Abstract
Background:
Dodonaea angustifolia is used in Ethiopian traditional medicine to treat malaria. The objective of this work was to conduct bioassay guided fractionation of the leaves of D. angustifolia using Plasmodium berghei infected mice.
Method:
The antiplasmodial activity of the extracts and pure compounds was evaluated using the standard Peter’s four-day suppressive method. The structures of isolated compounds were elucidated using chemi-cal and spectroscopic methods.
Results:
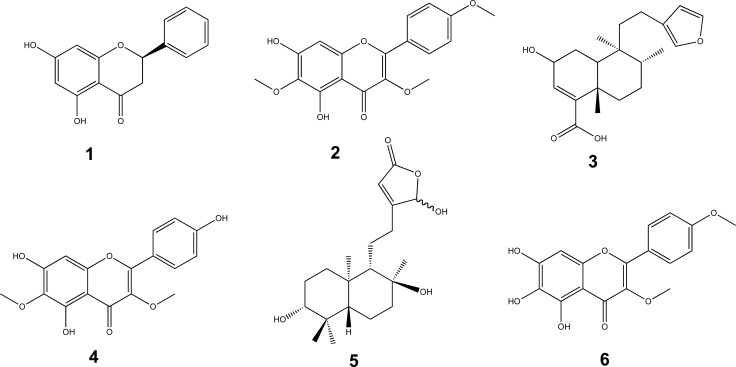
In this study, the ethyl acetate soluble portion of the 80% aqueous MeOH extract of the leaves significantly suppressed parasitaemia in Plasmodium berghei infected mice (80.28% at 150 mg/kg). Three active compounds which exhibited significant percent suppression of parasitaemia by 81% at 40 mg/kg, 80% at 50 mg/kg and 70% at 40 mg/kg, respectively were identified. These are the flavanone pinocembrin (1), the flavanol santin (2) and the clerodane diterpene 2-hydroxy-15,16-epoxyceloda-3,13(16),14-trien-18-oic acid (3). Under similar conditions, chloroquine suppressed parasitaemia by 100% at 25 mg/kg. Chemical study of the ethanol extract of the leaves yielded 5,7,4'-trihydroxy-3,6-dimethoxyflavone (4), ent-16-hydroxy-labdan-3α,8β-dihydroxy,13(14)-en-15,16-olide (5) and 5,6,7-trihydroxy-3,4'-dimethoxyflavone (6). Compound 6 has not been reported before as a natural product.
Conclusion:
From the leaves of D. angustifolia, three compounds with significant antiplasmodial activi-ties were isolated and characterized, with pinocembrin as the most active compound.
Keywords: Dodonaea angustifolia, Plasmodium berghei, pinocembrin, santin, anti-malarial, diterpene, flavonoid, Sapindaceae
1. Introduction
Malaria is a global disease prevalent in the tropics caused by Plasmodium parasites [1] affecting 40% of the world’s population [2]. Ethiopia is among the malaria-epidemic prone countries in Africa with nearly 70% of the population at risk [3]. Children under five years of age are the most vulnerable. The problem is also exacerbated due to increased incidences of the resistance of the parasite to anti-malarial drugs [4]. Consequently, it is still common for people to use traditional remedies to treat malaria.
Dodonaea, commonly called hop-bush or sand olive, is a genus that comprises around 70 species [5]. D. angustifolia L. f. (Sapindaceae), known as Ketketa in Ethiopia, is a shrub growing up to 3 m tall. It also occurs in different forms naturally from southern Africa to Arabia, as well as in Australia and New Zealand. All parts of the plant are hairless and resinous when young. Previous ethnopharmacological reports indicate therapeutic uses such as stem, leaf and root infusions to treat sore throats and colds [6]. The plant is also reported to exhibit antibacterial [7], antifungal [8], antidiabetic [9], anti-inflammatory [10] and antidiarreal properties [11].
The traditional use of the aerial parts of this plant to treat malaria persuaded us to undertake bioassay guided study of the leaves in order to test the efficacy of extracts and isolated compounds against mice infected with Plasmodium berghei.
2. Materials and Methods
2.1. Experimental Animals
Male Swiss albino mice weighing 25-35 g, 6-8 weeks of age were obtained from the Animal House of the College of Natural Sciences (CNS), Addis Ababa University (AAU), Addis Ababa, Ethiopia. The mice were housed in standard cages with saw dust as beddings and given a diet and tap water ad libitum. All animal care according to standard procedures was followed. Ethical clearance for use of animals in experiments was obtained from the Ethical Committee of the College of Natural Sciences of AAU ref no CNSDO/92/07/14 Date: Nov. 03, 2014.
