Abstract
Plants are excellent sources of nutrition and highly bioactive substances that might use in the development of new drugs and pharmaceutical agents. Three species of the Genus Euphorbia (Family Euphorpiaceae), namely; Euphorbia granulata Forssk, Euphorbia helioscobia L., and Euphorbia hirta Linn growing in Ryiadh, KSA were air-dried, powdered, and their active materials were extracted with alcohol. The nutritional value phytochemical constituents and antimicrobial activity of the plants were determined. The chemical contents were similar in the three species; however, lipid profile of the plants showed that the stearic acid and lignoceric acid were detected only in E. helioscopia and E. hirta, while palmitoleic acid was detected only in E. hirta. The percentage of unsaturated fatty acid methyl esters were 52.48%, 69.39% and 66.52% in Euphorbia granulate, Euphorbia helioscobia, E. hirta, respectively. Three compounds, 1-ethoxypentacosane, heptacosan-1-ol and β-sitosterol were isolated from the three plant extracts and identified using different spectroscopic analysis. The percentage of crude protein was 43.65%, 25.00% and 18.75% in E. granulata, E. helioscobia, and E. hirta, respectively. The free amino acids and amino acid composition were quantitatively determined using amino acid analyzer. All the plant extracts were active against bacterial and fungal test organisms, however, the antimicrobial activity were varied according to both the Euphorbia species and the test organism.
Keywords: Herbal therapies, Natural products, Euphorbia sp., Steroidal compounds, Biological activity, Amino acids, Fatty acids
1. Introduction
Nowadays, the use of herbal medicines and phytonutrients and/or nutraceuticals continues significantly to expand quickly around the world with many people now turning to these natural products for treatment of various health challenges in different national healthcare settings (WHO, 2004). Recently, there was a tremendous surge in interest in herbal therapies in developing and developed countries, with these natural products being available in drug stores, food stores and supermarkets as well. Interestingly, almost 80% of the world’s populations (4 billion) which are living in the developing world rely on natural products as a primary source of healthcare and traditional medical practice (Mukherjee, 2002, Bodeker et al., 2005).
Genus Euphorbia is important in herbal remedy due to its various phytochemical constituents as phenolic compounds (Duarte et al., 2008, Mueller and Pohl, 1970), terpenoids (Liu et al., 2002, Cao et al., 1992), tannins (Giordani et al., 2001, Yashida et al., 1994), and alkaloids, cyanogenic glycosides, flavonoids, and lipids (Uzair et al., 2009). Moreover, it is used for treatment of variable health problems including spasmolytic (Bondarenko, 1972), diuretic (Liu et al., 2002), increase capillary strength (Bondarenko, 1972), antileukemic (Kupchan, 1976), anti-inflammatory and analgesic (Heirmann and Bucar, 1994, Singh et al., 1984). The extract of Euphorbia stenoclada was proved to have positive effect on human airway smooth muscle cells (HASMC) (Chaabi et al., 2007). While the extract of Euphorbia esula showed a mild antiviral activity (Halowish et al., 2003).
The extract of different species of Euporbia; E. wallichii, E. neoboutonia mannii, E. fusiformis, granulate, helioscopia and E. hirta are biologically active and used in treatment of fever and intestinal disorders and wound, bacterial and fungal infections (Ali et al., 2009, Uzair et al., 2009, Ramezani et al., 2008, Tene et al., 2008). The current study was carried out to determine the phytochemical constituents and antimicrobial, antioxidant and anticancer activities of Euphorbia granulata, Euphorbia helioscobia and Euphorbia hirta.
2. Material and methods
2.1. Plant materials
The aerial parts of; Euphorbia granulata Forssk, Euphorbia helioscobia L., and Euphorbia hirta Linn were collected from territory desert of Riyadh, KSA in 2016. The plant samples were identified by Dr. Jacob T. Pandalayil (Assistant Professor of Plant Taxonomy, Botany and Microbiology Department, Faculty of science, King Saud University) and also compared with the published plant description (Migahid, 1996). A voucher specimen has been deposited in the herbarium of Faculty of Sciences, King Saud University. The plant materials were air-dried in shade, reduced to fine powder, packed in tightly closed containers and stored for phytochemical and biological studies.
2.2. Phytochemical screening
The air-dried powder of the E. granulata Forssk, E. helioscobia L., and E. hirta Linn were subjected to screening for their phytochemical constituents according to the method described by Khan et al. (2011).
