Abstract
The effect that cardiorespiratory fitness has on the dynamic cerebral autoregulatory capacity during changes in mean arterial pressure (MAP) remains equivocal. Using a multiple‐metrics approach, challenging MAP across the spectrum of physiological extremes (i.e., spontaneous through forced MAP oscillations), we characterized dynamic cerebral autoregulatory capacity in 19 male endurance athletes and eight controls via three methods: (1) onset of regulation (i.e., time delay before an increase in middle cerebral artery (MCA) conductance [MCA blood velocity (MCAv)/MAP] and rate of regulation, after transient hypotension induced by sit‐to‐stand, and transfer function analysis (TFA) of MAP and MCAv responses during (2) spontaneous and (3) forced oscillations (5‐min of squat‐stand maneuvers performed at 0.05 and 0.10 Hz). Reductions in MAP and mean MCAv (MCAV mean) during initial orthostatic stress (0‐30 sec after sit‐to‐stand) and the prevalence of orthostatic hypotension were also determined. Onset of regulation was delayed after sit‐to‐stand in athletes (3.1 ± 1.7 vs. 1.5 ± 1.0 sec; P = 0.03), but rate of regulation was not different between groups (0.24 ± 0.05 vs. 0.21 ± 0.09 sec−1; P = 0.82). While both groups had comparable TFA metrics during spontaneous oscillations, athletes had higher TFA gain during 0.10 Hz squat‐stand versus recreational controls (P = 0.01). Reductions in MAP (P = 0.15) and MCAV mean (P = 0.11) during orthostatic stress and the prevalence of initial orthostatic hypotension (P = 0.65) were comparable between groups. These results indicate an intact ability of the cerebral vasculature to react to spontaneous oscillations but an attenuated capability to counter rapid and large changes in MAP in individuals with elevated cardiorespiratory fitness.
Keywords: Cardiorespiratory fitness, cerebral pressure‐flow relationship, dynamic cerebral autoregulation
Introduction
Regular aerobic endurance exercise has been associated with higher resting cerebral blood velocity in men (Ainslie et al. 2008), while higher cardiorespiratory fitness (CRF) has been shown to improve cerebral blood velocity during exercise (Brugniaux et al. 2014), as well as cerebrovascular reactivity to carbon dioxide (Bailey et al. 2013b; Barnes et al. 2013). However, whether CRF positively influences the ability of the cerebrovasculature to respond to rapid changes in mean arterial pressure (MAP) (traditionally referred to as dynamic cerebral autoregulation) remains equivocal. A weakened dynamic cerebral autoregulatory capacity, characterized by a delayed onset of regulation following transient hypotension induced by thigh‐cuff deflation and larger normalized transfer function gain of spontaneous oscillations in MAP and blood velocity in the middle cerebral artery (MCAv), has been reported in endurance athletes compared to sedentary controls (Lind‐Holst et al. 2011). Conversely, comparable dynamic cerebral autoregulatory capacity between endurance athletes and sedentary controls has also been reported following thigh‐cuff deflation (RoR, rate of regulation) and via transfer function analysis (TFA; coherence – fraction of the MAP which is linearly related to MCAv, gain – amplitude of MCAv change for a given oscillation in MAP, and phase – difference in the timing of the MAP and MCAv waveforms) of spontaneous or forced oscillations in MAP and MCAv in young and elderly athletes (Aengevaeren et al. 2013; Ichikawa et al. 2013). Taking into consideration the notion that most metrics are unrelated to each other (Tzeng et al. 2012), direct comparison between studies using different analysis techniques may lead to inconsistent physiological information (Tzeng et al. 2012; Tzeng and Ainslie 2014). Additionally, maneuvers such as the sit‐to‐stand may provide more realistic data closer to daily life in comparison with data gathered with the thigh‐cuff deflation technique. To the best of our knowledge, there is no published report, which examined the dynamic cerebral autoregulatory capacity in endurance athletes using the sit‐to‐stand maneuver.
It is now recognized that spontaneous TFA has poor signal‐to‐noise ratio and poor reproducibility compared to TFA performed on forced oscillations (e.g., induced by repeated squat‐stand maneuvers) (Smirl et al. 2015). Of note, the thigh‐cuff deflation technique for quantifying the cerebral autoregulatory index has even worse reproducibility than spontaneous TFA metrics [reviewed in Smirl et al. (2015)]. The above‐mentioned issues could explain, at least partly, why findings related to the impact of CRF on dynamic cerebral autoregulatory capacity currently in the literature are equivocal (Lind‐Holst et al. 2011; Aengevaeren et al. 2013; Ichikawa et al. 2013). Accordingly, utilizing a multiple assessment approach to study dynamic cerebral autoregulatory capacity within the same individuals is required to improve physiological interpretation. Importantly, this approach should include forced MAP oscillations induced by squat‐stand maneuvers to enhance the interpretation of the linear association between arterial blood pressure and cerebral blood velocity (Smirl et al. 2015). Importantly, it is possible that the small magnitude and inconsistency of spontaneous MAP oscillations associated with the TFA approach fail to detect subtle abnormalities in dynamic cerebral autoregulatory capacity (Bailey et al. 2013a). These modifications may only become obvious when MAP oscillations are forced during sit‐to‐stand or repeated squat‐stand maneuvers.
With the understanding that each dynamic cerebral autoregulatory capacity assessment technique most likely reflects somewhat different components of the cerebral‐pressure flow relationship (Tzeng et al. 2012), the aims of this study were to address whether CRF affects different metrics using a multiple assessment strategy and hemodynamic stressors (sit‐to‐stand, TFA on spontaneous and forced MAP and MCAv oscillations). We addressed these aims via a comparative approach between endurance athletes and recreationally active controls and with varying CRF using a correlational approach. In order to examine if the change in dynamic cerebral autoregulatory capacity with CRF represents an adaptive or maladaptive consequence of the trained state, we assessed whether dynamic cerebral autoregulatory capacity was related to orthostatic tolerance in our participants. We hypothesized higher CRF would be associated with a delayed onset of regulation following sit‐to‐stand but comparable TFA metrics between spontaneous/driven MAP and MCAv oscillations. Finally, the delayed onset of regulation observed with higher CRF would not be associated with reduced orthostatic tolerance.
Materials and Methods
Participants
Nineteen endurance‐trained male athletes (V̇O2max: 55.9 ± 4.9 mL·kg·min−1, training history of 5–12 h·week−1 for at least 2 years) and eight inactive/moderately active control participants (V̇O2max: 39.2 ± 5.0 mL·kg·min−1; P < 0.001 vs. athletes, training history <3 h·week−1) (Table 1) volunteered to participate in this study. All participants provided written informed consent, and the study was approved by the Comité d’éthique de la recherche de l'IUCPQ‐Université Laval (CER:20869) according to the principles established by the Declaration of Helsinki. Endurance athletes were trained and competed in a variety of endurance sports, including cycling (n = 9), triathlon (n = 7), mountain biking (n = 2), and cross‐country skiing (n = 1). All participants were free from any medical conditions, demonstrated a normal ECG, and were not taking any medication. This study was part of a larger study characterizing cardiovascular and cerebrovascular function in endurance athletes, and examining high‐intensity interval training effects on performance and physiological function.
Table 1.