Plant Material: D. angustifolia L. f. leaves were collected from the outskirts of the eastern part of Addis Ababa in November 2012. The plant was identified by Mr Melaku Wondafrash and a voucher specimen (001/2012) of the plant was deposited at the Herbarium of the College of Natural Sciences, Addis Ababa University, Ethiopia.
Reagents and Instruments: Chloroquine (CQ) pharmaceutical tablets ready for human use (60% pure) were used as positive control. All solvents used were of analytical grade. Melting points were determined in capillary tube with a Thiele Tube Melting Point Apparatus. Analytical TLC was run on a 0.25 mm thick layer of silica gel GF254 (Merck) on aluminum plate. Spots were detected by observation under UV light (254 nm) followed by spraying with vanillin in H2SO4 and heating with a hot air gun. Column chromatography was performed using silica gel (230-400 mesh) Merck. Solvents were evaporated under reduced pressure using Rotavapor BUCHI, RE 121. NMR spectra were measured on a Bruker Avance instrument (Bruker Avance 400 NMR Spectrometer). UV-Vis spectra were recorded on a T60 UV-Visible Spectrophotometer. Optical rotations were measured using an Autopol®IV Automatic Polarimeter.
FT-MS: The high resolution positive and negative ion electrospray mass spectra were obtained from a Bruker Apex III Fourier transform ion cyclotron resonance mass equipped with an Infinity™ cell, a 7.0 Tesla superconducting magnet, an RF-only hexapole ion guide and an external electrospray ion source. Nitrogen was used as drying gas at 150°C. The sample solutions were introduced continuously via a syringe pump with a flow rate of 120 μL h-1. All data were acquired with 512 k data points and zero filled to 2048 k by averaging 32 scans. The data were evaluated by the Bruker XMASS 7.0.8 software.
2.2. High Performance Liquid Chromatography-Mass Spectrometry (HPLC/ESI-MS)
The positive ion ESI mass spectra of the samples and the collision-induced dissociation (CID) mass spectra were obtained from a TSQ Quantum Ultra AM system equipped with a hot ESI source (electrospray voltage 3.0 kV, sheath gas: nitrogen; vaporizer temperature: 50oC; capillary temperature: 250oC). The MS system is coupled with an Accela UHPLC system (Thermofisher Scientific), equipped with a Syncronis-C18 column (1.7 μm, 50 x 1 mm, Thermo Scientific). For the UHPLC, a gradient system was used starting from H2O:CH3CN 90:10 (each of them containing 0.2% formic acid) to 100% CH3CN within 30 min and was then held on 5% for further 5 min; flow rate was 150 μL min-1 and injection volume was 3 μL.
Extraction and Isolation for Antiplasmodial Assay: D. angustifolia leaves powder (100 g) was extracted with 80% aqueous MeOH (700 mL) by shaking for 6 h. The greenish filtrate obtained after filtration was diluted to 50% aqueous solution and extracted with ethyl acetate (200 mL x 3). The ethyl acetate phase was dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure to afford 4.5 g (4.5%) dark green solid. The ethyl acetate phase was found active against Plasmodium berghei infected mice and was pre-adsorbed and chromatographed over silica gel (80 g, 8 x 120 cm, 230-400 mesh; Merck) using n-hexane for packing. Elution started with n-hexane initially (20 mL, 30 mg, F1) followed by hexane:EtOAc step gradient (4:1, 90 mL, 180 mg, F2-4; 3:2, 60 mL, 400 mg, F5&6; 3:2, 60 mL, 120 mg, F7&8; 1:1, 60 mL, 80 mg, F9&10; 2:3, 40 mL, 120 mg, F11; and 1:4, 120 mL, 100 mg, F12-14), and then with EtOAc (40 mL, 20 mg, F15; 120 mL, 15 mg, F16-18; and 90 mL, 200 mg, F19-21), and finally ended with EtOAc:MeOH (4:1, 120 mL, 300 mg, F22-25; and 1:1, 60 mL, 2 mg, F26&-27). A total of 27 fractions were collected and those with the same TLC profiles were combined to afford 10 subfractions (F1, F2-4, F5&6, F7&8, F9&10, F11-15, F16-18, F19-21, F22-25 and F26&27).
Bioassay on experimental animals tested on P. berghei infected mice showed F1-6 to be inactive while combined fractions F7&8, F9&10, F11-15 and F19-21 were active. Washing F7&8 with diethyl ether resulted in yellow crystals identified as santin (2) (40 mg, 0.04%). The diethyl ether soluble portion was air dried and applied on Sephadex LH20 using MeOH:CHCl3 (1:1) as eluent to afford pinocembrin (1, 10 mg, 0.01%). Column chromatography of fraction F9&10, eluting using hexane:EtOAc (1:1) yielded 2-hydroxy-15,16-epoxyceloda-3,13(16),14-trien-18-oic acid (3, 30 mg, 0.03%). Likewise, washing F11-15 and F19-21 with EtOAc:diethyl ether (1:1, 5 mL x 3) yielded additional 2-hydroxy-15,16-epoxyceloda-3,13(16), 14-trien-18-oic acid (3, 80 mg, 0.1%) as a white solid.
