Abstract
The standardized ethanol extract (EE) of aerial parts of four Acacia species [A. salicina (ASEE), A. laeta (ALEE), A. hamulosa (AHEE), and A. tortilis (ATEE)] were examined in order to compare their cytotoxic and antimicrobial activities. All the extracts were standardized by UPLC- PDA method using rutin as standard compound. The extracts ALEE, AHEE and ATEE were found to contain rutin along with several other phytoconstituents while rutin was absent in ASEE. All the extracts showed varying level of antimicrobial activity with zone of inhibition ranged from 11 to 21 mm against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. The ALEE and ATEE showed relatively high antimicrobial potency (MIC = 0.2 to 1.6 mg mL−1) in comparison to other extracts. All the extracts were found to reduce the biofilm of P. aeruginosa PAO1 strain significantly in comparison to the untreated control. The cytotoxic property of ASEE, ALEE, AHEE, ATEE were evaluated against HepG2 (Liver), HEK-293 (Kidney), MCF-7 (Breast) and MDA-MB 231 (Breast) cancer cells. Of these, ALEE, AHEE and ATEE exhibited moderate cytotoxic property against human liver carcinoma cells (HepG2; IC50 = 46.2, 39.2 and 42.3 μg mL−1, respectively) and breast cancer cell lines (MCF-7; IC50 = 57.2, 55.3 and 65.7 μg mL−1, respectively). The ATEE and ALEE showed moderate cytotoxicity against HEK-293 (kidney) cells with IC50 = 49.1 and 53.5 μg mL−1, respectively. Since, Acacia species (A. laeta and A. hamulosa) contains numerous polyphenols which might prove to be highly cytotoxic and antimicrobial agents, we suggest that these species can be further subjected to the isolation of more cytotoxic and antimicrobial compounds.
Keywords: Acacia, Antimicrobial, Cytotoxicity, Rutin, Standardization
1. Introduction
Since many centuries the mankind has relied fully or partially on plants and plant extracts for healthcare. The traditional system of medicine based on plants continuously plays an important role in the health care system. At present more than 30% of the total plant families found in nature have been used at one time and other (Joy et al., 1998). Genus Acacia (belongs to subfamily Mimosoideae of Fabaceae or Leguminosae family) is pod-bearing shrubs and trees with leaves containing large amounts of tannins and condensed tannins. (Nadkarni, 2005, Dymock et al., 2005). Around 1300 species are reported in the genus Acacia, out of which 960 species are found in the Australia only and the remaining Acacia species are spread around the tropical to warm temperate regions including Arabia, Egypt, tropical Africa, Europe, America and southern Asia (Kritikar and Basu, 2003). Several phytoconstituents have been isolated and reported from different species of genus Acacia. The leaves and bark of Acacia plants contains highest amount of tannin as well as polyphenolic compounds such as (+) dicatechin, quercetin, gallic acid (Said, 1997, Asolkar et al., 2005). Pods of the Acacia plant was reported to contain various polyphenolic compounds like gallic acid, (+) catechin, robidandiol and chlorogenic acid (Gulco, 2001). The root and flowers contain several biologically important constituents like hentriacontane, sitosterol, betulin, β-amyrin, kaempferol-3 glucoside and isoquercetin (Chatterjee and Pakrashi, 2000). The A. nilotica extract obtained from wood were found very impressive in preventing the disease caused by the overproduction of radicals and illustrated the high cytotoxic potential against MCF-7 cell line (a breast cancer cell line) (Barapatre et al., 2016). Aqueous and methanol extracts of bark of A. karroo was found to inhibit virus HIV-type 1 reverse transcriptase significantly (Mulaudzi et al., 2011) while the A. salicina leaves extracts showed significant antioxidant activities (Boubaker et al., 2012) against superoxides radicals and also found to protect pKS plasmid DNA from hydroxyl radicals. The A. salicina leaves extracts also possessed significant anti-mutagenisity against Salmonella typhimurium TA98 and S. typhimurium TA 1535 strains (Chatti et al., 2011). A phytoconstituent Isorhamnetin 3-O-neohesperidoside isolated from A. salicina leaves protected the cells against oxidative stress by