Abstract
Psychological stress stimulates physiological responses releasing catecholamines and corticoids, which act via corresponding receptors on immune cells, producing a shift in the cytokine balance. These responses are variable depending on the nature of stressors. The effect of the academic stress on the production of the Th1-cytokines (TNF-α, IFN-γ, IL-1β, IL-2, IL-6 and IL-8) and Th2-cytokines (IL-1ra, IL-4, IL-5 and IL-10) on 35 medical/health sciences students after completing their questionnaires was investigated. Blood samples were taken at three stages; baseline stage at the beginning, midterm and final academic examination stages. Plasma cortisol and cytokines were measured during the three stages. The last two stages were compared with the baseline non-stress period. Results of the stress induced during the final examination stage were the highest with a significant increase in cortisol release, IL-4, IL-5 and IL-1ra release with a shift in Th1:Th2 cytokines balance towards Th2. Whereby, the midterm stage did not show significant reduction in Th1-cytokines except for TNF-α, with an increase in IFN-γ level that was reduced in the third stage. Th2 cytokine, IL-1ra, had positive correlations with Th1 cytokines; IL-2 and IFN-γ in the second stage and IL-6 cytokine in the third stage. Cortisol was positively correlated with IL-8 in the last stage and heart rates had negative correlation with IL-10 in the first and last stages. Findings of this study indicate that exam stress down-regulates Th1 with a selective up-regulation of Th2-cytokines. In conclusion, Cortisol might have a role in suppressing the release of Th1- mediated cellular immune response which could increase the vulnerability among the students to infectious diseases.
Keywords: Academic stress, Cortisol, Th1-cytokines, Th2-cytokines, Medical/health sciences students
1. Introduction
The relationship between psychological stressors and immunity has gained lots of interest among scientists and clinicians in the field of health psychology and psychoneuroimmunology. Studies on psychological stress found that depending on the type of stress, stress hormones may suppress or enhance the immune function. Some studies suggested that stress hormones such as glucocorticoids and catecholamines inhibit the production of proinflammatory cytokines. Whereby, they stimulate the production of the anti-inflammatory cytokines (Chrousos, 2000, Connor et al., 2005, Dhabhar, 2014, Elenkov and Chrousos, 2002). Other studies indicated that stress hormones in stress are potential mediators of exhaustive exercise-induced immunosuppression (Kohut et al., 2005). On the other hand, researchers also suggested that certain local responses and under certain conditions, stress hormones actually may boost regional immune responses by increasing the production rate of the pro-inflammatory cytokines such as Interleukin-6 (IL-6), Tumor necrosis factor - alpha (TNF-α) and Interferon (IFN)-gamma (Maes et al., 1998, Elenkov and Chrousos, 2002). Moreover, recent studies indicated that psychological stress causes sympathetic responses in the autonomic nervous system leading to several effects such as reducing heart rate (Föhr et al., 2016, Lee et al., 2014).
Cytokines are small proteins released by white blood cells and modulate the balance between humoral and cell-based immune responses. They have a specific effect on the interactions and communications between cells. CD4-expressing T helper cells are the major source for cytokine production and regulation and they are of two subtypes of cells known as helper T cells type 1 (Th1) and type 2 (Th2) (Chen, 2007). Th1 can evoke cell-mediated immunity and phagocyte-dependent inflammation and make disease worse by producing proinflammatory cytokines such as IFN-γ, IL-2, TNF-α, IL-6, IL-8 and IL-1β. Th2 cells are known to evoke strong humoral-mediated immunity with antibody production and eosinophil accumulation by producing anti-inflammatory cytokines such as IL-1ra, IL-4, IL-5 and IL-10 which counteract the Th1 mediated response (Chen, 2007, Shinkai et al., 2002). Excessive proinflammatory responses as in acute inflammation can lead to uncontrolled tissue damage, fever, inflammation and, in some cases, shock and death. Whereas, excessive Th2 response, as in chronic inflammation, is associated with allergies and atopy (asthma, eczema, allergic rhinitis & allergic conjunctivitis). The balance between Th1 and Th2 responses is needed for optimal health whereas any disturbance would influence many pathological processes (Chen, 2007, Dhabhar, 2014, Kidd, 2003, Kim and Maes, 2003). Therefore, it is very important to distinguish between the types of psychological stressors regarding their duration, frequency and severity to understand their impact on the Th1 and Th2 cytokines.
Several models have been studied in this field focusing on the immunosuppressive consequences of stress on the immune system (Dhabhar, 2014, Segerstrom and Miller, 2004). One of the studied models was on the academic stress such as on the effect of studying or of examination periods on immunity and cytokine release (Kim and Maes, 2003). Results were variable, some indicated that examination stress down-regulates immune functions such as the lymphocyte proliferation, the production of IFN-γ, the activity of natural killer (NK) cells, the mucosal wound healing and the salivary immunoglobulin A (IgA) (Glaser et al., 1986, Marucha et al., 1998, Murphy et al., 2010, Rojas et al., 2002). Other studies showed immune activation in response to examination stress such as enhancing salivary IgA in students during acute stress of an imminent examination (Murphy et al., 2010). Also, the levels of phytohemagglutinin (PHA)-stimulated IL-2 production and lymphocyte proliferative response to PHA, were higher during an examination period compared to a non-examination period (Koh et al., 2001, Koh et al., 2006). In 1998, Marshall indicated that stress induced by exams, among healthy medical students, changed the immune response by shifting the balance of Th1 and Th2 cytokines towards Th2 cytokine response.
Several types of academic stressors, such as too many assignments, academic commitments, financial pressure, students’ accommodation, taking and studying for exams, study loads, competition attitudes and other relative stressors, have been identified by researchers (Assaf, 2013, Fairbrother and Warn, 2003). The degree of stress experienced by students may differ from one university to another depending on the university system, environment and culture in the country. But, almost all, consider academic examination as a naturally occurring psychological stressor. In 2014, Dhabhar evaluated the range of effects of stress on immune function, and discussed how these effects may promote immunoprotection versus immunopathology. In our previous survey study done at The University of Jordan, the negative relations between academic stress and health outcomes on Pharmacy College students were determined (Assaf, 2013). In this study, we wanted to analyze the influence of the academic stress experienced by medical/health sciences students at The University of Jordan on their immune system, and to determine the direction of the immune balance whether it would enhance or suppress their immune response at the beginning, mid of the semester and in the final exam periods. So, the aim of this study is to determine the psychological academic stressors and measure the levels of the stress hormone (Cortisol) and the pro-inflammatory (Th1) and anti-inflammatory (Th2) cytokines released in the blood of the medical/health sciences students at The University of Jordan. The secretion of cortisol hormone was measured during the three stages as a marker of psychological stress. Then, to compare changes in the plasma levels of the stress hormone (cortisol) and cytokines (Th1 and Th2) released during the mid of the semester and in the final exam period with the baseline non-stress period at the beginning of the fall semester.
