Alvarez and Latorre describe the long-lasting contribution of Goldman’s equation to our understanding of membrane transport.
Abstract
In 1943, David Goldman published a seminal paper in The Journal of General Physiology that reported a concise expression for the membrane current as a function of ion concentrations and voltage. This body of work was, and still is, the theoretical pillar used to interpret the relationship between a cell’s membrane potential and its external and/or internal ionic composition. Here, we describe from an historical perspective the theory underlying the constant-field equation and its application to membrane ion transport.
Introduction
The Goldman (1943) paper is, perhaps, one of the most well-known articles published in The Journal of General Physiology. Because of the great impact that this article still has on the study of transport of ions across membranes, the JGP editorial board thought that it deserved some discussion and comments.
Goldman was interested in explaining the phenomenon of electrical rectification found at the time in several different biological preparations such as Valonia, a species of algae found in the oceans, the squid giant axon, and the frog muscle (Blinks, 1930a,b; Cole and Curtis, 1941; Katz, 1942). This phenomenon, in which the current is large when flowing in one direction and small in the reverse direction, was hard to understand. Thermodynamic equations are not applicable, because ion transport in cells is out of equilibrium. Goldman’s experimental work was an attempt to reproduce some of the electrical properties of biological membranes in artificial systems. However, his work is mostly remembered (and cited) for the section in which he considered that ions move according to their concentration (diffusion) and voltage gradients and that the electric field through the membrane is constant, hence the name “constant-field equation.” Under these assumptions, and considering that the ion concentrations at the internal and external membrane boundaries are directly proportional to those in the aqueous solutions, he was able to arrive at an explicit solution of the Nernst–Planck (NP) equation (Nernst, 1888; Planck, 1890). The NP equation describes the contribution of concentration and voltage gradients to the current density carried by single ionic species. Today, we know it as the constant-field current equation (Eq. 1):
| (1) |
where Ii is the current density carried by ion i, Pi is the permeability coefficient, zi the valence of ion I, V is the membrane voltage, Ci,in and Ci,out are the ion concentrations in the solutions bathing either side of the membrane, and R, T, and F have their usual meaning. In this equation, a positive current carries charge from the inside to the outside and the membrane voltage is the internal minus the external electric potential. Goldman concluded that the theory could account reasonably well for the variation of the resting potential of the giant axon of the squid with external K+ concentration as determined by Curtis and Cole (1942).
The Second World War interrupted the work of most scientists, and it was not until 1949 that Goldman’s analysis of the movement of ions across membranes reappeared in the literature. This time, it was invoked to show that Bernstein’s membrane hypothesis (Bernstein, 1902), although correct in the identification of the origin of the resting potential, was mistaken regarding the ionic basis of the action potential. Bernstein’s theory proposed that membrane excitation consisted of a large increase in membrane permeability to all ions, in which case the membrane potential would fall nearly to zero. Although in the late 1930s there were already squid axon experiments indicating that there was a transient reversal of the membrane potential during nerve activity (Hodgkin and Huxley, 1939), there was no clear theoretical basis to explain this result. Hodgkin and Katz (1949) took advantage of the Goldman current equation and obtained an expression for the membrane potential, Vr, in terms of the internal and external ion composition and the ion permeabilities: the Goldman–Hodgkin–Katz (GHK) voltage equation (Eq. 2, which is formally equivalent to Eq. 18 in Goldman’s 1943 paper).
| (2) |
The use of this equation allowed Hodgkin and Katz to show that under resting conditions, the K+ permeability is 20 times larger than Na+ permeability. During nerve activity, the situation is reversed, and Na+ permeability becomes 20 times greater than the K+ permeability at the peak of the action potential (Hodgkin and Katz, 1949). Notably, the experimental strategy used by Hodgkin and Katz (1949) to determine the changes in active membrane potential with external Na+ is essentially the same as the one used by Hille almost 20 yr later to determine the ion selectivity of different ion channels (see Eq. 2 in Hodgkin and Katz, 1949).
The sodium hypothesis could not explain, however, the fact that crustacean muscle fibers elicit action potentials after replacement of the external sodium by tetraethylammonium (Fatt and Katz, 1953). Some years later, Fatt and Ginsborg (1958) found that an isotonic solution of Sr2+ or Ba2+ could sustain large crustacean muscle action potentials. To interpret the data obtained in crustacean muscle fibers, Fatt and Ginsborg (1958) expanded the constant-field theory so as to include divalent cations, arriving at the conclusion that divalent cations determined the peak of the action potential in these muscle fibers. The GHK voltage equation that incorporates a mixture of monovalent and divalent cations predicted that in this type of preparation the permeability of Ba2+ at the peak of the action potential was 50 times larger than that of K+. This was the first indication of the existence of Ca2+ action potentials and hence voltage-dependent Ca2+ channels.
