Abstract
Psychological stress can have devastating and lasting effects on a variety of behaviors, especially those associated with mental illnesses such as anxiety and depression. Animal models of chronic stress are frequently used to elucidate the mechanisms underlying the relationship between stress and mental health disorders and to develop improved treatment options. The current study expands upon a novel chronic stress paradigm for mice: predatory stress. The predatory stress model incorporates the natural predator-prey relationship that exists among rats and mice and allows for greater interaction between the animals, in turn increasing the extent of the stressful experience. In this study, we evaluated the behavioral effects of exposure to 15 days of predatory stress on an array of behavioral indices. Up to 2 weeks after the end of stress, adult male mice showed an increase of anxiety-like behaviors as measured by the open field and social interaction tests. Animals also expressed an increase in depressive-like behavior in the sucrose preference test. Notably, performance on the novel object recognition task, a memory test, improved after predatory stress. Taken as a whole, our results indicate that 15 exposures to this innovative predatory stress paradigm are sufficient to elicit robust anxiety-like behaviors with evidence of co-morbid depressive-like behavior, as well as changes in cognitive behavior in male mice.
Keywords: Predatory stress, Depressive-like behavior, Anxiety-like behavior, Mice
1. Introduction
Anxiety and depression impact the lives of an estimated 5.2% and 13.3% of adults, respectively [1]. Chronic stress has been implicated in the development and severity of anxiety disorders, as evident in post-traumatic stress disorder [4,5]. In addition, a major stressful life event of an acute nature often precedes a depressive episode and chronic stress increases the overall lifetime risk for developing depression [2,3]. Given the relationship among stress, anxiety, and depression, and the need for additional therapeutic options, chronic stress models are often used to study anxiety-like and depressive-like behaviors in a variety of rodent species, including mice.
Chronic stress paradigms that precipitate lasting effects are limited in adult male animals [6,7]. In addition to behavioral changes, standard effects of chronic stress include changes in corticosterone levels, vasculature, hippocampal volume, amygdaloid plasticity, body weight, and neurochemistry [5,8–12]. A commonly used chronic stress paradigm is chronic mild unpredictable stress, which has both face validity in producing symptoms and predictive validity for response to antidepressants in rats [13]; however, the effects of this paradigm in mice have been less consistent [6,7]. Social defeat is another commonly used stress paradigm in rats [11], but a percentage of mice are resistant to the negative effects of chronic social defeat [14–16]. An alternative chronic stress paradigm is the use of predatory stress, which is more potent than exposure to chronic mild unpredictable stress in mice [17].
Predatory aggression, a type of psychological stressor, can be used to test the effects of stress on anxiety-like and depressive-like behaviors in rodents. Predatory scents such as cat odor have been presented to experimental mice as part of the stress paradigm [18]. An alternative source of predatory stress is the use of live predators, usually cats or snakes [19]. Barriers that curb the interaction of the predator with the prey are essential for the safety of the animals, but these safeguards limit the level of stress induced by exposure to a predator the predatory–prey experience. Furthermore, it may not be feasible to bring certain types of predator animals into the laboratory because of the need for specialized facilities that may not be readily available in many settings and potential inadvertent sensory contamination. Rats are natural predators of mice and these species already frequently share colony facilities (although in separate housing rooms). The accessibility of rats in the research setting, paired with the aggression that rats naturally express toward mice, led to the development of a rat-on-mouse predatory chronic stress paradigm [17]. This paradigm protects the mouse from injury by placing it in an activity ball which allows the rat to move the mouse around the cage but prevents direct physical contact. This design exposes the mouse to the sight, sound, smell, and physical effects of one of its natural predators, the rat, in a controlled laboratory environment. Previous use of the predatory stress model demonstrated that a 28-day period initiated during adolescence elicited anxiety-like and depressive-like behaviors in adult mice one week after the end of stress [17].
Here we expand the use of this predatory stress model to determine if the robust behavioral effects previously reported can be produced in adult male mice with a shorter duration of exposure to the stress paradigm (15 days). In addition, we assessed the behavioral effects of the paradigm as much as two weeks after the conclusion of the stress exposure and demonstrate lasting effects of the predatory stress exposure on behavior. Collectively, the data presented here demonstrate that the rat-on-mouse predatory stress paradigm causes lasting changes in behavior of adult male mice following a 15 day exposure to the chronic stress paradigm.