2. Materials and methods
2.1. Participants
The ethical approval for the study was obtained from the Institutional Review Board of Jordan University Hospital. The study was performed over the course of an academic semester between September 2010 and February 2011. Volunteers were recruited from a group of medical/health sciences students at The University of Jordan (Amman, Jordan), excluding asthma suffering students and the first-year students who have not yet adjusted to the University environment. A total of 40 students responded positively and met the inclusion criteria, of whom 35 complied fully with the requirements of this study in the three stages (34 students from school of Pharmacy and one student from school of Medicine) comprising 88.6% females and 11.4% males. The purpose and procedures of the study were explained to all potential subjects and an informed consent was obtained from those who decided to participate. The clinical parameters of resting blood pressure and heart rate for each participant were taken at the beginning and at the end of the study. They were measured twice each over a 5 min interval and the average of the readings was recorded.
The study was done along three stages, the first week of the semester, the mid of the semester and during the academic final examinations of the fall semester of 2010/2011. Volunteers were all unmarried, non-smokers, with no chronic disease and with no recent acute illness or use of antibiotic, steroidal, or anti-inflammatory medications. To protect their anonymity, designated numbers for the participants were placed on the questionnaire forms and on their blood samples labels.
2.2. Procedure
This study was carried out using a longitudinal design. Blood samples collection was carried out through the three stages during the period September 2010 - January 2011. The results of the last two stages were compared with the baseline first stage. An interviewer administered a questionnaire to the participants. The questionnaire was designed to reveal information about stress as perceived by participants at the beginning and end of the academic fall semester of 2010/2011. After providing a written informed consent, the respondent students completed a pre-validated short questionnaire survey. The questionnaire was developed by reviewing available surveys in the literature which used to measure students’ academic stress as indicated in the previous study (Assaf, 2013). Validity and reliability of the questionnaire were evaluated by an expert committee of one clinical immunologist, one clinical pharmacist and one statistician. This was to ensure its applicability relevant to the medical/health sciences students at The University of Jordan. Internal Consistency Reliability was tested by the Cronbach’s alpha Coefficient and they all were above 0.65.
The questionnaire was handed to the responding medical/health sciences students, after briefing, discussing and answering questions regarding this survey by the researcher and the research assistants. It was administered twice: once at the beginning of the semester and again in the academic final examinations period. The purpose was to indicate the psychological status of the respondents at the beginning of the semester and during the semester. Blood samples were collected within the three periods.
2.3. Psychological assessment
To detect the effect of academic stress on the students’ immune system taking into consideration that they may not be under the same amount of stress in every single semester, this study was conducted in the fall semester after students returning from their summer vacation. The clinical parameters of blood pressure, heart rates and body mass index (BMI) (calculated from self-reported height and weight) were recorded. The questionnaire was designed of four major parts, in addition to a part about general personal information including age, sex and marital status. The first part was mostly about study concerns regarding that fall semester. The second part was about family concerns, accommodation and employment if applicable. The third part was about the daily hassles that can contribute to a student’s health such as exercising, nutritional routines, smoking, having social activities and the amount of sleeping. To determine the impact of university stress on student life, participants were asked to indicate the frequency of changes in their physical and emotional states and their attitudes during the fall semester of 2010/2011. Students described their immune system responses in the last part of the survey by indicating the changes in their health (such as having cold/flu, tonsillitis, hair problems, skin problems, getting medical care and hospitalization) and the frequency of their occurrence during that fall semester. Two forms of the questionnaire, one for the first and one for the third stages, differ in some questions to determine the impact of the academic semester on the students. At the first stage and stage three, participants were asked to indicate if the final exam is the most stressful factor and midterm exams is the second most stressful factor compared to other periods and factors. Health-related data were all self-reported by the participants.
2.4. Blood collection
All subjects underwent a relaxation period for approximately 15 min before giving blood samples. 10–15 ml of blood samples were collected in heparinized vacutainers during the daytime of the three stages at almost equal time points, generally between 9:00 a.m. and 3:00 p.m, where plasma was separated within one hour of collection and stored at –80 °C until the end of the study. Although there had been some concern about potential circadian variations (e.g., cortisol levels) with physiological measures, variations were related mostly to the difference in levels between the waking and sleeping hours, early in the morning and late in the evening. On the other hand, daytime changes are very gradual and much less pronounced (Guyton, 2006). In order to capture the natural effect of stress at stages two and three more accurately, collection of blood samples was carried out during daytime hours closely matching each student's anticipation to the first baseline stage for the blood collection time. In the third stage, all blood samples were collected from students who already had at least one final exam and will still be having more exams. On the average, the time difference between baseline and exam samples was 1.5 h. At the time of the assay, samples were thawed and cytokine and cortisol assays were performed in duplicate in a single batch using commercially available ELISA kits.
2.5. Cortisol assay
To determine the cortisol production level in each stage, 10 µl of plasma from each stage was tested by ELISA assay according to the manufacturer’s protocol (Diagnostic automation, INC, USA). All incubation steps were performed at room temperature. The optical density was read at 450 nm against blank using the microplate reader (Biotek, USA). This assay has a sensitivity of 0.36 µg/dL. The intra-assay coefficient of variation (CV) was less than 10%. The interassay CV was less than 12%.
2.6. Cytokine assay
To determine the cytokine production level in each stage, 50 µl of plasma from each stage was tested. The concentrations of human TNF-α, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 (eBioscience, San, Diego, CA) and IL1ra (R&D systems, Oxford, UK) were determined using ELISA assay according to the manufacturer’s protocol. All incubation steps were performed at room temperature. The optical density at 450 nm, corrected by the reference wavelength of 570 nm, was measured with microplate reader (Biotek, USA). All cytokine assays were calibrated against the World Health Organization international standards by the kit manufacturer. The intra- and inter-assay coefficients of variation were less than 9% for all cytokine assays.
2.7. Statistical analysis
Responses to the questionnaire were coded and statistical analyses were calculated using SPSS, version 17 (SPSS, Inc, Chicago, IL). Data were examined for accuracy and for assumptions for parametric tests. Pearson's Chi-square was used to test for significant differences between responses during any two stages. Results were considered significant when p value was less than 0.05. In a few cases, some participants failed to answer some questions, resulting in missing data which were not estimated or used in analysis.