2.3. Extraction and isolation
The air-dried powder of E. granulata, E. helioscobia and E. hirta was extracted according to the method described by Awaad et al. (2016). One-hundred grams of the plant powder was extracted by percolation in 95% ethanol at room temperature for two days. The extract was then filtered and the residue was re-percolated for another two days. The re-percolation process was repeated four times during 8 days. The combined filtrates of each plant were concentrated under reduced pressure at low temperature. The obtained residue (20, 22 and 17 g for E. granulata, E. helioscobia and E. hirta, respectively) was suspended in 100 ml distilled water and then filtered. The un-dissolved pellets (9, 8 and 6 g; lipid contents (A1–A3) of E. granulata, E. helioscobia and E. hirta, respectively) were kept for further investigation. The aqueous layer which have been filtered off to give the polar components (P1–P3) were separately dried by lyophilization. The obtained dry residues were kept for determinations of its nutritional values.
2.3.1. Lipid contents
Lipid contents of the three plants (A1–A3) were separately saponified using the method described by Mathew et al. (2007) to obtain the saponifiable (S1–S3) and unsaponifiable (US1–US3) fractions (Percentages are recorded in Table 1).
Table 1.
GLC analysis of fatty acid methyl esters of Euphorbia granulata, E. helioscopia and E. hirta.
| Peak No. | tR | tRR | Authentic methyl ester of | No of carbons | E. granulata | E. helioscopia | E. hirta |
|---|---|---|---|---|---|---|---|
| 1 | 11.76 | 00.81 | Myristic acid | 14 | 01.61 | 03.43 | 03.18 |
| 2 | 14.18 | 00.97 | Palmitoleic acid | 16.1 | 00.00 | 00.00 | 00.18 |
| 3 | 14.59 | 01.00 | Palmitic acid | 16 | 50.45 | 60.72 | 57.92 |
| 4 | 16.22 | 01.11 | Heptadecanoic acid | 17 | 04.75 | 06.11 | 10.59 |
| 5 | 17.62 | 01.21 | Oleic acid | 18.1 | 36.88 | 18.87 | 13.49 |
| 6 | 18.02 | 01.22 | Stearic acid | 18 | 00.00 | 03.19 | 04.77 |
| 7 | 19.16 | 01.31 | Linoleic acid | 18.2 | 05.99 | 05.63 | 09.23 |
| 8 | 21.52 | 01.47 | Arachidic acid | 20 | 00.42 | 01.00 | 00.28 |
| 9 | 25.27 | 01.73 | Lignoceric acid | 24 | 00.00 | 01.05 | 00.37 |
| Unsaturated fatty acids | 52.48 | 69.39 | 66.52 | ||||
| Saturated fatty acids | 47.62 | 30.61 | 33.49 | ||||
| Total | 100% | 100% | 100% | ||||
tR; Retention time, tRR is relative retention time to Palmitic acid.
2.3.1.1. The saponifiable fractions (S1–S3)
The saponifiable fractions (S1–S3) were subjected to GLC (after methylation) to determine their fatty acids content according to the method described by Fakhry and Maghraby (2013). Results are represented in Table 2.
Table 2.
The free, protein- hydrolysate and total amino acids of Euphorbia granulata, E. helioscopia and E. hirta.
