Abstract
Preautonomic neurons in the paraventricular nucleus (PVN) of the hypothalamus play a large role in the regulation of hepatic functions via the autonomic nervous system. Activation of hepatic sympathetic nerves increases glucose and lipid metabolism and contributes to the elevated hepatic glucose production observed in the type 2 diabetic condition. This augmented sympathetic output could originate from altered activity of liver-related PVN neurons. Remarkably, despite the importance of the brain-liver pathway, the cellular properties of liver-related neurons are not known. In this study, we provide the first evidence of overall activity of liver-related PVN neurons. Liver-related PVN neurons were identified with a retrograde, trans-synaptic, viral tracer in male lean and db/db mice and whole-cell patch-clamp recordings were conducted. In db/db mice, the majority of liver-related PVN neurons fired spontaneously; whereas, in lean mice the majority of liver-related PVN neurons were silent, indicating that liver-related PVN neurons are more active in db/db mice. Persistent, tonic inhibition was identified in liver-related PVN neurons; although, the magnitude of tonic inhibitory control was not different between lean and db/db mice. In addition, our study revealed that the transient receptor potential vanilloid type 1-dependent increase of excitatory neurotransmission was reduced in liver-related PVN neurons of db/db mice. These findings demonstrate plasticity of liver-related PVN neurons and a shift toward excitation in a diabetic mouse model. Our study suggests altered autonomic circuits at the level of the PVN, which can contribute to autonomic dysfunction and dysregulation of neural control of hepatic functions including glucose metabolism.
SIGNIFICANCE STATEMENT A growing body of evidence suggests the importance of the autonomic control in the regulation of hepatic metabolism, which plays a major role in the development and progression of type 2 diabetes mellitus. Despite the importance of the brain-liver pathway, the overall activity of liver-related neurons in control and diabetic conditions is not known. This is a significant gap in knowledge, which prevents developing strategies to improve glucose homeostasis via altering the brain-liver pathway. One of the key findings of our study is the overall shift toward excitation in liver-related hypothalamic neurons in the diabetic condition. This overactivity may be one of the underlying mechanisms of elevated sympathetic activity known in metabolically compromised patients and animal models.
Keywords: liver-related neurons, patch-clamp, pseudorabies virus, PVN, TRPV1
Introduction
The CNS is a major contributor to the control of whole-body metabolism including hepatic glucose and lipid metabolism (Pocai et al., 2005; Yi et al., 2010; Schwartz et al., 2013). Traditionally, the hypothalamus, a key brain region in the regulation of homeostatic functions was valued for its neuroendocrine control systems; nevertheless, its role in governing the autonomic nervous system became well accepted (Sandoval et al., 2009; Kalsbeek et al., 2010; Seoane-Collazo et al., 2015; O'Hare and Zsombok, 2016; Ruud et al., 2017). In general, the autonomic nervous system modulates organ functions through opposing actions of the sympathetic and parasympathetic nervous systems. Stimulation of the sympathetic innervation of the liver elevates endogenous glucose production and glycogenolysis; whereas, activation of the parasympathetic nerves reduces glucose production and increases glucose storage (Shimazu and Fukuda, 1965; Shimazu, 1967, 1996; Uyama et al., 2004; Yi et al., 2010). Imbalance of the autonomic nervous system, primarily increased activity of the sympathetic nervous system (SNS) has been shown to play a role in the development of metabolic disorders including diabetes mellitus (Carnethon et al., 2003; Schlaich et al., 2015; Thorp and Schlaich, 2015). Sympathetic preganglionic neurons in the spinal cord transmit the information through the sympathetic nerves to the liver. These preganglionic neurons receive information from preautonomic neurons located in higher brain areas including the paraventricular nucleus of the hypothalamus (PVN). The PVN is considered a command component of the autonomic pathway that integrates signals from many different brain areas, including hypothalamic nuclei with well established roles in glucose and energy homeostasis (Yi et al., 2010; O'Hare and Zsombok, 2016). Excitation of PVN neurons with NMDA, a glutamate receptor agonist or removal of inhibition with a GABAA receptor antagonist elevated plasma glucose levels via the SNS (Kalsbeek et al., 2004). Classical neurotransmitters, hormones, and neuropeptides have been shown to influence glucose levels likely via PVN-dependent mechanisms (Ionescu et al., 1989); however, only limited amount of information is available about the cellular properties of preautonomic, liver-related PVN neurons (Derbenev and Zsombok, 2016). Our previous study demonstrated that activation of the transient receptor potential vanilloid type 1 (TRPV1), a ligand-gated nonselective cation channel increases excitatory neurotransmission to a subset of liver-related PVN neurons (Gao et al., 2012). This TRPV1-dependent regulation of liver-related PVN neurons was diminished in the streptozotocin-treated hyperglycemic, insulin-deficient mouse model (Gao et al., 2012), which suggests that TRPV1 plays a role in synaptic regulation of liver-related PVN neurons; however, the basic cellular properties including the excitability of liver-related PVN neurons remained to be determined.
In general, in vivo findings demonstrated that increasing the activity of preautonomic PVN neurons enhanced sympathetic activity and this increased hepatic sympathetic activity could contribute to elevated glucose production. Remarkably, despite the importance of the liver-related neurons in the neural control of hepatic functions, the cellular properties of these preautonomic PVN neurons, except their TRPV1-dependent excitation, has not yet been established. In this study, we used a multidisciplinary experimental approach including trans-synaptic viral tracing and patch-clamp electrophysiology to identify liver-related neurons in the PVN and test the hypothesis that a subset of liver-related PVN neurons are overactivated in the db/db mouse model of type 2 diabetes.
Materials and Methods
Animals.
Male (7–10 weeks old) db/db mice (homozygous, BKS.Cg-Dock7m+/+Leprdb/J, JAX, stock #000642) and age-matched, lean (heterozygous) mice were used throughout the study. In one set of experiments, age-matched, male C57BL/6J mice (JAX #000664) were used to reveal tonic currents in liver-related neurons and compare them to db/db and lean mice. Experiments were performed following the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Tulane University's Institutional Animal Care and Use Committee.
Injection of pseudorabies virus.
Pseudorabies virus 152 [PRV-152; reports enhanced green fluorescence protein (EGFP), supplied by NCRR CNNV Virus Center; Card, 1998; Cano et al., 2004], a retrogradely transported pseudorabies viral vector was used to identify liver-related neurons, as described previously (Gao et al., 2012). Briefly, under anesthesia, the liver was exposed and two injections (∼2 μl per site) were made into the parenchyma. A drop of adhesive “liquid bandage” was used to seal each injection site to prevent the leakage of the virus. The animals were maintained in a biosafety level 2 facility up to 114–116 h postinjection and used for patch-clamp studies.
Brain slice preparation and whole-cell patch-clamp recordings.