All blood samples were assayed in duplicate, and the mean µg/dL or pg/ml was calculated for the cortisol or cytokine assays respectively. To reduce variability within individual subjects, all samples from each subject were assayed at the same time. For comparison of quantitative variables of the three stages of plasma cytokine and cortisol concentrations, non-parametric One way ANOVA (Kruskal-Wallis test) was used. The Pearson correlation coefficient (r) and associated probability (P) were calculated for the data sets of the clinical parameter with the levels of plasma cytokines and cortisol in the third stage. The correlation coefficients between the biological assays and the first two most stressing factors during that semester were determined using Spearman's rank-order correlation. Values less than 0.05 were considered significant. Results to be expressed as mean ± S.E.M and 95% confidence intervals (CI) were given for all comparisons. All data were analyzed using GraphPad Prism 5 software (GraphPad Software, Inc, La Jolla, Calif) except for Pearson correlation coefficient was analyzed using SPSS, version 17 (SPSS, Inc, Chicago, IL).
3. Results
3.1. Demographic and psychological stressors
The majority of the participants were females with a ratio of 7.7:1 ranged between 19 and 25 years old with an average age of 22.11 years (S.E.M = 0.2017). Only two of the participants were not living with their parents during the referred semester as their families live in a different city. No demographic characteristics were significantly correlated with psychological stress or immunological responses. Also, there were no significant changes in their systolic or diastolic blood pressure values between the first and last stages. Whereas, a significant increase in the heart rate between the first stage (82.80 ± 1.986) and the third stage (89.46 ± 2.927) was noticed. The body mass index (BMI) of the students in this study ranged between 16.02 and 34.29 (Mean BMI 22.69 ± 0.6460), where males had a higher mean BMI (26.07 ± 1.080) than females (22.23 ± 0.6794), although the difference was not statistically significant (Table 1).
Table 1.
The Clinical parameters of blood pressure, heart rates and Body Mass Indices (BMI) of medical/health sciences students participating in the study to assess perceived stress and immune response.
| Clinical parameters | Mean ± S.E.M |
|---|---|
| N = 35 | |
| BMI | 22.69 ± 0.6460 |
| Systolic blood pressure (mm Hg) | |
| Stage One | 120.1 ± 3.410 |
| Stage Three | 122.0 ± 2.025 |
| Diastolic blood pressure (mm Hg) | |
| Stage One | 76.91 ± 2.186 |
| Stage Three | 77.12 ± 2.024 |
| Heart rate (BPM) (p = 0.028) | |
| Stage One | 82.80 ± 1.986 |
| Stage Three | 89.46 ± 2.927 |
At the beginning of the semester, the participants were asked to indicate if final exams is considered the most stressing factor for them followed by midterm exams. They were also asked the same by the end of the semester. At the beginning of the semester, only one student (2.9%) indicated that final exams are the most stressful factor while the majority of the students (74.3%) agreed that midterm exams is the second most stressful factor. On the other hand, the majority indicated that final examination period was the most stressful factor (65.7%) at end of the semester and the second most stressful factor was the midterm period (71.4%) during that study semester.
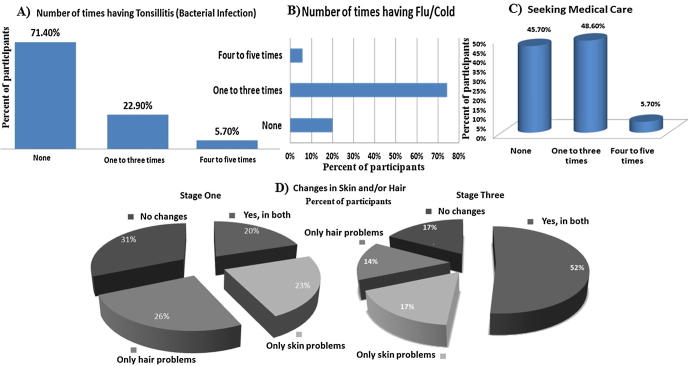
No health issues were significantly correlated with psychological stressors related to their study or family issues. Fig. 1 shows their answers related to the number of times they had some health problems during the semester. At the beginning of the study, (28) 80% of the participants indicated that they do not easily get cold/flu, while later during that semester almost 80% of the participants got cold/flu. On the other hand, the majority got ill during the mid of the semester and some during the final exams which might be due to the effect of the season. Also, at the beginning of the study, (7) 20% of them had problems in both hair and skin like dryness and this was increased to reach (18) 51.4% at the end of the study. More than 50% of the participants were at least once under medical care due to health issues during that fall semester. Also, 17 (48.6%) participants had health issues during the midterm period where nine (25.7%) of them, had been unable to attend university classes during that semester due to illnesses (data not shown).
Fig. 1.
Immune-related diseases among medical/health sciences students participating in the study (A, B and C) at the third stage and (D) comparing the first with the last stage.
3.2. Effect of academic stress on the cortisol level
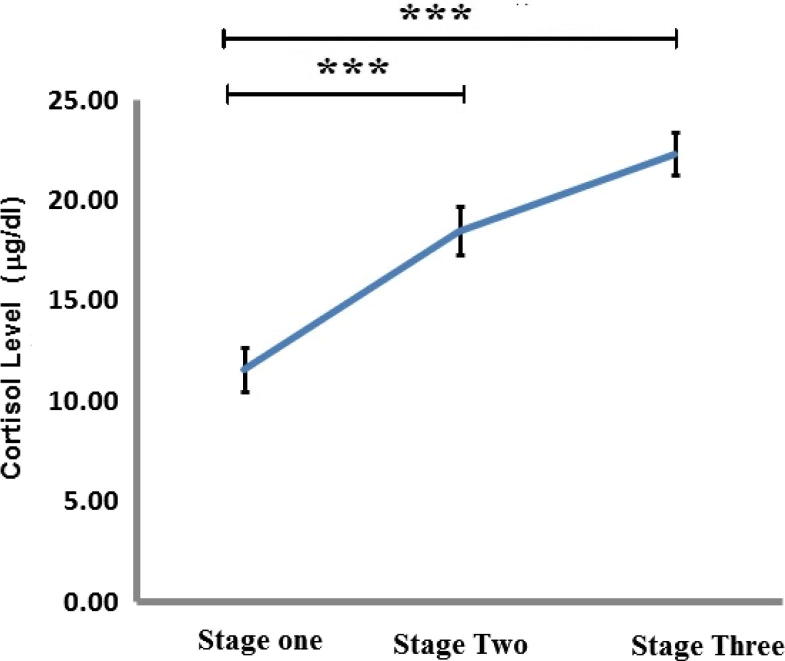
The levels of cortisol released during the three stages were significantly increased as illustrated in Fig. 2. The levels of cortisol increased from stage one to stage two with further increase in stage three. The mean of cortisol level during the third stage (22.35 ± 1.069) was the highest compared to the first stage (11.61 ± 1.098) and the second stage (17.98 ± 1.29).
Fig. 2.
The effect of academic stress on cortisol release (mg/dl) during the pre-stress period (stage one), the midterm period (stage two) and the final exam period (stage three). Each data point represents the mean ± SEM. Statistically significant differences with P < 0.05 were considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
To determine the stress-induced cortisol levels in the last two stages, they were compared with the first baseline stage mean value (11.6 μg/dl). Fig. 3 shows the percent of participants who had cortisol levels above the baseline mean value.
