However scrutiny of these studies led to recognition of a possible cause of lack of estrogen efficacy based on the design of these studies, as a result of the effort to enroll women who definitely were post-menopause, 10 years had elapsed on average between menopause and the onset of estrogen replacement in these studies. This realization led to the development of the timing hypothesis.(Barrett-Connor, 2007; Dubey, Imthurn, Barton, & Jackson, 2005; Grodstein, Clarkson, & Manson, 2003) This hypothesis proposed that the prolonged delay after the onset of menopause led to significant tissue and gene expression changes, such that late estrogen replacement post-menopause was occurring in a markedly different tissue state than 10 years earlier, when estrogen production ceased.
HRT Controversies
A second issue in hormone replacement therapy (HRT) is the type and route of hormone replacement. The current review focuses on estrogen; however the combination of progesterone and estrogen has been addressed elsewhere by others.(Tchaikovski & Rosing, 2010; Canonico et al., 2010) Estrogen is a potent steroid hormone with 17β-estradiol (E2) being the most active metabolite. There are two key issues in hormone replacement. First, the oral delivery of estrogen results in high hepatic levels of estrogen from first pass metabolism, which can stimulate protein synthesis and appears to be associated with increased risk of thrombosis compared to transdermal delivery, though this remains controversial. (Bagot et al., 2010; Canonico et al., 2010; Tchaikovski & Rosing, 2010; Olié, Canonico, & Scarabin, 2011) Estrogen will, through its nuclear properties, change hepatic gene expression and can potentially effect expression of genes involved in coagulation. Estrogen replacement using a transdermal patch avoids the first pass surge in hepatic estrogen levels and may have less complications, though some controversy remains. Second, conjugated equine estrogen (CEE) is the most commonly used estrogen compound for HRT. CEE is derived from the urine of pregnant mares and includes estrogen compounds not found in humans. The composition of CEE differs significantly from the estrogens found in premenopausal women. Different estrogens differ in their binding affinities and selectivity for the estrogen receptors (ER); consequently they have different downstream effects. These differences could have a significant impact on the effects of HRT. CEE contains sodium estrone sulfate, sodium equilin sulfate, and sodium 17α-dihydroequilinenin, but no 17β-estradiol, the major and most potent form of estrogen found in premenopausal women.(Booth & Lucchesi, 2008) Recently estrone levels have been linked with thrombin generation, a central step in the coagulation cascade, in postmenopausal women.(Bagot et al., 2010) Thus, estrones have the potential to greatly increase the risk of thrombosis, and as CEE contains a large amount of estrones, CEE would be expected to be associated with an increased risk of thrombotic events. However, the studies in this area are limited and it is premature to draw definitive conclusions.
Estrogen is a complex hormone with pleiotropic effects. Basic research is essential to understand the underlying cell and molecular mechanisms of estrogen’s actions and to gain insight into the clinical effects of estrogen. This review will focus on basic cardiovascular research on estrogen, which provides the underpinning for our understanding of the clinical effects of this hormone.
2. Aging, Inflammation and Fibrosis
Aging and estrogen loss are indelibly linked. Aging is associated with inflammation and increased inflammatory serum cytokines such as TNF and IL-6.(Donato, Black, Jablonski, Gano, & Seals, 2008; Chung et al., 2009) Aging is also associated with increased oxidative stress and a blunting of the protective heat shock response in males and females.(Locke & Tanguay, 1996; Gutsmann-Conrad, Heydari, You, & Richardson, 1998; Fawcett, Sylvester, Sarge, Morimoto, & Holbrook, 1994; Jackson & McArdle, 2011; Stice et al., 2011) In the cardiovascular system, aging is accompanied by increased stiffness, increased fibrosis, loss of contractile reserve, increased ROS and endothelial dysfunction. All of these factors contribute to cardiovascular dysfunction. It is in this setting that estrogen, which is an antioxidant through indirect upregulation of antioxidant gene expression and increasing eNOS activity while decreasing superoxide production, is lost through menopause.(Siow, Li, Rowlands, de Winter, & Mann, 2007; Barbacanne et al., 1999; Florian, Freiman, & Magder, 2004) A key question is whether estrogen or a synthetic estrogen receptor modulator (SERM) can ameliorate any of these changes. SERMs have differing receptor specificity and tissue responses. Two SERMs are used clinically, tamoxifen and raloxifene, and many more are under development. SERMs have the potential to provide a selective activation profile of estrogen targets.
Summary
Estrogen’s effects and the loss of estrogen must be considered in the setting of aging. There is potential for pharmotherapeutics to eventually ameliorate some of the changes that occur with estrogen loss. In this review, we will focus on current understanding of the cellular and molecular changes associated with aging and estrogen loss and their implications for cardiovascular health.
3. Estrogen Receptors
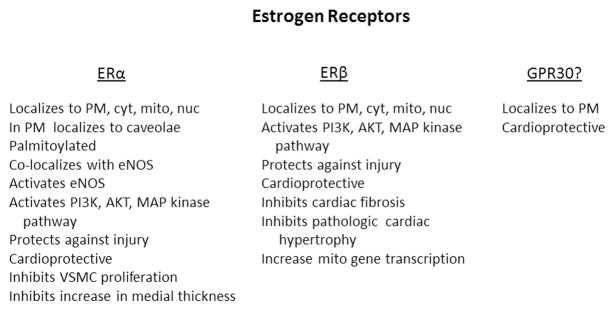
There are two established estrogen receptors, α and β (Figure 1). Differences in tissue distribution in these receptors are thought to modulate tissue response to estrogen, but this remains to be proven. Post-translational modifications may be important modulators of receptor function, but studies in this area have been limited. Several differences between ERα and β have been identified in the cardiovascular system, as will be discussed. These differences are summarized in Table I.
Figure 1.
Summary of key known functions of the ER. GPR30’s status as an estrogen receptor remains unresolved. PM -plasma membrane; cyt -cytosol; mito -mitochondrion; nuc -nucleus.
Table I.
Summary of identified differences between ERα and ERβ actions in cardiovascular system.
| ER α | ERβ |
|---|---|
| Activates eNOS in adult endothelial cells | Activates eNOS in embryonic cells |
| Prevents VSMC proliferation | Inhibits anti-proliferative effects of ERα in diabetes |
| No effect on pathologic cardiac hypertrophy | Reduces pathologic cardiac hypertrophy |
| Mitochondrial gene expression effect unknown | Regulates mitochondrial gene expression |
ERα and β are found both in association with the plasma membrane (not transmembrane), in the cytoplasm and in the nucleus. Palmitoylation of ERα regulates its localization to the plasma membrane, where it is found in caveolae.(Levin, 2010; Acconcia et al., 2005; Chambliss et al., 2000) ERα complexes with caveolin 1, c-Src, Akt, PI3K, HSP90 and eNOS in caveolae in the plasma membrane.(Kim & Bender, 2009; Haynes et al., 2003; Li, Haynes, & Bender, 2003) A number of studies indicate that an ERα splice variant, ER46, is present in this complex, rather than the full length receptor.(Li et al., 2003; Figtree, McDonald, Watkins, & Channon, 2003; Kim & Bender, 2009; Keung, Chan, Ho, Vanhoutte, & Man, 2011) ERα’s co-localization with HSP90 and eNOS within the caveolae facilitates activation of eNOS by E2.(Chambliss et al., 2000; Mineo & Shaul, 2006; Levin, 2010) The chaperone protein, HSP90, assists with the interaction between the ER and eNOS and other signaling enzymes. Caveolin-1 actually inhibits eNOS, and caveolin-1 knockout mice have increased eNOS activity.(Razani et al., 2001) The multiple proteins involved in the assembly of the ERα signaling complex in caveolae have been recently reviewed in detail.(Boonyaratanakornkit, 2011) Activation of eNOS requires dissociation from caveolin-1; frequently this is mediated by calcium/calmodulin, but in some circumstances, such as shear stress, eNOS activation is thought to be independent of calcium. Several excellent reviews have recently addressed the molecular mechanisms underlying eNOS activation.(Fleming, 2010; Dessy, Feron, & Balligand, 2010) In addition to activation of eNOS, E2 activation of PI3K, Akt, ER 1/2, and MAP kinases has a protective effect, which is discussed in more detail below.
Striatin-3, a caveolin-binding protein, associates with ERα and is necessary for the assembly of the membrane signaling complex leading to rapid activation of eNOS by E2.(Dan, Cheung, Scriven, & Moore, 2003) Striatin-3 actually binds to ERα and targets it to the membrane. Overexpression of striatin-3 was sufficient to target ERα to the plasma membrane in cancer cell lines. Interestingly, overexpression of a splicing variant of striatin-3 depressed ERα transcriptional activity in a number of cell lines including cancer cell lines, but had no effect on ERβ(Tan, Long, Nakshatri, Nephew, &. Bigsby, 2008) Whether any of these findings would extend to cardiovascular cells is unknown.
ERα, ERβ and Endothelial Cells
Most cardiovascular work on membrane ERs has focused on ERα. ERβ was not cloned until 1996, and as a result fewer investigations have focused on ERβ; however some studies have specifically addressed both receptors. ERα knockout reduced basal NO production in the aorta of E2 treated ovx mice, while ERβ knockout had no effect on NO production.(Darblade et al., 2002) Dan et al. observed by deconvolution of images that 1/3 of ERα co-localized with caveolin-1 at or near the plasma membrane in rat vascular endothelium (cerebral and coronary arteries).(Dan et al., 2003) In contrast, ERβ was predominantly in the nucleus. Similarly, studies on the ovine uterine artery found that ERα was present both in the nucleus and at the plasma membrane with caveolin-1 in uterine artery endothelial cells.(Liao, Magness, & Chen, 2005) ERβ mRNA levels were 1/8th those of ERα, and here again ERβ was not associated with caveolin-1. However, other investigators, using primary endothelial cells obtained from embryonic ovine intra-pulmonary arteries, have found both ERα and ERβ to be present at the plasma membrane, with ERα equally distributed between caveolae and non-caveolae.(Chambliss, Yuhanna, Anderson, Mendelsohn, & Shaul, 2002) In contrast, ERβ was present in the caveolae, but the majority of the protein was associated with the non-caveolar membrane. Even though more ERα was present in the caveolae of these embryonic endothelial cells, the investigators found that almost all eNOS activation was mediated by ERβ rather than ERα. This conclusion was based on the use of ICI 182,780, which is a non-specific inhibitor of both ERα and ERβ, and R,R-tetrahydrochrysene (THC), which is thought to be a specific inhibitor of ERβ. Using the membrane caveolae fraction, ICI 182,780 reduced E2 stimulated eNOS activity 70%, while THC reduced activity 92%, leading the investigators to conclude that nongenomic activation of eNOS in endothelial cells was largely mediated by ERβ.(Chambliss et al., 2002) Clearly, both ERα and ERβ can activate eNOS, but there is a difference in the results of these studies as to which receptor is responsible for eNOS activation in endothelial cells. Three studies using adultmodels (rat, mouse and pregnant sheep) all found that ERα was the predominant receptor associated with the endothelial cell membrane and with the caveolae. A fourth study, using embryonic ovine intra-pulmonary artery endothelial cells found ERβ to be the predominant activator of eNOS in endothelial cells. The primary difference in these models is that 3 studies used young adult models and one used embryonic. There are significant differences in the oxygenation of the fetus, which has a relatively hypoxic environment, and the adult; the differences in receptors between the embryonic cells and the adult cells may reflect adaption for different environments. Furthermore, studies in the adult models showed ERα to be the primary ER associated with caveolae in endothelial cells.(Liao et al., 2005)
ERα, ERβ and Vascular Smooth Muscle Cells
While the localization ERα and ERβ has been carefully investigated in endothelial cells, precise localization studies have not been done for vascular smooth muscle cells (VSMCs) or for cardiac myocytes. The presence of membrane associated and nuclear ERα and ERβ has been demonstrated by many studies, but direct work examining the localization of the receptors has been limited in VSMC and cardiac myocytes.(Stice et al., 2011) VSMCs from male SD rat mesenteric artery showed co-localization of both ERα, only as ER46, and ERβ with caveolin-1.(Keung et al., 2011) Interestingly, co-IP of caveolin-1 precipitated not only ERα and ER β, but also PKG.(Keung et al., 2011) ERα and ERβ are both present in VSMCs isolated from adult human aorta and both localized predominantly to the nucleus.(Nakamura et al., 2004) Similarly, RT-PCR demonstrated both ERα and ERβ in VSMCs from the aortic arch of 8 wk old rats.(Watanabe et al., 2003) Adult human primary VSMCs expressed both ERα and ERβ by RT-PCR, but more ERα was present than ERβ(Takahashi et al.,. 2003) Expression of ERα and ERβ in VSMC cells lines was distinctly different from primary cells or pathology specimens. CRL-2481 cells(American Tissue Culture Collection [ATCC]), a line derived from human umbilical vein smooth muscle cells, expressed only ERα while CRL-1999 cells (ATCC), a line derived from human aortic smooth muscle cells, expressed only ERβ(Nakamura et. al., 2004) A10 cells, a VSMC line derived from the embryonic rat thoracic aorta, expressed only ERβ by RT-PCR.(Takahashi et al., 2003) Thus, both ERα and ERβ are present in VSMC and localize predominantly in the nucleus; however both receptors are present at the plasma membrane and associate with caveolin-1. Some work suggests more ERα than ERβ is present in adult VSMCs. Three different cell lines derived from VSMCs expressed ERα or ERβ, but not both, making these cell lines not good models for investigation of the role of ERα and ERβ in VSMCs. Studies to date support that both ERα and ERβ are expressed in VSMCs, but the relative amounts of each receptor and the intracellular distribution of the receptors remains to be definitively defined.
ERα, ERβ and Cardiac Myocytes
In cardiac myocytes even less data exists as to intracellular distribution of the estrogen receptors. Both ERα and ERβ were present in cardiac myocytes isolated from adult and aged ovariectomized Norway Brown(NB) rats with and without immediate E2 replacement. Western blot showed no difference in the amount of ERα or ERβ present among the four groups.(Stice et al., 2011)
Nongenomic vs. Genomic Signaling
Membrane associated ERα and ERβ, when bound by estrogen, can each activate a signaling cascade that includes PI3K and Akt, as well as ERK 1/2, JNK and p38.(Wang et al., 2009; Wang, Crisostomo, Wairiuko, & Meldrum, 2006; Patten et al., 2004; Wu, Chambliss, Umetani, Mineo, & Shaul, 2011) This signaling cascade protects the cell from injury, except for JNK, which increases apoptosis. The signaling mediated by membrane associated ERα and ERβ has been termed nongenomic signaling and more recently nonnuclear signaling. ERα nongenomic signaling has recently been shown to provide cardiovascular protection without increasing uterine or breast cancer growth in mice.(Chambliss et al., 2010) This supports the idea that the increased risk of cancer with estrogen therapy is related to estrogen’s nuclear effects (gene expression) and that estrogen in a form that is not taken up by the cell (selecting nonnuclear effects), such as the estrogen-dendrimer conjugate used in this study, may be protective without the concomitant increased risk of cancer seen with oral estrogen.
