Abstract
Background
Papillary thyroid carcinoma (PTC) is associated with mutations of BRAFV600E and RET/PTC and high levels of expression of nuclear factor-κB (NF-κB). However, few studies have focused on the association between NF-κB expression and mutations in BRAFV600E and RET/PTC, especially regarding PTC cell proliferation and migration. The aim of this in vitro study was to investigate the effect of BRAFV600E or RET/PTC on NF-κB expression, cell proliferation and cell migration in four established PTC cell lines.
Material/Methods
Four cell lines included TPC-1 (BRAFWT/WT), BCPAP (BRAFV600E/V600E), PCCL3, and PTC3-5 (RET/PTC), were grown in culture in vitro with or without suppression of NF-κB using pyrrolidine dithiocarbamate (PDTC), and cell proliferation, and cell migration were evaluated.
Results
Expression of the BRAF gene was increased in the BCPAP cell line when compared with the TPC-1 cells. Expression of the RET gene was increased in the PTC3-5 cell line when compared with the PCCL3 cells. In the BCPAP and PTC3-5 cell lines, the relative expression of NF-κB protein, including phosphorylated p100/52, phosphorylated p65, phosphorylated IKKα/β, phosphorylated IκBα, and p65 nuclear translocation were increased compared with the TPC-1 and PCCL3 cells. Proliferation and migration of BCPAP and PTC3-5 cells were increased compared with the TPC-1 and PCCL3 cells. Suppression of NF-κB reduced NF-κB protein expression and inhibited the proliferation of cells in the TPC-1, BCPAP, PCCL3 and PTC3-5 cell lines, and migration of the BCPAP and PTC3-5 cells.
Conclusions
BRAFV600E and RET/PTC and the expression of NF-κB promote the proliferation and migration of papillary thyroid carcinoma cells in vitro.
MeSH Keywords: Protein Modification, Translational; Proto-Oncogene Proteins B-raf; Receptor Activator of Nuclear Factor-kappa B; Thyroid Neoplasms
Background
Thyroid carcinoma (TC) includes some of the most common cancers of the endocrine system. The major types of TC included papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), medullary thyroid carcinoma (MTC), and anaplastic thyroid carcinoma (ATC). PTC is the most frequent type of TC and is diagnosed in more than 80% of cases of thyroid malignancy [1]. Improvements in diagnostic techniques, including the use of Doppler ultrasound imaging, have refined and redefined the ability to stage of the aggressive forms of TC and to distinguish PTC from FTC, MTC, and ATC, more clearly [2].
Recent studies have focused on the molecular basis of thyroid carcinogenesis. It has now been shown that PTC is associated with genetic mutations related to the mitogen-activated protein (MAPK) signaling pathway, known to be involved in regulating cell growth, cell differentiation, and cell survival [3]. According to a study in 2003 by Kimura et al. [4], molecular alterations may be found in up to 70% of PTC, including the genes encoding receptor tyrosine kinase, RET, neurotrophic receptor tyrosine kinase-1 (NTRK1), and the two intracellular effector genes of the mitogen-activated protein kinase (MAPK) pathway, RAS and BRAF [4]. Up to 50% of cases of PTC demonstrate activation of the BRAF oncogene, which is commonly associated with the oncogenic V600E mutation [4,5].
The re-arranged RET gene is located on chromosome 10q11.2 and encodes for a cell membrane receptor tyrosine kinase [5]. RET is activated by chromosomal rearrangement, which is one of the most common molecular events occurring in PTC [6,7]. Currently, at least 11 types of RET/PTC have been reported, formed by the fusion of the RET gene to different partners [8]. Also, RET/PTC is tumorigenic in thyroid follicular cells, resulting in the transformation of thyroid cells and increasing the risk of TC, as well as down-regulating thyroid-specific gene expression [9–11].
Recent studies have shown that the activation of RET/PTC requires signaling along the MAPK pathway [12], as well as functional BRAF kinase [13]. According to Mitsutake et al. [13], BRAF gene silencing may reverse the effects induced by RET/PTC, such as ERK phosphorylation, inhibition of thyroid-specific gene expression, and promotion of cell proliferation. Some studies have shown that signals from the wild type RET receptor or RET/PTC activate other pathways, particularly the phosphatidylinositol-3 kinase/AKT pathway [14,15].
Adeniran et al. [16] found that PTC with RET/PTC rearrangements typically occurred in young adults, with a high rate of lymph node metastasis, classic papillary histology, and possibly more favorable prognosis. Previous studies have also suggested that the RET/PTC activates several functional clusters of genes involved in regulating inflammation and immune responses [17,18]. Therefore, the linkage between RET/PTC and signaling pathways might play an important role in the tumor progression and metastasis, as well as inflammation and immune responses.
The BRAF protein belongs to the family of RAF proteins, including ARAF, BRAF, and CRAF, as the intracellular effectors in MAPK signaling cascade [19]. BRAF has the highest basal kinase activity and the most potent activator of MEK [19]. Davies et al. [20] reported that the BRAF gene with point mutations within the kinase domain resulted in carcinogenesis, including in melanoma and colorectal cancer. Among the mutations, a thymine-to-adenine transversion in nucleotide 1799 (T1799A), which was also described as T1796A, occurred most commonly, resulting in a glutamic acid instead of a valine at residue 600 of the protein (V600E) [20]. Other studies have shown that a BRAF mutation had the greatest prevalence in PTC [4], and 45% of these mutations were V600E, followed by K601E and V599Ins [21–23].
