Abstract
Background
Despite advances in treatment, acute myocardial infarction (MI) is still associated with significant morbidity and mortality, especially in patients with extensive damage and scar formation. Based on some promising preclinical studies, there is interest in the use of mesenchymal stromal cells (MSCs) to promote cardiac repair after acute MI. However, there is a need for a systematic review of this evidence to summarize the efficacy and safety of MSCs in preclinical models of MI. This will better inform the translation of MSC therapy for acute MI and guide the design of a future clinical trial.
Methods/design
A systematic literature search of MEDLINE, Embase, and BIOSIS Previews will be conducted. We will identify comparative preclinical studies (randomized and non-randomized) of myocardial infarction that include animals given MSC therapy versus a vehicle/placebo. The primary outcome will be left ventricular ejection fraction. Secondary and tertiary outcomes will include death, infarct size, measures of cardiac function, biochemical outcomes, and MSC retention and differentiation. Risk of bias will be assessed using the Cochrane Risk of Bias Tool. Subgroup analyses will be performed to measure how various sources of preclinical study heterogeneity affect the direction and magnitude of the primary outcome. We will meta-analyze data using inverse variance random effects modeling.
Discussion
This systematic review of preclinical evidence will provide a summary of the efficacy and safety of MSCs in animal models of MI. The results will help determine whether sufficient evidence exists to conduct a clinical trial in humans and inform its design.
Electronic supplementary material
The online version of this article (10.1186/s13643-017-0601-9) contains supplementary material, which is available to authorized users.
Keywords: Mesenchymal stromal cells, Mesenchymal stem cells, Perioperative myocardial infarction, Myocardial infarction, Preclinical, Systematic review protocol
Background
Cardiovascular disease is the leading cause of mortality in the western world [1]. Acute myocardial infarction (MI) can lead to permanent loss of cardiomyocytes and scar tissue formation and, in the event of a large area of injury, may result in heart failure and life-threatening arrhythmia. Despite advances in treatment such as coronary revascularization, some MI patients are left with extensive cardiac damage and a poor prognosis, highlighting the need to develop novel therapies to repair non-functional myocardium.
Over the past decade, mesenchymal stromal cells (MSCs)—also known as adult stem cells, marrow stromal cells, or mesenchymal stem cells—have emerged as a potential new therapy for acute MI. These cells can be isolated from a variety of tissues including bone marrow, adipose tissue, and the umbilical cord and (because they appear to be relatively immune privileged) can be subsequently delivered as an allogeneic product to patients [2]. In individual studies using preclinical models of acute MI, MSCs have been demonstrated to augment tissue repair, improve cardiac function [3], dampen the inflammatory response [4], and potentially reduce mortality [5]. However, these preclinical studies have not been systematically summarized to examine the efficacy of these cells in acute MI. Members of our group and others have demonstrated that MSC therapy acts via a myriad of paracrine pathways to dampen inflammation and augment cytoprotection [6–8]. Moreover, MSCs can improve cellular energetics by transferring mitochondria [9]. This is unlike drug-based therapeutics which largely act via “lock-and-key” mechanisms in which a specific substrate binds to a single active site matching its structure.
We are particularly interested in perioperative MI, which is an MI that occurs in the setting of inflammation and increased oxygen consumption induced by surgery [10, 11]. Perioperative MI is associated with poor outcomes, including a 30-day mortality of ~ 12% (vs. 2% for post-surgical patients without perioperative MI) [12]. Given the cytoprotective effects of MSCs, they may be particularly beneficial in the highly pro-inflammatory and catabolic setting of perioperative MI (see Fig. 1). Prior to considering a first-in-human clinical trial of MSC therapy for perioperative MI, we propose a comprehensive synthesis of the published literature. These data will determine whether additional evidence gaps remain to warrant further preclinical work, as well as future directions of MI research.
Fig. 1.
