Abstract
Glycoside phosphorylases catalyse the reversible synthesis of glycosidic bonds by glyco-sylation with concomitant release of inorganic phosphate. The equilibrium position of such reactions can render them of limited synthetic utility, unless coupled with a secondary enzymatic step where the reaction lies heavily in favour of product. This article surveys recent works on the combined use of glycan phosphorylases with other enzymes to achieve synthetically useful processes.
Keywords: Phosphorylase, disaccharide, α-glucan, cellodextrin, high-value products, biofuel
1. INTRODUCTION
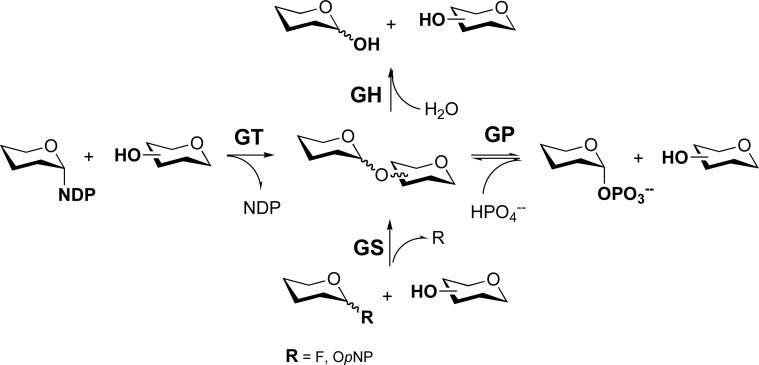
Glycoside phosphorylases (GPs) are carbohydrate-active enzymes (CAZymes) (URL: http://www.cazy.org/) [1] involved in the formation/cleavage of glycosidic bond together with glycosyltransferase (GT) and glycoside hydrolase (GH) families (Figure 1) [2-6]. GT reactions favour the thermodynamically more stable glycoside product [7]; however, these enzymes can be challenging to work with because of their current limited availability and relative instability, along with the expense of sugar nucleotide substrates [7]. GHs thermodynamically favour the hydrolysis of glycosidic bonds; however, active site mutation has allowed their transformation into glycosynthases (GSs), which use activated sugars (such as fluoro or p-nitrophenyl glycosides) to drive reaction in the synthetic direction [7, 8].
Figure 1.
Enzymes involved in glycosidic bond formation and cleavage. GT = glycosyltransferase; GP = glycoside phosphorylase; GH = glycoside hydrolase; GS = glycoside synthase; NDP = nucleotide diphosphate; OpNP = O-para-nitrophenyl.
In contrast to GTs and GHs, the equilibrium position for GP reactions tends to result in a mix of starting materials and products, rendering them of modest use in a synthetic sense. However, GPs can be used to generate sugar-1-phosphates; in addition, coupling GP reactions with a secondary glycosylation step provides a means of capitalizing on these robust enzymes, which employ cheap and accessible substrates.
1.1. Background on Glycoside Phosphorylases
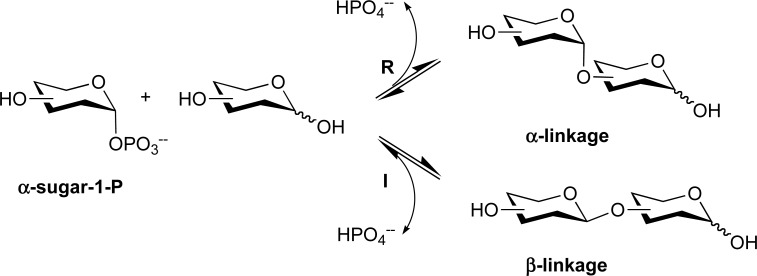
GPs use inorganic phosphate to breakdown glycosidic bonds (phosphorolysis), releasing sugar-1-phosphate and acceptor (Figure 1) [3-5]. The phosphorylase reaction is an equilibrium between the phosphorolysis and the reverse synthetic reaction, since the free energy released by the glycosidic bond cleavage is similar to that released by the sugar phosphate ester cleavage (Figure 2) [4, 5]. Phosphorylases have been classified into different families depending on the sequence similarity to GTs and GHs in CAZy database (Table 1) [4, 5]. Most of the GPs belong to GH families, [5] and they can be distinguished into retaining and inverting phosphorylases depending on whether the new glycosidic bond is formed with inversion or retention of the anomeric configuration with respect to the sugar-1-phosphate substrate (Figure 2, Table 1) [4, 5].
Figure 2.
Reaction mechanism of glycoside phosphorylases. R, retaining; I, inverting.
Table 1.
Classification of characterised glycoside phosphorylases [3].
| Mechanism | Family | Linkage | Donor | Acceptor | Product |
|---|---|---|---|---|---|
| Retaining | GT4 | α,α-(1→1) | α-D-Glc-1-P | D-Glc | Trehalose [14, 15] |
| GT35 | α-(1→4) | α-D-Glc-1-P | α-D-Glc-(1→4)- α-D-(Glc)n | Glycogen [16]/starch [17] | |
| GH13 | α,α-(1→2) | α-D-Glc-1-P | D-Fru | Sucrose | |
| α-(1→4) | α-D-Maltose-1-P | α-D-Glc-(1→4)-α-D-(Glc)n | α-D-Glc-(1→4)-α-D-(Glc)n+2 | ||
| Inverting | GH65 | α,α-(1→1) | β-D-Glc-1-P | D-Glc/D-Glc-6-P | Trehalose/trehalose-6-P |
| α-(1→2) | β-D-Glc-1-P | D-Glc | Kojibiose | ||
| α-(1→3) | β-D-Glc-1-P | D-Glc | Nigerose | ||
| α-(1→4) | β-D-Glc-1-P | D-Glc | Maltose | ||
| GH94 | β-(1→2) | α-D-Glc-1-P | D-Glc | Sophorose | |
| β-(1→3) | α-D-Glc-1-P | D-Glc | Laminaribiose | ||
| β-(1→4) | α-D-Glc-1-P | D-Glc/ β-D-Glc-(1→4)-β-D-(Glc)n | Cellobiose/cellodextrin | ||
| β-(1→4) | α-D-GlcNAc-1-P | D-GlcNAc | Chitobiose | ||
| GH112 | β-(1→3) | α-D-Gal-1-P | D-GalNAc/D-GlcNAc | Galacto-N-biose/lacto-N-biose | |
| β-(1→4) | α-D-Gal-1-P | L-Rha | β-D-Gal-(1→4)-L-Rha | ||
| GH130 | β-(1→4) | α-D-Man-1-P | D-Glc | β-D-Man-(1→4)-D-Glc | |
| α-D-Man-1-P | β-D-Man-(1→4)-β-D-(Man)n | Mannan | |||
| α-D-Man-1-P | D-GlcNAc | β-D-Man-(1→4)-D-GlcNAc |
The characterised GPs comprise enzymes that are able to act on a wide range of glycosidic linkages (Table 1), with the exception for α/β-(1→6) and β-(1→1) linkages, for which no specific GPs have been characterised at the moment. Most of the GPs known so far use D-Glc-1-phosphate as a donor substrate, forming disaccharides or oligosaccharides; far fewer GPs use D-Gal-1-phosphate [9], D-GlcNAc-1-phosphate [10] or D-Man-1-phosphate [11-13] as their donor substrates (Table 1) [3, 5, 14-17]. There are currently no known phosphorylases that transfer N-acetylneuraminic acid (Neu5Ac), L-fucose (L-Fuc), L-rhamnose (L-Rha), D-fructose (D-Fru), D-glucuronic acid (D-GlcA) or D-glucosamine (D-GlcN) as their physiological substrates. In this respect, GTs and GHs cover a broader range of carbohydrate specificities and have been much more extensively studied, characterised and modified in order to optimise their use in the synthesis of complex carbohydrates [8, 18].
While GP synthetic reactions can be used for carbohydrate/glyco-molecule synthesis, GP phosphorolysis reactions can be exploited for the degradation of glyco-polymers, leading to the production of useful sugar-1-phosphates. Due to the industrial interest in GPs as a result of their cheap and readily available substrates, in the last decade a big effort has been put into the discovery of new GP activities, as well as GP engineering to overcome the limitations associated with their strict specificity in terms of substrates or linkage [19]; efforts to improve their thermostability and catalytic efficiency with non-natural substrates have also been reported [20, 21].
GPs have been exploited in academia and also in industry for the synthesis of glycosylated compounds, high-value carbohydrates and both natural and unnatural polysaccharides. On the other hand, the phosphorolytic reaction of GPs is currently being investigated as an alternative route for waste recycling and biofuel production. The aim of the article is to provide an overview of recent developments in GP applications, especially where they have been used in conjunction with other GPs (phosphorylase coupling) [8] and/or with other enzymes. The review will be focused on three main classes of GPs: disaccharide phosphorylases for the production of small molecules [5, 8, 19]; α-glucan phosphorylases for starch synthesis and/or degradation [22, 23]; finally, cellodextrin/cellobiose phosphorylases (CDP/CBP) for cellulose synthesis and/or degradation [24]. All of these areas are being explored because of their potential industrial application in the production of more soluble glycosylated drugs, excipients, prebiotics, food additives, adhesives and new materials.