2.3. Extraction and Isolation of Compounds from the Ethyl Acetate Soluble Portion of the Ethanol Extract
Ground leaves of D. angustifolia (150 g) were extracted with ethanol (1 L) by shaking for 6 h, filtered and concentrated under reduced pressure at 40oC to afford 25 g (16%) of jelly solid. This extract was defatted with n-hexane (150 mL), the insoluble portion was taken up in EtOAc (150 mL) by shaking for 6 h, filtered and concentrated to afford 10 g (6%). This extract (6 g) was adsorbed and subjected to silica gel (80 g, 8 x 120 cm, 230-400 mesh; Merck) column chromatography. The column was eluted with petrol:EtOAc of increasing polarities to afford 26 fractions which were pooled together according to their TLC profile to afford nine combined fractions. Fr1&2 were eluted with 100% petrol (100 mL, 450 mg). The next six combined fractions were collected with petrol:EtOAc (9:1, 100 mL, 1 g, F3-5; 9:1, 150 mL, 1 g, F6-10; 4:1, 100 mL, 200 mg, F11-13; 4:1, 50 mL, 80 mg, F14; 7:3, 160 mL, 700 mg, F15-18; 1:1, 160 mL, 250 mg, F19-21; 100% EtOAc, 100 mL, 10 mg, F 22-24; F 25&26, 100% EtOAc, 100 mL, 100 mg).
F3-5 (700 mg) was adsorbed and applied on column chromatography over silica gel (13 g, 8 x 120 cm, 230-400 mesh; Merck). Elution was carried out first with petrol (10 mL, 20 mg, F1) and then petrol:EtOAc (9:1, 30 mL, 80 mg, F2&3; 4:1, 20 mL, 35 mg, F4; 1:1, 20 mL, 30 mg, F5; 2:3, 40 mL, 109 mg, F6&7; and 1:4, 10 mL, 80 mg, F8) of increasing polarity to afford eight subfractions. Fraction 8 was identified as santin (2) (80 mg, 0.08%).
F6-10 (700 mg) was adsorbed and chromatographed over silica gel (17 g, 8 x 120 cm, 230-400 mesh; Merck) column chromatography. Elution was carried out using petrol (10 mL, 10 mg, F1) first followed by petrol:EtOAc (9:1, 20 mL, 25 mg, F2; 4:1, 120 mL, 200 mg, F3; 3:2, 60 mL, 80 mg, F4; 3:2, 20 mL, 60 mg, F5; 1:1, 40 mL, 120 mg, F6) and finally with 100% EtOAc (40 mL, 68 mg, F7). The TLC profile of F6 and F7 showed one spot identified as santin (2, 190 mg, 1.9%).
F11-13 (100 mg) was combined and applied on Sephadex LH20 using MeOH:CHCl3 (1:1) as elute (each 5 mL) to afford seven fractions. The sixth and seventh fractions were identified as santin (2, 5 mg, 0.05%) and pinocembrin (1, 12 mg, 0.02%), respectively.
F15-18 (500 mg) was adsorbed and applied to silica gel (15 g) column chromatography. It was eluted first using petrol:EtOAc (9:1, 35 mL, 80 mg, F1; 4:1, 20 mL, 50 mg, F2; 3:2, 30 mL, 50 mg, F3; 1:1, 20 mL, 60 mg, F4, 2:3, 20 mL, 80 mg, F5; and 1:4, 20 mL, F6) followed by EtOAc (20 mL, 100 mg, F7) to afford seven subfractions. On TLC examination, F4 showed one spot identified as santin (80 mg). F7 was suspended in CHCl3 and filtered. The filtrate was concentrated to afford yellowish solid identified as 5,7,4'-trihydoxy-3,6-dimethoxyflavone (4, 18 mg, 0.02%).
F19-21 (250 mg) was adsorbed and chromatographed over silica gel (12 g, 8 x 120 cm, 230-400 mesh; Merck) column chromatography. Elution was carried out using petrol (20 mL, 20 mg, F1) followed by petrol:EtOAc (9:1, 20 mL, 30 mg, F2; 4:1, 30 mL, 188 mg, F3; and 1:1, 60 mL, 68 mg, F4) to afford four subfractions. On eluting the fourth fraction, a solid formed which was decanted to afford 5,6,7-trihydroxy-3,4'-dimethoxyflavone (6, 12 mg). The third fraction was applied on Sephadex LH20 eluted using MeOH:CHCl3 (1:1, 10 mL each) to afford two subfractions. The first subfraction was identified to be 2-hydroxy-15,16-epoxyceloda-3,13(16), 14-trien-18-oic acid (3, 160 mg, 0.16%).