2. Materials and methods
2.1. Animals
Adult male C57/Bl6 mice (Stress n = 10; Controls n = 8) were single-housed and maintained on a reverse 12:12 h light:dark cycle in a temperature and humidity controlled environment in an AAALAC-approved facility. Mice were given ad libitum food and water throughout the experiment, and were housed in a room separate from the rats. Twenty adult Long Evans rats were used as the predatory animals. These rats were pair-housed and maintained on a 12:12 h light:dark cycle. Rats had access to ad libitum water throughout the experiment. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Predatory stress
The predatory stress paradigm consisted of placing a mouse in a 5″ diameter plastic hamster ball (Super Pet, Elk Grove Village, IL; material # 1000079348). Each ball was then placed in the cage of two Long Evans predator rats that were fed a restricted diet of 8 standard lab chow pellets per day. For 30 min on 15 consecutive days, the rats were allowed to freely agitate the hamster balls. To ensure the safety of the mice, the lids were secured for the duration of the stress period. Mice in the control condition were exposed to daily cage transport and handling for 15 consecutive days.
2.3. Behavioral tests
We used a variety of established behavioral tests to measure patterns in anxiety-like and depressive-like behaviors in these mice. Behavioral tests were performed in the following order before and after stress: sucrose preference, open field, novel object, marble burying, and social interaction (see Fig. 1). Control mice were exposed to the same sequence of behavioral tests with a 21-day break between exposures to control for the duration of the stress exposure and waiting period.
Fig. 1.
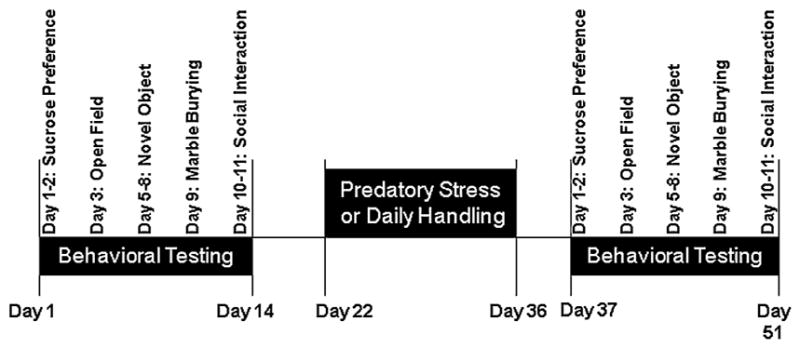
Timeline of behavioral testing and predatory stress. Behavioral testing was conducted prior and following chronic stress in order to measure the effects of the stress paradigm on anxiety- and depressive-like behaviors, as well as on cognition. Behaviors were completed in the following order before and after stress: sucrose preference, open field, novel object, marble burying, and social interaction. Predatory stress lasted 15 days and post-behavioral testing began the day after the end of stress.
2.3.1. Affective-like behaviors
Open field: The open field test is used as a measure for anxiety-like behavior and general locomotor activity [20–22]. Mice were placed in the center of a 45 cm × 45 cm square box and allowed to explore for 10 min. Mice were videotaped and scored using Cleversys, Inc. behavioral tracking software (Reston, VA). Social interaction: The social interaction test is a measure of anxiety-like behavior in mice [23,24]. Mice were placed in the open field box for 5 min along with a stimulus male mouse of similar size. The total time the experimental mouse spent in active contact with the stimulus animal was measured using Cleversys, Inc. behavioral tracking software. Marble burying: The marble burying test is used as a measure of anxiety-like behavior [25]. Mice were placed in a standard rat cage containing 5 inches of bedding and 20 black marbles arranged in 4 columns, and allowed to roam freely for 30 min. A marble was considered buried if 50% or less of the marble was visible after 30 min. Sucrose preference: The sucrose preference test is traditionally an assessment of anhedonia, a central symptom of depression in humans [26,27]. We used a highly palatable food paradigm in this study, in which mice were exposed to sucrose enriched pellets and their standard diet for 1 h on two consecutive days [28]. Pellets were weighed before and after the 1-h testing period and the weights were averaged together. The mice were also weighed in order to control for body mass.