NF-κB plays a critical role in regulating cell apoptosis, promoting inflammation and immune responses [24]. Previous studies have shown that NF-κB is expressed in primary TC, especially in ATC tissues [25,26]. Cerutti et al. [27] suggested that NF-κB was constitutively activated and uncontrolled in TC cells, influencing the tumor phenotype. Ludwig et al. [28] found that NF-κB showed increased expression in parafollicular C cells, and the activation of proto-oncogene RET was NF-κB-dependent in TT cells isolated from MTC tissues. Overall, the function of NF-κB is important in the pathogenesis and progression of TC, which may provide a future target for therapy.
However, there have been few previous studies that have focused on the relationship between PTC and NF-κB, especially to study PTC and BRAFV600E or RET/PTC. The aim of this in vitro study was to investigate the effect of BRAFV600E or RET/PTC on NF-κB expression, cell proliferation and cell migration in four established PTC cell lines.
Material and Methods
Cell culture
Human thyroid BCPAP (BRAFV600E/V600E) and TPC-1 (BRAFWT/WT) cell lines, and a rat thyroid PCCL3 cell line were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). BCPAP and TPC-1 cells were maintained in RPMI 1640 (Thermo Fisher Scientific, Inc. Shanghai, China) containing 10% fetal bovine serum (FBS) (Gibco, Thermo) and penicillin-streptomycin (1: 100) (Sigma-Aldrich, Co. LLC. Shanghai, China). PCCL3 cells were maintained in H4 medium that consisted of Coon’s medium-F12 with high zinc content (Seebio Biotech, Inc. Shanghai, China) supplemented with 5% FBS, 0.3 mg/ml L-glutamine (Sigma), 1m IU/ml thyroid stimulating hormone (TSH) (Sigma), 10 μg/ml insulin (Sigma), 5μg/ml apo transferrin (Sigma), 10 nmol/l hydrocortisone (Sigma), and penicillin-streptomycin. The expression of RET/PTC was obtained from PTC3-5 cells derived from PCCL3 by doxycycline (Sigma) induction (500 ng/ml) [29]. All cell lines were cultured under the conditions of 37 °C, 5% CO2, and saturated humidity. When 90~95% confluency was reached, cell lines were passaged for culture.
Inhibition of NF-κB
Pyrrolidine dithiocarbamate (PDTC) (Beyotime Biotechnology, Shanghai, China) was dissolved in medium, filter-sterilized and prepared for the following experiments. BCPAP, TPC-1, PCCL3 and PTC3-5 cells were cultured in 6-well plates (Corning, New York, USA) with medium without FBS. 100μM PDTC was used to inhibit NF-κB activation for 24 h.
Protein extraction and Western blotting (WB)
Total proteins were extracted from cell lysate with RIPA buffer (Thermo) and then quantified with a BCA protein assay kit (Beyotime), according to the specifications. Nuclear and cytoplasmic proteins were loaded into 10% sodium dodecyl sulfate (SDS) polyacrylamide gel, separately, and then transferred to polyvinylidene difluoride (PVDF) membranes (Merck Millipore Corporation, Shanghai, China). After blocking with 5% non-fat milk, following primary antibodies were used to blot the special proteins at 4°C overnight: rabbit anti-IKKα/β pS180/S181 (1: 10000, Abcam), rabbit anti-NF-κB p65 (1: 5000, Abcam), rabbit NF-κB p65 pS536 (1: 10000, Abcam), rabbit anti-IκBα pS32 (1: 10000, Abcam), rabbit anti-NF-κB p100/p52 pS866 (1: 2000, Abcam), mouse anti-BRAF (F-7) (1: 200, Santa Cruz Biotechnology, Inc. Shanghai, China), rabbit anti-RET (1: 1000, Abcam), mouse anti-β-actin (1: 1,000, Proteintech Group, Inc. Wuhan, China), mouse anti-α-tublin (1: 10000, Proteintech). Goat anti-mouse and goat anti-rabbit secondary antibodies (1: 3000, Jackson Immuno Research Laboratories, Inc. West Grove, PA, USA) were used to incubate the proteins at 25°C for 1 h. An electrochemiluminescence (ECL, Millipore) system was used to expose the proteins.
Immunofluorescence (IF)
Cells at 25% confluency were cultured in 6-well plates with coverslips for 24 h. The cells were fixed with 4% paraformaldehyde (Solarbio Technology Co., Ltd. Beijing, China) at 25 °C for 30 min, quenched with 30mM glycine (BGI, Shenzhen, China) in phosphate buffer saline (PBS, Boster Biological Technology Co., Ltd. Wuhan, China) at 25°C for 5min, and then permeabilized with 0.5% Triton-X (Solarbio) in PBS at 25°C for 5min. Pre-blocking was done with 5% non-fat milk and 2% bovine serum albumin (BSA, Gen-View Scientific Inc. USA) at 25°C for 1 h, and then the following primary antibodies were used to blot the specific proteins: rabbit anti-NF-κB p65 (1: 5000, Abcam), incubated with Fluor® 488 goat anti-rabbit antibody(1: 500, ZSGB-BIO, Beijing, China) at 25°C for 1 h. Staining was done with 4′, 6-diamidin-2-phenylindole (DAPI), and observed with a confocal laser scanning microscope (Olympus Corporation, Beijing, China).