Baseline characteristics and resting values between athletes and controls
| Athletes | Controls | P‐values | |
|---|---|---|---|
| Baseline characteristics | |||
| n | 19 | 8 | |
| Age (year) | 26 ± 5 | 31 ± 4 | 0.07 |
| Height (m) | 1.77 ± 0.08 | 1.80 ± 0.10 | 0.47 |
| Body mass (kg) | 72.6 ± 9.5 | 84.1 ± 12.2 | 0.02 |
| Body mass index (kg/m2) | 23 ± 2 | 26 ± 3 | 0.01 |
| Maximal oxygen uptake (mL/kg·min−1) | 55.9 ± 4.9 | 39.2 ± 5.0 | <0.001 |
| Resting values | |||
| n | 19 | 8 | |
| Heart rate (bpm) | 54 ± 8 | 58 ± 5 | 0.20 |
| Mean arterial pressure (mmHg) | 73 ± 10 | 74 ± 10 | 0.92 |
| Middle cerebral artery mean blood velocity (cm/sec) | 63.9 ± 11.7 | 65.5 ± 14.8 | 0.75 |
| Cerebrovascular conductance index (cm sec−1 mmHg−1) | 0.88 ± 0.14 | 0.91 ± 0.23 | 0.72 |
Values are mean ± SD.
Experimental design
Participants reported to the laboratory on two separate occasions to perform: (1) an incremental cycling test to determine V̇O2max and (2) anthropometric and resting measurements as well as an evaluation of cerebrovascular function and orthostatic tolerance (Fig. 1). Participants were asked to refrain from exercise training for at least 12 h and to avoid alcohol and caffeine consumption for 24 h before each visit. All sessions were performed in the same order for all participants and there was at least 24 h between testing sessions.
Figure 1.
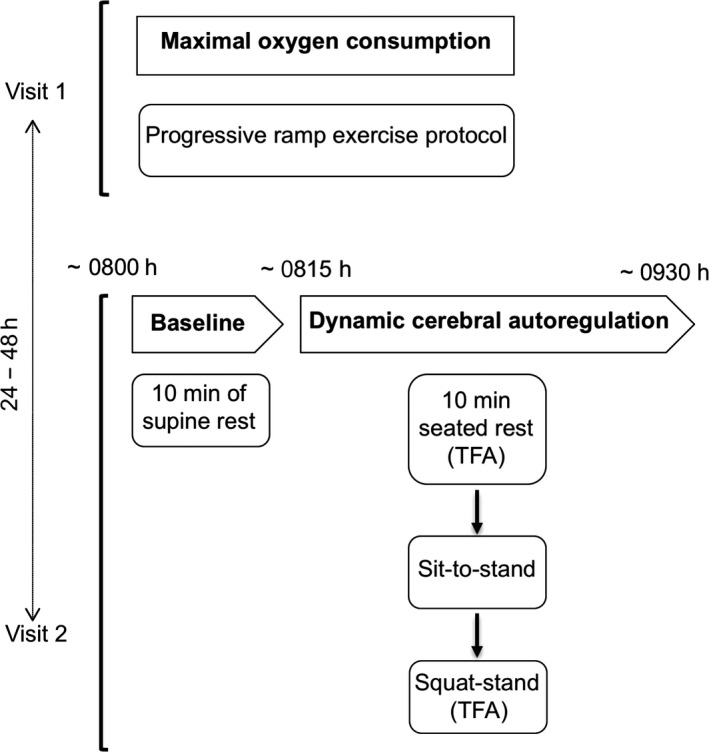
Experimental protocol.
Measurements
Heart rate and blood pressure
Heart rate was measured using a three‐lead electrocardiograph (ECG). Beat‐to‐beat blood pressure (BP) was measured by the volume clamp method using a finger cuff (Nexfin, Edwards Lifesciences, Ontario, Canada). The cuff was placed on the right middle finger and referenced to the level of the heart using a height correct unit for BP correction. MAP was obtained by integration of the pressure curve divided by the duration of the cardiac cycle. This method has been shown to reliably measure the dynamic changes in beat‐to‐beat BP which correlate well with the intraarterial BP recordings and can be used to describe the dynamic relationship between BP and cerebral blood velocity (Omboni et al. 1993; Sammons et al. 2007).
Cerebral blood flow
Cerebral blood flow was estimated by monitoring MCAv via a 2‐MHz pulsed transcranial Doppler ultrasound (Doppler Box; Compumedics DWL, Inc. San Juan Capistrano, CA). Identification and location of the left MCA was determined using standardized procedures (Willie et al. 2011). The probe was attached to a headset and secured with a custom‐made headband and adhesive conductive ultrasonic gel (Tensive, Parker Laboratory, Fairfield, NY) to ensure a stable position and angle of the probe throughout testing.
End‐tidal partial pressure of carbon dioxide
End‐tidal partial pressure of carbon dioxide (PetCO2) was measured during squat‐stand maneuvers through a breath‐by‐breath gas analyzer (Breezesuite, MedGraphics Corp., MN) calibrated following manufacturer instructions before each evaluation.
Data acquisition
For each evaluation, signals (except PetCO2) were analog‐to‐digital‐converted at 1000 Hz via an analog‐to‐digital converter (Powerlab 16/30 ML880; ADInstruments, Colorado Springs, CO) and stored for subsequent analysis using commercially available software (LabChart version 7.1; ADInstruments). PetCO2 was acquired separately with Breeze Suite (MedGraphics Corp., MN), and time‐aligned with the subsequent data.
Maximal oxygen consumption (V̇O2max)
V̇O2max was determined during a progressive ramp exercise protocol performed on an electromagnetically braked upright cycle ergometer (Corival, Lode, the Netherlands). The test started with 1 min of unloaded pedaling followed by an incremental ramp protocol (25 or 30 W/min) to volitional exhaustion. Expired air was continuously recorded using a breath‐by‐breath gas analyzer (Breezesuite, MedGraphics Corp., MN) for determination of V̇O2, carbon dioxide production (V̇CO2), and respiratory exchange ratio (RER: V̇CO2/V̇O2). Maximal V̇O2 was defined as the highest 30‐sec averaged V̇O2, concurrent with a RER ≥ 1.15. For more detail and interpretation of these results on gas exchange analysis during this maximal cycling protocol, refer to Paquette et al. (2017).
Anthropometric measurements and resting hemodynamics
Stature and body mass were measured in each participant. Resting hemodynamic measurements included MAP (volume clamp method using a finger cuff), which has been validated against intraarterial pressure (Martina et al. 2012), heart rate (HR; ECG), and mean MCAv (MCAvmean) (transcranial Doppler ultrasound), which were continuously monitored on a beat‐by‐beat basis during 10 min of supine rest. Cerebrovascular conductance index (CVCi; MCAvmean /MAP) was then calculated. The average values of the last 5 min of recording represented the baseline.
Integrative assessment of cerebrovascular function and orthostatic tolerance
To assess dynamic cerebral autoregulatory capacity to transient changes in MAP across the spectrum of physiological extremes (spontaneous to forced MAP oscillations), and given that there is no gold standard methodology for dynamic cerebral autoregulatory capacity quantification (Tzeng et al. 2012), we employed a multiple metric approach as outlined below in the chronological order that they were completed (Fig. 1).