Hodgkin and Huxley’s dissection of the ionic currents that underlie the action potential (Hodgkin and Huxley, 1952) identified voltage-dependent Na+ and K+ currents in the giant axon of the squid. On the other hand, in the early 1960s, Baker et al. (1962) were able to internally perfuse giant squid axons. This made it possible to learn about the selectivity of the Na+ conductance as the ionic composition of the internal medium could now be controlled. By having only K+ in the internal medium and mixtures of Rb+ and Cs+, or Li+ as a replacement for external Na+. Chandler and Meves (1965) were able to determine the relative selectivity of the sodium channel for alkali-metal ions using the GHK equation. They found that Li+(1.1) > Na+(1) > K+(0.08) > Rb+(0.025) > Cs+(0.016). Importantly, preference for Na+ of the Na+ system allowed Chandler and Meves (1965) to make inferences about the molecular nature of the structures defining ion selectivity, which, when considered in light of the theory developed by Eisenman (1962), were not far from the mark of what we know now about these proteins. Having only Na+ outside and K+ inside of the axon reduced the GHK to a simpler expression known as the bi-ionic potential, which give us a direct measurement of the PNa+/PK+ permeability ratio when the concentrations on both sides of the membrane are equal. In fact, when reduced to the bi-ionic case, the GHK equation is the most convenient way of determining the ratio between the permeabilities of the permeant ions. We should mention here that in the case of a membrane that is selective to either positive or negative ions, the constant-field assumption is not required and the voltage equation for the bi-ionic case can be obtained through integration, using the NP equation and the appropriate boundary conditions (see Appendix B; e.g., Sten-Knudsen, 1978).
The group of Hille (Hille, 1971, 1972, 1973; Dwyer et al., 1980; Wollmuth and Hille, 1992) determined the ion selectivity of several ion channels, including Na+, K+, HCN (Ih), and ACh receptor channels. Using a series of inorganic and organic ions of different size and by determining the permeability ratios relative to the reference ions (Na+, K+) by applying the GHK equation, they were able to obtain the approximate dimensions of the selectivity filter of these channels. Because the internal concentration of the reference ions was unknown in the different preparations, the experimental strategy used was to determine first the reversal potential in the presence of the reference ion and then in the presence of the test cation. They obtained the permeability ratio between the test ion and the reference ion from the difference of these potentials because the GHK equation contains only the permeability coefficients and concentrations of the test and reference ions.
The GHK voltage equation can also be used to calculate the membrane voltage when there is a contribution to the total current from electrogenic pumps, as is the case, for example, of the Na+/K+ pump or of electrogenic exchangers such as the Na+/Ca2+ exchanger (e.g., Sjodin, 1980; Mullins, 1981; Armstrong, 2003).
A quick review of the JGP archives revealed that the GHK equation has also been used to determine the relative ion permeability in mechanotransducer channels (Beurg et al., 2015), in Ca2+-activated Cl− channels (Jeng et al., 2016), and in T-type Ca2+ channels (Smith et al., 2017), to name just a few. In other words, after 74 yr, the GHK equation is still the theoretical framework to interpret the reversal potentials obtained in different ionic media. Despite the fact that several reviews and paper chapters dealt with the derivation of the constant-field equation, we thought it was of interest to discuss the theory underlying it and its approximations from an historical perspective.
A historical note about diffusion and the NP equation
Michael Faraday, the founder of electrochemistry, studied the process of electrolysis and introduced the terms: electrode, ions, cations, and anions (Faraday, 1834). Adolf Fick stated the empirical laws of diffusion and Svante Arrhenius established that salts dissociate in solution resulting in positively charged cations and negatively charged anions (Fick, 1855; Arrhenius, 1887). Jacobus van ’t Hoff discovered that dilute solutions behaved very much like ideal gases and introduced the concept of osmotic pressure (van ’t Hoff, 1887). All these concepts and experimental data were necessary to set up the scenario that led to the so-called NP equation, which when integrated assuming a constant field, results in the Goldman current equation.
Nonelectrolyte diffusion
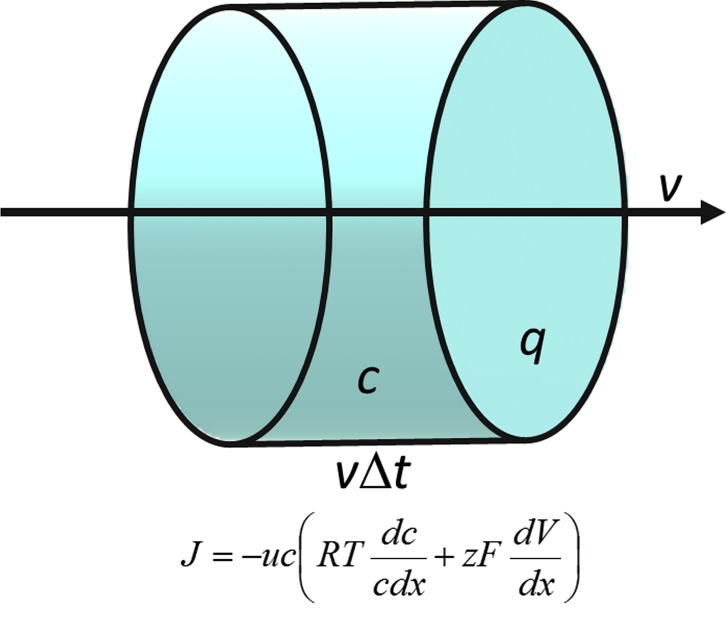
Walther Nernst (Nernst, 1888) compared the diffusion of gasses and ions in solution. Gas diffusion is much faster than ion diffusion, because ions undergo considerable friction when moving through a viscous medium. Therefore, the velocity of ion movement must be proportional to the force driving the diffusion. Nernst reformulated the Fick law of diffusion in physical terms. Nernst (1888) considered a slice in a diffusion cylinder of cross section q and height dx (Fig. 1). The volume of the slice is qdx. If there is an osmotic pressure difference dp across the slice, the force acting on the molecules in the slice is qdp. The number of moles contained in the slice is the concentration c times the volume qdx. Therefore, the force acting upon each mole, in N/mol, is
| (3) |
The number of molecules crossing the area q is the number of molecules contained in a 1-cm-high cylinder times the velocity of the molecules. Nernst (1888) defined K as the force required to drive the molecules at a velocity of 1 cm−1. Therefore, the velocity of the molecules will be (1/K)(1/c)dp/dx. On the other hand, the number of molecules, S, crossing area q in time interval Δt is the number of molecules contained in a cylinder of volume q times the distance they traveled in Δt at a velocity (1/K)(1/c)dp/dx:
| (4) |
According to van ’t Hoff (1887), the osmotic pressure is proportional to the concentration:
| (5) |
where p0 is a proportionality constant.