Acute, transverse hypothalamic slices containing the PVN (300 μm) were made using a vibrating microtome as previously described (Gao et al., 2012). The slices were stored in a holding chamber at 34–36°C, and then transferred to a recording chamber mounted on a fixed stage under an upright microscope (Nikon FN1 or Olympus BX51WIF). Whole-cell patch-clamp recordings were performed at 34–37°C from liver-related neurons in the PVN identified under a 40× water-immersion objective (NA = 0.8). EGFP-containing neurons were identified based on their epifluorescence, and infrared differential interference contrast optics to target specific cells. For whole-cell patch-clamp recordings, electrodes (3–7 MΩ) were filled with a solution containing the following (in mm): 130 K+ or Cs+ gluconate, 10 HEPES, 5 EGTA, 1 NaCl, 1 MgCl2, 1 CaCl2, 3 KOH or CsOH, 2–3 Mg-ATP, 0.2% biocytin, pH 7.3–7.4. Electrophysiological signals were recorded using an Axoclamp 700B amplifier (Molecular Devices) and acquired by pClamp (Molecular Devices). IPSCs were recorded at −10 mV and EPSCs at −60 mV. Synaptic currents were analyzed off-line using MiniAnalysis (Synaptosoft). Tetrodotoxin (TTX; 1 μm; Tocris Bioscience) in the bath solution was used to block action potentials and monitor miniature IPSCs (mIPSCs) and EPSCs (mEPSCs). Capsaicin (1 μm; Tocris Bioscience) was used to determine the effects of TRPV1 activation on the PSCs. To reveal the existence of tonic inhibition in liver-related PVN neurons, in the presence of kynurenic acid (1 mm; Sigma-Aldrich), the GABAA receptor antagonist bicuculline methiodide (30 μm; Tocris Bioscience) was applied. The bicuculline-dependent inward shift of the holding current was used to determine the tonic GABAA current in liver-related PVN neurons of lean, db/db and C57BL/6J mice (Park et al., 2007; Jiang et al., 2013). Membrane capacitance was used to determine the cell-surface area in liver-related PVN neurons in lean and db/db mice (Taylor, 2012; Duzhyy et al., 2015). To determine the excitability of liver-related PVN neurons, recordings were performed in current-clamp mode. The firing rates of liver-related PVN neurons were compared at resting membrane potential and following a step protocol or a ramp protocol. During the step protocol, the recorded neurons were hyperpolarized to −100 mV and then depolarizing current steps (duration 1 s) were applied and the firing activity of liver-related PVN neurons of lean and db/db mice was compared. The firing activity of neurons was also revealed after a depolarizing current injection that evoked a 10 mV depolarization from the resting membrane potential. During a ramp protocol, a linearly increasing depolarizing current was injected (duration 5 s) from membrane potential of −100 mV to at least 0 mV.
Experimental design and statistical analysis.
Male lean and db/db mice (n = 34 and 33) were inoculated with PRV and recordings were conducted from EGFP-labeled PVN neurons. The number of recorded cells and the number of mice used for these recordings are included into the results section for each experiment. Continuous recordings have been conducted and 2 min periods were analyzed with MiniAnalysis (Synaptosoft) to measure peak amplitude and frequency. Ten to 90% rise time, weighted decay time (τw), and area of postsynaptic currents (charge transfer, Q) were measured under curve fitting as described previously (Jiang et al., 2013). The mean inhibitory phasic current (Iphasic) was calculated: Iphasic= frequency × charge transfer (Nusser and Mody, 2002; Park et al., 2006). Comparison between groups was made with an unpaired two-tailed Student's t test and p < 0.05 was considered significant. Numbers are reported as mean ± SEM. Statistical analysis following the current-clamp studies were performed using GraphPad Prism 7 software. Kolmogorov–Smirnov test with Dallal–Wilkinson–Lillie for p values was used to test normality of values distribution. Unpaired t test was used to test two groups with Gaussian distributed values, whereas non-Gaussian values were tested with Mann–Whitney test. Comparisons of more than two groups of Gaussian distributed values were performed with one-way ANOVA followed by a multiple-comparison Tukey test. Comparisons of more than two groups of non-Gaussian distributed values were performed with Kruskal–Wallis test followed by a multiple-comparison Dunn test.
Measurements of plasma glucose, insulin, and glucagon levels.
Plasma glucose levels were measured with One Touch Ultra glucometer (Life Scan). Plasma insulin and glucagon concentrations were determined using a Mouse Ultrasensitive Insulin ELISA kit (ALPCO) and glucagon ELISA-10 μl kit (Mercodia AB), respectively.
Results
Physiological parameters
Male lean and db/db mice were used in the studies. The bodyweight and glucose levels of db/db mice were significantly higher compared with lean mice [39.4 ± 0.5 g (n = 33) vs 26.6 ± 0.4 g (n = 34), Mann–Whitney, p = 0.000; 540.3 ± 10.7 mg/dl (n = 32) vs 191.4 ± 6.7 mg/dl (n = 32), Mann–Whitney, p = 0.000]. In some cases, insulin and glucagon levels were detected with ELISA. As expected plasma insulin levels were significantly higher in db/db mice compared with lean mice [5.4 ± 0.7 ng/ml (n = 10) vs 0.59 ± 0.1 ng/ml (n = 8), unpaired t test, p = 0.000]. Glucagon levels were elevated in db/db mice compared with lean mice [31.2 ± 7.5 pmol/L (n = 10) vs 6.3 ± 2.0 pmol/L (n = 9), unpaired t test, p = 0.007].
Membrane properties of liver-related PVN neurons
In db/db mice, the majority of recorded liver-related PVN neurons had spontaneous firing, whereas in lean mice the majority of them were silent. In db/db mice (n = 4) 10 of 13 neurons fired spontaneously, whereas in lean mice (n = 5) only 5 of 13 recorded liver-related PVN neurons had spontaneous firing (Fig. 1A,B,E). Because in lean mice the majority of neurons were silent (∼60%), we separated the recorded cells into silent (lean silent) and spontaneously firing (lean spontaneous) groups and compared with spontaneously firing liver-related PVN neurons from db/db mice (db/db spontaneous). Because only three silent neurons were recorded in db/db mice with variable cellular properties, we did not include this group in the figures, but reported their values in the text where it is appropriate.
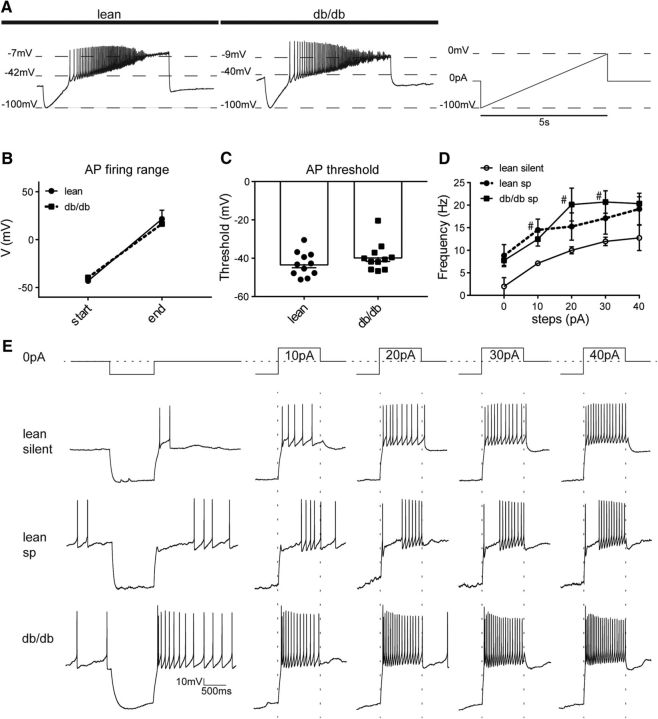
Figure 1.
