Abstract
Despite considerable advances in the understanding of thyroid gland biology, correctly diagnosing thyroid nodules and treating high-grade thyroid carcinoma remains challenging. Cancer/testis (CT) antigens have emerged as potential diagnostic tools as well as targets of potential cancer vaccinations. In the present study, a total of 117 patients who underwent surgical therapy for thyroid disease were available for analysis. The expression levels of melanoma-associated antigen (MAGE) A, MAGE-C1/CT7, cancer/testis antigen 1B (CTAG1B) and G antigen (GAGE) were analyzed by immunohistochemistry. None of the CT antigens were expressed in the normal thyroid or goiter. In papillary and follicular carcinoma, MAGE-A was present in 8.1% of cases, GAGE in 10.8% and CT/7MAGE-C1 and CTAG1B in 2.7% each. In medullary carcinoma, CT antigen expression was as follows: MAGE-A in 42.9% of patients; MAGE-C1/CT7 in 46.5%; GAGE in 92.9%; and CTAG1B in 3.6%. A statistically significant association was observed between the expression of G MAGE-C1/CT7 and patient gender as well as patient clinical stage (P=0.029 and 0.031, respectively). In poorly differentiated and anaplastic carcinoma cases, CT antigen expression was as follows: MAGE-A in 61.8% of cases; MAGE-C1 in 57.1%; GAGE in 66.7%; and CTAG1B in 14.4%. There was a statistically significant association between expression of GAGE and gender (P=0.043). However, there was no association between CT antigen expression and patient survival in any of the tumor entities analyzed. The current study identified a distinct expression pattern of CT antigens in malignant thyroid tumors indicating that CT antigens have the potential to outperform existing thyroid cancer biomarkers. The prevalence of CT antigens in high-grade carcinomas suggests that they serve an important biological role within malignant tumors.
Keywords: thyroid nodule, thyroid neoplasms, biological tumor markers, immunohistochemistry
Introduction
Nodular disease of the thyroid gland is a relatively common malignancy worldwide and is present in 4–7% of North-American adults (1,2). Clinical studies suggest that the diagnosis of thyroid nodules may increase from 20–70% in the general population, based on the increased use of ultrasound techniques, and their presence may reach up to 50% in patients undergoing autopsy (3). Although the majority of nodules are benign and asymptomatic, there is an ~10% risk of the presence of underlying malignant disease, which requires patients to undergo additional procedures, including surgical intervention (4). The majority of malignant thyroid neoplasms have a good prognosis; however, several studies have identified factors that significantly affect the patient survival rate and have long-term implications (5–7). Therefore, it is crucial that a distinction between benign and malignant lesions is reliably made pre-surgically using techniques including fine needle biopsy and/or post-surgically (8,9). Consequently, novel techniques that unambiguously aid distinguishing between benign and malignant disease are required.
The thyroid has been the focus of immunohistochemical studies comprising large numbers of antigens and antibodies in order to characterize benign and malignant lesions (10). While certain antibodies have demonstrated notable potential, particularly when used together to increase their impact, a marker with high sensitivity and specificity remains to be identified.
The search for tumor-associated antigens capable of eliciting an immune response and that may be used in the development of cancer vaccines has been the primary effort in the field of tumor immunology over the last 2 decades (11). Several tumor antigens have been identified as having the ability to elicit cellular and/or humoral immune responses in the autologous host (12). One such group of tumor-associated antigens is referred to as cancer/testis (CT) antigens. They are expressed in a number of types of cancer; however, in normal adult tissue, CT antigens are solely present in testicular germ cells and occasionally in the placenta (13). There have been >100 CT antigens and CT antigen-families identified to date and melanoma associated antigen (MAGE) A1 remains the prototype. Classical CT antigens that map to chromosome X and with largely unknown functions may be distinguished from non-classical CT antigens that have known functions and map to autosomes (14,15). CT antigens are considered valuable target antigens for vaccine-based immunotherapeutic approaches due to their cancer-associated expression pattern and their lack of expression in almost all normal tissues except germ cells (6,16). Their exclusive presence in malignant tumors has been confirmed in numerous studies and in various tumor types (17); however, little is known about the presence of CT antigens in thyroid neoplasms.
Among CT antigens, particular antigens have been studied more extensively. MAGE-A antigens are the most highly expressed in tumors, including head and neck cancer (18–23). In recent years, members of the MAGE-A family, particularly MAGE-A3, have been studied as target antigens in vaccine clinical trials for numerous types of cancer (24) and current data suggest that MAGE-C1 may serve an important role in tumorigenesis (22,25). In myeloma for example, the expression of MAGE-C1 is correlated with disease progression and resistance to apoptosis and its expression was reported to be a strong prediction marker for lymph node metastases in melanoma (26,27). New York esophageal squamous cell carcinoma 1 (NY-ESO-1) is not highly expressed compared with other CT antigens; however, it is a cytoplasmic highly immunogenic molecule present in numerous malignant cells and has been the subject of translational research in patients with melanoma (28–31). Additionally, it has been demonstrated that the G antigen (GAGE) family is associated with specific clinical characteristics in certain types of cancer, including poor prognosis and increasing cellular resistance to apoptosis (29,32).
Consequently, in the present study the in situ protein expression of the CT antigens MAGE-A, MAGE-C1/CT7, GAGE and CTAG1B were measured in benign and malignant lesions of the thyroid gland and the potential associations with clinicopathological and prognostic variables was analyzed.
Materials and methods
Patient group
In the present study, data from patients who underwent total thyroidectomy at the Departments of Head and Neck Surgery and Otorhinolaryngology of A.C. Camargo Cancer Center, São Paulo as well as the Medical Center of the University of São Paulo at Ribeirao Preto between January 1962 and December 2011 were analyzed. Inclusion criteria were: Availability for pathological specimens and complete clinical data, patient age and gender, nodule size, status of potential vascular and capsular invasions, extraglandular extension, presence of ganglionic metastasis and distant metastasis. A total of 117 patients were enrolled in the study based on the inclusion criteria; 86 patients were from the Ribeirao Preto Medical School Hospital and 31 patients from the AC Camargo Hospital. The 117 cases consisted of the following lesions: 22 colloid goiters; 9 follicular adenomas; 9 follicular carcinomas; 28 papillary carcinomas; 28 medullary carcinomas; 8 poorly differentiated carcinomas; and 13 anaplastic carcinomas. In addition, thyroid tissue from 8 necropsy cases without any thyroid disease was analyzed. All patients provided written informed consent and the study has been approved by the Ethical Committee of the Faculty of Medicine of Ribeirão Preto, University of São Paulo and A.C. Camargo Cancer Center (protocols no. 13.141/2009 and 1.645/12).