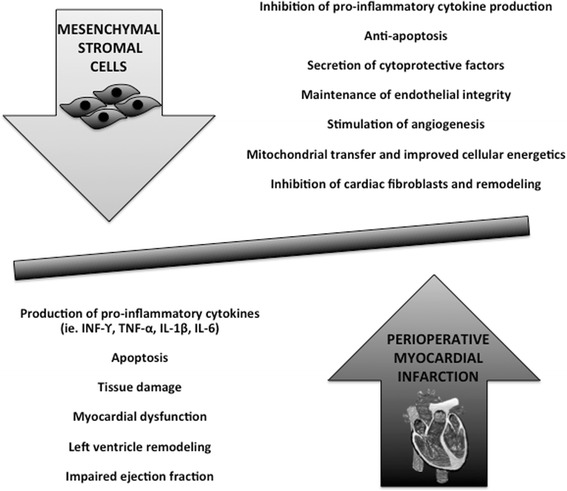
Therapeutic mechanisms of mesenchymal stromal cells in myocardial infarction
The specific aims of this systematic review are as follows:
To systematically compare the efficacy and safety of MSC therapy versus control in preclinical MI. Our primary outcome is left ventricular ejection fraction. Secondary endpoints include death, other measures of cardiac function, inflammatory markers, and vessel density. Tertiary endpoints will include cellular retention and differentiation.
Threats to internal validity will be evaluated using a modified version of the Cochrane Risk of Bias Tool for preclinical studies [13]. We will determine whether risk of bias influences the magnitude and direction of the primary endpoint.
External validity will be evaluated using subgroup analyses to measure how various sources of preclinical study heterogeneity (e.g., type of MI model, animal species, severity of MI) affect the direction and magnitude of the primary outcome.
Construct validity will be examined to evaluate the degree preclinical studies of MSC therapy for MI incorporate elements of clinical perioperative MI (e.g., pathophysiological elements), as this will be the focus of our future clinical trial.
Methods and design
Protocol
This systematic review protocol is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) reporting guidelines [14]. A summary of the protocol will be listed on the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) website (http://www.camarades.info). The final review will be reported using the PRISMA guidelines [15].
Data sources
We will search the following databases Ovid MEDLINE®, Ovid MEDLINE® In-Process & Other Non-Indexed Citations, Embase Classic + Embase, and BIOSIS. In addition, a manual review of the bibliographies of selected articles (e.g., reviews) will be performed.
Search strategy
Search strategies will be developed by our research team in collaboration with an information specialist. Prior to final implementation, all strategies will undergo Peer Review of Electronic Search Strategies (PRESS) by another senior information specialist [16, 17]. Search strategies will use controlled vocabulary (e.g., Mesenchymal Stromal Cells) and keywords (e.g., MSCs) with adjustment for each database. We will apply preclinical filters to increase search efficiency [18–20]. Duplicate citations will be removed. The example search strategy (see Additional file 1) was used to search in MEDLINE.
Eligibility criteria
Eligible studies include controlled comparative studies of preclinical MI or cardiac ischemia-reperfusion injury. We will include studies in which true randomization is performed using a method with a low risk of selection bias (such as computer random number generators and random number tables), as well as those that are quasi-randomized (i.e., by day of week or alternation) and non-randomized. This broad range of comparative studies will be included in order to answer our study question as terminology and methodology that is commonplace in clinical studies is not routinely employed in preclinical studies, and previous reviews have shown that randomization is reported in a third or less of animal studies [21]. Only peer-reviewed publications will be eligible with no restriction to publication year.
Population
We will include all preclinical in vivo models of experimentally induced MI that mimic pathophysiological aspects of clinical MI (see Table 1). Included studies will be perioperative (i.e., anesthetic provided before or concurrent to acute MI). In vitro studies, ex vivo studies, and neonatal MI models will be excluded.
Table 1.