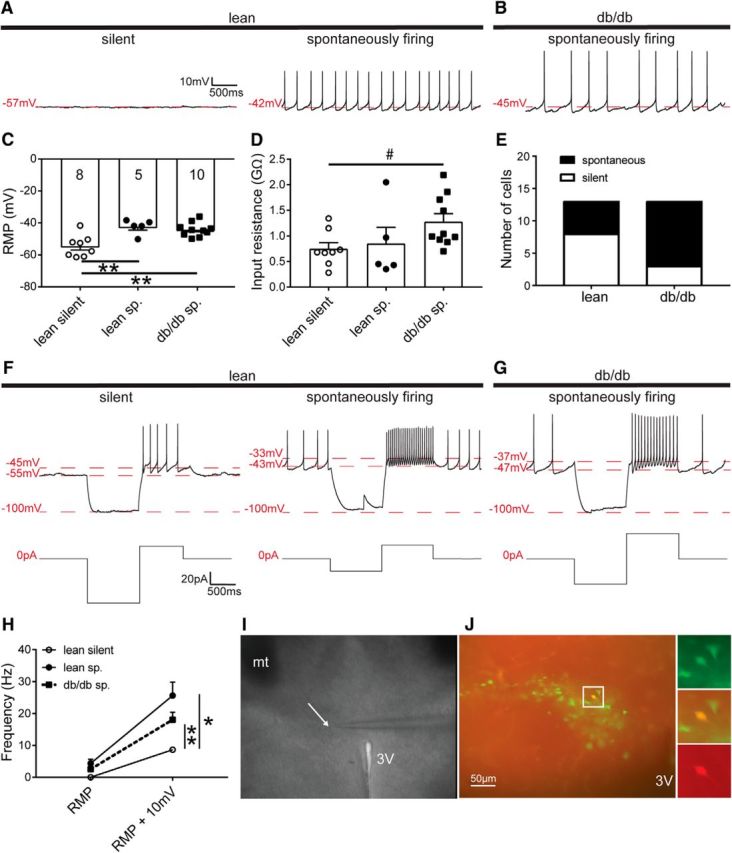
The excitability of liver-related PVN neurons is increased in db/db mice. A, Continuous recordings show silent and spontaneously firing liver-related PVN neurons in a lean mouse. B, Trace illustrates a spontaneously firing neuron from a db/db mouse. C, D, Bar graphs show the resting membrane potentials (C) and input resistance (D) of liver-related PVN neurons. E, Distribution of silent and spontaneously firing liver-related PVN neurons in lean and db/db mice. F, G, Firing activity of liver-related PVN neurons after a 10 mV depolarization compared with resting membrane potential in lean (F) and db/db (G) mice. H, Frequency of action potentials before and after a 10 mV depolarization compared with resting membrane potential. I, Differential interference contrast image of a brain slice containing PVN from a lean mouse during patch-clamp recording. J, Liver-related PVN neurons identified with PRV-152 (green) and a recorded liver-related PVN neuron visualized with biocytin (red). Significance: *p < 0.05, **p < 0.01, #p < 0.05, determined by uncorrected Dunn's multiple-comparison test. Lean sp, Lean spontaneously firing; db/db sp, spontaneously firing liver-related neurons in db/db mice; RMP, resting membrane potential; 3v, third ventricle, mt, mammillothalamic tract. The arrow points to the tip of a recording glass pipette.
At resting membrane potential, the firing rate of spontaneously active liver-related PVN neurons of lean mice was 4.3 ± 1.3 Hz (range: 0.3–7.2 Hz; n = 5). In db/db mice the firing rate was 2.5 ± 0.6 Hz (range: 0.2–5.1 Hz; n = 10), demonstrating no significant difference in activity at resting membrane potential between the spontaneously firing groups in lean and db/db mice (ANOVA, F = 4.2; Tukey, p = 0.27; Fig. 1H). Despite the similarity in firing, the observation that the majority of liver-related neurons in lean mice are silent compared with neurons from db/db mice indicates that in db/db mice liver-related PVN neurons rest in a more depolarized state. Therefore, we compared the resting membrane potentials among the groups. The resting membrane potential of the lean silent group was −54.5 ± 2.4 mV (n = 8), which is significantly different from liver-related PVN neurons in db/db mice (−44.1 ± 1.3 mV; range: −53.0 to −36.0 mV; n = 10; ANOVA, F = 11.26; Tukey, p = 0.0015), and from the lean spontaneously firing group (−42.4 ± 2.1 mV; n = 5; ANOVA, F = 11.26; Tukey, p = 0.0019; Fig. 1C). The resting membrane potential in the silent db/db group was −38.8 ± 8.5 mV (n = 3). These findings were consistent with the observed action potential frequency of spontaneously firing groups in lean and db/db mice (Fig. 1H).
Next, we determined the input resistance of liver-related PVN neurons in lean and db/db mice. The input resistance of the silent liver-related PVN neurons of lean mice was 0.75 ± 0.1 GΩ (n = 8), whereas the input resistance in the spontaneous db/db group was 1.28 ± 0.2 GΩ (n = 10; Kruskal–Wallis, Dunn, p = 0.029; Fig. 1D). The input resistance of the lean spontaneously firing group was 0.85 ± 0.3 GΩ (n = 5), which was not significantly different from the input resistance of db/db mice, although it showed a decreasing trend (Kruskal–Wallis, Dunn, p = 0.052). The input resistance in the silent db/db group was 1.46 ± 0.6 GΩ (n = 3). These data indicate significant difference in input resistance of liver-related PVN neurons between lean silent and spontaneously firing neurons in db/db mice (Fig. 1D).
We determined the firing rate of liver-related PVN neurons following current injections. First, as described previously (Luther and Tasker, 2000; Stern, 2001; Luther et al., 2002), after a hyperpolarizing current step, a 10 mV depolarization compared with resting membrane potential was evoked by current injection. In the lean silent group, we were able to evoke firing in three of the eight recorded neurons with an average frequency of 8.6 ± 0.7 Hz (range: 7.52–10.07 Hz), whereas the rest of the neurons remained silent. In the lean spontaneous group, the firing rate increased to 25.65 ± 4.20 Hz (range: 14.34–39.47 Hz; n = 5; ANOVA, F = 9.7, p = 0.034). In the spontaneous db/db group, the firing increased to 18.08 ± 2.30 Hz (range: 7.42–28.11 Hz; n = 10; ANOVA, F = 9.7, p = 0.0014; Fig. 1F–H), whereas in the silent db/db group we were able to evoke firing in one of the three recorded neurons. These data demonstrate that after a 10 mV depolarization liver-related PVN neurons in db/db mice fired more than the majority of liver-related PVN neurons in lean mice. Then, a ramp protocol was used to define the threshold of firing. Our data did not reveal difference in the threshold and the amplitude of the first evoked action potentials among the groups (Fig. 2A–C).
Figure 2.