Proliferation assay
BCPAP, TPC-1, PCCL3 and PTC3-5 cells were cultured in 96-well plates (Corning, USA) with a concentration of 1×104 cells/well. Cells were cultured for 48 h and then incubated using a cell counting kit-8 (CCK-8) (Thermo) at 37°C for 20min.
Wound healing assay
1×106 cells were cultured in 6-well plate to 90% confluency. Linear wounds were created with pipette tips and cells were cultured in medium without serum for 10 h, continuously. The wound healing results were observed under microscopy (Olympus)
Statistical analysis
All the data were obtained from three separate experiments, with triplicated samples, and analyzed by SPSS 20.0. The measurable data were shown as mean ± standard deviations (SD). Results of CCK-8 assays were performed with one-way ANOVA, and then pairwise comparison. For homogeneity of variance, least significance testing (LSD) was performed; Dunnett’s T3 test for original values was performed for heterogeneity of variance. P<0.05 was considered to be significant.
Results
Suppression of NF-κB inhibited βRAF and RET/PTC expression
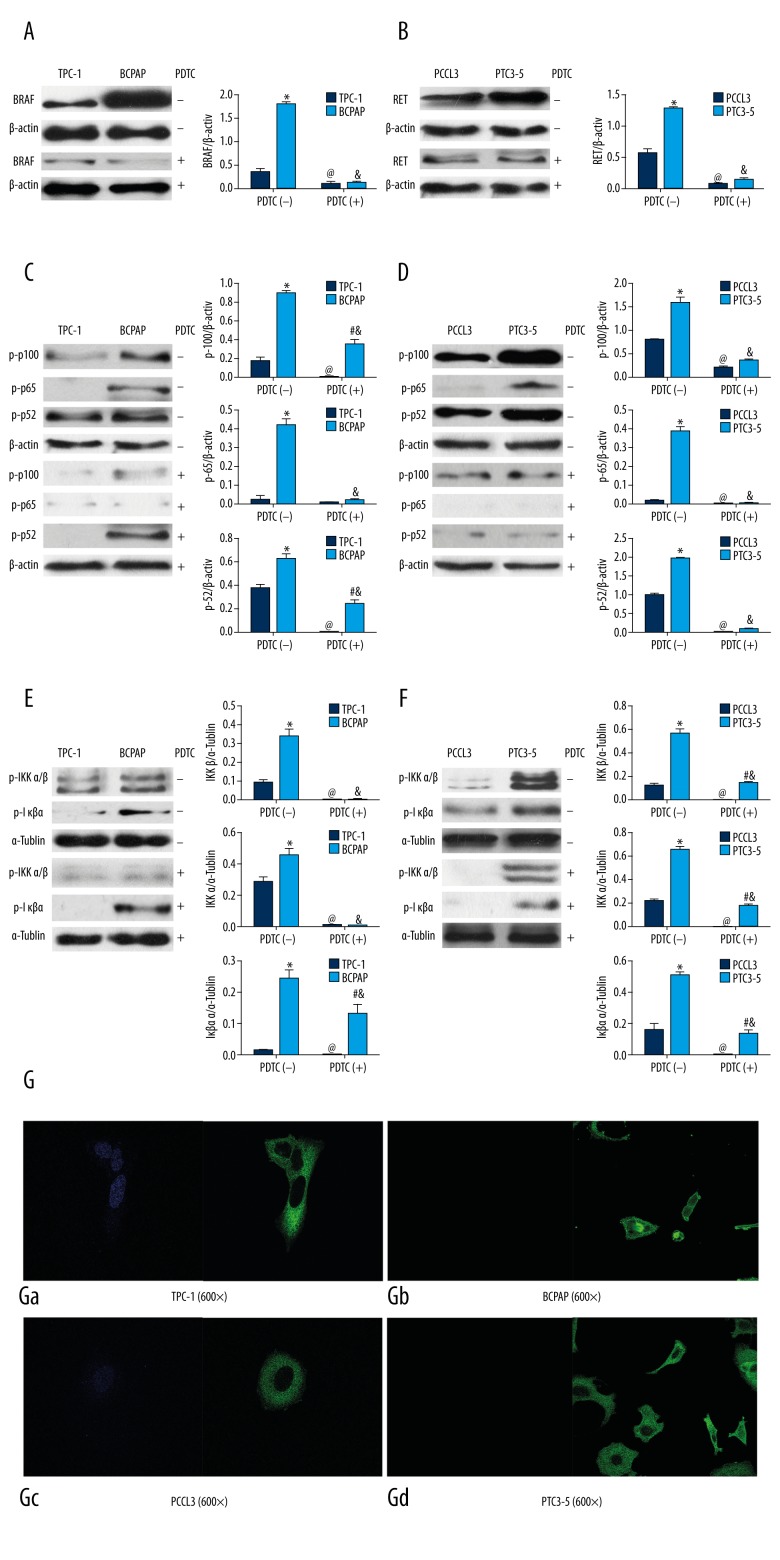
To investigate the effect of NF-κB on BRAF and RET/PTC, we used the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), to treat TPC-1 (BRAFWT/WT), BCPAP (BRAFV600E), PCCL3 and PTC3-5 (RET/PTC) cells. As showed in Figure 1A, in TPC-1 cells, BRAF expression was significantly lower than that in BCPAP (P<0.05). With PDTC treatment, BRAF expression in TPC-1 and BCPAP cells were significantly decreased after treatment, when compared with before treatment (P<0.05). In Figure 1B, RET/PTC expression in PTC3-5 cells was significantly greater than that in PCCL3 cells (P<0.05). After PDTC treatment, RET/PTC expression in PTC3-5 and PCCL3 cells were both significantly decreased, when compared with before treatment (P<0.05). The effect of PDTC on NF-κB activation is shown in Figure 1C and 1D, as decreasing phosphorylated p100, p65, and p52 expression after PDTC treatment when compared with before treatment. These results indicated that suppressing NF-κB effectively inhibited BRAF expressions in TPC-1 and BCPAP cells, as well as RET/PTC expression in PTC3-5 and PCCL3 cells.