Figure 1.
Discovering the NP equation. The cylinder in the figure represents a point located inside the membrane. Molecules are diffusing at mean velocity v. During time Δt, all molecules inside the cylinder cross the circular windows of area q. This number of molecules is the concentration c times the cylinder volume qvΔt. The number of molecules crossing per unit area of the window per unit time interval is the flow, J = cv. The velocity of the molecules is the mobility, u, times the driving force. The mobility is the velocity the molecules acquire under a force of one newton per mole. This driving force is minus the chemical potential gradient d(RTlnc + zFV)/dx, where zF is the charge per mole of molecules and V is the local electric potential. The flow, J = cv, is therefore described by Eq. A1 in Appendix A.
Combining Eqs. 2 and 3, we have
| (6) |
Also, the flow, J, the number of moles transported per unit area, and unit time is
| (7) |
We recast Eq. 5 by defining a mobility u ≡ 1/K and set p0 = RT using van ’t Hoff law. Using these definitions, we get
| (8) |
Recalling Fick’s empirical first law J = −Ddc/dx, where D is the diffusion coefficient, we obtain that D = uRT, which is the well-known Einstein relation (Einstein, 1905).
Electrolyte diffusion
According to Arrhenius, salts dissociate in solution to produce positively and negatively charged ions (Arrhenius, 1884, 1887). In addition to the osmotic pressure gradient, the electrostatic potential gradient (dV/dx) times the charge of the ions acts as an additional force on these ions. The electric potential gradient times F, the Faraday constant, is the force applied in N/mol. The following equation was stated by Nernst (1888) for the velocity of a pair of ions, a cation and an anion, with different mobility, u+ and u−, respectively, diffusing at the same speed under a common osmotic pressure and electric potential gradient:
| (9) |
Both ions must diffuse at the same speed to maintain electroneutrality in spite of their different mobilities. This is possible because of the opposite sign of the factor in front of the electric field. The flow of the cation J+ and the flow anion J− is the velocity times the concentration:
| (10a) |
| (10b) |
Introducing the van ’t Hoff equation and the Einstein relation into Eq. 8 produces
| (11) |
This is the NP equation (Nernst, 1888; Planck, 1890), where z is the valence of the charge carrier. Maex (2017) recently provided more historical intimacies on NP relationships.
The Goldman current equation
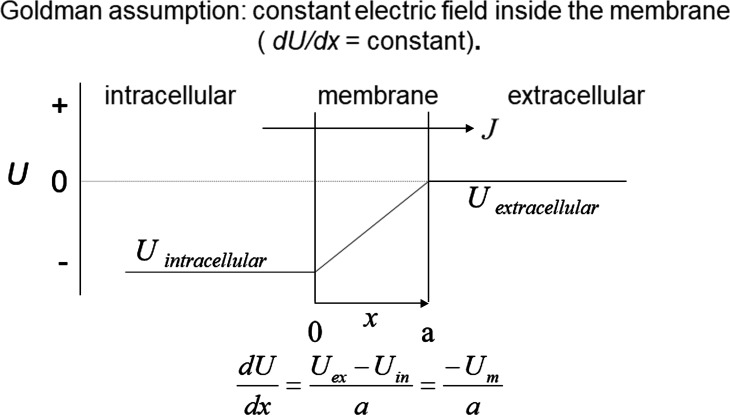
The starting point of the Goldman current equation is the NP equation (Eq. 11). Goldman introduced the assumption of a constant electric field within the membrane (dV/dx = −ΔV/a), where ΔV is the electric potential difference across the membrane and a is the membrane thickness (Fig. 2). Integrating Eq. 11 and taking as boundaries the edges of the membrane, we recover Eq. 1 (Sten-Knudsen, 1978) as
| (12) |
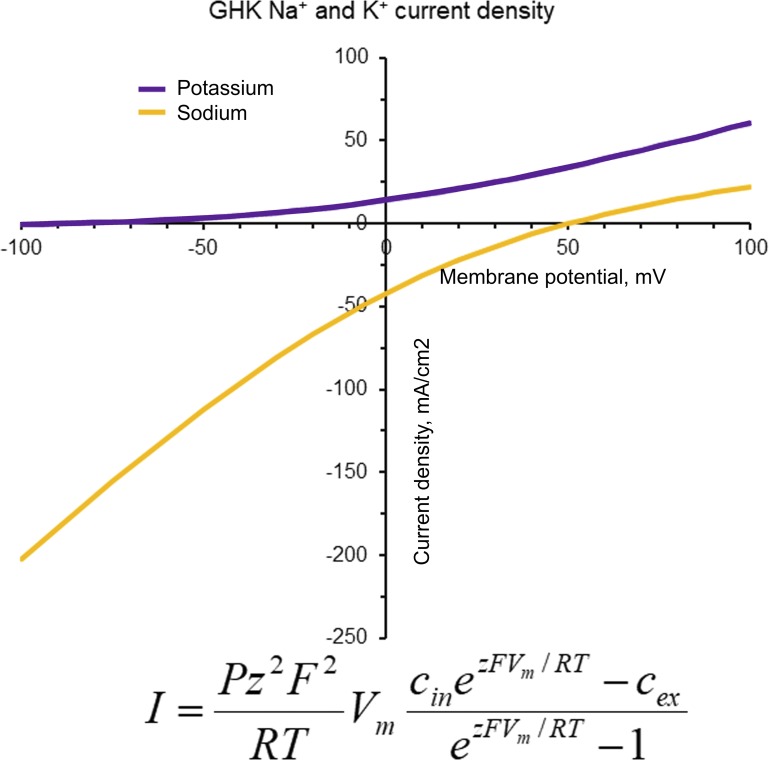
where n′ and n0 are the local concentration of the ion on either side of the membrane. Eq. 12 is the Goldman equation for the current. Here, ii is the current density carried by the ith ion and u′i is the velocity acquired by the ith ion when we applied a force of 1 N per coulomb on it (dV/dx = −ΔV/a). For details of the derivation, see Appendix A (Hodgkin and Katz, 1949; Sten-Knudsen, 1978; Johnston and Wu, 1995; Latorre et al., 1996; Hille, 2001; Matthews, 2013; Sterratt, 2015). In Fig. 3, we show squid giant axon potassium and sodium currents calculated using Eq. A12.