Firing rate of liver-related PVN neurons following depolarization. A, Liver-related PVN neurons were depolarized from −100 mV to at least 0 mV with a linearly increasing current in lean and db/db mice. B, C, The firing range (B) and threshold of action potentials (C) were not altered in db/db mice. D, E, Depolarizing current steps from 0 pA to 40 pA (E) revealed increased action potential frequency in liver-related PVN neurons from db/db mice (D). #p < 0.05, determined by uncorrected Dunn's multiple-comparison test, db/db vs lean silent. AP, Action potential; lean sp, lean spontaneously firing; db/db sp, spontaneously firing liver-related neurons in db/db mice.
To further investigate the excitability of liver-related PVN neurons in lean and db/db mice, a hyperpolarizing current step followed by depolarizing current steps (10–40 pA) were applied (Fig. 2E). In the lean silent group, we were able to evoke action potentials in four of the eight neurons, and the activity of these neurons were compared with the spontaneously firing liver-related PVN neurons both in lean and db/db mice. The firing rate of recorded PVN neurons gradually increased in each group; however, the frequency of lean silent group was significantly lower, than in the spontaneously firing neurons in db/db mice (Kruskal–Wallis, Dunn, p = 0.052 at 0 pA, p = 0.047 at 10 pA, p = 0.040 at 20 pA, p = 0.042 at 30 pA, p = 0.08 at 40 pA; Fig. 2D). Together, these findings suggest that liver-related PVN neurons in db/db mice are more excited compared with the majority of liver-related PVN neurons of lean mice.
Phasic excitatory and inhibitory currents in liver-related PVN neurons
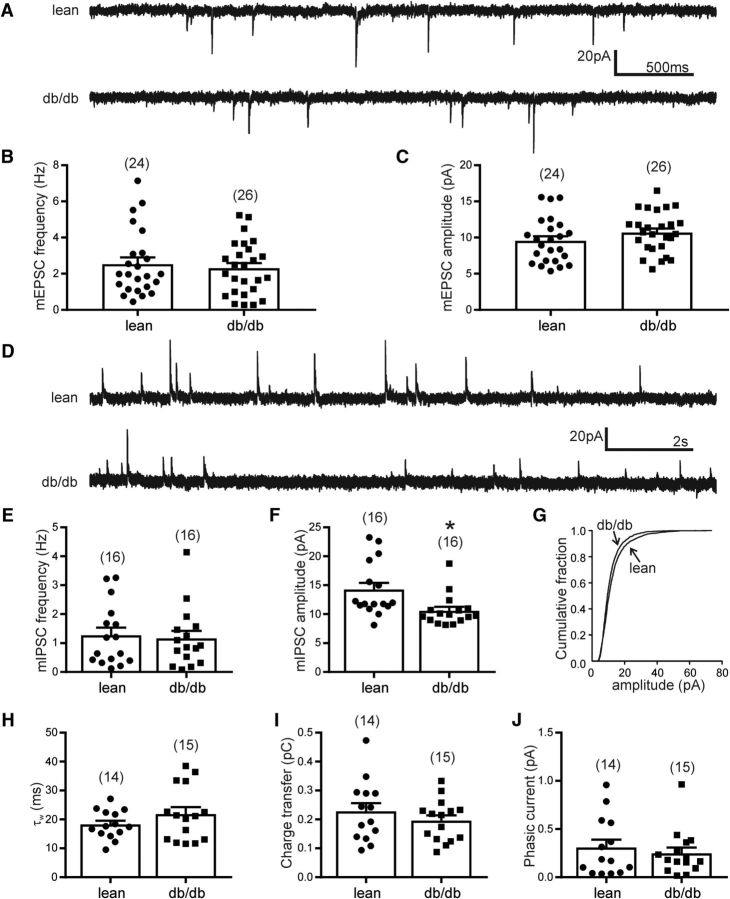
The frequencies and amplitude of mEPSCs and mIPSCs of liver-related PVN neurons were examined in the presence of TTX in slices from lean and db/db mice. In lean mice, the average mEPSC frequency of liver-related PVN neurons was 2.5 ± 0.4 Hz (range: 0.5–7.1 Hz; n = 24 cells from 9 mice). In db/db mice, the average frequency of mEPSCs in liver-related PVN neurons was 2.3 ± 0.3 Hz (range: 0.3–5.2 Hz; n = 26 cells from 10 mice; t test, p = 0.62). The amplitude of mEPSCs in lean mice was 9.5 ± 0.6 pA (range: 5.4–15.6 pA; n = 24), whereas 10.7 ± 0.6 pA (range: 5.6–16.5 pA; n = 26) in db/db mice (t test, p = 0.18). These data suggest no alteration of the basic excitatory neurotransmission of liver-related PVN neurons in db/db mice (Fig. 3A–C).
Figure 3.
Phasic excitatory and inhibitory neurotransmission in liver-related PVN neurons. A, Representative recordings of mEPSCs at a holding potential of −60 mV from a liver-related PVN neurons in a lean (top trace) and a db/db mouse (bottom trace). B, C, There was no significant difference in mEPSC frequency (B) or amplitude (C) of liver-related PVN neurons in lean and db/db mice (p > 0.05). D, Representative recordings of mIPSCs at a holding potential of −10 mV from a liver-related PVN neuron in lean (top trace) and db/db mice (bottom trace). E–J, Combined data showing mIPSC frequency (E), amplitude (F), cumulative fraction (G), weighted decay time (H), charge transfer (I), and mean phasic inhibitory current (J) in liver-related PVN neurons from lean and db/db mice. The numbers of recorded cells are shown in parentheses. *p < 0.05.
The average frequency of mIPSCs was not different in lean and db/db mice (1.3 ± 0.3 Hz, n = 16 cells from 5 mice vs 1.2 ± 0.3 Hz, n = 16 cells from 7 mice; t test, p = 0.78; Fig. 3D,E). In contrast, the amplitude of mIPSCs was significantly smaller in db/db mice compared with lean mice. The amplitude of mIPSCs in liver-related PVN neurons of lean mice was 14.2 ± 1.2 pA (range: 8.1–23.3 pA; n = 16), whereas 10.6 ± 0.7 pA (range: 8.1–18.7 pA; n = 16) in db/db mice (t test, p = 0.011; Fig. 3F). Because the amplitude of mIPSCs was significantly smaller in liver-related PVN neurons of db/db mice, we determined whether GABAergic synaptic strength is decreased in db/db mice by calculating charge transfer and mean synaptic current as described previously (Potapenko et al., 2011; Jiang et al., 2013). In liver-related PVN neurons of lean mice charge transfer was 0.23 ± 0.03 pC (range: 0.09–0.47 pC; n = 14). In db/db mice, the charge transfer was 0.19 ± 0.02 pC (range: 0.09–0.33 pC; n = 15), indicating no significant difference (t test, p = 0.34; Fig. 3I). In lean mice, the mean current was 0.31 ± 0.08 pA (range: 0.04–0.96 pA; n = 14), whereas in db/db mice 0.25 ± 0.06 pA (range: 0.02–0.96 pA; n = 15; t test, p = 0.55; Fig. 3J). Ten to 90% rise time and the weighted decay time (τw) of mIPSCs in liver-related PVN neurons did not show significant difference between lean and db/db mice (t test, p = 0.53, p = 0.21; Fig. 3H). These findings did not reveal significant difference in mean inhibitory current in liver-related PVN neurons of db/db mice, suggesting that the total synaptic inhibition was unaltered in db/db mice compared with lean mice.