Histological preparation and immunohistochemical staining
Surgical specimens were fixed in 10% buffered formalin for a maximum of 48 h at room temperature. Paraffin blocks with representative areas of tumor, in 4-µm sections, were selected for immunohistochemical analysis following confirmation of the presence of tumor on a hematoxylin and eosin stained section. Readings were performed by 2 independent observers, surgical pathologists with experience in the area who were unaware of the identity of the cases, prior to inclusion in the study without, and using tissue microarray technology.
For the detection of CT antigens, the following antibodies were employed. CTAG1B was detected by monoclonal antibody (mAb) E978 and mAb CT7-33 was used for MAGE-C1/CT7; the two mAbs had been previously generated by our group (28,33). GAGE was detected with a commercial reagent clone #26 (BD Transduction Laboratories; BD Biosciences, Franklin Lakes, NJ, USA). To analyze MAGE-A antigens, a cocktail consisting of mAb MA454 for MAGE-A1, mAb 57B for MAGE-A4 and mAb 6C1 for MAGE-A1, -A3/6, -A4, -A10 and A12 was used to detect a broad spectrum of MAGE-A antigens (18–20,34). All slides were subjected to heat-induced antigen retrieval prior to application of the primary antibodies. The antibodies, concentrations and conditions are listed in Table I. All primary antibodies, with the exception of mAb E978, were detected with a biotinylated horse-anti-mouse-secondary antibody (dilution, 1:200; Vector Labs, Inc., Burlingame, CA, USA) followed by an avidin-biotin-complex tertiary (dilution, 1:70; ABC-Elite, Vector Laboratories, Inc.). mAb E978 was detected with the PowerVision kit (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Diaminobenzidine (DAB) served as a chromogen (Biogenex, Fremont, CA, USA) and hematoxylin (Gill II) was used for counterstaining. Immunostaining was assessed semi-quantitatively and graded based on the estimated amount of immunopositive tumor cells as follows: Negative, no staining; focal (f), <5%; 1+, 5–25%; 2+, >25–50%; 3+, >50-75%; 4+, >75%.
Table I.
Primary antibodies.
| Monoclonal antibody | Antigen | Dilution | Buffer |
|---|---|---|---|
| MA454a,c | MAGE-A1 | 1:200 | EDTA, 1 mM, pH 8.0 |
| 57Bb,c | MAGE-A4 | 1:4,000 | EDTA, 1 mM, pH 8.0 |
| 6C1a,c | MAGE-A 1, −2, −3, −4, −6, −10 and −12 | 1:20 | Citrate, 10 mM, pH 6.0 |
| CT7-33a | CT7 (MAGE-C1) | 1:32,000 | Citrate, 10 mM, pH 6.0 |
| #26d | GAGE | 1:80,000 | EDTA, 1 mM, pH 8.0 |
| E978a | CTAG1B | 1:3,200 | High pH retrieval solution |
Source: Ludwig Institute for Cancer Research, New York, NY, USA
Dr. Giulio Spagnoli, Department of Biomedicine, Basel, Switzerland
Included in the ‘Multi MAGE-A primary cocktail solution’ to detect the expression of MAGE-A family antigens
Transduction Labs, BD Biosciences, Franklin Lakes, NJ, USA. CT, cancer/testis antigen; MAGE, melanoma-associated antigen; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen; EDTA, ethylenediaminetetraacetic acid.
Statistical analysis
Cases were divided into four groups: i) Benign diseases (colloid goiters and follicular adenomas); ii) follicular and papillary carcinomas; and iii) medullary carcinomas; and iv) poorly differentiated carcinomas.
Statistical analysis evaluated the significance of CT antigen expression and clinicopathological variables associated with the patient (gender and age) and tumor (histological type, size, tumor stage, positive lymph node, metastasis, stage grouping, angiolymphatic invasion and extra-thyroidal extension). Variables were grouped as follows: i) Age: Patients were divided into two groups, patients <45 years old and patients ≥45 years; ii) tumor classification: Tumors were analyzed in two separate groups, T1/T2 vs. T3/T4 tumors and T4 tumors, which were defined as poorly differentiated and anaplastic carcinomas; and iii) staging: Tumors were analyzed in two separate groups, stage I/II patients vs. stage III/IV patients (poorly differentiated and anaplastic carcinomas were all considered clinical stage IV). Following pathological analysis, the cases were divided into the 4 aforementioned groups. On the basis of the contingency table of the observed frequencies, the expected frequencies were calculated. The χ2 was used in the present study, involving the sum of all the results that are obtained by dividing the square result of the difference between the observed and expected frequencies by the expected frequency. The obtained value of the χ2 test is compared with the border value for the determined number of the degree of freedom and from the χ2 table, the probability of the zero hypotheses is read. The significance of the correlation of gene expression with histopathologic and clinical characteristics was analyzed by the Fisher exact test (P<0.05 was considered to indicate a statistically significant difference).
Results
Clinical and immunostaining variables
There were 31/117 patients with benign diseases consisting of 22 goiters and 9 follicular adenomas, of which the vast majority (30/31; 96.7%) were women. The average age in this group was 51.1 years, with a standard deviation (SD) of 19.46 years and median age of 55 years. Clinical evaluation demonstrated that the average nodule diameter was 3.0±2.15 cm (0.6–10 cm).
Benign samples, immunostaining variable
None of the 31 cases with benign lesions exhibited any expression of the CT antigens tested. The eight healthy thyroid tissue samples were negative for all CT antigens tested.
Papillary and follicular carcinomas, clinical and immunostaining variables
The clinical data as well as the immunohistochemical staining for patients with papillary and follicular carcinoma are presented in Table II and Fig. 1. There were 37 patients, of which 30 (81.5%) were women, with a ratio of women to men of 8.1:1.9. The average age of patients with this disease was 47.13 years, with an SD of 14.75 years and a median age of 46 years. Clinically, the average diameter of the nodules was 2.8±1.36 cm (0.5–5 cm). There were 9 follicular and 28 papillary carcinomas. In the group with follicular carcinoma, there was no predominance in tumor location between the right and left side (2:1). Among the 28 cases of papillary carcinomas, tumor location was in the left lobe in 10 and in the right lobe in 18 cases respectively. GAGE and MAGE-A were most commonly expressed in 4/37 (10.8%) and 3/37 (8.1%) cases, respectively. In 6/37 samples (16.2%), ≥1 CT antigen was present. One case of papillary carcinoma was positive for three CT antigens (MAGE-A, GAGE and MAGE-C1/CT7) and another papillary carcinoma was positive for two CT antigens (MAGE-A and CTAG1B). In papillary and follicular carcinomas, there was no association between the expression of any CT antigens and the variables analyzed.
Table II.