Spontaneous oscillations in MAP and MCAv
Participants rested in a seated position for 10 min. All systemic and cerebral hemodynamic variables were continuously monitored, and the last 5 min of the data from the quiet rest period were used to characterize the dynamic relationship between MAP and MCAv via TFA under spontaneous conditions (see the “Data analysis and statistical approach” section).
Sit‐to‐stand
Subsequent to the 10 min of seated quiet rest, participants quickly (0–3 sec) stood up and maintained this standing position for 5 min without moving. MAP and MCAvmean were continuously monitored during this evaluation of the sit‐to‐stand response. In addition to the characterization of the dynamic cerebral autoregulatory capacity (see the “Data analysis and statistical approach” section), this technique permitted us to examine the orthostatic tolerance of the participants. A participant was considered intolerant when criteria for initial orthostatic hypotension were reached. Initial orthostatic hypotension was defined as a decrease in systolic blood pressure ≥ 40 mmHg and/or a decrease in diastolic blood pressure ≥20 mmHg during the first 15 sec of standing (Wieling et al. 2007). At the end of the standing position, participants were asked to indicate if they experienced any orthostatic symptoms (dizziness, blurry vision, weakness, etc.) after standing. Furthermore, the sit‐to‐stand technique was used to focus on two principal phases of the orthostatic response: (1) the initial response (0–30 sec) and (2) the steady‐state response (120–180 sec) (Wieling and van Lieshout 1997). The first phase permitted us to analyze the immediate response following sit‐to‐stand, while the second phase was used to evaluate the recovery of hemodynamic variables.
Repeated squat‐stand maneuvers
Following 10 min of quiet rest, squat‐stand maneuvers were performed after confirmation of the recovery of baseline hemodynamics following sit‐to‐stand. Participants started in a standing position and then squatted down until the back of their legs attained a ~90 degree angle. This squat position was maintained for a specific period of time (5 or 10 sec–see below), after which they moved to the standing position. Following instructions and practice, participants performed 5‐min periods of repeated squat‐stand maneuvers at a frequency of 0.05 Hz (10‐sec squat, 10‐sec standing) and 0.10 Hz (5‐sec squat, 5‐sec standing) as previously described (Smirl et al. 2015) with 5 min of standing rest between 0.05 and 0.10 Hz maneuvers. These large oscillations in MAP (~25–30 mmHg) are extensively buffered by the cerebrovasculature when executed at frequencies within the high‐pass filter buffering range (<0.20 Hz). The repeated squat‐stand maneuvers augment the signal‐to‐noise ratio enhancing the reproducibility and interpretability of findings through a physiologically relevant MAP stimulus to the cerebrovasculature (Smirl et al. 2015). The sequence of the squat‐stand maneuvers was randomized between participants. During these maneuvers, participants were instructed to maintain normal breathing and to avoid Valsalva. The linear aspect of the dynamic MAP‐MCAv relationship was characterized via TFA (see the “Data analysis and statistical approach” section). MAP, HR, MCAvmean, and PetCO2 were continuously monitored during this evaluation. PetCO2 data were averaged over each 5‐min squat‐stand maneuvers.
Data analysis and statistical approach
Cerebrovascular responses to acute hypotension
In order to describe the cerebrovascular responses to acute hypotension following the sit‐to‐stand technique, we used the following metrics: (1) the time delay before onset of regulation, (2) the change in MCAvmean and MAP from baseline to nadir (absolute: ∆MCAvmean, ∆MAP; and relative to baseline: ∆ MCAvmean (%), ∆MAP(%)), and (3) the rate of regulation (RoR).
Time delay before onset of regulation is the time lapse between sit‐to‐stand and the increase in CVCi (Lind‐Holst et al. 2011). The onset of regulation was considered to have occurred the moment CVCi began increasing constantly without any subsequent transient decrease. This metric was evaluated by two different observers (LL and PB). The fall in MCAvmean and MAP is the difference between baseline MCAvmean or MAP and minimum MCAvmean or MAP recorded after the sit‐to‐stand. The physiological response to acute hypotension can be divided into two phases (Ogoh et al. 2008); Phase I is the time point after sit‐to‐stand where MCAvmean changes are independent of any arterial baroreflex correction (1–7 sec after sit‐to‐stand (van Beek et al. 2008; Deegan et al. 2009; Sorond et al. 2009; Tzeng et al. 2014)). Phase II is the time point starting at the onset of arterial baroreflex and continuing for 4 sec (Ogoh et al. 2008). Thus, during Phase I, the rate of change in CVCi is directly related to dynamic cerebral autoregulatory capacity, without arterial baroreflex regulation. Accordingly, RoR was calculated during Phase I as an index of dynamic cerebral autoregulatory capacity:
where ∆CVCi/∆t is the linear regression slope between CVCi and time (t) during Phase I (a 2.5‐sec interval (∆t) starting after individually determined onset of regulation was taken for the analysis of RoR), and ∆MAP is calculated by subtracting baseline MAP from averaged MAP during Phase I (Aaslid et al. 1989; Ogoh et al. 2008).
Assessment of the dynamic relationship between MAP and MCAv
Beat‐to‐beat MAP and MCAv signals were spline interpolated and resampled at 4 Hz for spectral analysis and TFA based on the Welch algorithm. Each 5‐min recording was first subdivided into five successive windows that overlapped by 50%. Data within each window were linearly detrended and passed through a Hanning window prior to discrete Fourier transform analysis. For TFA, the cross‐spectrum between MAP and MCAv was determined and divided by the MAP auto‐spectrum to derive the transfer function coherence, absolute gain (cm sec−1 mmHg−1), normalized gain (%/%), and phase (radians) in accordance with the recommendations of the Cerebral Autoregulation Research Network (CARNet)(Claassen et al. 2016).
The TFA coherence, gain, and phase of the spontaneous oscillations were band averaged across the very low‐ (0.02–0.07 Hz), low‐ (0.07–0.2 Hz), and high‐frequency (0.2–0.4 Hz) ranges, driven MAP oscillations were sampled at the point estimate of the driven frequency (0.05 and 0.10 Hz). These point estimates were selected as they are in the very low (0.02–0.07 Hz) and low (0.07–0.20 Hz) frequency ranges where cerebral autoregulation is thought to be most operant (Zhang et al. 1998). Only the TFA phase and gain values where coherence exceeded 0.50 were included in the analysis to ensure the measures were robust for subsequent analysis (Smirl et al. 2014). Phase wrap‐around was neither present when coherence exceeded 0.50 in the spontaneous data nor at any of the point estimate values for squat‐stand maneuvers.
Statistical analysis
After confirming normal distribution of data using Shapiro–Wilk normality tests, between‐group differences were analyzed using independent samples t‐tests, with Welch's correction in the presence of unequal variances between groups. Difference in the prevalence of initial orthostatic hypotension between groups was analyzed using the Fisher's exact test. Relationships between variables were determined using Pearson product‐moment or Spearman's Rho correlations. Statistical significance was established at P < 0.05 and data are expressed as mean ± SD.
Results
Participant characteristics and baseline systemic and cerebrovascular hemodynamics
Body mass (P = 0.02) and body mass index (P = 0.01) were lower in athletes as anticipated. Baseline systemic and cerebrovascular hemodynamics monitored during supine rest were similar between athletes and controls (Table 1). As expected, V̇O2max was higher in athletes (+17 mL·kg·min−1; P < 0.001) compared to the control group.