F25&26 (100 mg) was dissolved in CHCl3:MeOH (1:1) and applied on Sephadex LH-20 and eluted with CHCl3:MeOH (1:1) to afford seven fractions (each 10 mL). F2&3 were combined (30 mg) to afford ent-16-hydroxy-labdan-3α,8β-dihydroxy,13(14)-en-15,16-olide (5).
Malaria Parasite and Inoculation: For in vivo antiplasmodial assays, extracts of the plant and the mice infective CQ sensitive strain of P. berghei were used. Each mouse used in the experiment was infected intraperitoneally with 0.2 mL of infected blood containing about 1 x 106-107 P. berghei parasitized erythrocytes [12]. For each experiment, about 1 mL P. berghei infected blood sample was used by taking blood from the tail end of the donor mice with rising parasitaemia of about 25-35% in such a way that 1 mL blood contains 5 x 106-107 P. berghei-parasitized erythrocytes. This was prepared by determining the percentage of parasitaemia and diluting 1 mL of blood in 4 mL of physiological saline solution of 0.9% NaCl.
Evaluating the Antiplasmodial Activity of the Extracts and Pure Compounds: The antiplasmodial activities of the constituents were evaluated using Peter’s four-day suppressive method [13]. Each mouse used in the study was infected interaperitoneally on D0 and randomly divided into two experimental groups (each group treated with plant extract) and two control groups (group treated with CQ as positive control and 20% DMSO as a negative control). Five mice per cage as a group were assigned. Each test extract and pure compounds were prepared in two doses, CQ at 25 mg/kg in a volume of 0.2 mL/mouse and vehicles (20% DMSO) at 0.2 mL/mouse. Each extract was administered as a single dose per day. All the extracts, the drug (CQ) and DMSO were given through intragastric route by using a standard intragastric tube after 3 hours of infection on D0 and continued daily for the consecutive four days. On the fifth day (D4), blood samples were collected from tail snip of each mouse. Thin smears were prepared by fixing with methanol for 3-5 minutes and stained with 10% Geimsa solution at a pH of 7.2 for 20 minutes. Five uniform fields from the tailed region of each stained slide were examined under the microscope with an oil immersion objective of 100X magnifying lens power to evaluate the percent suppression of each extract with respect to the negative control group. Parasitaemia counts were made from fields of different slides. The average was taken to determine the percent parasitaemia. The percent parasitaemia in each field and percent suppression for a group were calculated as follows [14].
Percent parasitaemia as:
Percentage suppression for a group was calculated as:
2.4. Statistical Analysis
Results of the studies were expressed as mean plus or minus standard error of the mean (M±SEM). Comparison of percentage parasitaemia suppression and statistical significance was determined by one way ANOVA followed by Scheffe’s post-hoc test using SPSS Version 20.0. Level of significance was set as P<0.05.
3. Results and Discussion
Initially, the antiplasmodial activities of the ethanol extract of the leaves of D. angustifolia were screened at higher dose (300 mg/kg) against Plasmodium berghei infected mice. The extract inhibited the parasites by 77.20%. The active extract was fractionated into hexane and ethyl acetate fractions, with the latter being more active exhibiting percent suppression of parasitaemia by 82.00% at 300 mg/kg, while the hexane extract was much less active (19.84%).
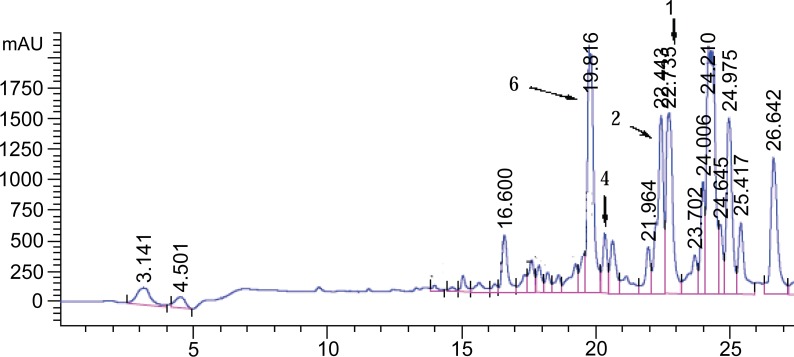
Chromatographic profiling of the ethyl acetate extract using thin layer chromatography (TLC) (Fig. 1) and liquid chromatography-mass spectrometry (LC-MS) (Fig. 2) revealed compounds 2 and 3 to be the major components.