Figure 1.
BRAFV600E and RET/PTC activated NF-κB and pyrrolidine dithiocarbamate (PDTC) suppressed NF-κB. * TPC-1+PDTC(−) vs. BCPAP+PDTC(−) or PCCL3+PDTC(−) vs. PTC3-5+PDTC(−), P<0.05; # TPC-1+PDTC(+) vs. BCPAP +PDTC(+) or PCCL3+PDTC(+) vs. PTC3-5+PDTC(+), P<0.05; @ TPC-1+PDTC(−) vs. TPC-1+PDTC(+) or PCCL3+PDTC(−) vs. PCCL3+PDTC(+), P<0.05; & BCPAP +PDTC(−) vs. BCPAP +PDTC(+) or PTC3-5+PDTC(−) vs. PTC3-5+PDTC(+), P<0.05.
BRAFV600E and RET/PTC activated NF-κB
In order to investigate the effects of BRAFV600E and RET/PTC on NF-κB activation, we compared NF-κB relative protein expression in TPC-1 (BRAFWT/WT), BCPAP (BRAFV600E), PCCL3 and PTC3-5 (RET/PTC) cells. As shown in Figure 1C and 1E, in TPC-1 cells, phosphorylated p100, p65 and p52 were both expressed at significantly lower levels in TPC-1 cells compared with BCPAP cells (P < 0.05). Similar findings were seen for PCCL3 cells with significantly lower levels of expression of phosphorylated p100, p65 and p52 compared with PTC3-5 cells (P<0.05). After PDTC treatment, the expression of phosphorylated p100, p65 and p52 in TPC-1, BCPAP, PCCL3 and PTC3-5 were significantly reduced when compared with before treatment, but phosphorylated p100 and p52 expressions in BCPAP were still significantly greater than in TPC-1 cells (P<0.05). These results indicated that BRAFV600E and RET/PTC both activated NF-κB, promoting phosphorylation of p100, p65 and p52. However, the effect of PDTC suppression on the phosphorylation of p100 and p52 was more obvious in PTC3-5 cells than in the BCPAP cells.
Also, BRAFV600E and RET/PTC promoted the phosphorylation of IκBα, as the inhibitor of NF-κB, and IKKα/β as the inhibitor of IκBα, appearing as a significantly increased expression of phosphorylated IKKα/β and IκBα in BCPAP when compared with that in TPC-1 cells, and in PTC3-5 cells when compared with that in PCCL3 cells (P<0.05). Following PDTC treatment, the expression of phosphorylated IKKα/β and IκBα in TPC-1, BCPAP, PCCL3 and PTC3-5 cells were significantly decreased (P<0.05). However, phosphorylated IκBα expression in BCPAP, or phosphorylated IKKα/β and IκBα expression in PTC3-5 cells was still significantly greater than in TPC-1 or PCCL3 cells (P<0.05). These results indicated that BRAFV600E and RET/PTC could both promote phosphorylation of IKKα/β and IκBα, resulting in activated NF-κB, but the effect of PDTC on the suppression of phosphorylation of IKKα/β in BCPAP was greater than that in PTC3-5 cells.
BRAFV600E and RET/PTC activated p65 nuclear translocation
To investigate the effect of BRAFV600E and RET/PTC on the activation of p65 nuclear translocation, we used immunofluorescence to locate the p65 expression. As shown in Figure 1G, p65 (green) was expressed in both the cytoplasm and nucleus in BCPAP cells (Figure 1Gb), while p65 was only expressed in the cytoplasm of TPC-1 cells (Figure 1Ga). Also, p65 was expressed in both the cytoplasm and nucleus in PTC3-5 cells (Figure 1Gd), while p65 was only expressed in the cytoplasm of PCCL3 cells (Figure 1Gc). These results indicated that both BRAFV600E and RET/PTC could promote p65 nuclear translocation, suggesting activation of NF-κB.