Effects of cardiorespiratory fitness on dynamic cerebral autoregulatory capacity
TFA of spontaneous oscillations in MAP and MCAv
MAP and MCAv power spectrum densities during spontaneous oscillations were not different between athletes and controls (Table 2). Athletes and controls also had comparable TFA coherence, gain and normalized gain, and phase (all P > 0.11).
Table 2.
Transfer function analysis of spontaneous oscillations in mean arterial pressure and middle cerebral artery blood velocity
| Athletes | Controls | P‐values | |
|---|---|---|---|
| Very low frequency (0.02–0.07 Hz) | |||
| n | 15 | 8 | |
| Mean arterial pressure power (mmHg2) | 10.811 ± 4.362 | 13.546 ± 13.630 | 0.48 |
| Middle cerebral artery blood velocity power (cm/sec2) | 8.992 ± 4.700 | 11.823 ± 12.070 | 0.43 |
| Coherence | 0.489 ± 0.202 | 0.475 ± 0.228 | 0.88 |
| Gain (cm sec−1 mmHg−1) | 0.765 ± 0335a | 0.798 ± 0.263b | 0.82 |
| Normalized gain (%/%) | 1.267 ± 0.417a | 1.263 ± 0.532b | 0.99 |
| Phase (radians) | 0.630 ± 0.539a | 0.674 ± 0.180b | 0.84 |
| Low frequency (0.07–0.20 Hz) | |||
| n | 15 | 8 | |
| Mean arterial pressure power (mmHg2) | 5.394 ± 4.171 | 7.648 ± 5.796 | 0.29 |
| Middle cerebral artery blood velocity power (cm/sec2) | 5.511 ± 4.759 | 4.972 ± 3.057 | 0.78 |
| Coherence | 0.652 ± 0.143 | 0.677 ± 0.152 | 0.70 |
| Gain (cm sec−1 mmHg−1) | 0.925 ± 0.191 | 0.802 ± 0.296 | 0.24 |
| Normalized gain (%/%) | 1.560 ± 0.291 | 1.315 ± 0.412 | 0.11 |
| Phase (radians) | 0.529 ± 0.172 | 0.460 ± 0.200 | 0.40 |
| High frequency (0.20–0.40 Hz) | |||
| n | 15 | 8 | |
| Mean arterial pressure power (mmHg2) | 1.076 ± 0.891 | 1.100 ± 0.584 | 0.96 |
| Middle cerebral artery blood velocity power (cm/sec2) | 1.345 ± 1.177 | 0.843 ± 0.297 | 0.25 |
| Coherence | 0.644 ± 0.176 | 0.620 ± 0.277 | 0.80 |
| Gain (cm sec−1 mmHg−1) | 1.00 ± 0.208 | 0.882 ± 0.258b | 0.26 |
| Normalized gain (%/%) | 1.701 ± 0.414 | 1.498 ± 0.389b | 0.29 |
| Phase (radians) | 0.055 ± 0.211 | −0.017 ± 0.204b | 0.46 |
Values are mean ± SD.
n = 14.
n= 7.
Responses to acute hypotension induced by sit‐to‐stand
MAP (95 ± 10 vs. 97 ± 13 mmHg; P = 0.69) and MCAvmean (61 ± 9 vs. 60 ± 13 cm·s−1; P = 0.83) were not different between athletes and controls at baseline. Upon standing, decreases in MAP and MCAvmean to their nadir were of similar amplitude in athletes compared to controls (Table 3). There were no group differences in the time taken to reach the nadir for MAP (13 ± 8 vs. 14 ± 9 sec; P = 0.67) and MCAvmean (7 ± 2 vs. 8 ± 1 sec; P = 0.54). The time delay before onset of regulation was more than twofold longer in athletes (P = 0.03; Table 3 and Fig. 2). This metric was inversely correlated with TFA phase during 0.05 Hz (r = −0.499; P = 0.02) and 0.1 Hz (r = −0.429; P = 0.046) squat‐stand, and positively correlated with TFA gain (r = 0.501; P = 0.02) and normalized gain (0.462; P = 0.04) during 0.1 Hz squat‐stand. Following onset of regulation, RoR was similar between groups (P = 0.82; Table 3).
Table 3.
Central and cerebrovascular hemodynamics in response to sit‐to‐stand
| Athletes | Controls | P‐values | |
|---|---|---|---|
| n | 16 | 7 | |
| Onset of regulation (sec) | 3.1 ± 1.7 | 1.5 ± 1.0 | 0.03 |
| ∆ Mean arterial pressure to nadir (mmHg) | −26 ± 10 | −21 ± 5 | 0.11 |
| ∆ Middle cerebral artery mean blood velocity to nadir (cm/sec) | −15 ± 5 | −11 ± 7 | 0.11 |
| ∆ Mean arterial pressure to nadir (%baseline) | −27 ± 10 | −22 ± 5 | 0.25 |
| ∆ Middle cerebral artery mean blood velocity to nadir (%baseline) | −25 ± 7 | −18 ± 1 | 0.09 |
| Rate of regulation (sec−1) | 0.24 ± 0.18 | 0.21 ± 0.22 | 0.82 |
Values are mean ± SD.
Figure 2.
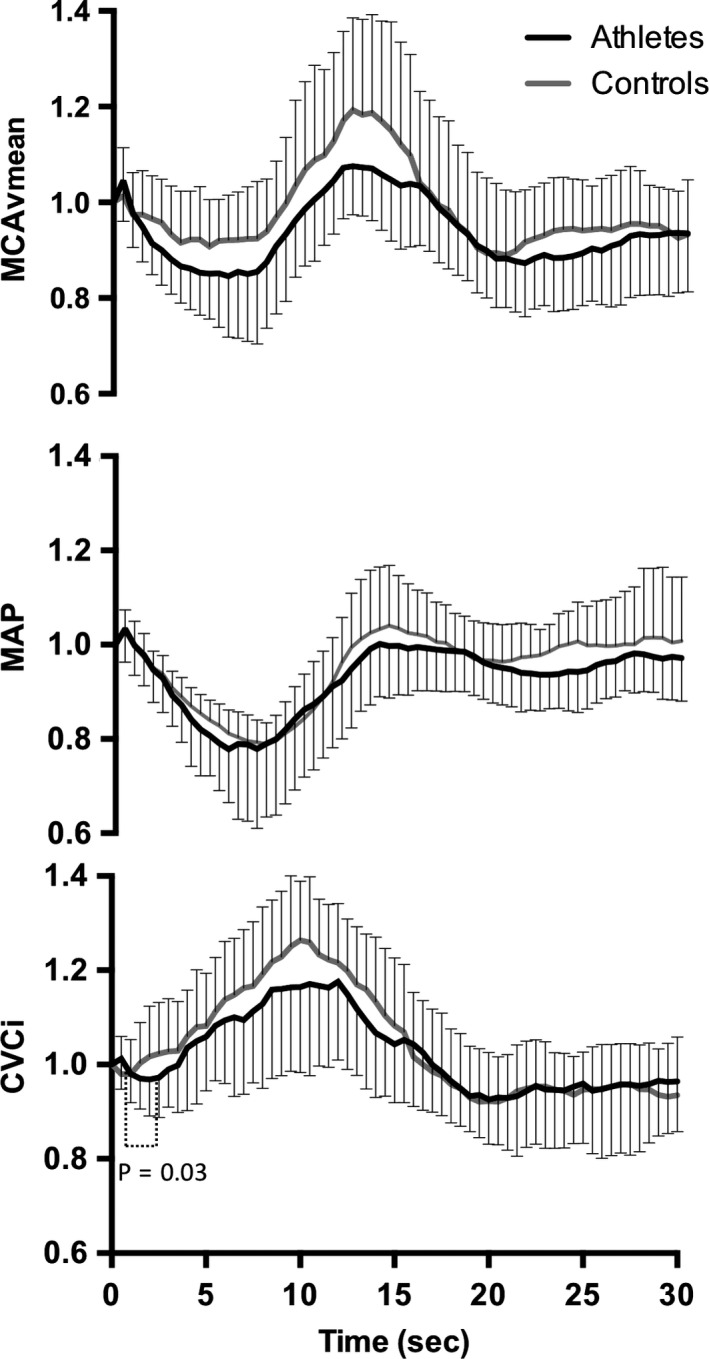
Normalized response of mean arterial pressure, middle cerebral artery mean blood velocity, and cerebrovascular conductance index in athletes (black line) and controls (gray line) after sit‐to‐stand. Time 0 indicates the transition from sitting to standing.
