Abstract
Triacylglycerols (TAG) serve as the predominant form of energy storage in mammalian cells, and TAG synthesis influences conditions such as obesity, fatty liver, and insulin resistance. In most tissues, the glycerol 3-phosphate pathway enzymes are responsible for TAG synthesis, and the regulation and function of these enzymes is therefore important for metabolic homeostasis. Here we review the sites and regulation of glycerol-3-phosphate acyltransferase (GPAT), acylglycerol-3-phosphate acyltransferase (AGPAT), lipin/phosphatidic acid phosphatase (PAP), and diacylglycerol acyltransferase (DGAT) enzyme action. We highlight the critical roles that these enzymes play in human health by reviewing Mendelian disorders that result from mutation in the corresponding genes. We also summarize the valuable insights that genetically engineered mouse models have provided into the cellular and physiological roles of GPATs, AGPATs, lipins and DGATs. Finally, we comment on the status and feasibility of therapeutic approaches to metabolic disease that target enzymes of the glycerol 3-phosphate pathway.
1. Overview of the glycerol 3-phosphate pathway for lipid synthesis
Triacylglycerols (TAG) play an important role in the maintenance of energy homeostasis [1]. In mammals, TAG serves as the predominant form of energy storage in adipose tissue, and can be hydrolyzed during fasting conditions to release fatty acids for energy generation in distant tissues [2]. Adipose tissue is designed to store large amounts of TAG, but when the storage capacity is exceeded (obesity) or is impaired (lipodystrophy), ectopic TAG storage may occur in tissues such as liver, skeletal and cardiac muscle, and pancreatic beta cells, contributing to conditions such as non-alcoholic fatty liver disease and insulin resistance [3–8]. TAG storage within tissues occurs in the form of lipid droplets (LDs), which typically contain a core of TAG and other neutral lipids (e.g., cholesteryl esters and retinyl esters) surrounded by a phospholipid monolayer [4]. The synthesis of TAG and their incorporation into LDs may prevent the accumulation of fatty acids and other lipids that could otherwise become toxic within cells [5]. In addition to a role as a lipid reservoir, LDs are linked to diverse cellular functions. It is becoming widely appreciated that LDs function beyond lipid storage, including serving as a reservoir for transcription factors and histone proteins, platforms for viral replication, and for protein degradation [6–9].
The regulation of TAG biosynthesis is an important determinant of LD biogenesis and is a key theme of this review. Early morphological studies revealed that LDs are tightly associated with the endoplasmic reticulum (ER) [10,11]. In most mammalian tissues, the majority of TAG is synthesized at the ER membrane through the glycerol 3-phosphate pathway (originally described by Kennedy), which involves the esterification of fatty acids to a glycerol 3-phosphate backbone [12–15]. The resulting TAG is then packaged into “initial LDs” (iLD) that are stored in the cytoplasm [16,17]. iLDs may undergo expansion through recruitment of TAG synthesis enzymes via ER-LD membrane bridges, a process that requires Arf1/COP-1 machinery. Local TAG synthesis occurs to convert the iLDs to “expanding LDs” (eLD) [18,19]. In the small intestine, the majority of TAG is derived from esterification of fatty acids to monoacylglycerol, which is abundant in the intestinal lumen as a result of hydrolysis of dietary lipids [20,21]. Excellent reviews have focused on the monoacylglycerol pathway [22,23], and here, we will focus largely on the glycerol 3-phosphate pathway, which is active in most tissue types.
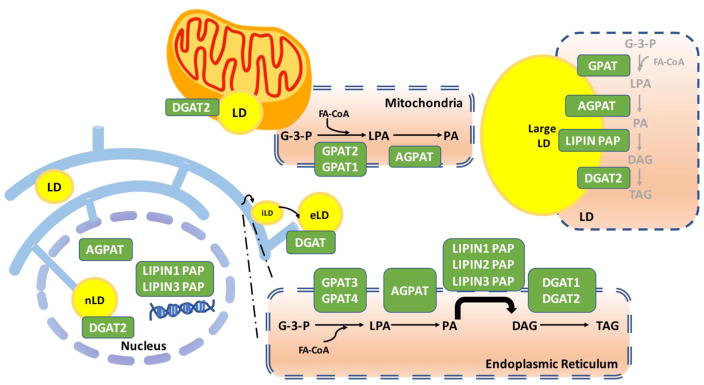
The glycerol 3-phosphate pathway is regulated by the sequential action of glycerol-3-phosphate acyltransferases (GPATs)], 1-acylglycerol-3-phosphate acyltransferases (AGPATs) [24], lipin phosphatidic acid phosphatase (PAP) proteins, and diacylglycerol acyltransferases (DGATs) (Fig. 1). GPATs, AGPATs and DGATs are resident membrane proteins, whereas lipins do not possess transmembrane domains and translocate to the ER membrane and other membranes transiently to catalyze their lipid phosphatase reaction [24–26]. A large proportion of TAG synthesis occurs at the ER, but these enzymes may also localize to LDs, the mitochondrial membrane, and in the nucleus (Fig. 1) [27].
Fig. 1.
Glycerolipid biosynthetic pathway enzyme localization to subcellular compartments. Four major enzyme families control lipid synthesis including glycerol phosphate acyltransferase (GPAT), acylglycerolphosphate acyltransferase (AGPAT), lipin (phosphatidate phosphatase), and diacylglycerol aceyltransferase (DGAT) enzymes. LD: lipid droplets; iLD: initial lipid droplets; eLD: expanding lipid droplets; nLD: Nuclear lipid droplets; G-3-P: Glycerol-3-phosphate; LPA: Lysophosphatidic acid; PA: Phosphatidic acid; DAG: Diacylglycerol; TAG: Triacylglycerol; FA-CoA: Fatty acyl-CoA.
1.1 TAG synthesis enzymes on the endoplasmic reticulum
The first and rate-limiting step of TAG synthesis in the ER is catalyzed by GPAT3 [28] or GPAT4 [29], which convert glycerol 3-phosphate (G-3-P) and fatty acyl CoA (FA-CoA) to lysophosphatidate (LPA). LPA is then converted to phosphatidate (PA) by AGPAT enzymes. Most AGPAT enzymes (AGPAT1, AGPAT2 [30], AGPAT3 [30], AGPAT4 [18] and AGPAT5 [31]) have been reported to reside in the ER, anchored there through a conserved transmembrane motif. PA is subsequently dephosphorylated to diacylglycerol (DAG) by lipin PAP enzymes (lipin 1, 2 or 3) [32,33]. The final step of TAG biosynthesis is the conversion of DAG to TAG, which is catalyzed by DGAT1 and DGAT2. The ER membrane localization of DGAT1 and DGAT2 may be distinct, as shown in both plants and mammals [34,35]. In addition to the modulation of TAG synthesis, enzymes of the glycerol 3-phosphate pathway also influence phospholipid synthesis. PA generated by AGPATs serves as a substrate for the synthesis of acidic phospholipids, phosphatidylglycerol, or phosphatidylinositol, and cardiolipin [36]. The DAG resulting from lipin PAP enzyme action may be converted to TAG or to zwitterionic phospholipids, phosphatidylcholine, phosphatidylethanolamine and phosphatidylserine [36].
1.2 TAG synthesis enzymes on lipid droplets
In addition to the ER, enzymes of the glycerol 3-phosphate pathway associate directly with LDs. The mechanism of LD formation and growth is still incompletely understood, but recent studies have been illuminating. Parton posited that LDs are formed in the ER and subsequently bud off into the cytoplasm [37]. Farese and Walter have identified populations of small and large LDs, and observed that small LDs remain constant in size, while large LDs have the capacity to grow larger [18]. The diversion point is still not fully understood, but it is thought that initial LDs (iLDs) are first formed in the ER, and some iLDs recruit enzymes through ER-LD membrane bridges to locally synthesize TAG and transform to expanding LDs [38]. GPAT4, DGAT1 and DGAT2 may be recruited to LDs through ER-LD membrane bridges, and this may be stimulated by lipid overloading [18,35,39]. AGPAT3 [18,40], lipin [41] and DGAT2 [35] have also been detected on the surface of LDs in flies or mammals.
1.3 TAG synthesis enzymes at the nuclear membrane
Nuclear LDs have been visualized in various cell types, including human liver, rat liver and HepG2 cells [42,43]. Since the ER is contiguous with the nuclear envelope, it was unclear whether these LDs are simply cytoplasmic LDs surrounded by nuclear membrane invaginations, or whether they truly reside within the nucleus. It was difficult to distinguish these possibilities using fluorescence microscopy. Recent studies using electron microscopy of serial sections has revealed that LDs may indeed reside within the nucleoplasm [42,44]. Nuclear LDs may be limited to specific cell types. They have been detected in human liver and human and mouse hepatic cell lines, but are much less abundant in fibroblasts and adipocytes [42,44]. Nuclear LDs appear to localize with protrusions from the inner nuclear cell membrane, and depend on SUN proteins and PML (promyelocytic leukemia) bodies for formation [44]. It appears that there is capacity for expansion of LDs locally within the nucleus, as nuclear LDs co-localize both with DGAT2 and with the rate-limiting enzyme for phosphatidylcholine synthesis in the CDP-choline pathway, phosphocholine cytidylyltransferase [44]. Fatty acyl-CoAs are also present in the nucleus [45,46], and AGPAT3, AGPAT5, lipin 1 and lipin 3 can all localize to the nucleus [26,44]. Lipin 1 has been shown to have a nuclear role outside of lipid synthesis, acting as a transcriptional modulator in conjunction with nuclear receptors and transcriptional coactivators. This role of lipin 1 has been reviewed elsewhere [47]. The function of nuclear LDs is not well understood, and possibilities include supplying lipids for lipid signaling, nuclear membrane expansion, or serving as reservoirs for histones and other proteins.