CT antigen expression in patients with papillary and follicular carcinoma.
| P | Gender | Age, years | Disease | Size, cm | Tumor stage | Positive lymph node | Metastasis | Stage grouping | Angiolymphatic invasion | Extra-thyroidal extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 23 | Papillary | 3.0 | T2N1aM0 | Yes | Yes | III | No | No | – | – | – | – |
| 2 | F | 54 | Papillary | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 3 | F | 34 | Papillary | 1.9 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 4 | F | 45 | Papillary | 3.2 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 5 | F | 47 | Papillary | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 6 | M | 76 | Papillary | 3.5 | T2N1aM0 | Yes | Yes | III | Yes | Yes | – | – | – | – |
| 7 | F | 49 | Papillary | 2.7 | T2N1bM0 | Yes | Yes | IV | Yes | No | – | – | – | – |
| 8 | F | 13 | Papillary | 3.5 | T2N0M0 | No | No | I | No | No | – | – | f | – |
| 9 | F | 33 | Papillary | 1.5 | T1N0M0 | No | No | I | Yes | No | 1+ | f | f | – |
| 10 | F | 43 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 11 | M | 45 | Papillary | 1.0 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 12 | F | 30 | Papillary | 1.4 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 13 | M | 37 | Papillary | 0.5 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 14 | F | 68 | Papillary | 4.5 | T3N0M0 | No | No | III | No | No | – | – | f | – |
| 15 | M | 39 | Papillary | 1.7 | T1N0M0 | No | No | I | Yes | No | – | – | – | – |
| 16 | M | 73 | Papillary | 7.0 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 17 | M | 47 | Papillary | 0.6 | T1N1aM0 | Yes | Yes | III | No | No | – | – | – | – |
| 18 | F | 51 | Papillary | 2.2 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 19 | F | 48 | Papillary | 1.1 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 20 | F | 71 | Papillary | 4.0 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 21 | M | 62 | Papillary | 5.0 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 22 | F | 28 | Papillary | 1.6 | T1N0M0 | No | No | I | Yes | No | – | – | – | – |
| 23 | F | 37 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 24 | F | 37 | Papillary | 2.3 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 25 | F | 66 | Papillary | 2.6 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 26 | F | 47 | Papillary | 1.7 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 27 | F | 46 | Papillary | 1.8 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 28 | F | 41 | Papillary | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 29 | F | 63 | Follicular | 4.0 | T2N0M0 | No | No | II | No | No | 1+ | – | – | 4+ |
| 30 | F | 61 | Follicular | 3.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 31 | F | 70 | Follicular | 1.4 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 32 | F | 44 | Follicular | 2.5 | T2N0M0 | No | No | II | No | No | – | – | – | – |
| 33 | F | 46 | Follicular | 4.5 | T3N0M0 | No | No | III | No | No | – | – | – | – |
| 34 | F | 43 | Follicular | 2.9 | T2N0M0 | No | No | II | No | No | 4+ | – | – | – |
| 35 | F | 33 | Follicular | 3.1 | T2N0M0 | No | No | I | Yes | Yes | – | – | – | – |
| 36 | F | 30 | Follicular | 2.8 | T2N0M0 | No | No | I | No | No | – | – | – | – |
| 37 | F | 50 | Follicular | 4.5 | T3N0M0 | No | No | III | No | No | – | – | – | – |
P, patient number; F, female; M, male; CT antigen, cancer/testis antigen; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen; TNM, tumor-node-metastasis; f, focal.
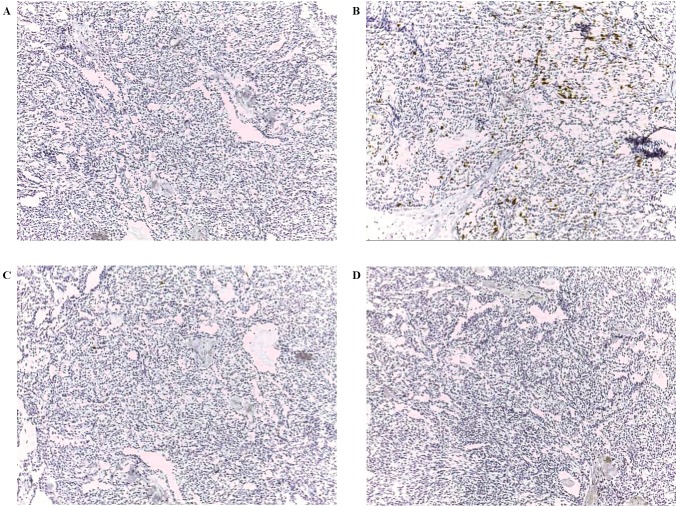
Figure 1.
Expression patterns of CT antigens in papillary carcinoma (magnification, ×100). (A) Immunohistochemical staining with mAb Multi MAGE-A with (+) immunoreactivity. (B) Immunohistochemical staining with mAb CT7-33 with focal immunoreactivity. (C) Immunohistochemical staining with mAb #26. (D) Immunohistochemical staining with mAb E978 with negative immunoreactivity. CT antigen, cancer/testis antigen; mAb, monoclonal antibody; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1.
Medullary carcinoma, clinical and immunostaining variables
Table III summarizes the clinical and CT antigen expression data for the 28 patients with medullary thyroid carcinoma. Distribution by gender indicates that 67.9% of patients were women, with a ratio of women to men of 6.8:3.2. The average patient age was 47.5 years, with an SD of 15.68 years and a median of 51 years and the average nodule diameter was 1.9±1.02 cm (0.5–4.2 cm). While the expression of CT antigens in papillary and follicular carcinoma was low, a completely different pattern was present in medullary carcinoma. GAGE was the most prevalent antigen and present in 26/28 (92.9%) cases. MAGE-A and MAGE-C1/CT7 were both expressed in ~50% of cases [MAGE-A, 12/28 (42.9%); MAGE-C1/CT7, 13/28 (46.4%)]. CTAG1B was poorly expressed and was detected in only 2/28 (7.1%) cases. Only 2 cases were completely negative. One (3.6%) case, tested positive for all four tested CT antigens and 9 cases (32.1%) expressed three CT antigens (Fig. 2 and Table IV). Among cases of medullary carcinoma, the variables patient gender as well as patient clinical stage exhibited a statistically significant association with the expression of MAGE-C1/CT7 (P=0.029 and 0.031, respectively). GAGE expression was observed in almost all cases, but there was no statistically significant association with any of the variables investigated. MAGE-A and MAGE-C1/CT7 were widely expressed, but without statistical significance.
Table III.