Fig. (1).
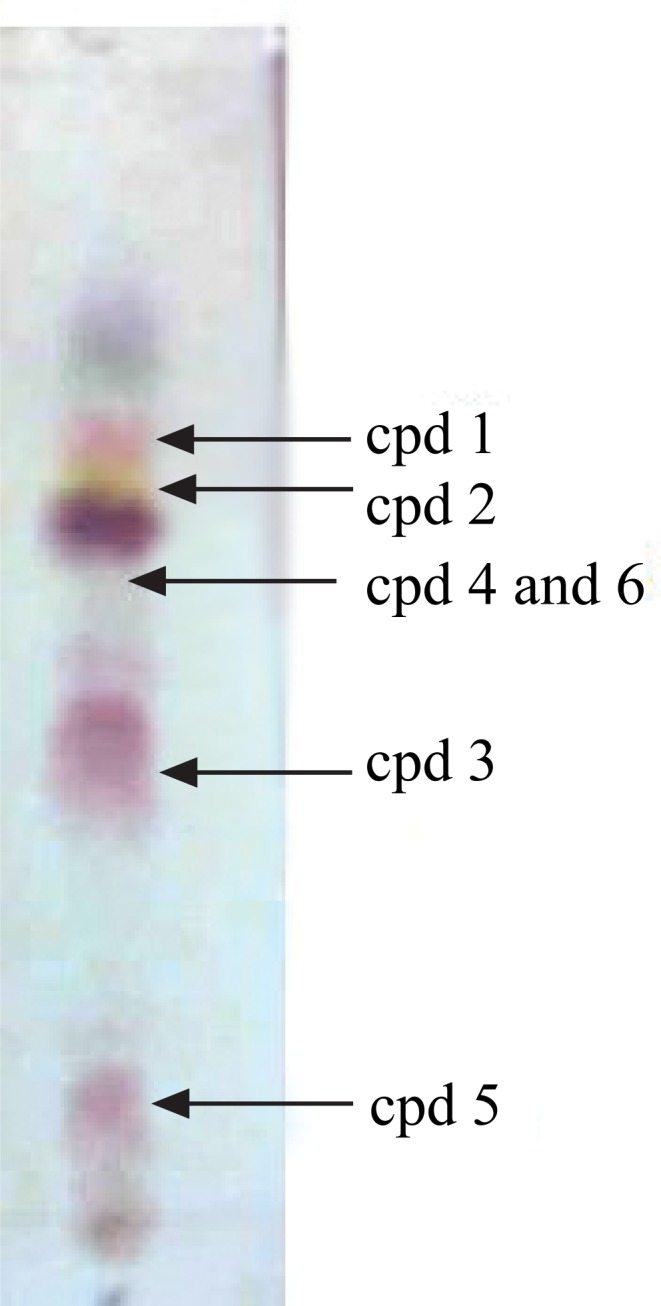
TLC profile of the ethyl acetate extracts of the leaves of D. angustifolia (eluent EtOAc:petrol, 3/2, sprayed with vanillin/H2SO4).
Fig. (2).
LC-MS chromatogram of the ethyl acetate extracts of the leaves of D. angustifolia in which compounds 1, 2, 4 and 6 were identified from the corresponding m/z ratios.
Compounds 4 and 6 stood out clearly in the HPLC (Fig. 2) at retention times of 19.816 and 20.142 min corresponding to 5,7,4’-trihydroxy-3,6-dimethoxyflavone and 5,6,7-trihydroxy-3,4’-dimethoxyflavone respectively. Compounds 1 (pinocembrin) and 2 (santin) appeared at retention times of 22.739 and 22.442 min respectively. The remaining compounds 3 and 5 were isolated and characterized although their respective peaks are not assigned in Fig. (2). The compounds were identified based on their m/z values and other spectroscopic (NMR, IR, HR-MS, and UV) and physical methods.
Similar activity was also exhibited by the ethyl acetate soluble fraction of the 80% aqueous methanol extract of the leaves (80.28% at 150 mg/kg and 74.00% at 300 mg/kg). However, the aqueous phase was found inactive. This indicates that the active fraction responsible for the antiplasmodial activity of the plant material is of medium polarity. Furthermore, the EtOAc soluble portion of 80% aqueous methanol extract of D. angustifolia leaves did not show much difference in their percent suppression when the dose was doubled. Hence, treating the mice with lower dose was effective, as maximizing the dose might cause toxicity without causing significant inhibition.