Fig. 3.
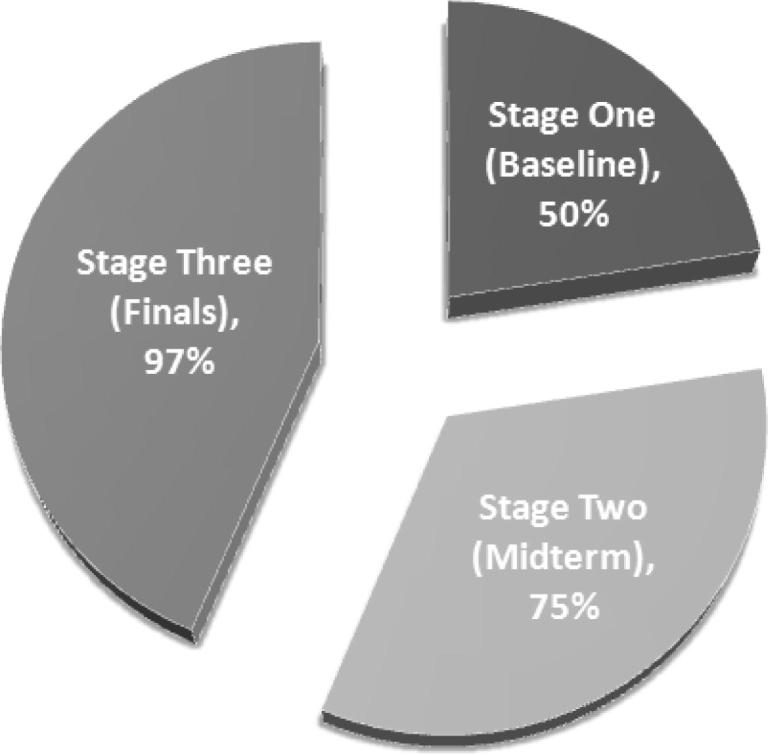
Percentage of participants who had cortisol levels above the baseline mean value (11.6 μg/dl) in the three stages.
3.3. Effect of academic stress on cytokine release
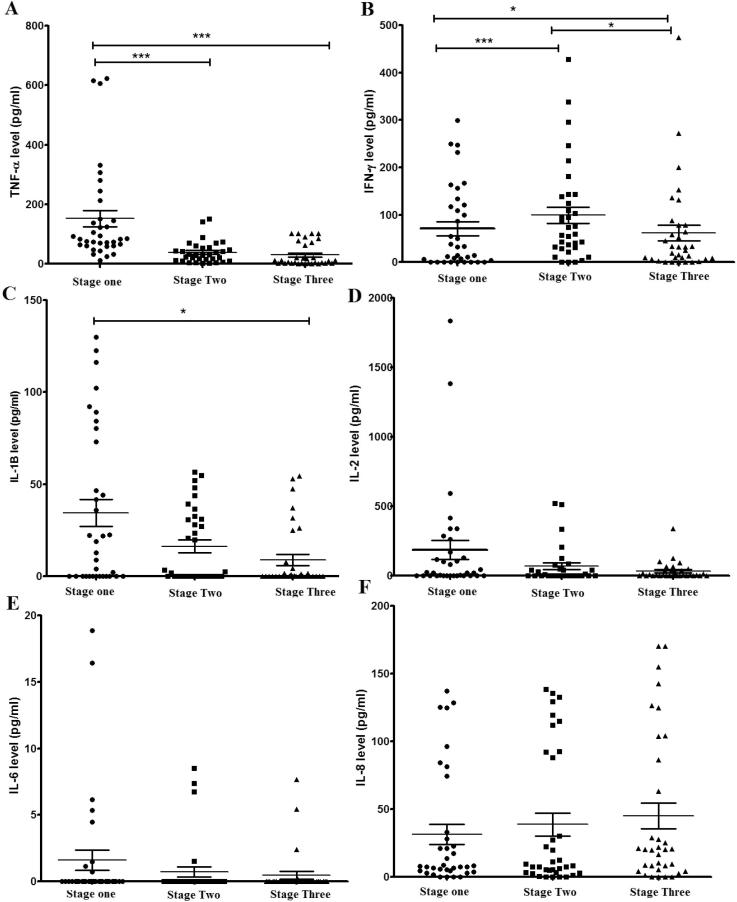
As shown in Fig. 4, Fig. 5, significant changes were noted in several cytokine levels between baseline and the other two stressful periods. In Fig. 4, Th1 proinflammatory cytokines (TNF-α, IFN-γ and IL-1β) showed a significant reduction during the final examination period (stage three), but not during the second stage except for TNF-α which showed a significant reduction in both the second and third stages when compared with the baseline first stage. On the other hand, IFN-γ, showed a significant reduction in the third stage compared with the second stage, with an induction in the second stage compared to the baseline first stage. No significant changes between stages were noticed in the release of pro-inflammatory cytokines IL-2, IL-6 and IL-8 although the trend of the reduction in the mean values of IL-2 and IL-6 is noticed in between the stages (Fig. 4).
Fig. 4.
The effect of academic stress on Th1 cytokine levels during the pre-stress period (stage one), the midterm period (stage two) and the final exam period (stage three). (A) TNF-α, (B) IFN-γ, (C) IL-1β, (D) IL-2, (E) IL-6 and (F) IL-8. Data represent the mean concentrations (pg/ml) of triplicates. Statistically significant differences with P < 0.05 were considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
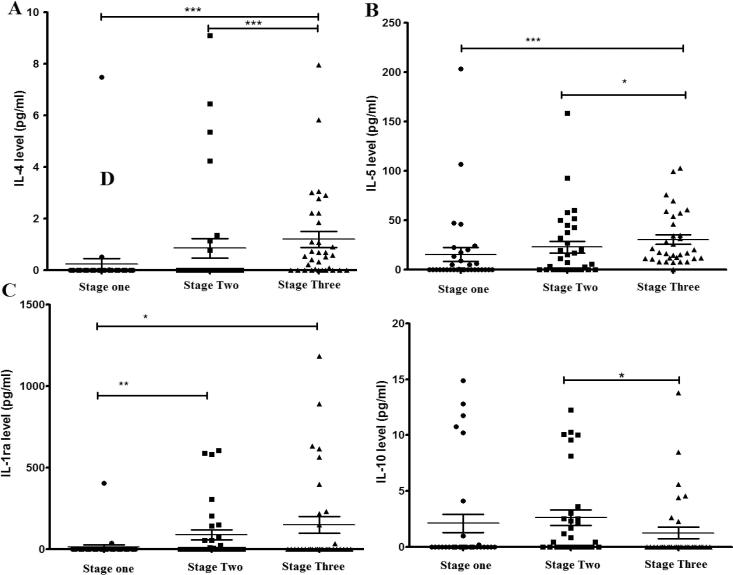
Fig. 5.