inhibiting xanthine oxidase and superoxide anion scavengers (Bouhlel et al., 2010). Aquoeus extract of A. tortilis at low doses showed potential anxiolytic activity and at high doses it exhibited antidepressant, sedative as well as anticonvulsant property which might be due to inhibitory mechanism of glycine and the action of constituents present in the extract on BZD or 5-HT(1A) receptor (Mohammad Alharbi and Azmat, 2015). The aqueous extract of A. tortilis polysaccharide (AEATP) was very effective in reducing the blood glucose at high doses (Kumar and Singh, 2014). The different fractions of A. etbaica, A. laeta, A. origena and A. pycnantha were found very active against Klebsiella oxytocain, Staphylococcus aureus and Klebsiella pneumoniae strains (Mahmoud et al., 2016). Several antimicrobial and cytotoxic biomarkers such as rutin and β-amyrin have been quantitatively estimated in different species of genus Acacia by HPTLC method (Alam et al., 2015, Alam et al., 2017). The diverse pharmacological property possessed by various Acacia species motivated us to compare the cytotoxic [against HepG2 (Liver), HEK-293 (Kidney) MCF-7 (Breast) and MDA-MB 231 (Breast)] and antimicrobial (against S. aureus, E. coli, P. aeruginosa and C. albicans) properties of standardized extracts of four Acacia species (Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis) grown in kingdom of Saudi Arabia.
2. Materials and methods
2.1. Plant material
The aerial parts of four Acacia species viz. Acacia salicina (Voucher No. 15007), Acacia laeta (Voucher No. 15081), Acacia hamulosa (Voucher No. 16221), and Acacia tortilis (Voucher No. 14977), were collected from estern region of Saudi Arabia and identified by field Taxonomist of Pharmacognosy Department, Pharmacy College, King Saud University, Saudi Arabia. Plant specimens were deposited in the departmental herbarium.
2.2. Plant material extraction by Ultrasonic method
The aerial parts of A. salicina (AS), A. laeta (AL), A. hamulosa (AH), and A. tortilis (AT) were dried in air, powdered and passed through a 0.75 mm sieve. The extraction process took place in a Transsonic-460/H ultrasonic cleaner (ELMA, Germany) according to the Xia et al., 2011. The powdered materials of AS, AL, AH, and AT (50.0 g, each) were extracted by ultrasonication (frequency 20 kHz, power 100 W) using ethanol (95%) for 30 min. The obtained ethanol extracts (ASEE, ALEE, AHEE, and ATEE, respectively) were centrifuged at 5000 rpm for 20 min, filtered through Whatman filter paper No. 1. Therefore, the obtained extracts (ASEE, ALEE, AHEE, and ATEE, respectively) of the four Acacia species (AS, AL, AH, and AT) were concentrated and dried under reduced pressure using rotary evaporator (R-210, BUCHI). The estimated yields (w/w) of ASEE, ALEE, AHEE, and ATEE were 4.81, 5.77, 5.92, and 6.52%, respectively.
2.3. In vitro cytotoxicity assay
Human cancer cell lines: HepG2 (Liver), HEK-293 (Kidney), MCF-7 (Breast) and MDA-MB 231 (Breast) were grown in DMEM media supplemented with 10% bovine serum, 1X penicillin–streptomycin (Sigma-Aldrich) at 37 °C in a humidified chamber with 5% CO2. 5-Flurourasil (sigma) was used as the reference drug (standard). Cells were seeded (1 × 105 cells/well in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Lab ware) a day before treatment and grown. Stocks of ASEE, ALEE, AHEE, and ATEE (1.0 mg mL−1) were made with 5% DMSO (Sigma-Aldrich) and further working solutions (100 μg mL−1) were prepared in serum-free culture media. Cells were treated with four different doses (25, 50, 100 and 200 μg mL−1; in triplicate) of the extracts, in complete growth media, including reference drug, was further incubated for 48 h. On day 2 of treatment, cell proliferation and viability test was performed using TACS MTT Cell Proliferation and Viability Assay Kit (TACS) as per manufacturer’s instructions. The relationship between surviving fraction and compound concentration was plotted to obtain the survival curve of cancer cell lines. The response parameter calculated was the IC50 value, which corresponds to the concentration required for 50% inhibition of cell viability. IC50 values were estimated using the best fit regression curve method in Excel.
