Introduction
Brugada syndrome is characterized by ST-segment elevation in the right precordial leads and associated with life-threatening arrhythmias originating in the right ventricular outflow tract (RVOT).1 Ablation of RVOT myocardium prevents episodes of arrhythmias in these patients.2 The mechanism underlying these arrhythmias, however, remains debated and may involve repolarization3 or conduction abnormalities.4
Catheter mapping in Brugada patients has shown delayed activation and highly fractionated local electrograms at the RVOT epicardium.2, 5 Fractionated electrograms result from discontinuous conduction caused by increased interstitial fibrosis,6 which may set the stage for activation block owing to current-to-load mismatch.7 Subsequent activation of the unexcited myocardium by a wavefront arising from a different direction may set the stage for reentry.4 However, when the myocardium remains unexcited it may serve as a systolic current sink that is manifested as ST-segment elevation on the body-surface electrocardiogram (ECG).7 This theory is supported by the observation that pre-excitation of unexcited RVOT myocardium reduced ST-segment elevation on the body-surface ECG in patients with Brugada syndrome.5 Whether excitation failure indeed occurs in the human RVOT myocardium in the presence of subtle structural abnormalities is unclear.
Case report
We studied a human right ventricular wedge preparation obtained from a nonfailing heart, recorded epicardial unipolar electrograms at different cycle lengths, and determined the level of fatty infiltration. To interpret experimental data, conduction in the RVOT was simulated in a sheet of 2 × 5 cm using the Ten Tusscher human ventricular ionic model for describing the action potentials8 and the CARP simulator for modeling conduction and repolarization patterns.9 The nonfailing donor heart was provided by Mid-America Transplant Services (St Louis, MO), as previously described.10 The procedures in this study were in accordance with legal regulations on the use of donor hearts in the United States. The Washington University School of Medicine ethics committee (Institutional Review Board) approved the use of hearts that were rejected for transplantation for research purposes. Furthermore, the study was performed in conformance to the Declaration of Helsinki and was approved by the Washington University Institutional Review Board.
Monophasic complexes recorded from the human RVOT
The donor heart was obtained from a 72-year-old woman who died of stroke. She did not have a history of cardiovascular disease; therefore, no echocardiogram or electrocardiographic information were available.
We made a wedge preparation from the right ventricular wall, including the RVOT, and cannulated the right coronary artery. One minute after mounting the preparation in the perfusion set-up, stimulation (1 Hz) on the right ventricular epicardium resulted in rigorous contraction of the preparation. Unipolar electrograms recorded from several epicardial locations of the RVOT showed monophasic-like complexes with superimposed fractionated deflections (Figure 1A). Bipolar electrograms, generated by subtraction of unipolar electrograms recorded at close locations, did not show monophasic-like components, indicating that these complexes did not represent local injury. However, sharp fractionated complexes remained, signifying propagation of an activation front through the myocardium.11
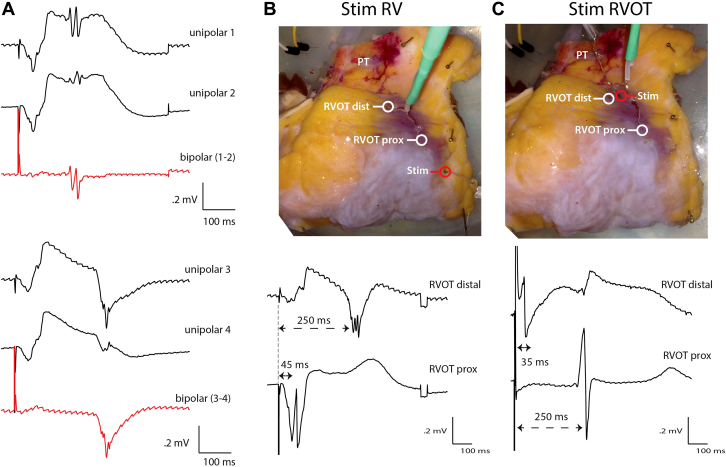
Figure 1.