The prevalence of initial orthostatic hypotension was comparable between groups (Athletes: 10/16 vs. Controls: 3/7, P = 0.65), though none of the participants reported symptoms consistent with neurogenic syncope. None of the participants were classified as orthostatically hypotensive during the steady‐state phase (120–180 sec) of standing. There were no correlations between metrics of the dynamic cerebral autoregulatory capacity and decreases in MAP or MCAvmean to their nadir upon standing (data not shown).
TFA of forced oscillations in MAP and MCAv
MAP and MCAv power spectrum densities during forced oscillations at 0.05 and 0.10 Hz were not different between athletes and controls (Table 4). Absolute TFA gain during 0.10 Hz squat‐stand was higher in athletes compared to controls (P = 0.01). Absolute and normalized TFA gain during 0.10 Hz squat‐stand were positively correlated with V̇O2max (TFA gain: r = 0.61, P < 0.01; Fig. 3A, TFA normalized gain: r = 0.73, P < 0.01; Fig. 3B; pooled data), while TFA phase during 0.10 Hz squat‐stand was inversely correlated with V̇O2max (r = −0.55; P < 0.001; Fig. 3C). All the other metrics were not different between groups (Table 4) and not correlated with V̇O2max. PetCO2 was comparable between athletes and controls during squat‐stand maneuvers performed at 0.05 (43 ± 5 vs. 43 ± 5 mmHg; P = 0.95) and 0.10 Hz (43 ± 5 vs. 44 ± 4 mmHg; P = 0.75).
Table 4.
Transfer function analysis of forced oscillations in mean arterial pressure and middle cerebral artery blood velocity using squat‐stand maneuvers
| Athletes | Controls | P‐values | |
|---|---|---|---|
| Squats 0.05 Hz | |||
| n | 13 | 7 | |
| Mean arterial pressure power (mmHg2)/Hz | 4558 ± 5632 | 3514 ± 4244 | 0.67 |
| Middle cerebral artery blood velocity power (cm/sec2)/Hz | 9351 ± 12072 | 7780 ± 7110 | 0.76 |
| Coherence | 0.975 ± 0.033 | 0.940 ± 0.070 | 0.24 |
| Gain (cm sec−1 mmHg−1) | 0.675 ± 0.185 | 0.553 ± 0.202 | 0.19 |
| Normalized gain (%/%) | 1.126 ± 0.300 | 0.951 ± 0.273 | 0.22 |
| Phase (radians) | 0.786 ± 0.252 | 0.965 ± 0.340 | 0.16 |
| Squats 0.10 Hz | |||
| n | 15 | 7 | |
| Mean arterial pressure power (mmHg2)/Hz | 7523 ± 5104 | 5863 ± 3482 | 0.45 |
| Middle cerebral artery blood velocity power (cm/sec2)/Hz | 10712 ± 8847 | 11634 ± 6165 | 0.81 |
| Coherence | 0.995 ± 0.008 | 0.994 ± 0.008 | 0.78 |
| Gain (cm sec−1 mmHg−1) | 0.871 ± 0.162 | 0.686 ± 0.116 | 0.01 |
| Normalized gain (%/%) | 1.431 ± 0.294 | 1.216 ± 0.189 | 0.09 |
| Phase (radians) | 0.541 ± 0.230 | 0.438 ± 0.238 | 0.31 |
Values are mean ± SD.
Figure 3.
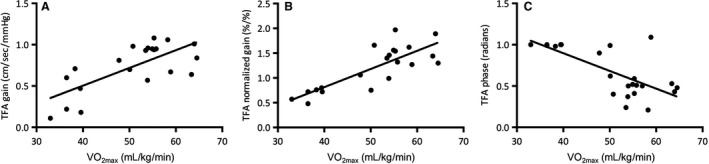
Relationships between maximal oxygen uptake (V̇O2max) and transfer function analysis gain (Panel A), normalized gain (Panel B), and phase (Panel C) during 0.10 Hz squat‐stand.
Discussion
The key findings from this study were that elevated CRF was associated with (1) delayed onset of regulation in response to transient hypotension induced by sit‐to‐stand, without affecting its response rate (i.e., RoR) and (2) augmented absolute TFA gain during 0.10 Hz squat‐stand. However, TFA metrics during spontaneous MAP oscillations were comparable between groups. Collectively, these results indicate an intact ability of the cerebral vasculature of trained individuals to react to spontaneous MAP oscillations, but a diminished capability to buffer rapid and large MAP changes. Importantly, these findings suggest that we may need to challenge the system in the trained state (through forced MAP oscillations) to reveal subtle modifications in the dynamic cerebral autoregulatory capacity. In addition, since higher CRF was not associated with a greater prevalence of initial orthostatic hypotension, and that the delayed onset of regulation or augmented TFA gain during 0.10 Hz squat‐stand failed to affect orthostatic tolerance, these variations in the dynamic cerebral autoregulatory capacity do not translate into orthostatic intolerance in healthy young male participants. These observations suggest that dynamic cerebral autoregulatory capacity metrics with forced oscillations in MAP is a more sensitive biomarker of impairment compared to the hypotensive challenge used in this study. Considering the differences in the autoregulatory stimulus between each technique, the multimodal approach applied in this study was able to identify subtle differences between these populations, which may have been overlooked if a singular approach was applied (Subudhi et al. 2015; Smirl et al. 2016).