Preclinical models of perioperative myocardial infarctiona
| Class | Example |
|---|---|
| Ligation of the left coronary artery | Open chest, closed chest |
| Ischemia-reperfusion | Global ischemia-reperfusion model |
| Cryoinjury | Liquid nitrogen cooled copper probe used to injure coronary vessel |
| Microembolism | Injection of automicrothrombotic particulates into coronaries |
| Electrocauterisation | Direct electorcauterization of a coronary vessel |
| Pharmacological induction | Isoproterenol |
| Genetic model | Watanabe heritable hyperlipidemic rabbits with acute induced infarction |
aAll included models must provide an anesthetic either pre-induction or concurrent with the induction of myocardial infarction
Intervention
Studies using MSCs will be included; the International Society of Cellular Therapy consensus statement defining criteria for MSCs will be used as a guide [22]. We will include MSCs from xenogeneic, syngeneic, or allogeneic sources of any tissue origin. All delivery routes, including direct myocardial injection, intravenous and intra-arterial, will be considered. To be eligible, MSCs must be administered as a pretreatment or no later than 7 days following the induction of MI. This timing has been chosen to reflect the possible interventional window for a perioperative clinical trial.
Our focus will be on non-manipulated cells as this will be the intervention in a potential future trial. We will exclude differentiated MSCs (e.g., differentiated into a myocyte), genetically engineered MSCs, and MSCs administered by a scaffold system. Studies using MSCs only modified for cellular identification (e.g., reporter gene systems or nanoparticles) will be included. We will also exclude studies that investigate another novel agent as a co-treatment.
Comparator
All studies with a control arm of animals that have had experimental MI or cardiac ischemia-reperfusion injury (diseased control animals) induced and were treated with placebo/vehicle will be included.
Outcomes
Primary endpoint
Left ventricular ejection fraction (LVEF), measured as a continuous variable at specific time points after MSC or control intervention, will be the primary endpoint. LVEF is a clinically meaningful endpoint since it has been linked to mortality following MI [23]. Physiologically, LVEF determines stroke volume, which together with heart rate determines cardiac output. It is also a feasible outcome as it is the most commonly reported cardiac function measure in preclinical studies [24, 25]. Various techniques are used to measure LVEF including two- or three-dimensional echocardiography, magnetic resonance imaging, and computed tomography. In our review, we will include and describe all techniques of LVEF measurement.
Secondary outcomes
Secondary outcomes will be a combination of dichotomous and continuous measures. A detailed listing of secondary/tertiary outcomes is provided in Table 2. Given the large number of outcomes, these results will be considered exploratory and interpreted cautiously. Secondary endpoints will include measures of cardiac function by echocardiography (e.g., cardiac output, fractional shortening, left ventricle end diastolic diameter, left ventricle end systolic diameter) and cardiac catheterization (e.g., left ventricular end diastolic pressure, left ventricular end systolic pressure, mean pulmonary artery pressure, right ventricular systolic pressure), biochemical outcomes (e.g., cytokines), infarct size, and vessel density. These measurements will provide additional support as to whether MSCs preserve ventricular function and prevent the pathological remodeling that occurs after MI. Furthermore, data on biochemical markers will help elucidate the role MSC therapy plays in regulating cellular and molecular mechanisms involved in the pro-inflammatory state following MI. Death will also be recorded; however, few studies use this endpoint due to considerations for animal welfare [26]. The occurrence of adverse events/negative effects with MSC administration will be recorded.
Table 2.
A priori defined secondary and tertiary outcome measures
| Outcomes | Comments and/or Examples of specific measures |
|---|---|
| Secondary outcomes: | |
| Death | Rarely used an outcome due to ethical concerns regarding animal welfare |
| Infarct size | Variety of quantifiable techniques (e.g. histological staining, nuclear imaging) |
| Cardiac function | Echocardiography |
| ◦ cardiac output | |
| ◦ left ventricle end diastolic diameter | |
| ◦ left ventricle end systolic diameter | |
| ◦ fractional shortening | |
| Cardiac catheterization | |
| ◦ cardiac output | |
| ◦ left ventricular end diastolic pressure | |
| ◦ left ventricular end systolic pressure | |
| ◦ mean pulmonary artery pressure | |
| ◦ right atrial pressure | |
| Biochemical outcomes | Proinflammatory cytokines |
| ◦ interleukin-1beta, 6 | |
| ◦ tumour necrosis factor-alpha | |
| Anti-inflammatory cytokines | |
| ◦ interleukin-10 | |
| ◦ transforming growth factor-beta1, 2, 3 | |
| Vessel density | Histological staining and quantification of vessels in cardiac tissue |
| Tertiary outcomes: | |
| Cellular retention | Imaging and quantification of labelled cells in host tissue |
| Cellular differentiation | Measurement of cardiac troponin in donor cells retained in host tissue |
Tertiary outcomes
Tertiary endpoints will include MSC retention and differentiation (see Table 2). While our primary and secondary endpoints focus on measures that evaluate the efficacy of MSCs, the homing and potential differentiation of MSCs in myocardial tissue is also of interest.
