Introduction
Key Teaching Points.
-
•
Concussions are a subset of mild traumatic brain injuries that typically result in transient impairment of neurological function, with or without loss of consciousness. Better recognition of their presentation and prevalence has increased awareness of the risk of concussions in active individuals.
-
•
Changes in heart rate variability could reflect changes in autonomic tone that often accompanies concussion.
-
•
The smartphone electrocardiogram could provide an accessible means of assessing heart rate variability in concussed individuals.
With an estimated 1.6 to 3.8 million occurrences in the United States annually, sports-related concussions are a major health concern.1, 2 Concussions are a subset of mild traumatic brain injuries that typically result in transient impairment of neurological function, with or without loss of consciousness.2 They are currently assessed using a variety of sideline measures, including symptom evaluation, the Glasgow Coma Scale, the Maddock’s score, the Standardized Assessment of Concussion, and the Balance Error Scoring System.3 Recently, there has been interest in how heart rate variability (HRV) changes after a concussion.4, 5, 6 In this case report, we present an analysis of HRV using a smartphone electrocardiogram (ECG) in a recently concussed collegiate football player to explore the potential future applications of HRV as a diagnostic and monitoring tool in concussed athletes.
HRV and concussion
HRV refers to the variation in beat-to-beat time intervals caused by both rhythmic and nonrhythmic fluctuations.7, 8 HRV can be analyzed using time-domain methods, based on the beat-to-beat intervals (RR intervals), to produce variables such as the standard deviation of RR intervals (SDRR).7 HRV can also be evaluated using frequency-domain methods by analyzing how the power (variance) distributes as a function of frequency.7 Total power represents the overall variability.7 For short-term recordings, 3 components are typically used: high frequency (HF) (0.15–0.40 Hz), low frequency (LF) (0.04–0.15 Hz), and very low frequency (VLF) (0.033–0.04 Hz).7 The LF and HF components can also be expressed in normalized units: the relative proportion of each component of the total power minus the VLF component.7 The HF component is largely associated with vagal activity, while the LF component is considered a marker of sympathetic activity, although it is also thought to have influences from both sympathetic and parasympathetic inputs.7 Despite the controversy regarding the interpretation of the LF component, the ratio between LF and HF components (LF/HF) has been used as an important marker of sympathovagal balance.6, 7 It is important to note, however, that the phenomenon of HRV is not simply the sum of sympathetic and parasympathetic effects, but rather a net effect of many other known and unknown factors.7 Nonlinear methods can also be used to analyze HRV, such as approximate entropy (ApEn), a useful measure of irregularity and complexity.5, 7
LF oscillations (<0.05 Hz) are linked to thermal regulation and mid-frequency oscillations (0.1 Hz) to changes in blood pressure.8 HF (>0.15 Hz) fluctuations in beat-to-beat intervals can be attributed to respiratory sinus arrhythmia, a naturally occurring variation in heart rate associated with respiration, in which heart rate increases on inspiration because of the suppression of vagal tone and decreases on expiration.8, 9 In addition, autonomic inputs can modify HRV in response to environmental stimuli, such as physical activity and stress, and certain disease states have been linked to changes in HRV.7
Previous research has shown that at rest, there are no significant differences in any of the HRV variables measured between concussed athletes and matched controls.4, 5 In response to low to moderate-intensity exercise, a significant reduction in mean RR interval, LF power, and HF power was observed.4 In recently concussed athletes compared with matched controls, ApEn was found to be significantly lower during sustained isometric grip contraction.5
A reduction in HRV can be a sign of autonomic dysfunction and is observed in various diseases such as myocardial infarction, diabetic neuropathy, and tetraplegia.7 This has led to research into potential clinical applications of HRV, such as using HRV to predict mortality risk after acute myocardial infarction; however, the accuracy in predicting outcomes is relatively low.10 Previous research has also shown that in traumatic and nontraumatic acute brain injury, there is a decrease in HRV on spectral analysis, particularly at low frequencies, which is proportional to the degree of neurological injury, with variability approaching zero during brain death.11 The proposed mechanism of this decrease is the physiological uncoupling of the autonomic and cardiovascular systems.11
Case report
An 18-year-old male collegiate football player complained of a headache after a spring practice and was diagnosed with a concussion by using the Standardized Assessment of Concussion, Balance Error Scoring System, and Glasgow Coma Scale along with a medical history and physical examination findings. Five days after his concussion, and while still symptomatic, 5-minute smartphone ECG (AliveCor, Inc., San Francisco, CA) readings were recorded at rest and immediately after 15 minutes on a stationary bike with workload to maintain a heart rate of 100 beats/min. This assessment was repeated 2 weeks postinjury when he was symptom free and was allowed to return to play. ECG readings were recorded with the athlete holding the device with 2 hands, giving the equivalent of lead I. The ECG was stored in PDF format.
HRV analysis was performed using Kubios HRV version 2.2 (Biosignal Analytics and Medical Imaging Group, Kuopio, Finland), a frequently used software program for HRV analysis.12 Time-domain variables included mean RR interval and SDRR, and frequency-domain parameters, calculated by fast Fourier transform, included LF power, HF power, and total power. Nonlinear methods were also used, specifically ApEn (Figures 1 and 2).