The effect of academic stress on Th2 cytokine levels during the pre-stress period (stage one), the midterm period (stage two) and the final exam period (stage three). (A) IL-4, (B) IL-5, (C) IL-1ra and (D) IL-10. Data represent the mean concentrations (pg/ml) of triplicates. Statistically significant differences with P < 0.05 were considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
In contrast, the levels of Th2 anti-inflammatory cytokines (IL-4, IL-5 and IL-1ra) showed a significant increase during the third stage when compared with the first baseline stage. IL-4 and IL-5 were also significantly increased between midterm and final exams stages, whereas IL-1ra significantly increased in the second stage compared with the first, while no changes were noticed between second and third stages. On the other hand, IL-10 (a potent anti-inflammatory cytokine which is secreted by lymphocytes of the Th2 sub-types) showed a significant reduction in its level in the third stage when compared with the second stage only (Fig. 5).
To assess the specific Th1:Th2 cytokine balance and to indicate the shift in the secreting cells, the ratio of the mean values for Th1 cytokines/Th2 cytokines were calculated for the three stages showing a decrease in Th1/Th2 ratio from the first to the last stage. Table 2 is showing one of them, IFN-γ/IL-4 ratio.
Table 2.
The ratio of the mean values for IFN-γ and IL-4 cytokines in the three stages.
| Th1/Th2 Ratio | Baseline (stage 1) | Midterm (stage 2) | Final exam (stage 3) |
|---|---|---|---|
| IFN-γ/IL-4 | 297.85 | 114.75 | 50.08 |
3.4. Correlations between questionnaire answers and the biological assays (cytokines and stress hormone)
The correlation coefficients between the biological assays and the first two stressing factors (final and midterm exams) that affected the participants’ life during that semester were determined using Spearman's rank-order correlation. The stressing factor was categorized into two groups, one with the majority who indicated final exams as the first most stressful factor or midterm exams as the second most stressful factor compared to those who chose other factors. The most stressing factor in the third stage, the final exams, showed a significant association only with IL-2 release in the third stage (rs = 0.439, p = 0.012) where 50% of them released IL-2. The second most stressful factor in the last stage, midterm exams, showed an association with another Th1 cytokine, INF-γ, but from the first stage (rs = 0.514, p = 0.002), where the majority (72%) released the cytokine. When they were asked about the time they had to seek out medical care during that semester, almost 22% of them indicated that they did not get ill while the rest got ill and the majority (63%) of those who got ill indicated that it was during the mid of the semester. This also showed an association with cortisol, IL-4 and IL-10 release. Cortisol release in the third stage was significantly associated with the increase in the need for seeking medical care during the midterm period (rs = 0.369, p = 0.03). Th2 cytokines, IL-4 and IL-10, from the first stage were significantly associated with the increase in the need for medical care during that semester (rs = 0.373, p = 0.039 and rs = 0.444, p = 0.09 respectively). Also, IL-4 release on the second stage was significantly associated with the need to seek for medical care (rs = 0.396, p = 0.02). Both cytokines showed no release in almost all of those who needed to seek medical care, whereas it was only released in those who indicated that they did not got ill during that semester.
3.5. Correlations between clinical parameters and the biological assays (cytokines and stress hormone)
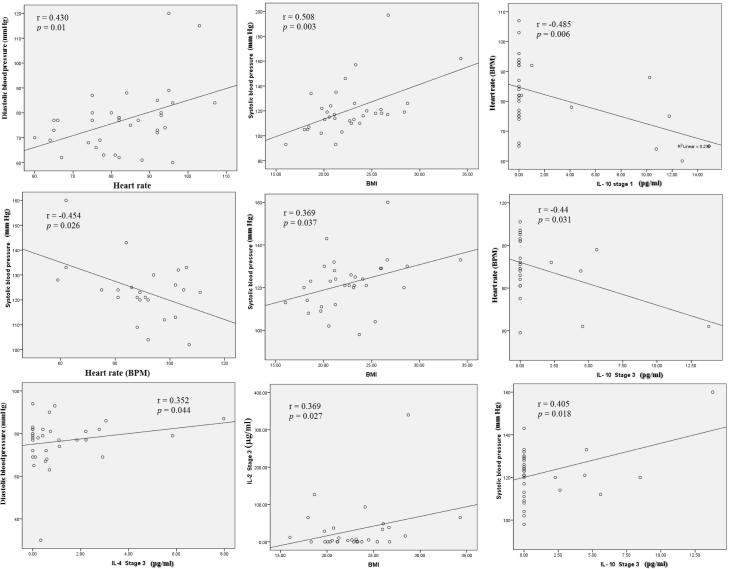
The correlations between the clinical parameters and the plasma levels of the released cytokines and cortisol during the baseline stage and the third stage were measured. No significant association was found between the release of cortisol and the clinical parameters. On the other hand, results revealed that production of plasma Th2 cytokine IL-4 was positively correlated with diastolic blood pressure in the third stage while it did not show any correlation in the baseline first stage. Systolic blood pressure was correlated with several clinical parameters and IL-10 cytokine. It showed a positive correlation with BMI in the first and third stages and IL-10 release in the third stage. On the other hand, it was negatively correlated with heart rate at the third stage as shown in Fig. 6. Heart rate was also negatively correlated with plasma Th2 cytokine IL-10 release in both the first and third stages. During the third stage, BMI also showed a positive correlation with plasma Th1 cytokine IL-2 release (Fig. 6).
Fig. 6.
Correlation between final examination third stage-induced changes in BMI (Body Mass Index), Heart rate, blood pressure and plasma cytokines and cortisol concentrations. Correlations between values were assessed by Pearson’s correlation confident (r). Statistically significant differences with P < 0.05 were considered significant.
3.6. Correlation between cytokines and stress hormone
As shown in Table 3, correlation coefficients between Th1 and Th2 cytokines and cortisol indicated that IL-1ra and IL-4 (Th2 cytokines) had the highest correlations with the other cytokines but not in the first stage. During the examination periods (stages two and three), different cytokines showed some significant correlations with other cytokines (Table 3B and C), but the least correlation was during the baseline first stage (Table 3A). In the second stage, the levels of IL-1ra showed positive correlations with IFN-γ and IL-2 cytokines and in the last stage it was also positively correlated with IL-6. Furthermore, IL-4 was positively correlated with IL-2, IL-6 and IL-8 release but in the second stage only. Meanwhile, IL-10 showed positive correlations with IL-6 in the first stage while it was negatively associated with IL-1β in the second stage. During the second stage, IL-5 showed a positive correlation with IL-8 release and cortisol was also positively correlated with IL-8, but only during the third stage.
Table 3.