A: The traces show 4 different unipolar electrograms recorded at the right ventricular outflow tract (RVOT) and 2 corresponding bipolar electrograms (red). B: A photograph of the epicardial side of the wedge preparation showing the site recording at the proximal and distal RVOT and the corresponding unipolar recordings. Stimulation occurred at the right ventricle. The proximal electrode was located 2 cm from the site of stimulation and recorded activation at ∼45 ms. The distal electrode was located twice as far but activated at ∼250 ms, suggesting delayed activation of RVOT. The monophasic morphology in the distal RVOT coincided with the electrogram at the proximal RVOT. C: Unipolar electrograms recorded from the proximal and distal RVOT when stimulated on the distal RVOT. Note the sharp deflection and normal ST segment in the unipolar electrogram recorded from the distal RVOT. Dist = distal; prox = proximal; PT = pulmonary trunk; RV = right ventricle; stim = stimulation.
Next, we recorded unipolar electrograms during right ventricular pacing at the proximal region of the RVOT, at the border with the right ventricle, and at the distal region of the RVOT close to the pulmonary trunk (Figure 1B). At the proximal RVOT we recorded fractionated potentials (2 deflections) with normal ST segment and T wave. At the distal RVOT, however, a monophasic complex occurred at the moment of activation of the proximal RVOT and sharp fractionated potentials (3 deflections) were present ∼250 ms after stimulation, indicating local activation. Monophasic complexes or ST-segment elevation can result from local ischemia.12 To exclude this possibility in our preparation, we paced next to the recording site on the distal RVOT (Figure 1C). This resulted in disappearance of the monophasic complexes and resulted in electrograms with sharp unfractionated deflections with a normal ST segment. Activation of the proximal RVOT, however, was delayed but showed a normal unfractionated electrogram. Interestingly, a monophasic-like complex was recorded at the distal RVOT following activation of the proximal RVOT (Figure 1C). The shortest pacing interval with capture of both the proximal RVOT and distal RVOT was 200 ms (data not shown).