βRAFV600E and RET/PTC promoted thyroid cell proliferation
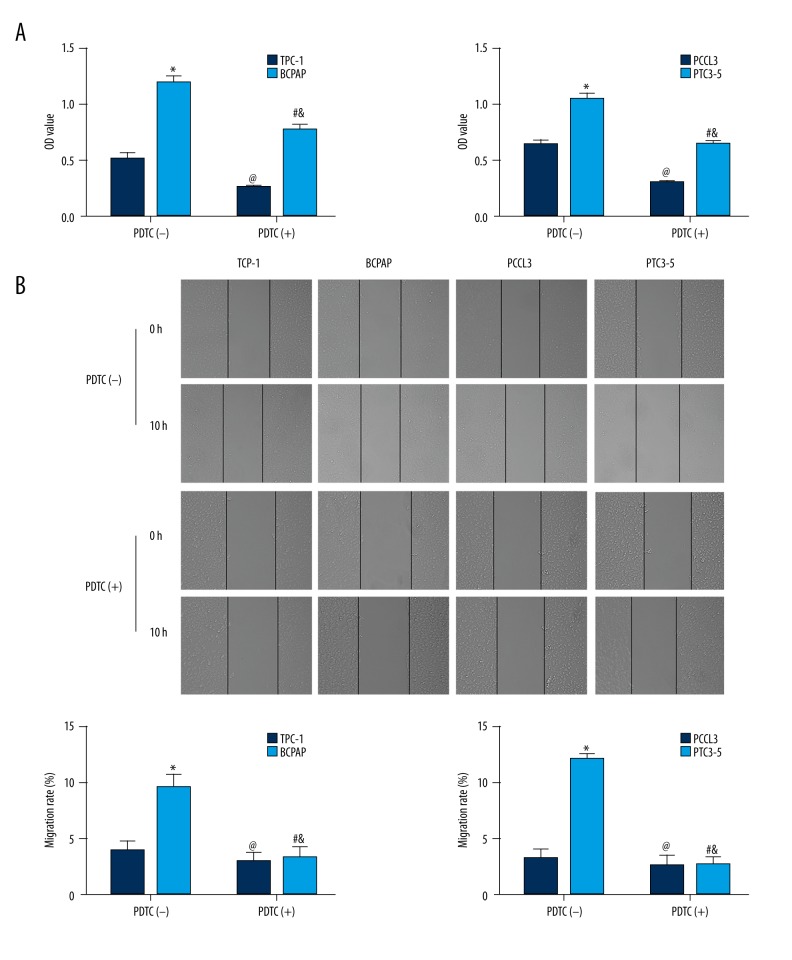
To investigate the effect of BRAFV600E and RET/PTC on promoting thyroid cell proliferation, we used the CCK-8 assay to detect the optical density (OD) value of cells. As shown in Table 1 and Figure 2A, the average OD values between TPC-1 and BCPAP, with or without PDTC treatment, were significantly different (F=275.432, P<0.05). TPC-1 proliferation was significantly lower than BCPAP (P<0.05). After PDTC treatment, both TPC-1 and BCPAP proliferation were significantly reduced compared with that before treatment (P<0.05), but BCPAP proliferation was still greater than TPC-1.
Table 1.
BRAFV600E and RET/PTC promoting thyroid cells proliferation (OD value, X±SDs).
| TPC-1 | BCPAP | PCCL3 | PTC3-5 | |
|---|---|---|---|---|
| PDTC (−) | 0.527±0.049 | 1.215±0.048* | 0.646±0.039 | 1.048±0.056* |
| PDTC (+) | 0.271±0.020@ | 0.781±0.045#& | 0.305±0.016@ | 0.643±0.029#& |
| F | 275.432 | 194.894 | ||
| P | <0.001 | <0.001 | ||
TPC-1+PDTC(−) vs. BCPAP+PDTC(−) or PCCL3+PDTC(−) vs. PTC3-5+PDTC(−), P<0.05;
TPC-1+PDTC(+) vs. BCPAP +PDTC(+) or PCCL3+PDTC(+) vs. PTC3-5+PDTC(+), P<0.05;
TPC-1+PDTC(−) vs. TPC-1+PDTC(+) or PCCL3+PDTC(−) vs. PCCL3+PDTC(+), P<0.05;
BCPAP +PDTC(−) vs. BCPAP +PDTC(+) or PTC3-5+PDTC(−) vs. PTC3-5+PDTC(+), P<0.05.
Figure 2.
BRAFV600E and RET/PTC promoted thyroid cell proliferation and migration through NF-κB. * TPC-1+PDTC(−) vs. BCPAP+PDTC(−) or PCCL3+PDTC(−) vs. PTC3-5+PDTC(−), P<0.05; # TPC-1+PDTC(+) vs. BCPAP +PDTC(+) or PCCL3+PDTC(+) vs. PTC3-5+PDTC(+), P<0.05; @ TPC-1+PDTC(−) vs. TPC-1+PDTC(+) or PCCL3+PDTC(−) vs. PCCL3+PDTC(+), P<0.05; & BCPAP +PDTC(−) vs. BCPAP +PDTC(+) or PTC3-5+PDTC(−) vs. PTC3-5+PDTC(+), P<0.05.
The average OD values between PCCL3 and PTC3-5, with or without PDTC treatment, showed no significant differences (F=194.894, P<0.05). PCCL3 cell proliferation was significantly lower than PTC3-5 (P<0.05). After PDTC treatment, both PCCL3 and PTC3-5 proliferation was significantly suppressed, compared with before treatment (P<0.05), but PTC3-5 cell proliferation was still greater than PCCL3. These results indicated that both BRAFV600E and RET/PTC promoted thyroid cell proliferation, and PDTC suppression of NF-κB could inhibit thyroid cell proliferation.