2.4. In vitro antimicrobial assay
The agar well diffusion method (Perez et al. 1990) as adopted earlier (Ahmad and Beg, 2001) was used. 0.1 ml of diluted inoculum (105 CFU mL−1) of test organism was spread on Muller-Hinton agar plates. Wells of 8 mm diameter were punched into the agar medium and filled with 100 µl of plant extracts (ASEE, ALEE, AHEE, and ATEE) of 100 mg mL−1 concentration and solvent blank (DMSO) separately. The plates were incubated for overnight at 37 °C. The antibacterial activity was evaluated by measuring the zone of inhibition against test organism. Antibiotics (Ampicillin and Doxycycline) were used as positive control against bacteria while nystatin was used as the control antifungal drugs. Each experiment was performed in triplicate.
2.4.1. Determination of minimal inhibitory concentrations (MICs)
The minimal inhibitory concentrations (MICs) of the plant extracts were determined using a rapid p-Iodonitrotetrazolium chloride (INT; Sigma-Aldrich, St Quentin Fallavier, France) colorimetric assay (Eloff, 1998). Briefly, the test samples were first dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich)-Mueller Hinton Broth (MHB; Sigma-Aldrich). The solution obtained was then added to MHB and serially diluted two fold (in a 96-well microtilter plate). One hundred microliters of inoculums (1.5 × 106 CFU mL−1) prepared in MHB were then added. The plates were covered with a sterile plate sealer and then agitated with a shaker to mix the contents of the wells and incubated at 37 °C for 18 h. Wells containing MHB, 100 μL of inoculum, and DMSO at a final concentration of 2.5% served as the negative control. The MICs of each extract were detected after 18 h of incubation at 37°C following addition of 40 μL INT (0.2 mg mL−1) and incubation at 37°C for 30 min. Viable bacteria reduced this yellow dye to pink. The MIC of each sample was defined as its lowest concentration that prevented this change and then resulted in the complete inhibition of microbial growth.
2.4.2. Assay for biofilm inhibition
The effect of test agents on biofilm formation was measured using the microtitre plate assay (O’Toole and Kotler, 1998). Briefly, 1% overnight cultures (0.4 OD at 600 nm) of test pathogens were added into 1 mL of fresh LB medium in the presence and the absence of sub-MICs of test agents. Bacteria were allowed to adhere and grow without agitation for 24 h at 30 °C. After incubation, microtitre plate was emptied by removing the media along with free-floating planktonic cells and the wells were gently rinsed twice with sterile water. The surface-attached cells (biofilm) were stained with 200 µL of 0.1% crystal violet (CV) (Hi-media, Mumbai, India) solution. After 15 min, CV solution was discarded completely and wells were filled with 200 µL of 95% ethanol to solubilize CV from the stained cells. The biofilm biomass was then quantified by measuring the absorbance at OD 470 nm in a microplate reader (Thermo Scientific Multiskan Ex, India).
2.5. Standardization of extracts of all Acacia species by UPLC-PDA method using rutin as reference standard
Varying ratios of acetonitrile (Solvent-A) and water (Solvent-B) were used as mobile phase, column oven temperature, detection wavelength and mobile phase flow rate were investigated for better separation of the analyte. A gradient program was employed for the separations of reference compound rutin in one run within the reasonably short run time. The gradient was set up such that for the first 2 min the solvent-A was linearly increased from 0% to 10% followed by a steeper gradient, 10 to 35% of solvent-A in the next 3 min. Again the gradient was decreased from 35% to 20% in the next 2 min and finally, an additional 2 min of separation was extended with 100% solvent-B. The UV-detection wavelength of 332 nm was set as per the absorption maxima (λmax) for rutin.