Both ERα and ERβ have important roles as transcription factors, activated by estrogen binding. In the nucleus these receptors in their transcription factor role both promote and inhibit the expression of an array of genes. Investigation of gene expression regulated by ERα vs. ERβ has been limited, with just a handful of studies on cancer cell lines with stably over expressed ERβ. This work supports distinct sets of genes regulated by the two receptors with some overlap.(Zhao, Dahlman-Wright, & Gustafsson, 2010) ERβ is able to at least partially inhibit ERα induced gene transcription and also ERα induced cell proliferation.(Zhao et al., 2010; Lindberg et al., 2003) Furthermore, ERβ2, a variant of ERβ that has been proposed to be a dominant negative inhibitor, can induce proteosomal degradation of ERα(Zhao et al., 2010).
GPER
Recent work has identified a third, membrane associated ER, G-protein-coupled estrogen receptor (GPER).(Deschamps & Murphy, 2009) This receptor is also known as G-protein coupled receptor 30 (GPR30) and as the membrane estrogen receptor (mER). GPER has been shown to contribute to E2 mediated vasodilation.(Lindsey, Carver, Prossnitz, & Chappell, 2011) A second group of investigators demonstrated that GPER activation by the selective agonist, G1, relaxed porcine aortic rings and human coronary artery VSMCs in an endothelial independent manner.(Yu et al., 2011) RT-PCR and western blotting confirmed the expression of GPER in both porcine coronary arteries and human coronary artery VSMCs. The specificity of the findings was confirmed with G15, a selective inhibitor of GPER.(Yu et al., 2011) Additional studies demonstrated that GPER activation specifically activated the large-conductance calcium and voltage activated potassium channel (BKca ), which is expressed in coronary artery VSMCs. G1 had no direct effect on BKca (Yu et al., 2011). This work provides a new mechanism by which GPER can promote vascular relaxation.
GPER may activate the nonnuclear protective response (PI3-kinase, Akt, etc.) via a truncated 36 kDa ERα(Kang et al., 2010). Others have reported that GPER activation by G1 reduces infarct size in the isolated perfused male mouse heart.(Bopassa, Eghbali, Toro, & Stefani, 2010) GPER activation also inhibited opening of the mitochondrial permeability transition pore (mPTP) in the same study. The protective effects of GPER activation were abolished by treatment with the ERK inhibitor, PD98059. GPER activation can attenuate diastolic dysfunction and ventricular remodeling after ovx.(Wang et al., 2012b) There still remains some controversy as to whether GPER is an estrogen receptor, and more studies are needed to define the role of GPER in estrogen signaling.(Wu et al., 2011) However, overall there is greater acceptance of GPER as an important mediator of estrogen signaling.
Lastly, the estrogen related receptors (ERRs) should be briefly mentioned. The ERRs are orphan nuclear receptors, with three isoforms – alpha, beta and gamma. All of the ERRs are involved in energy metabolism and mitochondrial biogenesis.(Dufour et al., 2007; Giguère, 2008; Bookout et al., 2006) Their activity depends on coactivator proteins, principally those in the peroxisome proliferator-activated receptor coactivator-1 family.(Giguère, 2008; Schreiber, Knutti, Brogli, Uhlmann, & Kralli, 2003) Estrogen binding to ERα has been reported to upregulate ERRα expression, and there are intriguing similarities between the ER and ERR response elements.(Shigeta, Zuo, Yang, DiAugustine, & Teng, 1997; Giguère, 2002) To date, the ligand for these interesting receptors remains to be identified, but there is no known interaction between the ERRs and estrogen.
4. Genomics of Estrogen and the Cardiovascular System
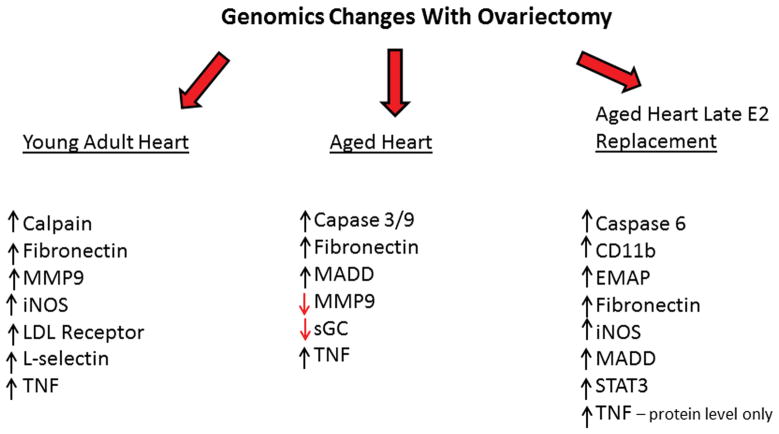
Estrogen is an important modulator of gene expression; both estrogen receptors act both as transcription factors and as inhibitors of gene expression. Despite these important functions, studies of the effect of estrogen on gene expression have been surprisingly limited. In Sprague Dawley rat hearts, heat shock protein (HSP)72 levels are increased in the female heart vs. the male. It takes 9 weeks post-ovariectomy (ovx) for the level of HSP72 in the female heart to drop to the level in male hearts.(Voss et al., 2003) This slow change in HSP72 levels suggests that HSP72 is indirectly regulated by estrogen and that a cascade of changes occurs post-ovx such that the full effect of ovariectomy on gene expression is not seen until at least 9 weeks post-surgery. In the young adult Sprague Dawley, ovariectomy resulted in increased expression of a number of pro-inflammatory genes in the heart at 9 weeks post ovx, including L-selectin, SOCS2, SOCS3, calpain, TNF, iNOS, fibronectin, and the LDL receptor compared to ovariectomy with immediate E2 replacement.(Hamilton, Lin, Wang, & Knowlton, 2007) However, changes of gene expression with aging and estrogen loss are of greater relevance. In aged Norway Brown (NB) rats, considered a good model of aging as this inbred strain does not develop obesity with aging, 9 weeks post-ovx cardiac expression of caspase 3, caspase 9, calpain 2, MADD, fibronectin, MMP9 and TNF mRNA increased.(Pechenino et al., 2011) Immediate estrogen replacement prevented many of these changes. In contrast, late E2 replacement, modeling many of the clinical trials, had a pro-inflammatory effect, increasing expression of CD11b, iNOS, STAT3, caspase 6, MADD and fibronectin (Figure 2). Protein levels of both iNOS and TNF were increased in the late replacement group. Thus ovx with loss of estrogen and progesterone had sustained pro-inflammatory effects long after surgery in both young and aged rat hearts. These changes were greatly attenuated by E2 replacement. Most importantly, the changes seen with late replacement in TNF, CD11b, and iNOS among other genes, would be expected to promote inflammation, monocyte adhesion and transmigration leading to increased atherosclerosis. These changes in gene expression provide insight into the underlying mechanism of the increased incidence of cardiovascular events in the clinical trials with late estrogen replacement.
Figure 2.
Key genomic changes with ovariectomy in adult heart, aged heart, and aged heart with late estrogen replacement. Increased TNF in aged heart with late replacement only seen at protein level.
5. Estrogen and the Vasculature
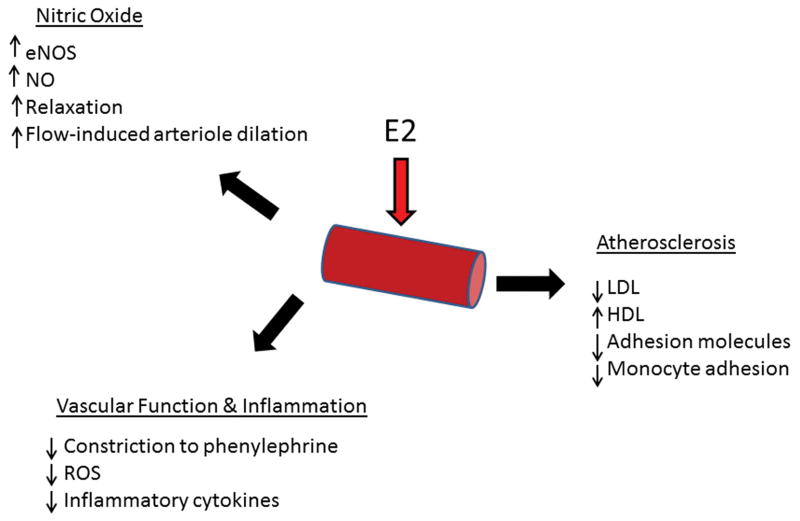
The vasculature is the site of critical changes with aging and estrogen loss including increased atherosclerosis and abnormal function (relaxation and constriction). Endothelial cells as well as VSMCs are important targets of estrogen. Thus, changes in estrogen and aging particularly impact the vasculature (Figure 3).
Figure 3.
Summary of major effects of E2 on vasculature.
Vascular Function and Nitric Oxide
It is well established that estrogen enhances NO production and vascular relaxation.(Schulz, Anter, Zou, & Keaney, Jr., 2005; Simoncini, Rabkin, & Liao, 2003; Mendelsohn, 2000) Overwhelmingly estrogen loss occurs with the onset of menopause in middle age, at which point women have an acceleration of atherosclerosis and changes in vascular function. Yet only a limited number of studies have focused on the effect of estrogen loss with aging.(Xing, Nozell, Chen, Hage, & Oparil, 2009; Chakrabarti, Lekontseva, & Davidge, 2008) In endothelial cells E2 activates eNOS via the PI3-kinase Akt pathway leading to the production of NO, and thus has an important role in regulation of vascular tone.(Haynes et al., 2000; Hisamoto et al., 2001) CEE impairs eNOS expression and NO production compared to treatment with E2.(Novensa et al., 2010) Estrone was able to increase NO production, but the other metabolites of equine estrogen in CEE had little effect on NO production.(Novensa et al., 2010) In other work, E2, but not estrone or 17α-dihydroequilenin, increased eNOS expression and NO in cultured human coronary artery endothelial cells.(Wingrove, Garr, Pickar, Dey, & Stevenson, 1999) The role of hormones in regulation of eNOS and the synthesis of NO has recently been reviewed.(Duckles & Miller, 2010)
Aging, Estrogen and Vascular Constriction
Aging is associated with impaired vascular function, and this is compounded by loss of estrogen. Intact female spontaneously hypertensive (SHR) rats (16 mo) had greater contraction to both phenylephrine and KCl compared to younger rats.(Wynne, Payne, Cain, Reckelhoff, & Khalil, 2004) In response to phenylephrine ovx Norway Brown rats, both adult (6 mo) and aged (22 mo), had greater vascular constriction than ovx with E2 replacement.(Stice, Eiserich, & Knowlton, 2009) Increased response to vasconstrictive agents has also been reported in the mesenteric arteries of 13–16 mo old ovx Sprague Dawley rats.(Arenas, Armstrong, Xu, & Davidge, 2006) This correlated with increased expression of angiotensin II type 1 receptor (AT1R), which could be ameliorated by E2 treatment. Similar results were found in both young Wistar-Kyoto ovx rats and young SHR ovx rats, which had increased AT1R and increased superoxide production (only measured in SHR rats), both of which were attenuated by E2 replacement.(Wassmann et al., 2001; Nickenig et al., 1998) These results indicate that increased constriction is not a function of aging, but secondary to loss of estrogen.
Aging, Estrogen and Vascular Relaxation
The intact female spontaneously hypertensive (SHR) rats (16 mo) had decreased relaxation compared to younger rats.(Wynne et al., 2004) In old ovx NB rats, only the aged ovx without E2 replacement had impaired relaxation, and this was not corrected by treatment with an NO donor.(Stice et al., 2009) In fact there was no evidence of decreased eNOS or iNOS expression nor of a change in circulating NO. This is in contrast to aging male models where a decrease in expression and/or activity of eNOS occurred in the vasculature.(Long et al., 2005; Soucy et al., 2006) An important variable in these studies may be aging related obesity, which is found in a number of rat strains, including Sprague Dawleys. Aged ovx NB rats, which do not develop obesity, had no change in eNOS, but decreased expression of soluble guanylyl cyclase (sGC) α and β, the two subunits which constitute a receptor for NO in smooth muscle cells.(Stice et al., 2009) In this model, treatment with an sGC agonist corrected the impaired relaxation. sGC also was decreased in aged ovx NB rats by microarray study, but there was no change in sGC expression in young ovx SD rats.(Hamilton et al., 2007; Pechenino et al., 2011) The reduction in sGC in the aged ovx NB rats was prevented by E2 replacement. Interestingly, there is work demonstrating that NFκB inhibits expression of sGC, and increased NFκB activity has been found in the aging cardiovascular system.(Marro et al., 2008; Stice et al., 2011; Csiszar, Wang, Lakatta, & Ungvari, 2008) Increased NFκB activation can lead to increased TNF. Provocatively, Etanercept (the truncated TNF receptor used to reduce TNF activity in rheumatoid arthritis) treatment resulted in significant improvement in vascular relaxation in aged rats, and this correlated with a reduction in the elevated TNF serum levels post ovx, suggesting that TNF may be an important contributor to post-menopausal vascular disease.(Arenas, Armstrong, Xu, & Davidge, 2005)
Similar impairment in relaxation has been found in humans. In human studies of forearm endothelium-dependent vasodilation, aging was associated with impaired function and estrogen improved relaxation post-menopause.(Chan & Fiscus, 2004; Saitta et al., 2001)
Flow-Induced Dilation
Ovx and aging both impair flow-induced arteriole dilation.(Kang, Reyes, & Muller-Delp, 2009) In young Fischer-344 rats impaired arteriole dilation post-ovx can be reversed by E2 replacement, while in aged rats ovx had no effect on arteriole dilation.(LeBlanc et al., 2009) However, E2 treatment markedly improved flow-induced arteriole dilation in the aged rats. This improvement in aged intact and aged ovx with E2 treatment is likely secondary to the decrease in endogenous E2 levels with aging with supplemental E2 resulting in higher circulating levels.
E2, ERα and ERβ, and Angiogenesis
E2 regulated the expression of ERα and ERβ in both bovine and human endothelial cells.(Ihionkhan et al., 2002) E2 increased ERα would be expected to lead to increased NO, as ERα activates eNOS, as discussed above. ERα expression in endothelial cells fluctuated with stages of the human cycle with the highest expression at peak estrogen levels.(Gavin, Seals, Silver, & Moreau, 2009) Investigators have found that ERα also has a direct effect on key vascular proteins and on angiogenesis.(Jesmin et al., 2010) ERα knockout was associated with a striking decrease in VEGF levels and capillary density in the heart.
Thus, E2 enhances vascular relaxation via increased NO production and also enhances angiogenesis with evidence supporting ERα as mediating these effects. Aging is associated with an increase in inflammatory cytokines such as IL-6 and TNF, and a single study supports a role for TNF in depressed vascular relaxation. Aging increases vascular constriction, decreases relaxation, and decreases flow-mediated vasodilation. E2 replacement and raloxifene have been shown to attenuate these changes.