The membrane capacitance was not significantly different in liver-related PVN neurons of lean and db/db mice. In lean mice, the membrane capacitance was 16.26 ± 1.53 pF (n = 36 cells from 9 mice), whereas 16.77 ± 1.40 pF (n = 42 cells from 10 mice) in db/db mice (Mann–Whitney, p = 0.38). Because the measurement of capacitance is often used to measure the cell-surface area (Taylor, 2012; Duzhyy et al., 2015), our data indicate that liver-related PVN neurons preserved their size in 7- to 10-week-old db/db mice.
Tonic inhibitory currents in liver-related PVN neurons
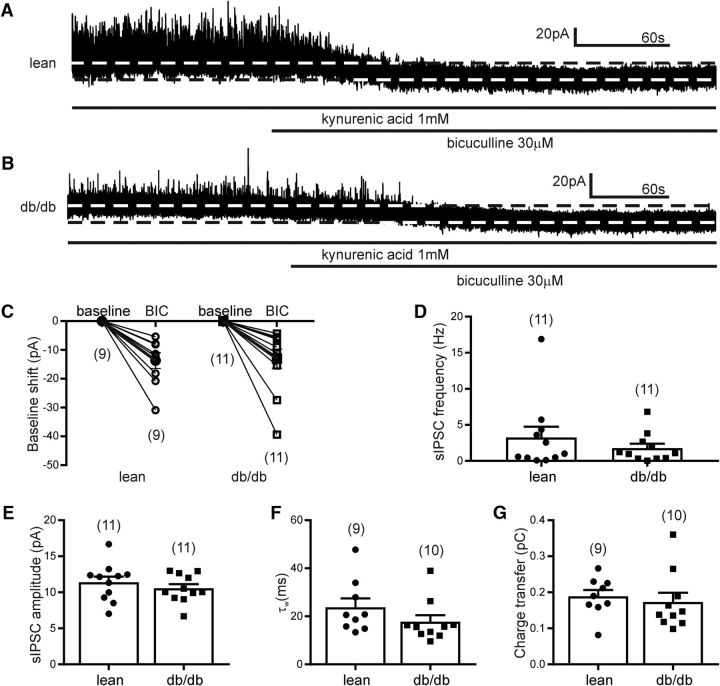
Presympathetic PVN neurons are under tonic inhibition (Park et al., 2007; Jiang et al., 2013); therefore, we determined the existence of tonic inhibitory current in liver-related PVN neurons. Application of bicuculline revealed a tonic inhibitory current with an average amplitude of 13.7 ± 2.7 pA (range: 5.4–30.9 pA) in 9 of 12 recorded liver-related PVN neurons of lean mice (n = 9 cells from 5 mice; Fig. 4). No tonic inhibitory current was revealed in the remaining three liver-related PVN neurons (<3 pA shift in baseline). In db/db mice, administration of bicuculline resulted in a 13.0 ± 3.3 pA shift (range: 4.3–39.4 pA; n = 11 cells from 5 mice; p = 0.88). One additional recorded neuron did not have tonic inhibitory current. The tonic inhibitory current of liver-related PVN neurons was similar in lean, db/db and C57BL/6J mice (13.7 ± 2.7 pA, n = 9; 13.0 ± 3.3 pA, n = 11; or 17.9 ± 4.4 pA, n = 6). These data indicate that the majority of liver-related PVN neurons are under tonic GABAA-dependent inhibition; however, the tonic inhibitory control of liver-related PVN neurons was not altered in db/db mice (Fig. 4C).
Figure 4.
Tonic inhibition of liver-related PVN neurons was not affected in db/db mice. A, B, Representative recordings of sIPSCs in a liver-related PVN neuron from lean (A) and db/db (B) mice. In the presence of kynurenic acid, bicuculline application revealed tonic inhibitory currents indicated by a baseline shift. C, Combined data showing the range in baseline shift in each recorded liver-related PVN neurons in lean and db/db mice. The filled circle (lean) and square (db/db) represent the average baseline shift. D–G, There were no significant difference in frequency (D), amplitude (E), weighted decay time (F), and charge transfer (G) of sIPSCs in liver-related PVN neurons. The numbers of recorded cells are shown in parentheses.
In addition, the frequency and amplitude of spontaneous IPSCs were determined in the presence of kynurenic acid. Our data did not show significant difference neither in frequency (3.3 ± 1.5 Hz, n = 11 vs 1.8 ± 0.6 Hz, n = 11; t test, p = 0.37) nor in amplitude (11.4 ± 0.8 pA, n = 11 vs 10.5 ± 0.6 pA, n = 11; t test, p = 0.40) between the groups. The charge transfer was also not different between groups (0.19 ± 0.02 pC, n = 9 vs 0.17 ± 0.03 pC, n = 10; t test, p = 0.62; Fig. 4D–F). The weighted decay time (τw) in liver-related PVN neurons of lean mice was 23.8 ± 3.7 ms (n = 9) and 17.7 ± 2.7 ms (n = 10) in db/db mice (t test, p = 0.21; Fig. 4F), which indicated no significant difference in τw of sIPSCs between lean and db/db mice in the presence of kynurenic acid.
TRPV1-dependent excitatory neurotransmission in liver-related PVN neurons
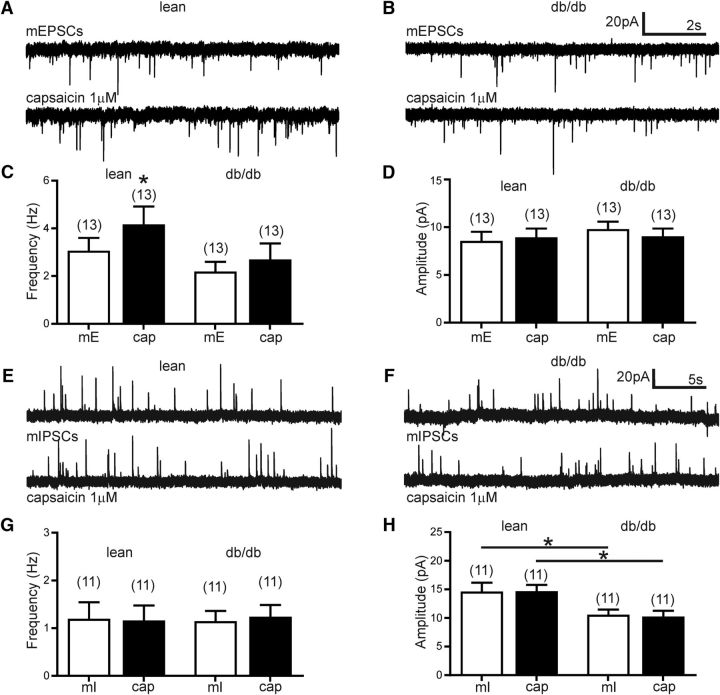
To analyze potential changes in TRPV1-dependent neurotransmission, capsaicin was bath applied while mEPSCs and mIPSCs were recorded. Capsaicin significantly increased the overall mEPSC frequency in liver-related PVN neurons from lean mice (Fig. 5). The average mEPSCs frequency increased from 3.1 ± 0.5 Hz (range: 0.7–7.1 Hz; n = 13 cells from 5 mice) to 4.2 ± 0.7 Hz (range: 1.5–10.8 Hz; n = 13) following capsaicin application (t test, p = 0.01; Fig. 5C). Additional analyzes of individual cells with the Kolmogorov–Smirnov test determined that 4 of 13 cells did not show significant increase in mEPSC frequency, indicating that a subgroup of liver-related neurons is not controlled by TRPV1-dependent excitatory neurotransmitter release, which is consistent with previous findings (Gao et al., 2012). On the other hand, in db/db mice capsaicin did not increase the overall mEPSC frequency in liver-related PVN neurons (Fig. 5B). The average frequency of mEPSCs was 2.2 ± 0.4 Hz (range: 0.3–5.1 Hz; n = 13 cells from 6 mice) before, whereas 2.7 ± 0.67 Hz (range: 0.14–8.3 Hz) after capsaicin application (t test, p = 0.17). The normalized percentage increase of mEPSC frequency in liver-related PVN neurons from lean mice was 42.5 ± 15.2% (n = 13), whereas it was 8.7 ± 10.8% in db/db mice (n = 13), suggesting that the TRPV1-dependent regulation of liver-related PVN neurons is reduced in db/db mice compared with lean mice.