Figure 2.
The constant-field assumption. Goldman assumed that dV/dx, the electric field, was the same at all the points along the membrane thickness. Therefore, the field is just the electric potential difference across the membrane divided by the membrane width. Knowing the voltage–distance relationship the integration of the NP equation is possible, as explained in Appendix A. As Goldman did, we define the electric potential difference as the intracellular potential minus the extracellular potential. Hodgkin and Huxley (1952) reversed this convention.
Figure 3.
Goldman rectification. Rectification is a consequence of the asymmetry of the ion concentrations of the solution bathing the membrane. The figure displays the potassium and sodium current densities as a function of voltage calculated using the Goldman current equation, with GK = 36 mScm−2 and GNa = 120 mScm−2. Potassium concentrations are 440 mM (intracellular) and 10 mM (extracellular). Sodium concentrations are 50 mM (intracellular) and 440 mM (extracellular).
Goldman generalized Eq. 12 to any number of uni-univalent salts by defining Λ+ as the conductance per unit areas in one direction and Λ− as the conductance per unit area in the reverse direction (Goldman, 1943).
| (13a) |
| (13b) |
where Σ+ and Σ− are sums of the contributions from all the cations and anions to Λ+ and Λ−, respectively. Thus, the total current density, I, across the membrane when bathed with arbitrary mixtures of uni-univalent salts is
| (14) |
Goldman remarked that Λ+ and Λ− are the limiting conductances in either direction at extreme positive or negative voltages (Goldman, 1943).
The Goldman equation for zero-current potential
Nernst calculated the nonequilibrium steady-state potential, V0, for a mixture of uni-univalent salts setting I = 0 in Eq. 14:
| (15) |
Eq. 15 is Eq. 18 in Goldman’s paper (Goldman, 1943). Hodgkin and Katz (1949) applied this equation to calculate the resting potential of the giant squid axon as a function of Na+, K+, and Cl− concentrations and mobilities. Combining Eqs. 15 and 13, we have
| (16) |
The ion concentrations in Eq. 16 are those in the intracellular and extracellular borders of the membrane. Hodgkin and Katz (1949) assumed that the concentration ni at the outer edges of the membrane was directly proportional to the concentration of the ion i in the external fluid (e.g., nNa = β[Na]). Hodgkin and Katz (1949) also introduced the concept of membrane permeability, Pi, which relates ion mobility within the membrane, the partition coefficient, βi, and the membrane thickness, a:
| (17) |
This definition turns Eq. 14 into the familiar GHK equation for the membrane potential, Vr (Eq. 1).
The assumptions implicit in Eq. 18 as summarized by Hodgkin and Katz (1949) were that (1) ions in the membrane move under the influence of diffusion and the electric field in a manner that is essentially similar to that in free solution, (2) the electric field may be regarded as constant throughout the membrane, (3) the concentrations of ions at the edges of the membrane are directly proportional to those in the aqueous solutions bounding the membrane, and (4) the membrane is homogeneous.
Walz et al. (1969) discuss that the constant-field assumption is physically reasonable and show that it is correct for very thin membranes and low ion concentrations. It is important to note here that assumption 2 (constant electric field inside the membrane) is not required for the case of a membrane selective to either monovalent cations or anions (see Appendix B). Armstrong (2003) also derived the GHK equation for anions and cations without the constant-field assumption.
Caveats of the Goldman current density equation in real channels
For the Goldman equation to be valid, conductance must be a linear function of ion concentration; however, there are examples where this condition does not hold and conductance does depend on ion concentration, saturating at high concentration (French and Adelman, 1976). Also, in some instances, conductance tends toward a limiting value at very low concentrations because of the influence of charges on the membrane surface (McLaughlin, 1977). Other systems display anomalous mole fraction effects on mixtures of ions (Nonner et al., 1998). Deviations of the expected NP behavior of ions channels arise from their ability to hold more than one ion in their conduction systems. In particular, K+ channels are multi-ion channels in which ions move in single file (Hodgkin and Keynes, 1955). Also, current will deviate from the GHK equation in narrow channels, where ions interact with binding sites along the pathway (e.g., potassium channels; Doyle et al., 1998). When ions interact with the channel walls or other ions in the pore while diffusing, the strategy is to use Eyring rate theory (Eyring et al., 1949), which assumes that ions must overcome a sequence of energy barriers to move through the channel. The cases of one-ion and multi-ion channels are discussed in detail in the classical papers of Peter Läuger and Bertil Hille (Läuger, 1973; Hille and Schwarz, 1978) However, we should mention here that these limitations of the electrofusion theory based on the NP equation also prompted the development of the Poisson-NP electrofusion theory, which can handle such cases (Nonner and Eisenberg, 1998).