The active components present in the ethyl acetate soluble fraction of the 80% aqueous methanol extract were achieved by silica gel column chromatography which led to four active subfractions (F7&8, F9&10, F11-15 and F19-21). F7&8 suppressed parasitaemia by 56.25% at 50 mg/kg and 63.12% at 100 mg/kg. On the other hand, the percent parasitaemia of those mice treated with F11-15 and F19-21 was found to be 13.40±0.54 and 12.60±0.52, 11.80±0.59 and 10.80±0.58 at 50 mg/kg and 100 mg/kg doses, respectively. Likewise, the parasitaemia suppression of F11-15 and F19-21 was 60.30% and 61.10%, 63.50% and 66.60% at doses of 50 mg/kg and 100 mg/kg, respectively, demonstrating that these fractions contribute to the activity of the extracts. Furthermore, there was no significant difference in mean body weight in all groups of mice including those groups of mice treated with the standard drug. The active fractions yielded compounds 1 (10 mg, 0.01%), 2 (40 mg, 0.04%), and 3 (30 mg, 0.03%), which were identified by comparison of the physical and spectroscopic data as the known compounds pinocembrin (1) [15], santin (2) [16] and 2-hydroxy-15,16-epoxyceloda-3,13(16),14-trien-18-oic acid (3) [17], respectively (Fig. 3). The isolated compounds were validated for their antimalarial properties. To our knowledge, there is no prior report in the literature on the antimalarial or antiplasmodial activities of compounds 1-3.
Fig. (3).
Chemical structures of compounds isolated from the leaves of D. angustifolia.
The percent parasitaemia of pinocembrin (1) administered at a dose of 20 mg/kg and 40 mg/kg was 77.03% and 81.00%, respectively. Likewise, santin (2) was found to induce inhibition of parasitaemia by 85.50% and 80.95% at doses of 100 mg/kg and 50 mg/kg, respectively, whereas compound 3 (2-hydroxy-15,16-epoxyceloda-3,13(16),14-trien-18-oic acid) inhibited the parasite by 60.35% and 70.81% at doses of 20 mg/kg and 40 mg/kg, respectively.
In addition, there was no significant difference in mean body weight between D0 and D4 in all groups of mice including those mice treated with the standard drug (CQ). A significant reduction in parasitaemia inhibition was observed in mice administered with compounds 1-3 at low dosage indicating that the isolated compounds 1-3 contribute significantly to the effects of the crude extracts.
Mean survival time (MST) of the mice was an additional parameter used to evaluate the efficacy of antiplasmodial activity of the 80% aqueous methanol extract [18]. In this study, most test groups had statistically significant MST advancement than their corresponding negative control. All of the test mice were dead between 7-12 days except those group of mice treated with CQ which is in agreement with Piguet et al. (2011) [19]. The mice treated with the extract, subfractions (7&8, F9&10, F11-15 and F19-21) and pure constituents showed prolonged MST of the treated mice compared to their negative control treated with DMSO. This may be because the natural product suppresses P. berghie and also reduces the overall pathogenic effect of the parasite on the treated mice.
More compounds from the leaves of D. angustifolia were obtained by following another extraction protocol as follows. The ethyl acetate soluble portion of the ethanol extract yielded the above described compounds 1, 2 and 3 as well as two known (4 and 5) and one new (6) natural products (Fig. 3). These were identified as 5,7,4'-trihydroxy-3,6-dimethoxyflavone (4), and ent-16-hydroxy-labdan-3α,8β-dihydroxy,13(14)-en-15,16-olide (5) by comparison of their physical and spectroscopic data with literature reports [20, 21]. However, compounds 4-6 were not subjected to the antimalarial activity tests.
Compound 6 was isolated as a yellow solid. The UV-Vis spectrum (MeOH) showed absorption maxima at 285 and 346 nm which is distinctive of compounds having a flavonoid skeleton. The (+)-ESI-FT-MS displayed a molecular ion peak at m/z 331.0803 (calcd 331.2968 for C17H15O7) corresponding to the molecular formula C17H14O7. A prominent peak was observed at m/z 316 accounting for the loss of a methyl group from the parent ion. The 1H-NMR spectrum of compound 6 disclosed the presence of two methoxy signals at δ 3.80 (3H, s) and 3.90 (3H, s). The methine proton signal at δ 6.63 (1H, s) was assigned to H-8 of the A-ring of the flavonoid. The doublets at δ 8.02 and 7.12, showing only ortho coupling (each 2H, J = 8.80 Hz) were due to H-2′, 6′ and H-3′, 5′, respectively. The most downfield sharp singlet signal at δ 12.60 was characteristic of a chelated hydroxyl group establishing the presence of 5-OH.