Estrogen, VSMC Proliferation and Diabetes Mellitus
E2 is known to inhibit VSMC proliferation. E2 (100 pM and 10 nM) inhibited proliferation of VSMC, derived from atherosclerotic human arteries, positive for ERα(Nakamura et al., 2004). Both E2 and raloxifene caused G1 cycle arrest of VSMC (primary human VSMC, gender unknown).(Takahashi et al., 2003) Premenopausal diabetic women lose the protective effects of estrogen against atherosclerosis.(Sowers, 1998) Basic investigations have addressed the role of high glucose in modulating the responses to E2. High glucose (25 mM) promotes VSMC proliferation compared to normal glucose levels (5 mM).(Ling et al., 2002) Previously, it has been demonstrated that E2 (1–100 nM) inhibited platelet derived growth factor (PDGF)-BB induced DNA synthesis and VSMC proliferation in studies on VSMC derived from the internal mammary arteries of two non-diabetic women with coronary artery disease.(Ling et al., 2002) PKC-β activation under high glucose conditions blocked inhibition of VSMC proliferation by E2, and inhibition of PKC-β with LY-379196 restored E2’s anti-proliferative effects.(Ling et al., 2002) However, in studies of VSMCs derived from pre-menopausal women only the selective ERα agonist, PPT, prevented high glucose induced VSMC proliferation.(Ortmann et al., 2011) E2, the selective ERβ agonist, DPN, and the selective GPER agonist, G-1, did not prevent VSMC proliferation in high glucose conditions. ERα activation prevented the increase in H2O2 and ROS that occurred in high glucose conditions. In addition, selective ERα activation increased expression of MnSOD and inhibited the sustained phosphorylation of ERK, which occurred under high glucose conditions.(Ortmann et al., 2011) Significantly, co-activation of ERβ blocked the protective effects of ERα activation.(Ortmann et al., 2011) Thus, E2 inhibits VSMC proliferation and this is abrogated by high glucose, which increases ROS and leads to sustained ERK phosphorylation. Interestingly, estrones, major components of CEE, do not inhibit VSMC proliferation.(Dubey et al., 2000) Thus, the most common form of estrogen replacement fails to prevent VSMC proliferation. Selective activation of ERα blocks high glucose mediated VSMC proliferation and signaling changes, including the increase in ROS and the prolonged phosphorylation of ERK. ERβ activation prevented the protective effects of ERα activation. These interesting studies suggest that in premenopausal diabetic women that estrogen signaling is a dichotomy with ERα protecting the vasculature and ERβ inhibiting the protective signaling by ERα. More work is needed to confirm these very intriguing results.
E2 and Prevention of Arterial Injury
Estrogen -mediated protective responses are found in all cells and tissues that have been studied so far. However, there are many injury studies involving estrogen, and pathways involved in cardiovascular protection after chronic estrogen replacement vs. immediate estrogen treatment likely differ. There had been different reports with regard to which ER mediated these protective responses. In part the differences likely stem from the study of different types of estrogen mediated protection. Vascular injury, induced most commonly by denudation of a portion of the carotid artery, was found to have reduced medial thickness and VSMC proliferation regardless of the presence of ERα, ERβ or neither receptor.(Karas et al., 2001) However, this study used the Chapel Hill ERαknockout mouse, and this mouse has a residual splice variant or truncated ERα(Couse et al., 1995). A repeat of this study using a total knockout of ERα demonstrated that loss of ERα resulted in loss of protection against VSMC proliferation and increased medial thickness after vascular injury, consistent with the finding discussed above that ERα activation prevented hyperglycemia induced VSMC proliferation.(Pare et al., 2002) Differentiating the effects mediated by ERα and ERβ has become a complex task with similar studies of cardiovascular injuries finding beneficial effects from each receptor.(Patten et al., 2004; Pelzer et al., 2005; Wang et al., 2006; Wang et al., 2009; Favre et al., 2010) The availability of more specific agonists for the two receptors and greater understanding of the knockout models should contribute greatly to the dissection of the specific functions of each receptor.
E2, NFκB and Endothelial Cell Protection
In human coronary endothelial cells E2, at pharmacologic levels (nM to :M vs. physiologic), activates NFκB, heat shock factor (HSF) 1 and leads to increased expression of heat shock protein (HSP) 72.(Hamilton, Mbai, Gupta, & Knowlton, 2004) This activation of NFκB is associated with protection against hypoxia/reoxygenation, as blocking the downstream signaling of NFκB with DNA binding decoys resulted in a loss of the protective effect of pretreating with E2. In contrast, inhibition with downstream signaling of HSF1 with DNA binding decoys had no effect on the protective effects of pretreatment with E2, suggesting that the protective response to E2 is redundant. Activation of NFκB is mediated by simultaneous activation of P38, JNK and Akt, which activate ERK 1/2 leading to activation of NFκB (J.P. Stice and A.A. Knowlton, unpublished).
Experimental Issues
An issue in endothelial cell, as well as cardiac myocyte studies, is the concentration of E2 used in cell culture. Plasma concentrations of E2 are in the picomolar range, but culture models predominantly use nanomolar and sometimes higher concentrations of E2. Several different groups have reported that aromatase contributes to heart and vascular tissue estrogen levels. (Grohé, Kahlert, Löbbert, & Vetter, 1998) Thus, it is possible that local levels of E2 exceed those measured in plasma samples. However, much more evidence is needed to prove there are substantially higher tissues levels of estrogen.
Estrogen, Apoptosis and Injury
Apoptosis is an important mechanism of endothelial cell loss in the aging vasculature.(Csiszar, Ungvari, Koller, Edwards, & Kaley, 2005; Razandi, Pedram, & Levin, 2000; Alvarez et al., 1997) This increase in apoptosis is exacerbated by the loss of endothelial progenitor cells with aging, which may prime the vessel for atherosclerosis.(Goldschmidt-Clermont, 2003) Estrogen has known anti-apoptotic properties.(Patten et al., 2004; Kim, Pedram, Razandi, & Levin, 2006a; Liu, Pedram, & Kim, 2011; Hsieh et al., 2006b) E2 prevents both endothelial cell and endothelial progenitor cell (EPC) apoptosis.(Razandi et al., 2000; Alvarez et al., 1997; Strehlow et al., 2003; Masuda et al., 2007) The anti-apoptosis effects of E2 are likely mediated by increased activation of the protective PI3K/Akt pathway and also by decreased inflammation and cytokine production. Therefore menopause, through increased endothelial and EPC apoptosis, further impairs the function of the vasculature.
Summary
Thus, these studies demonstrate that E2 increases NO p roduction by endothelial cells, and this will lead to vascular relaxation; however in the setting of aging and ovx there is impaired relaxation as well as an increased response to vasocontrictive agents. At a cellular level, aging and ovx was associated with decreased expression of sGCα and β, which comprise a receptor for NO in VSMCs. E2 via ERα increases angiogenesis and protects against both vascular injury and endothelial cell injury. ERα activation blocks VSMC proliferation in response to hyperglycemia, but activation of ERβ will inhibit this response. CEE does not activate many of these protective responses. In the vasculature, ERα has the primary role in a range of estrogen mediated protective responses.
6. Atherosclerosis
Premenopausal women have a much lower incidence of cardiovascular disease than males. Estrogen has a very positive effect on lipoprotein profiles, lowering LDL and raising HDL. This has been estimated to contribute about 30% of the cardiovascular benefit in premenopausal women.(Mendelsohn & Karas, 1999) Post-menopause women have a rapid acceleration of atherosclerosis and an increase in LDL and decrease in HDL, all accompanied by an increase in the prevalence of coronary artery disease. Vital to understanding the mechanisms underlying the increase in atherosclerosis, beyond the change in lipid profile, is to understand the effect of estrogen on the actual cellular and molecular events of atherosclerosis. As discussed, E2 decreases VSMC proliferation via ERα, but this is blocked if ERβ is also activated. GPER has no effect on VSMC proliferation.(Ortmann et al., 2011) ERα and ERβ expression decrease in atherosclerosis, and given that ERα and ERβ activate eNOS, reduction in these receptors would be detrimental.(Nakamura et al., 2004) Another critical initial event in the development of atherosclerosis is abnormal monocyte adhesion and transmigration in the vasculature.(Libby, 2002; Gower et al., 2011) Here, too, estrogen plays a role.
Aging, Estrogen and Adhesion
Monocyte adhesion is a manifestation of inflammation and an essential step in atherosclerosis. Studies of estrogen and monocyte adhesion have been limited. Estrogen is known to inhibit VCAM-1 expression. TNF and LPS are commonly used to prime the system in order to assess the effect of another treatment on adhesion. In human saphenous vein endothelial cells, E2 inhibited VCAM-1 expression and blocked the increased adhesiveness of a monocyte cell line after LPS treatment.(Simoncini, De Caterina, & Genazzani, 1999) Pharmacologic concentrations of E2 inhibited LPS-induced VCAM-1 expression, and decreased U937 cell (monocytic cell line) adhesion to a monolayer of human saphenous vein endothelial cells.(Simoncini et al., 2000b) A more physiologic dose (for culture conditions), 10 nM, of E2 decreased TNF stimulated U-937 adhesion to cultured human umbilical vein endothelial cells ( HUVECs).(Rodriguez, Lopez, Paez, Masso, & Montano, 2002) Furthermore, E2 inhibited the increase in IL-8 and MCP-1 secretion into the media. E2 (10 nM) inhibited angiotensin II induced leukocyte adhesion via eNOS and cyclooxygenase activation.(Álvarez, Hermenegildo, Issekutz, Esplugues, & Sanz, 2002) Estrogen inhibited MCP-1 induced migration of THP-1 cells.(Yada-Hashimoto et al., 2006) The utilization of HUVECs as a model for many of these studies limits applicability of the results, as it is becoming increasingly apparent that HUVECs differ significantly in their responses from endothelial cells derived from adult arteries.(Tan et al., 2004; Lucas, Rose, & Morris, 2000)
A number of in vivo studies have provided further insight into the actions of estrogen on the vasculature. In a young in vivo rabbit model, E2 replacement at physiologic concentrations post ovx blocked increased monocyte adhesion and subendothelial migration in the setting of a diet-induced hyper-cholesterolemia.(Nathan, Pervin, Singh, Rosenfeld, & Chaudhuri, 1999) E2 also blocked the induction of MCP-1 in this model.(Pervin et al., 1998) Similarly, 12 months of hormone replacement treatment in post-menopausal women decreased VCAM-1 and e-selectin.(Yeboah, Klein, Brosnihan, Reboussin, & Herrington, 2008) Thus, E2 can block rolling, adhesion and transmigration induced by LPS, TNF and hypercholesterolemia. Many endothelial cell studies use high concentrations of E2 (nM vs. pM in plasma samples), a common issue for endothelial cell culture studies, as discussed above. There are some studies that suggest that tissue levels of E2 may be significantly higher than plasma levels, possibly related to aromatase, but this has yet to be proven.(Nickenig et al., 1998) Thus it is not known if similar effects to those seen with nanomolar concentrations of E2 would be seen in vivo with physiologic concentrations in the picomolarrange.
Summary
Estrogen has key actions in the vasculature enhancing relaxation through increased NO production, inhibiting VSMC proliferation, and decreasing adhesion molecule expression and monocyte adhesion. E2 also protects endothelial cells and EPCs from injury and apoptosis, thus maintaining the integrity of the vasculature. However the expression of ERα and ERβ is decreased in atherosclerosis, reducing the positive effects of estrogen in diseased vasculature.(Nakamura et al., 2004)
7. Estrogen and the Heart
Gender Differences in Cardiac Disease
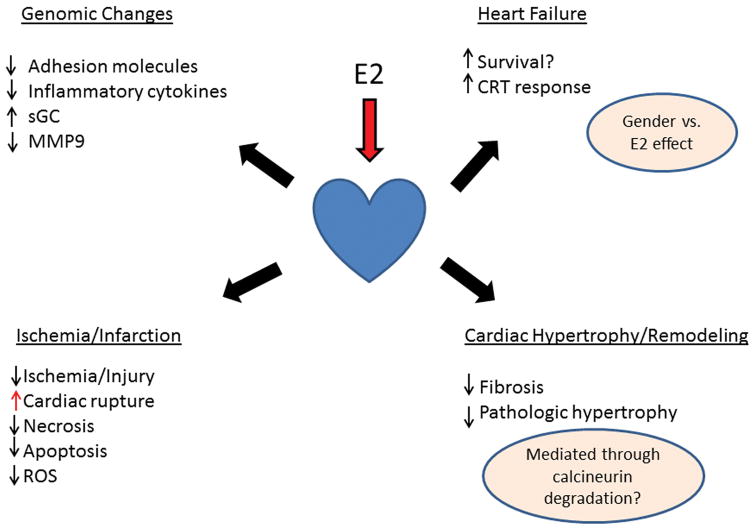
Premenopause women have a much lower rate of myocardial infarction than men, but post-menopause the rate of atherosclerosis and infarction greatly increases. Although estrogen is well known to improve lipid profiles, as discussed, other factors are involved in the gender differences in cardiovascular mortality (Figure 4). For cardiac rupture the gender differences are reversed. Female patients with myocardial infarction are more likely to have cardiac rupture, an often deadly complication.(Wehrens & Doevendans, 2004; Shapira, Isakov, Burke, & Almog, 1987) In animal models of myocardial infarction, rupture is exceedingly rare, except in the male mouse where it can be quite common. In the mouse several different mechanisms have been identified for increased cardiac rupture in male mice. Testosterone increased cardiac dilation and neutrophil infiltration in intact males mice and in female mice, compared to castrated male mice.(Cavasin, Tao, Yu, & Yang, 2006) Similarly, intact female mice had less inflammation, more myofibroblast transformation and more neovascularization at the border zone than male mice after infarct.(Wang et al., 2012a) Other investigators have demonstrated that this reversal of the gender ratio of rupture in the mouse is strain dependent and related to higher blood pressure in the male mice.(van den Borne et al., 2009) So, the etiology of the increase in cardiac rupture post-MI in male mice is multi-factoral.
Figure 4.
Summary of key cardiac effects of E2. Questions remain with regards to the effect of gender vs. estrogen on heart failure. Inhibition of fibrosis and pathologic cardiac hypertrophy is thought to be mediated through ERβ leading to calcineurin degradation.
Heart Failure, Gender and Estrogen
Heart failure with decreased systolic function is often a sequella to myocardial infarction (s). A number of studies beginning in the late 1990’s and early 2000’s suggested a gender difference in systolic heart failure with improved course and survival in females (Table II).(O’Meara et al., 2007; Adams et al., 1999) A retrospective analysis of the Vesnarinone database demonstrated improved survival for women on estrogen compared to women not taking estrogen, while analysis of the CHARM database showed overall improved survival for women, but no difference between those on HRT and those not.(Reis et al., 2000; O’Meara et al., 2007) Some studies have found no gender difference in heart failure survival.(Opasich et al., 2000) A pooled analysis of data from 5 randomized controlled heart failure trials also supported longer survival for female patients.(Frazier et al., 2007)
Table II.