1.4 TAG synthesis enzymes on mitochondria
Another organelle that is associated with LDs and TAG synthesis is the mitochondrion. There is evidence that representative enzymes for each of the steps of the glycerol 3-phosphate pathway may associate with mitochondria. GPAT1 and GPAT2 both are resident in the outer mitochondrial membrane [13,48–50]. Whereas GPAT1 has well characterized substrate preference for saturated fatty acids, GPAT2 has no apparent specificity for fatty acid substrates [13]. Proteomic analyses of mitochondria in white and brown adipocytes suggests that AGPAT2 and AGPAT3 are mitochondrial forms [51]. Ectopically expressed AGPAT5 has also been associated with mitochondria, but it is not clear if the endogenous protein also associates with this organelle [52]. Lipin 1 may be recruited from the cytosol or other cellular compartments to the mitochondrial membrane in response to the action of mitochondrial-resident phospholipase D, which hydrolyzes cardiolipin to generate PA, the substrate for lipin PAP enzyme activity [53]. DGAT enzymes are also known to associate with mitochondria. DGAT2 co-localizes with mitochondria in cultured adipocytes [35]. Furthermore, DGAT activity is enriched in mitochondria-associated membranes, and it is possible that DGAT2 translocates from the ER to LDs through these membranes [54,55]. It is not clear at present whether the role of glycerol 3-phosphate enzymes on mitochondria is to produce mass synthesis of glycerolipids, to modulate levels of lipid intermediates, or some other function. This merits additional investigation.
2 Critical physiological roles for glycerolipid synthesis enzymes in man
Spontaneous mutations in human genes for AGPAT2, LPIN1, LPIN2, DGAT1, and D GAT2 have been extremely informative about the role of these glycerolipid synthetic enzymes in normo- and pathophysiology.
2.1 AGPAT2 mutation and lipodystrophy
AGPAT2 homozygous or compound heterozygous inactivating mutations cause congenital generalized lipodystrophy, type 1 [56–58]. Subjects with AGPAT2 deficiency lack metabolically active adipose tissue in abdominal and thoracic fat depots, as well as bone marrow, but retain mechanical fat tissue in sites such as the scalp, orbits, palms and soles [59]. This phenotype underscores a critical role for AGPAT2 enzyme activity in the generation of metabolically functional adipose tissue, which cannot be compensated by other AGPAT family members. The inability to store lipids within adipose tissue leads to triglyceride accumulation in liver and skeletal muscle. The very low leptin and adiponectin levels that result from lack of functional adipose tissue likely contribute to increased appetite and metabolic dysfunction leading to diabetes. Indeed, treatment with leptin or synthetic leptin analogs is effective at reducing metabolic dysfunction in individuals with congenital general lipodystrophy caused by AGPAT2 deficiency and other genetic defects, working in large part through action in the hypothalamus to reduce appetite [59,60].
2.2 LPIN1 mutation and childhood rhabdomyolysis
LPIN1 mutations cause childhood rhabdomyolysis, severe muscle degradation that leads to complications such as kidney failure and may prove fatal [61–63]. LPIN1 is expressed in many human tissues, but the very severe muscle phenotype may relate to the fact that human skeletal muscle has among the highest LPIN1 expression levels, with little or no expression of LPIN2 or LPIN3 [27]. Several pathogenic LPIN1 mutations have been identified, including deletions, nonsense, missense or frame-shift changes in the protein coding sequence. In vitro assessment of a pathogenic missense mutation (R725H) suggests that the disease is related to the loss of lipin 1 PAP enzyme activity, and not related to a deficit in the coactivator function of lipin 1 [64]. The disease is typically asymptomatic until lipin 1-deficient individuals experience metabolic stress such as fever, fasting or anesthesia. Heterozygous carriers of LPIN1 missense mutations may experience muscle symptoms in response to statin drug use [61], which suggests that LPIN1 genotype may be a determinant for the development of statin-related myopathy, the most common side effect of statin drugs [65]. The analysis of lipids in muscle or myoblasts of lipin 1-deficient subjects revealed mild changes in phospholipid content, increased free fatty acids, and an increase in the lipin 1 enzyme substrate, phosphatidic acid [61,66]. Histology of patient myoblasts grown in culture revealed an accumulation of lipid droplets, and this was enhanced by treatment with cytokines (as a proxy for inflammation that may occur during rhabdomyolytic episodes in vivo) [66]. Lipin 1-deficient myoblasts also exhibited increased expression of the gene encoding acetyl-CoA carboxylase 2, which could reduce fatty acid oxidation in these cells, contributing to lipid accumulation [66].
2.3 LPIN2 mutation and Majeed syndrome
LPIN2 mutations cause Majeed syndrome, a rare autoinflammatory disease typically detected in affected individuals during early childhood [67,68]. Key symptoms are osteomyelitis in the absence of infection and dyserythropoietic anemia. Affected children also may exhibit failure to thrive, hepatomegaly, and neutrophilic dermatosis. It has been shown that the loss of lipin 2 PAP enzyme activity is responsible for Majeed syndrome symptoms, as one pathogenic mutation (S734L) encodes a lipin 2 protein that lacks PAP activity, but retains coactivator activity [69]. As is the case with LPIN1-associated rhabdomyolysis, the symptoms in Majeed syndrome occur episodically. The triggers of inflammatory episodes have not been well established, although one hypothesis suggests saturated fatty acids may activate toll-like receptors and lead to production of pro-inflammatory cytokines [68].
2.3 DGAT1 mutation and congenital diarrhea disorder
DGAT1 mutations have been identified by exome sequencing in two families, and found to be associated with severe diarrhea resembling a group of congenital diarrheal disorders [70]. In the initial family identified, two siblings carried homozygous DGAT1 loss-of-function mutations and developed intractable diarrhea within 3 days of birth [71]. This proved fatal in one of the affected individuals, but resolved in the other by 12 months of age. Although DGAT1 is expressed in most tissues, the severe intestinal phenotype in DGAT1-deficient individuals may relate to the fact that DGAT1 expression is highest in human intestine [72]. A plausible mechanism for the observed severe diarrhea is impaired fat absorption and accumulation of lipid intermediates within the intestinal mucosa [71]. The basis for the distinct clinical outcomes of the two siblings carrying the same mutation is unknown; possibilities include differential compensation by DGAT2 or other genetic modifiers.
In a second family, two siblings carrying homozygous missense DGAT1 mutations (L105P) exhibited watery diarrhea shortly after birth that was less severe than in the initial family studied [73]. The mutant DGAT1 protein in these individuals retained partial function, and could catalyze triglyceride synthesis in patient fibroblasts, albeit at lower levels than in fibroblasts from unaffected individuals. Thus, the severity of diarrhea associated with DGAT1 mutations depends upon the degree of protein function remaining. These findings confirm a critical role for DGAT1 in intestinal lipid homeostasis, highlight DGAT1 mutations as a contributor to congenital diarrheas with a range of severity, and raise caution about the therapeutic use of DGAT1 inhibitors.
2.5 DGAT2 mutation and dominantly inherited Charcot-Marie-Tooth disease
A family has been identified with DGAT2 missense mutation (Y223H) associated with dominantly inherited Charcot-Marie-Tooth disease [74]. The affected individual had peripheral neuropathy and decreased serum triglyceride levels. Although it is not fully understood how the mutant DGAT2 protein confers neuropathy symptoms, it is possible that the missense mutation confers gain-of-function, as it is inherited in an autosomal dominant fashion. Furthermore, expression of the variant DGAT2 protein inhibited axonal branching of peripheral nerves in zebrafish, and also inhibited the proliferation of mouse motor neuron cells in vitro [74].
3 Mouse models to study the physiology of glycerolipid metabolism
Several mouse models with mutations in enzymes of the glycerol 3-phosphate pathway have been characterized. These models have allowed mechanistic studies that are not feasible in humans and have greatly augmented our understanding of the physiological and cellular roles of these enzymes. Here we summarize key features of these models, and refer the reader to several excellent reviews for additional information [13,27,47,75–79].