CT antigen expression in patients with medullary carcinoma.
| P | Gender | Age, years | Size, cm | Tumor stage | Positive lymph node | Metastasis | Stage grouping | Angiolymphatic invasion | Extra-thyroidal extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 40 | 3.0 | T1N0M0 | No | No | I | No | No | 1+ | 1+ | f | – |
| 2 | F | 57 | 2.4 | T1N1bM0 | No | No | II | No | No | – | – | f | – |
| 3 | F | 38 | 2.7 | T1N1aM0 | No | No | II | No | No | f | f | f | – |
| 4 | F | 67 | 3.4 | T3N0M0 | Yes | Yes | III | No | No | 1+ | f | f | – |
| 5 | F | 67 | 2.5 | T1N0M0 | No | No | II | No | No | – | f | f | – |
| 6 | F | 40 | 3.0 | T2N1bM1 | No | No | I | Yes | Yes | f | – | f | – |
| 7 | M | 56 | 1.5 | T1N0M0 | No | No | I | No | No | f | f | f | – |
| 8 | M | 54 | 0.5 | T2N1bM0 | Yes | No | IV | No | No | f | f | f | – |
| 9 | M | 53 | 1.2 | T2N0M0 | Yes | No | III | Yes | No | f | f | f | f |
| 10 | F | 48 | 4.2 | T1N0M0 | No | No | III | No | No | f | f | f | – |
| 11 | F | 42 | 1.0 | T1N0M0 | No | No | I | No | No | 1+ | – | f | – |
| 12 | M | 65 | 3.0 | T4bN0M1 | Yes | Yes | IV | Yes | No | – | f | f | – |
| 13 | F | 17 | 0.5 | T1N0M0 | No | No | I | No | No | – | – | – | – |
| 14 | F | 50 | 2.0 | T1N1bM0 | Yes | No | IV | Yes | No | – | – | f | – |
| 15 | F | 33 | 2.3 | T1N0M0 | No | No | II | No | No | – | – | – | – |
| 16 | F | 60 | 1.7 | T1N0M0 | No | No | I | No | Yes | – | – | f | – |
| 17 | F | 63 | 1.3 | T2N1aM0 | No | No | I | No | No | – | – | f | – |
| 18 | M | 29 | 1.0 | T2N1bM0 | No | Yes | IV | Yes | Yes | 1+ | f | f | – |
| 19 | F | 77 | 0.6 | T2N1aM0 | No | No | I | No | No | – | – | f | – |
| 20 | M | 14 | 1.2 | T2N0M0 | Yes | No | IV | Yes | Yes | f | f | f | – |
| 21 | M | 53 | 0.5 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 22 | M | 35 | 0.6 | T2N0M0 | No | No | I | No | No | – | – | f | – |
| 23 | F | 31 | 2.5 | T1N0M0 | Yes | No | III | Yes | No | f | – | f | – |
| 24 | F | 25 | 2.5 | T1N1bM0 | Yes | No | IV | Yes | No | – | f | f | – |
| 25 | F | 49 | 3.2 | T1N1aM0 | Yes | No | III | Yes | Yes | – | – | f | – |
| 26 | F | 60 | 2.8 | T3N0M0 | No | No | II | No | No | – | f | f | – |
| 27 | M | 55 | 1.4 | T1N0M0 | No | No | I | No | No | – | – | f | – |
| 28 | F | 54 | 2.0 | T2N1bM1 | No | No | II | No | No | – | – | f | – |
P, patient number; F, female; M, male; CT antigen, cancer/testis antigen; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen; TNM, tumor-node-metastasis; f, focal.
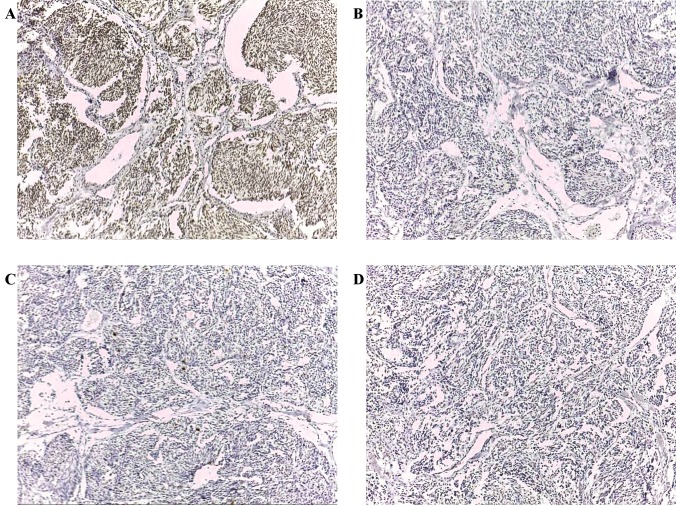
Figure 2.
Expression patterns of CT antigens in medullary carcinoma with focal immunoreactivity (magnification, ×100). (A) Immunohistochemical staining with mAb Multi MAGE-A. (B) Immunohistochemical staining with mAb CT7-33. (C) Immunohistochemical staining with mAb #26. (D) Immunohistochemical staining with mAb E978. CT antigen, cancer/testis antigen; mAb, monoclonal antibody; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1.
Table IV.
Summary of CT antigen expression patients with medullary carcinoma.
| Antigen | Expression, % |
|---|---|
| Multi MAGE-A | 42.9 |
| CT7 (MAGE-C1) | 46.4 |
| GAGE | 92.9 |
| CTAG1B | 3.6 |
CT antigen, cancer/testis antigen; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen.
Poorly differentiated carcinomas, clinical and immunostaining variables
Clinical and protein expression data for the 21 cases of poorly differentiated and anaplastic carcinoma are summarized in Table V. There were 10 women and 11 men (1.0:1.1). The mean age of patients with this disease was 65.3 years, with a SD of 11.4 years and a median of 65 years. The mean tumor size was 2.7±1.49 cm (0.8–7.0 cm). Among the tested CT antigens, GAGE demonstrated the highest incidence (14/21; 66.7%), which was similar to the incidence of MAGE-A (13/21; 61.9%) and MAGE-C1/CT7 (12/21; 57.1%); 19/21 (90.5%) tumors expressed ≥1 CT antigen (Fig. 3 and Table VI). Notably, there were 3 cases that were positive for all four tested CT antigens and 5 cases expressed three CT antigens. Among the cases of poorly differentiated and anaplastic carcinomas, there was an association between GAGE expression and gender (P=0.043). An increased expression of MAGE-A and MAGE-C1/CT7 in all variables was observed, but the difference was not significant.
Table V.