2.3.2. Novel object recognition task
The novel object recognition task assesses learning and memory in rats and mice [29,30]. Mice were placed in the center of a 45 cm × 45 cm open field box. Two identical objects were placed diagonally from each other and animals were allowed to explore for 10 min. During the no-delay task, one object was removed and replaced by a different object that was novel to the animal. During the hour- and 24-h-delay tasks, the mice were returned to their home cage for 1 or 24 h, respectively, after the initial exposure to the objects. Time spent with each object was recorded and assessed using Cleversys, Inc. behavioral tracking software.
2.4. Statistics
All statistical analyses were computed and graphed in Graph Pad 6.0. Change scores were calculated by subtracting the first round of scores from the second round. Unpaired two-tailed t-tests were used to assess differences in change scores between the control and stress groups. The alpha value was set to p < 0.05.
3. Results
3.1. Predatory stress increases negative affective-like behaviors in the open field, social interaction, and sucrose preference tests
3.1.1. Open field
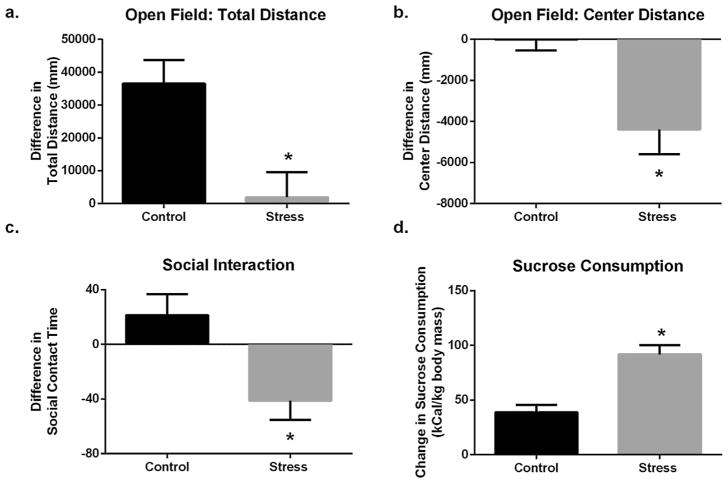
Control and stress mice behaved differently after repeated testing in the open field. Control mice travelled a greater distance during the second round of open field test, whereas the mice exposed to predatory stress traveled similar distances during the pre- and post-stress rounds, resulting in a between-group difference (t15 = 3.263; p = 0.0052). Additionally, stressed mice travelled significantly less distance in the center after predatory stress compared to controls (t15 = 3.161; p = 0.0065; Fig. 2a, b).
Fig. 2.
Predatory stress increased affective behaviors. (a) Control mice travelled a greater distance in the open field during the second round. However, mice exposed to predatory stress did not alter the distance travelled between the two rounds of testing. (b) Stressed mice travelled significantly less distance in the center after predatory stress. (c) Total time spent in social contact was decreased by predatory stress but unaltered by repeated testing. (d) When compared to the control group, mice exposed to stress significantly increased their consumption of sucrose. Data shown are mean ± SEM; asterisk indicates significance at p < 0.05 in an unpaired two-tailed t-test.
3.1.2. Social interaction
There were no differences in social contact between the first and second exposure for the control group of mice. In contrast, predatory stress decreased the total time spent in social contact with a novel conspecific (t15 = 3.004; p = 0.0089; Fig. 2c).
3.1.3. Marble burying
Marble burying is used as a measure of anxiety-like behavior in mice. The number of marbles buried between the first and second exposures to the task was not influenced by chronic stress as compared to mice from the control condition (t8 = 0.1521; p = 0.8829; data not shown).
3.1.4. Sucrose preference
After chronic predatory stress, adult males increased consumption of sucrose on the second day of post-stress testing when compared to the control group (t15 = 4.798; p = 0.0002; Fig. 2d).