Resting cerebral hemodynamics
The literature related to the cerebrovascular benefits of aerobic exercise training is limited and conflicting. Research to date has shown regular exercise is associated with an attenuated decline in cerebral blood velocity with normal aging (Lautenschlager et al. 2012), while regular endurance exercise is associated with increased cerebral blood velocity (Ainslie et al. 2008) and cerebrovascular reactivity to carbon dioxide (Bailey et al. 2013b; Barnes et al. 2013). Interestingly, Brugniaux et al. (2014) showed that the fitness effect for cerebral blood velocity was only apparent during incremental cycling exercise, but not at rest. With this in mind, reduced TFA phase at 0.10 Hz and elevated TFA normalized gain at 0.05 Hz have also been reported with driven oscillations in MAP during moderate exercise with old versus young adults, while these values were also comparable at rest (Smirl et al. 2016). Overall, these findings indicate that cerebrovascular benefits associated with higher CRF may only be discernable when the cerebrovasculature's regulatory capacity is challenged (Bailey et al. 2013a). Our findings of comparable baseline MCAvmean and CVCi between athletes and controls support this notion, albeit within a relatively young and healthy adult population.
Impact of cardiorespiratory fitness on dynamic cerebral autoregulatory capacity
The influence of CRF on dynamic cerebral autoregulatory capacity remains ambiguous and poorly understood. Lind‐Holst et al. (2011) reported a delayed onset of regulation following transient hypotension induced by thigh‐cuff deflation and increased TFA normalized gain of spontaneous oscillations in MAP and MCAv in endurance athletes. In contrast, similar dynamic cerebral autoregulatory capacity between young athletes and controls has been reported following thigh‐cuff deflation and via TFA of spontaneous oscillations in MAP and MCAv (Ichikawa et al. 2013). Further, Aengevaeren et al. (2013) did not observe a difference in dynamic cerebral autoregulatory capacity, characterized by TFA of spontaneous oscillations in MAP and MCAv as well as repeated sit‐to‐stand, in Masters athletes compared to elderly controls. Such discrepancies may be a consequence, at least partly, of methodological differences in the assessment of dynamic cerebral autoregulatory capacity (Tzeng et al. 2012; Smirl et al. 2015).
Most metrics of dynamic cerebral autoregulatory capacity appear unrelated to each other or show only weak/moderate correlations (Tzeng et al. 2012). As there exists no gold standard to examine dynamic cerebral autoregulatory capacity as a whole entity, several investigators strongly suggest the use of a multiple assessment approach, such as utilized in our study, to improve physiological interpretation of dynamic cerebral autoregulatory capacity (Tzeng et al. 2012; Subudhi et al. 2015). In support of the findings from Lind‐Holst et al. (2011), but with the utilization of a maneuver which represents a physiological stress closer to daily life in comparison with the thigh‐cuff deflation technique, we observed that CRF was associated with nearly a 2‐sec delayed onset of regulation following transient hypotension induced by sit‐to‐stand (Table 3). However, and similarly to what has been reported by others (Lind‐Holst et al. 2011), CRF did not influence RoR. Overall, these observations indicate that CRF may affect the latency of cerebrovascular counterregulation in response to transient hypotension, without disturbing the cerebral autoregulatory control integrity. The physiological and/or clinical significance of a delayed onset of regulation in endurance athletes, or in any other population, remains to be determined. Hypothetically, a postponed initiation of autoregulation in response to an abrupt decrease in ABP could extend the time period associated with pressure‐passive cerebral blood velocity without counterregulation, which in turn would increase to risk of presyncope, especially in individuals with an affected cerebral autoregulatory control integrity.
We did not observe any significant difference in TFA metrics during spontaneous oscillations in MAP and MCAv between athletes and controls as reported by some (Aengevaeren et al. 2013; Ichikawa et al. 2013), but not all (Lind‐Holst et al. 2011). Interestingly, a higher absolute TFA gain became apparent in athletes during driven oscillations at 0.10 Hz for the squat‐stand protocol. These results indicate the cerebral vasculature of individuals with higher CRF has an intact ability to regulate spontaneous oscillations in MAP, but becomes less capable of dampening efficiently large and rapid changes in MAP. These findings could be due to the cardiac baroreceptors aiding in the regulation of MAP for the slower driven oscillations. Indeed, cardiac baroreceptors tend to engage and counterregulate at ~7 sec (Aaslid et al. 1989), which is therefore effective for 0.05 Hz, whereas it does not have time to counterregulate at 0.10 Hz. Also, 0.10 Hz oscillations are associated with Mayer waves, representing an index of sympathetic activity in the peripheral vasculature, which could play a role in the frequency dependent nature of this response (Hyndman et al. 1971; Preiss and Polosa 1974). Contradictory results are present in the literature regarding the influence of CRF on TFA gain. For example, higher (Lind‐Holst et al. 2011), lower (Ichikawa et al. 2013), or similar (Aengevaeren et al. 2013) TFA gain has been reported in athletes compared to untrained controls. Differences in body position during evaluation, age of participants, the method used to characterize this metric, as well as fitness levels may all contribute, to some extent, to these discrepancies. Importantly, taking into consideration that spontaneous TFA has rather poor signal‐to‐noise ratio and reproducibility compared to TFA on driven oscillations (Smirl et al. 2015), further research is necessary to better understand whether the changes measured via TFA of spontaneous oscillations in MAP and MCAv reflect a physiologically significant modification in the ability of the cerebral vasculature to regulate MAP. The opposing conclusions in regards to the influence of CRF on dynamic cerebral autoregulatory capacity show the importance of characterizing the latter with multiple metrics, along with the inclusion of various frequencies for repeat‐driven data methods such as squat‐stand maneuvers, in order to take into account the complete range of physiological information instead of focusing on specific metrics (Tzeng et al. 2012; Tzeng and Ainslie 2014). In addition, the direction of MAP changes should also be considered when studying the dynamic cerebral autoregulatory capacity, as the brain seems to be better adapted to dampen transient hypertension compared to transient hypotension (Tzeng et al. 2010; Numan et al. 2014; Brassard et al. 2017). Future studies to determine how CRF affects this hysteresis phenomenon are needed.
Variations in the dynamic cerebral autoregulatory capacity in the trained state: adaptation or maladaptation?
CRF is accepted as an important indicator of cardiovascular health (Ross et al. 2016). In addition, elevated CRF is associated with higher resting and exercising cerebral blood velocity across the aging spectrum (Ainslie et al. 2008; Brugniaux et al. 2014), and cerebrovascular reactivity to carbon dioxide (Bailey et al. 2013b). Accordingly, the slower dynamic cerebral autoregulatory capacity in response to transient hypotension as well as subtle changes in the cerebral‐pressure flow relationship during squat‐stand maneuvers with elevated CRF reported in this study may seem counterintuitive, since this would place the brain in a disadvantageous position during hemodynamic stress of large amplitude. On the other hand, our findings may require a reappraisal of what we expect of the cerebral circulation in response to clear cardiorespiratory adaptation. Of interest, we examined whether these changes in the dynamic cerebral autoregulatory capacity represented adaptive or maladaptive consequences of the trained state by studying relations between dynamic cerebral autoregulatory capacity metrics and orthostatic tolerance in our participants. The prevalence of initial orthostatic hypotension was comparable between athletes and controls, and we did not observe any correlations between dynamic cerebral autoregulatory capacity metrics and reductions in MAP and MCAvmean during orthostatic stress in our participants. Therefore, our findings indicate the functional impairment in dynamic cerebral autoregulatory capacity accompanying higher CRF does not seem to be associated with, nor is critical enough to worsen, orthostatic tolerance. As such, we suggest this functional impairment represents an adaptive, instead of a maladaptive, consequence of the trained state.