Correlations between Th1 and Th2 cytokines and cortisol release. (A) Baseline, stage one, (B) Midterm period, stage two, (C) Final examination period, stage three (*p < 0.05 and **p < 0.01).
| IL-10 | IL-5 | IL-4 | IL-1ra | Cortisol | |
|---|---|---|---|---|---|
| (A) Stage one | |||||
| Cortisol | −0.051 | 0.235 | −0.061 | 0.117 | 1 |
| IFN-γ | 0.012 | 0.059 | −0.027 | 0.162 | 0.013 |
| TNF-α | 0.079 | −0.172 | 0.056 | −0.022 | −0.13 |
| IL-8 | −0.157 | −0.211 | −0.047 | 0.248 | 0.076 |
| IL-6 | 0.388* | 0.039 | −0.071 | −0.048 | −0.23 |
| IL-2 | −0.106 | 0.067 | −0.079 | −0.078 | 0.012 |
| IL-1β | −0.114 | −0.193 | −0.155 | 0.026 | 0.332 |
| (B) Stage two | |||||
| Cortisol | −0.116 | −0.235 | −0.007 | −0.134 | 1 |
| IFN-γ | −0.151 | 0.142 | 0.130 | 0.588** | −0.105 |
| TNF-α | 0.035 | −0.080 | −0.037 | 0.253 | −0.115 |
| IL-8 | −0.147 | 0.372* | 0.345* | 0.092 | −0.158 |
| IL-6 | 0.069 | 0.085 | 0.492** | −0.146 | −0.191 |
| IL-2 | −0.189 | −0.108 | 0.642** | 0.626** | −0.032 |
| IL-1β | −0.396* | 0.243 | −0.117 | −0.037 | 0.260 |
| (C) Stage three | |||||
| Cortisol | 0.238 | −0.015 | −0.186 | −0.002 | 1 |
| IFN-γ | −0.075 | 0.169 | 0.237 | −0.131 | −0.062 |
| TNF-α | 0.036 | 0.148 | −0.063 | 0.065 | −0.001 |
| IL-8 | −0.166 | −0.100 | 0.022 | −0.016 | 0.346* |
| IL-6 | 0.038 | 0.306 | −0.133 | 0.667** | 0.251 |
| IL-2 | −0.141 | −0.100 | −0.016 | 0.031 | 0.249 |
| IL-1β | 0.074 | −0.164 | −0.127 | −0.148 | 0.184 |
4. Discussion
The study investigated the mechanism of stress-related cytokine alteration by determining the alteration in the production of the stress hormones and the cytokines among medical/health sciences students under midterm and final examination periods compared to a non-stressful period. Our results showed that the concerned medical/health sciences students underwent psychological and physiological academic-stress changes where the majority of them reported having cold/flu, tonsillitis and hair and skin deteriorations during that semester. Having cold/flu could also be related to the effect of the season having higher probability to catch the flu rather than the effects of stress. On the other hand, they indicated that they do not easily catch cold/flu. Changes in hair and skin could also be related to the seasonal effect, but the percentage of those having both hair and skin deteriorations in the third stage compared to the first stage (52% vs 20% respectively) would probably eliminate this assumption.
At the third stage, participants indicated that the final examination period was the most stressful factor followed by the midterm period, whereas at the first stage only one indicated that final exam period is considered the most stressful time in general. The percentage of change in their response between the first and last stages was clearly noticed, but not significant. On the other hand, comparing their responses with the second most stressful factors from stage one to three, most participants indicated that midterm exam period is the most stressful factor in both stages (p = 0.009). The clinical results of this study showed the same; their plasma released the highest concentrations of cortisol during the final examination period followed by the midterm period. This was also supported by the increase in the heart pulse rate during the third stage.
Physiological and psychological stresses were found to activate the sympathetic nervous system (SNS) and increase the activity of the hypothalamic-pituitaryadrenal (HPA) axis leading to increasing levels of plasma catecholamines (CAs) (noradrenaline and adrenaline) and glucocorticoid (GCs) (cortisol in human) respectively (Smith and Vale, 2006, Stephens and Wand, 2012). This effect was with wide individual differences and consequences. Stress also was found to interact with the immune system and alter the immune response (Glaser and Kiecolt-Glaser, 2005). The secreted stress hormones like cortisol were found to function through separate receptors on different types of cells including the immune cells modulating cytokine production (Chrousos, 2000, Glaser and Kiecolt-Glaser, 2005, Hänsel et al., 2010, Miller et al., 2011, Smith and Vale, 2006, Stephens and Wand, 2012, Tsigos and Chrousos, 2002, Webster Marketon and Glaser, 2008).
Studies have found that such stress-induced changes in the immune response shifts the balance of Th1 and Th2 cytokines towards Th2 cytokine response (Marshall et al., 1998). This is done by activating the HPA axis resulting in the release of plasma cortisol which is an important neuroendocrine mediator of the stress-induced cytokine alteration (Stephens and Wand, 2012, Tian et al., 2014). Many studies have shown the impact of different types of stressors on the immune system where academic stress was one of the stress models used (Chen, 2007, Kang and Fox, 2001, Kim and Maes, 2003, Maes et al., 1997, Matalka et al., 2000).
In this study, academic stress was significantly associated with changes in Th1 and Th2 cytokines by showing down-regulation in the release of some Th1 cytokines and an up-regulation of selective Th2 cytokines, which is in line with the previous studies (Chen, 2007, Chrousos, 2000, Connor et al., 2005, Elenkov and Chrousos, 2002, Kang and Fox, 2001, Matalka, 2003). The findings of this study indicated that the stress during the final examination period induced significant changes in cytokine production, although the final examinations for university students were normally relatively mild, common and repetitive. The release of cytokines during the second stage (the midterm period) was variable, this may indicate that the psychological status of the students during the mid of the term is less stressed compared to the final exam stage.
In this study, the significant reduction in Th1 cytokines IFN-γ, TNF- α and IL-1β is consistent with findings from other studies (Chen, 2007, Glaser et al., 1986, Kang and Fox, 2001, Miller et al., 2002). Although some studies showed an increase in TNF- α release, but that was from mitogen stimulated whole blood cells (Maes et al., 1998, Paik et al., 2000). Although IL-2 did not show a significant reduction in its release, it showed the same trend as the rest and was reduced among the stages. For that, the need for larger sample size to detect the significant changes is requested. Kang and fox, 2001, indicated that a reduction in IFN-γ and IL-2 production may down-regulate one's defense against viral infection, which in turn may influence disease activities.
Among the Th2 cytokines, IL-10 showed a significant reduction in its release during the third stage. This was consistent with Maes and colleagues study, 1998. They found that stress-induced anxiety is accompanied by increased production of IFN-γ with diminished production of IL-10 and IL-4. On the other hand, this study showed an increased production of IL-4 with a decrease in production of IFN-γ in stage three, while IFN-γ had a significant increase in its release during the midterm stage two. To resolve this paradox, some researchers have chosen to focus on how stress might shift the balance of the immune response by studying the ratios between selective cytokines from each type. Thl-type cytokine (IFN-γ) and Th2-type cytokine (IL-4) are known to regulate the cellular and humoral components of the adaptive immune system respectively and their ratio can assess the specific Th1:Th2 cytokine balance.