3.2. Predatory stress alters behavior in the 24-h delay novel object test
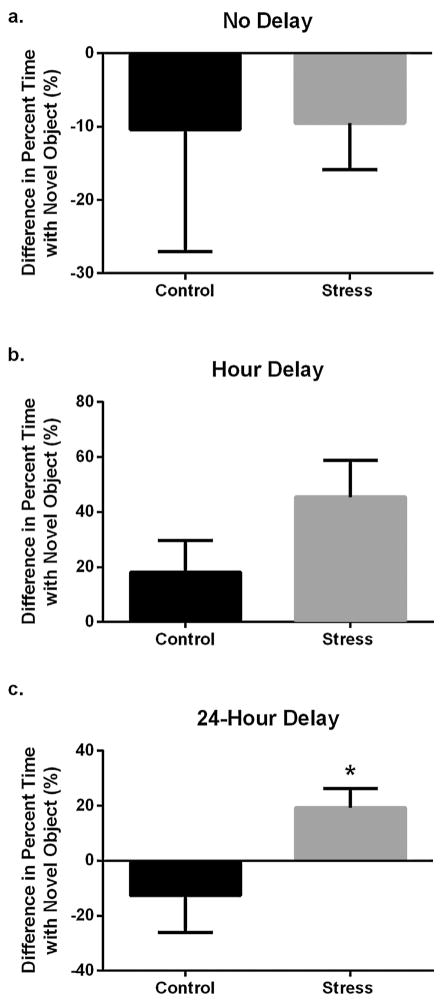
During the no-delay and hour-delay tasks, predatory stress did not affect percent time spent with the novel object (p > 0.05; Fig. 3a, b). In the 24-h delay trial, stress increased the percentage of time the mice spent with the novel object as compared to the control group (t15 = 2.184; p = 0.0453; Fig. 3c).
Fig. 3.
Behavior in the novel object test after the 24-h delay was altered by predatory stress. (a) Percentage of time spent with the novel object was unaltered by predatory stress during the no-delay task (p > 0.05). (b) Percentage of time spent with the novel object was unaltered by predatory stress during the hour-delay task (p > 0.05). (c) Chronic predatory stress increased the percentage of time spent with novel object in the 24-h delay task. Data shown are mean ± SEM; asterisk indicates significance at p < 0.05 in a paired two-tailed t-test.
4. Discussion
Given the ubiquitous nature of stress and its impact on numerous diseases, most notably mental health disorders such as anxiety and depression, the search for effective, reliable, and valid mouse models of stress have received significant attention. The present study demonstrated that predatory stress has face validity and reliability in producing anxiety- and depressive-like behaviors in mice. Moreover, we demonstrate here that 15 days of stress is sufficient to produce changes in behavior that last up to two weeks after stress. This extends previous findings by demonstrating that a shorter exposure to stress (15 vs. 28 days) produces robust behavioral changes for weeks following the conclusion of the stress exposure. Numerous previous studies have shown behavioral changes after chronic stress paradigms lasting between 5 and 21 days [17,31–34]. Collectively, these data demonstrate that the predatory stress paradigm produces robust and sustained increases in anxiety-like and depressive-like behaviors in adult male mice, as well as changes in memory retention.
Stress and anxiety are behaviorally as well as biologically linked in both humans and rodents [35,36]. In mice, anxiety-like behaviors can be assessed by examining behavioral change in the open field test, the social interaction test, and the marble-burying test [20–25,37–40]. In this study we show that adult mice travelled similar distances in the open field before and after predatory stress (Fig. 2a). In contrast, control mice that were not exposed to chronic stress travelled more in the second round of open field testing, as illustrated by the large change score (Fig. 2a). These data indicate that whereas control mice increased their exploration of the open field box on the second exposure, stressed mice did not increase their exploration of the environment, suggesting an anxiety-like phenotype. Similarly, mice after predatory stress travelled less distance in the center of the open field and this change was not observed in the controls, again indicative of anxiety-like behavior (Fig. 2b). Predatory stress additionally altered behavior in the social interaction test. The change in total time spent interacting between the first and second round of the test was significantly different in the two groups, with stress reducing the duration of social interaction (Fig. 2c). Because the social interaction test is an established measure of anxiety-like behaviors in mice, the changes observed in this study are evidence for an anxiety-like phenotype in mice after 15 days of predatory stress [23].