CT antigen expression in patients with poorly differentiated and anaplastic carcinomas.
| P | Gender | Age | Disease | Size (cm) | Tumor stage | Positive lymph node | Metastasis | Angiolymphatic invasion | Extra-thyroidal extension | Multi MAGE-A | CT7 (MAGE-C1) | GAGE | CTAG1B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 61 | anaplastic | 2.0 | T4aN0M0 | No | No | No | No | f | f | f | – |
| 2 | M | 73 | anaplastic | 1.5 | T4aN0M0 | No | No | No | No | f | f | – | – |
| 3 | F | 82 | anaplastic | 0.8 | T4bN0M0 | No | No | Yes | Yes | 4+ | f | f | – |
| 4 | F | 83 | anaplastic | 4.7 | T4bN0M0 | No | No | Yes | Yes | – | – | – | – |
| 5 | M | 53 | anaplastic | 1.2 | T4bN0M0 | No | No | No | Yes | 1+ | f | – | – |
| 6 | F | 70 | anaplastic | 2.5 | T4aN1bM0 | Yes | No | Yes | Yes | f | 1+ | 2+ | – |
| 7 | F | 61 | anaplastic | 1.5 | T4bN0M0 | No | Yes | Yes | Yes | 4+ | f | f | f |
| 8 | F | 59 | anaplastic | 2.5 | T4aN0M0 | No | No | No | No | – | – | f | – |
| 9 | M | 59 | anaplastic | 4.0 | T4aN0M0 | No | Yes | No | No | – | – | f | – |
| 10 | M | 57 | anaplastic | 2.2 | T4aN1bM0 | Yes | No | Yes | No | – | f | f | – |
| 11 | M | 70 | anaplastic | 3.5 | T4aN0M0 | No | No | No | No | 4+ | – | – | – |
| 12 | M | 79 | anaplastic | 4.0 | T4aN0M0 | No | No | No | No | – | – | f | – |
| 13 | F | 78 | anaplastic | 7.0 | T4bN1bM1 | Yes | Yes | Yes | Yes | – | – | 3+ | – |
| 14 | F | 74 | p. differentiated | 3.2 | T4aN0M0 | No | No | No | No | f | f | f | – |
| 15 | M | 44 | p. differentiated | 1.8 | T4bN0M0 | No | No | No | Yes | 1+ | f | f | – |
| 16 | F | 57 | p. differentiated | 2.4 | T4bN1bM0 | Yes | No | Yes | Yes | 4+ | f | f | 4+ |
| 17 | M | 80 | p. differentiated | 1.2 | T4aN0M1 | No | Yes | No | No | – | – | – | – |
| 18 | M | 52 | p. differentiated | 1.8 | T4aN0M0 | No | No | Yes | No | 4+ | – | – | – |
| 19 | M | 65 | p. differentiated | 1.6 | T4aN0M0 | No | No | No | No | 4+ | – | – | – |
| 20 | F | 66 | p. differentiated | 4.3 | T4aN0M0 | No | No | No | No | – | f | f | – |
| 21 | F | 50 | p. differentiated | 3.0 | T4aN1bM0 | Yes | No | Yes | Yes | 4+ | f | f | 3+ |
P, patient number; F, female; M, male; CT antigen, cancer/testis antigen; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen; TNM, tumor-node-metastasis; f, focal.
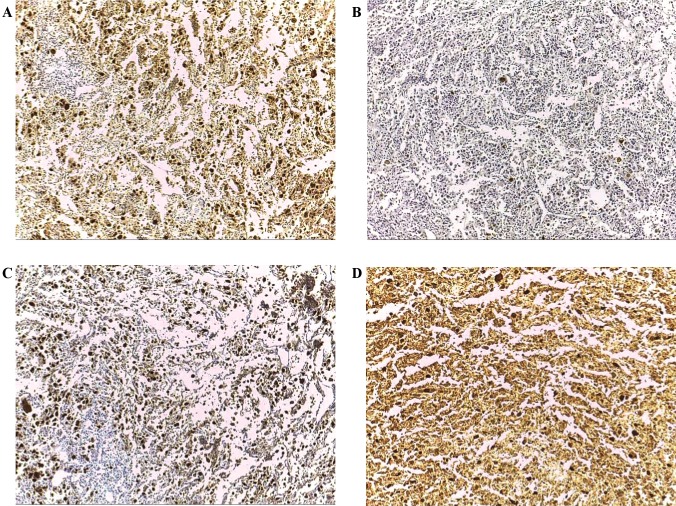
Figure 3.
Expression patterns of CT antigens in poorly differentiated carcinoma (magnification, ×100). (A) Immunohistochemical staining with mAb Multi MAGE-A with (++++) immunoreactivity. (B) Immunohistochemical staining with mAb CT7-33 with focal immunoreactivity. (C) Immunohistochemical staining with mAb #26 with (++++) immunoreactivity. (D) Immunohistochemical staining with mAb E978 with (++++) immunoreactivity. CT antigen, cancer/testis antigen; mAb, monoclonal antibody; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1.
Table VI.
Summary of CT antigen expression in patients with poorly differentiated and anaplastic carcinomas.
| Antigen | Expression (%) |
|---|---|
| Multi MAGE-A | 61.9 |
| MAGE-C1 | 57.1 |
| GAGE | 66.7 |
| CTAG1B | 14.3 |
CT antigen, cancer/testis antigen; MAGE-A, melanoma-associated antigen A; MAGE-C1/CT7, melanoma-associated antigen C1; CTAG1B, cancer/testis antigen 1B; GAGE, G antigen.
Patients were followed from the day of surgery (stipulated as the start of follow-up) until June 2012; the average follow-up period was 73.8 months (range, 1–168 months). The analysis of evolution and survival was assessed in four steps: i) patients who did not express any CT antigens vs. those who expressed 1 CT antigen; ii) patients who did not express any CT antigens vs. those who expressed 2 CT antigens; iii) patients who did not express any CT antigens vs. those who expressed 3 CT antigens; and iv) patients who did not express any CT antigen vs. those who expressed 4 CT antigens. Furthermore, the association between the extent of immunopositive areas based on the immunohistochemical grading for each CT antigen and clinical data was assessed. However, there was no association between the extent of protein expression for any of the tested CT antigens and clinical variables.
During the follow-up period, regional recurrence occurred in 3 cases of papillary carcinoma, 1 case of follicular carcinoma and 2 cases of medullary carcinoma. Distant metastasis was identified in 4 cases of papillary carcinoma, 3 cases of medullary carcinoma, 1 case of poorly differentiated carcinoma and 3 cases of anaplastic carcinoma. Regarding patient mortality, 2 patients with papillary carcinoma, 3 patients with medullary carcinoma and all 21 patients with poorly differentiated and anaplastic carcinoma succumbed during the follow-up period.
A statistically significant association between clinical variables including recurrence, metastases or survival and the presence of any CT antigen, including co-expression was not identified. This lack of association was observed for all samples studied.