2. Disaccharide phosphorylases
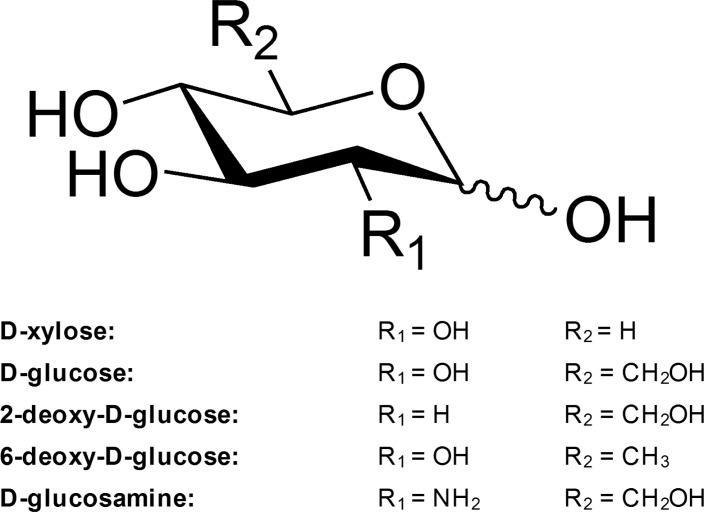
Disaccharide phosphorylases carry out a reversible phosphorolytic reaction using a glycosyl phosphate and a monosaccharide as sugar donor and acceptor, respectively. They are all members of GH and GT families that are mentioned in Table 1, except for GT35 which contains exclusively oligosaccharide phosphorylases (starch and glycogen phosphorylases). Since the CAZy classification is based largely on amino acid sequence homology [1], disaccharide phosphorylases with different substrate specificities are often classified in the same family. For example, GH94 family contains disaccharide phosphorylases acting on β-D-Glc-(1→2)-D-Glc (sophorose), β-D-Glc-(1→4)-D-Glc (cellobiose), β-D-GlcNAc-(1→4)-D-GlcNAc (N,N´-diacetyl-chitobiose) and β-D-Glc-(1→3)-D-Glc (laminaribiose). Research in recent years has focused on the acceptor and donor preferences of disaccharide phosphorylases, since these govern their potential for use in the production of not only novel disaccharides, but also small glyco-conjugated compounds with potentially useful pharmacological properties. Broad acceptor preference is a key feature that is shared by several disaccharide phosphorylases. For example, cellobiose phosphorylase (CBP) (EC 2.4.1.20) shows relaxed specificity at the C2- and C6-positions of the acceptor sugar, thus allowing the use of 2-deoxy-D-glucose, 6-deoxy-D-glucose, D-glucosamine (2-amino-2-deoxy-D-glucose), and D-xylose (6-dehydroxymethyl-D-glucose) as its substrate (Figure 3) [25]. Another powerful example is the acceptor promiscuity of sucrose phosphorylase (SP) (EC 2.4.1.7), which not only accepts a variety of monosaccharides but also other non-carbohydrate molecules, details of which will be discussed in section 2.2.
Figure 3.
Structure illustration for CBP acceptors [25]. R1 and R2, which are linked to C-2 and C-6 respectively, are substituted by different functional groups for each of the aforementioned acceptors.
In the following section, we will explore the potential applications of disaccharide phosphorylases either as a sole catalyst and/or in combination with other phosphorylases or other carbohydrate-active enzymes to produce small glyco-conjugated molecules and rare disaccharides.
2.1. Production of Valuable/Rare Sugars
A single disaccharide phosphorylase can be used as a sole catalyst for the production of a disaccharide of interest with anomeric stereochemistry and regiocontrol determined by the choice of enzyme specificity and monosaccharide compositions of the product by the supplied substrates. As previously mentioned, relaxed acceptor preference is the key property that has been used by many research groups for the production of rare disaccharides. Morimoto and co-workers have shown that SP from Leuconostoc mesenteroides exhibits activity towards eight different ketohexose acceptors while using α-D-Glc-1-phosphate as a donor, allowing the production of eight corresponding D-glucosyl-ketohexoses, which are rare or absent in nature [26].
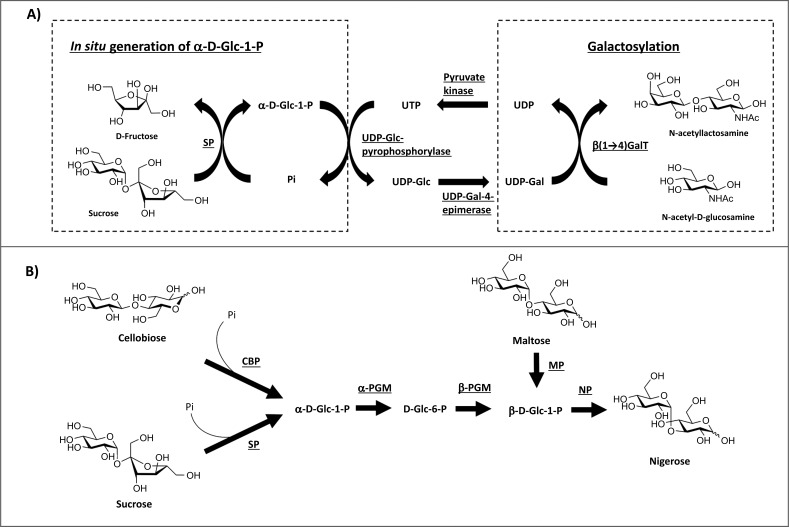
Disaccharide phosphorylases can also be used in conjunction with other biocatalysts to expand the range of possible products through a “one-pot” enzymatic approach. A common strategy is the use of SP as an in situ generator of α-D-Glc-1-phosphate, which can then be used as substrate for the downstream reaction catalysed by other phosphorylases and/or other biocatalysts. Such an approach has been demonstrated in a one-pot enzymatic galactosylation of N-acetyl-D-glucosamine, which produced N-acetyllactosamine in 85% yield (Figure 4A) [27].
Figure 4.
A) In situ generation of α-D-Glc-1-phosphate by SP coupling with galactosylation of N-acetylglucosamine [27]. β-(1→4)-GalT = β-(1→4)-galactosyltransferase; UDP = uridine diphosphate; UTP = uridine triphosphate; Pi = inorganic phosphate; SP = sucrose phosphorylase. B) Enzymatic production of nigerose from 4 different starting materials (cellobiose, starch, sucrose and maltose) [28]. Cellobiose, starch and sucrose were used for the production of α-D-Glc-1-phosphate, which was converted to β-anomer, and subsequently used by nigerose phosphorylase for nigerose production. Maltose was also phosphorolysed by maltose phosphorylase to generate β-D-Glc-1-phophate which can be used directly by nigerose phosphorylase. Pi = inorganic phosphate; CBP = cellobiose phosphorylase; SP = sucrose phosphorylase; MP = maltose phosphorylase; NP = nigerose phosphorylase; PGM = phosphoglucomutase.
By substituting SP with other disaccharide phosphorylases that are specific to substrates of choice, other cheap substrates can be used as starting materials for the one-pot enzymatic approach. Nihira and co-workers have demonstrated this idea by the use of either CBP or SP in the first step of catalysis to generate α-D-Glc-1-phosphate from cellobiose or sucrose, respectively (Figure 4B).
α-D-Glc-1-phosphate was sequentially transformed by α-phosphoglucomutase (α-PGM) (EC 5.4.2.2) into D-Glc-6-phosphate, then by β-phosphoglucomutase (β-PGM) (EC 5.4.2.6) to produce β-D-Glc-1-phosphate, which was then used by nigerose phosphorylase (NP) (EC 2.4.1.279) to generate α-(1→3)-linked nigerose as a final product of the reaction. Alternatively, maltose can be phosphorolysed by maltose phosphorylase (MP) (EC 2.4.1.8) for the generation of β-D-Glc-1-phosphate [28]. These combinatorial approaches may be adapted for the production of other disaccharides by substituting nigerose phosphorylase with other β-D-Glc-1-phophate-generating phosphorylases with appropriate carbohydrate acceptors.
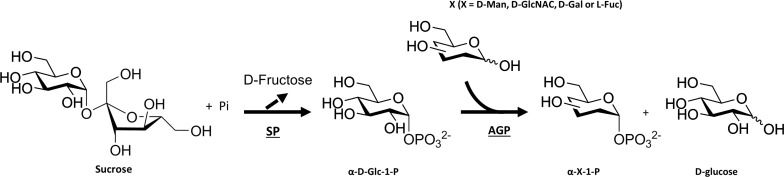
It is possible to expand the range of sugar-1-phosphates beyond Glc-1-phosphate by coupling SP with α-Glc-1-phosphatase (AGP) (EC 3.1.3.10) either sequentially or simultaneously, which allowed phosphorylation of D-Man, D-GlcNAc, D-Gal and L-Fuc at the anomeric hydroxyl group with absolute axial selectivity (Figure 5) [29].
Figure 5.
Coupling SP with AGP for the enzymatic production of a variety of sugar-1-phosphate with axial selectivity at the anomeric position [29]. Sucrose was used by SP to generate α-D-Glc-1-phosphate, which was subsequently or simultaneously used in combination with 4 different acceptors, namely D-Man, D-GlcNAc, D-Gal or L-Fuc, by AGP for the production of the corresponding sugar-1-phosphates. SP = sucrose phosphorylase and AGP = α-glucose-1-phosphatase.
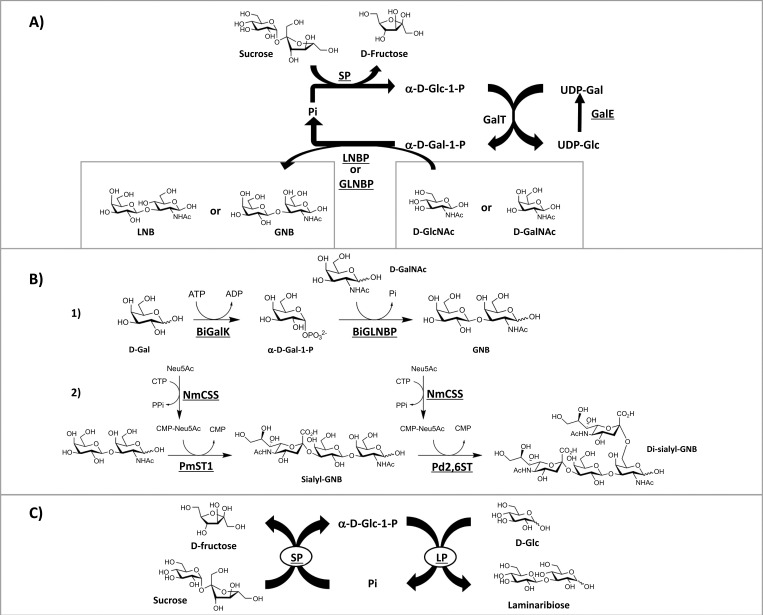
Lacto-N-biose I (β-D-Gal-(1→3)-D-GlcNAc; LNB) has been shown to have prebiotics effects on in vitro Bifidobacterial growth, suggesting a link between the prevalence of these bacterial groups in breast-fed infants and the existence of gene cluster encoding the unique metabolic pathway for LNB in Bifidobacteria [30]. Since Bifidobacteria are relatively well-known probiotic species which positively correlate with several health benefits in human, such as reducing inflammatory responses [31, 32] and improved gastrointestinal tract well-being [33, 34], the synthesis of LNB is of great interest as an alternative source of prebiotic LNB to enhance the presence of these probiotic bacteria. Nishimoto and Kitaoka have developed an in vitro enzymatic synthesis of LNB from sucrose and D-GlcNAc using a combination of 4 enzymes in 10-liter of the reaction mixture with an overall reaction yield of 83%; 1.4kg of crystalline LNB was recovered from the reaction with 99.6% purity (Figure 6A) [35].