Summary of reported analyses of heart failure clinical trial databases for gender related differences in survival.
| Study | Year | Total Patients | %F | F/U Yrs. | Overall Survival | CV | HF | ICM | DCM | Population | Hormone Status | ref # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Database | 1999 | 471 | 23.8 | 1.5 | F>M | F>M | NS | F>M | end-stage HF | none | 1 | |
| New Idiopathic Cardiomyopathy | 2000 | 1230 | 40 | 4.4 | F>M | F>M | New DCM only | none | 2 | |||
| Charm Database | 2007 | 7599 | 31.6 | 3.2 | F>M | F>M | F>M | F>M | Systolic & Diastolic HF | No difference HRT vs. No HRT | 3 | |
| 5 Pooled Trials | 2007 | 11642 | 24.5 | 0.6–2.5 | F>M | F>M | F>M | F>M | systolic HF | none | 4 | |
| Cleveland Clinic Scar & Survival | 2009 | 339 | 24 | 4 | M>F | M>F | M>F | NA | established CAD/MI only | none | 5 | |
| CV Health Study Database | 2009 | 1264 | 51 | 3 | F>M | F>M | ND | 65 or over Diastolic & systolic HF 80% dead by 3 yrs | none | 6 | ||
| Digitalis Trial Group Database | 2009 | 3338 | 50 | 3.2 | F>M | F>M | ND | HF | none | 7 | ||
| HF Clinic Database | 2011 | 531 | 26 | 1 | F>M | ND | outpatient, systolic & valve disease | none | 8 |
HF -heart failure; F -female; M -male; ND -not done; NA -Not applicable; DCM -dilated cardiomyopathy (not ischemic); ICM -ischemic cardiomyopathy; column on far right, numbers correlate with following references: 1. Adams et al., 1999; 2. Kong et al., 2004; 3. O’Meara et al., 2007; 4. Frazier et al., 2007; 5. Kwon, Halley, & Popovic, 2009; 6. Parashar et al., 2009; 7. Ahmed et al., 2011; 8. Feldman et al., 2011.
The clinical data is difficult to draw conclusion from, given that almost all of the data is retrospective analysis of databases collected for other reasons and that only two databases had any estrogen/HRT use data. One might theorize that the lack of difference between those receiving HRT and those not in the CHARM database would suggest no benefit from estrogen replacement per se, but a gender related benefit. The type of estrogen replacement is important, and benefit might not be seen with CEE, but again there is little or no data on actual estrogen therapy. One might hypothesize that women had better compliance with medications leading to a better outcome in these studies, but the CHARM study found women were less compliant than men with medications. The combination of systolic and diastolic dysfunction (normal ejection fraction) further complicates analysis of some of the trials. These are quite distinct diseases, although data from the Mayo Clinic suggests similar prognosis with a 50% five year survival.(Owan et al., 2006) Furthermore, some of the trials followed patients for a year or less, which is too short a time to detect survival differences. Overall studies on women and survival with heart failure are limited by the lack of data on estrogen status or the use of HRT in many of these reports (see Table II). Most of the patients in the study groups are post-menopausal, based on age, but patients may be using HRT, and this information is important for understanding the role of hormones vs. gender in prolonged survival. Overall the data is insufficient to definitively conclude there is a gender benefit in survival with systolic heart failure.
There are a number of other gender differences in systolic heart failure. Women are more likely to improve with cardiac resynchronization therapy (CRT) than men, and these findings have been supported by a number of studies, but whether this is related to estrogen treatment vs. gender is unknown.(Verhaert et al., 2010) Explanted end-stage failing hearts, removed at the time of transplant, were examined for the amount of cardiac myocyte apoptosis vs. necrosis. Overall cell death was two-fold higher in male hearts vs. female with significant increases in both apoptosis and necrosis.(Guerra et al., 1999) Of course both male and female end-stage failing hearts had greater apoptosis and necrosis than normal hearts. The gene expression profile was examined in new onset heart failure to determine if there was a gender difference early on in the disease.(Heidecker et al., 2010) Surprisingly, comparing male to female gene expression based on cardiac biopsies from patients with idiopathic dilated cardiomyopathy (no significant coronary disease), identified only 35 transcripts that were over-expressed in males and 16 that were down-regulated. Most of the over-expressed genes related to the Y chromosome, including CD24, which helps regulate the balance between immune defense and autoimmunity. Female hearts had increased expression of XIST and an X-linked zinc finger protein. Three other genes of interest, which were increased in females, were GATAD1(binds proteins regulating adrenergic/angiotensin receptor intracellular trafficking), SLC2A12 (GLUT12, a glucose transport) and PDE6b (cardio-protective effects). These findings begin to elucidate possible molecular mechanisms by which gender could influence survival with heart failure.
As a whole, basic investigations into gender and heart failure have been limited. ERα has been found to be upregulated almost two-fold in nonischemic cardiomyopathy regardless of gender.(Mahmoodzadeh et al., 2006) In normal human hearts ERα localized to the cytosol, sarcolemma, intercalated discs, and nuclei. In end-stage heart failure neither ERα or connexin 43 were found at the intercalated discs, and the investigators proposed that ERα has a role in phosphorylation of connexin 43, which is normally in the disc, and loss of ERα localization to the disc led to loss of connexin 43 phosphorylation and localization to the disc.
Myocytes
E2 indirectly regulates cardiac HSP 72 expression in both male and female adult rat cardiac myocytes.(Knowlton & Sun, 2001), (Hamilton, Gupta, & Knowlton, 2004; Voss et al., 2003) In addition to its role in protein folding, HSP72 has cardioprotective effects in transgenic models, and reduces apoptosis by stabilizing the mitochondrial membrane and preventing apoptosome formation.(Marber et al., 1995; Trost et al., 1998; Suzuki et al., 2002) In isolated adult Sprague Dawley female cardiomyocytes, E2 treatment, at pharmacologic levels, increased the expression of HSP 72 through sequential activation of the transcription factors, NFκB and HSF-1.(Hamilton et al., 2004) E2 pre-treatment also protected against hypoxia/reoxygenation.(Knowlton & Sun, 2001; Hamilton et al., 2004) Although E2 can induce cardiac HSP 72 expression in adults, little is known how the loss of estrogen that occurs naturally during menopause in aging female rats affects the cardiac HSP response and the adaptive response to stress.
To better understand the effects of estrogen in aging cardiac myocytes, we investigated the effect of immediate estrogen replacement in cardiac myocytes derived from aged (22 mo) and adult (6 mo) NB rats 9 weeks post ovx. Both groups of adult cardiac myocytes showed activation of NFκB and increased HSP72 expression with estrogen treatment.(Stice et al., 2011) However, neither aged group had a response, and in fact NFκB was activated at baseline in the aged ovx cardiac myocytes. The heat shock response is normally activated in response to hypoxia and reoxygenation, and this is followed by increased HSP expression, a protective response. In both groups of adult cardiac myocytes this response was seen, but in the aged cardiac myocytes regardless of estrogen replacement there was no activation of HSF1 and no increase in HSP72. HSF1 expression was not changed, but in the aged ovx phosphorylation at serine 303/307, which inhibits activation of transcription, but not binding to the promoter by HSF1, was present.(Stice et al., 2011)
IL-6 and TNF levels did not differ amongst the adult and aged rats with and without E2 replacement; however, in culture, much higher expression of IL-6 and TNF occured at the mRNA level. This increase in IL-6 and TNF could be ameliorated by acute treatment with estrogen. The aged ovx cardiac myocytes had a greatly impaired ability to handle ROS, the production of which is known to increase with age.(Ungvari et al., 2008; Podlutsky, Ballabh, & Csiszar, 2010) Thus for the cardiac myocytes there was a loss of the heat shock response, and increase in inflammatory cytokine expression and a markedly reduced ability to handle ROS with aging and loss of estrogen. Estrogen replacement improved many of these changes.
Summary
In the heart, there is a possible gender difference in heart failure morbidity and mortality, but it is premature to draw conclusions. However, female patients with heart failure have a greater response to cardiac resynchronization therapy than males. Some gender differences have been identified in gene expression early in the course of nonischemic cardiomyopathy, but more work is needed to determine if these differences are a product of gender, the presence of estrogen, or the etiology of nonischemic cardiomyopathy, which encompasses a wide variety of diagnoses with often the etiology unknown. ERα expression is double in heart failure regardless of gender. At the cellular level, E2 increases expression of the protective heat shock proteins and improves cardiac myocyte survival. In vivo studies have confirmed these findings in the intact heart in a trauma hemorrhage model. In contrast, with aging there is a loss of the heat shock response with inactivation of the transcriptional activity of HSF1, which controls the heat shock response. The aged rat has increased NFκB activity regardless of estrogen status. Aged cardiac myocytes from ovx rats without estrogen replacement have increased cytokine production and markedly increased ROS. Thus aging is associated with increased inflammation, and some of this can be offset by E2 replacement.
8. Estrogen, the Extracellular Matrix and Cardiac Remodeling
Following injury such as myocardial infarction, the heart undergoes extensive adverse remodeling. This is also seen in the pathologic (vs. physiologic hypertrophy with exercise or pregnancy) hypertrophy associated with hypertension. Prevention or modification of this pathologic hypertrophy can have significant benefits in terms of symptom relief and potentially prolongation of survival. Estrogen has a significant and important effect on myocardial hypertrophy and remodeling, which is just beginning to be understood.
MMPs and TIMPs
Estrogen overall has been found to have an inhibitory effect on MMP expression, although there is cell and tissue variation. In adult rat cardiac fibroblasts (9–11 week old Wistar rats), E2 inhibited MMP-2 expression.(Mahmoodzadeh, Dworatzek, Fritschka, Pham, & Regitz-Zagrosek, 2010) This effect was nongenomic, mediated by rapid signaling from ERα via the MAP kinase pathway and ERK 1/2 to phosphorylate Elk1, which acted as a repressor of MMP-2 transcription. The inhibition of MMP-2 transcription by E2 was blocked by ICI 182,780 and by PD98059. In vivo E2 blocked MMP-9 activation, but not MMP-2 in a cardiac volume overload model (aortocaval fistula) in 8 week old female SD rats with ovx with and without E2 replacement.(Voloshenyuk & Gardner, 2010) E2 decreased the increase in the TIMP-2/MMP-2 ratio in acute and chronic volume overload. Ovx with volume overload was associated with decreased MMP-2 and ER expression as well as upregulation of MMP-9 and MT1-MMP. E2 replacement also prevented perivascular fibrosis in the volume overloaded ovx rats. However, in cultured human smooth muscle cells, derived from an 11 mo old female, estrogen had no effect on MMP2 or MMP3 expression.(Natoli et al., 2005) Overall these studies support that in adult models E2 inhibits MMP2 expression, MMP9 activation and reduces fibrosis.
Fibrosis
A number of studies have demonstrated that E2 ( 1 nM – 1uM ) can inhibit angiotensin II mediated neonatal cardiac fibroblast proliferation, and that this is blocked by the ER receptor antagonist, ICI182,780.(Zhou, Shao, Huang, Yao, & Lu, 2007; Pedram, Razandi, O’Mahony, Lubahn, & Levin, 2010) Angiotensin II treatment increased the synthesis of type I and III collagen 5-fold, and this also was inhibited by E2.(Zhou et al., 2007) Ovx is associated with increased ACE activity and AT1R binding densities.(Dean, Tan, O’Brien, & Leenen, 2005) E2 replacement post ovx prevented these changes. Similarly, estrogen and progesterone inhibited collagen deposition in cultured human smooth muscle cells.(Natoli et al., 2005) Both also markedly increased elastin deposition. Likewise, in the volume overloaded ovx rat heart, immediate E2 replacement was associated with decreased perivascular fibrosis compared to ovx without replacement.(Voloshenyuk & Gardner, 2010) Angiotensin II and endothelin-1 activated TGFβ and stimulated the transition from cardiac fibroblast to myofibroblast.(Pedram et al., 2010) Fibronectin, vimentin and collagens I and III were produced. E2 or DPN, an ERβ agonist, blocked these changes. Activation of cAMP and PKA were important steps in preventing the transition to myofibroblast and activation of cJNK, a key element in fibrosis. Similar changes were found in vivo where angiotensin II treatment in ovx mice led to cardiac hypertrophy and fibrosis, which could be inhibited by E2 treatment of WT ovx mice, but not in the ERβ KO ovx mice.(Pedram et al., 2010) Thus loss of estrogen promotes increased Angiotensin II and fibrosis.
Estrogen and Pathologic Cardiac Hypertrophy
Cardiac hypertrophy is more prevalent in males. WT, ERα and ERβ knockout mice underwent ovx with half receiving E2 replacement. E2 attenuated TAC (transverse aortic constriction) induced cardiac hypertrophy in ERα-/-, but not in ERβ −/− mice.(Babiker et al., 2006) Reduction in TAC induced hypertrophy by E2 treatment had no effect on cardiac function. Interestingly, uterine growth was blocked by ERα knockout, but not by ERβ knockout. In WT and ERα, but not ERβ, knockout mice, E2 replacement blocked increased phosphorylation of p38 and increased ANP expression, as well as reducing hypertrophy. In studies of intact female mice (no ovx) vs. male mice, males had greater hypertrophy and more heart failure 9 wks post TAC. TAC was associated with increased expression of matrix and mitochondrial genes, and these changes were greater in males than females. ERβ, but not ERα, knockout, resulted in greater hypertrophy, including increased cardiac myocyte diameter, in both males and females.(Fliegner et al., 2010) ERβ knockout mice also had a greater induction of pro-apoptotic genes, and this was more pronounced in males than females.
Several recent studies have found that estrogen increases degradation of calcineurin via an ER dependent pathway, and this pathway appears to be only activated by ERβ(Pedram et al., 2008;. Donaldson et al., 2009) E2 signaling targets calcineurin for increased degradation via ubiquitination and the 26s proteasomal system. In calcineurin −/− mice, E2 had no effect on LV hypertrophy.(Donaldson et al., 2009) Loss of calcineurin removes downstream signaling via the NFAT family of transcription factors, which are major targets of calcineurin, suggesting that this may be a major pathway for the inhibition of hypertrophy by E2.
Summary
Overall, estrogen has been found to attenuate cardiac hypertrophy in numerous studies ranging from human to mouse. E2 has positive effects on cardiac remodeling. Multiple different studies indicate that ERβ mediates the beneficial effects of E2 on ventricular remodeling.