| No | tR | Amino acid | Percentage of amino acid (mg/g) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. granulata |
E. helioscopia |
E. hirta |
|||||||||
| Free | Protein hydolysate | Total | Free | Protein hydolysate | Total | Free | Protein hydolysate | Total | |||
| 1 | 11.53 | Aspartic | 04.70 | 10.48 | 18.18 | 04.50 | 11.19 | 13.69 | 01.25 | 13.84 | 15.09 |
| 2 | 14.85 | Therionine | 03.89 | 09.12 | 12.01 | 02.54 | 06.45 | 08.99 | 01.67 | 07.44 | 09.11 |
| 3 | 16.26 | Serine | 01.55 | 06.99 | 08.54 | 01.03 | 03.05 | 04.08 | 02.31 | 00.68 | 05.31 |
| 4 | 18.47 | Glutamic acid | 02.67 | 03.81 | 06.48 | 02.20 | 08.69 | 10.89 | 05.40 | 04.06 | 09.46 |
| 5 | 25.54 | Glycine | 01.66 | 04.55 | 06.20 | 01.15 | 05.43 | 06.58 | 02.63 | 06.18 | 08.81 |
| 6 | 26.87 | Alanine | 03.01 | 01.40 | 04.41 | 00.50 | 01.59 | 02.09 | 01.52 | 03.14 | 04.64 |
| 7 | 30.22 | Valine | 00.41 | 00.50 | 00.91 | 00.10 | 00.09 | 00.19 | 01.20 | 04.06 | 05.26 |
| 8 | 32.57 | Methionine | 02.30 | 03.32 | 05.62 | 02.20 | 07.12 | 09.32 | 01.04 | 00.11 | 00.15 |
| 9 | 34.20 | Isoleucine | 02.96 | 06.01 | 08.90 | 00.91 | 04.03 | 04.94 | 01.20 | 04.05 | 04.25 |
| 10 | 35.44 | Leucine | 03.45 | 07.13 | 10.50 | 00.44 | 05.01 | 05.45 | 03.20 | 01.60 | 04.83 |
| 11 | 39.70 | Tyrosine | 01.54 | 01.73 | 03.27 | 01.50 | 05.16 | 06.66 | 01.00 | 00.91 | 01.91 |
| 12 | 42.40 | Phenyl alanine | 00.95 | 00.76 | 01.71 | 02.02 | 02.82 | 04.84 | 02.03 | 05.02 | 07.05 |
| 13 | 50.57 | Histidine | 00.90 | 02.03 | 02.93 | 04.08 | 05.03 | 09.21 | 02.18 | 04.22 | 06.40 |
| 14 | 54.19 | Lysine | 01.77 | 01.04 | 02.81 | 02.00 | 01.02 | 03.02 | 02.30 | 05.12 | 07.52 |
| 15 | 63.02 | Argenine | 04.79 | 02.82 | 07.61 | 03.03 | 07.02 | 10.05 | 2.00 | 06.01 | 08.01 |
| Total% | 100.00 | 100 | 99.9 | ||||||||
tR; Retention time.
2.3.1.2. Unsaponifiable fractions (US1–US3)
Unsaponifiable fractions (US1–US3) were applied simultaneously on top of 3 glass column (120 × 2 cm) packed with silica gel (120 g) and eluted using hexane: ethyl acetate (95:5). Eighty fractions were collected (40 ml. each) all similar fractions (according to color, number and Rf of spots), from each column, were collected and combined together. In the end, three subfractions were obtained and used for isolation of three compounds (T1–T3) by purifications and recrystallization from methanol. The isolated compounds were identified and screened for their antimicrobial activity (Results are recorded in Table 3).
Table 3.
Antimicrobial activities of Euphorbia granulata, E. helioscopia and E. hirta.
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| Microorganism | Diameter of the inhibition zone (mm) |
||||||
| E. granulata | E. helioscopia | E. hirta | T1 | T2 | T3 | Standard | |
| Gram negative bacteria: | Gentamycin | ||||||
| Proteous vulgaris (RCMB 010085) | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 20.3 ± 0.30 |
| Klebsiella pneumoniae (RCMB 0010093) | 18.9 ± 0.44 | 16.7 ± 0.35 | 24.4 ± 0.19 | 18.3 ± 0.44 | 23.7 ± 0.58 | 00.0 | 26.3 ± 0.15 |
| Escherichia coli (RCMB 010056) | 14.2 ± 0.35 | 13.5 ± 0.58 | 21.4 ± 0.35 | 15.8 ± 0.58 | 18.9 ± 0.39 | 00.0 | 25.3 ± 0.18 |
| Gram positive bacteria: | Ampicillin | ||||||