Fatty infiltration of the right ventricular wall
Extracellular monophasic action potentials typically arise when the underlying myocardium does not excite while being electrically coupled to surrounding myocardium that does. This can be achieved by pressing the recording electrode on the myocardium or by generating injury.13 We hypothesized that the monophasic-like complexes recorded in our preparation share this mechanism and were recorded from myocardium that failed to excite but was sufficiently coupled to surrounding activated myocardium. For excitation failure to occur, subtle structural abnormalities are required.7 To test this hypothesis, we inspected the transmural wall of the RVOT at the locations where local electrograms were recorded. The photograph in Figure 2 shows fatty infiltration throughout the transmural wall. A detailed investigation using a Masson’s trichrome stain confirmed the presence of fat, which divided the myocardium into separate strands, predominantly at the epicardial side.
Figure 2.
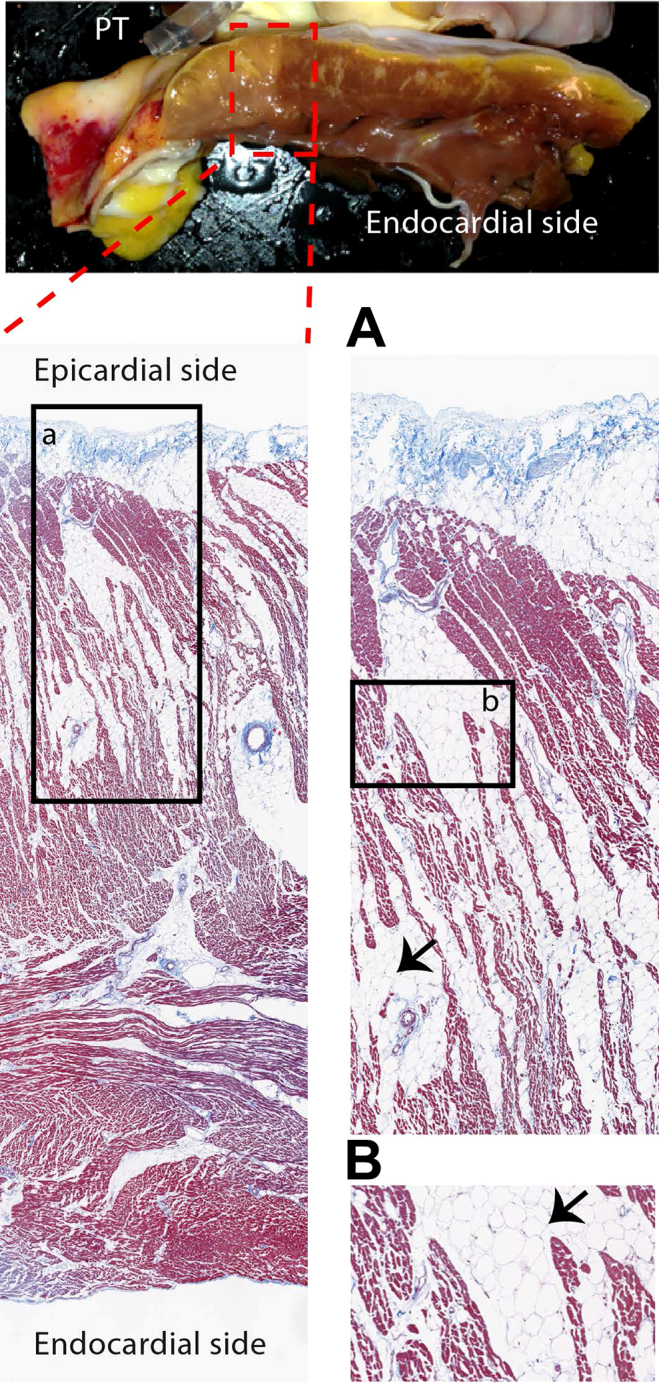
Top image shows a photograph of the transmural wall of the right ventricular outflow tract (RVOT) preparation at the location where we recorded electrograms. The images below show Masson’s trichrome staining, indicating fatty infiltration into the transmural wall of the RVOT. Panels a and b represent magnifications. PT = pulmonary trunk.
Computer simulations
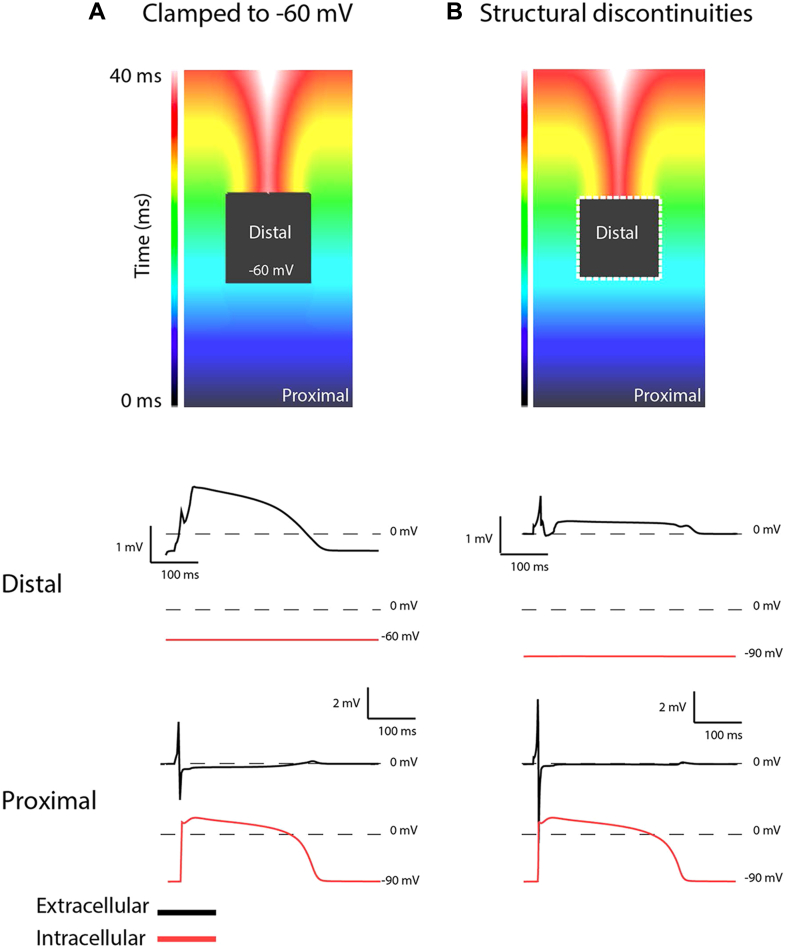
To investigate whether these structural abnormities can lead to excitation failure and monophasic-like electrograms, we performed computer simulations (Figure 3). First, we clamped the membrane potential of cells in a 1 × 1-cm area to -60 mV in the middle of a 2-dimensional sheet, thus rendering them unexcitable but fully coupled with the surrounding cells (Figure 3A). During activation, a monophasic-like electrogram occurred in the clamped area (panel A, distal black electrogram). Secondly, we removed the clamp but interrupted the intercellular coupling between alternating cells at the margin of the distal area, thereby simulating structural discontinuities (dotted line in Figure 3B). This resulted in excitation failure of the distal area and local electrograms with ST-segment elevation.