Figure 5.
TRPV1-dependent excitation in liver-related PVN neurons is reduced in db/db mice. A, B, Representative recordings of mEPSCs from liver-related PVN neurons in lean (A) and db/db mice (B). Capsaicin increased mEPSC frequency in liver-related PVN neurons of lean mice (A), whereas the frequency of mEPSCs did not show a significant increase in db/db mice (B). C, Bar graphs demonstrate that TRPV1-dependent control of liver-related PVN neurons is decreased in db/db mice. D, Capsaicin did not alter the amplitude of mEPSCs neither in lean nor in db/db mice. E, F, Representative recordings of mIPSCs from liver-related PVN neurons in lean mice (E) and db/db mice (F). G, H, Combined data illustrate that capsaicin did not modulate the frequency (G) and amplitude (H) of mIPSCs in liver-related PVN neurons of lean and db/db mice. However, the average amplitude of mIPSCs in liver-related PVN neurons of db/db mice was significantly smaller compared with lean mice. The numbers of recorded cells are shown in parentheses. *p < 0.05.
Our data did not show significant difference in the amplitude of mEPSCs. The average amplitude of mEPSCs was 8.5 ± 0.9 pA (range: 5.3–15.5 pA; n = 13) before and 8.9 ± 0.9 pA (range: 5.4–16.6 pA) after capsaicin application in lean mice (t test, p = 0.59). Similarly, capsaicin did not change mEPSC amplitude of liver-related PVN neurons in db/db mice. The average amplitude of mEPSCs was 9.8 ± 0.8 pA (range: 5.6–14.4 pA; n = 13) before and 9.0 ± 0.8 pA (range: 5.0–14.0 pA) after capsaicin application (t test, p = 0.07; Fig. 5D).
In the dorsal motor nucleus of the vagus, capsaicin was reported to increase mIPSC frequency; however, its effect on mIPSC in the PVN is not known. Therefore, we determined the effect of TRPV1 activation on the frequency and amplitude of mIPSCs (Fig. 5E–H). The average mIPSC frequency was not significantly affected by capsaicin application neither in lean nor in db/db mice. In lean mice, the average frequency of mIPSCs was 1.20 ± 0.35 Hz (n = 11) before and 1.16 ± 0.31 Hz after capsaicin application (n = 11; t test, p = 0.63). In liver-related PVN neurons of db/db mice the average frequency of mIPSCs was 1.15 ± 0.21 Hz before and 1.24 ± 0.24 Hz after capsaicin application (n = 11; t test, p = 0.49; (Fig. 5G). Our recordings did not reveal significant difference in the amplitude of mIPSCs after capsaicin application neither in lean nor in db/db mice (14.6 ± 1.53 pA vs 14.7 ± 1.10 pA, t test, p = 0.94 and 10.6 ± 0.67 pA vs 10.3 ± 0.99 pA, t test, p = 0.63; n = 11); however, the average mIPSC amplitude was significantly smaller in db/db mice compared with lean mice as reported above and shown in Figure 3 (Figs. 5H, 3F).
Discussion
This study provides the first evidence of overall activity of liver-related PVN neurons in lean and db/db mice. The following novel findings emerged: (1) the excitability of liver-related PVN neurons was increased in db/db mice; (2) liver-related PVN neurons were more depolarized and had higher input resistance in diabetic condition; (3) the firing activity following current injections was higher in db/db mice; (4) there was no difference in excitatory and inhibitory neurotransmission between groups; however, the amplitude of inhibitory currents was smaller in db/db mice; (5) liver-related PVN neurons received tonic inhibition, although the magnitude of inhibition was not affected by diabetic conditions; and (6) the TRPV1-dependent control of liver-related PVN neurons was reduced in db/db mice. These findings suggest altered autonomic circuitry in db/db mice, which may contribute to increased activity of the SNS leading to elevation of hepatic glucose production.
Preautonomic PVN neurons and liver function
The involvement of PVN in the control of glucose homeostasis is well established (Kalsbeek et al., 2004, 2010; Uyama et al., 2004; Yi et al., 2010). Increasing the activity of PVN neurons either through activation of NMDA receptors or inhibition of GABAA receptors resulted in hyperglycemia, which was absent in rats with sympathectomy, indicating that the hyperglycemia is due to PVN-originated activation of the SNS (Kalsbeek et al., 2004). Similarly, norepinephrine administration into the PVN resulted in hyperglycemia and inhibition of insulin release, an effect which was independent of corticosterone and was blunted by administration of a sympathetic ganglionic blocker (Ionescu et al., 1989). The effects of stimulation of the PVN were similar to the findings of electrical stimulation of the splanchnic nerve (Shimazu and Fukuda, 1965; Kaneto et al., 1975; Hartmann et al., 1982), which further demonstrates that the PVN relays information through the autonomic nervous system to alter liver function. These in vivo studies provide convincing evidence for the importance of the PVN in the control of liver function; however, specific details including the activity of liver-related neurons remained undetermined. Preautonomic PVN neurons regulate the sympathetic and parasympathetic tone through sympathetic neurons in the spinal cord and parasympathetic brainstem neurons, respectively (Rogers and Nelson, 1984; Yamashita et al., 1984; Lawrence and Pittman, 1985). Increased sympathetic activity was shown in the db/db mouse model (Rahmouni et al., 2003), which could contribute to elevated hepatic glucose production reported in db/db mice (Zhang et al., 2013). Because modulation of the synaptic balance and/or altering postsynaptic properties of liver-related PVN neurons is likely one of the underlying mechanisms of increased sympathetic activity, determining the cellular properties of liver-related PVN neurons is crucial to the understanding of the brain-liver circuit. In this study, we established the cellular properties of liver-related PVN neurons and provided evidence for altered brain-liver circuitry at the cellular level.