Timing
The primary outcome of left ventricle ejection fraction and secondary biochemical outcomes and death will be collected at baseline, < 6 h, 6–24 h, > 24–72 h, > 72 h–1 week, > 1–3 weeks, > 3–4 weeks, and > 4 weeks after the administration of MSCs versus controls. These detailed intervals reflect the evolution of inflammation and remodeling in MI, described by our group and others [27–31]. In preclinical models of myocardial infarction, robust increases in expression of cytokines such as TNF-α, IL-1β, and IL-6 have been noted immediately after myocardial injury and up to 24 h later [32]. This is followed by a chronic remodeling phase in which cardiomyocytes are replaced by granulation tissue and a scar is formed at the infarct. Scar formation has been demonstrated by approximately day 14 post-infarct in mice, while a canine infarct is still evolving at this time point [29]. Therefore, our prespecified time intervals will capture outcomes during the post-infarct inflammatory response and repair of cardiac function in both small and large animal models of MI. All other secondary outcomes of cardiac function and tertiary outcomes of retention and engraftment will be collected at the latest time point, > 4 weeks after administration, to capture these measurements after the pro-inflammatory state and repair has occurred.
Study selection and data extraction
Studies will be screened independently by two reviewers using dedicated cloud-based software (DistillerSR, Evidence Partners, Ottawa, Canada). Using the previously described a priori inclusion criteria, first level screening (title/abstract) will be liberal with both reviewers needed to exclude an article and one reviewer needed to include. We will be using an accelerated screening method for the title and abstracts in which the second reviewer will review records excluded by the first reviewer [33]. Second level screening (full study) will be performed independently in duplicate. If there are disagreements, the two individuals involved will review the case. If they cannot come to an agreement, a senior team member will provide the final decision. Reasons for exclusion will be recorded to enable a transparent selection process [34, 15].
Information from included studies will be collected on electronic data extraction forms. General categories include study characteristics (e.g., design), study population (e.g., species), MI model (e.g., cryoinjury), intervention and comparison (e.g., MSC dose), co-interventions (e.g., immunosuppressants, antibiotics, and cardiac medications), and preclinical outcomes (e.g., ejection fraction). Data extraction forms will be prepared a priori, and a calibration exercise will pilot five studies to refine the forms and ensure inter-rater consistency. Examples of data collection elements can be seen in Table 3.
Table 3.
A priori defined data collection elements
| Data collection element | Items |
|---|---|
| Study Characteristics | Author |
| Year of publication | |
| Funding support | |
| Country | |
| Study design | |
| Total number of animals used | |
| N per independent intervention group | |
| Species | |
| Strain | |
| Gender | |
| Weight | |
| Mean age | |
| MI model (i.e. LAD ligation, ischemia reperfusion, cryoinjury, microembolism) | |
| Intercurrent illness of animal | |
| Anesthetic administered | |
| Intervention Characteristics | Route of MSC delivery (intravenous, intracoronary, intramyocardial) |
| Timing of MSC delivery | |
| Frequency of MSC delivery | |
| Source of MSCs (syngenic, allogeneic, xenogenic) | |
| Tissue origin of MSCs (bone marrow, adipose, Wharton’s jelly) | |
| Condition of MSCs (fresh, cryopreserved) | |
| Vehicle | |
| Defining criteria for MSCs |
Risk of bias assessment
As there is no validated tool to assess risk of bias in animal studies, we will describe potential biases using a modified version of The Cochrane Risk of Bias Assessment Tool [13]. Items include concealment of allocation, random sequence generation, blinding of personnel and endpoint measurements, and completeness of endpoint reporting. We will include additional domains relevant to animal studies such as source of funding, conflict of interest, sample size calculations, similarity of groups or adjustment for confounders at baseline, random housing of animals, and animal selection at random for outcome assessment. Risk of bias assessment will be carried out in duplicate by two independent reviewers. Disagreements will be resolved using the same process listed above. Each criterion will be assigned a value of low, high, or unclear risk of bias for each included study. A summary for all included studies will be presented in a table format. We have planned an analysis to determine the effects of high vs. low risk of bias on the effect size of the primary outcome.