Figure 3.
A: Simulation of a 2-dimensional myocardial sheet where the intracellular potential in the black region is clamped to -60 mV while the surrounding region is activated, showing a monophasic-like potential in the extracellular space. B: The black region is not clamped but is connected to the surroundings by small connections leading to conduction blocks, causing an ST-segment elevation in the extracellular space.
Discussion
Our data indicate that excitation failure in the RVOT myocardium occurs in the presence of structural abnormalities. Unexcited myocardium may subsequently become the source of ST-segment elevation and set the stage for reentrant-based arrhythmias.7
The donor from whom we obtained the wedge preparation was not diagnosed with a cardiac pathology and was not known to have a sodium channel mutation. The infiltration of fat into the transmural wall of the RVOT indicates severe structural abnormalities. As a result, the myocardium was divided into separate bundles, giving rise to discontinuous conduction.6 This explains the fractionated extracellular potentials we recorded, which is common for patients with fibrofatty infiltration in the right ventricle wall.14 However, as we observed mainly infiltration of fat, and not fibrosis, this most likely represents a common aged heart and suggests the donor did not suffer from arrhythmogenic right ventricular dysplasia.15
Previous simulation studies indicate that abnormalities within the myocardium cause excitation failure at high pacing frequency and lead to ST-segment elevation on the body-surface ECG in the presence of sodium channel dysfunction.7 The absence of a Brugada phenotype or evidence of arrhythmia in the donor is probably explained by the fact that a sodium channel dysfunction was not present. Moreover, the presence of unexcited tissue in the wedge preparation is likely related to the absence of alternative activation pathways such as the RVOT septal wall, which has been cut in our preparation. This means that in the intact heart the RVOT could have been activated by these alternative pathways and, therefore, did not form the basis for reentrant arrhythmias.
Local electrograms recorded from the epicardium of the RVOT of Brugada syndrome patients often show a monophasic-like morphology.2 Excitation failure of parts of the RVOT myocardium owing to current-to-load mismatch is proposed as the underlying mechanism.5, 7 Our data show that parts of the distal RVOT myocardium failed to excite during right ventricular stimulation while being excitable by direct stimulation. For current-to-load mismatch to occur the adjacent excited and unexcited regions must be electrically coupled. Our simulations show that the monomorphic potentials in the extracellular space of the distal RVOT myocardium are representative of the intracellular potentials of the adjacent right ventricular myocardium.13 Thus, the presence of monophasic complexes in the extracellular space of the distal RVOT indicates that it was electrically coupled with the right ventricular myocardium. Our observations lend support to the idea that structural abnormalities in RVOT myocardium are a common phenomenon in aging adults and, when causing conduction block, may be sufficient for generating the Brugada phenotype. However, in the presence of a sodium channel–blocking drug or a mutation in the sodium channel, the structurally altered myocardium may lead to the Brugada phenotype and arrhythmia at a younger age than in those individuals without sodium channel blockade or SCN5A mutation.4
In conclusion, we show monophasic-like local electrograms at the epicardial side of the RVOT as a result of current-to-load mismatch owing to structural abnormalities. The same may occur in the RVOT of Brugada syndrome patients and may, at least in part, explain ST-segment elevation and the onset of arrhythmias.