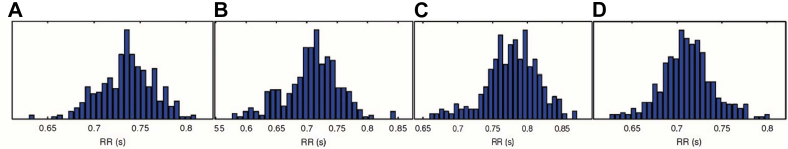
Figure 1.
Time-domain results—RR interval distributions: (A) concussion at rest, (B) concussion postexercise, (C) recovered at rest, and (D) recovered postexercise.
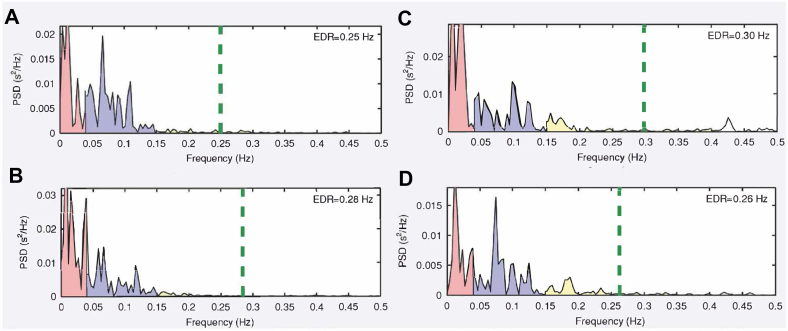
Figure 2.
Frequency-domain results: (A) concussion at rest, (B) concussion postexercise, (C) recovered at rest, and (D) recovered postexercise. The most noticeable change is in high-frequency power (yellow), which was markedly higher in recovery (both at rest and postexercise). EDR = ECG-derived respiration; PSD = power spectral density.
At rest, mean RR interval and SDRR were lower at 5 days (when the athlete was still symptomatic) than at 2 weeks (when he had recovered). HF power and total power were also lower at 5 days than at 2 weeks, while LF/HF was higher. Finally, there was minimal difference in LF power and ApEn at 5 days compared with at 2 weeks (Table 1). After exertion, there was minimal difference in mean RR interval and a notably higher SDRR, LF power, LF/HF, and total power at 5 days than at 2 weeks. HF power and ApEn were lower at 5 days than at 2 weeks (Table 1).
Table 1.
HRV in a concussed vs recovered athlete at rest and postexercise
| Variable | Concussion (5 d) at rest | Concussion (5 d) postexercise | Recovered (2 wk) at rest | Recovered (2 wk) postexercise |
|---|---|---|---|---|
| Mean RR interval (ms) | 735.6 | 708 | 777.5 | 710.8 |
| SDRR (ms) | 29.6 | 45.4 | 38.1 | 29 |
| LF (ms2) | 533 | 469 | 473 | 337 |
| HF (ms2) | 44 | 52 | 157 | 105 |
| LF/HF | 12.076 | 9.059 | 3.017 | 3.209 |
| Total power (ms2) | 986 | 1331 | 1377 | 725 |
| ApEn | 1.095 | 0.782 | 1.159 | 1.104 |
ApEn = approximate entropy; HF = high-frequency power; HRV = heart rate variability; LF = low-frequency power; SDRR = standard deviation of RR intervals.
Discussion
Previous studies have shown that exercise induces an overall decrease in HRV, with a decrease in total power and HF power and a constant or decreased LF power due to an increase in sympathetic activity and a decrease in parasympathetic activity.4, 8, 13 Not surprisingly in our case, 2 weeks postinjury, mean RR interval, SDRR, LF power, HF power, and total power were lower after exertion than at rest (Table 1). Five days postinjury, mean RR interval and LF power were lower after exertion than at rest. SDRR, HF power, and total power, however, were higher after exertion (Table 1). This deviation from the expected pattern suggests that there may have been some dysfunction in autonomic cardiovascular regulation when the athlete was symptomatic at 5 days.
The AliveCor smartphone ECG and other low-cost mobile devices have been increasingly popular as health screening tools.14, 15 In athletes, the AliveCor monitor has shown potential to aid in the real-time evaluation of potential arrhythmias.14 It has also been proven to be accurate when compared with the 12-lead ECG in measuring corrected QT intervals and capable of detecting corrected QT interval prolongation.15
Only findings from our subject at rest can be compared with findings of previous research since our methods for recording the athlete’s ECG in the exercise setting were slightly different. (We chose to record the ECG immediately after physical exertion rather than during physical activity as was done in previous studies.) Previous research showed that there are insignificant differences between concussed athletes and their matched controls at rest.4, 5 While there were minimal differences in LF power and ApEn in our case, we found that RR interval, SDRR, HF power, and total power were substantially lower when the athlete was still symptomatic than when he had recovered (Table 1). These differences also led to a higher LF/HF at 5 days. Gall et al4 attributed the lack of differences in HRV parameters between concussed athletes and their matched controls at rest to the mild nature of their injury. Prior research on traumatic brain injury has shown that the degree of physiological uncoupling between the autonomic and cardiovascular systems that causes abnormal changes in HRV is proportional to the severity of the neurological damage.4, 11 Thus, it is possible that the athlete in our case suffered a more severe brain injury.