2.6. Statistical analysis
“The statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnet’s test for the estimation of total variation in a set of data”. “Results were communicated in terms of mean values with (±SD) where the probability (p < 0.01) of getting the result was considered as significant”.
3. Results and discussion
3.1. Extraction of plant material by Ultrasonic method
The estimated yields (w/w) of ASEE, ALEE, AHEE, and ATEE were found as 4.81, 5.77, 5.92, and 6.52%, respectively.
3.2. Cytotoxicity activity of ethanol extract of Acacia species
The ethanol extract of A. salicina (ASEE), A. laeta (ALEE), A. hamulosa (AHEE), and A. tortilis (ATEE) were assayed for their in vitro anticancer activity against HepG2, HEK-293, MCF-7, and MDA-MB-231 cancer cells and were found to exhibit marked toxicity (Table 1). The ethanol extracts of A. laeta (ALEE), A. hamulosa (AHEE) and A. tortilis (ATEE) were found to possess moderate cytotoxic properties against human liver carcinoma cells (HepG2; IC50 = 46.2, 39.2 and 42.3 μg mL−1, respectively) and breast cancer cell lines (MCF-7; IC50 = 57.2, 55.3 and 65.7 μg mL−1, respectively). The A. tortilis (ATEE) and A. laeta (ALEE) exhibited moderate anticancer property against HEK-293 (Kidney cells) with IC50 = 9.1 and 53.5 μg mL−1, respectively. The other species extracts were found to be ineffective against liver and kidney cancer cells. Among these species the extract of A. tortilis (ATEE) was found to contain maximum cytotoxic property against human liver carcinoma cells (HepG2) while A. hamulosa (AHEE) showed maximum cytotoxic property against breast cancer cell lines (MCF-7). A. tortilis (ATEE) exhibited highest anticancer property against Kidney carcinoma cells (HEK-293) while A. salicina (ASEE) and A. hamulosa (AHEE) were found to be ineffective against HEK-293 cells. Among all the four species of Acacia, A. laeta and A. tortilis were found effective against all the cancer lines.
Table 1.
The estimated IC50 (μg mL−1 ± SD) values of ASEE, ALEE, AHEE and ATEE.
| Sample code | HepG2 (Liver) | HEK-293 (Kidney) | MCF-7 (Breast) | MDA-MB-231 (Breast) |
|---|---|---|---|---|
| ASEE | 89.5 ± 3.81 | 75.3 ± 3.65 | 98.3 ± 5.02 | 103.1 ± 5.58 |
| ALEE | 46.2 ± 1.59 | 53.5 ± 2.23 | 57.2 ± 2.61 | 60.3 ± 2.48 |
| AHEE | 39.2 ± 1.52 | 92.1 ± 4.55 | 55.3 ± 2.57 | 59.1 ± 2.70 |
| ATEE | 42.3 ± 1.78 | 49.1 ± 1.92 | 65.7 ± 2.49 | 52.2 ± 1.99 |
| 5-Flurourasil | 3.1 ± 0.07 | 2.5 ± 0.05 | 3.7 ± 0.07 | 3.9 ± 0.09 |
3.3. Antimicrobial activity of ethanol extracts of Acacia species
All the extracts (ASEE, ALEE, AHEE, ATEE) exhibited excellent antimicrobial activity with zone of inhibition ranged from 14 to 20 mm against S. aureus, E. coli, P. aeruginosa and C. albicans (Table 2). The minimum inhibitory concentration (MIC) values of ASEE, ALEE, AHEE, and ATEE were found to be in the range of 0.2 to 3.2 mg mL−1 (Table 3). Among all these active extracts, ALEE showed relatively high potency with MIC values ranged from 0.2 to 1.6 mg mL−1 against all the microbial strains. The MIC of crude extracts of individual plants varies against different test strains. The relationship between zone of inhibition and MIC value may or may not be related. Since the crude extracts have mixture of phytoconstituents, which may influence the diffusion power of the active constituents. Several workers have made similar observations by using extracts from 15 higher plants used in Indian traditional medicine (Ahmad and Aqil, 2007). These differences could also be due to the differences in the chemical composition of these extracts as the secondary metabolites of plants have many effects including antibacterial and anticandidal properties. All the extracts (ASEE, ALEE, AHEE, ATEE) were further assayed for their biofilm inhibitory property against the P. aeruginosa PAO1 strain. All extracts exhibited statistically significant reduction in biofilm as compared to the untreated control (Fig. 1). Similar reduction in biofilm of PAO1 has previously been described with the extracts of edible plants and fruits, Capparis spinosa and T. foenumgraceum (Musthafa et al., 2010, Abraham et al., 2012, Husain et al., 2015).