3.1 GPAT1–deficient mice
Gpat1 expression levels are highest in brown and white adipose tissues and liver, with substantial levels also in muscle, heart, and lower levels in kidney, brain, and lung [50]. To study the role of the mitochondrial GPAT1 enzyme, multiple mouse strains have been engineered. Gpat1−/− mice exhibit reduced body weight and gonadal fat pad weight in females, likely resulting from reduced TAG synthesis [80,81]. Of particular interest has been the contribution of GPAT1 activity in the regulation of TAG content in liver, where this enzyme accounts for 30–50% of total GPAT activity [13]. Gpat1−/− mice exhibit reduced hepatic TAG levels when fed either chow or high fat diets, and when bred onto the highly steatotic leptin-deficient ob/ob background [81–83]. The latter model of combined GPAT1 and leptin deficiency revealed that while lack of GPAT1 successfully normalizes liver TAG and DAG content, this is not sufficient to improve insulin resistance that occurs in ob/ob mice [83]. GPAT1 deficiency also lessens TAG accumulation in the heart in response a high fat/high sucrose diet [84]. The reduced TAG storage observed in Gpat1−/− mice in tissues such as adipose tissue, liver, and heart may be related to a combination of reduced TAG synthesis and increased fatty acid oxidation [81,85]. Conversely, adenoviral overexpression of Gpat1 in mouse liver produces steatosis within 7 days [49,86].
Owing to the propensity of GPAT1 to incorporate unsaturated fatty acids into acyl glycerol phosphate, GPAT1 deficiency reduces the amount of palmitic acid that is incorporated into glycerolipids, including phosphatidylcholine and phosphatidylethanolamine, in liver and heart, as well as in isolated liver mitochondria [80,84,85]. GPAT1 deficiency reduces the occurrence of liver tumors in mice treated with a carcinogen, likely related to altered lipid content and composition [87]. Altered membrane phospholipid content may underlie the observed effects of GPAT1 deficiency on T cell proliferation and cytokine production [88–90]. Additional details about GPAT1 function have been recently reviewed [13,79].
3.2 GPAT4–deficient mice
The ER-associated GPAT4 enzyme is expressed at highest levels in brown adipose tissue and testis, and a moderate levels in white adipose tissue, liver, intestine, kidney, mammary epithelium and brain [91–93]. Gpat4−/− mice were generated using gene-trap insertional mutagenesis into the gene originally named Agpat6, based on sequence similarity to genes encoding AGPAT proteins [91,93]. Subsequent analysis of enzyme activity revealed that the enzyme is indeed a GPAT, and does not have detectable AGPAT activity [29,92]. Gpat4−/− mice were initially analyzed on a C57BL/6J background that still contained a small proportion of 129 strain background. These mice have reduced body weight and fat pad mass in the gonadal and subdermal depots, and small adipocytes, indicating reduced TAG storage per cell [91]. GPAT4-deficient mice resist weight gain when fed a high fat diet, and female mice also exhibit about 20% reduction in body weight when bred onto the ob/ob background (male mice were not analyzed) [91]. The reduced body weight is associated with increased energy expenditure, which may be secondary to enhanced thermogenic requirements due to the reduced storage of insulating subdermal adipose tissue. Liver TAG accumulation is reduced by about 50% in Gpat4−/− mice. Besides reduced TAG accumulation in adipose tissues and liver, the other major phenotype of Gpat4−/− mice is impaired lactation and inability to produce milk with sufficient TAG content to suckle their progeny [93]. The mammary alveoli and ducts that produce milk are undeveloped, pointing to a critical role for GPAT4 in mammary epithelium. Thus, GPAT4 has key roles in adipose tissue, mammary gland and liver, but the liver effects are likely buffered by GPAT1 activity in this tissue.
3.3 AGPAT2–deficient mice
Agpat2 is highly expressed in visceral white adipose tissue depots, brown adipose tissue, and liver [91]. Agpat2−/− mice exhibit generalized lipodystrophy, as observed in their human counterparts [94]. 3T3-L1 preadipocytes with Agpat2 knockdown, and preadipocytes from Agpat2−/− mice, exhibit impaired differentiation characterized by reduced expression of the adipogenic differentiation program, including reduced levels of PPARγ and C/EBPα [95,96]. Thus, defects in differentiation may be the fundamental defect in Agpat2−/− preadipocytes, prior to a need for TAG synthesis. There is also evidence that brown adipose tissue of Agpat2−/− mice exhibits massive necrosis and is ablated in young mice, whereas white adipose tissue undergoes apoptosis and/or necrosis as the mice age [96,97]. White adipose tissue in newborn Agpat2−/− mice has several ultrastructural abnormalities, including an absence of caveolae, aberrant mitochondria, and an accumulation of autophagic organelles [96]. In addition to reduced neutral lipid accumulation, adipose tissue from Agpat2−/− mice exhibits altered phospholipid content, with an unexpected increase in the levels of the AGPAT2 product, phosphatidic acid [96]. Disruption of phospholipid homeostasis is a common feature in human and mouse tissues with defects in components of the glycerol 3-phosphate pathway.
As observed in human generalized lipodystrophy, Agpat2−/− mice develop hepatic steatosis, insulin resistance, and diabetes [94]. The TAG that accumulates in liver of these mice is associated with increased expression of Mogat1 (encoding monoacylglycerol acyltransferase), raising the possibility that activity of this enzyme contributes to the increased TAG [94]. However, this appears not to be the case, as mice that are deficient for both Agpat2 and Mogat1 still accumulate hepatic TAG [98]. Additionally, hepatic steatosis in Agpat2−/− mice cannot be reversed by adenoviral expression of AGPAT2 directly in liver, suggesting that steatosis does not result from local AGPAT deficiency, but rather is a secondary result of the insulin resistance that occurs due to lipodystrophy and reduced leptin levels [99,100]. In addition to hepatic abnormalities, Agpat2−/− mice develop pancreatic islet hypertrophy, possibly in response to chronic hyperglycemia [97].
3.4 Lipin 1–deficient mice
Lpin1 is expressed at highest levels in white and brown adipose tissues, skeletal muscle, and testis, with lower levels in heart, liver, kidney and other tissues [101]. The Lpin1 gene was isolated through positional cloning of the mutation responsible for the fatty liver dystrophy (fld) spontaneous mouse mutant [101]. The fld mouse was originally characterized as having a fatty liver and hypertriglyceridemia during the neonatal period, and peripheral neuropathy that progressively worsens throughout the lifetime of the mouse [102,103]. The mice were also found to have reduced body weight, an 80% reduction in white and brown adipose tissue mass, and insulin resistance—characteristics of generalized lipodystrophies [104]. The fld mouse is homozygous for a Lpin1 gene rearrangement that leads to complete lack of protein product; a second mutant allele containing a Lpin1 point mutation (G84R) resulted in the same phenotype, confirming the identity of the gene [101]. Analysis of the Lpin1 gene sequence revealed two additional family members in mammals (Lpin2 and Lpin3), and orthologs in invertebrates, plants and yeast [101]. The identification of lipin proteins as PAP enzymes was made by Carman and colleagues through purification and peptide sequencing of yeast PAP enzyme activity, and matching the peptide sequence to the mammalian lipin sequences [32].
The identification of lipin proteins as PAP enzymes provided a lens through which to interpret phenotypes of mutant mouse models. The lack of normal adipose tissue in lipin 1-deficient fld mice was shown to result from impaired adipocyte differentiation in addition to reduced TAG content in adipocytes [105,106]. Conversely, expression of a lipin 1 transgene in adipose tissue causes increased fat storage per adipocyte, consistent with a role for lipin 1 in promoting TAG synthesis in adipocytes [107,108]. Lipin 1 is also required for lipid storage and development of mature myelinated nerves, providing an explanation for the peripheral neuropathy of lipin 1-deficient fld mice [109]. The impaired differentiation of preadipocytes and myelinated peripheral nerve are associated with an accumulation of the lipin 1 PAP enzyme substrate, phosphatidic acid, which leads to activation of ERK signaling [106,109]. The role of lipin PAP activity in regulation of phosphatidic acid levels and cell signaling has been previously reviewed [77].
Tissue-specific engineered Lpin1 alleles have been studied to address the role of lipin 1 in specific tissues. An adipose tissue-specific mutant allele, which deletes the first 115 amino acids of the 890 amino acid lipin 1 protein and abolishes PAP enzyme activity, leads to substantially reduced white adipose tissue weight and TAG synthesis, similar to global lipin 1 deficiency [110]. This mutant allele retains the transcriptional coactivator activity that is present in full-length lipin 1, demonstrating that the lipodystrophic phenotype is primarily due to loss of PAP activity. This model also revealed a role for lipin 1 in the regulation of TAG hydrolysis through modulation of phosphatidic acid levels and cAMP signaling through protein kinase A [110]. Hepatocyte-specific lipin 1 deletion causes increased susceptibility to alcohol-induced hepatic steatosis, potentially through reduced activation of PGC-1α and fatty acid oxidation [111]. It was also shown that loss of lipin 1 PAP activity does not prevent TAG accumulation in liver in response to dietary lipid overload, possibly related to compensation by the other lipin family members expressed in liver [112]. In contrast to the results with manipulation of lipin 1 in hepatocytes, deletion of lipin 1 in myeloid cells reduces inflammation associated with alcohol-induced steatosis [113].