All together, these results indicate that chronic predatory stress elicited anxiety-like behaviors in mice up to 2 weeks after the conclusion of the stress exposure and these changes were not accounted for by repeated exposure. These data are consistent with the previous report investigating the effects of predatory stress that demonstrated an increase in anxiety-like behavior; however, the stress paradigm lasted 28 consecutive days and behavioral assessment concluded one week following stress [17]. This prior study showed that predatory stress, like chronic unpredictable stress, produced anxiety-like behavior in the marble-burying test [17]. Interestingly, this was the only test out of the battery of tests of anxiety-like behavior conducted in the current study that did not produce a significant finding. However, it is possible that a ceiling effect influenced the results of the current study, because before stress exposure the mice were already burying over 50% of the marbles. An alternative explanation for this inconsistency is that the mice in the previous study of this predatory stress paradigm were adolescents, and adolescent and adult rodent behavior has been shown to differ in multiple facets [41,42]. Notably, however, the overall effects remain the same, in that the paradigm produced anxiety-like behaviors in both cases.
Other chronic stress paradigms have produced increases in anxiety-like behavior to varying degrees and of varying durations. Similar to the data presented here, chronic unpredictable stress has been shown to elicit changes in open field behavior [6] and social interaction [43]. In contrast, the majority of the chronic unpredictable stress studies used paradigms lasting at least three weeks, and tested animals during or immediately after the stress paradigm [6,13,43]. Inescapable foot shock has been shown to produce long lasting effects on open field behavior and fecal boli production for 14–21 days, respectively [40], but this type of stressor lacks the ethological validity of predatory stress [44]. In this study, we demonstrate the ability for a shorter stress period (15 days) and an ethologically relevant stressor to produce robust anxiety-like behaviors.
In addition to the role stress plays in anxiety disorders, stress is implicated in the onset and severity of depressive symptoms [45]. Importantly, 50% of individuals with generalized anxiety display signs of depression, illustrating the correlation between anxiety and depression, as well as stress [46,47]. Chronic stress induces several behavioral changes in mice, many of which are interpreted as depressive-like [6]. In this study, we used sucrose preference to assess depressive-like behaviors. Anhedonia is cited as a key symptom of depression in humans and provides a model for measuring depressive-like behaviors in rodents [13]; however, either a decrease or an increase in appetite is part of the criteria for diagnosis of major depressive disorder in humans [48]. Recent studies have found that chronic stress increases consumption of palatable foods, potentially mediated by glucocorticoids and/or ghrelin [34,49–51]. In our study, we found that predatory stress increased consumption of the sucrose-enriched pellets, indicating the ability of predatory stress to induce feeding of a highly palatable food (Fig. 2d). Control mice did not increase their consumption of sucrose pellets to the same degree as the stressed animals (Fig. 2d). Because our study examines consumption of sucrose-enriched pellets over the course of two days and not throughout the entire experiment, the results presented here demonstrate a novel effect of stress on consumption of palatable foods.
Cognitive changes can be coincident with mood disorders [52,53] and cognitive performance in general may be affected by stress [54]. Generally, an inverted U-shaped curve has been thought to describe the relationship between stress intensity and memory formation [54]. In this study we used the novel object recognition task to measure memory formation and retention before and after chronic stress. In the no-delay and hour-delay tasks, mice spent similar amounts of time exploring the novel object both before and after stress, indicating that the animals recognized the familiar object and stress did not impact short-term memory. In contrast, predatory stress increased time spent with the novel object after the 24-h delay (Fig. 3), suggesting that the chronic stress enhanced recognition memory at this time point. These data contradict another study that demonstrated a detrimental effect of predatory stress on recognition memory in mice [55]. However, this previous study used a different predatory stress paradigm that consisted of a single acute exposure to a pair of rats underneath the novel object apparatus during the acquisition phase of the task [55]. Importantly, several other studies of chronic stress in mice and rats have either failed to elicit memory impairments or have shown improvements in memory after chronic stress [10,33,56].