Figure 6.
A) Two biosynthetic routes for GNB and LNB production proposed by Nishimoto and Kitaoka [35, 40]. For lacto-N-biose (LNB) production, LNBP and D-GlcNAc were used as the final catalyst and substrate respectively. LNBP and D-GlcNAc are substituted by GLNBP and D-GalNAc, respectively, for the production of GNB. B) Enzymatic production of GNB and sialylated-GNB proposed by Li and co-workers [41]. (1) BiGalK was used to convert D-Gal to α-D-Gal-1-P, which was utilised as a sugar donor by BiGLNBP with D-GalNAc as an acceptor to produce GNB. (2) Sialyl-GNB was formed by transferring Neu5Ac from CMP-Neu5Ac onto D-Gal of GNB via α-(2→3) linkage by the activity of PmST1. The sialyl-GNB was further sialylated at C-6 of D-GalNAc by Pd2,6ST via α-(2→6) linkage to form disialyl-GNB. BiGLNBP = GNB/LNB phosphorylase from Bifidobacterium infantis; BiGalK = galactokinase from Bifidobacterium infantis; NmCSS = CMP-Sia synthetase from Neisseria meningitides; PmST1 = α-(2→3)-sialyltransferase 1 from Pasteurella multocida; Pd2,6ST = α-(2→6)-sialyltransferase from Photobacerium damselae. C) Combination of immobilised LP from Euglena gracilis and SP can be used for the production of laminaribiose using sucrose as a starting material [42]. SP = sucrose phosphorylase; Pi = inorganic phosphate; LP = laminaribiose phosphorylase.
Galacto-N-biose (β-D-Gal-(1→3)-D-GalNAc; GNB) is an O-glycan disaccharide that is a major core component of mucin in the gastrointestinal tract [36] and is metabolised by Bifidobacteria, similarly to lacto-N-biose [37]. Serine or threonine-linked GNB, so called T-antigen, can be found at high levels on human cancer cells [38], while derivatives of GNB, such as sialyl- and disialyl-GNB, are present on gangliosides and serve as receptors for influenza viral infection [39]. Given the physiological significance of GNB and its sialylated forms, synthesis of these molecules is important for therapeutic and diagnostic purposes. Two enzymatic routes for GNB production involving disaccharide phosphorylases have been explored. The first approach was modified from the aforementioned synthesis of LNB by substituting LNBP with GNB/LNB phosphorylase (GLNBP) (EC 2.4.1.211) in the presence of UDP-glucose, sucrose, D-GalNAc and inorganic phosphate as substrates (Figure 6A). Large scale preparation using this approach yielded gram quantities of GNB with more than 90% purity [40].
The second route is a bi-enzymatic system using two enzymes: a galactokinase (EC 2.7.1.6) catalyses the synthesis of α-D-Gal-1-phosphate from D-Gal in the presence of ATP, and GLNBP transfers D-Gal to D-GalNAc to create GNB with up to 95% yield (Figure 6B, 1), which can then be sialylated by an α-(2→3)-sialyltransferase (EC 2.4.99.4) and α-(2→6)-sialyltransferase (EC 2.7.99.1) to form sialyl- or disialyl-GNB (Figure 6B, 2) [41].
Apart from the one-pot enzymatic approach in solution, immobilised-enzyme combinations can also be explored as an alternative for the use of disaccharide phosphorylases as biocatalysts. One recent example shows that production of laminaribiose could be accomplished by a bi-enzymatic system containing individually immobilised Euglena gracilis protein extract with laminaribiose phosphorylase activity (LP, EC 2.4.1.31) and recombinant SP onto Sepabeads (Figure 6C) [42]. The advantages of enzyme immobilisation are the extension of operational life time and reusability of the enzymes, which were explicitly demonstrated in Müller’s work, where the immobilised laminaribiose phosphorylase retained more than 50% activity when used seven times, providing a proof-of-concept that could potentially be implemented for other phosphorylases.
2.2. Glycosylation of Non-Carbohydrate Small Molecules
Glycosylation of small non-carbohydrate molecules can have positive impact on their physical/chemical/biological activity, such as improved solubility of hydrophobic compound and stability of labile molecules [8]. The acceptor promiscuity of the phosphorylases, in particular of SP, can be exploited in this respect to glycosylate non-carbohydrate molecules, such as aromatics, sugar alcohols, phenolic compounds and carboxylic acids [8, 43]. The advantage of such promiscuity was demonstrated for an enzymatic production of 2-O-(α-D-glucopyranosyl-sn-glycerol (αGG), an additive for cosmetic agents (Glycoin®), using sucrose phosphorylase from Leuconostoc mesenteroides as a biocatalyst and glycerol as an acceptor, with the final yield of 10.2g in 90-ml reaction mixture [44, 45].
SP not only can be applied as a phosphorylase, but also as a trans-glycosidase, in which case the glucosyl group is transferred directly from sucrose to acceptor alcohol, rather than with the intermediary of α-D-Glc-1-phosphate. This property of SP was exploited for mono and bis-glucosylation of hydroxyl groups in (-)-epigallocatechin gallate [46], which is a prospective anti-cancer agent [44].
Despite the relaxed aglycone specificity of SP, several desirable acceptors such as flavonoids or stillbenoids remain poor substrates due to their bulky size and hydrophobicity of the molecules. These barriers may be overcome by exploiting knowledge of available 3D structures of SPs, which enables the acceptor specificity to be tailored by site-directed mutagenesis to accommodate non-native acceptors.
Kraus, Grimm and Seibel have implemented this strategy by redesigning the active site of SP from Bifidobacterium adolescentis, exchanging Gln345 for Phe (Q345F), which resulted in a wider access channel with a nonpolar aromatic surface that is likely to contribute to the improved activity towards glucosylation of resveratrol, (+)-catechin and (-)-epicatechin: glycosylation yields of up to 97% were dramatically improved with respect to those achievable with the wild type enzyme [47].
De Winter and co-workers have demonstrated α-glycosylation of a variety of antioxidants such as pyrogallol methyl gallate, resveratrol, and epicatechin by SP from Bifidobacterium adolescentis or β-glucosylation by cellodextrin phosphorylase (CDP) (EC 2.4.1.49) from Clostridium sterococarium to produce cellobiosylated and cellotriosylated antioxidants. The glycosylation improves the stability and solubility of most of modified antioxidants without substantial loss of the antioxidant potency [48]. The increase in solubility and stability may provide significant advantages for the commercial application of such compounds.
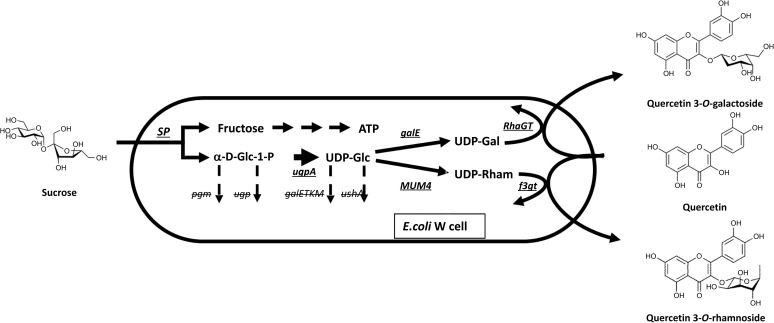
Apart from substantial evidence for in vitro use of disaccharide phosphorylases for disaccharide production and aglycone glycosylation, there are recent examples of in vivo glycosylation platforms. Specifically, metabolic engineering of E. coli introducing phosphorylases in combination with other enzymes has been used to alter the existing metabolic pathway to favour the production of desirable sugar-nucleotides. In vivo glucosylation and rhamnosylation of quercetin, a plant flavonol bioactive metabolite, which naturally occurs in different glycosylated forms with varying pharmacological properties, have been demonstrated by constructing an alternative sucrose metabolic pathway in E. coli. Introducing SP as a key enzyme enabled the use of sucrose for the production of α-D-Glc-1-phosphate as a precursor for UDP-glucose, while the by-product fructose could be used as a carbon source for cell growth. The produced UDP-glucose was converted to UDP-galactose or UDP-rhamnose by introducing UDP-glucose-4-epimerase (GalE) or UDP-rhamnose synthase (MUM4) genes, respectively, into the E. coli strain. UDP-galactose and rhamnose were then used for galactosylation and rhamnosylation of quercetin, respectively (Figure 7) [49]. The advent of such in vivo glycosylation platform provides a great alternative to the extraction of these glycosylated compounds from their original sources, which are laborious and low yielding processes.
Figure 7.
Metabolic engineering of E. coli for in vivo glycosylation platform of a flavonol molecule [49]. Underlined genes were introduced into E. coli for overexpression of the enzymes that are essential for production of desirable sugar metabolites, whereas erased genes were deleted from E. coli genome to prevent the breakdown of α-D-Glc-1-phosphate and UPD-Glc.
3. Α-glucan phosphorylase
α-Glucan phosphorylases are a diverse group of retaining enzymes that generate α-D-Glc-1-phosphate from polymeric glucans being widely distributed amongst prokaryotic and eukaryotic organisms [50]. This class of phosphorylases are members of the GT35 family, as per the CAZy database (Table 1), behaving as oligosaccharide phosphorylases, mostly specific to the biopolymers starch and glycogen. The catalytic behavior of α-glucan phosphorylases has been extensively studied with respect to mammalian glycogen metabolism, wherein the breakdown/synthesis of polyglucans dependent on the relative Pi/α-D-Glc-1-phosphate concentrations and equilibria, was first discovered [50].