As noted in a previous section, human lipin 1 deficiency is characterized by episodes of rhabdomyolysis, rather than lipodystrophy. It is not fully understood why lipin 1 deficiency has a dramatic effect on adipose tissue in mouse and not in humans, but may be related to compensation for TAG synthesis by alternative means in human adipose tissue. In cultured human Simpson-Golabi-Behmel syndrome preadipocytes, knockdown of lipin 1 resulted in a 95% reduction in N-ethyl maleimide-sensitive PAP activity and some alterations in early adipogenesis. Nevertheless, after depletion of lipin 1, lipin 2 and lipin 3 in these cells, they maintained the ability to differentiate and accumulate neutral lipids [114]. It is proposed that this may be a result of adaptive induction of additional PAP activities and reduced lipolysis [114].
Despite some species differences in lipin 1-deficient mice and humans, the mouse has provided mechanistic insight into the role of lipin 1 in skeletal muscle. Analysis of muscle from fld mice revealed that there is substantial myocyte turnover and an accumulation of abnormal mitochondria, which exhibit impaired respiratory capacity [115]. In response to injury, muscle from fld mice has reduced capacity for regeneration owing to impaired differentiation [116]. The accumulation of damaged mitochondria is associated with impaired autophagy to remove these organelles, and subsequent impaired muscle function [115]. The defect in autophagy in lipin 1-deficient muscle can be traced to impaired generation of DAG from PA at the surface of lysosomes, which is required for fusion with autophagosomes and effective degradation of damaged proteins and organelles [115,117]. Both human and mouse skeletal muscle is characterized by altered phospholipid content, which likely influences cellular membranes and may contribute to the impaired muscle function [61,115]. The action of lipin 1 at multiple membrane sites has been recently reviewed [27].
3.5 Lipin 2–deficient mice
Lpin2 is expressed in a distinct array of tissues compared to Lpin1, although there is some overlap. Lpin2 expression is prominent in liver, small intestine, kidney, brain, and macrophages, with very little expression in adipose tissues and muscle [69]. Similar to individuals with Majeed syndrome, Lpin2−/− mice develop dyserythropoietic anemia [118]. Lpin2−/− mice exhibit reduced trabecular bone density, but under standard laboratory conditions do not show evidence of inflammatory bone lesions that are typically observed in Majeed syndrome [118]. Osteomyelitis in lipin 2-deficient humans occurs episodically in response to unknown metabolic stressors, which laboratory mice presumably do not experience. Recent work has identified lipin 2 as a regulator of NLRP3 inflammasome activation [119]. Lipin 2 influences MAP kinase signaling to regulate interleukin (IL)-1β production during inflammasome priming. Lipin 2 knockdown in human macrophages led to enhanced IL-1β production, as is observed in Majeed syndrome [119]. Lipin 2-deficient mice injected with high doses of lipopolysaccharide had an exaggerated response, producing high levels of inflammatory cytokines (IL-1β, IL-18, tumor necrosis factor α), and reduced expression of anti-inflammatory cytokines (IL-10) [119].
Studies of lipin 2-deficient mice revealed a relationship between lipin 1 and lipin 2 in vivo. Lipin 2 deficiency in liver and cerebellum are compensated by upregulation of lipin 1, which maintains PAP activity and normal lipid profile in these tissues under basal conditions [118]. However, upon feeding Lpin2−/− mice a high fat diet, the increased lipin 1 levels lead to enhanced hepatic triglyceride accumulation [118]. Additionally, as mice age, lipin 1 levels in the cerebellum diminish, leaving Lpin2−/− mice with a deficit of PAP activity and abnormal phospholipid content that is associated with age-related ataxia [118]. Finally, crosses between Lpin1−/− and Lpin2−/− mice revealed that deficiency for the two lipins is incompatible with life and results in embryonic lethality [118].
3.6 Lipin 3–deficient mice
Lpin3 is expressed at low levels across several tissues, with most prominent expression in small intestine, and lower levels in kidney, liver, heart and adipose tissues [33]. Despite the dominant role of lipin 1 as the key PAP activity in mouse and human adipocytes, lipin 3 is also induced during differentiation of mouse 3T3-L1 preadipocytes and primary human preadipocytes [26]. In a panel of 114 mouse strains, Lpin3 expression levels are highly significantly correlated with adiposity traits such as total body fat and weights of femoral, gonadal, retroperitoneal and mesenteric fat pads [77]. Mice deficient for either lipin 1 or lipin 3 showed >85% reduction in adipose tissue PAP activity [77]. Nevertheless, Lpin3−/− mice do not exhibit lipodystrophy, presumably due to compensation by lipin 1, although lipin 3 cannot compensate for lipin 1 in vivo. Mice made deficient for both lipin 1 and lipin 3 exhibit severe generalized lipodystrophy that is even more pronounced that that in Lpin1−/− mice [77]. Lipin 1 and lipin 3 proteins influence the levels and subcellular localization of one another when expressed in cultured cells [77]. When expressed together from heterologous promoters, levels of both proteins are dramatically increased compared to levels when expressed independently. Lipin 1 and lipin 3 proteins show substantial co-localization when expressed in combination, with enhanced perinuclear localization of both proteins compared to their localization when expressed independently. These observations suggest that a complex interactive relationship exists between lipin family members within some cell types and may influence their in vivo function.
3.7 DGAT1–deficient mice
Dgat1 expression is prominent in small intestine, adipose tissue, mammary gland, skeletal muscle, heart, and other tissues [78]. Dgat1−/− mice are leaner than littermates and exhibit reduced weight gain on a high fat diet [120]. However, unlike Agat2−/− and Lpin1−/− mice, Dgat1−/− mice are not lipodystrophic. Their lean phenotype is associated with enhanced energy expenditure and altered intestinal fat absorption, which can be corrected by replacing Dgat1 expression specifically in small intestine [121,122]. When bred onto the genetically obese agouti yellow (AY/a) background, DGAT1 deficiency results in a 20% reduction in body weight and increased insulin sensitivity [123]. Interestingly, DGAT1 deficiency does not improve adiposity or insulin sensitivity on the ob/ob background, suggesting that leptin may be required for this effect. Conversely, expression of a Dgat1 transgene in adipose tissue leads to larger fat pads and hypertrophic adipocytes on a chow diet, and 20% increased body weight on a high fat diet with improved insulin sensitivity compared to wild-type mice [124]. Expression of a Dgat1 transgene in muscle replicates the “athlete’s paradox,” with increased intramyocellular fat stores, but increased insulin sensitivity [125]. The improved insulin sensitivity with enhanced DGAT1 may be associated with channeling fatty acid substrates into TAG, preventing the accumulation of DAG and ceramides.
DGAT1 is also required for normal sebaceous gland physiology and for lipid levels [126]. As a result, Dgat1−/− mice have impaired thermoregulation when challenged by conditions such as water immersion. Dgat1−/− mice that also lack apolipoprotein E develop fewer atheroslcerotic lesions than their Apoe−/− counterparts [127]. This may be due to reduced intestinal cholesterol uptake and absorption, as well as increased cholesterol efflux from macrophages.
3.8 DGAT2–deficient mice
Dgat2 is expressed in liver, adipose tissue, mammary gland, heart, peripheral white blood cells, and testis [78]. The Dgat2−/− mouse has a severe deficiency in skin barrier function, leading to dehydration, hypothermia and death shortly after birth [128]. Altered skin physiology in Dgat2−/− mice is associated with abnormalities in the lamellar body secretory system. Mice are born with reduced body weight, which suggests additional problems during intrauterine development. Newborn mice fail to suckle, leading to hypoglycemia, which is likely to be a main contributor to early death.
3.9 Comparison of mouse and human deficiencies in glycerolipid synthesis
Phenotypes associated with enzyme deficiencies in the glycerol 3-phosphate pathway exhibit some differences between mouse and human. Evidence available at present suggests that these species differences reflect the degree to which mice and humans compensate for the loss of specific enzymes. For example, lipin 1 accounts for ≥ 95% of PAP enzyme activity in both human and mouse adipose tissue, yet the occurrence of lipodystrophy exclusively in mouse appears to be due to induction of a compensatory mechanism for TAG synthesis in human adipocytes [33,114]. Despite these differences, there also exist strongly convergent mechanisms across mouse and human deficiencies for glycerolipid biosynthetic enzymes. For example, AGPAT2-deficient humans and mice, as well as lipin 1-deficient mice, exhibit impaired phospholipid homeostasis in adipose tissue, including an accumulation of the lipid intermediate, phosphatidic acid. Phosphatidic acid accumulation also occurs in adipose tissue of the lipodystrophic seipin-deficient mouse [129], and in muscle of lipin 1-deficient humans and mice. Disturbed phospholipid homeostasis may underlie impaired lipid droplet expansion during adipocyte differentiation and altered membrane remodeling, with deleterious effects on organelles such as mitochondria and autophagososmes, as well as dysregulation of intracellular signaling pathways [130]. Further evidence for conserved roles of glycerolipid synthetic enzymes in human and mouse physiology comes from the association of human genetic variants with traits observed in mouse models. For example, although humans with lipin 1 deficiency do not develop lipodystrophy as observed in the mouse, genetic variants in LPIN1 are associated with adiposity traits such as body mass index and waist circumference in four different human populations [131].