We were not able to compare findings from our subject after exertion with those from previous research because of a difference in methods, but nonetheless there were noticeable differences between the athlete at 5 days when he was symptomatic and at 2 weeks when he was asymptomatic, which may suggest some autonomic dysfunction due to concussion. SDRR, LF power, LF/HF, and total power were higher at 5 days than at 2 weeks, while HF power and ApEn were lower.
In our case, HRV evaluation on consecutive days during and after the symptomatic phase would have helped determine whether changes in HRV parameters were simply by chance. In addition, we were only able to obtain ECG readings 5 days postinsult and it would have been useful to have readings at the time of the injury. We plan to further investigate the possibility of using HRV analysis as a sideline assessment tool for concussion in a prospective study examining the changes in HRV postexertion at multiple time points in concussed athletes by using preconcussion measurements as controls. Changes in HRV in concussed athletes compared with controls have been shown to persist past the symptomatic phase and after an athlete is allowed to return to play.6 In addition, assessing how the degree of exertion affects HRV parameters in the context of concussion would be important in order to evaluate the clinical applicability of HRV as a diagnostic tool.
Conclusion
We believe our case to be the first to use a smartphone ECG for HRV assessment in a concussed athlete. HRV may be a promising tool, especially via wearable technologies (eg, smartphones and watches), for the sideline assessment of concussion. Limitations to such a protocol are factors that affect HRV, such as stress, respiration, medications, level of exertion, and baroreceptor response.
A baseline ECG/HRV measurement of each athlete recorded beforehand (eg, during preseason) could be used as a comparison for when an athlete does suffer a suspected concussion because HRV measurements may vary from individual to individual. Repeated HRV measurements over consecutive days with symptoms and also after recovery would improve reliability of HRV measurements. Although current protocols on return-to-play guidelines recommend that an athlete must be asymptomatic at rest and with exercise before allowed to return to play, future research may lead to the inclusion of HRV analysis in the evaluation of concussions.2
Footnotes
Dr Albert is the cofounder and president of AliveCor.
References
- 1.Daneshvar D.H., Nowinski C.J., Mckee A.C., Cantu R.C. The epidemiology of sport-related concussion. Clin Sports Med. 2011;30:1–17. doi: 10.1016/j.csm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrory P., Meeuwisse W., Dvorak J. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 3.Harmon K.G., Drezner J.A., Gammons M., Guskiewicz K.M., Halstead M., Herring S.A., Kutcher J.S., Pana A., Putukian M., Roberts W.O. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47:15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 4.Gall B., Parkhouse W., Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med Sci Sports Exerc. 2004;36:1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 5.La Fountaine M.F., Heffernan K.S., Gossett J.D., Bauman W.A., De Meersman R.E. Transient suppression of heart rate complexity in concussed athletes. Auton Neurosci Basic Clin. 2009;148:101–103. doi: 10.1016/j.autneu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Abaji J.P., Curnier D., Moore R.D., Ellemberg D. Persisting effects of concussion on heart rate variability during physical exertion. J Neurotrauma. 2015;33:811–817. doi: 10.1089/neu.2015.3989. [DOI] [PubMed] [Google Scholar]
- 7.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 8.Carter J.B., Banister E.W., Blaber A.P. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch J.A., Bishop B. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Physiology. 1981;241:H620–H629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- 10.Song T., Qu X.F., Zhang Y.T., Cao W., Han B.H., Li Y., Piao J.Y., Yin L.L., Da Cheng H. Usefulness of the heart-rate variability complex for predicting cardiac mortality after acute myocardial infarction. BMC Cardiovasc Disord. 2014;14:59. doi: 10.1186/1471-2261-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein B., Toweill D., Lai S., Sonnenthal K., Kimberly B. Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am J Physiol. 1998;275:R1287–R1292. doi: 10.1152/ajpregu.1998.275.4.R1287. [DOI] [PubMed] [Google Scholar]
- 12.Tarvainen M.P., Niskanen J.P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P.A. Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 13.James D.V.B., Barnes A.J., Lopes P., Wood D.M. Heart rate variability: response following a single bout of interval training. Int J Sport Med. 2002;44:247–251. doi: 10.1055/s-2002-29077. [DOI] [PubMed] [Google Scholar]
- 14.Peritz D.C., Howard A., Ciocca M., Chung E.H. Smartphone ECG AIDS real time diagnosis of palpitations in the competitive college athlete. J Electrocardiol. 2015;48:896–899. doi: 10.1016/j.jelectrocard.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Garabelli P., Stavrakis S., Albert M., Koomson E., Parwani P., Chohan J., Smith L., Albert D., Xie R., Xie Q., Reynolds D., Po S. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol. 2016;27:827–832. doi: 10.1111/jce.12976. [DOI] [PubMed] [Google Scholar]