10. Mitochondria and Estrogen Receptors: Signaling Role?
A number of studies indicate that estrogen may affect the cardiac mitochondria directly. In addition to their presence in the nuclei and plasma membrane, ERα and ERβ also localize to the mitochondria of a number of cell types and tissues in both males and females. ERβ has been studied in a wider variety of cells, including rat primary cardiomyocytes.(Yang et al., 2004; Simpkins, Yang, Sarkar, & Pearce, 2008; Pedram, Razandi, Wallace, & Levin, 2006) The known actions of estrogen on mitochondria are predominately carried out by ERβ, and most of the studies have focused on this receptor.
Transcription of mitochondrial proteins, particularly those encoded in mitochondrial DNA (mtDNA), can be regulated by ERβ(Simpkins et al., 2008; O’Lone et al., 2007; Chen,. Yager, & Russo, 2005) E2 treatment increases transcript levels of many mtDNA genes in various cell types. In addition, electron transport chain activity and O2 consumption are increased with estrogen treatment.(Chen et al., 2005) In a trauma-hemorrhage model, E2 treatment led to increased complex IV expression and activity, but not complex I.(Hsieh et al., 2006b) As complex IV (cytochrome c oxidase) is encoded by mtDNA while complex I is encoded in nuclear DNA, this suggests that estrogen is effecting mitochondrial(mt) gene transcription.
Nuclear respiratory factor (NRF)-1, a critical transcription factor for nuclear encoded mitochondrial genes, has increased expression following E2 treatment. Expression of mitochondrial transcription factor A (Tfam), a key mtDNA transcription factor, is modulated by NRF-1. Increases in Tfam expression lead to increased transcription of mtDNA encoded genes. These changes were blocked by ER antagonists, but not PI3K or MAPK inhibitors, demonstrating that this regulation is mediated by activation of ERs by estrogen.(Mattingly et al., 2008) Though this finding was in cancer cell lines derived from breast and lung, similar findings are likely in other tissues. The presence of estrogen in female hearts (or 2 week pre-treatment of males) caused increased phosphorylation and activity of aldehyde dehydrogenase (ALDH) 2 as well as the phosphorylation of α-ketoglutarate dehydrogenase (α-KGDH) post I/R injury. ALDH2 metabolizes toxic ROS-generated adducts, while the increased phosphorylation of α-KGDH is believed to reduce ROS production.(Lagranha, Deschamps, Aponte, Steenbergen, & Murphy, 2010)
E2 has cardioprotective effects after injury, and much of this is likely mediated via the mitochondria. E2 treatment prevented cytochrome c release from isolated heart mitochondria subjected to high calcium levels.(Morkuniene, Jekabsone, & Borutaite, 2002) Similar results were found in an isolated perfused heart study using global ischemia; in addition treatment with E2 improved mitochondrial respiration and prevented DNA strand breaks.(Morkuniene, Arandarcikaite, & Borutaite, 2006) In cultured neonatal myocytes, after hypoxia and reoxygenation, E2 reduced apoptosis and attenuated ROS production by inhibiting p53 phosphorylation and translocation to mitochondria.(Liu et al., 2011) In global ischemia of isolated perfused hearts, the benefits of estrogen (decreasing cytochrome c release, caspase 3-like activity and DNA strand breaks) were largely abolished by inhibition of protein kinase G, though benefits also decreased with inhibition of Akt and NOS.(Morkuniene, Arandarcikaite, Ivanoviene, & Borutaite, 2010) Estrogen protection in a trauma-hemorrhage model was mediated via ERβ, activation of which attenuated the reduction of cardiac mitochondrial ATP, prevented lipid accumulation and normalized levels of a number of proteins; of these, inhibition of PGC-1α abrogated the cardioprotective effects, via a Tfam dependent pathway.(Hsieh et al., 2006a)
11. Estrogen, Ischemia and Cardiac Protection
There is a substantial literature demonstrating that estrogen can protect the heart from ischemic injury and this has been the subject of several excellent reviews.(Deschamps, Murphy, & Sun, 2010; Booth & Lucchesi, 2008; Ostadol, Netuka, Maly, Besik, & Ostadalova, 2009) Table III summarizes the results of many of the studies investigating whether estrogen protects against cardiac injury. Both ERα and ERβ have been shown to mediate cardiac protection against ischemia/reperfusion injury through both knockout models and the use of selective agonists (see Table III).(Wang et al., 2006; Wang et al., 2009; Deschamps et al., 2010; Zhai, Eurell, Cooke, Lubahn, & Gross, 2000; Booth, Marchesi, Kilbourne, & Lucchesi, 2003) Recent work has identified a third, membrane associated ER, G-protein-coupled estrogen receptor (GPER).(Deschamps & Murphy, 2009) Activation of GPER with a selective agonist (G-1) reduced cardiac infarct size and improved functional recover in a Langendorff model. GPER activated Akt and ERK 1/2, similar to ERα.(Simoncini et al., 2000a) Although there is some disagreement, it appears that both ERα and ERβ activate PI3K, Akt and a protective, anti -apoptotic signaling cascade.(Wang et al., 2009)
Table III.
Summary of studies investigating protective effect of E2 in myocardial and myocyte injury. Table limited primarily to studies of ischemia, MI, hypoxia/reoxygenation for isolated cardiac myocytes.
| timing | species | strain | sex | age | injury | endpoint | E2 effect | reference |
|---|---|---|---|---|---|---|---|---|
| 9 wk, 8 wk, 6 wk | mice | CBL | f | 8–10 wk | MI | infarct size | improved | 1 |
| 10 wk, 10 wk, 8wk | rat | wistar | f | 12 wk | MI | hemodynamics, infarct size | improved | 2 |
| 10 wk, 10 wk, 8 wk | rat | wistar | f | 160–180g | MI | infarct, hemodynamics | no effect | 3 |
| N/A, N/A | mice | CBL | m/f | 12–15 wk | MI | infarct, survival, remodeling | mixed | 4 |
| unknown, 60 min | rabbit | NZW | f | 2.6–3.2 kg | MI | infarct size, morphology | improved | 5 |
| 3 wk, 2 wk | rat | SD | m/f | 11–13 wk | MI | infarct size | improved | 6 |
| 14 d, 7 d | mice | CBL | f | 6–8 wk | MI | infarct size, apoptosis | mixed | 7 |
| N/A, 0, 0 | dog | I/R | infarct size | improved | 8 | |||
| 21 d, 14 d | mice | CBL | f | 8 wk | isch | infarct size | improved | 9 |
| 33 d, 5 d | rat | SD | f | 2 mo | isch | pressure measurements | improved | 10 |
| 1 mo, 1 mo | rat | SD | f | 12–14 mo | isch | cardiac work | improved | 11 |
| N/A, N/A | mice | CBL; ERbKO | m/f | 16 wk | isch | LVDP | improved | 12 |
| N/A, 10 min | rat | SD | m/f | 200–350 g | isch | infarct size | improved | 13 |
| 10 wk, 10 wk | rat | SD | f | 6 mo | isch | LV function, morphology | improved | 14 |
| 1 mo, 1 mo | rat | SD | f | 11–12 mo | isch | remodeling | improved | 15 |
| N/A, cult | rat | wistar | 2 day | chem | apoptosis | improved | 16 | |
| N/A, cult | rat | SD | 1–3 day | H/R | apoptosis | improved | 17 | |
| N/A, cult | rat | SD | 1–3 day | H/R | apoptosis | improved | 18 | |
| N/A, 2 hr, 2 hr | rat | SD | m | 275–325 g | T/H | function, apoptosis, PI3K levels | mixed | 19 |
| N/A, 0, 0 | rat | SD | m | 275–325 g | T/H | function | improved | 20 |
Timing: ovx, E2 administration, injury; time before death. Abbreviations: Cult=day 1culture, SD=Sprague-dawley, NB=Norway-brown, CBL=C57BL/6, m=male, f=female, m/f=both, MI=in vivo ischemia, I/R=ischemia/reoxygenation, isch=global ischemia, H/R=hypoxia/reoxygenation, T/H=trauma/hemorrhage, chem=treated with staurosporine, d=day, wk=week, mo=month
Column on far right lists numbers for following references: 1. Eickels, M., Patten, R., et al. 2003. 2. Beer, S., Reincke, M., et al. 2007. 3. Hügel, S., Reincke, M., et al. 1999. 4. Chen, Q., Williams, R., et al. 2010. 5. Booth, E., Marchesi, M., et al. 2003. 6. Lagranha, C., Deschamps, A., et al. 2010. 7. Patten, R., Pourati, I., et al. 2004. 8. Node, K., Kitakaze, M., et al. 1997. 9. Lin, J., Steenbergen, C., et al. 2009. 10. Bolego, C., Rossoni, G., et al. 2010. 11. Xu, Y., Armstrong, S., et al. 2004. 12. Wang, M., Wang, Y., et al. 2009. 13. Deschamps, A., & Murphy, E. 2009. 14. Zhai, P., Eurell, T., et al. 2000. 15. Xu, Y., Arenas, I., et al. 2003. 16. Pelzer, T., Schumann, M., et al. 2000. 17. Kim, J., Pedram, A., et al. 2006. 18. Liu, H., Pedram, A., et al. 2011. 19. Yu, H., Hsieh, Y., et al. 2007. 20. Ba, Z., Hsu, J., et al. 2008.
Estrogen and Protection in Trauma Hemorrhage
A single treatment of high dose estrogen has been found to reduce morbidity and mortality in a male rat model of trauma.(Yu & Chaudry, 2009) Multiple variables including inflammatory cytokine levels and heat shock protein levels are improved.(Szalay et al., 2006) Furthermore, heme oxygenase (HO)-1 levels are increased via E2 activation of AKt. (Hsu et al., 2009) In other work, female rats at peak estrus were found to have improved morbidity and mortality with trauma.(Jarrar, Wang, Cioffi, Bland, & Chaudry, 2000) More recently female patients less than 50, suggesting the presence of estrogen, have been shown to have better outcomes and less complications than males of similar age following trauma.(Frink et al., 2007) Such findings are intriguing, but a true randomized trial of single dose estrogen treatment after trauma, such as studied extensively by Chaudry in the rodent model, is needed to prove the value of this intervention.
12. Experimental Modelsand Issues
Aging and estrogen status are inexorably linked. Estrogen loss occurs with aging. Menopause has onset on average at age 54, middle age in women, who generally are expected to live into their 80’s (www.socialsecurity.gov). However basic estrogen research predominantly uses adolescent models, for example 6–8 week old mice. The relevance of these models to changes that occur with aging is questionable. Even elective ovx for medical indications occurs most commonly in a middle aged population. Thus, it is important to study models that address both aging as well as changes in estrogen.
A second issue is the knockout models. The first ERα and ERβ knockout models were created very early after the development of this powerful technique. The Chapel Hill ERα knockout mouse, also known as the Lubahn model, has a residual splice variant or truncated ERα(Couse et al., 1995). This splice variant has ER activity, and thus the ERa knockout is incomplete. The Strasbourg ERα knockout mouse used later techniques, which resulted in full deletion of ERα. (Dupont et al., 2000) A colony of the Chapel Hill ERβ knockout mouse was transferred to the Karolinska Institute; these mice, while retaining the genetics of the Chapel Hill mouse, demonstrated a different phenotype, while subsequently generated ERβ knockouts (Strasbourg and Wyeth) are very similar to the Chapel Hill mouse.(Harris, 2007) The various knockout models are summarized in Table IV.
Table IV.
Summary of ER knockout mice.
| Gene | Knockout | Mutation | Company | Original Paper | Names | Used by Review References |
|---|---|---|---|---|---|---|
| esr1 | ERα | disruption, exon 2 | Lubhan, 1993 | Chapel Hill (CH), αERKO | 1, 2, 3, 4 | |
| esr1 | ERα | disruption, exon 2 | Taconic (discontinued) | Lubhan, 1993 | 5 | |
| esr1 | ERα | disruption, exon 2 | Jackson Labs | Lubhan, 1993 | ||
| esr1 | ERα | deletion, exon 3 | Dupont, 2000 | Strasbourg (ST), ERαKO, Ex3αERKO | 6 | |
| esr2 | ERβ | disruption, | Krege, 1998 | Chapel Hill, | 2, 3, 7, 8 | |
| exon 3 | ERβKOCH, βERKO | |||||
| esr2 | ERβ | disruption, exon 3 | Taconic (discontinued) | Krege, 1998 | 5, 9, 10 | |
| esr2 | ERβ | disruption, exon 3 | Jackson Labs | Krege, 1998 | ||
| esr2 | ERβ | disruption, exon 3 | Krege, 1998 | Transfer to Karolinska Institute, ERβKOKI | ||
| esr2 | ERβ | disruption, exon 3 | Dupont, 2000 | Strasbourg, ERβKOST | 6 | |
| esr2 | ERβ | Translational block, post-codon 19 | Shughrue, 2002 | Wyeth, ERβKOWY | ||
| esr2 | ERβ | deletion, exon 3 | Antal, 2008 | ERβSTL-/L- | ||
| esr1+esr2 | ERα+ERβ | cross α, β KOCH | Couse, 1999 | Chapel Hill | 11, 12 | |
| esr1+esr2 | ERα+ERβ | cross α, β KOST | Dupont, 2000 | Strasbourg | ||
| esr1+esr2 | ERα+ERβ | cross αKOCH, βKOWY | Shughrue, 2002 | Wyeth |
Column on far right, numbers correlate with following references, which used the respective mouse models: 1. Couse, J., Curtis, S., et al. 1995. 2. Babiker, F., Lips, D. et al. 2006. 3. Pedram, A., Razandi, M. et al. 2008. 4. Zhai, P., Eurell, T. et al. 2000. 5. Jesmin, S., Mowa C., et al. 2010. 6. Darblade, B., Pendaries, C. et al. 2002. 7. Pelzer, T., Arias Loza, P., et al. 2005. 8. Pedram, A., Razandi, M. et al. 2010. 9. Wang, M., Wang, Y. et al. 2009. 10. Fliegner, D., Schubert, C., et al. 2010. 11. Lindberg, M., Moverare, S., et al. 2003. 12. Karas, R., Schulten, H., et al. 2001.
Agonists and Antagonists
There are a number of selective ER agonists and antagonists. The most commonly used agonists are PPT (4,4′,4′-(4-propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol), DPN (2,3-bis(4-hydroxyphenyl)-propionitrile), and G-1 ((″)-1-[(3aR*,4S*,9bS*)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c] quinolin-8-yl]-ethanone) for ERα, ERβ, and GPER, respectively. ICI 182,780 (7α,17β-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]estra-1,3,5(10)-triene-3,17-diol) is one of the most commonly used ER antagonists; it was developed as an anti-estrogen, but has been found to act as an antagonist for ERα and ERβ while as an agonist for GPER.(Meyer, Baretella, Prossnitz, & Barton, 2010) Antagonists for individual receptors include MPP (1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride), THC ((R,R)-5,11-diethyl-5,6,11,12-tetrahydro-2,8-chrysenediol) and the recently developed G15 (4-(6-Bromo-benzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolone). For most compounds however, GPER selectivity hasn’t been studied. In addition there are the various SERMs. The two most frequently used in studies are tamoxifen and raloxifene. The activity of these compounds as agonists and antagonists, including which receptor is affected, is dependent on the tissue. All of these common agonists and antagonists have a fairly high selectivity for one isoform over the other. These are usually the most selective compounds, though they are frequently not the most potent. The most commonly used agonists and antagonists, particularly those that have been used in vivo, are summarized in Table V. A comprehensive review of ER (ant)agonists from a medicinal chemistry standpoint has been written by Veeneman.(Veeneman, 2005)
Table V.