4. Regulation of glycerolipid synthesis enzymes
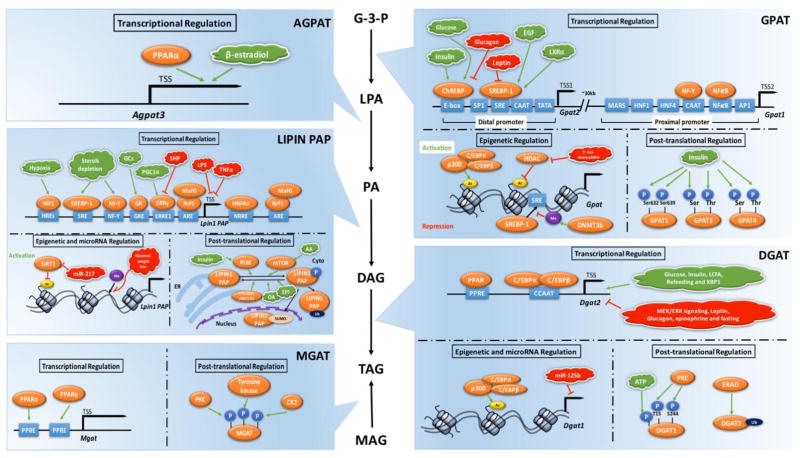
4.1 GPAT transcriptional and post-translational regulation
GPAT isoforms reside primarily in the mitochondrial membranes (GPAT1 and GPAT2) or ER membrane (GPAT3 and GPAT4). The transcriptional regulation of Gpat1 has been well studied. Gpat1 is regulated by proximal and distal promoters located 30 kb apart (Fig. 2) [132,133]. SREBP (sterol response element binding protein), ChREBP (carbohydrate response element binding protein), SP1, and CTF1 binding sites are present in the distal promoter region, whereas hepatocyte nuclear factor (HNF-1 and HNF-4), NFκB, NFY binding reverse CAAT box, activating protein binding site (AP1 and AP4), myocyte enhancer factor (MEF2), upstream stimulatory factor (USF), and matrix attachment regions (MARS) are present in the proximal promoter. Insulin and glucose activate the binding of ChREBP to promote Gpat1 transcription, and EGF and LXRα stimulate the binding of SREBP-1 to sterol response elements to increase Gpat1 expression [134]. On the other hand, glucagon and leptin exert inhibitory effects [134].
Fig. 2.
Regulation of key enzymes in glycerolipid biosynthesis, including GPAT, AGPAT, Lipin, DGAT and MGAT enzymes. Transcriptional regulation, epigenetic and miroRNA regulation, and post-translational regulation are summarized. Activation processes are marked in green and inhibition processes are marked in red. ChREBP: Carbohydrate-responsive element binding protein; SRE: Sterol regulatory element; SREBP-1: Sterol regulatory element-binding protein 1; NF-Y: Nuclear factor-Y; TSS: Transcription start site; Ac: Histone acetylaiton; Me: DNA methylation; P: Phosphorylation; Ser: Serine; Thr: Threonine; GRE: Glucocorticoid response element: GR: Glucocorticoid receptor; GCs: Glucocorticoids; HREs: Hormone-response elements; ERRE1: ERR response element 1; ERRγ: Estrogen related receptor gamma; ARE: Antioxidant response element; NRRE: Nuclear receptor response element; OA: Oleic acid; AA: Amino acid; EPI: Epinephrine; Ub: Ubiquitination; SUMO: Sumoylation; ER: Endoplasmic reticulum; Cyto: Cytoplasm.
Mechanisms underlying epigenetic regulation of genes encoding GPAT enzymes have also been investigated (Fig. 2). It has been shown that p300-C/EBPα/β complex binds to the promoter of Gpat1 gene and increases Gpat1 transcription [135]. p300 is a histone acetyltransferase enzyme which is associated with gene activation [136]. Treatment of human cell lines with 5-aza-2′ deoxycytidine (a histone deacetylase inhibitor) induces GPAT2 expression [137]. DNA methylation is another important epigenetic player, which normally associates with decreased gene expression. DNA methylation, which is typically associated with repression of gene expression, has been shown to inhibit the recruitment of SREBP-1c to the Gpat1 promoter in mouse liver, potentially mediated by DNA methyltransferase 3b [138].
At the post-translational level, GPAT protein levels and activity are regulated by phosphorylation. Insulin affects mitochondrial GPAT1 activity by increasing Vmax and KM for its glycerol-3-phosphate and palmitoyl-CoA substrates [139]. In vivo, insulin treatment of primary adipocytes induces phosphorylation at serine 632 and serine 639 of GPAT1 [139]. GPAT3 and GPAT4 are also phosphorylated by insulin at serine and threonine residues, leading to increased GPAT activity in adipocytes [140].
4.2 AGPAT transcriptional regulation
A limited amount of information is available regarding transcriptional regulation of AGPAT enzymes. Analysis of the Agpat1 promoter shows binding sites for NOR1 and PPARα, which are both involved in muscle development [141]. Consistent with this, cardiac AGPAT activities are 25% lower in PPARα null mice compared with wild-type [142]. In addition, mRNA expression of cardiac Agpat3 is regulated by PPARα activation [142]. The mRNA expression of Agpat3 also responds to gonadal hormones, with increased expression in response to β-estradiol [143].
4.3 Lipin transcriptional and post-translational regulation
The Lpin1 gene is highly responsive to transcriptional stimuli, and several specific factors that modulate expression levels have been identified. Lpin1 expression is stimulated by glucocorticoids, sterol depletion, or hypoxia, acting through specific transcription factor binding sites in the proximal promoter region (Fig. 2) [144–148]. PGC-1α regulates cardiac Lpin1 expression through transcription factors ERRα/γ, which bind to an estrogen related receptor element 1 (ERRE1) [149,150]. Hepatocyte nuclear factor 4α (HNF4α) also cooperates with PGC-1α to regulate Lpin1 expression in liver cells [151]. The regulation of Lpin1 by NF-E2-related factor 1 (Nrf1) occurs through binding to antioxidant response elements (AREs) [152]. Ethanol increases Lpin1 gene expression, which is mediated through inhibition of AMPK and activation of SREBP-1 [153]. Inhibitors of Lpin1 gene expression include small heteromeric partner (SHP), lipopolysaccharide, and tumor necrosis factor α [131].
Lpin1 expression is also regulated indirectly through the action of the microRNAs, miR-217. miR-217 promotes ethanol-induced fat accumulation through down-regulating SIRT1 in vitro and in vivo [154]. SIRT1 is a histone deacetylase that inhibits Lpin1 gene expression in mouse AML-12 hepatocytes [154]. miR-217 action is therefore associated with increased Lpin1 gene expression in hepatocytes. The miR-217–SIRT1–lipin 1 axis also plays a role in macrophage-dependent inflammation in response to ethanol and LPS [154]. DNA methylation of the Lpin1 gene has been shown to be decreased in the offspring of maternal weight-loss dams, which agrees with increased Lpin1 mRNA levels [155].
Lipin 1 levels and activity are also regulated through mRNA splicing and post-translational mechanisms, which have been reviewed recently [27] and only selected aspects are briefly described here. One of the key mechanisms for regulation of lipin 1 protein function is through reversible protein phosphorylation. Lipin 1 protein phosphorylation can be induced through mTOR signaling and the phosphatidylinositol-3 kinase pathway, leading to cytosolic localization [156–160]. Conversely, the phosphatase complex CTDNEP1/NEP1-R1 dephosphorylates lipin 1 and promotes its association with the ER membrane [161,162]. Oleic acid and epinephrine promote lipin 1 de-phosphorylation and translocation to the ER [158]. Lipin 1 is also modified by sumoylation, which promotes entry into the nucleus [163]. Lipin 1 protein degradation occurs through ubiquitination and proteasomal degradation [115,164,165]. At present, little is known about the transcriptional and post-translational regulation of lipin 2 and lipin 3.
4.4 DGAT transcriptional and post-translational regulation
DGAT enzymes are regulated at the transcriptional, epigenetic, and post-translational levels under specific conditions. Dgat1 and Dgat2 mRNA levels are induced during adipocyte differentiation in NIH 3T3-L1 cells, with corresponding increases in DGAT activity [72]. This may be mediated, in part, through a PPAR response element (PPRE) in the Dgat1 promoter region [166]. The PPRE also likely mediates increased Dgat1 expression in vitro and in vivo in response to thiazolidinediones, which act as PPARγ agonists [167]. C/EBPα and C/EBPβ increase Dgat2 mRNA expression during adipogenesis [168]. Dgat gene expression is also enhanced by glucose, insulin, and long-chain fatty acids [169,170]. In liver, the transcriptional factor X-box binding protein 1 (XBP1), which is induced during the unfolded protein response, increases Dgat2 expression in liver [171]. MEK-ERK signaling appears to repress Dgat1 and Dgat2 mRNA expression in hepatocytes, as MEK-ERK inhibition induces Dgat expression [172]. Leptin deficiency induces Dgat2 expression in white adipose tissue, skeletal muscle and small intestine in mouse, suggesting that leptin may negatively regulate Dgat expression [123,126,173]. DGAT activity is decreased by administration of glucagon [174] and epinephrine [175] in rats. Furthermore, fasting and refeeding regulate Dgat1 and Dgat2 expression differently. Dgat2 expression is decreased by fasting and increased by refeeding in white adipose tissue, while Dgat1 exhibits the opposite pattern [169].