| Staphylococcus aureus (RCMB 010027) | 20.3 ± 0.35 | 16.8 ± 0.19 | 23.8 ± 0.25 | 18.2 ± 0.44 | 22.4 ± 0.44 | 00.0 | 28.9 ± 0.14 |
| Staphylococcus epidermidis (RCMB 010024) | 21.1 ± 0.44 | 18.9 ± 0.25 | 21.2 ± 0.19 | 16.5 ± 0.35 | 20.8 ± 0.19 | 00.0 | 25.4 ± 0.18 |
| Streptococcus pyogenes (RCMB 010015) | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 00.0 | 26.4 ± 0.34 |
| Fungi | Amphotericin B | ||||||
| Aspergillus fumigatus (RCMB 02564) | 18.4 ± 0.58 | 15.9 ± 0.58 | 19.8 ± 0.25 | 15.3 ± 0.25 | 18.2 ± 0.44 | 00.0 | 23.7 ± 0.10 |
| Candida albicans (RCMB 05035) | 16.8 ± 0.63 | 12.9 ± 0.25 | 18.9 ± 0.25 | 12.7 ± 0.39 | 15.4 ± 0.58 | 00.0 | 21.9 ± 0.12 |
| Candida tropicalis (RCMB 05042) | 15.4 ± 0.25 | 13.4 ± 0.35 | 19.8 ± 0.58 | 13.6 ± 0.63 | 18.6 ± 0.44 | 00.0 | 25.4 ± 0.16 |
| Geotricum candidum (RCMB 05096) | 19.8 ± 0.25 | 17.6 ± 0.25 | 21.6 ± 0.58 | 16.7 ± 0.58 | 20.4 ± 0.44 | 00.0 | 26.4 ± 0.20 |
| Microsporum canis (RCMB 0834) | 17.5 ± 0.58 | 13.6 ± 0.44 | 22.6 ± 0.19 | 14.5 ± 0.19 | 20.4 ± 0.58 | 00.0 | 22.2 ± 0.34 |
| Trichophyton mentagrophytes (RCMB 0925) | 19.2 ± 0.44 | 15.8 ± 0.35 | 16.8 ± 0.44 | 13.9 ± 0.25 | 15.7 ± 0.19 | 00.0 | 24.1 ± 0.18 |
2.3.1.2.1. T1: 1-ethoxypentacosane
White crystals (198.7 mg) with Rf = 0.45 (in system Benzene: ethyl acetate 86/14 v/v). 1HNMR (CDCl3) showed signals at δ; 3.62 ppm (2H q, J = 4.98, H-26) its position indicate that its CH3 occurs next to Oxygen atom; δ 1.55 ppm (3H t, J = 5.34, H-27); multiple δ 1.27 ppm (48H, m, (CH2)24H-1 → 24); δ 0.86 ppm (3H t, J = 6.48, H-25) for the terminal CH3 group. 13C-NMR (CDCl3) showed 27 carbons 14 carbons of them were similar. HMQC, DEPT-135 and HMQC confirmed the structure in addition to comparing with published data (Awaad et al., 2013).
2.3.1.2.2. T2: Heptacosan-1-ol
White crystals (700 mg) with Rf 0.33 (in system Benzene: ethyl acetate 86/14 v/v). 1HNMR (CDCl3) showed signals at δ: 3.62 ppm (2H q, J = 5.52, H-2) this proton near to —OH group, quintet δ 1.55 ppm (2H q, J = 7.38, H-3) this proton between two CH2, multiplet δ 1.28 ppm (48H m, (CH2) 24 H-3 → 26), and triplet at δ 0.86 ppm (3H t, J = 7.08, H-28). 13C-NMR (CDCl3) showed 13 carbons 9 of them were similar. HMQC, DEPT-135 and HMQC confirmed the structure in addition to comparing with published data (Awaad et al., 2013).
2.3.1.2.3. T3: β-sitosterol
Whitish crystal residue (300 mg) with Rf 0.45 (in system Benzene: ethyl acetate 86/14 v/v). 1HNMR (CDCl3) showed signals at δ δ 5.34 ppm (1H d, J = H-7) this proton double bound, singlet at δ 3.51 ppm (1H, s, —OH), at δ 2.26 ppm (2H q, J = H-3) nearest from —OH, triplet at δ 1.98 ppm (2H,t,J = H-5 & H-8), at δ 1.83 ppm (3H, t, H-28), sextet at 1.63 ppm (1H,s, H-18), singlet at 1.57 ppm (8H, s, H-1, H-2, H-15 & H-16), at 1.33 ppm (5H, m, H-9, H-11, & H-12), multiplet at 1.14 ppm (6H, m, H-4, 24, 21, 17, & 22), at 1.12 ppm (6H, d, H-29 & H-30), at 0.91 ppm (4H d, J = H-19 & H-20), singlet at 0.81 ppm (9H, s, H-24, H-25 & H-26), singlet at 0.66 ppm (3H, s, H-23). 13C-NMR (CDCl3) showed 30 carbons. HMQC, DEPT-135 and HMQC confirmed the structure in addition to comparing with published data (Awaad et al., 2013).