Summary of the most common and selective ER agonists and antagonists.
| name | agonist for | antagonist for |
|---|---|---|
| PPT | ERα | |
| 16α-LE2 | ERα | |
| DPN | ERβ | |
| 8β-VE2 | ERβ | |
| ERB-041 (WAY-202041) | ERβ | |
| WAY-202196 | ERβ | |
| WAY-200070 | ERβ | |
| G-1 | GPER | |
| ICI 182,780 | GPER | ERα, ERβ |
| ICI 164,384 | ERα, ERβ | |
| Fulvestrant | ERα, ERβ | |
| MPP | ERα | |
| (R,R)-THC | ERα (weak) | ERβ |
| G15 | GPER |
New Models
Non -surgical Ovary Shut-off-4-vinylcyclohexene diepoxide (VCD), which acts as an ovotoxin selective for primary and primordial follicles, treatment leads to ovarian failure as the mature follicle population is depleted through natural cycling.(Mayer et al., 2002; Van Kempen, Milner, & Water, 2011) This model is proposed to better mimic human menopause as the estrogen levels in the body decline gradually (simulating perimenopause) over several months while leaving the ovaries intact. A potential disadvantage of this model is the time taken to reach full menopause – approximately 110 days after the end of the 15 day treatment period. Studies thus far have been primarily in rodents, though cynomolgus macaques have also been demonstrated as a useful model.(Appt et al., 2006) Mice treated with VCD are commercially available from Jackson Laboratory (JAX VCD-Induced Menopause). Cardiovascular investigations using the VCD model have been very limited. Lesion development following a high cholesterol diet has been studied, and it was similar in OVX and VCD-treated mice; estradiol treatment was more effective at reducing lesion size in VCD-treated mice than OVX.(Mayer, Dyer, Eastgard, Hoyer, & Banka, 2005) This is a more natural model of menopause, but given the months it takes to reach menopause with VCD treatment, the costs to using this approach could be prohibitive. An excellent review of the strengths and weaknesses of the VCD model has been recently published.(Van Kempen et al., 2011)
12. Summary
Estrogens are complex hormones with pleiotropic effects. E2 is the most potent estrogen found in humans. Two ERs regulate the nuclear responses to E2, leading to significant changes in gene expression. Essential non-nuclear responses to E2 include eNOS activation with increased production of NO and activation of a cardio-protective signaling cascade including Akt and MAP kinases. Late estrogen replacement, similar to recent clinical trials, increased expression of inflammatory genes and those involved in early stages of atherosclerosis. E2 has a major impact on the vasculature, influencing vascular function, expression of adhesion proteins and the inflammatory state. Aging and estrogen loss are closely linked and lead to impaired vascular function, which can be improved with E2 replacement. E2 has a wide range of effects on the heart, including preventing apoptosis and reducing damage in settings of cardiac ischemia and reperfusion. Retrospective analyses of heart failure databases have suggested a gender benefit with longer survival in heart failure patients, but more rigorous primary investigations are needed to prove this point. On the other hand, more basic work into gender differences and estrogen in heart failure has led to some novel findings. E2 can protect both isolated cardiac myocytes and the intact heart from ischemia-related injury. In isolated aged cardiac myocytes, estrogen loss is associated with increased expression of inflammatory cytokines and increased susceptibility to ROS. After injury, such as myocardial infarction or hypertension, the heart undergoes pathologic remodeling, which leads to deleterious effects on cardiac function. E2 has an inhibitory effect on this adverse remodeling and the salutary effects of E2 appear to be mediated primarily through ERβ. Estrogen has numerous beneficial effects on the mitochondria including increased mitochondrial gene transcription and increased activation of proteins involved in ROS removal. Basic investigations of the use of a single pharmacologic dose of E2 for protection against injury have had intriguing results, suggesting another route by which estrogen can be beneficial.
Acknowledgments
Supported by a VA Merit Award (AAK), HHMI- MIG (ARL) and T32-86350 (ARL).
Abbreviations
- CEE
conjugated equine estrogen
- DPN
Dipropylnitrile, an ERβ agonist
- E2
17β-estradiol, the most potent form of estrogen
- ER
Estrogen receptor
- HRT
hormone replacement therapy
- HSF
heat shock factor
- HSP
heat shock protein
- HUVECs
Human umbilical vein endothelial cells
- mt
mitochondria
- NB
Norway brown rat strain
- NRF-1
Nuclear respiratory factor 1
- ovx
ovariectomy or ovariectomized
- ROS
reactive oxygen species
- sGC
soluble guanylyl cyclase
- SHR
spontaneously hypertensive rat strain
- TAC
transverse aortic constriction
- Tfam
mitochondrial transcription factor A
- VSMCs
vascular smooth muscle cells
Footnotes
Conflict of Interest Statement
The authors declare there are no conflicts of interest.
Reference List
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, et al. Palmitoylation-dependent Estrogen Receptor α Membrane Localization: Regulation by 17β-Estradiol. Molecular Biology of the Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KF, Sueta CA, Gheorghiade M, O’Connor CM, Schwartz TA, Koch GG, et al. Gender Differences in Survival in Advanced Heart Failure. Circulation. 1999;99:1816–1821. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- Ahmed MI, Lainscak M, Mujib M, Love TE, Aban I, Pina I, et al. Gender-related dissociation in outcomes in chronic heart failure: Reduced mortality but similar hospitalization in women. International Journal of Cardiology. 2011;148:42. doi: 10.1016/j.ijcard.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez A, Hermenegildo C, Issekutz A, Esplugues J, Sanz M. Estrogens inhibit angiotensin II-induced leukocyte-endothelial cell interactions in vivo via rapid endothelial nitric oxide synthase and cyclooxygenase activation. Circulation Research. 2002;91:1142–1150. doi: 10.1161/01.res.0000046018.23605.3e. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Gips S, Moldovan N, Wilhide C, Milliken E, Hoang A, et al. 17β-estradiol inhibits apoptosis of endothelial cells. Biochemical and Biophysical Research Communications. 1997;237:372–381. doi: 10.1006/bbrc.1997.7085. [DOI] [PubMed] [Google Scholar]
- Antal MC, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proceedings of the National Academy of Sciences. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appt S, Kaplan J, Clarkson T, Line CJ, Christian P, Hoyer P. Destruction of primoridial ovairan follicles in adult cynomolgus macaques after exposure to 4-vinylcyclohexene diepoxide: a nonhuman primate model of menopause transition. Fetility and Sterility. 2006;86:1210–1216. doi: 10.1016/j.fertnstert.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Chronic Tumor Necrosis Factor-{alpha} Inhibition Enhances NO Modulation of Vascular Function in Estrogen-Deficient Rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Armstrong SJ, Xu Y, Davidge ST. Tumor Necrosis Factor-{alpha} and Vascular Angiotensin II in Estrogen-Deficient Rats. Hypertension. 2006;48:497–503. doi: 10.1161/01.HYP.0000235865.03528.f1. [DOI] [PubMed] [Google Scholar]
- Ba Z, Hsu J, Chen J, Kan W, Schwacha M, Chaudry IH. Systematic Analysis of the Salutary Effect of Estrogen on Cardiac Performance After Trauma-Hemorrhage. Shock. 2008;30:585–589. doi: 10.1097/SHK.0b013e31816f1a45. [DOI] [PubMed] [Google Scholar]
- Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, et al. Estrogen Receptor β Protects the Murine Heart Against Left Ventricular Hypertrophy. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1524–1530. doi: 10.1161/01.ATV.0000223344.11128.23. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Marsh MS, Whitehead M, Sherwood R, Roberts L, Patel RK, et al. The effect of estrone on thrombin generation may explain the different thrombotic risk between oral and transdermal hormone replacement therapy. Journal of Thrombosis and Haemostasis. 2010;8:1736–1744. doi: 10.1111/j.1538-7836.2010.03953.x. [DOI] [PubMed] [Google Scholar]
- Barbacanne MA, Rami J, Michel JB, Souchard JP, Philippe M, Besombes JP, et al. Estradiol increases rat aorta endothelium-derived relaxing factor (EDRF) activity without changes in endothelial NO synthase gene expression: possible role of decreased endothelium-derived superoxide anion production. Cardiovascular Research. 1999;41:672–681. doi: 10.1016/s0008-6363(98)00254-5. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. American Journal of Epidemiology. 2007;166:506–510. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- Beer S, Reincke M, Kral M, Callies F, Strömer H, Dienesch C, et al. High-dose 17β-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Research in Cardiology. 2007;102:9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]
- Bolego C, Rossoni G, Fadini G, Vegeto E, Pinna C, Albiero M, et al. Selective estrogen receptor-αagonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilazation in the absence of uterotrophic action. The FASEB Journal. 2010;24:2262–2272. doi: 10.1096/fj.09-139220. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical Profiling of Nuclear Receptor Expression Reveals a Hierarachical Transcriptional Network. Cell. 2006;125:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011;76:877–884. doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Booth EA, Lucchesi BR. Estrogen-Mediated Protection in Myocardial Ischemia-Reperfusion Injury. Cardiovascular Toxicology. 2008;8:101–113. doi: 10.1007/s12012-008-9022-2. [DOI] [PubMed] [Google Scholar]
- Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17{beta}-Estradiol as a Receptor-Mediated Cardioprotective Agent. Journal of Pharmacology and Experimental Therapeutics. 2003;307:395–401. doi: 10.1124/jpet.103.054205. [DOI] [PubMed] [Google Scholar]
- Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition port opening and protects the heart against ischemia-reperfusion injury. American Journal of Physiology - Heart and Circulatory Physiology. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico M, Fournier A, Caracaillon L, Olié V, Plu-Bureau G, Oger E, et al. Postmenopausal Hormone Therapy and Risk of Idiopathic Venous Thromboembolism: Results From E3N Cohort Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:340–345. doi: 10.1161/ATVBAHA.109.196022. [DOI] [PubMed] [Google Scholar]
- Cavasin MA, Tao Z, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction causing rupture and degrading cardiac function. American Journal of Physiology - Heart and Circulatory Physiology. 2006;290:H2043–H2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a Modulator of Vascular Inflammation. Life. 2008;60:376–382. doi: 10.1002/iub.48. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, et al. Non-nuclear estrogen receptor-α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. The Journal of Clinical Investigation. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RGW, Mendelsohn ME, Shaul PW. ER{beta} Has Nongenomic Action in Caveolae. Molecular Endocrinology. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, et al. Estrogen Receptor {alpha} and Endothelial Nitric Oxide Synthase Are Organized Into a Functional Signaling Module in Caveolae. Circulation Research. 2000;87:44e–452. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- Chan G, Fiscus R. Exaggerated production of nitric oxide (NO) and increases in inducible NO-synthase mRNA levels induced by the pro-inflammatory cytokine interleukin-1β in vascular smooth muscle cells of elderly rats. Experimental Gerontology. 2004;39:387–394. doi: 10.1016/j.exger.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2005;1746:1–17. doi: 10.1016/j.bbamcr.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Chen Q, Williams R, Healy C, Wright C, Wu S, O’Connell T. An association between gene expression and better survival in female mice following myocardial infarction. J Mol Cell Cardiol. 2010;49:801–811. doi: 10.1016/j.yjmcc.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo A, et al. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Research Reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Molecular Endocrinology. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiological Genomics. 2005;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta E, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. Journal of Applied Physiology. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan P, Cheung JCY, Scriven DRL, Moore EDW. Epitope-dependent localization of estrogen receptor α, but not-β in en fase arterial endothelium. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1295–H1306. doi: 10.1152/ajpheart.00781.2002. [DOI] [PubMed] [Google Scholar]
- Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, et al. Estradiol Alters Nitric Oxide Production in the Mouse Aorta Through the α-, but not the β-, Estrogen Receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- Dean SA, Tan J, O’Brien ER, Leenen FHH. 17{beta}-Estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. AJP - Regulatory, Integrative and Comparative Physiology. 2005;288:R759–R766. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E, Sun J. Estrogen Receptor Activation and Cardioprotection in Ischemia Reperfusion Injury. Trends in Cardiovascular Medicine. 2010;20:73–78. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessy C, Feron O, Balligand JL. The regulation of endothelial nitric oxide synthase by caveolin: a paradigm validated in vivo and share by the ‘endothelium-derived hyperpolarizing factor’. Pflugers Archives - European Journal of Physiology. 2010;459:817–827. doi: 10.1007/s00424-010-0815-3. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, et al. Estrogen Attenuates left Ventricular and Cardiomyocyte Hypertrophy by an Estrogen Receptor-Dependent Pathway That Increases Calcineurin Degradation. Circ Res. 2009;104:265–275. doi: 10.1161/CIRCRESAHA.108.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008;7:805–812. doi: 10.1111/j.1474-9726.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: Importance of timing of treatment and type of estrogen. Cardiovascular Research. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ. Clincally Used Estrogens Differentially Inhibit Human Aortic Smooth Muscle Cell Growth and Mitogen-Activated Protein Kinase Activity. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:964–972. doi: 10.1161/01.atv.20.4.964. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Archives - European Journal of Physiology. 2010;459:841–851. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, et al. Genome-wide Orchestration of Cardiac Functions by the Orphan Nuclear Receptors ERRα and γ. Cell Metabolism. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gasmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Favre J, Gao J, Henry JP, Remy-Jouet I, Fourquaux I, Billon-Gales A, et al. Endothelial Estrogen Receptor αPlays an Essential Role in the Coronary and Myocardial Protective Effects of Estradiol in Ischemia/Reperfusion. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:2562–2567. doi: 10.1161/ATVBAHA.110.213637. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Sylvester SL, Sarge KD, Morimoto RI, Holbrook NJ. Effects of neurohormonal stress and aging on the activation of mammalian heat shock factor 1. Journal of Biological Chemistry. 1994;269:32272–32278. [PubMed] [Google Scholar]
- Feldman DE, Ducharme A, Giannetti N, Frennette M, Michel C, Grondin F, et al. Outcomes for Women and Men Who Attend a Heart Failure Clinic: Results of a 12-Month Longitudinal Study. Journal of Cardiac Failure. 2011;17:540–546. doi: 10.1016/j.cardfail.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Figtree GA, McDonald D, Watkins H, Channon KM. Truncated Estrogen Receptor {alpha} 46-kDa Isoform in Human Endothelial Cells: Relationship to Acute Activation of Nitric Oxide Synthase. Circulation. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Archives - European Journal of Physiology. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, et al. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in presssure overload. AJP - Regulatory, Integrative and Comparative Physiology. 2010;298:R1597–R1606. doi: 10.1152/ajpregu.00825.2009. [DOI] [PubMed] [Google Scholar]