This being acknowledged, endurance athletes are exposed, albeit intermittently, to aggressive oscillations in different types of stresses, including shear stress, hypocapnia and hypercapnia, sympathetic nervous activity, inflammation‐oxidation‐nitrosylation, and blood volume. Accordingly, their cerebral vasculature is intermittently exposed to a very different hemodynamic and metabolic stress milieu compared to nonathletes, and the different impact on cerebrovascular function of each of these stressors remains unclear. The lack of influence of the functional impairment in dynamic cerebral autoregulatory capacity on orthostatic tolerance observed in this study could represent a desensitization of their cerebral vasculature due to chronic exposure to stress (Thomas et al. 2013).
Still, we cannot rule out the possibility that this functional impairment in dynamic cerebral autoregulatory capacity could be amplified, and eventually affect orthostatic tolerance, in endurance athletes with higher aerobic capacity. In addition, there is likely a confounding variable at play which is the notion these groups are generally young healthy males and the subtle changes reported in the current study would be exacerbated if these measures were performed in older adults or at‐risk populations (e.g., hypertension, early stage heart failure, or acute mild traumatic brain injury).
Limitations
There are some limitations to our study that need to be acknowledged. Only young male participants have been studied and our findings cannot be generalized to other populations. Although the PetCO2 levels reported in this study are elevated compared to expected values, they were unchanged throughout the experimental conditions and therefore would have had limited if any impact on the current findings. We acknowledge that the small and unequal sample sizes of our study is associated with interpretive limitations, although a retrospective power analysis revealed that we were adequately powered to observe statistical differences at P < 0.05 for the main variables of interest examined in this study. Another limitation of this study is its cross‐sectional design and a longitudinal study is needed to confirm our findings. Only one sit‐to‐stand was used for the RoR calculation. The inclusion of more than one trial may be necessary for upcoming studies given the poor reproducibility of this metric. Since age was not perfectly matched between groups (although both were relatively “young” compared to typical older cohorts, e.g., >60 years), our findings may have been influenced by this variable. However, previous work indicates that aging has no influence on dynamic cerebral autoregulatory capacity (Carey et al. 2000), although differences may be revealed during exercise (Smirl et al. 2016). Cerebral blood velocity in the MCA measured with transcranial Doppler ultrasound will represent flow only if the diameter of the MCA remains constant. Changes in MAP and PetCO2 have been associated with changes in the diameter of internal carotid artery and MCA (Coverdale et al. 2014; Verbree et al. 2014; Lewis et al. 2015). We consider that the physiological range of variation in MAP and PetCO2 (when available) from this study will be associated with a negligible impact on the diameter of the MCA (Ainslie and Hoiland 2014). However, further research using a multimodal imaging approach (e.g., combination of transcranial and carotid Doppler ultrasound, where possible), with PetCO2 recordings for each procedure, is strongly recommended to support our findings.
Conclusions
These results indicate an intact ability of the cerebral vasculature to react to spontaneous oscillations but an attenuated capability to counter rapid and large changes in MAP in aerobically fit individuals. Considering the subtle differences in the three techniques used in this study, we strongly suggest other investigators to consider employing a multimetrics approach for the assessment of dynamic cerebral autoregulatory capacity to improve interpretation and understanding of this measure. Using BP stimuli of diverse natures and magnitudes, the subtle differences in the ability of the cerebral vasculature to respond to MAP changes (whether it is within the context of CRF, or any other physiological situation) are more likely to be revealed.
Conflict of Interests
The authors declare that there is no conflict of interest.
Labrecque L. , Rahimaly K. , Imhoff S. , Paquette M. , Le Blanc O. , Malenfant S. , Lucas S. J. E. , Bailey D. M. , Smirl J. D. , Brassard P.. Diminished dynamic cerebral autoregulatory capacity with forced oscillations in mean arterial pressure with elevated cardiorespiratory fitness. Physiol Rep, 5 (21), 2017, e13486, https://doi.org/10.14814/phy2.13486
Funding Information
This study has been supported by the Ministère de l’Éducation, du Loisir et du Sport du Québec and the Foundation of the Institut universitaire de cardiologie et de pneumologie de Québec. D.M.B. is supported by a Reichwald Family UBC Southern Medical Program Chair in Preventive Medicine.
References
- Aaslid, R. , Lindegaard K. F., Sorteberg W., and Nornes H.. 1989. Cerebral autoregulation dynamics in humans. Stroke 20:45–52. [DOI] [PubMed] [Google Scholar]
- Aengevaeren, V. L. , Claassen J. A., Levine B. D., and Zhang R.. 2013. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly Masters athletes. J. Appl. Physiol. (1985) 114:195–202. [DOI] [PubMed] [Google Scholar]
- Ainslie, P. N. , and Hoiland R. L.. 2014. Transcranial Doppler ultrasound: valid, invalid, or both? J. Appl. Physiol. (1985) 117:1081–1083. [DOI] [PubMed] [Google Scholar]
- Ainslie, P. N. , Cotter J. D., George K. P., Lucas S., Murrell C., Shave R., et al. 2008. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 586:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, D. M. , Jones D. W., Sinnott A., Brugniaux J. V., New K. J., Hodson D., et al. 2013a. Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin. Sci. (Lond.) 124:177–189. [DOI] [PubMed] [Google Scholar]
- Bailey, D. M. , Marley C. J., Brugniaux J. V., Hodson D., New K. J., Ogoh S., et al. 2013b. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44:3235–3238. [DOI] [PubMed] [Google Scholar]
- Barnes, J. N. , Taylor J. L., Kluck B. N., Johnson C. P., and Joyner M. J.. 2013. Cerebrovascular reactivity is associated with maximal aerobic capacity in healthy older adults. J. Appl. Physiol. (1985) 114:1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek, A. H. , Claassen J. A., Rikkert M. G., and Jansen R. W.. 2008. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow Metab. 28:1071–1085. [DOI] [PubMed] [Google Scholar]
- Brassard, P. , Ferland‐Dutil H., Smirl J. D., Paquette M., Le Blanc O., Malenfant S., et al. 2017. Evidence for hysteresis in the cerebral pressure‐flow relationship in healthy men. Am. J. Physiol. Heart Circ. Physiol. 312:H701–H704. [DOI] [PubMed] [Google Scholar]
- Brugniaux, J. V. , Marley C. J., Hodson D. A., New K. J., and Bailey D. M.. 2014. Acute exercise stress reveals cerebrovascular benefits associated with moderate gains in cardiorespiratory fitness. J. Cereb. Blood Flow Metab. 34:1873–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, B. J. , Eames P. J., Blake M. J., Panerai R. B., and Potter J. F.. 2000. Dynamic cerebral autoregulation is unaffected by aging. Stroke 31:2895–2900. [DOI] [PubMed] [Google Scholar]