2.3.2. Polar components (P1–P3)
Polar components (P1–P3) including proteins, carbohydrates, phenols, flavonoids and tannins of the three plants were determined using the procedures published by Bhumi and Savithramma (2014).
3. Antimicrobial activities
3.1. Test organisms
Different microorganisms including six bacterial strains; Gram-negative bacteria, Escherichia coli (RCMB 010056), Klebsiella pneumonia (RCMB 0010093), and Proteus vulgaris (RCMB 010085), Gram-positive bacteria, Staphylococcus aureus, Staphylococcus epidermidis (RCMB 010027) and Stroptococcus byogenes (RCMB 010015); and six fungal strains fungal strains including Aspergillus fumigatus (RCMB 02564), Candida albicans (RCMB 05035), C. tropicalis (RCMB 05042), Geotricum candidum (RCMB 05096), Microsporum canis (RCMB 0834) and Trichophyton mentagrophytes (RCMB 0925) were used. The test organisms were obtained from the Microbiology Laboratory, Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt.
3.2. Antimicrobial activity assay
The antimicrobial activity, for ethanolic extract and the isolated compounds, of Euphorbia granulata, E. helioscobia and E. hirta was determined using the well diffusion method according to National Committee for Clinical Laboratory Standards (NCCLS) (Zain et al., 2012). Petri plates containing 20 ml of, nutrient (for bacteria) or malt extract (for fungi), agar medium were seeded with 1–3 day cultures of microbial inoculums. Wells (6 mm in diameter) were cut off from agar and 50 µl of plant extracts were tested in a concentration of 100 mg/ml and incubated at 37 °C for 24–48 h (bacterial strains) and at 25 °C for 3–5 days (fungal strains). The antimicrobial activity was determined by measurement of the diameter of the inhibition zone around the well.
3.3. Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) was determined by micro-dilution method using serially diluted (2 folds) plant extracts and the isolated compounds according to the National Committee for Clinical Laboratory Standards (NCCLS) (Zain et al., 2012). The MIC of Euphorbia granulata, E. helioscobia and E. hirta extracts and isolated compounds were determined by dilution of concentrations from 0.0 to 100 mg/ml. Equal volume of each extract and nutrient broth were mixed in a test tube. Specifically, 0.1 ml of standardized inoculum (1–2 × 107 cfu/ml) was added in each tube. The tubes were incubated at 25 °C and 37 °C for 24–48 h and/or 3–5 days. Two control tubes, containing the growth medium, saline and the inoculum were maintained for each test batch. The lowest concentration (highest dilution) of the extract that produced no visible microbial growth (no turbidity) when compared with the control tubes were regarded as MIC.
4. Results and discussion
4.1. The primary phytochemical
The primary phytochemical screening showed that the E. granulata, E. helioscopia and E. hirta were similar in their chemical contents, particularly, carbohydrates and/or glycosides, flavonoids, tannins, sterols and/or triterpenes, and proteins and/or amino acids, traces of anthraquinones. On the other hand, alkaloids and/or nitrogenous bases, cardinolides, saponins, anthraquinones and oxidase enzyme were absent.
4.2. Lipid contents
4.2.1. The saponifiable fractions isolated compounds
The saponifiable fractions of E. granulate, E. helioscobia, and E. hirta (S1–S3) were analyzed using GLC for the methyl esters derivatives of the fatty acids. The major fatty acids were palmitic acid (50.45%, 60.72% and 57.92%) and oleic acid (36.88%, 18.87% and 13.49%) for E. granulata, E. helioscopia, and E. hirta, respectively (Table 1). On the other hand, the lowest fatty acid percentage was arachidic acid (0.42%, 1.00% and 0.28%) for E. granulata, E. helioscopia and E. hirta, respectively. Interestingly, stearic acid and lignoceric acid were present only in E. helioscopia and E. hirta, while palmitoleic acid was detected only in E. hirta (Table 1).
The percentage of unsaturated fatty acids methyl esters (52.48, 69.39 and 66.52) is remarkable compared to that of saturated fatty acids methyl esters (47.62, 30.61 and 33.49) in E. granulate, E. helioscobia, and E. hirta, respectively. The amount of unsaturated fractions is between 1.00% and 60.72%, while that of saturated is between 36.88% and 0.18%.