Applications of the Goldman equation
Squid axon resting and action potential
Curtis and Cole (1942) measured the effect of changing the external potassium concentration on the squid axon resting potential. They found that the resting potential deviates from that expected for a potassium-selective membrane, as stated in the Bernstein hypothesis (Bernstein, 1902). Hodgkin and Katz (1949) explained Curtis and Cole’s results (Curtis and Cole, 1942) using Eq. 1 with PK/PNa/PCl = 1:0.04:0.45. Most importantly, the experiments done on squid giant axons using internal electrodes showed that during an action potential the membrane voltage went well above that expected from the simple depolarization predicted by Bernstein’s membrane theory (Hodgkin and Huxley, 1939, 1952; Curtis and Cole, 1942). Hodgkin and Katz (1949) measured the effect of changing the external sodium concentration on the potential at the peak of the action potential. They explained their results using Eq. 1 with PK/PNa/PCl = 1:20:0.45. The conclusion was that during the action potential, the sodium permeability increased 500 times compared with that at rest. This result explained the observation of Cole and Curtis (1939) that the membrane resistance decreased dramatically during the action potential. What is really impressive is the fact that Hodgkin and Katz (1949) concluded that during the refractory period, the Na+ membrane permeability coefficient was 0, PK/PNa/PCl = 1:0:0.45. During the refractory period, they claimed that “the sodium permeability must therefore be reduced by exhaustion or inactivation of the special mechanism which comes into play when the nerve is first depolarized.”
Anomalous rectification
Bernard Katz in 1949 (Katz, 1949) found that inward potassium current in frog muscles was larger than the outward current, even in symmetric potassium concentrations. GHK equations revealed that the mechanism underlying this anomalous rectification was a potassium permeability increase upon membrane hyperpolarization.
Rectification of frog skin sodium current
The GHK current equation gives a straightforward explanation for the nonlinear current–voltage curve observed in frog skin (Fuchs et al., 1977).
Monovalent cation selectivity measured in bi-ionic potential experiments
The system for measuring bi-ionic potential consists of a membrane bathed by two solutions, either of different cations, X and Y, sharing the same impermeant anion, or different anions, X and Y, sharing a common cation. Under these experimental conditions, Eq. 1 reduces to
| (18) |
Hille (1971) devised an experimental strategy to obtain the ion selectivity of Na+ channels in myelinated fibers, a preparation in which one does not have access to the cytoplasmic milieu. He measured the zero-current voltage by exposing sodium channels of myelinated nerve fibers to different external solutions containing either sodium or different inorganic and organic monovalent cations, X. If the change of external solution does not alter the intracellular ionic composition, Eq. 19 produces the permeability ratio PX/PNa. VX and VNa are the zero-current potentials under conditions in which the external cation is Na or X.
| (19) |
Using inorganic and organic ions of different sizes, Hille concluded that the selectivity filter of the nerve sodium channel has a rectangular section of 3 × 5 Å. Dwyer et al. (1980), applying similar methods to the nicotinic acetylcholine receptor, discovered a good correlation of the organic cation size and permeability, and explained the correlation using a pore model with a square cross section of 6.5 × 6.5 Å. Notably, the pore dimensions obtained from the crystal structures of Na+ and Ach-receptor channels are consistent with those predicted by permeability determinations. Indeed, the narrower part of the pore of a voltage-gated sodium channel is 3 Å in diameter (Shen et al., 2017) and that of the acetylcholine receptor is 6 Å in diameter (Miyazawa et al., 2003; Hilf and Dutzler, 2008)
GHK voltage equation for mixtures of monovalent and divalent cations
Fatt and Ginsborg (1958) derived the GHK voltage equation for the case in which K+ is the only intracellular cation and Sr2+ the only extracellular cation. In this case, the Goldman currents equations for IK and ISr are
where U is the dimensionless reduced voltage, U = FV/RT. In steady state, the sum of the potassium current plus the strontium current must be zero:
The denominator on the calcium current may be written as (eU − 1)(eU + 1) and the expression for the sodium current multiplied and divided by (eU + 1; so as to have a common denominator), which can thus be eliminated to produce
Finally, Fatt and Ginsborg (1958) solved for the permeability ratio:
| (20) |
Eq. 20 is a convenient way of obtaining permeability ratios between mono- and divalent cations. In the case of divalent cations, it is mandatory to take into consideration the activity coefficient, because it can be as low as 0.4 when the divalent concentration is 0.1 M. Fatt and Ginsborg (1958) found PSr/PK = 13 at the peak of the action potential in muscle fibers of crayfish.
A bird’s eye review of the literature indicates that Eq. 20 is the standard formula used to obtain the selectivity of ion channels permeable to Ca2+. We give a few examples in the following paragraphs.