Assessment of external and construct validity
In preclinical studies, external validity describes the ability to generalize findings to different experimental conditions. External validity will be assessed by subgroup analysis of the primary outcome based on species, strain, age, sex, presence of intercurrent illness, MI model, ischemic time (if an ischemia-reperfusion model), MSC source (animal/tissue), timing of MSC administration (pretreatment vs. rescue) administration route, type of control, use of co-interventions (antibiotic, immunosuppressant, antihypertensive, statin, β-blocker, antiplatelet, anticoagulant therapies, all yes vs. no), and single versus multicenter study. Given the large number of analyses planned, they will be used in an exploratory manner and the results interpreted with caution. Examining the effect of differences in experimental design will inform aspects of a future clinical trial.
In preclinical studies, construct validity refers to the extent an animal model corresponds to the clinical entity it is intended to represent [35]. Construct validity will be assessed in relation to the extent the experimental systems model the clinical entity of perioperative MI using a framework based on expert opinion (see Table 4). It will help determine whether the included studies enable reliable causal inference and generalization to a potential clinical study of MSCs for perioperative MI.
Table 4.
Checklist of construct validity for preclinical perioperative myocardial infarction (PeriopMI)
| Construct validity domain | Criteria from guidelinesa,b | Specific application to PeriopMI | Justification | Yes/No |
|---|---|---|---|---|
| Animal Subjects | Matching model to age of patients in clinical setting | Middle aged to elderly animal model used | Incidence of PeriopMI increases over age 50; age >75 is an independent risk factor for PeriopMI [46] | |
| Matching model to co-morbidities in clinical setting | Animal model has ≥ 1 co-morbidity risk factor for PeriopMI, either chronic or acute (e.g. atherosclerosis, diabetes, chronic kidney disease, hypotension, acute blood loss) | Co-morbidities listed are independent risk factors for PeriopMI [47] | ||
| Outcome Measures | Matching of outcome measure to clinical setting | Late outcome measures performed (e.g. >3 weeks when scar formation and acute changes are complete) | A longer follow-up duration may reflect chronic effects of an acute therapy for PeriopMI | |
| Modeling of Disease | Matching model to human manifestation of disease | Model reflects elements of Type 1 MI (e.g. plaque rupture) and/or Type 2 (e.g. supply demand imbalance) | Clinical PeriopMI displays aspects of Type 1 and Type 2 MI [30, 42] | |
| A pro-inflammatory state is reported | Clinical PeriopMI has a large inflammatory burden [19] | |||
| Administration of Intervention | Treatment response along mechanistic pathway | Therapy given as a pretreatment (i.e. preventative) or within the first 48 h after anesthesia | Majority of PeriopMI occurs within the first 48 h after surgery | |
| Environment | Address confounds associated with setting, experimental setting | Post-operative analgesia provided | Inadeqaute post-operative analgesia increases systemic inflammation |
Abbreviations: MI myocardial infarction, PeriopMI perioperative myocardial infarction
aRecommendations to reduce threats to construct validity were identified by Henderson et al. [19]
bConstruct validity criteria suggested by ≥40% of included guidelines included in checklist
Strategy for data synthesis
Search results will be presented in a PRISMA study flow diagram [15]. Categorical variables will be summarized by frequencies/percentages, and continuous variables will be summarized by means and standard deviations or median and interquartile ranges, depending on data distribution.