- Florian M, Freiman A, Magder S. Treatment with 17-β-estradiol reduces superoxide production in aorta of ovariectomized rats. Steroids. 2004;69:779–787. doi: 10.1016/j.steroids.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Frazier CG, Alexander KP, Newby K, Anderson S, Iverson E, Packer M, et al. Associations of Gender and Etiology With Outcomes in Heart Failure with Systolic Dysfunction. Journal American College of Cardiology. 2007;49:1450–1458. doi: 10.1016/j.jacc.2006.11.041. [DOI] [PubMed] [Google Scholar]
- Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of Sex and Age on Mods and Cytokines After Multiple Injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular Endothelial Estrogen Receptor α Is Modulated by Estrogen Status and Related to Endothelial Function and Endothelial Nitric Oxide Synthase in Healthy Women. Journal of Clinical Endocrinology and Metabolism. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V. To ERR in the estrogen pathway. Trends in Endocrinology and Metabolism. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Giguère V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocrine Reviews. 2008;124:789–799. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. American Heart Journal. 2003;146:S5–S12. doi: 10.1016/j.ahj.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement study follow-up (HERS II) JAMA: The Journal of the American Medical Association. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Clarkson T, Manson JE. Understanding the Divergent Data on Postmenopausal Hormone Therapy. New England Journal of Medicine. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- Grohé C, Kahlert S, Löbbert K, Vetter H. Expression of oestrogen receptor α and β in rat heart: role of local oestrogen synthesis. Journal of Endocrinology. 1998;156:R1–R7. doi: 10.1677/joe.0.156r001. [DOI] [PubMed] [Google Scholar]
- Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, et al. Myocyte death in the failing human heart is gender dependent. Circulation Research. 1999;85:856–866. doi: 10.1161/01.res.85.9.856. [DOI] [PubMed] [Google Scholar]
- Gutsmann-Conrad A, Heydari AR, You S, Richardson A. The expression of heat shock protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Experimental Cell Research. 1998;241:404–413. doi: 10.1006/excr.1998.4069. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFκB signaling. Journal of Molecular and Cellular Cardiology. 2004;36:577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Lin L, Wang Y, Knowlton AA. Effect of Ovariectomy on Cardiac Gene Expression: Inflammation and Changes in SOCS Genes Expression. Physiological Genomics. 2007;32:254–263. doi: 10.1152/physiolgenomics.00039.2007. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, Heat Shock Proteins, and NF{kappa}B in Human Vascular Endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- Harris HA. Estrogen Receptor-b: Recent Lessons from in Vivo Studies. Molecular Endocrinology. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-Kinase-Akt pathway in human endothelial cells. Circulation Research. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, et al. Src Kinase Mediates Phosphatidylinositol 3-Kinase/Akt-dependent Rapid Endothelial Nitric-oxide Synthase Activation by Estrogen. Journal of Biological Chemistry. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- Heidecker B, Lamirault G, Kasper EK, Wittstein IS, Champion HC, Breton E, et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. European Heart Journal. 2010;31:1188–1196. doi: 10.1093/eurheartj/ehp549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, et al. Estrogen Induces the Akt-dependent Activation of Endothelial Nitric-oxide Synthase in Vascular Endothelial Cells. Journal of Biological Chemistry. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Choudhry MA, Yu HP, Shimizu T, Yang S, Suzuki T, et al. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist-mediated cardioprotection following trauma-hemorrhage. The FASEB Journal. 2006a;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, et al. Upregulation of mitochondrial respiratorycomplex IV by estrogen receptor -β is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J Mol Cell Cardiol. 2006b;41:511–521. doi: 10.1016/j.yjmcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hsu JT, Kan WH, Hsieh CH, Choudry MA, Bland KI, Chaudry IH. Mechanisms of salutary effects of estrogen on cardiac function following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. Critical Care Medicine. 2009;37:2338–2344. doi: 10.1097/CCM.0b013e3181a030ce. [DOI] [PubMed] [Google Scholar]
- Hugel S, Reincke M, Stromer H, Winning J, Horn M, Dienesch C, et al. Evidence against a role of physiological concentrations of estrogen in post-myocardial infarction remodeling. Journal of the American College of Cardiology. 1999;34:1427–1434. doi: 10.1016/s0735-1097(99)00368-x. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA: The Journal of the American Medical Association. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen Causes Dynamic Alterations in Endothelial Estrogen Receptor Expression. Circulation Research. 2002;91:814–820. doi: 10.1161/01.res.0000038304.62046.4c. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. Journal of Physiology. 2011;589(9):2139–2145. doi: 10.1113/jphysiol.2011.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. AJP - Heart and Circulatory Physiology. 2000;279:H1015–H1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Sultana SN, Shimojo N, Togashi H, Iwashima Y, et al. VEGF signaling is disrupted in the hearts of mice lacking estrogen receptor alpha. European Journal of Pharmacology. 2010;641:168–178. doi: 10.1016/j.ejphar.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of Estrogen Receptor Variant ER-α36, Not GPR30, in Nongenomic Signaling. Molecular Endocrinology. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LS, Reyes R, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H1087–H1095. doi: 10.1152/ajpheart.00356.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas RH, Schulten H, Pare G, Aronovitz M, Ohlsson C, Gustafsson JA, et al. Effects of Estrogen on the Vascular Injury Response in Estrogen Receptor α,β (Double) Knockout Mice. Circ Res. 2001;89:534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- Keung W, Chan MLY, Ho EYW, Vanhoutte PM, Man RYK. Non-genomic activation of adenylyl cyclase and protein kinase G by 17β-estradiol in vascular smooth muscle of the rat superior mesenteric artery. Pharmacological Research. 2011;64:509–516. doi: 10.1016/j.phrs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen Prevents Cardiomyocyte Apoptosis through Inhibition of Reactive Oxygen Species and Differential Regulation of p38 Kinase Isoforms. Journal of Biological Chemistry. 2006a;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen Prevents Cardiomyocyte Apoptosis through Inhibition of Reactive Oxygen Species and Differential Regulation of p38 Kinase Isoforms. Journal of Biological Chemistry. 2006b;281:6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bender JR. Membrane-initiated actions of estrogen on the endothelium. Molecular and Cellular Endocrinology. 2009;308:3–8. doi: 10.1016/j.mce.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AA, Sun L. Heat shock factor-1, steroid hormones, and regulation of heat shock protein expression in the heart. American Journal of Physiology. 2001;280:H455–H464. doi: 10.1152/ajpheart.2001.280.1.H455. [DOI] [PubMed] [Google Scholar]
- Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, et al. Enhanced Inhibition of Neointimal Hyperplasia by Genetically Engineered Endothelial Progenitor Cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor b. Proceedings of the National Academy of Sciences. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon DH, Halley CM, Popovic ZB. Gender differences in survival in patients with severe left venricular dysfunction despite similar exent of myocardial scar mesured on cardiac magnetic resonance. European Journal of Heart Failure. 2009;11:937–944. doi: 10.1093/eurjhf/hfp118. [DOI] [PubMed] [Google Scholar]
- Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex Differences in the Phosphorylation of Mitochondrial Proteins Result in Reduced Production of Reactive Oxygen Species and Cardioprotection in Females. Circulation Research. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, et al. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. AJP - Regulatory, Integrative and Comparative Physiology. 2009;297:R1713–R1723. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends in Endocrinology and Metabolism. 2010;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proceedings of the National Academy of Sciences. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WX, Magness RR, Chen D. Expression of Estrogen Receptors-α and -β in the Pregnant Ovine Uterine Artery. Biology of Reproduction. 2005;72:530–537. doi: 10.1095/biolreprod.104.035949. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in Atherosclerosis. Nature. 2002;420:868–878. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lin J, Steenbergen C, Murphy E, Sun J. Estrogen Receptor -β Activation Results in S - Nitrosylation of Proteins Involved in Cardioprotection. Circulation. 2009;120:245–254. doi: 10.1161/CIRCULATIONAHA.109.868729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, et al. Estrogen Receptor (ER)-β Reduces ERα-Regulated Gene Transcription, Supporting a “Ying Yang” Relationship between ERα and ER β in Mice. Molecular Endocrinology. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 Agonist G-1 is Not Different from Estradiol in the mRen2. Lewis Female Rat. Journal of Cardiovascular Pharmacology. 2011;57:598–603. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Little PJ, Williams MR, Dai A, Hashimura K, Liu J, et al. High glucose abolishes the antiproliferative effect of 17β-estradiol in human muscle cells. American Journal of Physiology - Endocrinology And Metabolism. 2002;282:E746–E751. doi: 10.1152/ajpendo.00111.2001. [DOI] [PubMed] [Google Scholar]
- Liu H, Pedram A, Kim JK. Oestrogen prevents cardiomyocyte apoptosis by suppressing p38α-mediated activation of p53 and by down-regulating p53 inhibition on p38β. Cardiovascular Research. 2011;89:119–128. doi: 10.1093/cvr/cvq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke M, Tanguay RM. Diminished heat shock response in the aged myocardium. Cell Stress and Chaperones. 1996;1:251–260. doi: 10.1379/1466-1268(1996)001<0251:dhsrit>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DA, Newaz MA, Prabhakar SS, Price KL, Truong LD, Feng L, et al. Loss of nitric oxide and endothelial-derived hyperpolarizing factor-mediated responses in aging. Kidney Int. 2005;68:2154–2163. doi: 10.1111/j.1523-1755.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Rose PE, Morris AG. Contrasting effects of HSP72 expression on apoptosis in human umbilical vein endothelial cells and an angiogenic line, ECV304. British Journal of Haematology. 2000;110:957–964. doi: 10.1046/j.1365-2141.2000.02255.x. [DOI] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V. 17β-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovacular Research. 2010;85:719–728. doi: 10.1093/cvr/cvp350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, et al. Estrogen receptor alpha up-regulation and redistribution in human heart failure. The FASEB Journal. 2006;20:926–934. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. Journal of Clinical Investigation. 1995;95:1854–1860. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro ML, Peiró C, Panayiotou CM, Baliga RS, Meurer S, Schmidt H, et al. Characterization of the Human α1β1Soluble Guanylyl Cyclase Promoter. Journal of Biological Chemistry. 2008;283:20027–20036. doi: 10.1074/jbc.M801223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, et al. Estrogen-Mediated Endothelial Progenitor Cell Biology and Kinetics For Physiological Postnatal Vasculogenesis. Circulation Research. 2007;101:598–606. doi: 10.1161/CIRCRESAHA.106.144006. [DOI] [PubMed] [Google Scholar]
- Mattingly KA, Ivanova MM, Rigss KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Molecular Endocrinology. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L, Dyer C, Eastgard R, Hoyer P, Banka C. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1910–1916. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- Mayer L, Pearsall N, Christion P, Devine P, Payne C, McCuskey M, et al. Long-term effects of varian follicular depletion in rats by 4-vinylcyclohexene diepoxide. Reproductive Toxicology. 2002;16:775–781. doi: 10.1016/s0890-6238(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. Nongenomic, estrogen receptor-mediated activation of endothelial nitric oxide synthase. Circulation Research. 2000;87:677–682. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New England Journal of Medicine. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of Epicardial Coronary Arteries by the G Protein-Coupled Estrogen Receptor Agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovascular Research. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Morkuniene R, Arandarcikaite O, Borutaite V. Estradiol prevents release of cytochromec from mitochondria and inhibits ischemia-induced apoptosis in perfused heart. Experimental Gerontology. 2006;41:704–708. doi: 10.1016/j.exger.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Morkuniene R, Arandarcikaite O, Ivanoviene L, Borutaite V. Estradiol-induced protection against ischemia-induced heart mitochondrial damage and caspase activation is mediated by protein kinase G. Biochimica et Biophysica Acta. 2010;1797:1012–1017. doi: 10.1016/j.bbabio.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Morkuniene R, Jekabsone A, Borutaite V. Estrogens prevent calcium-induced release of cytochrome c from the heart. FEBS Letters. 2002;521:53–56. doi: 10.1016/s0014-5793(02)02820-x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Suzuki T, Miki Y, Tazawa C, Senzaki K, Moriya T, et al. Estrogen receptors in atherosclerotic human aorta: inhibition of human vascular smooth muscle cell proliferation by estrogens. Molecular and Cellular Endocrinology. 2004;219:17–26. doi: 10.1016/j.mce.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol Inhibits Leukocyte Adhesion and Transendothelial Migration in Rabbits In Vivo : Possible Mechanisms for Gender Differences in Atherosclerosis. Circulation Research. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, et al. Sex Steroids Modulated Human Aortic Smooth Muscle Cell Matrix Protein Deposition and Matrix Metalloproteinase Expression. Hypertension. 2005;46:1129–1134. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baumer AT, Grohe C, Strehlow K, Rosenkranz S, Stablein A, et al. Estrogen Modulates AT1Receptor Gene Expression in Vitro and in Vivo. Circulation. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Masatsugu H. Amelioration of ischemia-and reperfusion -induced myocardial injury by 17β-estradiol. Circulation. 1997;96:1953–1963. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- Novensa L, Selent J, Pastor M, Sandberg K, Heras M, Dantas AP. Equine Estrogens Impair Nitric Oxide Production and Endothelial Nitric Oxide Synthase Transcription in Human Endothelial Cells Compared with the Natural 17β-estradiol. Hypertension. 2010;56:405–411. doi: 10.1161/HYPERTENSIONAHA.110.151969. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, et al. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Molecular Endocrinology. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- O’Meara E, Clayton T, McEntegart MB, McMurray JJV, Pina I, Granger CB, et al. Sex Differences in Clinical Characteristics and Prognosis in a Broad Spectrum of Patients With Heart Failure: Results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- Olié V, Canonico M, Scarabin PY. Postmenopausal hormone therapy and venous thromboembolism. Thrombosis Research. 2011;127:S26–S29. doi: 10.1016/S0049-3848(11)70008-1. [DOI] [PubMed] [Google Scholar]
- Opasich C, Tavazzi L, Lucci D, Gorini M, Albanese MC, Cacciatore G, et al. Comparison of One-Year Outcome in Women Versus Men With Chronic Congestive Heart Failure. American Journal of Cardiology. 2000;86:353–357. doi: 10.1016/s0002-9149(00)00934-6. [DOI] [PubMed] [Google Scholar]