Iino et al. (1997) used the GHK equation to calculate the reversal potential of the current carried by Na+ and Ca2+ ions in GluN1/GluN2B NMDA receptors expressed in Xenopus laevis oocytes. They found PCa/PNa = 17 for these NMDA receptors. They used sodium and calcium ion concentrations corrected for activity coefficient using the Guggenheim formula (Robinson and Stokes, 1959). Schneggenburger et al. (1993) used the GHK current equation to determine the fraction of the current carried by Ca2+ through glutamate receptor channels.
For the TRP channel TRPV1, PCa/PNa measured using Eq. 20 is 9.6 (Caterina et al., 1997). This channel, as well as other channels such as TRPV3 and ATP-activated P2X receptor channels, present dynamic changes of selectivity (e.g., the TRPV1 permeability ratio PCa/PNa, changes from 17.5 to 5.9 during capsaicin activation; Chung et al., 2008). Lewis (1979) derived the zero-current voltage for a membrane separating solutions containing sodium, potassium, and calcium on both sides. Using this equation, McKemy et al. (2002) calculated PCa/PNa = 3.3 for TRPM8 channels.
Coda
By integrating the NP equation, which relates ion fluxes to ion concentration and voltage gradients, Goldman (1943) was able to arrive at a concise expression for the membrane current as a function of ion concentrations and voltage. To integrate the NP equation, Goldman assumed that the electric field across the membrane is constant and that ions move under the influence of diffusion and the electric field. This equation, now known as the Goldman current equation, is the basis of the also famous GHK voltage equation, which is universally used to calculate permeability ratios between different ions and, hence, the ion selectivity of ion channels of the most different nature. In this tribute to Goldman’s paper, we showed how this relatively simple theory has influenced the ion transport field using examples from the literature over time and by discussing how the GHK equation has been adapted to different experimental circumstances. Thanks to the determination of different channels and transporter structures and molecular modeling, the mechanisms that determine ion selectivity and transport in them is much better understood at present. However, the elegance, simplicity, and usefulness of the GHK equation are something difficult to beat.
Appendix A
Integration of the NP equation for a single ionic species is as follows:
| (A1) |
Because the diffusion coefficient, D, is D = uRT, and defining the reduced potential U as U ≡ FV/RT, we have
| (A2) |
Multiplying both sides of Eq. A2 by the integration factor eZU, we rewrite Eq. A2 as
| (A3) |
separating variables
| (A4) |
Assuming the electric field dU/dx is constant across the membrane:
| (A5) |
where a is the membrane thickness. Now we can integrate both sides as follows:
| (A6) |
Boundary conditions definitions are as follows: Extracellular edge of the membrane x = a, c(a) = c′ex, U(a) = 0; Intracellular edge of the membrane x = 0, c(0) = c′in, U(0) = Um:
| (A7) |
| (A8) |
Introducing the partition coefficient, β, relating the membrane and solution concentrations, c =βc′, and defining the permeability coefficient P as P = Dβ/a.
| (A9) |
The electric current density, I, is zF times the mass flow, J:
| (A10) |
Expanding the reduced potential, we get the electric current density in terms of membrane potential, Vm, and ion concentrations (Eq. 1):
| (A11) |
For the condition I = 0 and from Eq. A10, we can define a Nernst reduced potential, UNernst. UNernst = cex/cin.
We introduce the Nernst reduced potential as
| (A12) |
for cin ≈ cex, UNersnt ≈ 0 and for Um ≈ UNernst. The approximation ex − 1 = x is accurate for x→0, so the current density becomes a liner function of voltage:
| (A13) |
which is the widely used linear form, a variation of Ohm’s law
| (A14) |
where the conductance, G, is
| (A15) |
Appendix B
There is no need for the assumption that the field is constant within the membrane for the case of univalent cations alone (e.g., Na+ and K+ in the case of a cation- or anion-selective membrane), as long as the ions are of the same valence.
From Eq. A4, we get
| (B1) |
We prepare for integration
| (B2) |
We integrate the right side of Eq. B2 using the boundary conditions defined in Appendix A.
| (B3) |
| (B4) |
Kramers (1940) first derived Eq. B4. Making zero the sum of the flows of sodium and potassium, the integral on the common denominator in Eq. B4 disappears as well as the constants to get P from D, so we can write
| (B5) |
Finally, we solve for the zero-current potential Vm:
| (B6) |
| (B7) |
Thus, we recover the GHK equation for sodium and potassium without using the constant-field assumption.
Acknowledgments
We thank John Ewer for critical reading of the manuscript.
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (grant 1150273 to R. Latorre) and the Centro Interdisciplinario de Neurociencia de Valparaíso, a Millennium Institute (P09-022-F). This work was partially supported by the Air Force Office of Scientific Research under award number FA9550-16-1-0384 to R. Latorre.
The authors declare no competing financial interests.
Olaf S. Andersen served as editor.