Physiologically, phosphorylases are attributed to catabolism of glycogen reserves as well as its biogenesis, which also involves branching enzymes [51]. Starch metabolism on the other hand is more complex, reflecting its intricate architectural construction, also requiring a battery of other enzymes. These enzymes are comprised of numerous branching enzymes (BEs) that transfer variable glycan chain lengths, forming α-(1→6) branch points and thus are classified type I (long chains) and type II (short chains). Additionally, the debranching enzymes (DBEs) eventually trim the hyperbranched amylopectin molecules, forming semi-crystalline and insoluble starch granules [51]. Over the years, α-glucan phosphorylases (particularly the easily isolated potato enzyme) have been used in isolation or in combination to engineer various forms of synthetic amylose or glycogen derivatives through enzymatic routes. In recent times, the focus of phosphorylases as enzymatic tools has shifted to the plant and bacterial orthologues as they are more diverse and are devoid of the allosteric regulation seen in mammalian glycan phosphorylases [3]. With respect to the use of the ubiquitous GH13 family BEs [52] for similar applications, several variants are currently in use owing to their functional diversity, robust nature and suitability for synthetic reaction routes.
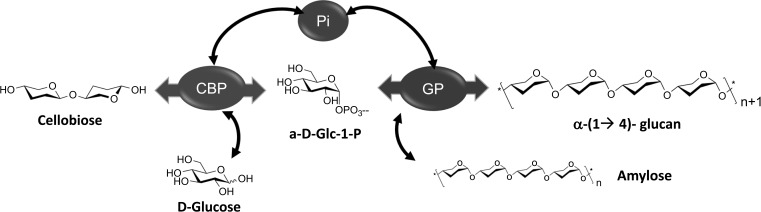
Although the conventional approaches were restricted to the use of glycan phosphorylases for modification of their natural substrates, Ohdan et al. have expanded their scope by using them in combination with glucan transferases to engineer synthetic amyloses [53]. This group has demonstrated the use of cellobiose phosphorylase to catalyse the phosphorolysis of cellobiose into α-D-Glc-1-phosphate and glucose, followed by the in situ α-glucan phosphorylase-based polymerization of a glucan primer (Figure 8). The resulting synthetic amylose assumes a helical conformation, forming inclusion complexes with diverse guest molecules, thereby providing scope for application in nanoscale entrapment and drug delivery [23].
Figure 8.
Reaction scheme depicting the combination of cellobiose phosphorylase (CBP) and α-glucan phosphorylase (GP) for the synthesis of synthetic amylose [53]. Pi = inorganic phosphate.
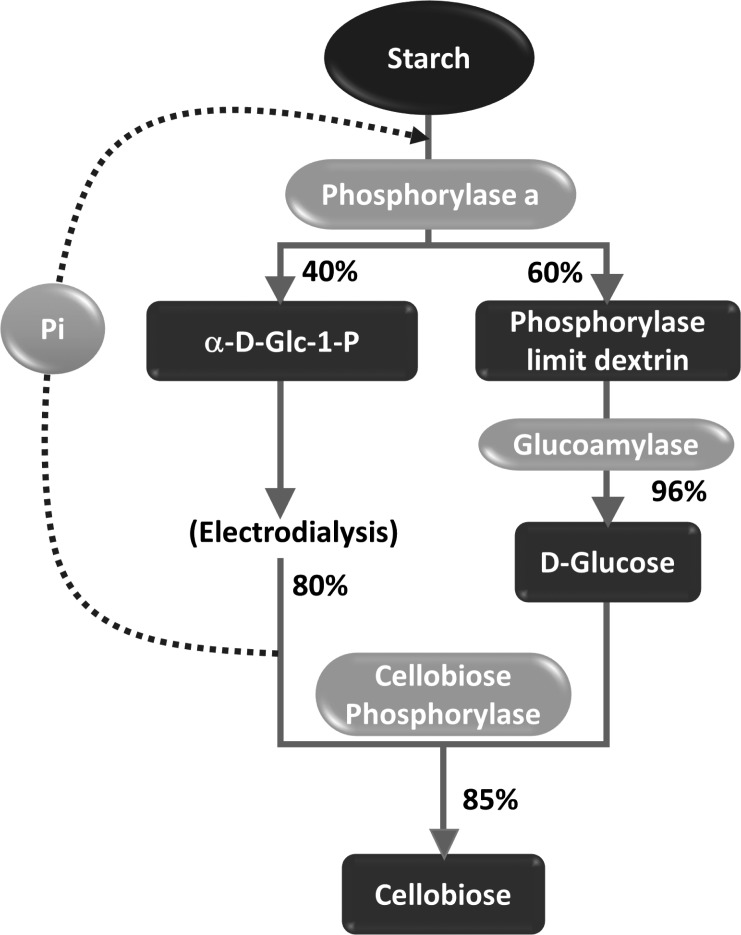
In an reverse approach, Suzuki et al. established an efficient system for the enzymatic conversion of starch into cellobiose using α-glucan phosphorylase alongside cellobiose phosphorylase (Figure 9) [54]. The process was driven by a high concentration of Pi, effecting the conversion of starch to α-D-Glc-1-phosphate, followed by the selective precipitation of Pi as insoluble magnesium ammonium phosphate in order to drive the equilibrium in favour of cellobiose phosphorylase-mediated conversion of α-D-Glc-1-phosphate to cellobiose. The enzymatic conversions, however, had to be performed in isolation owing to the different conversion rates and reaction parameters for the α-glucan phosphorylase and cellobiose phosphorylase. Interestingly, this process represented the first attempt to address the challenge of large scale production of α-D-Glc-1-phosphate, from one of the most economical and abundantly available agricultural products, starch. A major limitation in this regard was the partial conversion of starch into α-D-Glc-1-phosphate (~40%) and the generation of the high molecular weight by-products, owing to presence of highly branched amylopectin within starch.
Figure 9.
Reaction scheme depicting the conversion of starch to cellobiose using a combination of α-glucan phosphorylase and cellobiose phosphorylase [54]. Pi = inorganic phosphate.
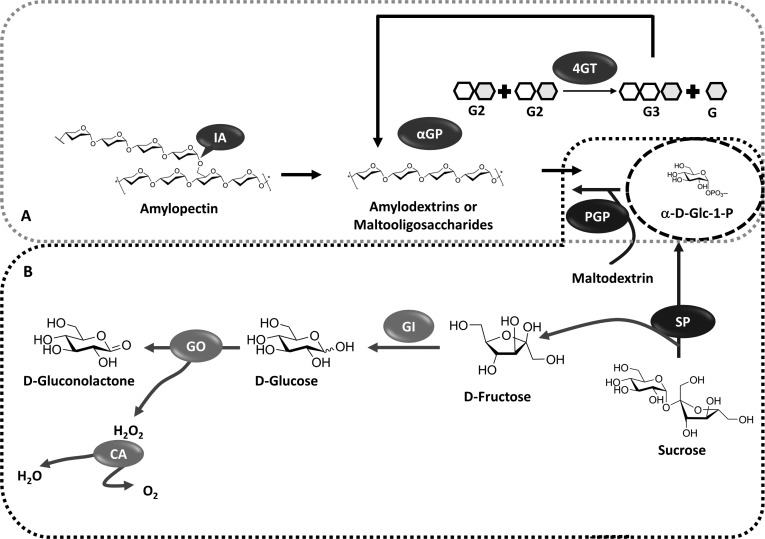
The partial hydrolysis of starch was addressed by Zhou and co-workers, involving three hyper-thermostable enzymes to engineer a one-pot approach, resulting in increased α-D-Glc-1-phosphate generation (Figure 10A) [55]. Isoamylase (IA) from Sulfolobus tokodaiienables was used for the simultaneous gelatinization of starch and starch hydrolytic debranching. Coupled with the isoamylase, the Thermotoga maritima α-glucan phosphorylase for α-D-Glc-1-phosphate production, and Thermococcus litoralis 4-glucanotransferase (4GT) for transfer of glucose units from maltose and short maltodextrins (to the ends of other maltodextrins), were used achieving an efficient utilisation of glucose units in the reaction. An unusually high yield of ~200 mM α-D-Glc-1-phosphate was achieved from corn starch, despite the fact that starch suffers from lower solubility and equilibrium constant compared to sucrose-SP system.
Figure 10.
Multi-enzyme one-pot reaction schemes for generation of α-D-Glc-1-phosphate. A) Enzymatic synthesis of α-D-Glc-1-phosphate from corn starch [55]. α-D-Glc-1-phosphate synthesis corn starch-mediated by thermostable α-glucan phosphorylase (αGP) from Thermotoga maritima. The hyper-thermostable isoamylase (IA) from Sulfolobus tokodaii was added for enhancing the α-D-Glc-1-phosphate yield. A third enzyme, 4-glucanotransferase (4GT) from Thermococcus litoralis was added to the reaction mixture for increasing the α-D-Glc-1-phosphate titre through maltose and maltotriose utilisation. B) One-pot scheme for enzymatic synthesis of α-D-Glc-1-phosphate from sucrose [56]. SP = sucrose phosphorylase from Thermoanaerobacterium thermosaccharolyticum (EC 2.4.1.7); PGP = potato α-glucan phosphorylase (EC 2.4.1.1); GI = glucose isomerase (EC 5.3.1.5); GO = glucose oxidase (EC 1.1.3.4); CA = catalase (EC 1.11.1.6).