Key Teaching Points.
-
•
In an explanted heart from a healthy donor we documented fatty infiltration in the right ventricular outflow tract (RVOT) myocardium.
-
•
Fatty infiltration in the RVOT myocardium causes activation delay and fractionated local unipolar electrograms.
-
•
Epicardial unipolar electrograms recorded from the RVOT showed local ST-segment elevation as a result of current-to-load mismatch at sites of fatty infiltration (and not related to ischemia).
Footnotes
Dr. Vigmond was supported by ANR-10-IAHU-04. Dr Rentschler holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. Support from the Dutch Heart Foundation was highly appreciated (2016T047). This research was also supported by NIH grant R01 HL115415, NIH R01 HL114395, and Foundation Leducq (RHYTHM).
References
- 1.Sieira J., Dendramis G., Brugada P. Pathogenesis and management of Brugada syndrome. Nat Rev Cardiol. 2016;13:744–756. doi: 10.1038/nrcardio.2016.143. [DOI] [PubMed] [Google Scholar]
- 2.Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasirirojanakorn K., Likittanasombat K., Bhuripanyo K., Ngarmukos T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C. The Brugada syndrome: ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12:272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoogendijk M.G., Opthof T., Postema P.G., Wilde A.A., de Bakker J.M., Coronel R. The Brugada ECG pattern: a marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the Brugada syndrome. Circ Arrhythm Electrophysiol. 2010;3:283–290. doi: 10.1161/CIRCEP.110.937029. [DOI] [PubMed] [Google Scholar]
- 5.Ten Sande J.N., Coronel R., Conrath C.E., Driessen A.H., de Groot J.R., Tan H.L., Nademanee K., Wilde A.A., de Bakker J.M., van Dessel P.F. ST-segment elevation and fractionated electrograms in Brugada syndrome patients arise from the same structurally abnormal subepicardial RVOT area but have a different mechanism. Circ Arrhythm Electrophysiol. 2015;8(6):1382–1392. doi: 10.1161/CIRCEP.115.003366. [DOI] [PubMed] [Google Scholar]
- 6.Nademanee K., Raju H., de Noronha S.V. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoogendijk M.G., Potse M., Linnenbank A.C. Mechanism of right precordial ST-segment elevation in structural heart disease: excitation failure by current-to-load mismatch. Heart Rhythm. 2010;7:238–248. doi: 10.1016/j.hrthm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Ten Tusscher K.H., Panfilov A.V. Cell model for efficient simulation of wave propagation in human ventricular tissue under normal and pathological conditions. Phys Med Biol. 2006;51:6141–6156. doi: 10.1088/0031-9155/51/23/014. [DOI] [PubMed] [Google Scholar]
- 9.ten Tusscher K.H., Panfilov A.V. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006;291:H1088–1100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 10.Glukhov A.V., Fedorov V.V., Lou Q., Ravikumar V.K., Kalish P.W., Schuessler R.B., Moazami N., Efimov I.R. Transmural dispersion of repolarization in failing and nonfailing human ventricle. Circ Res. 2010;106:981–991. doi: 10.1161/CIRCRESAHA.109.204891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spach M.S., Dolber P.C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986;58:356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- 12.Kleber A.G., Janse M.J., van Capelle F.J., Durrer D. Mechanism and time course of S-T and T-Q segment changes during acute regional myocardial ischemia in the pig heart determined by extracellular and intracellular recordings. Circ Res. 1978;42:603–613. doi: 10.1161/01.res.42.5.603. [DOI] [PubMed] [Google Scholar]
- 13.Franz M.R. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- 14.Garcia F.C., Bazan V., Zado E.S., Ren J.F., Marchlinski F.E. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 15.Burke A.P., Farb A., Tashko G., Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998;97:1571–1580. doi: 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]