4.2.2. The unsaponifiable fractions isolated compounds
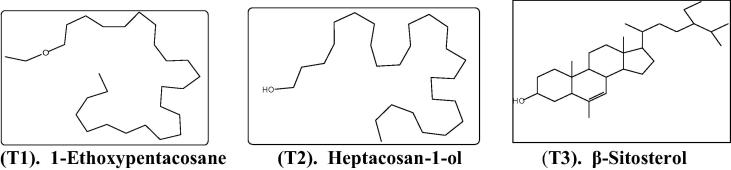
Three compounds (Fig. 1) were isolated from each plant under investigation and identified as; (T1: 1-ethoxypentacosane, T2: heptacosan-1-ol and T3: β-sitosterol). Identifications were carried out using different spectroscopic analysis and compare with published data (Awaad et al., 2013).
Fig. 1.
The isolated compounds from E. granulata, E. helioscopia, and E. hirta.
4.3. Polar components (P1–P3)
4.3.1. Protein content
The percentage of crude protein, as determined by the A.O.A.C method, was found to be 43.65, 25.00 and 18.75% for E. granulata, E. helioscobia, and E. hirta respectively. The free amino acids and amino acid composition of protein hydrolysates were quantitatively studied using amino acid analyzer (Table 2).
Aspartic acid was found to be the major component in the three plants; 3.69, 18.18 and 4.09 mg/g for E. granulata, E. helioscobia, and E. hirta, respectively. On the other hand, Valine concentration was the lowest; 0.91, 0.19 and 0.26 mg/g, for E. granulata, E. helioscobia, and E. hirta, respectively.
4.3.1.1. Antimicrobial activity
The antimicrobial activities of total alcohol and isolated compounds of Euphorbia granulata, E. helioscobia, and E. hirta extracts were determined using well-diffusion method (Table 3, Table 4). All the plant extracts and isolated compounds were active against different bacterial and fungal species. The highest antimicrobial activity of the extract; 24.4 ± 0.19 mm (01.95 ± 00.41 μg/ml), 23.7 ± 0.58 mm (01.95 ± 00.35 μg/ml) and 22.6 ± 0.19 mm (01.95 ± 00.19 μg/ml) were detected by Euphorbia hitra against Klebsiella pneumonia, Staphylococcus aureus and Microsporum canis, respectively (Table 3, Table 4).
Table 4.
The minimum inhibitory concentration (MIC) of Euphorbia granulata, E. helioscopia and E. hirta.
| Sample | ||||||
|---|---|---|---|---|---|---|
| Microorganism | MIC μg/ml |
|||||
| E. granulata | E. helioscopia | E. hirta | T1 | T2 | Standard | |
| Gram negative bacteria: | Gentamycin | |||||
| Klebsiella pneumoniae (RCMB 0010093) | 15.62 ± 00.23 | 62.50 ± 00.11 | 03.90 ± 00.41 | 15.62 ± 00.43 | 03.90 ± 0.07 | 00.97 ± 00.23 |
| Escherichia coli (RCMB 010056) | 62.50 ± 00.12 | 250.00 ± 00.15 | 15.62 ± 00.43 | 62.50 ± 00.23 | 15.62 ± 00.11 | 00.06 ± 00.11 |
| Gram positive bacteria: | Ampicillin | |||||
| Staphylococcus aureus (RCMB 010027) | 03.90 ± 00.33 | 15.62 ± 0.22 | 01.95 ± 00.35 | 03.90 ± 00.19 | 01.95 ± 00.44 | 00.06 ± 00.12 |
| Staphylococcus epidermidis (RCMB 010024) | 03.90 ± 00.09 | 07.80 ± 00.25 | 03.90 ± 00.22 | 07.80 ± 00.11 | 03.90 ± 00.18 | 00.24 ± 00.32 |
| Fungi | Amphotericin B | |||||
| Aspergillus fumigatus (RCMB 02564) | 07.80 ± 00.24 | 07.80 ± 00.09 | 03.90 ± 00.31 | 15.62 ± 00.13 | 03.90 ± 00.44 | 00.24 ± 00.12 |
| Candida albicans (RCMB 05035) | 125.00 ± 00.25 | 250.00 ± 00.58 | 31.25 ± 00.24 | 250.00 ± 00.39 | 125.00 ± 00.16 | 03.9 ± 00.54 |
| Candida tropicalis (RCMB 05042) | 500.00 ± 00.25 | 500.00 ± 00.35 | 31.25 ± 00.58 | 500.00 ± 00.63 | 31.25 ± 00.44 | 00.97 ± 00.29 |
| Geotricum candidum (RCMB 05096) | 03.90 ± 00.37 | 07.80 ± 00.35 | 03.90 ± 00.06 | 15.62 ± 00.22 | 03.90 ± 00.23 | 01.95 ± 00.19 |