Dichotomous endpoints (e.g., death) from each included study will be pooled and described as odds ratios and 95% confidence intervals. Results from outcomes with discrete data will be pooled, and meta-analysis will be performed with inverse variance random effects modeling. Continuous endpoints will be pooled using the ratio of weighted means method with inverse variance random effects modeling [36]. Ratio of means allows for pooling of outcomes expressed in different units and comparisons of effect sizes across interventions. As ratio of means is well suited for the small sample sizes of animal studies, and provides a result in a form similar to a risk ratio, we have chosen this method because of its simplified clinical interpretation. Statistical heterogeneity will be examined using I 2 tests with 95% uncertainty intervals [37]. Planned sensitivity analyses will examine heterogeneity of the primary outcome. These will be carried out according to risk of bias assessments. Selective outcome reporting will be assessed using the excess significance test (comparing the expected percentage of significant results vs. actual reported effects) [38]. An evaluation for the presence of publication bias will be conducted with funnel plot techniques and Egger’s regression test [39].
Knowledge translation
Several knowledge users of the results of this systematic review have been identified. These include the Canadian Perioperative Anesthesia Clinical Trials (PACT) Group, a network of academic perioperative medicine researchers that develop team-based approaches to investigate perioperative clinical and basic science questions (www.canadianpact.ca). Our other knowledge users include the Canadian Council on Animal Care, Canadian Society for Atherosclerosis Thrombosis, and Vascular Biology (www.csatvb.ca) and the Stem Cell Foundation of Canada (www.stemcellfoundation.ca). Through these users, our research will reach key perioperative researchers, preclinical and translational scientists, and health professionals as well as the lay community.
This work will identify gaps in the current knowledge of MSC therapy of MI. Publication of our results will also identify potential future directions of MSC for MI research as they specifically relate to perioperative MI. Most importantly, the publication of key findings of the review and meta-analysis will directly inform a potential clinical trial.
Discussion
This review proposes to systematically identify and summarize preclinical evidence that exists regarding MSC therapy in myocardial infarction models, using a rigorous methodology. We will assess the effect of MSC therapy on clinically important outcomes including cardiac function, infarct size, inflammation, and death.
In our pilot searches, we have identified two published preclinical reviews that investigated stem cells in both acute and chronic ischemia models [24, 25]. Our proposed preclinical review differs significantly from these studies in several respects. Both previous studies combined results from various stem cell types and were restricted to large animal models, whereas our review will focus on MSCs and consider both small and large animal models. The search strategies used in these papers identified 39 studies that used MSCs; however, based on our pilot search using a comprehensive strategy, we have identified approximately 200 studies to be included in our review. Most importantly, the data from these reviews included cell therapy for chronic heart failure (48% of studies); therapy of established chronic heart failure has little construct validity for the acute treatment of MI. Thus, our review will provide novel evidence to determine if a clinical study of MSCs for MI is warranted.
Given that less than 5% of high impact preclinical reports are clinically translated [40] and only 11% of clinically tested agents receive licensing, rigorous appraisal of preclinical data is needed prior to clinical testing of novel therapeutics. Historically, failed translation (preclinical to clinical) of specific therapies for stroke [41] and heart failure [42] could have been predicted by systematic reviews of animal data. Thus, our review is critical prior to conducting a resource intensive trial.
Furthermore, since the design of these preclinical studies wil include administration of anesthetic and disease induction that likely differs from spontaneous MI, this review also provides a unique opportunity to determine if MSCs may have efficacy in clinical perioperative MI. This question is of particular interest to our group as therapies that are effective in prevention of non-operative MI have failed to show benefit in perioperative MI [43–45] and there are currently few therapies for established perioperative MI. Given what is known about the mechanism of action of MSCs, they may be highly effective in the pro-inflammatory state that occurs with perioperative MI.