Table 2.
Antimicrobial activity of ASEE, ALEE, AHEE and ATEE.
| S. No | Plant extracts | Zone of inhibition (mm) |
|||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. albicans | ||
| 1. | ASEE | 14 ± 0.4 | 19 ± 1.1 | 16 ±1.9 | 14 ± 0.6 |
| 2. | ALEE | 21 ± 0.5 | 16 ± 2.1 | 17 ± 1.7 | 20 ± 0.5 |
| 3. | AHEE | 17 ± 1.8 | 20 ± 1.9 | 20 ± 0.7 | 18 ± 1.3 |
| 4. | ATEE | 17 ± 0.9 | 19 ± 0.8 | 16 ± 1.5 | 15 ± 1.0 |
| 5. | Ampicillin | 21 ± 1.9 | – | – | – |
| 6. | Doxycycline | – | 25 ± 1.2 | 24 ± 1.7 | – |
| 7. | Nystatin | – | – | – | 23 ± 1.1 |
Table 3.
Minimum inhibitory concentration (MIC) of ASEE, ALEE, AHEE and ATEE against bacterial and fungal strains.
| S. No | Plant extracts | Minimum inhibitory concentration (mg mL−1) |
|||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. albicans | ||
| 1. | ASEE | 0.8 | 0.8 | 0.8 | 0.8 |
| 2. | ALEE | 0.8 | 0.2 | 0.8 | 1.6 |
| 3. | AHEE | 1.6 | 1.6 | 1.6 | 3.2 |
| 4. | ATEE | 0.4 | 0.8 | 0.8 | 0.8 |
Fig. 1.
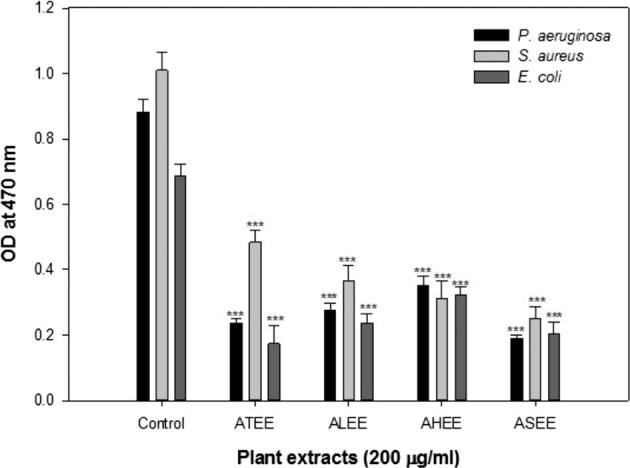
Effect of plant extracts on biofilm formation in pathogenic bacteria at sub-inhibitory concentrations. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.001.