Dgat gene expression is also subject to regulation through histone acetylation and microRNA. It has been shown that the p300-C/EBPα/β complex binds to the Dgat gene promoter and increases Dgat transcription [135]. miR-125b reduces the transcriptional activity of Dgat1 [176].
DGAT1 phosphorylation and DGAT2 ubiquitination may regulate DGAT protein levels and activity. DGAT1 phosphorylation inactivates DGAT enzymatic activity [177]. Protein kinase C phosphorylates T15 and S244 residues on DGAT1 [178]. DGAT2 can be degraded by the ubiquitin-proteasome pathway [179]. Specifically, DGAT2 degradation is regulated by gp78, an E3 ligase involved in ERAD (endoplasmic reticulum-associated protein degradation).
4.5 MGAT transcriptional and post-translational regulation
Although MGATs function in an alternative pathway for TAG synthesis, it is interesting to compare MGAT regulation with that of the enzymes of the glycerol 3-phosphate pathway. Transcriptional and post-translational regulation of MGAT have been reported. PPARγ regulates MGAT1 promoter activity in human primary hepatocytes, likely acting through PPAR response elements in the promoter (Fig. 2) [180]. MGAT protein phosphorylation sites are predicted, including consensus sites for phosphorylation sites for protein kinase C, tyrosine kinase, and/or casein kinase 2 [181].
5. Structural insights into GPAT and AGPAT function
5.1 Crystallization of non-mammalian GPAT/AGPAT–related enzymes
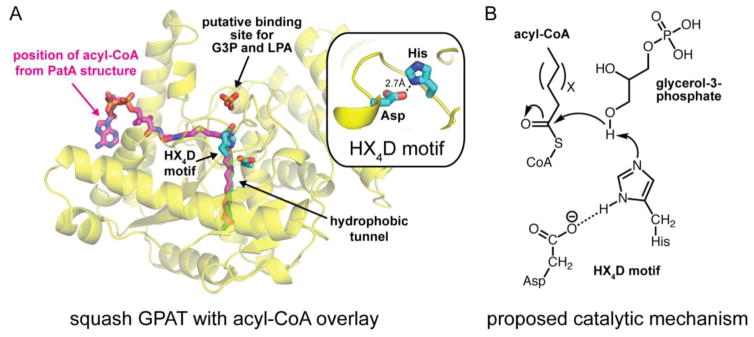
To date, mammalian glycerolipid biosynthetic enzymes have not been crystallized. However, analysis of related enzymes from plants and lower organisms has begun to provide some insights about the structural basis for their function. GPATs and AGPATs belong to a large and diverse superfamily of proteins known as the lysophospholipid acyltransferases (LPLATs) that catalyze an acyl-transfer reaction primarily using acyl-Coenzyme A (acyl-CoA) as an acyl-donor [182]. Members of this family acylate a diverse set of substrates. For example, GPATs catalyze the esterification of glycerol-3-phosphate (G3P) at the sn-1 position and AGPATs catalyze the esterification of lyso-phosphatidic acid (lyso-PA) at the sn-2 position. LPLATs all share a canonical HX4D motif with the histidine and aspartate residues predicted to be critical components for the enzymes’ catalytic mechanism [183,184].
The first structure of an LPLAT family member was of the GPAT from Cucurbita moschata (squash plant) [185]. This structure revealed an α/β fold with a hydrophobic cleft ~20Å in length that was suggested as the acyl-CoA binding site (Fig. 3A). The HX4D motif was located at one end of the cleft near a cluster of positively charged residues that was suggested to be important for G3P binding [185] and later confirmed biochemically [186]. Three years later, another group determined a new structure of squash GPAT with a sulfate ion, which can occupy phosphate-binding sites, bound by the cluster of positively charged residues [187]. Using the position of the sulfate ion as a guide for the phosphate moiety of G3P, investigators were able to model the position of G3P with the sn-1 hydroxyl group of G3P within hydrogen bonding distance of the catalytic histidine residue. A plausible catalytic mechanism was proposed that was similar to serine proteases [187,184]. In this proposed mechanism, the histidine and aspartate residues of the HX4D motif would participate in a charge-relay system to increase the nucleophilicity of the sn-1 hydroxyl group of G3P, which attacks the acyl-CoA to form lyso-PA (Fig. 3B).
Fig. 3.
Proposed structure and catalytic mechanism of GPAT enzymes from studies of lower organisms. (A) Cartoon structure of squash GPAT (pdb code: 1IUQ) with sulfate ion occupying the putative binding site for the phosphate moieties of G3P and LPA. The non-hydrolyzable acyl-CoA analog (magenta sticks) and palmitic acid (green sticks) from structures of M. smegmatis PatA (pdb codes: 5F34 and 5F2Z) are overlaid with the squash GPAT structure to highlight the putative hydrophobic active site cleft for acyl-CoA. The HX4D catalytic motif is shown in blue sticks. (B) Proposed catalytic mechanism for GPAT enzymes involving charge relay between the Asp and His residues of the HX4D motif. G3P, glycerol 3-phosphate; LPA, lyso-phosphatidic acid.
Since these initial studies, additional structures of LPLAT family members have been determined that have provided new insight into the binding site of acyl-CoA [188,189]. These include the structure of Mycobacterium smegmatis PatA, which uses palmitoyl-CoA in the biosynthesis of mycobacterial phosphatidyl-myo-inositol mannosides [188]. Several structures of PatA were determined bound to either palmitate or a nonhydrolyzable palmitoyl-CoA analog [188]. The structures revealed that palmitoyl-CoA binds within a bending hydrophobic cleft, previously suggested as the acyl-CoA binding site from the squash GPAT structure [185]. Importantly, the thioester of the acyl-CoA is adjacent to the catalytic histidine residue for nucleophilic attack and consistent with the previously proposed catalytic mechanism.
An important goal for the future is to elucidate GPAT and AGPAT structures in mammals. Only then will it be possible to test the structure-based hypotheses derived from the squash GPAT structure. In addition, it will be interesting to visualize how presumably subtle differences in the structure of GPATs and AGPATs allow them to specifically recognize and acylate the sn-1 and sn-2 positions of the glycerol backbone of G3P and lyso-PA to carryout their respective functions. Lastly, structural studies of PAP and DGAT enzymes remain to be carried out.
5.2 Membrane topology of DGAT enzymes
Several groups have experimentally determined the membrane topology of DGATs from different species and examined the role of highly conserved residues in catalytic function. This has established distinct membrane topologies for DGAT1 and DGAT2, with the most prominent differences being in the number of transmembrane spanning segments and the location of their respective catalytic sites on different sides of the ER membrane.
DGAT2 belongs to the DGAT2 and MGAT enzymes belong to the same acyl-CoA: monoacylglycerol acyltransferase gene family. Studies of mouse, S. cerevisiae, and tung tree DGAT2 have all found DGAT2 to be an integral membrane protein with both the N- and C-termini oriented towards the cytosol [34,190,191]. Proteolytic protection analysis and indirect immunofluorescence suggested that a long-hydrophobic region near the N-terminus of murine DGAT2 forms two transmembrane segments or a single membrane-embedded domain [190]. The majority of the protein was found to reside in the cytoplasm, which suggests the catalytic site generates TAG on the cytosolic leaflet of the ER. In contrast, using cysteine-accessibility assays, the S. cerevisiae homolog of DGAT2 was proposed to have four transmembrane domains, with the first corresponding to murine DGAT and the latter three being unique [191]. In this model, the active site was proposed to be embedded in the membrane.
A highly conserved HPHG motif in DGAT2 enzymes represents the most conserved motif and has been shown to be important for catalytic activity [190,191]. This motif is also conserved in all of the related MGAT enzymes [192,193]. Mutation of the individual His residues in yeast DGAT2 completely abolished catalytic activity [191], while in murine DGAT2, ~50% and ~10% catalytic activity was retained upon mutation of the first and second His residues, respectively, of the HPHG motif [190]. Notably, the HPHG motif is conserved among yeast and animal DGAT2 enzymes, but plant DGAT2 enzymes contain a slightly modified ESHG motif that only conserves the latter His and Gly residues [191]. Together, the current data suggest that the latter His residue is the most critical residue for catalysis and may be directly involved in the catalytic mechanism for the DGAT2 and MGAT enzymes.
DGAT1 does not share significant sequence homology with DGAT2 and belongs to a distinct family of enzymes designated as the membrane-bound O-acyltransferases (MBOATs), which also contain the acyl-CoA:cholesterol acyltransferases (ACATs). DGAT1 has a large number of hydrophobic sequences, which may form several transmembrane segments. Using protease protection assays and indirect immunofluorescence, it was suggested that murine DGAT1 contains three transmembrane domains, with most of the C-terminus residing in the ER lumen [194]. A conserved His residue within the ER-luminal facing C-terminal domain was essential for catalytic activity [194]. Importantly, this His residue is conserved in the related ACAT enzymes [193] and is also essential for activity [195]. Interestingly, a separate study found that DGAT1 activity resides on both the cytosolic and luminal sides of the ER membrane [196]. This conclusion was based on differential sensitivities to two specific DGAT1 inhibitors [196]. Notably, they found that DGAT1 accounted for all luminal DGAT activity, but also a substantial portion of the cytosolic DGAT activity [196]. Thus, the membrane topology or active site residues of DGAT1 may be able to invert in some instances or cell types.