- Ortmann J, Veit M, Zingg S, Di Santo S, Traupe T, Yang Z, et al. Estrogen Receptor-α But Not -β or GPER Inhibits High Glucose -Induced Human VSMC Proliferation: Potential Role of ROS and ERK. Journal of Clinical Endocrinology Metabolism. 2011;96:220–228. doi: 10.1210/jc.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostadol B, Netuka I, Maly J, Besik J, Ostadalova I. Gender Differences in Cardiac Ischemic Injury and Protection: Experimental Aspects. Experimental Biology and Medicine. 2009;234:1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. The New England Journal of Medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- Parashar S, Katz R, Smith NL, Arnold AM, Vaccarino V, Wenger N, et al. Race, Gender, and Mortality in Adults >65 Years of Age With Incident Heart Failure (from the Cardiovascular Health Study) American Journal of Cardiology. 2009;103:1120–1127. doi: 10.1016/j.amjcard.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, et al. Estrogen Receptor-α Mediates the Protective Effects of Estrogen Against Vascular Injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, et al. 17{beta}-Estradiol Reduces Cardiomyocyte Apoptosis In Vivo and In Vitro via Activation of Phospho-Inositide-3 Kinase/Akt Signaling. Circulation Research. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- Pechenino AS, Lin L, Mbai FN, Lee AR, He XM, Stallone JN, et al. Impact of Aging vs. Estrogen Loss on Cardiac Gene Expression: Late Estrogen Replacement and Inflammation. Physiological Genomics. 2011;43:1065–1073. doi: 10.1152/physiolgenomics.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Wallace DC, Levin ER. Functional esrogen receptors in the mitochondria of breast cancer cells. Molecular Biology of the Cell. 2006;17:2125–2137. doi: 10.1091/mbc.E05-11-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen Inhibits Cardiac Hypertrophy: Role of Estrogen Receptor-β to Inhibit Calcineurin. Endocrinology. 2008;149:3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER. Estrogen Receptor-{beta} Prevents Cardiac Fibrosis. Molecular Endocrinology. 2010;24:2152–2165. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer T, Schumann M, Neumann M, deJager T, Stimpel M, Serfling E, et al. 17β-estradiol prevents programmed cell death in cardiac myocytes. Biochemical and Biophysical Research Communications. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- Pelzer T, Arias Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, et al. Increased Mortality and Aggravation of Heart Failure in Estrogen Receptor-{beta} Knockout Mice After Myocardial Infarction. Circulation. 2005:01. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- Pervin S, Singh R, Rosenfeld ME, Navab M, Chaudhuri G, Nathan L. Estradiol Suppresses MCP-1 Expression In Vivo : Implications for Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1575–1582. doi: 10.1161/01.atv.18.10.1575. [DOI] [PubMed] [Google Scholar]
- Podlutsky A, Ballabh P, Csiszar A. Oxidative stress and endothelial dysfunction in pulmonary arteries of aged rats. AJP - Heart and Circulatory Physiology. 2010;298:H346–H351. doi: 10.1152/ajpheart.00972.2009. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Estrogen Signals to the Preservation of Endothelial Cell Form and Function. Journal of Biological Chemistry. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 Null Mice Are Viable but Show Evidence of Hyperproliferative and Vascular Abnormalities. Journal of Biological Chemistry. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Reis SE, Holubkov R, Young JB, White BG, Cohn JN, Feldman AM. Estrogen is associated with improved survival in aging women with congestive heart failure: Analysis of the vesnarinone studies. Journal of the American College of Cardiology. 2000;36:529–533. doi: 10.1016/s0735-1097(00)00738-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Lopez R, Paez A, Masso F, Montano LF. 17[beta]-estradiol inhibits the adhesion of leukocytes in TNF-[alpha] stimulated human endothelial cells by blocking IL-8 and MCP-1 secretion, but not its transcription. Life Sciences. 2002;71:2181–2193. doi: 10.1016/s0024-3205(02)01999-9. [DOI] [PubMed] [Google Scholar]
- Saitta A, Altavilla D, Cucinotta D, Morabito N, Frisina N, Corrado F, et al. Randomized, Double-Blind, Placebo-Controlled Study on Effects of Raloxifene and Hormone Replacement Therapy on Plasma NO Concentrations, Endothelin-1 Levels, and Endothelium-Dependent Vasodilation in Postmenopausal Women. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:1512–1519. doi: 10.1161/hq0901.095565. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor ERRα. Journal of Biological Chemistry. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-Mediated Endothelial Nitric Oxide Synthase Association With Heat Shock Protein 90 Requires Adenosine Monophosphate-Dependent Protein Kinase. Circulation. 2005 doi: 10.1161/CIRCULATIONAHA.105.546812. CIRCULATIONAHA. [DOI] [PubMed] [Google Scholar]
- Shapira I, Isakov A, Burke M, Almog C. Cardiac rupture in patients with acute myocardial infarction. Chest. 1987;92:219–223. doi: 10.1378/chest.92.2.219. [DOI] [PubMed] [Google Scholar]
- Shigeta H, Zuo W, Yang N, DiAugustine R, Teng CT. The mouse estrogen receptor-related orphan receptor α1: molecular cloning and estrogen responsiveness. Journal of Molecular Endocrinology. 1997;19:299–309. doi: 10.1677/jme.0.0190299. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- Simoncini T, De Caterina R, Genazzani AR. Selective Estrogen Receptor Modulators: Different Actions on Vascular Cell Adhesion Molecule-1 (VCAM-1) Expression in Human Endothelial Cells. Journal of Clinical Endocrinology Metabolism. 1999;84:815. doi: 10.1210/jcem.84.2.5570. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000a;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, et al. Estrogens and Glucocorticoids Inhibit Endothelial Vascular Cell Adhesion Molecule-1 Expression by Different Transcriptional Mechanisms. Circulation Research. 2000b;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Rabkin E, Liao JK. Molecular Basis of Cell Membrane Estrogen Receptor Interaction With Phosphatidylinositol 3-Kinase in Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:198–203. doi: 10.1161/01.atv.0000053846.71621.93. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria - physiological and pathological implications. Molecular and Cellular Endocrinology. 2008;290:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow RCM, Li FYL, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phytoestrogens: Transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radical Biology and Medicine. 2007;42:909–925. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, et al. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. Journal of Applied Physiology. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Sowers JR. Diabetes Melitus and Cardiovascular Disease in Women. Archives of Internal Medicine. 1998;158:617–621. doi: 10.1001/archinte.158.6.617. [DOI] [PubMed] [Google Scholar]
- Stice JP, Chen L, Kim SC, Chen L, Tran AL, Liu TT, et al. 17β-Estradiol, Aging, Inflammation and the Stress Response in the Female Heart. Endocrinology. 2011;152:1589–1598. doi: 10.1210/en.2010-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice JP, Eiserich JP, Knowlton AA. Role of Aging vs. the Loss of Estrogens in the Reduction in Vascular Function in Female Rats. Endocrinology. 2009;150:212–219. doi: 10.1210/en.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, et al. Estrogen Increases Bone Marrow-Derived Endothelial Progenitor Cell Production and Diminishes Neointima Formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Sammut IA, Latif N, Jayakumar J, Smolenski RT, et al. Heat Shock Protein 72 Enhances Manganese Superoxide Dismutase Activity During Myocardial Ischemia-Reperfusion Injury, Associated With Mitochondrial Protection and Apoptosis Reduction. Circulation. 2002;106:270I–276. [PubMed] [Google Scholar]
- Szalay L, Shimizu T, Suzuki T, Yu HP, Choudry MA, Schwacha MG, et al. Estradiol improves cardiac and hepatic function after trauma-hemorrhage: role of enhanced heat shock protein expression. American Journal of Physiology. 2006;290:R812–R818. doi: 10.1152/ajpregu.00658.2005. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ohmichi M, Yoshida M, Hisamoto K, Mabuchi S, Arimoto-Ishida E, et al. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. Journal of Endocrinology. 2003;178:319–329. doi: 10.1677/joe.0.1780319. [DOI] [PubMed] [Google Scholar]
- Tan B, Long X, Nakshatri H, Nephew KP, Bigsby RM. Striatin-3γinhibits estrogen receptor activity by recruiting protein phosphatase. Journal of Molecular Endocrinology. 2008;40:199–210. doi: 10.1677/JME-07-0132. [DOI] [PubMed] [Google Scholar]
- Tan PH, Chan C, Xue SA, Dong R, Ananthesayanan B, Manunta M, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173:171–183. doi: 10.1016/j.atherosclerosis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Tchaikovski SN, Rosing J. Mechanisms of Estrogen-Induced Venous Thromboembolism. Thrombosis Research. 2010;126:5–11. doi: 10.1016/j.thromres.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Trost SU, Omens JH, Karlon WJ, Meyer M, Mestril R, Covell JW, et al. Protection Against Myocardial Dysfunction After a Brief Ischemic Period in Transgenic Mice Expressing Inducible Heat Shock Protein 70. Journal of Clinical Investigation. 1998;101:855–862. doi: 10.1172/JCI265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. AJP - Heart and Circulatory Physiology. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- van den Borne SWM, van de Schans VAM, Strzelecka AE, Vervoort-Peters HTM, Lijnen PM, Cleutjens JPM, et al. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovacular Research. 2009;84:273–282. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, et al. 17-Beta-Estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. Journal of the American College of Cardiology. 2003;41:2084–2092. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- Van Kempen T, Milner T, Water E. Accelerted Ovarian Failure: A novel, chemically induced animal model of menopause. Brain Research. 2011;1379:176–187. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeneman GH. Nonsteroidal subtype selective estrogens. Current Medicinal Chemistry. 2005;12:1077–1136. doi: 10.2174/0929867053764662. [DOI] [PubMed] [Google Scholar]
- Verhaert D, Grimm RA, Puntawangkoon C, Wolski K, De S, Wilkoff BL, et al. Long-Term Reverse Remodeling with Cardiac Resynchronization Therapy. Journal American College of Cardiology. 2010;55:1788–1795. doi: 10.1016/j.jacc.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Voloshenyuk TG, Gardner JD. Estrogen improves TIMP-MMP balance and collagen distribution inn volume-overloaded hearts of ovariectomized females. AJP - Regulatory, Integrative and Comparative Physiology. 2010;299:R683–R693. doi: 10.1152/ajpregu.00162.2010. [DOI] [PubMed] [Google Scholar]
- Voss MR, Stallone JN, Li M, Cornelussen RNM, Kneufermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: The effect of estrogen. AJP - Heart and Circulatory Physiology. 2003;285:H687–H692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- Wang F, Keimig T, He Q, Ding J, Zhang Z, Pourabdollah-Nejad S, et al. Augmented Healing Process in Female Mice with Acute Myocardial Infarction. Gender Medicine. 2012a;4:230–247. doi: 10.1016/s1550-8579(07)80043-x. [DOI] [PubMed] [Google Scholar]
- Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. Activation of GPR30 Attenuates Diastolic Dysfunction and Left Ventricle Remodeling in Oophorectomized mRen2. Lewis Rats. Cardiovascular Research. 2012b doi: 10.1093/cvr/cvs090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Crisostomo PR, Wairiuko GM, Meldrum DR. Estrogen receptor-α mediates acute myocardial protection in females. American Journal of Physiology. 2006;290:H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, et al. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. AJP - Regulatory, Integrative and Comparative Physiology. 2009;296:R972–R978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann S, Baumer AT, Strehlow K, van Eickels M, Grohé C, Ahlbory K, et al. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation. 2001;103:435–441. doi: 10.1161/01.cir.103.3.435. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Akishita M, Nakaoka T, Kozaki K, Miyahara M, He H, et al. Estrogen receptor β mediates the inhibitory effect of estradiol on vascular smooth muscle cells proliferation. Cardiovacular Research. 2003;59:734–744. doi: 10.1016/s0008-6363(03)00496-6. [DOI] [PubMed] [Google Scholar]
- Wehrens XH, Doevendans PA. Cardiac rupture complicating myocardial infarction. International Journal of Cardiology. 2004;95:285–292. doi: 10.1016/j.ijcard.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Wingrove CS, Garr E, Pickar JH, Dey M, Stevenson JC. Effects of equine oestrogens on markers of vasoactive function in human coronary artery endothelial cells. Molecular and Cellular Endocrinology. 1999;150:33–37. doi: 10.1016/s0303-7207(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA: The Journal of the American Medical Association. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Wu Q, Chambliss KL, Umetani M, Mineo C, Shaul PW. Non-nuclear Estrogen Receptor Signaling in the Endothelium. Journal of Biological Chemistry. 2011;286:14737–14743. doi: 10.1074/jbc.R110.191791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne FL, Payne JA, Cain AE, Reckelhoff JF, Khalil RA. Age-Related Reduction in Estrogen Receptor-Mediated Mechanisms of Vascular Relaxation in Female Spontaneously Hypertensive Rats. Hypertension. 2004;43:405–412. doi: 10.1161/01.HYP.0000111833.82664.0c. [DOI] [PubMed] [Google Scholar]
- Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and Mechanisms of Vascular Protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovascular Research. 2003;57:388–394. doi: 10.1016/s0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. AJP - Heart and Circulatory Physiology. 2004;287:H165–H171. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]
- Yada-Hashimoto N, Nishio Y, Ohmichi M, Hayakawa J, Mabuchi S, Hisamoto K, et al. Estrogen and raloxifene inhibit the monocytic chemoattractant protein-1-induced migration of human monocytic cells via nongenomic estrogen receptor α. Menopause. 2006;13:935–941. doi: 10.1097/01.gme.0000248732.78698.a7. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, et al. Mitchondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeboah J, Klein K, Brosnihan B, Reboussin D, Herrington DM. Effects of hormone therapy on soluble cell adhesion moleculres in postmenopausal women with coronary artery disease. Menopause. 2008;15:1060–1064. doi: 10.1097/gme.0b013e31816d8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Hsieh Y, Choudhry MA, Schwacha M, Bland KI, Chaudry IH. The PI3K Pathway Mediates the Nongenomic Cardioprotective Effects of Estrogen Following Trauma-hemorrhage. Annals of Surgery. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HP, Chaudry IH. The Role of Estrogen and Receptor Agonists in Maintaining Organ Function After Trauma-Hemorrhage. Shock. 2009;31:1–20. doi: 10.1097/SHK.0b013e31818347e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Handong M, Barman SA, Liu AT, Sellers M, Stallone JN, et al. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. American Journal of Physiology - Endocrinology And Metabolism. 2011;301:E882–E888. doi: 10.1152/ajpendo.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cooke PS, Lubahn DB, Gross DR. Myocardial ischemia-reperfusion injury in estrogen receptor-alpha knockout and wild type mice. American Journal of Physiology. 2000;278:H1640–H1647. doi: 10.1152/ajpheart.2000.278.5.H1640. [DOI] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. AJP - Heart and Circulatory Physiology. 2000;279:H2766–H2775. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via Estrogen Receptor β. Journal of Biological Chemistry. 2010;285:39575–39579. doi: 10.1074/jbc.R110.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Shao Y, Huang Y, Yao T, Lu LM. 17β-Estradiol inhibits angiotensin II-induced collagen synthesis of cultured rat cardiac fibroblasts via modulating angiotensin II receptors. European Journal of Pharmacology. 2007;567:186–192. doi: 10.1016/j.ejphar.2007.03.047. [DOI] [PubMed] [Google Scholar]