3.4. Identification of rutin, A biflavonoid in Acacia species extract
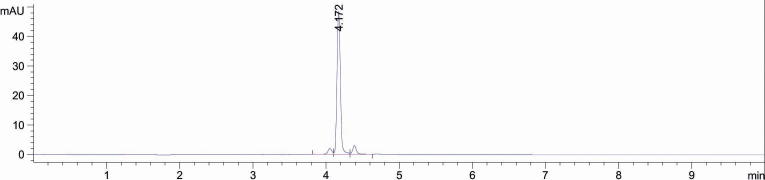
The developed UPLC- PDA method was found to furnish a sharp and intense peak of rutin at Rt = 4.172 (Fig. 2) with high resolution baseline using acetonitrile and water as suitable mobile phase (in gradient system) at flow rate of 0.18 mL min−1. Also, a good separation of different phytoconstituents present in the ethanol extract (ALEE, AHEE and ATEE) using the same mobile phase was achieved. The dried extract of A. laeta (ALEE), A. hamulosa (AHEE) and A. tortilis (ATEE) was found to contain 2.135, 1.797 and 0.446%, w/w of rutin (Table 4). The recovery % of rutin was found to be ranged from 98.61–99.20 (Table 5). Rutin has a high therapeutic value as it helps to relieve the symptoms of diseases like lymphatic and venous insufficiency, hemorrhagic diseases, and hypertension. Rutin was found to induce apoptosis in LNCaP cells (Prostate cells) at low concentration and in HepG2 cells (human hepatoma) at very high concentration by acting as pro-oxidant instead of an antioxidant which resulted in programmed cell death. It inhibited the tumor cell growth by blocking cells amelioration through G0-G1 transition which is medicated by down regulation of cyclin D1 and E. Rutin also found to exhibit excellent antibacterial property against E. coli strain (incorporated with envA1 allele) by inhibiting the action of enzyme type II topoisomerase as well as its selective promotion of E. coli topoisomerase IV-dependent DNA cleavage which is very essential for the survival of the cell. The enzyme inhibition by rutin took place due to enzyme interaction with various parts of rutin such as carbohydrate, phenyl ring, phenol and benzopyrone ring (Cushnie et al., 2006). The highest zone of inhibition and low MIC value of ALEE containing highest amount of rutin in comparison to other Acacia species supported the role of rutin as a potential antimicrobial agent. High rutin content of A. laeta (ALEE) and A. hamulosa (AHEE) also supported its strong cytotoxic activity.
Fig. 2.
The chromatogram of rutin showing retention time 4.172 min: (10 μg mL−1) [Conditions: Eclipse XDB 80Å C18 column (4.6 × 100 mm, 3.5 µm); mobile phase, acetonitrile: water (gradient system); flow rate, 0.18 mL min−1; UV-detection of rutin at 332 nm at temperature (25 ± 1 °C)].
Table 4.
Detailed analysis report of rutin in ASEE, ALEE, AHEE and ATEE.
| Name of extract | Theoretical concentration of extracts (μg mL−1) | Concentration of rutin found in extracts (μg mL−1) ± SD | % RSD | Rutin content (%) | Retention time (Rt) (min) |
|---|---|---|---|---|---|
| ASEE | 1000.0 | Not available | – | – | – |
| ALEE | 1000.0 | 21.352 ± 0.91 | 4.294 | 2.135 | 4.178 |
| AHEE | 1000.0 | 17.973 ± 0.53 | 2.954 | 1.797 | 4.169 |
| ATEE | 1000.0 | 4.464 ± 0.06 | 1.141 | 0.446 | 4.179 |
Table 5.
Recovery of rutin for the accuracy of the developed method (mean ± SD, n = 3).