Footnotes
Abbreviations used:
- GHK
- Goldman–Hodgkin–Katz
- NP
- Nernst–Planck
References
- Armstrong C.M. 2003. The Na/K pump, Cl ion, and osmotic stabilization of cells. Proc. Natl. Acad. Sci. USA. 100:6257–6262. 10.1073/pnas.0931278100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrhenius S. 1884. Recherches sur la Conductivité Galvanique des Électrolytes. Royal Publishing House, P.A. Norstedt & söne, Stockholm. [Google Scholar]
- Arrhenius S. 1887. Über die Dissociation der in Wasser gelösten Stoffe. Z. Phys. Chem. 1:631–648. [Google Scholar]
- Baker P.F., Hodgkin A.L., and Shaw T.I.. 1962. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J. Physiol. 164:355–374. 10.1113/jphysiol.1962.sp007026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J. 1902. Untersuchungen zur Thermodynamik der bioelektrischen Strome. Pflfigers Arch. ges. Physiol. 92:521–562. [Google Scholar]
- Beurg M., Goldring A.C., and Fettiplace R.. 2015. The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J. Gen. Physiol. 146:233–243. 10.1085/jgp.201511458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks L.R. 1930a The variation of electrical resistance with applied potential: I. Intact Valonia ventricosa. J. Gen. Physiol. 13:793–806. 10.1085/jgp.13.6.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks L.R. 1930b The variation of electrical resistance with applied potential: III. Impaled Valonia ventricosa. J. Gen. Physiol. 14:139–162. 10.1085/jgp.14.1.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., and Julius D.. 1997. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 389:816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chandler W.K., and Meves H.. 1965. Voltage clamp experiments on internally perfused giant axons. J. Physiol. 180:788–820. 10.1113/jphysiol.1965.sp007732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M.K., Güler A.D., and Caterina M.J.. 2008. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat. Neurosci. 11:555–564. 10.1038/nn.2102 [DOI] [PubMed] [Google Scholar]
- Cole K.S., and Curtis H.J.. 1939. Electric impedance of the squid giant axon during activity. J. Gen. Physiol. 22:649–670. 10.1085/jgp.22.5.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K.S., and Curtis H.J.. 1941. Membrane potential of the squid giant axon during current flow. J. Gen. Physiol. 24:551–563. 10.1085/jgp.24.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis H.J., and Cole S.C.. 1942. Membrane and action potentials from the squid giant axon. J. Cell. Comp. Physiol. 19:135–144. 10.1002/jcp.1030190202 [DOI] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., and MacKinnon R.. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- Dwyer T.M., Adams D.J., and Hille B.. 1980. The permeability of the endplate channel to organic cations in frog muscle. J. Gen. Physiol. 75:469–492. 10.1085/jgp.75.5.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein A. 1905. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 17:549–560. 10.1002/andp.19053220806 [DOI] [Google Scholar]
- Eisenman G. 1962. Cation selective glass electrodes and their mode of operation. Biophys. J. 2:259–323. 10.1016/S0006-3495(62)86959-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring H., Lumry R., and Woodbury J.W.. 1949. Some applications of modern rate theory to physiological systems. Rec. Chem. Prog. 10:100–114. [Google Scholar]
- Faraday M. 1834. Experimental researches in electricity. Seventh series. Trans. Roy. Soc. London. 124:77–79. 10.1098/rstl.1834.0008 [DOI] [Google Scholar]
- Fatt P., and Ginsborg B.L.. 1958. The ionic requirements for the production of action potentials in crustacean muscle fibres. J. Physiol. 142:516–543. 10.1113/jphysiol.1958.sp006034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P., and Katz B.. 1953. The electrical properties of crustacean muscle fibres. J. Physiol. 120:171–204. 10.1113/jphysiol.1953.sp004884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick A. 1855. Ueber Diffusion. Ann. Phys. 94:59–86. 10.1002/andp.18551700105 [DOI] [Google Scholar]
- French R.J., and Adelman W.J.. 1976. Competition, saturation, and inhibition-ionic interactions shown by membrane ionic currents in nerve, muscle, and bilayer systems. Curr. Top. Membr. Trans. 8:161–207. 10.1016/S0070-2161(08)60197-5 [DOI] [Google Scholar]
- Fuchs W., Larsen E.H., and Lindemann B.. 1977. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J. Physiol. 267:137–166. 10.1113/jphysiol.1977.sp011805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D.E. 1943. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 27:37–60. 10.1085/jgp.27.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf R.J.C., and Dutzler R.. 2008. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 452:375–379. 10.1038/nature06717 [DOI] [PubMed] [Google Scholar]
- Hille B. 1971. The permeability of the sodium channel to organic cations in myelinated nerve. J. Gen. Physiol. 58:599–619. 10.1085/jgp.58.6.599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 1972. The permeability of the sodium channel to metal cations in myelinated nerve. J. Gen. Physiol. 59:637–658. 10.1085/jgp.59.6.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 1973. Potassium channels in myelinated nerve. Selective permeability to small cations. J. Gen. Physiol. 61:669–686. 10.1085/jgp.61.6.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. 2001. Ionic Channels of Excitable Membranes. Third edition Sinauer, Sunderland, MA. 814 pp. [Google Scholar]
- Hille B., and Schwarz W.. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72:409–442. 10.1085/jgp.72.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., and Huxley A.F.. 1939. Action potentials recorded from inside a nerve fibre. Nature. 144:710–711. 10.1038/144710a0 [DOI] [Google Scholar]