The decrease in IFN-γ/IL-4 ratio from the first stage to the third stage indicates a decrease in production of IFN-γ secreting cells while increasing production of IL-4 secreting cells (Chen, 2007) and a shift away from cellular immunity (Th1) toward humoral immunity (Th2). This Th1:Th2 shift was consistent with the increase in cortisol release during the three stages. It was also indicated in other studies showing that cortisol suppresses Th1 cytokines production with its ability to increase the release of some Th2 cytokines depending on the stimulatory signals to T cells (DeKruyff et al., 1998, Elenkov, 2004, Franchimont et al., 2000).
Interestingly, heart rate was negatively correlated with the production of the Th2 cytokines, IL-10 in first and last stages. It was also negatively correlated with systolic blood pressure while positively correlated with diastolic blood pressure. This might indicate that the increase in the release of the stress hormone caused heart rate to speed up. Systolic blood pressure reflects the force of blood in the circulatory system when the heart contracts while diastolic blood pressure reflects this force when the heart is at rest. The increase in heart rate was associated with higher resting diastolic pressure and lower systolic blood pressure. This indicates that the increase in stress was accompanied with the heart rate speed up and more strongly related to blood pressure when the heart is at rest than when the heart contracts. The decrease in the release of the anti-inflammatory cytokine IL-10 in acute stress was associated with the increase in heart rate and cortisol in both the baseline and third stages. Our results are consistent with a recent study showing an evidence for the role of IL-10 in up- and downregulating the cortisol response to acute stressors between days. They indicated that IL-10 was negatively correlated with stress as it increased in the morning of the first day of stimulation which was related to a decrease in cortisol, while both increased during the second day of stimulation (Wolkow et al., 2015). Further research should include larger samples with the statistical power to determine the role of IL-10 in the academic stress.
Another Th2 anti-inflammatory cytokine (IL-4) showed a positive correlation with the resting diastolic pressure in the last stage. During final exams period, the increase in stress hormone accompanied with the heart rate speed up and higher resting diastolic pressure was correlated with IL-4 release. Previous studies showed evidence that IL-4 affects cortisol release by inducing the expression of enzymes involved in regulating this hormone (Woods and Judd, 2008, Wolkow et al., 2015).
As expected, almost similar to what have been shown in Assaf (2013) survey study, this project showed a significant association between academic stressing factors and the biological assays. The release of the pro-inflammatory cytokines IL-2 and IFN-γ was associated with the two most stressing factors, final exams and midterm exams respectively. The release of IFN-γ in the first stage was significantly higher in those who indicated that midterm exams are the second most stressful factors. In the first baseline stage, the participants were not exposed to the academic stress and Th1 cytokines were high in most of them whereas upon exposure to final exams, IL-2 cytokine was released in only half of those who indicated that final exams are the most stressful period. On the other hand, the release of Th2 cytokines (IL-4 and IL-10) showed an association with the need to seek medical care during the midterm period. In the baseline stage, they showed almost no release on those who needed medical care during the mid of the semester. It was also significant with IL-4 release during the mid of the semester in stage two. Participants who indicated that they did not get ill or needed medical care were mostly those who released IL-4 and IL-10 cytokines with higher Th2 humoral mediated immunity. Furthermore, the high cortisol release on the third stage associated with the increase in the need for seeking medical care during the mid of the semester indicates that participants are exposed to an academic stress that is shifting the Th1/Th2 balance towards Th2.
In conclusion, the results of this study showed that the stress induced during the final exam was the highest among the medical/health sciences students with a high increase in cortisol release which might be the reason for shifting Th1/Th2 cytokines balance towards Th2, resulting in an immune dysregulation rather than overall immunosuppression. Cortisol might have a role in suppressing the release of Th1- mediated cellular immune response, which could increase the vulnerability among the students to infectious diseases. In the meantime, it could enhance Th2-mediated humoral immune response which could increase vulnerability to autoimmune and allergic diseases. The effect of cortisol on the release of cytokines could be due to the direct regulation by glucocorticoids. For that, further studies should be done to check for glucocorticoid responsive element in their gene promoter to confirm this direct regulation. On the other hand, Kunz-Ebrecht et al. (2003) indicated that repeated episodes of acute stress might initiate a down-regulation in the glucocorticoid receptors which might lead to the development of a chronic inflammatory state. This was not indicated among the referred to medical/health sciences students. But it should be taken seriously into consideration to protect them from development of any chronic inflammations. Further studies with large sample size are needed to determine the links between cortisol and immunity.
5. Limitations and future directions
Despite the richness of the questionnaire content and the clinical parameters tested, this study has several limitations. The collected data in this study was utilized as responses to each question and was not computed as scores and health-related data were all self-reported by the participants. Another limitation lies in the observation that most of the medical/health sciences students who participated in this survey were from pharmacy and being females. This was due to the location of the study which was at the faculty of pharmacy, University of Jordan. It also represents almost the actual distribution of genders at the Faculty of Pharmacy. In future studies, more medical/health sciences and male participants should be included. Although circadian variations in cortisol were taken into consideration, seasonal variations in cortisol were not considered as recent studies indicated that cortisol does not have seasonal differences (Smith et al., 2015).
This study was a pilot one, limited by a small sample size. Therefore, a randomized, controlled trial with a larger sample size is needed to validate the variations in cortisol and cytokine levels in participants at rest and under stress. Also, using larger samples should further investigate the mechanism of exam stress-induced changes in Th1- and Th2-type cytokines and their balance and to relatively evaluate their correlations with diseases that occur during exam stress to help controlling them. Linking stress, cytokines and diseases should help to find the appropriate method for reducing stress.
Funding
The author wish to thank the Deanship of Academic Research (DAR) at the University of Jordan for their financial support.
Disclosure Statement
The author reports no conflict of interest to declare.
Acknowledgements
The authors would like to thank the participated medical/health sciences students who kindly devoted their time to this study and to thank Mr. Mashhour T. Assaf for his valuable contribution while writing and editing the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Assaf A.M. Stress-induced immune-related diseases and health outcomes of pharmacy students: a pilot study. SPJ. 2013;21(1):35–44. doi: 10.1016/j.jsps.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, E., 2007, Psychological stress and its relationship to cytokines and inflammatory diseases. In: Cytokines: Stress and Immunity, 2nd ed. Taylor & Francis Group, Boca Raton, FL.