| Percentage of rutin added (%) | Theoretical concentrations of rutin (μg mL−1) | Concentrations of rutin found (μg mL−1) ± SD | % RSD | % Recovery |
|---|---|---|---|---|
| 0.0 | 20.0 | 19.841 ± 0.22 | 1.118 | 99.20 |
| 50.0 | 30.0 | 29.601 ± 0.35 | 1.188 | 98.67 |
| 100.0 | 40.0 | 39.446 ± 0.81 | 2.055 | 98.61 |
| 150.0 | 50.0 | 49.568 ± 1.10 | 2.235 | 99.13 |
4. Conclusion
The results obtained from the present experiment are very exciting, taking into consideration the pharmacological importance of the studied microbial strains and cancer cell lines. The observed results justify the screening of different species of genus Acacia traditionally used in folk medicine to treat several ailments like infections and cancer. These data provides evidence that the aerial parts of A. laeta and A. hamulosa possess moderate cytotoxicity against breast, kidney and liver cancer cells while A. salicina extract was inactive against all the cell lines. A. tortilis extract was found to exhibit mild cytotoxic property. The extracts of all tested Acacia species showed excellent antimicrobial activity against the tested microbial and fungal strains and found as a potential cure for the infection. The high content of rutin in A. laeta ethanol extract (ALEE; as reported by the UPLC method) supported its high cytotoxic property as well as excellent antimicrobial property [comparatively maximum zone of inhibition (21 mm) and minimum IC50 value (0.2 mg mL−1)]. Since, Acacia species (A. laeta and A. hamulosa) contains numerous phytoconstituents which might prove to be highly cytotoxic and antimicrobial agents, we suggest that these species can be further subjected to the isolation of more cytotoxic and antimicrobial compounds.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-150.
Acknowledgments
Conflict of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed F. Alajmi, Email: alajmister@gmail.com.
Perwez Alam, Email: aperwez@ksu.edu.sa.
Saleh I. Alqasoumi, Email: sqasoumi@ksu.edu.sa.
Nasir Ali Siddiqui, Email: nasiratksu@gmail.com.
Omer A. Basudan, Email: omer_basodan@yahoo.com.
Afzal Hussain, Email: afzal.hussain.amu@gmail.com.
Fohad Mabood Husain, Email: fahadamu@gmail.com.
Azmat Ali Khan, Email: azmatbiotech@gmail.com.
References
- Abraham I.S.V.P., Agilandeswari P., Musthafa K.S., Pandian S.K., Ravi A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against gram negative bacterial pathogens. Food Res. Int. 2012;45:85–92. [Google Scholar]
- Ahmad I., Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESbetaL-producing multidrug-resistant enteric bacteria. Microbiol Res. 2007;162:264–275. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Ahmad I., Beg A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi drug resistant human pathogens. J. Ethnopharmacol. 2001;74:113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- Alam, P., Alajmi, M.F., Arbab, A.H., Parvez, M.K., Siddiqui, N.A., Alqasoumi, S.I., Al-Rehaily, A.J., Al-Dosari, M.S., Basudan, O.A., 2017. Comparative study of antioxidant activity and validated RP-HPTLC analysis of rutin in the leaves of different Acacia species grown in Saudi Arabia. Saudi Pharm. J. 25 (5), 715–723. [DOI] [PMC free article] [PubMed]
- Alam P., Alajmi M.F., Siddiqui N.A., Al-Rehaily A.J., Alharbi H., Basudan O.A., Hussain A. Densitometric validation and analysis of biomarker β-amyrin in different Acacia species (leaves) grown in Kingdom of Saudi Arabia by high performance thin-layer chromatography. Pak J Pharm Sci. 2015;28:1485–1491. [PubMed] [Google Scholar]
- Asolkar, L.V., Kakkar, K.K., Chakre, O.J., 2005. Glossary of Indian medicinal plants with active principles. Part I (A-K). New Delhi NISCAIR and CSIR, 10-11.