6. Glycerol 3-phosphate pathway enzymes as therapeutic targets
Since excess TAG storage is associated with obesity and co-morbidities such as hepatic steatosis and insulin resistance, the past decade has seen efforts to develop therapeutic treatments that target enzymes of the glycerol 3-phosphate pathway (Table 1). The most widely investigated drugs are acyltransferase inhibitors [197]. The first GPAT small molecule inhibitor (known as FSG67) was designed in 2009 to target the GPAT active site based on the GPAT crystal structure reported for the squash enzyme [198,199]. FSG67 exhibited promising effects in studies performed in mice, leading to reduced food intake, body weight, hepatic steatosis, and adiposity and improving insulin sensitivity. However, FSG67 induced hypophagia during short-term administration to mice, raising concerns for long-term use. Furthermore, the FSG67 inhibits the activity of all four GPAT isoforms, which would be expected to produce adverse effects, especially with chronic use.
Table 1.
Glycerolipid biosynthetic enzymes as therapeutic targets
| Target | Compound | Model | Function/effects | Reference |
|---|---|---|---|---|
| GPAT | FSG67 | Mouse | Decreases lipid accumulation in white and brown adipose tissues and | [198] |
|
| ||||
| Lipin 1 | Troxerutin | Mouse | Enhances nuclear lipin-1 localization, decreases cytoplasmic lipin-1. Reduces obesity, normalizes hyperglycemia and hyperlipidemia in high-cholesterol diet-induced diabetic mice. | [199] |
|
| ||||
| DGAT1 | Compound-A | mouse | Inhibits TAG synthesis in adipocytes and skeletal myotubes, increases fatty acid oxidation in skeletal myotubes. In mice, decreases visceral fat pad mass and hepatic lipid content. | [205] |
| Compound 2 | Rat | Decreases body weight, increases intestinal fatty acid oxidation and ketogenesis when dietary fat is available. | [206,207] | |
| Compound 3x | mouse | Reduces body weight gain and white adipose tissue gain without affecting food intake. | [208] | |
| Compound 4a | mouse | Reduces body weight and liver TAG in diet-induced obese mice; reduces serum TAG in lipid challenge test. | [209] | |
| Compound 9e | mouse | Reduces TAG in high fat diet-fed Swiss mice. | [210] | |
| Compound 10 | mouse | Prevents plasma TAG increase follows oil challenge at oral doses of <3 mg/kg. | [211] | |
| Compound 14 | mouse | Oral doses ≥0.03 mg/kg reduce postprandial TAG in mice following an oral lipid challenge. | [212] | |
| Compound 25 | rat | Inhibits body weight gain and plasma TAG elevation in a dose- dependent manner by a high-fat diet. | [213] | |
| Compound 29 | rat | Dose-dependent inhibition of weight gain in diet-induced rats and improves glucose tolerance. | [214] | |
| Compound 33 | mouse | Reduces plasma TAG by 87% in mouse. | [215] | |
| Compound 53 | mouse | Reduces plasma TAG, decreases TAG synthesis in adipose tissue, and prevents body weight gain when mice are fed a high fat diet. | [201] | |
| T-863 | mouse | Decreases body weight, improves insulin sensitivity, and alleviates hepatic steatosis in diet-induced obese mice. | [216] | |
| PF-04620110 | mouse | Reduces plasma TAG levels in rodents at doses of ≥0.1 mg/kg following a lipid challenge. | [217,218] | |
| JTT-553 | mouse | Reduces body weight, improves insulin resistance in adipose tissues and systemic glucose metabolism. | [219] | |
| A-922500 | rat, hamster, mouse | Reduces postprandial serum TAG levels. | [220,221] | |
|
| ||||
| DGAT2 | Compound 122 | HepG2 cells | Decreases de novo TAG synthesis. | [203] |
|
| ||||
| MGAT2 | Compound-A | mouse | Inhibits postprandial increases in plasma TAG levels, and TAG synthesis in small intestine; improves insulin sensitivity. | [204] |
At present, no drugs directly targeting lipin enzyme activity have been developed. In addition to the lack of information regarding lipin protein structure, a key challenge in using lipin proteins as therapeutic target is that it has dual function as PAP enzyme and transcriptional coregulator, which have distinct, sometimes reciprocal, physiological consequences [47]. Thus, whereas lipin 1 PAP activity promotes glycerolipid synthesis, the coactivation of PGC-1α in liver promotes fatty acid oxidation. It may be desirable to enhance the coactivator activity in the nucleus while inhibiting TAG synthesis activity of lipin 1 at the ER. One potential strategy would be to identify compounds that influence lipin 1 subcellular localization. Troxerutin is a naturally occurring polyphenol that exhibits beneficial effects towards reducing hepatic TAG accumulation. It has been shown that troxerutin enhances lipin 1 nuclear localization and decreases cytoplasmic localization [200]. Additional challenges in using lipin proteins as therapeutic targets are that it may be desirable to increase activity in some tissues, but inhibit in other tissues, and the complexities of having multiple lipin proteins in several tissues.
At present, DGAT1 is probably the most promising therapeutic target among the enzymes of the glycerol 3-phosphate pathway. Table 1 summarizes some of the DGAT1 inhibitors that have been studied, including some that have been used in clinical trials. Beneficial effects of inhibitors directly targeting DGAT1 in rodents include reduced postprandial serum TAG levels, reduced body weight gain, and improved insulin sensitivity. Some DGAT1 inhibitors have been investigated in clinical trials and exhibit promising outcomes. A phase II clinical trial of LCQ-908 in patients with familial chylomicronemia syndrome led to 40% reductions in fasting TAG levels [201]. However, the side effects of DGAT1 inhibitors have not been fully vetted, and recent characterization of patients with DGAT1 deficiency showing severe diarrhea raise concerns about potential adverse effects [71,73]. Indeed, the DGAT1 inhibitor AZD7687 was shown to improve insulin sensitivity and induce weight loss in rodent models [202]. However, a randomized clinical trial indicates that AZD7687 induces serious gastrointestinal side effects including diarrhea [203]. DGAT2 [204] and MGAT2 [205] inhibitors are still in the early stages of development. Additional basic research on glycerolipid synthesis and enzymes of the glycerol 3-phosphate pathway will increase the chances of rational design of therapeutic compounds in the future.
Highlights.
The regulation of triacylglycerol (TAG) synthesis is relevant to metabolic disease
The glycerol 3-phosphate pathway enzymes have roles in TAG homeostasis
Mutations in AGPAT2, LPIN1, LIPN2, DGAT1 and DGAT2 cause human disease
TAG synthetic enzymes act in diverse tissues and cellular processes
TAG synthetic enzymes may have potential as targets for anti-obesity therapeutics
Acknowledgments
We gratefully acknowledge support from Public Health Service P01 HL90553 and P01 HL028481 (K.R) and AHA 17SDG33410860 (M.V.A.).