- Hodgkin A.L., and Huxley A.F.. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117:500–544. 10.1113/jphysiol.1952.sp004764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., and Katz B.. 1949. The effect of sodium ions on the electrical activity of giant axon of the squid. J. Physiol. 108:37–77. 10.1113/jphysiol.1949.sp004310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., and Keynes R.D.. 1955. The potassium permeability of a giant nerve fibre. J. Physiol. 128:61–88. 10.1113/jphysiol.1955.sp005291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Ciani S., Tsuzuki K., Ozawa S., and Kidokoro Y.. 1997. Permeation properties of Na+ and Ca2+ ions through the mouse ε2/ζ1 NMDA receptor channel expressed in Xenopus oocytes. J. Membr. Biol. 155:143–156. 10.1007/s002329900166 [DOI] [PubMed] [Google Scholar]
- Jeng G., Aggarwal M., Yu W.P., and Chen T.Y.. 2016. Independent activation of distinct pores in dimeric TMEM16A channels. J. Gen. Physiol. 148:393–404. 10.1085/jgp.201611651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., and Wu S.-S.. 1995. Foundations of Cellular Neurophysiology. MIT Press, Cambridge, MA. 710 pp. [Google Scholar]
- Katz B. 1942. Impedance changes in frog’s muscle associated with electrotonic and “endpalte” potentials. J. Neurophysiol. 5:169–184. [Google Scholar]
- Katz B. 1949. Les constantes electriques de la membrane du muscle. Arch. Sci. Physiol. (Paris). 2:285–299. [Google Scholar]
- Kramers H.A. 1940. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica. 7:284–304. 10.1016/S0031-8914(40)90098-2 [DOI] [Google Scholar]
- Latorre R., Lopez-Barneo J., Bezanilla F., and Llinas R.. 1996. Biofísica y Fisiología Celular. Universidad de Sevilla, Sevilla. 705 pp. [Google Scholar]
- Läuger P. 1973. Ion transport through pores: a rate-theory analysis. Biochim. Biophys. Acta. 311:423–441. 10.1016/0005-2736(73)90323-4 [DOI] [PubMed] [Google Scholar]
- Lewis C.A. 1979. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J. Physiol. 286:417–445. 10.1113/jphysiol.1979.sp012629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maex R. 2017. On the Nernst–Planck equation. J. Integr. Neurosci. 16:73–91. 10.3233/JIN-170008 [DOI] [PubMed] [Google Scholar]
- Matthews G.G. 2013. Cellular Physiology of Nerve and Muscle. Fourth edition Wiley-Blackwell, Malden, MA. 248 pp. [Google Scholar]
- McKemy D.D., Neuhausser W.M., and Julius D.. 2002. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 416:52–58. 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- McLaughlin S. 1977. Electrostatic potentials at membrane–solution interfaces. Curr. Top. Membr. Trans. 9:71–144. 10.1016/S0070-2161(08)60677-2 [DOI] [Google Scholar]
- Miyazawa A., Fujiyoshi Y., and Unwin N.. 2003. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 423:949–955. 10.1038/nature01748 [DOI] [PubMed] [Google Scholar]
- Mullins L.J. 1981. Ion Transport in the Heart. Raven Press, New York. 136 pp. [Google Scholar]
- Nernst W. 1888. Zur Kinetik der in Lösung befindlichen Körper. Z. Phys. Chem. 2:613–637. 10.1515/zpch-1888-0174 [DOI] [Google Scholar]
- Nonner W., and Eisenberg B.. 1998. Ion permeation and glutamate residues linked by Poisson–Nernst–Planck theory in L-type calcium channels. Biophys. J. 75:1287–1305. 10.1016/S0006-3495(98)74048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W., Chen D.P., and Eisenberg B.. 1998. Anomalous mole fraction effect, electrostatics, and binding in ionic channels. Biophys. J. 74:2327–2334. 10.1016/S0006-3495(98)77942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planck M. 1890. Ueber die Erregung von Elektricität und Wärme in Elektrolyten. Ann. Phys. 275:161–186. 10.1002/andp.18902750202 [DOI] [Google Scholar]
- Robinson R.A., and Stokes R.H.. 1959. Electrolyte Solutions. Butterworths, London. 559 pp. [Google Scholar]
- Schneggenburger R., Zhou Z., Konnerth A., and Neher E.. 1993. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 11:133–143. 10.1016/0896-6273(93)90277-X [DOI] [PubMed] [Google Scholar]
- Shen H., Zhou Q., Pan X., Li Z., Wu J., and Yan N.. 2017. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science. 355:eaal4326 10.1126/science.aal4326 [DOI] [PubMed] [Google Scholar]
- Sjodin R.A. 1980. Contribution of Na/Ca transport to the resting membrane potential. J. Gen. Physiol. 76:99–108. 10.1085/jgp.76.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.L., Abdallah S., Wong Y.Y., Le P., Harracksingh A.N., Artinian L., Tamvacakis A.N., Rehder V., Reese T.S., and Senatore A.. 2017. Evolutionary insights into T-type Ca(2+) channel structure, function, and ion selectivity from the Trichoplax adhaerens homologue. J. Gen. Physiol. 149:483–510. 10.1085/jgp.201611683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sten-Knudsen O. 1978. Passive transport processes. In Membrane Transport in Biology. Concepts and Models. Biebisch G.H., Tosteson D.C., and Ussing H.H., editors. Springer-Verlag, New York: 5–113. 10.1007/978-3-642-46370-9_2 [DOI] [Google Scholar]
- Sterratt D.C. 2015. Goldman–Hodgkin–Katz equations. In Encyclopedia of Computational Neuroscience. Jaeger D. and Jung R., editors. Springer-Verlag, New York: 1300–1302. [Google Scholar]
- van ’t Hoff J.H. 1887. Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen. Z. Phys. Chem. 1:481–508. [Google Scholar]
- Walz D., Bamberg E., and Läuger P.. 1969. Nonlinear electrical effects in lipid bilayer membranes. I. Ion injection. Biophys. J. 9:1150–1159. 10.1016/S0006-3495(69)86442-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth L.P., and Hille B.. 1992. Ionic selectivity of Ih channels of rod photoreceptors in tiger salamanders. J. Gen. Physiol. 100:749–765. 10.1085/jgp.100.5.749 [DOI] [PMC free article] [PubMed] [Google Scholar]