βRAFV600E and RET/PTC promoted thyroid cell migration
In order to investigate the effect of BRAFV600E and RET/PTC on thyroid cell migration, we used wound healing assay to estimate migration rates. As shown in Table 2 and Figure 2B, the average migration rate of TPC-1 and BCPAP cells, with or without PDTC treatment, were significantly different (F=275.432, P<0.05). The cell migration rate of BCPAP cells was significantly greater than TPC-1 cells (P<0.05). After PDTC treatment, BCPAP migration was significantly suppressed, when compared with before treatment (P<0.05). Although TPC-1 migration was reduced following PDTC treatment, this comparison did not reach statistical significance.
Table 2.
BRAFV600E and RET/PTC promoting thyroid cells migration (X±SDs)%.
| TPC-1 | BCPAP | PCCL3 | PTC3-5 | |
|---|---|---|---|---|
| PDTC (−) | 3.943±0.831 | 9.717±1.183* | 3.110±0.886 | 12.070±0.540* |
| PDTC (+) | 2.997±0.752 | 3.280±0.910& | 2.480±1.011 | 2.623±0.689& |
| F | 34.829 | 101.865 | ||
| P | <0.001 | <0.001 | ||
TPC-1+PDTC(−) vs. BCPAP+PDTC(−) or PCCL3+PDTC(−) vs. PTC3-5+PDTC(−), P<0.05;
BCPAP +PDTC(−) vs. BCPAP +PDTC(+) or PTC3-5+PDTC(−) vs. PTC3-5+PDTC(+), P<0.05.
The average migration rate of the PCCL3 and PTC3-5 cells, with or without PDTC treatment were with significantly different (F=194.894, P<0.05). The migration rate of PTC3-5 was significantly greater than PCCL3 (P<0.05). Following PDTC treatment, PTC3-5 cell migration was significantly suppressed, when compared with that before treatment (P<0.05). Although PCCL3 cell migration was reduced following PDTC treatment, this comparison did not reach statistical significance. These results indicated that both BRAFV600E and RET/PTC could promote thyroid cell migration, and PDTC suppression of NF-κB could inhibit thyroid cell migration.
Discussion
Previous studies have shown that several gene mutations or gene re-arrangements are found in thyroid carcinoma (TC). In the meta-analysis of the published literature by Li et al. [30], rs6983267 was one of the frequent single nucleotide polymorphisms (SNPs) resulting in an increased risk of TC. The aim of this in vitro study was to investigate the effect of BRAFV600E or RET/PTC on NF-κB expression, cell proliferation and cell migration in four established papillary thyroid carcinoma (PTC) cell lines. The findings of this study showed that the BRAFV600E and RET/PTC genes, and the expression of NF-κB promoted the proliferation and migration of papillary thyroid carcinoma cells in vitro.
The BRAF gene is known to activate NF-κB [31], and BRAF V600E has previously been shown to be related to NF-κB activity in PTC [32]. However, NF-κB can be activated by RET/PTC, inducing oncogene expression [33]. In this study, we detected BRAF and RET expression in thyroid cells, and the results showed increased expression of BRAF and RET in the cell lines BCPAP (BRAF V600E) and PTC3-5 (RET/PTC), respectively. We assumed that BRAF V600E could stimulate BRAF expression, while RET/PTC could stimulate RET expression.
By detecting the proteins related to NF-κB, we also found hyperactivation of NF-κB system in both BCPAP (BRAF V600E) and PTC3-5 (RET/PTC) cells. This study also showed that the expression of the proteins associated with NF-κB, including phosphorylated p100/52, phosphorylated p65, phosphorylated IKKα/β, and phosphorylated IκBα, were promoted, and that p65 nuclear translocation was stimulated, which are supported by previous studies [33]. However, in this study, only one inhibitor, pyrrolidine dithiocarbamate (PDTC) was used to inhibit the NF-κB signaling pathway. Whether BRAF V600E and RET/PTC activated NF-κB by stimulating IκB phosphorylation or by stimulating IKK to activate IκB phosphorylation and then stimulating NF-κB, was not confirmed in this study.
Increased NF-κB activity has been shown to stimulate thyroid carcinogenesis and tumor progression in previous studies [26,34]. As previously reported [35,36], BRAF V600E and RET/PTC stimulate thyroid cells growth and proliferation. In this study, we found that the proliferation of BCPAP (BRAF V600E) and PTC3-5 (RET/PTC) cells were both greater than TPC-1 and PCCL3 cells, which supported the findings of previous studies. Furthermore, with PDTC treatment, the proliferation of both cell lines was suppressed. These findings support the view that both BRAF V600E and RET/PTC promote thyroid cell proliferation, but also that they do so by regulating NF-κB, which are findings that we have reported for the first time in this study.
Also, previous studies have shown that NF-κB is related to the growth and degree of aggressive behavior of not only thyroid tumors [37,38], but also of other common malignant tumors [39–43]. Therefore, the aim of this study was to detect and compare the migration of PTC cells in vitro using several established cell lines and showed that both BRAFV600E and RET/PTC could promote thyroid cells migration when compared with normal thyroid cells. Additionally, with PDTC treatment, the migration of BCPAP (BRAFV600E) and PTC3-5 (RET/PTC) was inhibited, but there were no significant effects found in normal thyroid cells. Therefore, we concluded that BRAFV600E and RET/PTC promote thyroid cells migration by upregulating NF-κB activity.