In summary, this review will be the first to provide an estimate of efficacy and safety of MSC therapy in preclinical models of MI. This will ultimately help determine whether sufficient evidence exists to support a first-in-human evaluation of MSC therapy for perioperative MI. Additionally, the results of this study will identify knowledge gaps and potential future areas of study in MI research.
Acknowledgements
CB was funded by a Faculty of Medicine Canadian Institutes of Health Research Summer Studentship and an Undergraduate Research Opportunity Award both from the University of Ottawa, ON, Canada.
MML was a post-doctoral fellow supported by the Heart and Stroke Foundation of Canada and The Ottawa Hospital Anesthesia Alternate Funds Association. We thank Risa Shorr (Librarian and Information Specialist, The Ottawa Hospital, Ottawa, ON, Canada) for providing assistance with the generation of a systematic search strategy.
Funding
This work received no external funding.
Availability of data and materials
Not applicable
Abbreviations
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
- MSCs
Mesenchymal stromal cells
Additional file
Description: representative search strategy. (DOCX 13 kb)
Authors’ contributions
MML, LM, and CCB conceived the study design and were responsible for the initial drafting and manuscript revisions. DJS, DF, HY, DMo, PL, DMa and PJD provided critical feedback for protocol development and the final manuscript. MML is the guarantor of the review. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
David Moher is the chief editor of Systematic Reviews. Duncan Stewart is the President CEO of Northern Therapeutics. The remaining authors have no competing interests to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13643-017-0601-9) contains supplementary material, which is available to authorized users.
Contributor Information
Carly C. Barron, Email: carly.c.barron@gmail.com
Manoj M. Lalu, Email: manojlalu@gmail.com
Duncan J. Stewart, Email: djstewart@ohri.ca
Dean Fergusson, Email: dafergusson@ohri.ca.
Homer Yang, Email: hyang@toh.on.ca.
David Moher, Email: dmoher@ohri.ca.
Peter Liu, Email: pliu@ottawaheart.ca.
David Mazer, Email: mazerd@smh.ca.
P. J. Devereaux, Email: philipj@mcmaster.ca
Lauralyn McIntyre, Phone: +1-613-737-8899, Email: lmcintyre@ottawahospital.on.ca, Email: lmcintyre@ohri.ca.
On Behalf of the Canadian Perioperative Anesthesia Clinical Trials Group, https://canadianpact.ca/.
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Lalu MM, McIntyre L, Pugliese C, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashman TJ, Gouon-Evans V, Costa KD. Mesenchymal stem cells for cardiac therapy: practical challenges and potential mechanisms. Stem Cell Rev. 2013;9:254–265. doi: 10.1007/s12015-012-9375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Akker F, Deddens JC, Doevendans PA, et al. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830:2449–2458. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 5.Llano R, Epstein S, Zhou R, et al. Intracoronary delivery of mesenchymal stem cells at high flow rates after myocardial infarction improves distal coronary blood flow and decreases mortality in pigs. Catheter Cardiovasc Interv. 2009;73:251–257. doi: 10.1002/ccd.21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.dos Santos CC, Murthy S, Hu P, et al. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. Am J Pathol. 2012;181:1681–1692. doi: 10.1016/j.ajpath.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 8.Togel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 9.Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landesberg G, Beattie WS, Mosseri M, et al. Perioperative myocardial infarction. Circulation. 2009;119:2936–2944. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373:2258–2269. doi: 10.1056/NEJMra1502824. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1,4053-4-1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.McGowan J. An evidence based checklist for the Peer Review of Electronic Search Strategies (PRESS EBC) Evid Based Libr Inf Pract. 2010;5(1):149. doi: 10.18438/B8SG8R. [DOI] [Google Scholar]