In control conditions (lean mice) the majority of neurons were silent, whereas in db/db mice the majority of liver-related PVN neurons fired spontaneously. This finding indicates that in db/db mice, liver-related PVN neurons are more active. The observation was consistent with the difference in resting membrane potential and input resistance among the groups. In addition, following depolarizing current injections liver-related PVN neurons of db/db mice had higher firing rate compared with lean silent neurons. Functional changes may originate from neuronal atrophy; however, the size of liver-related PVN neurons was preserved in db/db mice, indicating that the increased excitability is not due to alteration of cell-surface area.
PVN is a heterogeneous nucleus containing different types of neurons; therefore, identification of organ-related neurons is crucial. PRV-152 was used to identify liver-related PVN neurons. The time point used in our recordings results in labeling of liver-related PVN neurons and allows for recordings from identified neurons as shown in previous studies (Gao et al., 2012; Boychuk et al., 2013). PRV-related potential technical considerations have been extensively investigated, and addressed in numerous publications previously (Card, 1998; Smith et al., 2000; Ch'ng et al., 2007). PRV reliable identifies liver-related neurons (Stanley et al., 2010); however, it cannot distinguish between a sympathetic and parasympathetic neuron, which is the caveat of this study. In lean mice, we have observed more silent than spontaneously firing liver-related PVN neurons, whereas more spontaneously active neurons were identified in db/db mice. This observation suggests a shift toward excitation in db/db mice; therefore, we can speculate that the increased activity of liver-related PVN neurons is one of the contributors of elevated sympathetic activity in db/db mice; however, differentiating presympathetic and pre-parasympathetic neurons would be the subject of future studies. We used db/db mice; therefore, we need to keep in mind that these mice have impaired leptin signaling. This model was chosen, because it is a well described model with indication of enhanced gluconeogenesis (Zhang et al., 2013); however, we acknowledge that future studies are needed to dissect the contributing factors including but not limited to glucose, insulin, leptin, and potential developmental compensations.
Phasic and tonic control of liver-related PVN neurons
Increased excitatory neurotransmission and/or decreased inhibitory neurotransmission may lead to increased activity of the neurons. Shifts in excitatory and inhibitory balance in hypothalamic neurons of heart failure rats and streptozotocin-treated hyperglycemic mice have been shown (Han et al., 2010; Potapenko et al., 2011; Jiang et al., 2013), indicating that synaptic plasticity may be a common feature in diseases involving autonomic dysfunction. Furthermore, Kalsbeek et al. (2004) demonstrated that GABAergic inputs from the suprachiasmatic nuclei to preautonomic PVN neurons play a role in the control of glucose levels through sympathetic regulation of the liver. Therefore, we investigated the excitatory and inhibitory PSCs to liver-related PVN neurons. We did not find significant difference in frequency and amplitude of mEPSCs. The frequency of mIPSCs was also unaltered, but the amplitude of mIPSCs was significantly smaller in db/db mice. This could indicate decreased neurotransmitter release or modulation of postsynaptic receptors. Our analysis of total inhibitory phasic current did not reveal significant difference between the groups, which suggests that synaptic inputs are likely not responsible for the difference in spontaneous firing activity in liver-related PVN neurons of db/db mice. Indeed, the more depolarized resting membrane potential together with increased input resistance suggests that the increased firing of liver-related PVN neurons in db/db mice is likely due to postsynaptic changes. These changes might be mediated by alteration in potassium or chloride conductance; however, to delineate the potential underlying mechanisms requires further studies.
In addition to fast synaptic control, presympathetic PVN neurons are under tonic inhibition (Park et al., 2007; Jiang et al., 2013). The role of tonic current is still under investigation; however, it likely plays an important role in synaptic integration by altering membrane conductance and neuronal excitability (Nusser and Mody, 2002; Semyanov et al., 2004). Our data revealed the existence of tonic inhibition in the majority of liver-related PVN neurons; however, we did not find significant difference in the magnitude of tonic inhibitory currents between lean and db/db mice. In contrast, reduced tonic inhibitory currents were identified in kidney-related PVN neurons of streptozotocin-treated mice (Jiang et al., 2013). The difference may suggest differential control of PVN neurons [e.g., organ-related manner (kidney vs liver) or autonomic branch-related (sympathetic vs parasympathetic)]. In addition, the different mouse model of diabetes (streptozotocin vs db/db) may influence the regulation of liver-related PVN neurons.
TRPV1-dependent control of liver-related PVN neurons
TRPV1, a nonselective cation channel is associated with a variety of physiological functions and linked to the development and progression of diabetes mellitus (Razavi et al., 2006; Suri and Szallasi, 2008; Zsombok, 2013; Zsombok and Derbenev, 2016). Activation of TRPV1 increases neurotransmission in autonomic brain areas and functional plasticity of TRPV1-dependent neurotransmitter release was observed in streptozotocin-treated hyperglycemic mice (Zsombok et al., 2011; Boychuk et al., 2013). Our previous study demonstrated that capsaicin increases mEPSC frequency in liver-related PVN neurons and this TRPV1-dependent regulation was diminished in the type 1 diabetic condition (Gao et al., 2012). Our current findings further demonstrate that activation of TRPV1 increases excitatory neurotransmission to liver-related PVN neurons in lean mice, whereas this effect is reduced in db/db mice. Interestingly, our studies also indicate that TRPV1 does not play a role in controlling inhibitory neurotransmission to liver-related PVN neurons. This indicates a difference between neurons in the PVN and in the dorsal motor nucleus of the vagus (Derbenev et al., 2006), and could suggest area (e.g., hypothalamus vs brainstem) or species-related (mouse vs rat) differences. Nevertheless, these data suggest that TRPV1 plays role in the control of excitatory, but not inhibitory mechanisms at the level of PVN.
In summary, our study fills an existing gap by establishing the cellular properties of liver-related PVN neurons and demonstrates that liver-related PVN neurons are more active in db/db mice. We found that liver-related neurons in the PVN are controlled by phasic and tonic GABAergic inhibition and the TRPV1-dependent excitatory neurotransmission is reduced in db/db mice. These data demonstrate plasticity of the brain-liver pathway and further support the hypothesis that disruption of the autonomic circuitry in the PVN is a crucial factor in the development of autonomic imbalance, which contributes to increased glucose production.
Footnotes
This work was supported by research Grants from the National Institutes of Health (DK099598 to A.Z.), and core Grant support (NIH CoBRE in Hypertension, P30GM103337). The viral tracer was obtained from the Center for Neuroanatomy with Neurotropic Viruses P40 OD010996).
The authors declare no competing financial interests.