Discussion
The present study aimed to characterize the expression of various CT antigens in thyroid neoplasms. Though numerous studies have been performed to investigate the presence of CT antigens in a wide variety of malignant tumors, little is known about the expression of these antigens in thyroid tumors, particularly about any associations with clinical parameters. In the present study, the antibodies selected were previously generated by our group or by collaborators, the majority of which are now available commercially and have been used in a wide variety of studies (18,19,25,28,35). Only the antibody to GAGE antigens was a commercial product, which has been employed in several previous studies (29,36,37). To detect MAGE-A, a cocktail of several antibodies was used, thus covering a wide spectrum of MAGE-A antigens. As in previous studies, the lack of specificity and the ability to identify single MAGE-A antigens was intended to ensure the detection of any low-level MAGE-A expression in the present tumor series (34,38).
The current study confirms the cancer-restricted expression of classical CT antigens in the thyroid. No expression of any of the CT antigens was detected in normal thyroid tissue or benign lesions. As with tumors in other organs, this has important implications since the expression of any CT antigens in thyroid tissue would indicate malignancy. Though malignancy-associated expression has been demonstrated in a number of neoplasms, the diagnostic potential of CT antigens as immunohistochemical markers of malignancy has yet to be exploited by pathologists (21,39,40).
The most striking finding of the current study is the apparent dichotomy of high and low CT antigen-expressing thyroid cancer. Extremely low expression of all tested antigens in papillary and follicular carcinoma was observed. In this group, GAGE and MAGE-A were the most prevalent and present in ~11 and 8% of cases respectively. Expression of MAGE-C1/CT7 and CTAG1B was even lower. This level of expression is similar to other tumors that express low levels of CT antigens, such as colon carcinoma, renal cell carcinoma and lymphoma (28,25,41). The results of the current study are supported by Melo et al (42), who identified a lack of expression of MAGE-A and MAGE-C1/CT7 in a series of papillary and follicular thyroid carcinomas. Since an association of CT antigens with the biology of tumor stem cells was being assessed and due to the scarcity of potential stem cells within tumor tissue, a threshold level was not set and any number of immunostained tumor cells was regarded as positive in previous studies and the present study (43,44). The majority of positive cases demonstrated focal expression (expression in <5% of tumor cells) only, which may explain the slightly higher number of positive cases in the current study. The current study and the previous study by Melo et al (42) identified low CT antigen levels in papillary and follicular carcinoma, which contrasts with results from two previous studies detecting a much higher level of MAGE-A expression of up to 80% in the two tumor types (21,39), despite employing the same antibodies used in the current study. There is no clear explanation for these major discrepancies, except perhaps geographical differences in the patient population. However, it is unlikely that ethnic differences are the reason for such large differences in CT antigen expression.
Cheng et al (39) demonstrated CT antigen expression in healthy tissue, a feature not consistent with the present study and numerous previous analyses of the expression of classical CT antigens, including in the thyroid (15). Milkovic et al reported extremely high MAGE-A expression in thyroid tumors exceeding measurements of MAGE-A expression in any other study of epithelial cancer to date (21). However, each study employed high antibody concentrations, and figures provided in the studies suggest unspecific immunoreactivity.
The low expression of CT antigens in papillary and follicular neoplasms contrasts with the high expression detected in medullary and anaplastic/poorly differentiated thyroid carcinomas. The highest prevalence was observed for GAGE, which was present in ~90% of all medullary carcinomas analyzed. To the best of our knowledge, no previous analyses of GAGE antigens have demonstrated a similar high expression on a protein level (22,29,45). The present study used a commercial reagent used in several previous studies and was generated to a consensus region of the GAGE-family. Consequently, it cannot be determined if a particular GAGE antigen was the most prevalent. Notably, all GAGE-positive medullary carcinomas exhibited exclusively focal immunopositivity, occasionally comprising only a single positive tumor cell. The same predominant focal expression pattern was present for the other antigens in the majority of the tested medullary tumors. GAGE was again the most prevalent antigen in anaplastic/poorly different carcinomas and its expression pattern was mostly focal. Immunohistochemistry has demonstrated that the majority of CTs are focally expression, meaning that tumor cells are heterogeneous (18). There are a number of studies demonstrating that immune targets may include surface or cytoplasmic antigens, which are different in tumor cells and normal cells (46). Previous studies have demonstrated that the same CT antigen may be expressed in different subcellular compartments, nuclear and/or cytoplasmic, of tumor cells and this pattern of expression has been observed regarding MAGE, CTAG1B and MAGE-C1/CT7 (24,29,31). Furthermore, patients with plasma cell myeloma and only cytoplasmic MAGE-C1/CT7 expression had a better prognosis than patients with nuclear or combined nuclear and cytoplasmic MAGE-C1/CT7 expression (47). The high expression of MAGE-C1/CT7 and MAGE-A in medullary and anaplastic/poorly differentiated carcinomas was in the range of what has been reported in other malignant tumors (23,25,48). Notably, CTAG1B exhibited the lowest expression of all tested CT antigens in medullary as well as anaplastic/poorly differentiated tumors. This matches the previous expression pattern in epithelial tumors, where CTAG1B is among the lowest expressed CT antigens (34,36,38). Its low incidence of expression in numerous tumors is associated with high immunogenicity, as CTAG1B is the most immunogenic CT antigen in various types of cancer (30,40). The reverse pattern is observed in CT antigens, including MAGE-A antigens that exhibit high expression but low immunogenicity in several tumor types (49,50). Unfortunately, there was no serum available to test for immunity in the present tumor series. However, a protocol has been initiated that will allow for the collection of tissue specimens as well as peripheral blood in patients with thyroid tumors.
Notably, no association between CT antigen expression and the major clinical parameters was observed. The current study did identify two associations: One between the cytoplasmic expression of MAGE-A and the number of lymph node metastasis, and one between gender and the presence of MAGE-C1/CT7 or GAGE. However in the current study there was no association between CT antigen immunostaining and recurrence, metastasis or mortality. One possible reason could be the good overall prognosis of papillary/follicular carcinomas and the extremely low expression of CT antigens in these types of tumors. By contrast, there was high expression of particular CT antigens in medullary and anaplastic/poorly differentiated carcinomas and GAGE was present in almost all tumors. However, the survival time of patients with medullary and anaplastic/poorly differentiated tumors is extremely short and the sample size of the current study may have been too small to identify any associations between clinicopathological parameters and the presence of CT antigens.