| Microsporum canis (RCMB 0834) | 15.62 ± 00.58 | 500.00 ± 00.44 | 01.95 ± 00.19 | 125.00 ± 00.19 | 03.90 ± 00.58 | 01.95 ± 00.59 |
| Trichophyton mentagrophytes (RCMB 0925) | 03.90 ± 00.34 | 07.8 ± 0.35 | 07.8 ± 0.44 | 31.25 ± 0.25 | 07.80 ± 00.19 | 01.95 ± 00.23 |
The best activity of the isolated compounds; 23.7 ± 0.58 mm (03.90 ± 0.07 μg/ml), 22.4 ± 0.44 mm (01.95 ± 00.44 μg/ml), 20.8 ± 0.19 mm (03.90 ± 00.18 μg/ml), 20.4 ± 0.58 mm (03.90 ± 00.58 μg/ml) and 20.4 ± 0.44 mm (03.90 ± 00.23 μg/ml) were obtained by compound (T2) against Klebsiella pneumonia, Staphylococcus aureus, S. epidermidis, Microsporum canis and Geotricum candidum, respectively (Table 3, Table 4).
Acknowledgment
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ali I., Naz R., Khan W.N., Gul R., Choudhary M.I. Biological screening of different root extractes of Euphorbia wallichii. Pak. J. Bot 2009;. 41(4):1737–1741. [Google Scholar]
- Awaad A.S., Al-Refaie A., El-Meligy R.M., Zain M.E., Soliman H., Marzoke M.S., El-Sayed N.H. Novel compounds with new anti-ulcergenic activity from Convolvulus pilosellifolius using bio-guided fractionation. Phytother. Res. 2016;30:2060–2064. doi: 10.1002/ptr.5730. [DOI] [PubMed] [Google Scholar]
- Awaad A.S., El-Meligy R.M., Al-Jaber N.A., Al-Muteeri H.S., Zaina M.E., Alqasoumi S.I., Alafeefy A.M., Donia A.M. Anti-ulcerative colitis activity of the total alcohol extracts and isolated compounds from Euphorbia granuleta Forssk. Phytother. Res. 2013;27:1729–1734. doi: 10.1002/ptr.4985. [DOI] [PubMed] [Google Scholar]
- Bhumi G., Savithramma N. Screening of pivotal medicinal plants for qualitative and quantitative phytochemical constituents. Int. J. Pharm. Pharmaceut. Sci. 2014;6:3. [Google Scholar]
- Bodeker C., Bodeker G., Ong C.K., Grundy C.K., Burford G., Shein K. World Health Organization; Geneva, Switzerland: 2005. WHO Global Atlas of Traditional, Complementary and Alternative Medicine. [Google Scholar]
- Bondarenko O.M., Chogovets R.K., Litvinenko V.I., Obolentseva G.V., Sila V.I., Kigel T.B. Euphorbia palustris and Euphorbia stepposa flavonoids and their pharmacological properties. Farm, Zh. 1972;26(6):46–48. [PubMed] [Google Scholar]
- Cao D., Su Y.L., Yang T.S. Triterpene constituents from Euphorbia nematocypha. Hand-muzz. Yaoxuo Xuebao. 1992;27(6):445–451. [PubMed] [Google Scholar]
- Chaabi M., Michel V.F., Frossard N., Randriantsoa A., Andriantsitohaina R., Lobstein A. Anti-proliferative effect of Euphorbia stenoclada in human airway smooth muscle cells in culture. J. Ethnopharmacol. 2007;109(1):134–139. doi: 10.1016/j.jep.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Duarte N., Kayser O., Abreu P., Ferreira M.J. Antileishmanial activity of piceatannol isolated from Euphorbia lagascae seeds. Phytother. Res. 2008;22(4):455–457. doi: 10.1002/ptr.2334. [DOI] [PubMed] [Google Scholar]
- Fakhry E.M., El Maghraby D.M. Fatty acids composition and biodiesel characterization of Dunaliella salina. J. Water Resour. Protect. 2013;5:894–899. [Google Scholar]