The proton decoupled 13C-NMR spectrum of compound 6 with the aid of DEPT135 showed ten quaternary, three methine, and two methoxy carbon signals. The two methoxy carbon signals were observed at δ 54.9 and 59.3. The methine carbons at δ 113.9 (C-3', 5') and 130.0 (C-2', 6') were due to symmetrically placed aromatic carbons on unsymmetrically para substituted B-ring of a flavonoid. The carbon signal at δ 93.5 was due to C-8. The diagnostic signal in the 13C-NMR spectrum at δ 178.7 was suggestive for the presence of an α,β-unsaturated carbonyl carbon. The compound exhibited oxygenated aromatic quaternary carbon signals at δ 161.7 (C-4'), 155.8 (C-2), 152.8 (C-7), 149.7 (C-9), 146.7 (C-5), 138.2 (C-3), and 128.5 (C-6). Quaternary carbon signals were also observed at δ 123.0 and 105.2 due to C-1' and C-10, respectively. The HMBC spectrum indicated connectivity between the proton signal at δ 12.60 with the carbon signals at δ 105.5 (C-10), 128.6 (C-6) and 146.4 (C-5). The proton signal at δ 6.63 (H-8) showed HMBC correlation with the carbons at δ 105.5 (C-10), 128.5 (C-6) and 152.8 (C-9). The methoxy proton signals at δ 3.80 and 3.90 showed HMBC correlations with the olefinic carbon signals at δ 138.2 (C-3) and δ 161.7 (C-4'), respectively. According to the physical and spectroscopic findings, the structure of 6 can be assigned as 5,6,7-trihydroxy-3,4'-dimethoxyflavone. To the best of our knowledge, 5,6,7-trihydroxy-3,4'-dimethoxyflavone (6) has not been reported before from any natural source. The 1H and 13C-NMR data is summarized in Table 1.
Table 1.
NMR spectral data (CD3COCD3) of 5,6,7-trihydroxy-3,4'-dimethoxyflavone.
| Position | 5,6,7-trihydroxy-3,4'-dimethoxyflavone | Position | 5,6,7-trihydroxy-3,4'-dimethoxyflavone | ||
|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | ||
| 2 | 155.8 | 10 | 105.2 | ||
| 3 | 138.2 | 1' | 123.0 | ||
| 4 | 178.7 | 2' and 6' | 7.12 (2H, d, J = 8.80 Hz) | 130.0 | |
| 5 | 146.7 | 3' and 5' | 8.02 (2H, d, J = 8.80 Hz) | 113.9 | |
| 6 | 128.5 | 4' | 161.7 | ||
| 7 | 152.8 | OCH3 | 3.80 (3H, s) | 59.4 | |
| 8 | 6.42 (1H, s) | 93.5 | OCH3 | 3.90 (3H, s) | 59.9 |
| 9 | 149.7 | ||||
conclusion
In conclusion, bioassay guided fractionation showed the ethyl acetate soluble portion of the 80% aqueous MeOH extract of the leaves of D. angustifolia suppressed parasitaemia in Plasmodium berghei infected mice significantly (80.28% at 150 mg/kg). Three compounds isolated from the active EtOAc fractions, pinocembrin (1), the flavanol santin (2) and the clerodane diterpene 2-hydroxy-15,16-epoxyceloda-3,13(16),14-trien-18-oic acid (3), showed pronounced antiplasmodial activities. The result corroborates the traditional use of this plant as an antimalarial agent in Ethiopia.
ACKNOWLEDGEMENTS
This study was supported by a research grant (to E.D.) from the International Science Programs (ISP), Uppsala University, Sweden.