As mentioned previously, SP demonstrates notable substrate promiscuity. This property has encouraged the engineering of robust SPs from thermophilic organisms, such as Thermoanaerobacterium thermosaccharolyticum JW/SL-YS485, to facilitate the catalytic conversion of sucrose into synthetic amylose (Figure 10B) [56], with immense potential in the biomedical, food and polymer industries. The limitations of large scale amylose production were addressed by optimising chemo-enzymatic conditions with up to five different enzymes, in an assemblage of different one-pot reaction schemes. The core reaction had three different modules comprising of the SP-mediated phosphorylation of sucrose to α-D-Glc-1-phosphate and D-fructose; α-(1→4)-glucan phosphorylase mediated extension of amylose-like chains on a maltodextrin primer; and a third optional mechanism for the removal of fructose to prevent SP inhibition through the combined action of glucose isomerase, glucose oxidase, and catalase. A comprehensive comparison was made between the two and five enzyme, one-pot systems with respect to the α-D-Glc-1-phosphate and amylose generation, concluding that the SP α-(1→4)-glucan phosphorylase two-enzyme system is most suited for the generation of synthetic amyloses. The highlight of this reaction scheme was the effective recycling of inorganic phosphate to maintain steady state levels, enabling a novel alternative to the natural amylose biosynthesis pathway.
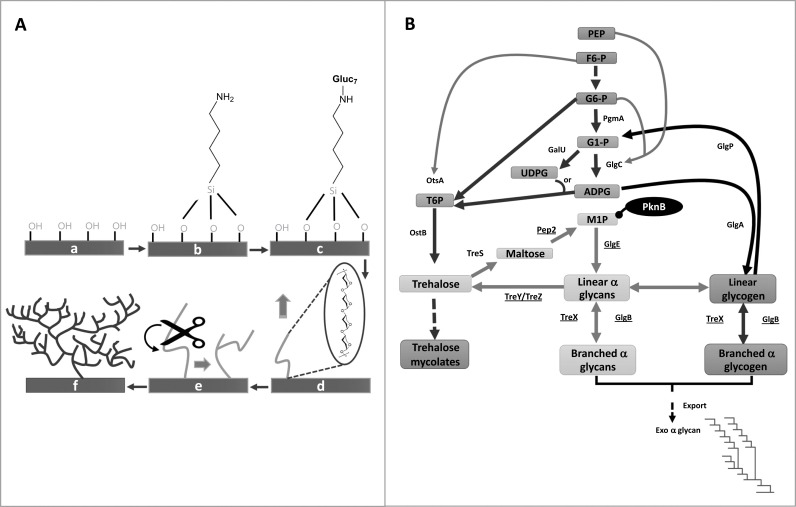
Advances in the combined use of glucan phosphorylases, branching enzymes and debranching enzymes for glucan-based material production have come from studies on different heterologous systems established in vivo [52, 57]. For instance, the Loos group has employed recombinant α-(1→4)-glucan branching enzyme from the extremophile Deinococcus geothermalis to access amylopectin-like polymers from long chain, amylose-based glucans. The BE transferred α-(1→4)-linked glucan chains from the non-reducing terminus of long chain linear α-(1→4)-glucans, creating β-(1→6)-linked branch-points at internal glucose residues in α-(1→4)-glucan chains. Optimisation of reaction conditions for the simultaneous α-(1→4) extension of glucan primer by potato α-glucan phosphorylase and the α-(1→6) side chain branching, through the action of BE thus generated branched polymeric glucans, with scope to tune the branching by modulating the reaction conditions [58]. The Loos group further extended the scope of the α-glucan phosphorylase-GBE enzyme system to derive glycocalyx-mimicking, hyperbranched polysaccharides (Figure 11A). These hyperbranched polysaccharide structures were developed on silicon surfaces, with tunable degrees of branching, making them amenable to further surface modifications [59].
Figure 11.
A) Combined use of potato α-glucan phosphorylase (αGP) and glycogen branching enzyme (GBE) from Deinococcus geothermalis for the generation of hyperbranched polysaccharides on silicon wafer [59]. (a) Oxidized Si wafer; (b) aminosilanized wafer; (c) maltoheptaose functionalised surface; (d) enzyme-catalysed growth of linear chain by αGP; (e) catalytic action of GBE; (f) resulting hyperbranched polysaccharide after the combined biocatalysis. B) Metabolic pathways associated with α-glucan and glycogen metabolism in Mycobacterium tuberculosis [61, 63]. The genes involved in the GlgE pathway are underlined. The pathway is negatively regulated by the serine/threonine kinase, PknB. The pathway has been exploited for generation of branched glycans using a combination of the GlgE and GlgB enzymes.
Additionally, Kobayashi and co-workers have established numerous chemo-enzymatic synthetic pathways using thermostable, α-glucan phosphorylase to obtain a variety of complex non-natural heteroaminopolysaccharides, amylose-grafted polysaccharides as well as amphoteric α-glucan dendrimers [60].
In connection with their work on an alternate bacterial glycogen synthesis pathway [61], Bornemann’s group has discovered an interesting route for the production of linear and branched α-glucans (Figure 11B). The pathway is unique in that it employs a maltosyltransferase, GlgE [62], that uses α-maltose-1-phosphate donor to generate α-(1→4)-linkages alongside a single branching enzyme, GlgB, which is shared with the classical glycogen synthesis pathway [63]. The in vitro synthesis of linear and branched glucan structures generated by recombinant GlgE and GlgB enzymes has been achieved since. The products concerned were significantly different to those produced by the classical glycogen biosynthesis pathway, having shorter chain lengths and a higher proportion of β-(1→6) linkages. The α-maltose-1-phosphate required for this alternate glycogen synthesis pathway is produced in vivo through the isomerization of the disaccharide trehalose [64]. Trehalose, being cheap and readily available, presents an interesting alternate feedstock for enzymatic routes to generate novel α-glucans.
4. cellodextrin phosphorylase
β-(1→4)-Glucans are abundant natural polysaccharides, involved mainly in structural roles due to their stiffness and high resistance to chemical and enzymatic degradation; the most common β-(1→4)-glucan is cellulose, the main component of plant cell wall, composed of β-(1→4)-linked glucose [65]. Its chemical structure, characterised by extensive linear chains interacting by H-bonds to form microfibrils with alternated amorphous and highly ordered crystalline regions, is responsible for its robustness and structural role [65]. The most common and ancient use of cellulose has been in paper production but recently it has been re-evaluated as eco-friendly and renewable material and its application has expanded in a variety of areas [65-67]. The
main source of cellulose is plants, where it is found in association with hemicellulose and lignin, from which it is difficult to isolate. By contrast, bacterial cellulose is produced in a much purer form, even if in a different crystalline state compared to plants, which makes its isolation easier and consequently, its application more popular [68]. Pure cellulose has been synthesised by isolated enzymes, such as cellulases used in the reverse synthetic reaction [69]; however, bacterial cellodextrin phosphorylase (CDP) (EC. 2.4.1.49) has emerged in the last years as a powerful enzymatic tool for the synthesis of cellulose-like materials.
CDP belongs to the GH94 family. It is an inverting enzyme that catalyses the synthesis of β-(1→4)-linked cello-oligomers from α-D-Glc-1-phosphate and β-glucoside acceptors to generate insoluble celluloses with different degrees of polymerization depending on the primer [70]. From the first discovery of CDP from Clostridium thermocellum (CtCDP) by Sheth and Alexander [70] and its expression in recombinant form in E. coli [71], CDP has been extensively studied especially in terms of characterisation of its cellulose product, such as degree of polymerization and crystalline form [24, 72, 73]. This information has contributed to the production of cellulosic materials with controlled degree of polymerization, peculiar molecular assemblies and catalytic activities which can be useful in a variety of applications [74-76].
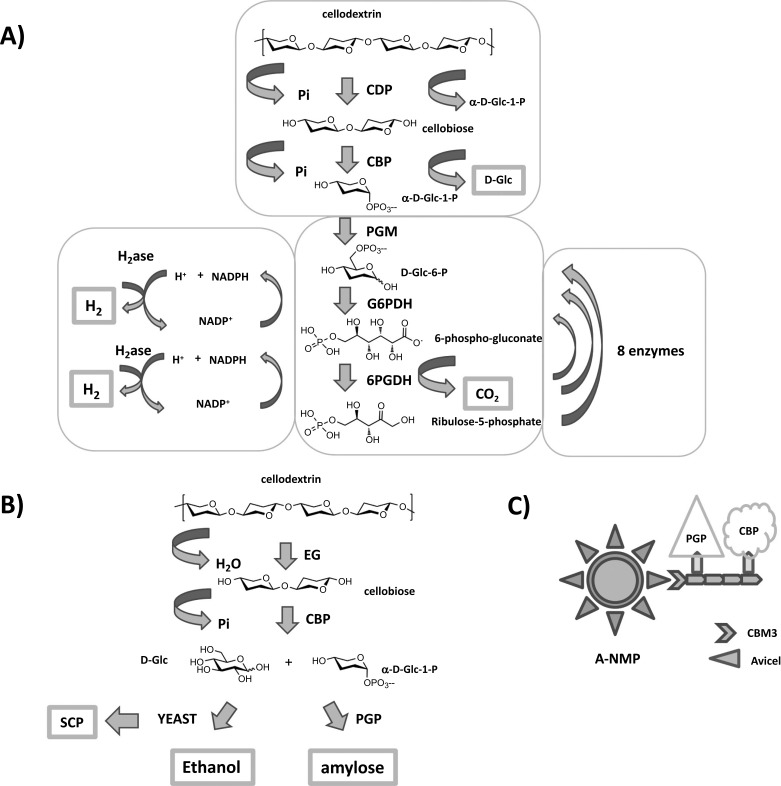
Because of the insolubility of cellulose products, the use of CDP in combination with other enzymes is generally limited to a top-down approach, where CDP is the last coupled enzyme to produce cellulose as a final product from a β-glucoside acceptor and α-D-Glc-1-phosphate; a bottom-up approach, where CDP is used at the beginning of a process for the degradation of cellulosic material to produce α-D-Glc-1-phosphate, can be used for a variety of applications, such as synthesis or biofuel generation. The application of CDP in the production of biofuels from biomass is quite an expanding and exciting area due to the fact that cellulose is the most abundant renewable polysaccharide on earth and, as such, could be a major source of renewable energy and biofuel, such as hydrogen and ethanol. The major obstacle of using cellulose for biofuel production is its breakdown to shorter chains/monosaccharides, which is difficult due to the high chemical/enzymatic resistance and restricted accessibility of its compact semi-crystalline structure. For this reason, CDP has been engineered to tether it by a peptide linker to a carbohydrate binding module (CBM), which could facilitate its interaction and activity onto solid cellulose, for which CDP otherwise has a very low affinity [77]. Ye et al. have reported the effect of the fusion of four different CBMs to CtCDP. They have shown that the fusion of CBM 9-2 from Thermatoga maritima Xyn10A to CDP significantly improved CDP activity on amorphous and crystalline cellulose without decreasing its affinity for shorter oligosaccharides [77]. This discovery has been applied to the synthetic pathway biotransformation (SyPaB) for hydrogen production, where a cocktail of 13 enzymes is used to produce efficiently hydrogen from cellobiose (Figure 12A) [78].