Molecular approaches to the treatment of PTC may be helpful in the future. According to Sun et al. [44], the CTGF gene was shown to be the target of miR-199a-5p, indicating that miR-199a-5p could be a novel therapeutic target for the treatment of follicular thyroid carcinoma (FTC). Therefore, from the findings of this study, we suggest that the molecular mechanisms of BRAFV600E and RET/PTC could be developed as therapeutic targets to treat PTC.
Conclusions
In conclusion, this study has shown that both BRAFV600E and RET/PTC could promote the proliferation and migration of papillary thyroid carcinoma cells in vitro by regulating NF-κB activity and relative protein expression. The findings of this study support the view that NF-κB activity could be important in the pathogenesis of PTC. Further studies are required to investigate the significance of the relationship between NF-κB activity and BRAFV600E and RET/PTC.
Footnotes
Source of support: Zhujiang Hospital, Southern Medical University
Conflict of interest statement
The authors declare no conflict of interest. All the authors contributed equally to the study. There was no external funding support. Financial support for the study was from the corresponding author and Zhujiang Hospital, Southern Medical University.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Marcy PY, Thariat J, Chevenet C, Lacout A. Jugular vein invasion diagnosis and prognosis in thyroid carcinomas. Pol J Radiol. 2016;81:268–69. doi: 10.12659/PJR.896757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–86. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 4.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63(7):1454–57. [PubMed] [Google Scholar]
- 5.Takahashi M. Structure and expression of the RET transforming gene. IARC Sci Publ. 1988;92(92):189–97. [PubMed] [Google Scholar]
- 6.Fusco A, Grieco M, Santoro M, et al. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature. 1987;328(6126):170–72. doi: 10.1038/328170a0. [DOI] [PubMed] [Google Scholar]
- 7.Grieco M, Santoro M, Berlingieri MT, et al. PTC is a novel re-arranged form of the RET proto-oncogene and frequently detected in vivo in human thyroid papillary carcinomas. Cell. 1990;60(4):557–63. doi: 10.1016/0092-8674(90)90659-3. [DOI] [PubMed] [Google Scholar]
- 8.Jhiang SM. The RET proto-oncogene in human cancers. Oncogene. 2000;19(49):5590–97. doi: 10.1038/sj.onc.1203857. [DOI] [PubMed] [Google Scholar]
- 9.Santoro M, Melillo RM, Grieco M, et al. The TRK and RET tyrosine kinase oncogenes cooperate with ras in the neoplastic transformation of a rat thyroid epithelial cell line. Cell Growth Differ. 1993;4(2):77–84. [PubMed] [Google Scholar]
- 10.Powell DJ, Jr, Russell J, Nibu K, et al. The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998;58(23):5523–28. [PubMed] [Google Scholar]
- 11.Trapasso F, Iuliano R, Chiefari E, et al. Iodide symporter gene expression in normal and transformed rat thyroid cells. Eur J Endocrinol. 1999;140(5):447–51. doi: 10.1530/eje.0.1400447. [DOI] [PubMed] [Google Scholar]
- 12.Knauf JA, Kuroda HS, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003;22(28):4406–12. doi: 10.1038/sj.onc.1206602. [DOI] [PubMed] [Google Scholar]
- 13.Mitsutake N, Knauf JA, Mitsutake S, et al. Conditional BRAFV600E expression induces DNA synthesis, apoptosis, dedifferentiation, and chromosomal instability in thyroid PCCL3 cells. Cancer Res. 2005;65(6):2465–73. doi: 10.1158/0008-5472.CAN-04-3314. [DOI] [PubMed] [Google Scholar]
- 14.Miyagi E, Braga-Basaria M, Hardy E, et al. Chronic expression of RET/PTC 3 enhances basal and insulin-stimulated PI3 kinase/AKT signaling and increases IRS-2 expression in FRTL-5 thyroid cells. Mol Carcinog. 2004;41(2):98–107. doi: 10.1002/mc.20042. [DOI] [PubMed] [Google Scholar]
- 15.De Falco V, Guarino V, Malorni L, et al. RAI(ShcC/N-Shc)-dependent recruitment of GAB 1 to RET oncoproteins potentiates PI 3-K signalling in thyroid tumors. Oncogene. 2005;24(41):6303–13. doi: 10.1038/sj.onc.1208776. [DOI] [PubMed] [Google Scholar]
- 16.Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30(2):216–22. doi: 10.1097/01.pas.0000176432.73455.1b. [DOI] [PubMed] [Google Scholar]
- 17.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Nat Acad Sci. 2005;102(41):14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puxeddu E, Knauf JA, Sartor MA, et al. RET/PTC-induced gene expression in thyroid PCCL3 cells reveals early activation of genes involved in regulation of the immune response. Endocr Relat Cancer. 2005;12(2):319–34. doi: 10.1677/erc.1.00947. [DOI] [PubMed] [Google Scholar]
- 19.Marais R, Light Y, Paterson HF, et al. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272(7):4378–83. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 20.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 21.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Nat Cancer Inst. 2003;95(8):625–27. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 22.Trovisco V, Vieira de Castro I, Soares P, et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202(2):247–51. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 23.Carta C, Moretti S, Passeri L, et al. Genotyping of an Italian papillary thyroid carcinoma cohort revealed high prevalence of BRAF mutations, absence of RAS mutations and allowed the detection of a new mutation of BRAF oncoprotein (BRAF(V599lns)) Clin Endocrinol. 2006;64(1):105–9. doi: 10.1111/j.1365-2265.2005.02401.x. [DOI] [PubMed] [Google Scholar]