list of Abbreviations
- CQ
Chloroquine
- D0
Day Zero
- ESI-FT-MS
Electro spray Ionization Fourier Transform Mass Spectrometry
- F
Fraction
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Cropper M.L., Haile M., Lampietti J., Poulos C., Whittington D. The demand for malaria vaccine: evidence from Ethiopia. J. Dev. Econ. 2009;75:303–318. [Google Scholar]
- 2.Ayoola G.A., Coker H., Adesegun S., Adepoju-Bello A., Obaweya K., Ezennia E., Atangbayila T. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern nigeria. Trop. J. Pharm. Res. 2008;7:1019–1024. [Google Scholar]
- 3.WHO . World Malaria Report. Geneva, Switzerland: 2012. [Google Scholar]
- 4.Nigatu T., Haileselassie B., Hailu S., Seyum D. Involving communities in the fight against malaria in Ethiopia. African Medical and Research Foundation Case Studies; 2009. pp. 1–26. [Google Scholar]
- 5.Shepherd K.A., Rye B.L., Meissner R.A., West J.G. Two new Western Australian species of Dodonaea (Sapindaceae) from northern Yilgarn ironstones. Nuytsia. 2007;17:375–384. [Google Scholar]
- 6.Oliveira S.Q., Almeida M.T., Maraslis F., Silva I.T., Sincero T.C., Palermo J.A., Cabrera C.M., Caro M.S., Simoes C.M., Schenkel E.P. Isolation of three new ent-labdane diterpenes from Dodonaea viscosa Jacq. (Sapindaceae): Preliminary evaluation of antiherpes activity. Phytochem. Lett. 2012;5:500–505. [Google Scholar]
- 7.Teffo L.S., Aderogba M.A., Eloff J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010;76:25–29. [Google Scholar]
- 8.Pirdada A.J., Shaikh W., Usmanghani U., Mohiuddin E. Antifungal activity of Dodonaea viscosa extract on fungi isolated from superficial skin infection. Pak. J. Pharm. Sci. 2010;23:337–340. [PubMed] [Google Scholar]
- 9.Veerapar V.P., Prabhakar K.R., Thippeswamy B.S., Pansal P., Sirinvasan K.K., Unikrshanan M.K. Antidiabetic effect of D. viscosa aerial parts in high fructose-fedinsulin resistant rats: A mechanism based study. Indian J. Exp. Biol. 2010;48:800–810. [PubMed] [Google Scholar]
- 10.Huschka C., Schmidtke M., Abate A., Neubert R.H., Getie M., Gebre-Mariam T., Rietz R., Hohne C. Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74:139–143. doi: 10.1016/s0367-326x(02)00315-5. [DOI] [PubMed] [Google Scholar]
- 11.Rajamanickam V., Rajasekaran A., Anandarajagopal K., Sridharan D., Selvakumar K., Rathinaraj B.S. Antidiarreal activity of Dodonaea viscosa root. IJPBS. 2010;2:182–185. [Google Scholar]
- 12.Shittu I., Emmanuel A., Nok J.A. Antimalarial effect of the ethanolic stem bark extracts of Ficus platyphylla. J. Parasitol. Res. 2011. pp. 1–5. [DOI] [PMC free article] [PubMed]
- 13.Peters W., Portus H., Robinson L. The four day suppressive in vivo antimalarial test. Ann. Trop. Med. Parasitol. 1975;69:155–171. [Google Scholar]
- 14.Chen Y., Li S., Sun F., Han H., Zhang X., Fan Y., Tai G., Zhou Y. In vivo antimalarial activities of glycoalkaloids isolated from Solanaceae plants. Pharm. Biol. 2010;48:1018–1024. doi: 10.3109/13880200903440211. [DOI] [PubMed] [Google Scholar]
- 15.Ching A.Y.L., Wah T.S., Sukari M.A., Lian G.E.C., Rahmani M., Khalid K. Characterization of Flavonoid Derivatives from Boesenbergia rotunda (L.). MJAS, 2007;11:154–159. [Google Scholar]
- 16.Riaz T., Abbasi M.A., Rehman A.A., Shahzadi T., Ajaib M., Khan K.M. Phytochemical screening, free radical scavenging, antioxidant activity and phenolic content of Dodonaea viscosa Jacq. J. Serb. Chem. Soc. 2012;77:423–435. [Google Scholar]
- 17.Khan R., Alam F., Anwar S., Khan B.M., Shah Z. Isolation of flavones from indigenous Dodonaea viscosa. J. Chem. Soc. Pak. 2012;34:195–201. [Google Scholar]
- 18.David F., Philip R., Simon C., Reto B., Solomon N. Antimalarial drug discovery: Efficacy models for compound screening. Natl. Rev. 2005;3:509–520. doi: 10.1038/nrd1416. [DOI] [PubMed] [Google Scholar]
- 19.Piguet P.F., Kan C., Vesin C., Rochat A., Donati Y., Barazzone C. Role of CD40-CD40L in mouse sever malaria. Am. J. Pathol. 2011;159:733–742. doi: 10.1016/s0002-9440(10)61744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omosa L.K., Midiwo J.O., Derese S., Yenesew A., Peter M.J., Heydenreich M. Neo-clerodane diterpenoids from the leaf exudate of Dodonaea angustifolia. Phytochem. Lett. 2010;3:217–220. [Google Scholar]
- 21.Oliveira S.Q., Almeida M.T., Maraslis F., Silva I.T., Sincero T.C., Palermo J.A., Cabrera C.M., Caro M.S., Simoes C.M., Schenkel E.P. Isolation of three new ent-labdane diterpenes from Dodonaea viscosa (Sapindaceae): Preliminary evaluation of antiherpes activity. Phytochem. Lett. 2012;5:500–505. [Google Scholar]