References
- Boychuk CR, Zsombok A, Tasker JG, Smith BN (2013) Rapid glucocorticoid-induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric-related hypothalamic preautonomic neurons. Front Neurosci 7:3. 10.3389/fnins.2013.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF (2004) Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol 471:462–481. 10.1002/cne.20040 [DOI] [PubMed] [Google Scholar]
- Card JP. (1998) Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev 22:685–694. 10.1016/S0149-7634(98)00007-4 [DOI] [PubMed] [Google Scholar]
- Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D (2003) Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the atherosclerosis risk in communities study, 1987–1998. Circulation 107:2190–2195. 10.1161/01.CIR.0000066324.74807.95 [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Spear PG, Struyf F, Enquist LW (2007) Glycoprotein D-independent spread of pseudorabies virus infection in cultured peripheral nervous system neurons in a compartmented system. J Virol 81:10742–10757. 10.1128/JVI.00981-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev AV, Zsombok A (2016) Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol 38:397–406. 10.1007/s00281-015-0529-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbenev AV, Monroe MJ, Glatzer NR, Smith BN (2006) Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci 26:9666–9672. 10.1523/JNEUROSCI.1591-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzhyy DE, Viatchenko-Karpinski VY, Khomula EV, Voitenko NV, Belan PV (2015) Upregulation of T-type Ca2+ channels in long-term diabetes determines increased excitability of a specific type of capsaicin-insensitive DRG neurons. Mol Pain 11:29. 10.1186/s12990-015-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A (2012) Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 61:1381–1390. 10.2337/db11-0820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TH, Lee K, Park JB, Ahn D, Park JH, Kim DY, Stern JE, Lee SY, Ryu PD (2010) Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol 299:R129–R139. 10.1152/ajpregu.00391.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann H, Beckh K, Jungermann K (1982) Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem 123:521–526. 10.1111/j.1432-1033.1982.tb06562.x [DOI] [PubMed] [Google Scholar]
- Ionescu E, Coimbra CC, Walker CD, Jeanrenaud B (1989) Paraventricular nucleus modulation of glycemia and insulinemia in freely moving lean rats. Am J Physiol 257:R1370–R1376. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Gao H, Krantz AM, Derbenev AV, Zsombok A (2013) Reduced GABAergic inhibition of kidney-related PVN neurons in streptozotocin-treated type 1 diabetic mouse. J Neurophysiol 110:2192–2202. 10.1152/jn.00013.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM (2004) Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24:7604–7613. 10.1523/JNEUROSCI.5328-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E (2010) Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci 1212:114–129. 10.1111/j.1749-6632.2010.05800.x [DOI] [PubMed] [Google Scholar]
- Kaneto A, Kajinuma H, Kosaka K (1975) Effect of splanchnic nerve stimulation on glucagon and insulin output in the dog. Endocrinology 96:143–150. 10.1210/endo-96-1-143 [DOI] [PubMed] [Google Scholar]
- Lawrence D, Pittman QJ (1985) Interaction between descending paraventricular neurons and vagal motor neurons. Brain Res 332:158–160. 10.1016/0006-8993(85)90399-3 [DOI] [PubMed] [Google Scholar]
- Luther JA, Tasker JG (2000) Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523:193–209. 10.1111/j.1469-7793.2000.t01-1-00193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG (2002) Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol 14:929–932. 10.1046/j.1365-2826.2002.00867.x [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87:2624–2628. [DOI] [PubMed] [Google Scholar]
- O'Hare JD, Zsombok A (2016) Brain-liver connections: role of the preautonomic PVN neurons. Am J Physiol Endocrinol Metab 310:E183–189. 10.1152/ajpendo.00302.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE (2006) Characterization of a novel tonic gamma-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology 147:3746–3760. 10.1210/en.2006-0218 [DOI] [PubMed] [Google Scholar]
- Park JB, Skalska S, Son S, Stern JE (2007) Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J Physiol 582:539–551. 10.1113/jphysiol.2007.133223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L (2005) A brain–liver circuit regulates glucose homeostasis. Cell Metab 1:53–61. 10.1016/j.cmet.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE (2011) Inhibitory–excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol 106:1545–1557. 10.1152/jn.00218.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL (2003) Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23:5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM (2006) TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127:1123–1135. 10.1016/j.cell.2006.10.038 [DOI] [PubMed] [Google Scholar]
- Rogers RC, Nelson DO (1984) Neurons of the vagal division of the solitary nucleus activated by the paraventricular nucleus of the hypothalamus. J Auton Nerv Syst 10:193–197. 10.1016/0165-1838(84)90057-2 [DOI] [PubMed] [Google Scholar]
- Ruud J, Steculorum SM, Brüning JC (2017) Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun 8:15259. 10.1038/ncomms15259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Obici S, Seeley RJ (2009) Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov 8:386–398. 10.1038/nrd2874 [DOI] [PubMed] [Google Scholar]
- Schlaich M, Straznicky N, Lambert E, Lambert G (2015) Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol 3:148–157. 10.1016/S2213-8587(14)70033-6 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Tschöp MH, Woods SC, Morton GJ, Myers MG, D'Alessio D (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503:59–66. 10.1038/nature12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci 27:262–269. 10.1016/j.tins.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Seoane-Collazo P, Fernø J, Gonzalez F, Diéguez C, Leis R, Nogueiras R, López M (2015) Hypothalamic-autonomic control of energy homeostasis. Endocrine 50:276–291. 10.1007/s12020-015-0658-y [DOI] [PubMed] [Google Scholar]
- Shimazu T. (1967) Glycogen synthetase activity in liver: regulation by the autonomic nerves. Science 156:1256–1257. 10.1126/science.156.3779.1256 [DOI] [PubMed] [Google Scholar]
- Shimazu T. (1996) Innervation of the liver and glucoregulation: roles of the hypothalamus and autonomic nerves. Nutrition 12:65–66. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Fukuda A (1965) Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science 150:1607–1608. 10.1126/science.150.3703.1607 [DOI] [PubMed] [Google Scholar]
- Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE (2000) Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A 97:9264–9269. 10.1073/pnas.97.16.9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S, Pinto S, Segal J, Pérez CA, Viale A, DeFalco J, Cai X, Heisler LK, Friedman JM (2010) Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc Natl Acad Sci U S A 107:7024–7029. 10.1073/pnas.1002790107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE. (2001) Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537:161–177. 10.1111/j.1469-7793.2001.0161k.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri A, Szallasi A (2008) The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci 29:29–36. 10.1016/j.tips.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Taylor AL. (2012) What we talk about when we talk about capacitance measured with the voltage-clamp step method. J Comput Neurosci 32:167–175. 10.1007/s10827-011-0346-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp AA, Schlaich MP (2015) Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015:341583. 10.1155/2015/341583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama N, Geerts A, Reynaert H (2004) Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280:808–820. 10.1002/ar.a.20086 [DOI] [PubMed] [Google Scholar]
- Yamashita H, Inenaga K, Koizumi K (1984) Possible projections from regions of paraventricular and supraoptic nuclei to the spinal cord: electrophysiological studies. Brain Res 296:373–378. 10.1016/0006-8993(84)90077-5 [DOI] [PubMed] [Google Scholar]
- Yi CX, la Fleur SE, Fliers E, Kalsbeek A (2010) The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim Biophys Acta 1802:416–431. 10.1016/j.bbadis.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Zhang F, Xu X, Zhang Y, Zhou B, He Z, Zhai Q (2013) Gene expression profile analysis of type 2 diabetic mouse liver. PloS One 8:e57766. 10.1371/journal.pone.0057766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A. (2013) Vanilloid receptors: do they have a role in whole body metabolism? Evidence from TRPV1. J Diabetes Complications 27:287–292. 10.1016/j.jdiacomp.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Derbenev AV (2016) TRP channels as therapeutic targets in diabetes and obesity. Pharmaceuticals 9:50. 10.3390/ph9030050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN (2011) Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31:14024–14031. 10.1523/JNEUROSCI.2081-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]