- 24.Tak PP, Firestein GS. NF-kappaB: A key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacifico F, Mauro C, Barone C, et al. Oncogenic and anti-apoptotic activity of NF-kappa B in human thyroid carcinomas. J Biol Chem. 2004;279(52):54610–19. doi: 10.1074/jbc.M403492200. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiades CS, McMillin D, Kotoula V, et al. Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab. 2006;91(10):4013–21. doi: 10.1210/jc.2005-2472. [DOI] [PubMed] [Google Scholar]
- 27.Cerutti J. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NF|[kappa]|B p65 protein expression. Oncogene. 1997;15(16):1987–94. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig L, Kessler H, Wagner M, et al. Nuclear factor-kappaB is constitutively active in C-cell carcinoma and required for RET-induced transformation. Cancer Res. 2001;61(11):4526–35. [PubMed] [Google Scholar]
- 29.Wang J, Knauf JA, Basu S, et al. Conditional expression of RET/PTC induces a weak oncogenic drive in thyroid PCCL3 cells and inhibits thyrotropin action at multiple levels. Mol Endocrinol. 2003;17(7):1425–36. doi: 10.1210/me.2003-0041. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Wang X, Dong J. Association of rs6983267 polymorphism and thyroid cancer susceptibility: A systematic review and meta-analysis. Med Sci Monit. 2016;22:1866–71. doi: 10.12659/MSM.896507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Suresh Kumar KG, Yu D, et al. Oncogenic BRAF regulates β-Trcp expression and NF-κB activity in human melanoma cells. Oncogene. 2007;26(13):1954–58. doi: 10.1038/sj.onc.1209994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bommarito A, Richiusa P, Carissimi E, et al. BRAFV600E mutation, TIMP-1 upregulation, and NF-κB activation: Closing the loop on the papillary thyroid cancer trilogy. Endocr Relat Cancer. 2011;18(6):669–85. doi: 10.1530/ERC-11-0076. [DOI] [PubMed] [Google Scholar]
- 33.Neely RJ1, Brose MS, Gray CM, et al. The RET/PTC-3 oncogene activates classical NF-κB by stabilizing NIK. Oncogene. 2011;30(1):87–96. doi: 10.1038/onc.2010.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iannetti A, Pacifico F, Acquaviva R, et al. The neutrophil gelatinase-associated lipocalin (NGAL), a NF-kappaB-regulated gene, is a survival factor for thyroid neoplastic cells. Proc Natl Acad Sci USA. 2008;105(37):14058–63. doi: 10.1073/pnas.0710846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D1, Liu Z, Condouris S, Xing M. BRAFV600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab. 2007;92(6):2264–71. doi: 10.1210/jc.2006-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanzi C, Cassinelli G, Cuccuru G, et al. Inactivation of RET/PTC1 oncoprotein and inhibition of papillary thyroid carcinoma cell proliferation by indolinone RPI-1. Cell Mol Life Sci. 2003;60(7):1449–59. doi: 10.1007/s00018-003-2381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyo JS, Kang G, Kim DH, et al. Activation of nuclear factor-κB contributes to growth and aggressiveness of papillary thyroid carcinoma. Pathol Res Pract. 2013;209(4):228–32. doi: 10.1016/j.prp.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Bauerle KT, Schweppe RE, Lund G, et al. Nuclear factor κB-dependent regulation of angiogenesis, and metastasis in an in vivo model of thyroid cancer is associated with secreted interleukin-8. J Clin Endocrinol Metab. 2014;99(8):1436–44. doi: 10.1210/jc.2013-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho HH, Chang CS, Ho WC, et al. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem Toxicol. 2010;48(9):2508–16. doi: 10.1016/j.fct.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Lin Z, Sun L, et al. Akt/Ezrin Tyr353/NF-κB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer. 2014;110(3):695–705. doi: 10.1038/bjc.2013.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Yang JL, Yu KK, et al. Activation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinoma. Mol Cancer. 2015;14(1):1–14. doi: 10.1186/s12943-014-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhi Y, Duan Y, Zhou X, et al. NF-κB signaling pathway confers neuroblastoma cells migration and invasion ability via the regulation of CXCR4. Med Sci Monit. 2014;20:2746–52. doi: 10.12659/MSM.892597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H, Ma D, Wang C, et al. Triptolide inhibits invasion and tumorigenesis of hepatocellular carcinoma MHCC-97H cells through NF-κB signaling. Med Sci Monit. 2016;22:1827–36. doi: 10.12659/MSM.898801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun D, Han S, Liu C, et al. Microrna-199a-5p functions as a tumor suppressor via suppressing connective tissue growth factor (CTGF) in follicular thyroid carcinoma. Med Sci Monit. 2016;22:1210–17. doi: 10.12659/MSM.895788. [DOI] [PMC free article] [PubMed] [Google Scholar]