In conclusion, the present study identifies a distinct expression pattern of CT antigens in malignant thyroid tumors. The expression of CT antigens is low in papillary and follicular carcinoma, whereas in medullary and anaplastic/poorly differentiated carcinomas the expression of particular CT antigens is extremely high, with GAGE being the most prevalent. A GAGE commercial reagent clone is commercially available, which means that GAGE proteins could be used to predict cancer prognosis; high GAGE expression is correlated with poor prognosis in stomach cancer, esophageal carcinoma and neuroblastoma (32). However, this correlation between GAGE expression and clinical characteristics is controversial, since it has not been identified in a previous study (29). Thus, the reliability of commercial GAGE monoclonal antibody as a prognostic marker is unclear and additional studies are required. Though the current study did not identify an association with clinical parameters in the individual patient, the prevalence of CT antigens in high-grade carcinomas suggests a biological role within the more malignant tumor entities.
Acknowledgment
This study was supported by São Paulo Research Foundation (FAPESP) (grant nos. 2012/00588-5 and 13/08135-2).
Glossary
Abbreviations
- CT antigens
cancer/testis antigens
- MAGE-A
melanoma associated-antigen A
- MAGE-C1/CT7
melanoma-associated antigen C1
- CTAG1B
cancer/testis antigen 1B
- GAGE
G antigen
- mAb
monoclonal antibody
References
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. American Thyroid Association Guidelines Taskforce: Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 2.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.Tan GH, Gharib H. Thyroid incidentalomas: Management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, Braverman LE, Clark OH, McDougall IR, Ain KV, Dorfman SG. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American thyroid association. Arch Intern Med. 1996;156:2165–2172. doi: 10.1001/archinte.1996.00440180017002. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung and bone: Analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–1278. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 6.Huang IC, Chou FF, Liu RT, Tung SC, Chen JF, Kuo MC, Hsieh CJ, Wang PW. Long-term outcomes of distant metastasis from differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2012;76:439–447. doi: 10.1111/j.1365-2265.2011.04231.x. [DOI] [PubMed] [Google Scholar]
- 7.Konturek A, Barczyński M, Nowak W, Richter P. Prognostic factors in differentiated thyroid cancer-a 20-year surgical outcome study. Langenbecks Arch Surg. 2012;397:809–815. doi: 10.1007/s00423-011-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung CC, Carydis B, Ezzat S, Bedard YC, Asa SL. Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab. 2001;86:2187–2190. doi: 10.1210/jcem.86.5.7504. [DOI] [PubMed] [Google Scholar]
- 9.Pizzolanti G, Russo L, Richiusa P, Bronte V, Nuara RB, Rodolico V, Amato MC, Smeraldi L, Sisto PS, Nucera M, et al. Fine-needle aspiration molecular analysis for the diagnosis of papillary thyroid carcinoma through BRAF V600E mutation and RET/PTC rearrangement. Thyroid. 2007;17:1109–1115. doi: 10.1089/thy.2007.0008. [DOI] [PubMed] [Google Scholar]
- 10.Faggiano A, Caillou B, Lacroix L, Talbot M, Filetti S, Bidart JM, Schlumberger M. Functional characterization of human thyroid tissue with immunohistochemistry. Thyroid. 2007;17:203–211. doi: 10.1089/thy.2006.0174. [DOI] [PubMed] [Google Scholar]
- 11.Beatty PL, Cascio S, Lutz E. Tumor immunology: Basic and clinical advances. Cancer Res. 2011;71:4338–4343. doi: 10.1158/0008-5472.CAN-11-0717. [DOI] [PubMed] [Google Scholar]
- 12.Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol. 1997;9:681–683. doi: 10.1016/S0952-7915(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 13.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 14.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 15.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–625. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 16.Bodey B. Cancer-testis antigens: Promising targets for antigen directed antineoplastic immunotherapy. Expert Opin Biol Ther. 2002;2:577–584. doi: 10.1517/14712598.2.6.577. [DOI] [PubMed] [Google Scholar]
- 17.Fratta E, Coral S, Covre A, Parisi G, Colizzi F, Danielli R, Nicolay HJ, Sigalotti L, Maio M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Mol Oncol. 2011;5:164–182. doi: 10.1016/j.molonc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jungbluth AA, Stockert E, Chen YT, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Busam KJ, Old LJ. Monoclonal antibody MA454 reveals a heterogeneous expression pattern of MAGE-1 antigen in formalin-fixed paraffin embedded lung tumours. Br J Cancer. 2000;83:493–497. doi: 10.1054/bjoc.2000.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landry C, Brasseur F, Spagnoli GC, Marbaix E, Boon T, Coulie P, Godelaine D. Monoclonal antibody 57B stains tumor tissues that express gene MAGE-A4. Int J Cancer. 2000;86:835–841. doi: 10.1002/(SICI)1097-0215(20000615)86:6<835::AID-IJC12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Rimoldi D, Salvi S, Schultz-Thater E, Spagnoli GC, Cerottini JC. Anti-MAGE-3 antibody 57B and anti-MAGE-1 antibody 6C1 can be used to study different proteins of the MAGE-A family. Int J Cancer. 2000;86:749–751. doi: 10.1002/(SICI)1097-0215(20000601)86:5<749::AID-IJC24>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Milkovic M, Sarcevic B, Glavan E. Expression of MAGE tumor-associated antigen in thyroid carcinomas. Endocr Pathol. 2006;17:45–52. doi: 10.1385/EP:17:1:45. [DOI] [PubMed] [Google Scholar]