- Claassen, J. A. , Meel‐van den Abeelen A. S., Simpson D. M., Panerai R. B.; international Cerebral Autoregulation Research N . 2016. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the International cerebral autoregulation research network. J. Cereb. Blood Flow Metab. 36:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale, N. S. , Gati J. S., Opalevych O., Perrotta A., and Shoemaker J. K.. 2014. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J. Appl. Physiol. (1985) 117:1090–1096. [DOI] [PubMed] [Google Scholar]
- Deegan, B. M. , Sorond F. A., Lipsitz L. A., Olaighin G., and Serrador J. M.. 2009. Gender related differences in cerebral autoregulation in older healthy subjects. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009:2859–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman, B. W. , Kitney R. I., and Sayers B. M.. 1971. Spontaneous rhythms in physiological control systems. Nature 233:339–341. [DOI] [PubMed] [Google Scholar]
- Ichikawa, D. , Miyazawa T., Horiuchi M., Kitama T., Fisher J. P., and Ogoh S.. 2013. Relationship between aerobic endurance training and dynamic cerebral blood flow regulation in humans. Scand. J. Med. Sci. Sports 23:e320–e329. [DOI] [PubMed] [Google Scholar]
- Lautenschlager, N. T. , Cox K., and Cyarto E. V.. 2012. The influence of exercise on brain aging and dementia. Biochim. Biophys. Acta 1822:474–481. [DOI] [PubMed] [Google Scholar]
- Lewis, N. C. , Smith K. J., Bain A. R., Wildfong K. W., Numan T., and Ainslie P. N.. 2015. Impact of transient hypotension on regional cerebral blood flow in humans. Clin. Sci. (Lond.) 129:169–178. [DOI] [PubMed] [Google Scholar]
- Lind‐Holst, M. , Cotter J. D., Helge J. W., Boushel R., Augustesen H., van Lieshout J. J., et al. 2011. Cerebral autoregulation dynamics in endurance‐trained individuals. J. Appl. Physiol. (1985) 110:1327–1333. [DOI] [PubMed] [Google Scholar]
- Martina, J. R. , Westerhof B. E., van Goudoever J., de Beaumont E. M., Truijen J., Kim Y. S., et al. 2012. Noninvasive continuous arterial blood pressure monitoring with Nexfin(R). Anesthesiology 116:1092–1103. [DOI] [PubMed] [Google Scholar]
- Numan, T. , Bain A. R., Hoiland R. L., Smirl J. D., Lewis N. C., and Ainslie P. N.. 2014. Static autoregulation in humans: a review and reanalysis. Med. Eng. Phys. 36:1487–1495. [DOI] [PubMed] [Google Scholar]
- Ogoh, S. , Brothers R. M., Eubank W. L., and Raven P. B.. 2008. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 39:1979–1987. [DOI] [PubMed] [Google Scholar]
- Omboni, S. , Parati G., Frattola A., Mutti E., Di Rienzo M., Castiglioni P., et al. 1993. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra‐arterial recordings. Hypertension 22:26–33. [DOI] [PubMed] [Google Scholar]
- Paquette, M. , Le Blanc O., Lucas S. J., Thibault G., Bailey D. M., and Brassard P.. 2017. Effects of submaximal and supramaximal interval training on determinants of endurance performance in endurance athletes. Scand. J. Med. Sci. Sports 27:318–326. [DOI] [PubMed] [Google Scholar]
- Preiss, G. , and Polosa C.. 1974. Patterns of sympathetic neuron activity associated with Mayer waves. Am. J. Physiol. 226:724–730. [DOI] [PubMed] [Google Scholar]
- Ross, R. , Blair S. N., Arena R., Church T. S., Despres J. P., Franklin B. A., et al. 2016. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation 134:e653–e699. [DOI] [PubMed] [Google Scholar]
- Sammons, E. L. , Samani N. J., Smith S. M., Rathbone W. E., Bentley S., Potter J. F., et al. 2007. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J. Appl. Physiol. (1985) 103:369–375. [DOI] [PubMed] [Google Scholar]
- Smirl, J. D. , Lucas S. J., Lewis N. C., duManoir G. R., Smith K. J., Bakker A., et al. 2014. Cerebral pressure‐flow relationship in lowlanders and natives at high altitude. J. Cereb. Blood Flow Metab. 34:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirl, J. D. , Hoffman K., Tzeng Y. C., Hansen A., and Ainslie P. N.. 2015. Methodological comparison of active‐ and passive‐driven oscillations in blood pressure; implications for the assessment of cerebral pressure‐flow relationships. J. Appl. Physiol. (1985) 119:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirl, J. D. , Hoffman K., Tzeng Y. C., Hansen A., and Ainslie P. N.. 2016. Relationship between blood pressure and cerebral blood flow during supine cycling: influence of aging. J. Appl. Physiol. (1985) 120:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond, F. A. , Serrador J. M., Jones R. N., Shaffer M. L., and Lipsitz L. A.. 2009. The sit‐to‐stand technique for the measurement of dynamic cerebral autoregulation. Ultrasound Med. Biol. 35:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi, A. W. , Grajzel K., Langolf R. J., Roach R. C., Panerai R. B., and Davis J. E.. 2015. Cerebral autoregulation index at high altitude assessed by thigh‐cuff and transfer function analysis techniques. Exp. Physiol. 100:173–181. [DOI] [PubMed] [Google Scholar]
- Thomas, B. P. , Yezhuvath U. S., Tseng B. Y., Liu P., Levine B. D., Zhang R., et al. 2013. Life‐long aerobic exercise preserved baseline cerebral blood flow but reduced vascular reactivity to CO2. J. Magn. Reson. Imaging 38:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, Y. C. , and Ainslie P.. 2014. Blood pressure regulation IX: cerebral autoregulation under blood pressure challenges. Eur. J. Appl. Physiol. 111:545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, Y.‐C. , Willie C. K., Atkinson G., Lucas S. J. E., Wong A., and Ainslie P. N.. 2010. Cerebrovascular regulation during transient hypotension and hypertension in humans. Hypertension 56:268–273. [DOI] [PubMed] [Google Scholar]
- Tzeng, Y. C. , Ainslie P. N., Cooke W. H., Peebles K. C., Willie C. K., MacRae B. A., et al. 2012. Assessment of cerebral autoregulation: the quandary of quantification. Am. J. Physiol. Heart Circ. Physiol. 303:H658–H671. [DOI] [PubMed] [Google Scholar]
- Tzeng, Y. C. , MacRae B. A., Ainslie P. N., and Chan G. S.. 2014. Fundamental relationships between blood pressure and cerebral blood flow in humans. J. Appl. Physiol. (1985) 117:1037–1048. [DOI] [PubMed] [Google Scholar]
- Verbree, J. , Bronzwaer A. S., Ghariq E., Versluis M. J., Daemen M. J., van Buchem M. A., et al. 2014. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra‐high‐field MRI. J. Appl. Physiol. (1985) 117:1084–1089. [DOI] [PubMed] [Google Scholar]
- Wieling, W. , and van Lieshout J. J.. 1997. Maintenance of postural normotension in humans Pp. 73–82 Clinical Autonomic Disorders. Lippincott‐Raven, Philadelphia. [Google Scholar]
- Wieling, W. , Krediet C. T., van Dijk N., Linzer M., and Tschakovsky M. E.. 2007. Initial orthostatic hypotension: review of a forgotten condition. Clin. Sci. (Lond.) 112:157–165. [DOI] [PubMed] [Google Scholar]
- Willie, C. K. , Colino F. L., Bailey D. M., Tzeng Y. C., Binsted G., Jones L. W., et al. 2011. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J. Neurosci. Methods 196:221–237. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Zuckerman J. H., Giller C. A., and Levine B. D.. 1998. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 274:H233–H241. [DOI] [PubMed] [Google Scholar]