- Giordani R., Trebaux J., Masi M., Regli P. Enhanced antifungal activity of ketoconzole by Euphorbia characias latex against Candida albicans. Ethnopharmacology. 2001;78(1):1–5. doi: 10.1016/s0378-8741(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Haloweish F., Kronberg S., Rice J.A. Rodent and Ruminant ingestive response to flavonoids in Euphorbia esula. J. Chem. Ecol. 2003;29(5):1073–1082. doi: 10.1023/a:1023869220586. [DOI] [PubMed] [Google Scholar]
- Heirmann A., Bucar F. Influence of some traditional medicinal plants of Senegal on prostaglandin biosynthesis. J. Ethnophamacol. 1994;42(2):111–116. doi: 10.1016/0378-8741(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Khan F.A., Hussain I., Farooq S., Ahmad M., Arif M., Ur Rehman I. Phytochemical screening of some Pakistanian medicinal plants. Middle-East J. Sci. Res. 2011;8:575–578. [Google Scholar]
- Kupchan S.M., Uchida I., Branfman A.R. Antileukemic principles isolated from Euphorbia plants daily. Science. 1976;191:571–572. doi: 10.1126/science.1251193. [DOI] [PubMed] [Google Scholar]
- Liu L.G., Meng J.C., Wu S.X., Li X.Y., Zhoo X.C., Tan R.X. New macrocyclic diterpenoids from Euphorbia esula. Planta Med. 2002;68(3):244–248. doi: 10.1055/s-2002-23135. [DOI] [PubMed] [Google Scholar]
- Mathew B., Toome Y., Kevin J. Modified saponification and HPLC methods from analyzing carotenoids from the retina of quail: implications for its use as a non- primate model species. Invest. Ophthalmol. Vis. Sci. 2007;48(9):3976–3982. doi: 10.1167/iovs.07-0208. [DOI] [PubMed] [Google Scholar]
- Migahid, A.M., 1996. Flora of Saudi Arabia. fourth ed. Vol. 1, King Saud University Press, pp. 127.
- Mueller R., Pohl R. Flavonol glycosides of Euphorbia amygdaloides and their quantitative determination at various stages of plant development. Planta Med. 1970;18(2):114–129. doi: 10.1055/s-0028-1099757. [DOI] [PubMed] [Google Scholar]
- Mukherjee P.W. Business Horizons Publishers; New Delhi, India: 2002. Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. [Google Scholar]
- Ramezani M., Behravan J., Arab M., Farzad S.A. Antiviral activity of Euphorbia helioscopia extract. J. Biol. Sci. 2008;8(4):809–813. [Google Scholar]
- Singh G.B., Kaur S., Satti N.K., Atal C.K., Maheshweri J.K. Anti-inflammatory activity of the aerial parts of E. peplus. J. Ethnopharmacol. 1984;10:233–255. doi: 10.1016/0378-8741(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Tene M., Tane P., Tamokou J.D., Kuiate J.R., Connolly J.D. Degraded diterpenoids from the stem bark of Neoboutonia mannii. Phytochem. Lett. 2008;1(2):120–124. [Google Scholar]
- Uzair M., Loothar B.A., Choudhary B.A. Biological screening of Euphorbia helioscobia L. Pak. J. Pharm. Sci. 2009;22(2):184–186. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2004. WHO Guide lines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems. [Google Scholar]
- Yashida T., Namba O., Kurokawa K., Amakuka Y., Liu Y., Okuda T. Tannins and related polyphenol of Euphorbiaceae plants XII, Euphorbin G and H, New dimeric hydrolysable tannin from Euphorbia chamaesyce. Chem. Pharm. Bull. 1994;42(10):10. [Google Scholar]
- Zain M.E., Awaad A.S., Al-Outhman M.R., El-Meligy R.M. Antimicrobial activities of Saudi Arabian desert plants. Phytopharmacology. 2012;2(1):106–113. [Google Scholar]