- 22.Inaoka RJ, Jungbluth AA, Baiocchi OC, Assis MC, Hanson NC, Frosina D, Tassello J, Bortoluzzo AB, Alves AC, Colleoni GW. An overview of cancer/testis antigens expression in classical Hodgkin's lymphoma (cHL) identifies MAGE-A family and MAGE-C1 as the most frequently expressed antigens in a set of Brazilian cHL patients. BMC Cancer. 2011;11:416. doi: 10.1186/1471-2407-11-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jungbluth AA, Busam KJ, Kolb D, Iversen K, Coplan K, Chen YT, Spagnoli GC, Old LJ. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer. 2000;85:460–465. doi: 10.1002/(SICI)1097-0215(20000215)85:4<460::AID-IJC3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Krishnadas DK, Shusterman S, Bai F, Diller L, Sullivan JE, Cheerva AC, George RE, Lucas KG. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother. 2015;64:1251–1260. doi: 10.1007/s00262-015-1731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungbluth AA, Chen YT, Busam KJ, Coplan K, Kolb D, Iversen K, Williamson B, Van Landeghem FK, Stockert E, Old LJ. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer. 2002;99:839–845. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- 26.Curioni-Fontecedro A, Knights AJ, Tinguely M, Nuber N, Schneider C, Thomson CW, von Boehmer L, Bossart W, Pahlich S, Gehring H, et al. MAGE-C1/CT7 is the dominant cancer-testis antigen targeted by humoral immune responses in patients with multiple myeloma. Leukemia. 2008;22:1646–1648. doi: 10.1038/leu.2008.43. [DOI] [PubMed] [Google Scholar]
- 27.Curioni-Fontecedro A, Nuber N, Mihic-Probst D, Seifert B, Soldini D, Dummer R, Knuth A, van den Broek M, Moch H. Expression of MAGE-C1/CT7 and MAGE-C2/CT10 predicts lymph node metastasis in melanoma patients. PLoS One. 2011;6:e21418. doi: 10.1371/journal.pone.0021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 29.Gjerstorff MF, Pøhl M, Olsen KE, Ditzel HJ. Analysis of GAGE, NY-ESO-1 and SP17 cancer/testis antigen expression in early stage non-small cell lung carcinoma. BMC Cancer. 2013;13:466. doi: 10.1186/1471-2407-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ademuyiwa FO, Bshara W, Attwood K, Morrison C, Edge SB, Karpf AR, James SA, Ambrosone CB, O'Connor TL, Levine EG, et al. NY-ESO-1 cancer testis antigen demonstrates high immunogenicity in triple negative breast cancer. PLoS One. 2012;7:e38783. doi: 10.1371/annotation/5cdf6105-2a52-497a-86b3-db8f4a4e439c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cilensek ZM, Yehiely F, Kular RK, Deiss LP. A member of the GAGE family of tumor antigens is an anti-apoptotic gene that confers resistance to Fas/CD95/APO-1, interferon-gamma, taxol and gamma-irradiation. Cancer Biol Ther. 2002;1:380–387. doi: 10.4161/cbt.1.4.11. [DOI] [PubMed] [Google Scholar]
- 33.Gnjatic S, Nishikawa H, Jungbluth AA, Güre AO, Ritter G, Jäger E, Knuth A, Chen YT, Old LJ. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 34.Curigliano G, Viale G, Ghioni M, Jungbluth AA, Bagnardi V, Spagnoli GC, Neville AM, Nolè F, Rotmensz N, Goldhirsch A. Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol. 2011;22:98–103. doi: 10.1093/annonc/mdq325. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang R, Zhu Y, Fang L, Liu XS, Tian Y, Chen LH, Ouyang WM, Xu XG, Jian JL, Güre AO, et al. Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun. 2006;6:7. [PubMed] [Google Scholar]
- 36.Sharma P, Shen Y, Wen S, Bajorin DF, Reuter VE, Old LJ, Jungbluth AA. Cancer-testis antigens: Expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res. 2006;12:5442–5447. doi: 10.1158/1078-0432.CCR-06-0527. [DOI] [PubMed] [Google Scholar]
- 37.Jungbluth AA, Silva WA, Jr, Iversen K, Frosina D, Zaidi B, Coplan K, Eastlake-Wade SK, Castelli SB, Spagnoli GC, Old LJ, Vogel M. Expression of cancer-testis (CT) antigens in placenta. Cancer Immun. 2007;7:15. [PMC free article] [PubMed] [Google Scholar]
- 38.Grigoriadis A, Caballero OL, Hoek KS, da Silva L, Chen YT, Shin SJ, Jungbluth AA, Miller LD, Clouston D, Cebon J, et al. CT-X antigen expression in human breast cancer; Proc Natl Acad Sci USA; 2009; pp. 13493–13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng S, Liu W, Mercado M, Ezzat S, Asa SL. Expression of the melanoma-associated antigen is associated with progression of human thyroid cancer. Endocr Relat Cancer. 2009;16:455–466. doi: 10.1677/ERC-09-0002. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi T, Kato T, Wang L, Maeda Y, Ikeda H, Sato E, Knuth A, Gnjatic S, Ritter G, Sakaguchi S, et al. Intracellular tumor-associated antigens represent effective targets for passive immunotherapy. Cancer Res. 2012;72:1672–1682. doi: 10.1158/0008-5472.CAN-11-3072. [DOI] [PubMed] [Google Scholar]
- 41.Inaoka RJ, Jungbluth AA, Gnjatic S, Ritter E, Hanson NC, Frosina D, Tassello J, Etto LY, Bortoluzzo AB, Alves AC, Colleoni GW. Cancer/testis antigens expression and autologous serological response in a set of Brazilian non-Hodgkin's lymphoma patients. Cancer Immunol Immunother. 2012;61:2207–2214. doi: 10.1007/s00262-012-1285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo DH, Mamede RC, Neder L, Saggioro FP, Figueiredo DL, da Silva WA, Jr, Jungbluth AA, Zago MA. Expression of MAGE-A4 and MAGE-C1 tumor-associated antigen in benign and malignant thyroid diseases. Head Neck. 2011;33:1426–1432. doi: 10.1002/hed.21616. [DOI] [PubMed] [Google Scholar]
- 43.Sigalotti L, Covre A, Nicolay HJ, Coral S, Maio M. Cancer testis antigens and melanoma stem cells: New promises for therapeutic intervention. Cancer Immunol Immunother. 2010;59:487–488. doi: 10.1007/s00262-009-0785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saldanha-Araujo F, Haddad R, Zanette DL, De Araujo AG, Orellana MD, Covas DT, Zago MA, Panepucci RA. Cancer/Testis antigen expression on mesenchymal stem cells isolated from different tissues. Anticancer Res. 2010;30:5023–5017. [PubMed] [Google Scholar]
- 45.Chen YT, Ross DS, Chiu R, Zhou XK, Chen YY, Lee P, Hoda SA, Simpson AJ, Old LJ, Caballero O, Neville AM. Multiple cancer/testis antigens are preferentially expressed in hormone-receptor negative and high-grade breast cancers. PLoS One. 2011;6:e17876. doi: 10.1371/journal.pone.0017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunda V, Frederick DT, Bernasconi MJ, Wargo JA, Parangi S. A potential role for immunotherapy in thyroid cancer by enhancing NY-ESO-1 cancer antigen expression. Thyroid. 2014;24:1241–1250. doi: 10.1089/thy.2013.0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tinguely M, Jenni B, Knights A, Lopes B, Korol D, Rousson V, Fontecedro A Curioni, Cogliatti SB, Bittermann AG, Schmid U, et al. MAGE-C1/CT-7 expression in plasma cell myeloma: Sub-cellular localization impacts on clinical outcome. Cancer Sci. 2008;99:720–725. doi: 10.1111/j.1349-7006.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 49.Groeper C, Gambazzi F, Zajac P, Bubendorf L, Adamina M, Rosenthal R, Zerkowski HR, Heberer M, Spagnoli GC. Cancer/testis antigen expression and specific cytotoxic T lymphocyte responses in non small cell lung cancer. Int J Cancer. 2007;120:337–343. doi: 10.1002/ijc.22309. [DOI] [PubMed] [Google Scholar]
- 50.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]