- 17.Sampson M, McGowan J, Cogo E, et al. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62:944–952. doi: 10.1016/j.jclinepi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 18.de Vries RB, Hooijmans CR, Tillema A, et al. A search filter for increasing the retrieval of animal studies in Embase. Lab Anim. 2011;45:268–270. doi: 10.1258/la.2011.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooijmans CR, Tillema A, Leenaars M, et al. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim. 2010;44:170–175. doi: 10.1258/la.2010.009117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leenaars M, Hooijmans CR, van Veggel N, et al. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim. 2012;46:24–31. doi: 10.1258/la.2011.011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 23.El Aidi H, Adams A, Moons KG, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031–1045. doi: 10.1016/j.jacc.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 24.Jansen Of Lorkeers SJ. Eding JE, Vesterinen HM, et al. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: systematic review and meta-analysis of large animal studies. Circ Res. 2015;116:80–86. doi: 10.1161/CIRCRESAHA.116.304872. [DOI] [PubMed] [Google Scholar]
- 25.van der Spoel TI, Jansen of Lorkeers SJ. Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 26.Emerson M. Refinement, reduction and replacement approaches to in vivo cardiovascular research. Br J Pharmacol. 2010;161:749–754. doi: 10.1111/j.1476-5381.2010.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho YH, Cha MJ, Song BW, et al. Enhancement of MSC adhesion and therapeutic efficiency in ischemic heart using lentivirus delivery with periostin. Biomaterials. 2012;33:1376–1385. doi: 10.1016/j.biomaterials.2011.10.078. [DOI] [PubMed] [Google Scholar]
- 28.Deten A, Volz HC, Briest W, et al. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–340. doi: 10.1016/S0008-6363(02)00413-3. [DOI] [PubMed] [Google Scholar]
- 29.Dewald O, Ren G, Duerr GD, et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughey CC, James FD, Ma L, et al. Diminishing impairments in glucose uptake, mitochondrial content, and ADP-stimulated oxygen flux by mesenchymal stem cell therapy in the infarcted heart. Am J Physiol Cell Physiol. 2014;306:C19–C27. doi: 10.1152/ajpcell.00156.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richardson JD, Psaltis PJ, Frost L, et al. Incremental benefits of repeated mesenchymal stromal cell administration compared with solitary intervention after myocardial infarction. Cytotherapy. 2014;16:460–470. doi: 10.1016/j.jcyt.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Ono K, Matsumori A, Shioi T, et al. Cytokine gene expression after myocardial infarction in rat hearts: possible implication in left ventricular remodeling. Circulation. 1998;98:149–156. doi: 10.1161/01.CIR.98.2.149. [DOI] [PubMed] [Google Scholar]
- 33.Khangura S, Konnyu K, Cushman R, et al. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1:10,4053-1-10. doi: 10.1186/2046-4053-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Henderson VC, Kimmelman J, Fergusson D, et al. Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Med. 2013;10:e1001489. doi: 10.1371/journal.pmed.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol. 2008;8:32,2288-8-32. doi: 10.1186/1471-2288-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin. Trials. 2007;4:245–253. doi: 10.1177/1740774507079441. [DOI] [PubMed] [Google Scholar]
- 39.Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15:235–243. doi: 10.2188/jea.15.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contopoulos-Ioannidis DG, Ntzani E, Ioannidis JP. Translation of highly promising basic science research into clinical applications. Am J Med. 2003;114:477–484. doi: 10.1016/S0002-9343(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 41.Horn J, de Haan RJ, Vermeulen M, et al. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke. 2001;32:2433–2438. doi: 10.1161/hs1001.096009. [DOI] [PubMed] [Google Scholar]
- 42.Lee DS, Nguyen QT, Lapointe N, et al. Meta-analysis of the effects of endothelin receptor blockade on survival in experimental heart failure. J Card Fail. 2003;9:368–374. doi: 10.1054/S1071-9164(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 43.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 44.Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1504–1513. doi: 10.1056/NEJMoa1401106. [DOI] [PubMed] [Google Scholar]
- 45.POISE Study Group. Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 46.Ashton CM, Petersen NJ, Wray NP, et al. The incidence of perioperative myocardial infarction in men undergoing noncardiac surgery. Ann Intern Med. 1993;118:504–510. doi: 10.7326/0003-4819-118-7-199304010-00004. [DOI] [PubMed] [Google Scholar]
- 47.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