Figure 12.
A) Production of H2 from enzymatic degradation of cellulosic biomass [78]. Reaction products are in bold boxes. Pi = inorganic phosphate; CDP = cellodextrin phosphorylase; CBP = cellobiose phosphorylase; PGM = phosphoglucomutase; G6PDH = Glc-6-phosphate dehydrogenase; 6PGDH = 6-phosphogluconate dehydrogenase; NADP+ = nicotinamide adenine dinucleotide phosphate (oxidised form); NADPH = nicotinamide adenine dinucleotide phosphate (reduced form). B) Enzymatic degradation of cellulosic waste into valuable products [79]. Cellulose enzymatic hydrolysis leads to the production of ethanol by yeast fermentation, amylose by potato a-glucan phosphorylase (PGP), and single cell protein (SCP). All products are in bold boxes. EG = endo-glucanase; Pi = inorganic phosphate; CBP = cellobiose phosphorylase. C) Co-immobilisation of CBM3, PGP and CBP onto Avicel-nano magnetic particles (A-NMPs). CBM3 = carbohydrate binding module 3.
In this system, cellobiose is phosphorolysed to D-Glc and α-D-Glc-1-phosphate by cellobiose phosphorylase (CBP). α-D-Glc-1-phosphate can therefore be transformed by a phosphoglucomutase (PGM) to D-Glc-6-phosphate; the latter is oxidised to 6-phospho-gluconate, which can be further oxidised/decarboxylated to ribulose-5-phosphate by the action of 2 consecutive dehydrogenases, Glc-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH) (enzymes from the oxidative pentose phosphate pathway, PPP) respectively, with reduction of two molecules of nicotinamide adenine dinucleotide phosphate – oxidised form (NADP+) to two molecules of nicotinamide adenine dinucleotide phosphate – reduced form (NADPH). Both NADPH are therefore oxidised back to NADP+ by hydrogenase (H2ase) with production of 2 molecules of hydrogen. Then, ribulose-5-phosphate can be regenerated to D-Glc-6-phosphate by the action of 8 enzymes from the non-oxidative PPP to restart the cycle [78]. The overall H2 yield starting from cellobiose was 93% (11.2 moles of H2 per mole of anhydroglucose units of cellobiose); the overall reaction was endothermic but still spontaneous, pushed forward by the production of gases easy to remove and purify avoiding any product inhibition. When CDP was added to the system for the production of H2 from cellopentaose, the yield was 68% due to incomplete reaction. Therefore, the system requires optimisation of time, concentration of starting materials, enzymes and their ratio, but it has the potential for producing large amounts of H2 from cellulosic biomass. Of course, for its application into an industrial large scale production, the biggest problem to overcome is the stability of the enzymes to high temperatures and their recycle to decrease the cost for their large scale production. These are common problems for many biocatalysts and are being addressed by the discovery of enzymes from thermophilic organisms, or mutagenesis/direct evolution.
Another example of a top-down approach where CDP could be used for degradation of cellulose leading to eco-friendly production of biofuel and food/feed is the work of You et al. [79]. Here, cellulosic waste was transformed in synthetic amylose and ethanol by simultaneous enzymatic biotransformation and microbial fermentation (SEBF) (Figure 12B) [79]. Solid cellulose is attacked by endo-glucanase (EG) and degraded down to cellobiose, which can be phosphorolysed to α-D-Glc-1-phosphate and D-Glc by cellobiose phosphorylase (CBP). The degradation carried out by glucanase/cellulase could potentially be taken up by CDP, thanks to the improvement of CDP activity onto solid cellulose by fusion with a CBM domain shown by Ye et al. [77]. Thereafter, α-D-Glc-1-phosphate can be used by potato α-glucan phosphorylase (PGP) to generate amylose, while released D-Glc can be taken up by yeast to generate ethanol. The yeast used in the process could potentially be used as single cell protein (SCP) for animal feed or protein dietary supplement. The You group has also developed an original way to recover the key enzymes, CBP and PGP, from the enzymatic cellulosic hydrolysate and fermentation broth. Avicel-containing Nano Magnetic Particles (A-NMPs, where Avicel is crystalline cellulose), are coated with CBM3, which binds tightly to the A-NMPs because of its strong affinity to crystalline cellulose. Then, PGP and CBP recombinant proteins stick together because of very high affinity for each other (Figure 12C). These co-immobilised enzymes show the same activity as those in solution and can be easily recovered by magnetic separation. Overall, this work shows how the
optimisation of waste recycling can be achieved and improved to the point where every single reaction component or waste product can be used and regenerated for some useful purposes.
CONCLUSION
Glycoside phosphorylases have been recognised in the last few years as valuable enzymatic tools for a vast variety of applications. Combining their activity with that of other enzymes has dramatically improved the yields of GP reactions and expanded the range of products which can be obtained. Disaccharide phosphorylases have been exploited in the enzymatic production of valuable disaccharides and small glyco-conjugates, some of which have already been successfully used in industry, while others are proof-of-concepts that manifest exciting potential. α-Glucan phosphorylases have mainly been applied to the synthesis of amylose, natural or chemo-enzymatically modified, for its application as food additive, adhesive, drug delivery system, etc. At the same time, amylose degradation by the same class of enzymes has allowed for the production of high value α-D-Glc-1-phosphate at scale. Cellodextrin phosphorylase can be used for the production of very clean cellulose and a variety of related chemically modified materials, which are useful due to their unique chemical and physical properties. However, its use in a cellulose degradation/recycle by phosphorolysis, which would be extremely relevant due to the high abundance of cellulosic waste, is still under investigation due to the low affinity of CDP for insoluble cellulose.
Despite their exploitation and relative success, there are still numerous improvements and challenges that need to be overcome in the use of GPs. Firstly, the narrow substrate specificity remains a limitation, especially in disaccharide phosphorylases, with only a dozen native acceptors being identified and the donor specificity largely restricted to D-Glc- and D-Gal-1-phosphate [19]. Screening for phosphorylases with novel substrate specificities, structural-based modifications and/or directed evolution of the existing phosphorylases will be crucial steps towards the application of these enzyme for production of novel valuable sugars and glyco-conjugates. Secondly, the stability of the biocatalysts must be improved in order to tolerate the industrial-operating conditions. The search for phosphorylases from thermophilic organisms is helping to overcome the thermostability problem common to many enzymes. Moreover, enzyme immobilisation provides one of the key promising steps that could substantially reduce the cost of enzyme purification and product isolation, extend the operational shelf life as well as enable multiple uses of the enzymes. Finally, specificity towards non-native substrates must be improved to further increase yield of the desired products. This challenge has partly been addressed by imprinting a substrate of choice onto a chemically cross-linked sucrose phosphorylase aggregate (CLEA), which result in a two-fold increase in specific activity towards the substrate (in this case, α-glucosyl glycerol) [80]. Moreover, glycosylation of various alcohols, both in vitro and in vivo, provides exciting alternatives for the production of glycosylated bioactive molecules, which are otherwise extracted from natural sources. These approaches may also allow the production of non-natural compounds which could have novel pharmacological activities or greater potency compared to naturally occurring materials. Despite all of these exciting opportunities, challenges still remain for the use of phosphorylases for glycosylation of hydrophobic compounds due to limited water solubility of the substrates, which will require addition of organic co-solvents or ionic liquids to the reaction, which can attenuate the activity of the phosphorylase enzymes [43].
There is still substantial scope for the expansion or research and development in the glycan phosphorylase field: new discoveries and numerous improvements are on the way to address the limitations and to employ these enzymes to their maximum capacity.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
These studies were supported by the UK BBSRC Institute Strategic Program on Understanding and Exploiting Metabolism (MET) [BB/J004561/1] and the John Innes Foundation; BBSRC, EPSRC and InnovateUK: IBCatalyst [BB/M02903411]; Royal Thai Government Scholarship; DBT Overseas Associateship (NER), Govt. of India, [BT/20/NE/2011].