One limitation of the current study is the use of only male mice. Previous studies from our lab [31,32,57–59] and others [10,60–62] have demonstrated distinct sex differences in the effects chronic stress on both physiology and behavior; therefore, the current results cannot be generalized to females. Given the prevalence of depression among women [63], an important future direction for this line of research will be to establish the effects of predatory stress in female mice. Despite this limitation, the current data set extends previous findings to establish predatory stress as a valid model of chronic stress exposure in adult male mice.
Taken together, these data demonstrate that predatory stress is able to elicit anxiety- and depressive-like behaviors after only 15-days of stress, behavioral changes that are observed up to 2 weeks after the end of stress. The wide range of behavioral changes shown in this study indicates that this relatively new predatory stress paradigm can reliably induce sustained anxiety- and depressive-like behaviors in adult male mice. With the high prevalence of anxiety and depression in society, mouse models of chronic stress that result in anxiety-like and depressive-like phenotypes are critical in advancing our understanding of the mechanisms of these disorders and in finding effective treatments. Given its potent and long-lasting effects, the predatory stress model has the potential to be a powerful tool in advancing the field of stress physiology.
HIGHLIGHTS.
Predatory stress elicits anxiety-like and depressive-like behaviors in male mice.
Two-weeks of predatory stress induce sustained alterations in behavior.
Predatory stress is a validated chronic stress paradigm in mice.
Acknowledgments
Role of funding source
Funding for the studies in this manuscript was provided by the NASA grant NNX08BA08G. Salary support for J Burgado was provided by the NIH grant R25GM097636 and salary support for CJ Barnum was provided by the grant for Graduate and Postdoctoral Training in Toxicology 5T32 ES 012870-09. Funding sources did not have a role in the study design, data collection, analysis and interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
The authors would like to thank Mandy Bekhbat and Leah Vaughan for their technical assistance.
References
- 1.Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115–23. doi: 10.1001/archpsyc.59.2.115. [DOI] [PubMed] [Google Scholar]
- 2.Ravindran AV, Griffiths J, Waddell C, Anisman H. Stressful life events and coping styles in relation to dysthymia and major depressive disorder: variations associated with alleviation of symptoms following pharmacotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:637–53. doi: 10.1016/0278-5846(95)00108-8. [DOI] [PubMed] [Google Scholar]
- 3.Dura JR, Stukenberg KW, Kiecolt-Glaser JK. Chronic stress and depressive disorders in older adults. J Abnormal Psychol. 1990;99:284–90. doi: 10.1037//0021-843x.99.3.284. [DOI] [PubMed] [Google Scholar]
- 4.Pynoos RS, Steinberg AM, Piacentini JC. A developmental psychopathology model of childhood traumatic stress and intersection with anxiety disorders. Biol Psychiatry. 1999;46:1542–54. doi: 10.1016/s0006-3223(99)00262-0. [DOI] [PubMed] [Google Scholar]
- 5.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–57. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–9. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 9.Neigh GN, Owens MJ, Taylor WR, Nemeroff CB. Changes in the vascular area fraction of the hippocampus and amygdala are induced by prenatal dexamethasone and/or adult stress. J Cereb Blood Flow Metabol. 2010;30:1100–4. doi: 10.1038/jcbfm.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–73. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- 11.Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, et al. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neurosci Biobehav Rev. 2005;29:83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 14.Meduri JD, Farnbauch LA, Jasnow AM. Paradoxical enhancement of fear expression and extinction deficits in mice resilient to social defeat. Behav Brain Res. 2013;256:580–90. doi: 10.1016/j.bbr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Han Q, Yang L, Liu Y, Lv N, Yu J, Wu G, et al. Resiliency to social defeat stress relates to the inter-strain social interaction and is influenced by season variation. Neurosci Lett. 2013;561:13–7. doi: 10.1016/j.neulet.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–73. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnum CJ, Pace TW, Hu F, Neigh GN, Tansey MG. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J Neuroinflamm. 2012;9:9. doi: 10.1186/1742-2094-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev. 2005;29:1123–44. doi: 10.1016/j.neubiorev.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Miura H, Ando Y, Noda Y, Isobe K, Ozaki N. Long-lasting effects of inescapable-predator stress on brain tryptophan metabolism and the behavior of juvenile mice. Stress. 2011;14:262–72. doi: 10.3109/10253890.2010.541539. [DOI] [PubMed] [Google Scholar]
- 20.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 21.Carola V, D’Olimpio F, Brunamonti E, Mangia F, Renzi P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res. 2002;134:49–57. doi: 10.1016/s0166-4328(01)00452-1. [DOI] [PubMed] [Google Scholar]