- Barapatre A., Meena A.S., Mekala S., Das A., Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int J Biol Macromol. 2016;86:443–453. doi: 10.1016/j.ijbiomac.2016.01.109. [DOI] [PubMed] [Google Scholar]
- Boubaker J.B., Mansour H., Ghedira K., Chekir G.L. Polar extracts from (Tunisian) Acacia salicina Lindl. Study of the antimicrobial and antigenotoxic activities. BMC Complement Altern Med. 2012;12:37. doi: 10.1186/1472-6882-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhlel I., Limem I., Skandrani I., Nefatti A., Ghedira K., Dijoux-Franca M.G., Leila C.G. Assessment of isorhamnetin 3-O-neohesperidoside from Acacia salicina: protective effects toward oxidation damage and genotoxicity induced by aflatoxin B1 and nifuroxazide. J. Appl. Toxicol. 2010;30:551–558. doi: 10.1002/jat.1525. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Pakrashi S.C. NISCAIR Press; New Delhi: 2000. The Treatise on Indian Medicinal Plants; pp. 51–53. [Google Scholar]
- Chatti I.B., Boubaker J., Skandrani I., Bhouri W., Ghedira K., Chekir G.L. Antioxidant and antigenotoxic activities in Acacia salicina extracts and its protective role against DNA strand scission induced by hydroxyl radical. Food Chem. Toxicol. 2011;49(8):1753–1758. doi: 10.1016/j.fct.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. Review. Erratum Int J Antimicrob Agents. 27, 2006, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymock W., Warden C.J.H., Hooper D. Shrishti book distributors; New Delhi: 2005. Pharmacographica Indica. A History of the Principal Drugs of Vegetable Origin. p. 556. [Google Scholar]
- Eloff J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- Gulco P. CCRUM; New Delhi: 2001. Medicinal Plants in Folklores of Bihar and Orissa; p. 25. [Google Scholar]
- Husain F.M., Ahmad I., Khan M.S., Al-Shabib N. Trigonella foenum-graceum (Seed) extract interferes with quorum sensing regulated traits and biofilm formation in the strains of Pseudomonas aeruginosa and Aeromonas hydrophila. Evid. Based Complement Alternat. Med. 2015:879540. doi: 10.1155/2015/879540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy P.P., Thomas J., Mathew S., Skaria P. Medicinal. Plants Kerala Agricultural University Publications; Kerala, India: 1998. p. 3. [Google Scholar]
- Kritikar K.R., Basu B.D. Indian Medicinal Plants with Illustrations. Uttaranchal Oriental Press; 2003. pp. 1289–1292. second ed. [Google Scholar]
- Kumar B.P., Singh R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. Biomed. Res. Int. 2014;2014:572013. doi: 10.1155/2014/572013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud M.F., Alrumman S.A., Hesham Ael-L. Biological activities of some Acacia spp. (Fabaceae) against new clinical isolates identified by ribosomal RNA gene-based phylogenetic analysis. Pak. J. Pharm. Sci. 2016;29:221–229. [PubMed] [Google Scholar]
- Mohammad Alharbi W.D., Azmat A. Anticonvulsant and neuroprotective effects of the Acacia tortilis growing in KSA. Pak. J. Pharm. Sci. 2015;28:531–534. [PubMed] [Google Scholar]
- Mulaudzi R.B., Ndhlala A.R., Kulkarni M.G., Finnie J.F., Van Staden J. Antimicrobial properties and phenolic contents of medicinal plants used by the Venda people for conditions related to venereal diseases. J. Ethnopharmacol. 2011;135:330–337. doi: 10.1016/j.jep.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Musthafa K.S., Ravi A.V., Annapoorani A., Packiavathy I.S.V., Pandian S.K. Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl-homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy. 2010;56:333–339. doi: 10.1159/000320185. [DOI] [PubMed] [Google Scholar]
- Nadkarni K.M. The Indian Plants and Drugs. New Delhi: Shrishti Book Distributors. 2005;4:5. [Google Scholar]
- O’Toole G.A., Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Perez C., Pauli M., Bazerque P. An antibiotic assay by the well agar method. Acta Biol. Med. Exp. 1990;15:113–115. [Google Scholar]
- Said H.M. Hamdard Pharmacopeia of Eastern Medicine. New Delhi Sri Satguru Publications; 1997. p. 353. second ed. [Google Scholar]
- Xia E.Q., Ai X.X., Zang S.Y., Guan T.T., Xu X.R., Li H.B. Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason Sonochem. 2011;18:549–552. doi: 10.1016/j.ultsonch.2010.09.015. [DOI] [PubMed] [Google Scholar]