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Lombard V., Ramulu H.G., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:490–495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field R.A. Glycobiology: Challenging reaction equilibria. Nat. Chem. Biol. 2011;7:658–659. doi: 10.1038/nchembio.668. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill E.C., Field R.A. Enzymatic synthesis using glycoside phosphorylases. Carbohydr. Res. 2015;403:23–37. doi: 10.1016/j.carres.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puchart V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015;33:261–276. doi: 10.1016/j.biotechadv.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Nakai H., Kitaoka M., Svensson B., Ohtsubo K.I. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013;17:301–309. doi: 10.1016/j.cbpa.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Kitaoka M., Hayashi K. Carbohydrate-processing phosphorolytic enzymes. Trends Glycosci. Glycotechnol. 2002;14:35–50. [Google Scholar]
- 7.Schmaltz R.M., Hanson S.R., Wong C.H. Enzymes in the synthesis of glycoconjugates. Chem. Rev. 2011;111:4259–4307. doi: 10.1021/cr200113w. [DOI] [PubMed] [Google Scholar]
- 8.Desmet T., Soetaert W., Bojarová P., Křen V., Dijkhuizen L., Eastwick-Field V., Schiller A. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chemistry. 2012;18:10786–10801. doi: 10.1002/chem.201103069. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima M., Nishimoto M., Kitaoka M. Characterization of three β-galactoside phosphorylases from Clostridium phytofermentans: Discovery of D-galactosyl-β-1,4-L-rhamnose phosphorylase. J. Biol. Chem. 2009;284:19220–19227. doi: 10.1074/jbc.M109.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.K., Keyhani N.O., Roseman S. Chitin catabolism in the marine bacterium Vibrio furnissii: Identification, molecular cloning and characterization of a N,N′-diacetylchitobiose phosphorylase. J. Biol. Chem. 2000;275:33077–33083. doi: 10.1074/jbc.M001042200. [DOI] [PubMed] [Google Scholar]
- 11.Nihira T., Suzuki E., Kitaoka M., Nishimoto M., Ohtsubo K.I., Nakai H. Discovery of β-1,4-D-mannosyl-N-acetyl-D-glucosamine phosphorylase involved in the metabolism of N-glycans. J. Biol. Chem. 2013;288:27366–27374. doi: 10.1074/jbc.M113.469080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senoura T., Ito S., Taguchi H., Higa M., Hamada H., Matsui S., Ozawa T., Jin S., Watanabe J., Wasaki J., Ito S. New microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem. Biophys. Res. Commun. 2011;408:701–706. doi: 10.1016/j.bbrc.2011.04.095. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara R., Saburi W., Odaka R., Taguchi H., Ito S., Mori H., Matsui H. Metabolic mechanism of mannan in a ruminal bacterium, Ruminococcus albus, involving two mannoside phosphorylases and cellobiose 2-epimerase: discovery of a new carbohydrate phosphorylase, β-1,4-mannooligosaccharide phos-phorylase. J. Biol. Chem. 2012;287:42389–42399. doi: 10.1074/jbc.M112.390336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitamoto Y., Akashi H., Tanaka H., Mori N. α-Glucose-1-phosphate formation by a novel trehalose phosphorylase from Flammulina velutipes. FEMS Microbiol. Lett. 1988;55:147–149. [Google Scholar]
- 15.Eis C., Nidetzky B. Characterization of trehalose phosphorylase from Schizophyllum commune. Biochem. J. 1999;341:385. [PMC free article] [PubMed] [Google Scholar]
- 16.Cori C.F., Cori G.T. Mechanism of formation of hexose monophosphate in muscle and isolation of a new phosphate ester. Proc. Soc. Exp. Biol. Med. 1936;34:702–705. [Google Scholar]
- 17.Lee Y.P. Potato phosphorylase I. Purification, physicochemical properties and catalytic activity. Biochim. Biophys. Acta. 1960;43:18–24. doi: 10.1016/0006-3002(60)90401-7. [DOI] [PubMed] [Google Scholar]
- 18.Yu H., Chen X. One-pot multienzyme (OPME) systems for chemoenzymatic synthesis of carbohydrates. Org. Biomol. Chem. 2016;14:2809–2818. doi: 10.1039/c6ob00058d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmet T., Soetaert W. Broadening the synthetic potential of disaccharide phosphorylases through enzyme engineering. Process Biochem. 2012;47:11–17. [Google Scholar]
- 20.Cerdobbel A., Desmet T.D., Winter K.D., Maertens J., Soetaert W. Increasing the thermostability of sucrose phosphorylase by multipoint covalent immobilization. J. Biotechnol. 2010;150:125–130. doi: 10.1016/j.jbiotec.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Chomvong K., Lin E., Blaisse M., Gillespie A.E., Cate J.H. Relief of xylose binding to cellobiose phosphorylase by a single distal mutation. ACS Synth. Biol. 2016 doi: 10.1021/acssynbio.1026b00211. [DOI] [PubMed] [Google Scholar]
- 22.Yanase M., Takaha T., Kuriki T. α-Glucan phosphorylase and its use in carbohydrate engineering. J. Sci. Food Agric. 2006;86:1631–1635. [Google Scholar]
- 23.Nishimura T., Akiyoshi K. Amylose engineering: phosphorylase-catalyzed polymerization of functional saccharide primers for glycobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016 doi: 10.1002/wnan.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrović D.M., Kok I., Woortman A.J., Ćirić J., Loos K. Characterization of oligocellulose synthesized by reverse phosphorolysis using different cellodextrin phosphorylases. Anal. Chem. 2015;87:9639–9646. doi: 10.1021/acs.analchem.5b01098. [DOI] [PubMed] [Google Scholar]
- 25.Hamura K., Saburi W., Matsui H., Mori H. Modulation of acceptor specificity of Ruminococcus albus cellobiose phosphorylase through site-directed mutagenesis. Carbohydr. Res. 2013;379:21–25. doi: 10.1016/j.carres.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto K., Yoshihara A., Furumoto T., Takata G. Production and application of a rare disaccharide using sucrose phosphorylase from Leuconostoc mesenteroides. J. Biosci. Bioeng. 2015;119:652–656. doi: 10.1016/j.jbiosc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa M., Schnaar R.L., Ichikawa Y. Application of sucrose phosphorylase reaction in one-pot enzymatic galactosylation: Scavenger of phosphate and generation of glucose 1-phosphate in situ. Tetrahedron Lett. 1995;36:8731–8732. [Google Scholar]
- 28.Nihira T., Miyajima F., Chiku K., Nishimoto M., Kitaoka M., Ohtsubo K., Nakai H. One pot enzymatic production of nigerose from common sugar resources employing nigerose phosphorylase. J. Appl. Glycosci. 2014;61:75–80. [Google Scholar]
- 29.Wildberger P., Pfeiffer M., Brecker L., Nidetzky B. Diastereoselective synthesis of glycosyl phosphates by using a phosphorylase-phosphatase combination catalyst. Angew. Chem. Int. Ed. 2015;54:15867–15871. doi: 10.1002/anie.201507710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyohara M., Tachizawa A., Nishimoto M., Kitaoka M., Ashida H., Yamamoto K. Prebiotic effect of lacto-N-biose I on bifidobacterial growth. Biosci. Biotechnol. Biochem. 2009;73:1175–1179. doi: 10.1271/bbb.80697. [DOI] [PubMed] [Google Scholar]
- 31.Whorwell P.J., Altringer L., Morel J., Bond Y., Charbonneau D., O’Mahony L., Kiely B., Shanahan F., Quigley E.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Mahony L., McCarthy J., Kelly P., Hurley G., Luo F., Chen K., O’Sullivan G.C., Kiely B., Collins J.K., Shanahan F., Quigley E.M. Lactobacillus and Bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Marteau P., Guyonnet D., Lafaye de Micheaux P., Gelu S. A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol. Motil. 2013;25:331–340. doi: 10.1111/nmo.12078. [DOI] [PubMed] [Google Scholar]
- 34.Guyonnet D., Schlumberger A., Mhamdi L., Jakob S., Chassany O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: A randomised, double-blind, parallel, controlled study. Br. J. Nutr. 2009;102:1654–1662. doi: 10.1017/S0007114509990882. [DOI] [PubMed] [Google Scholar]
- 35.Nishimoto M., Kitaoka M. Practical preparation of lacto-N-biose I, a candidate for the bifidus factor in human milk. Biosci. Biotechnol. Biochem. 2007;71:2101–2104. doi: 10.1271/bbb.70320. [DOI] [PubMed] [Google Scholar]
- 36.Moran A.P., Gupta A., Joshi L. Sweet-talk: Role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut. 2011;60:1412–1425. doi: 10.1136/gut.2010.212704. [DOI] [PubMed] [Google Scholar]
- 37.Nishimoto M., Kitaoka M. Identification of N-acetylhexosamine 1-kinase in the complete lacto-N-bioseI/galacto-N-biose metabolic pathway in Bifidobacterium longum. Appl. Environ. Microbiol. 2007;73:6444–6449. doi: 10.1128/AEM.01425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dube D.H., Bertozzi C.R. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y., Matsunaga M., Nagao Y., Taki T., Hirabayashi Y., Matsumoto M. Ganglioside GM1b as an influenza virus receptor. Vaccine. 1985;3:201–203. doi: 10.1016/0264-410x(85)90104-5. [DOI] [PubMed] [Google Scholar]
- 40.Nishimoto M., Kitaoka M. One-pot enzymatic production of β-D-galactopyranosyl-(1→3)-2-acetamido-2-deoxy-D-galactose (galacto-N-biose) from sucrose and 2-acetamido-2-deoxy-D-galactose (N-acetylgalactosamine). Carbohydr. Res. 2009;344:2573–2576. doi: 10.1016/j.carres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Liu Y., Li T., Wang W., Yu Z., Ma C., Qu J., Zhao J., Chen X., Wang P.G. Efficient chemoenzymatic synthesis of novel galacto-N-biose derivatives and their sialylated forms. Chem. Commun. (Camb.) 2015;51:10310–10313. doi: 10.1039/c5cc03746h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller C., Ortmann T., Abi A., Hartig D., Scholl S., Jördening H.J. Immobilization and characterization of E. gracilis extract with enriched laminaribiose phosphorylase activity for bienzymatic production of laminaribiose. Appl. Biochem. Biotechnol. 2016 doi: 10.1007/s12010-12016-12320-12014. [DOI] [PubMed] [Google Scholar]