- 22.Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–60. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 23.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 24.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 25.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–7. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 26.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 27.Hales CA, Stuart SA, Anderson MH, Robinson ES. Modelling Cognitive Affective Biases in Major Depressive Disorder using Rodents. British journal of pharmacology. 2014 doi: 10.1111/bph.12603. http://dx.doi.org/10.1111/bph.12603. [DOI] [PMC free article] [PubMed]
- 28.Ingallinesi M, Rouibi K, Le Moine C, Papaleo F, Contarino A. CRF2 receptor-deficiency eliminates opiate withdrawal distress without impairing stress coping. Mol Psychiatry. 2012;17:1283–94. doi: 10.1038/mp.2011.119. [DOI] [PubMed] [Google Scholar]
- 29.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–9. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 30.Sargolini F, Roullet P, Oliverio A, Mele A. Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res. 2003;138:153–63. doi: 10.1016/s0166-4328(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 31.Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–8. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowman RE, Maclusky NJ, Diaz SE, Zrull MC, Luine VN. Aged rats: sex differences and responses to chronic stress. Brain Res. 2006;1126:156–66. doi: 10.1016/j.brainres.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 34.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–62. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 35.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–8. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 36.Negron-Oyarzo I, Perez MA, Terreros G, Munoz P, Dagnino-Subiabre A. Effects of chronic stress in adolescence on learned fear, anxiety, and synaptic transmission in the rat prelimbic cortex. Behav Brain Res. 2014;259:342–53. doi: 10.1016/j.bbr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Nicolas LB, Kolb Y, Prinssen EP. A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol. 2006;547:106–15. doi: 10.1016/j.ejphar.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 40.Van Dijken HH, Van der Heyden JA, Mos J, Tilders FJ. Inescapable footshocks induce progressive and long-lasting behavioural changes in male rats. Physiol Behav. 1992;51:787–94. doi: 10.1016/0031-9384(92)90117-k. [DOI] [PubMed] [Google Scholar]
- 41.Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2007;176:210–5. doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 43.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–20. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 44.Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–46. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Hammen C. Stress generation in depression: reflections on origins, research, and future directions. J Clin Psychol. 2006;62:1065–82. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- 46.Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3:244–54. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackenzie CS, Reynolds K, Chou KL, Pagura J, Sareen J. Prevalence and correlates of generalized anxiety disorder in a national sample of older adults. Am J Geriatr Psychiatry. 2011;19:305–15. doi: 10.1097/JGP.0b013e318202bc62. [DOI] [PubMed] [Google Scholar]
- 48.Association AP; Association AP, editor. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 49.Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 50.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–92. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Aran A, Vieta E, Reinares M, Colom F, Torrent C, Sanchez-Moreno J, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry. 2004;161:262–70. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 53.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–6. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 54.Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–30. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- 55.Urani A, Philbert J, Cohen C, Griebel G. The corticotropin-releasing factor 1 receptor antagonist, SSR125543, and the vasopressin 1b receptor antagonist, SSR149415, prevent stress-induced cognitive impairment in mice. Pharmacol Biochem Behav. 2011;98:425–31. doi: 10.1016/j.pbb.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 56.Kirshenbaum GS, Saltzman K, Rose B, Petersen J, Vilsen B, Roder JC. Decreased neuronal Na+, K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behav. 2011;10:542–50. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 57.Harrell CS, Hardy E, Boss-Williams K, Weiss JM, Neigh GN. Sex and lineage interact to predict behavioral effects of chronic adolescent stress in rats. Behav Brain Res. 2013;248:57–61. doi: 10.1016/j.bbr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pyter LM, Kelly SD, Harrell CS, Neigh GN. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immunity. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Gluco-corticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 61.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–16. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 62.Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]