- Chrousos G.P. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. JACI. 2000;106(5):S275–S291. doi: 10.1067/mai.2000.110163. [DOI] [PubMed] [Google Scholar]
- Connor T.J., Brewer C., Kelly J.P., Harkin A. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 2005;159(1–2):119–128. doi: 10.1016/j.jneuroim.2004.10.016. [DOI] [PubMed] [Google Scholar]
- DeKruyff R.H., Fang Y., Umetsu D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. JI. 1998;160:2231–2237. [PubMed] [Google Scholar]
- Dhabhar F.S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 2014;58(2–3):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Elenkov I.J., Chrousos G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Fairbrother K., Warn J. Workplace dimensions, stress and job satisfaction. J. Manag. Psychol. 2003;18(1):8–21. [Google Scholar]
- Franchimont, D., Galon, J., Gadina, M., Visconti, R., Zhou Y.J., Aringer, M., Frucht, Chrousos, G.P., O’Shea, J.J., 2000. Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced stat4 phosphorylation in T lymphocytes. JI, 164,1768–1774. [DOI] [PubMed]
- Föhr T., Pietilä J., Helander E., Myllymäki T., Lindholm H., Rusko H., Kujala U.M. Physical activity, body mass index and heart rate variability-based stress and recovery in 16 275 Finnish employees: a cross-sectional study. BMC Public Health. 2016;2(16):701. doi: 10.1186/s12889-016-3391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R., Rice J., Speicher C.E., Stout J.C., Kiecolt-Glaser J.K. Stress depresses interferon production and natural killer (NK) cell activity in humans. Behav. Neurosci. 1986;100:675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- Guyton, A.C., 2006. Medical physiology, 11th ed., W.B. Elsevier Saunders, Philadelphia, PA.
- Hänsel A., Hong S., Cámara R.J.A., von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 2010;35(1):115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kang D.H., Fox C. Th1 and Th2 cytokine responses to academic stress. Res. Nurs. Health. 2001;24(4):245–257. doi: 10.1002/nur.1027. [DOI] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8(3):223–246. [PubMed] [Google Scholar]
- Kim Y.K., Maes M. The role of the cytokine network in psychological stress. Acta Neuropsychiatr. 2003;15(3):148–155. doi: 10.1034/j.1601-5215.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Koh K.B., Choe E., Song J.E., Lee E.H. Effect of coping on endocrinoimmune functions in different stress situations. Psychiatry Res. 2006;143:223–234. doi: 10.1016/j.psychres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Koh K.B., Park J.K., Kim C.H., Cho S. Development of the stress response inventory and its application in clinical practice. Psychosom. Med. 2001;63:668–678. doi: 10.1097/00006842-200107000-00020. [DOI] [PubMed] [Google Scholar]
- Kohut M.L., Martin A.E., Senchina D.S., Lee W. Glucocorticoids produced during exercise may be necessary for optimal virus-induced IL-2 and cell proliferation whereas both catecholamines and glucocorticoids may be required for adequate immune defense to viral infection. Brain Behav. Immun. 2005;19(5):423–435. doi: 10.1016/j.bbi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht S.R., Mohamed-Ali V., Feldman P.J., Kirschbaum C., Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Immun. 2003;17(5):373–383. doi: 10.1016/s0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Kim H.C., Kang J.I. Association between stressful life events and resting heart rate. BMC Psychol. 2014;8(2):29. doi: 10.1186/s40359-014-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, M., Hendriks, D., Van Gastel, A., Demedts, P., Wauters, A., Neel, H., Janca, A., Scharp’e, S., 1997. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology, 22,397–409. [DOI] [PubMed]
- Maes M., Song C., Lin A., De Jongh R., Van Gastel A., Kenis G., Smith R. The effects of psychological stress on humans: Increased production of pro-infl ammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Marshall G.D., Agarwal S.K., Lioyd C., Cohen L., Henninger E.M., Morris G.J. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav. Immun. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- Marucha D.T., Kiecolt-Glaser J.K., Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom. Med. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- Matalka K.Z. Neuroendocrine and cytokines-induced responses to minutes, hours, and days of mental stress. Neuro Endocrinol. Lett. 2003;24(5):283–292. [PubMed] [Google Scholar]
- Matalka, K.Z., Sidki, A., Abdul-Malik, S., Thewaini, A., 2000. Academic Stress- Infl uence on Epstein Bar virus and cytomegalovirus reactivation, cortisol, and prolactin. LAB. MED31, pp. 163–168.
- Miller G.E., Chen E., Parker K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Cohen S., Ritchey A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Murphy L., Denis R., Ward C.P., Tartar J.L. Academic stress differentially influences perceived stress, salivary cortisol, and immunoglobulin-A in undergraduate students. Stress. 2010;13(4):365–370. doi: 10.3109/10253891003615473. [DOI] [PubMed] [Google Scholar]
- Paik I.H., Toh K.Y., Lee C., Kim J.J., Lee S.J. Psychological stress may induce increased humoral and decreased cellular immunity. Behav. Med. 2000;26:139–141. doi: 10.1080/08964280009595761. [DOI] [PubMed] [Google Scholar]
- Rojas I.G., Padgett D.A., Sheridan J.F., Marucha P.T. Stress-induced susceptibility to bacterial infection during cutaneous wound healing. Brain Behav. Immun. 2002;16:74–84. doi: 10.1006/brbi.2000.0619. [DOI] [PubMed] [Google Scholar]
- Segerstrom S.C., Miller G.E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai K., Mohrs M., Locksley R.M. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420(6917):825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.N., Wilder C.S., Griffith W.C., Workman T., Thompson B., Dills R., Onstad G., Vredevoogd M., Vigoren E.M., Faustman E.M. Seasonal variation in cortisol biomarkers in Hispanic mothers living in an agricultural region. Biomarkers. 2015;20(5):299–305. doi: 10.3109/1354750X.2015.1068863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M.A., Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol. Res. 2012;34(4):468–483. [PMC free article] [PubMed] [Google Scholar]
- Tian R., Hou G., Li D., Yuan T.F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. ScientificWorldJournal. 2014;2014:780616. doi: 10.1155/2014/780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Webster Marketon J.I., Glaser R. Stress hormones and immune function. Cell. Immunol. 2008;252(1–2):16–26. doi: 10.1016/j.cellimm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wolkow, A., Aisbett, B., Reynolds, J., Ferguson, S.A., Main, L.C., 20015. Relationships between inflammatory cytokine and cortisol responses in firefighters exposed to simulated wildfire suppression work and sleep restriction. Physiol. Rep. 3(11). pii: e12604. [DOI] [PMC free article] [PubMed]
- Woods A.M., Judd A.M. Interleukin-4 increases cortisol release and decreases adrenal androgen release from bovine adrenal cells. Domest. Anim. Endocrinol. 2008;34:372–382. doi: 10.1016/j.domaniend.2007.10.004. [DOI] [PubMed] [Google Scholar]