- 43.Aerts D., Verhaeghe T.F., Roman B.I., Stevens C.V., Desmet T., Soetaert W. Transglucosylation potential of six sucrose phosphorylases toward different classes of acceptors. Carbohydr. Res. 2011;346:1860–1867. doi: 10.1016/j.carres.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Du G.J., Zhang Z., Wen X.D., Yu C., Calway T., Yuan C.S., Wang C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4:1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedl C., Sawangwan T., Mueller M., Schwarz A., Nidetzky B. A high-yielding biocatalytic process for the production of 2-O-(α-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew. Chem. Int. Ed. 2008;47:10086–10089. doi: 10.1002/anie.200803562. [DOI] [PubMed] [Google Scholar]
- 46.Kitao S., Matsudo T., Saitoh M., Horiuchi T., Sekine H. Enzymatic syntheses of two stable (−)-epigallocatechin gallate-glucosides by sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1995;59:2167–2169. [Google Scholar]
- 47.Kraus M., Grimm C., Seibel J. Redesign of the active site of sucrose phosphorylase through a clash-induced cascade of loop shifts. ChemBioChem. 2016;17:33–36. doi: 10.1002/cbic.201500514. [DOI] [PubMed] [Google Scholar]
- 48.De Winter K., Dewitte G., Dirks-Hofmeister M.E., De Laet S., Pelantová H., Křen V., Desmet T. Enzymatic glycosylation of phenolic antioxidants: phosphorylase-mediated synthesis and characterization. J. Agric. Food Chem. 2015;63:10131–10139. doi: 10.1021/acs.jafc.5b04380. [DOI] [PubMed] [Google Scholar]
- 49.De Bruyn F., Van Brempt M., Maertens J., Van Bellegem W., Duchi D., Mey M.D. Metabolic engineering of Escherichia coli into a versatile glycosylation platform: Production of bio-active quercetin glycosides. Microb. Cell Fact. 2015;14:138–149. doi: 10.1186/s12934-015-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso M.D., Lomako J., Lomako W.M., Whelan W.J. A new look at the biogenesis of glycogen. FASEB J. 1995;9:1126–1137. doi: 10.1096/fasebj.9.12.7672505. [DOI] [PubMed] [Google Scholar]
- 51.Boyer L., Roussel X., Courseaux A., Ndjindji O.M., Lancelon-Pin C., Putaux J.L., Tetlow I.J., Emes M.J., Pontoire B., D’ Hulst C., Wattebled F. Expression of Escherichia coli glycogen branching enzyme in an Arabidopsis mutant devoid of endogenous starch branching enzymes induces the synthesis of starch-like polyglucans. Plant Cell Environ. 2016;39:1432–1447. doi: 10.1111/pce.12702. [DOI] [PubMed] [Google Scholar]
- 52.Sawada T., Nakamura Y., Ohdan T., Saitoh A., Francisco P.B., Jr, Suzuki E., Fujita N., Shimonaga T., Fujiwara S., Tsuzuki M., Colleoni C., Ball S. Diversity of reaction characteristics of glucan branching enzymes and the fine structure of α-glucan from various sources. Arch. Biochem. Biophys. 2014;562:9–21. doi: 10.1016/j.abb.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Ohdan K., Fujii K., Yanase M., Takaha T., Kuriki T. Phosphorylase coupling as a tool to convert cellobiose into amylose. J. Biotechnol. 2007;127:496–502. doi: 10.1016/j.jbiotec.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki M., Kaneda K., Nakai Y., Kitaoka M., Taniguchi H. Synthesis of cellobiose from starch by the successive actions of two phosphorylases. N. Biotechnol. 2009;26:137–142. doi: 10.1016/j.nbt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Zhou W., You C., Ma H., Ma Y., Zhang Y.H. One-pot biosynthesis of high-concentration α-glucose 1-phosphate from starch by sequential addition of three hyperthermophilic enzymes. J. Agric. Food Chem. 2016;64:1777–1783. doi: 10.1021/acs.jafc.5b05648. [DOI] [PubMed] [Google Scholar]
- 56.Qi P., You C., Zhang Y.H. One-pot enzymatic conversion of sucrose to synthetic amylose by using enzyme cascades. ACS Catal. 2014;4:1311–1317. [Google Scholar]
- 57.Seo B.S., Kim S., Scott M.P., Singletary G.W., Wong K.S., James M.G., Myers A.M. Functional interactions between heterologously expressed starch-branching enzymes of maize and the glycogen synthases of brewer’s yeast. Plant Physiol. 2002;128:1189–1199. doi: 10.1104/pp.010756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciric J., Loos K. Synthesis of branched polysaccharides with tunable degree of branching. Carbohydr. Polym. 2013;93:31–37. doi: 10.1016/j.carbpol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 59.van der Vlist J., Schönen I., Loos K. Utilization of glycosyltransferases for the synthesis of a densely packed hyperbranched polysaccharide brush coating as artificial glycocalyx. Biomacromolecules. 2011;12:3728–3732. doi: 10.1021/bm2009763. [DOI] [PubMed] [Google Scholar]
- 60.Shoda S.I., Uyama H., Kadokawa J.I., Kimura S., Kobayashi S. Enzymes as green catalysts for precision macromolecular synthesis. Chem. Rev. 2016;116:2307–2413. doi: 10.1021/acs.chemrev.5b00472. [DOI] [PubMed] [Google Scholar]
- 61.Bornemann S. α-Glucan biosynthesis and the GlgE pathway in Mycobacterium tuberculosis. Biochem. Soc. Trans. 2016;44:68–73. doi: 10.1042/BST20150181. [DOI] [PubMed] [Google Scholar]
- 62.Syson K., Stevenson C.E., Rejzek M., Fairhurst S.A., Nair A., Bruton C.J., Field R.A., Chater D.M., Lawson K.F., Bornemann S. Structure of Streptomyces maltosyltransferase glge, a homologue of a genetically validated anti-tuberculosis target. J. Biol. Chem. 2011;286:38298–38310. doi: 10.1074/jbc.M111.279315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rashid A.M., Batey S.F., Syson K., Koliwer-Brandl H., Miah F., Barclay J.E., Findlay K.C., Nartowski K.P., Khimyak Y.Z., Kalscheuer R., Bornemann S. Assembly of α-glucan by GlgE and GlgB in mycobacteria and streptomycetes. Biochemistry. 2016;55:3270–3284. doi: 10.1021/acs.biochem.6b00209. [DOI] [PubMed] [Google Scholar]
- 64.Miah F., Koliwer-Brandl H., Rejzek M., Field R.A., Kalscheuer R., Bornemann S. Flux through trehalose synthase flows from trehalose to the alpha anomer of maltose in mycobacteria. Chem. Biol. 2013;20:487–493. doi: 10.1016/j.chembiol.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klemm D., Heublein B., Fink H.P., Bohn A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005;44:3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
- 66.Ummartyotin S., Manuspiya H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015;41:402–412. [Google Scholar]
- 67.Kaushik M., Moores A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016;18:622–637. [Google Scholar]
- 68.Jozala A.F., de Lencastre-Novaes L.C., Lopes A.M., de Carvalho Santos-Ebinuma V., Mazzola P.G., Pessoa-Jr A., Grotto D., Gerenutti M., Chaud M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016;100:2063–2072. doi: 10.1007/s00253-015-7243-4. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi S., Kashiwa K., Shimada J., Kawasaki T., Shoda S.I. Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase.; Macromol. Chem. Macromol. Symp.; 1992. pp. 54–55. [Google Scholar]
- 70.Sheth K., Alexande J.K. Cellodextrin phosphorylase from Clostridium thermocellum. Biochim. Biophys. Acta. 1967;148:808–810. [Google Scholar]
- 71.Krishnareddy M., Kim Y.K., Kitaoka M., Mori Y., Hayashi K. Cellodextrin phosphorylase from Clostridium thermocellum YM4 strain expressed in Escherichia coli. J. Appl. Glycosci. 2002;49:1–8. doi: 10.1093/oxfordjournals.jbchem.a003210. [DOI] [PubMed] [Google Scholar]
- 72.Hiraishi M., Igarashi K., Kimura S., Wada M., Kitaoka M., Samejima M. Synthesis of highly ordered cellulose II in vitro using cellodextrin phosphorylase. Carbohydr. Res. 2009;344:2468–2473. doi: 10.1016/j.carres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Nakai H., Hachem M.A., Petersen B.O., Westphal Y., Mannerstedt K., Baumann M.J., Dilokpimol A., Schols H.A., Duus J.Ø., Svensson B. Efficient chemoenzymatic oligosaccharide synthesis by reverse phosphorolysis using cellobiose phosphorylase and cellodextrin phosphorylase from Clostridium thermocellum. Biochimie. 2010;92:1818–1826. doi: 10.1016/j.biochi.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 74.Yataka Y., Sawada T., Serizawa T. Enzymatic synthesis and post-functionalization of two-dimensional crystalline cellulose oligomers with surface-reactive groups. Chem. Commun. 2015;51:12525–12528. doi: 10.1039/c5cc04378f. [DOI] [PubMed] [Google Scholar]
- 75.Yataka Y., Sawada T., Serizawa T. Multidimensional self-assembled structures of alkylated cellulose oligomers synthesized via in vitro enzymatic reactions. Langmuir. 2016;32:10120–10125. doi: 10.1021/acs.langmuir.6b02679. [DOI] [PubMed] [Google Scholar]
- 76.Serizawa T., Kato M., Okura H., Sawada T., Wada M. Hydrolytic activities of artificial nanocellulose synthesized via phosphorylase-catalyzed enzymatic reactions. Polym. J. 2016;48:539–544. [Google Scholar]
- 77.Ye X., Zhu Z., Zhang C., Zhang Y.H. Fusion of a family 9 cellulose-binding module improves catalytic potential of Clostridium thermocellum cellodextrin phosphorylase on insoluble cellulose. Appl. Microbiol. Biotechnol. 2011;92:551–560. doi: 10.1007/s00253-011-3346-8. [DOI] [PubMed] [Google Scholar]
- 78.Ye X., Wang Y., Hopkins R.C., Adams M.W., Evans B.R., Mielenz J.R., Zhang Y.H. Spontaneous high-yield production of hydrogen from cellulosic materials and water catalyzed by enzyme cocktails. ChemSusChem. 2009;2:149–152. doi: 10.1002/cssc.200900017. [DOI] [PubMed] [Google Scholar]
- 79.You C., Chen H., Myung S., Sathitsuksanoh N., Ma H., Zhang X.Z., Li J., Zhang Y.H. Enzymatic transformation of nonfood biomass to starch. Proc. Natl. Acad. Sci. USA. 2013;110:7182–7187. doi: 10.1073/pnas.1302420110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Winter K., Soetaert W., Desmet T. An imprinted Cross-linked enzyme aggregate (iCLEA) of Sucrose phosphorylase: Combining improved stability with altered specificity. Int. J. Mol. Sci. 2012;13:11333–11342. doi: 10.3390/ijms130911333. [DOI] [PMC free article] [PubMed] [Google Scholar]