Footnotes
Transparency document
The transparency document associate with this article can be found in online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birsoy K, Festuccia WT, Laplante M. A comparative perspective on lipid storage in animals. J Cell Sci. 2013;126 doi: 10.1242/jcs.104992. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2:229–237. doi: 10.2217/17460875.2.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krahmer N, Farese RV, Walther TC, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013;5:973–83. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther TC, Farese RV. Lipid Droplets and Cellular Lipid Metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welte MA. Expanding Roles for Lipid Droplets. Curr Biol. 2015;25:R470–R481. doi: 10.1016/j.cub.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford SE, Desselberger U. Lipid droplets form complexes with viroplasms and are crucial for rotavirus replication. Curr Opin Virol. 2016;19:11–15. doi: 10.1016/j.coviro.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herker E, Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol Metab. 2011;22:241–248. doi: 10.1016/j.tem.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. [accessed February 22, 2017];J Lipid Res. 1995 36:1211–26. http://www.ncbi.nlm.nih.gov/pubmed/7665999. [PubMed] [Google Scholar]
- 11.Cushman SW. STRUCTURE-FUNCTION RELATIONSHIPS IN THE ADIPOSE CELL. J Cell Biol. 1970;46 doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy E, Smith S, Weiss S. New synthesis of lecithin in an isolated enzyme system. [accessed April 24, 2017];Nature. 1956 178:594–5. doi: 10.1038/178594a0. http://www.ncbi.nlm.nih.gov/pubmed/13369471. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RA, Mashek DG. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem Rev. 2011;111:6359–86. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol - Endocrinol Metab. 2009;296 doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy E. Biosynthesis of phospholipides. [accessed April 24, 2017];Fed Proc. 1957 16:847–53. http://www.ncbi.nlm.nih.gov/pubmed/13480372. [PubMed] [Google Scholar]
- 16.Pol A, Gross SP, Parton RG. Biogenesis of the multifunctional lipid droplet: Lipids, proteins, and sites. [accessed April 24, 2017];J Cell Biol. 2014 204 doi: 10.1083/jcb.201311051. http://jcb.rupress.org/content/204/5/635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilfling F, Haas JT, Walther TC, Farese RV. Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Fröhlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV, Walther TC. Triacylglycerol Synthesis Enzymes Mediate Lipid Droplet Growth by Relocalizing from the ER to Lipid Droplets. Dev Cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilfling F, Thiam AR, Olarte MJ, Wang J, Beck R, Gould TJ, Allgeyer ES, Pincet F, Bewersdorf J, Farese RV, Walther TC, Buhman K, Coleman R, Bewersdorf J, Jnr RF, Walther T. Arf1/COPI machinery acts directly on lipid droplets and enables their connection to the ER for protein targeting. Elife. 2014;3:e01607. doi: 10.7554/eLife.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen CLE, Stone SJ, Cases S, Zhou P, Farese RV. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc Natl Acad Sci U S A. 2002;99:8512–7. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J, Lockwood J, Burn P, Shi Y. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J Biol Chem. 2003;278:13860–6. doi: 10.1074/jbc.M300139200. [DOI] [PubMed] [Google Scholar]
- 22.Yen CLE, Nelson DW, Yen MI. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res. 2015;56:489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Aquila T, Hung YH, Carreiro A, Buhman KK. Recent discoveries on absorption of dietary fat: Presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim Biophys Acta - Mol Cell Biol Lipids. 2016;1861:730–747. doi: 10.1016/j.bbalip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Sanz P, Hopewell R, Brindley DN. Long-chain fatty acids and their acyl-CoA esters cause the translocation of phosphatidate phosphohydrolase from the cytosolic to the microsomal fraction of rat liver. [accessed February 23, 2017];FEBS Lett. 1984 175:284–8. doi: 10.1016/0014-5793(84)80752-8. http://www.ncbi.nlm.nih.gov/pubmed/6090213. [DOI] [PubMed] [Google Scholar]
- 25.Grimsey N, Han GS, O’Hara L, Rochford JJ, Carman GM, Siniossoglou S. Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. J Biol Chem. 2008;283:29166–74. doi: 10.1074/jbc.M804278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csaki LS, Dwyer JR, Li X, Nguyen MHK, Dewald J, Brindley DN, Lusis AJ, Yoshinaga Y, de Jong P, Fong L, Young SG, Reue K. Lipin-1 and lipin-3 together determine adiposity in vivo. Mol Metab. 2014;3:145–154. doi: 10.1016/j.molmet.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Reue K. Lipin proteins and glycerolipid metabolism: Roles at the ER membrane and beyond. Biochim Biophys Acta. 2017 doi: 10.1016/j.bbamem.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Li JL, Li D, Tobin JF, Gimeno RE. Molecular identification of microsomal acyl-CoA:glycerol-3-phosphate acyltransferase, a key enzyme in de novo triacylglycerol synthesis. Proc Natl Acad Sci U S A. 2006;103:19695–700. doi: 10.1073/pnas.0609140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagle CA, Vergnes L, Dejong H, Wang S, Lewin TM, Reue K, Coleman RA. Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6−/− mice. J Lipid Res. 2008;49:823–31. doi: 10.1194/jlr.M700592-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt JA, Yvone GM, Brown WJ. Membrane topology of human AGPAT3 (LPAAT3) 2010 doi: 10.1016/j.bbrc.2010.05.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LU B, JIANG YJ, ZHOU Y, XU FY, HATCH GM, CHOY PC. Cloning and characterization of murine 1-acyl-sn-glycerol 3-phosphate acyltransferases and their regulation by PPARα in murine heart. Biochem J. 2005;385 doi: 10.1042/BJ20041348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han GS, Wu WI, Carman GM. The Saccharomyces cerevisiae Lipin Homolog Is a Mg2+-dependent Phosphatidate Phosphatase Enzyme. J Biol Chem. 2006;281:9210–9218. doi: 10.1074/jbc.M600425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282:3450–7. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 34.Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuerschner L, Moessinger C, Thiele C. Imaging of Lipid Biosynthesis: How a Neutral Lipid Enters Lipid Droplets. Traffic. 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 36.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 37.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–8. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 38.Farese RV, Walther TC. Lipid droplets go nuclear. J Cell Biol. 2016;212 doi: 10.1083/jcb.201512056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C. Human Lysophosphatidylcholine Acyltransferases 1 and 2 Are Located in Lipid Droplets Where They Catalyze the Formation of Phosphatidylcholine. J Biol Chem. 2011;286:21330–21339. doi: 10.1074/jbc.M110.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–46. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Zhang J, Qiu W, Han GS, Carman GM, Adeli K. Lipin-1γ isoform is a novel lipid droplet-associated protein highly expressed in the brain. FEBS Lett. 2011;585:1979–1984. doi: 10.1016/j.febslet.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uzbekov R, Roingeard P. Nuclear lipid droplets identified by electron microscopy of serial sections. n.d doi: 10.1186/1756-0500-6-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Layerenza JP, González P, García de Bravo MM, Polo MP, Sisti MS, Ves-Losada A. Nuclear lipid droplets: A novel nuclear domain. Biochim Biophys Acta - Mol Cell Biol Lipids. 2013;1831:327–340. doi: 10.1016/j.bbalip.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Ohsaki Y, Kawai T, Yoshikawa Y, Cheng J, Jokitalo E, Fujimoto T. PML isoform II plays a critical role in nuclear lipid droplet formation. J Cell Biol. 2016;212 doi: 10.1083/jcb.201507122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elholm M, Garras A, Neve S, Tornehave D, Lund TB, Skorve J, Flatmark T, Kristiansen K, Berge RK. Long-chain acyl-CoA esters and acyl-CoA binding protein are present in the nucleus of rat liver cells. [accessed February 24, 2017];J Lipid Res. 2000 41:538–45. http://www.ncbi.nlm.nih.gov/pubmed/10744774. [PubMed] [Google Scholar]
- 46.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:349–361. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 47.Harris TE, Finck BN. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab. 2011;22:226–233. doi: 10.1016/j.tem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Igal RA, Wang S, Gonzalez-Baró M, Coleman RA. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J Biol Chem. 2001;276:42205–12. doi: 10.1074/jbc.M103386200. [DOI] [PubMed] [Google Scholar]
- 49.Lindén D, William-Olsson L, Rhedin M, Asztély AK, Clapham JC, Schreyer S. Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J Lipid Res. 2004;45:1279–88. doi: 10.1194/jlr.M400010-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Wang S, Lee DP, Gong N, Schwerbrock NMJ, Mashek DG, Gonzalez-Baró MR, Stapleton C, Li LO, Lewin TM, Coleman RA. Cloning and functional characterization of a novel mitochondrial N-ethylmaleimide-sensitive glycerol-3-phosphate acyltransferase (GPAT2) Arch Biochem Biophys. 2007;465:347–358. doi: 10.1016/j.abb.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome Differences between Brown and White Fat Mitochondria Reveal Specialized Metabolic Functions. Cell Metab. 2009;10:324–335. doi: 10.1016/j.cmet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Prasad SS, Garg A, Agarwal AK. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria. J Lipid Res. 2011;52:451–62. doi: 10.1194/jlr.M007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA. piRNA-Associated Germline Nuage Formation and Spermatogenesis Require MitoPLD Profusogenic Mitochondrial-Surface Lipid Signaling. Dev Cell. 2011;20:376–387. doi: 10.1016/j.devcel.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rusiñol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. [accessed April 24, 2017];J Biol Chem. 1994 269:27494–502. http://www.ncbi.nlm.nih.gov/pubmed/7961664. [PubMed] [Google Scholar]
- 55.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–61. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal AK, Arioglu E, de Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- 57.Fu M, Kazlauskaite R, de Paiva Baracho MF, Do Nascimento Santos MG, Brandão-Neto J, Villares S, Celi FS, Wajchenberg BL, Shuldiner AR. Mutations in Gng3lg and AGPAT2 in Berardinelli-Seip Congenital Lipodystrophy and Brunzell Syndrome: Phenotype Variability Suggests Important Modifier Effects. J Clin Endocrinol Metab. 2004;89:2916–2922. doi: 10.1210/jc.2003-030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomes KB, Fernandes AP, Ferreira ACS, Pardini H, Garg A, Magré J, Pardini VC. Mutations in the Seipin and AGPAT2 Genes Clustering in Consanguineous Families with Berardinelli-Seip Congenital Lipodystrophy from Two Separate Geographical Regions of Brazil. J Clin Endocrinol Metab. 2004;89:357–361. doi: 10.1210/jc.2003-030415. [DOI] [PubMed] [Google Scholar]
- 59.Patni N, Garg A. Congenital generalized lipodystrophies--new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11:522–34. doi: 10.1038/nrendo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oral E, Chan J. Rationale for Leptin-Replacement Therapy for Severe Lipodystrophy. Endocr Pract. 2010;16:324–333. doi: 10.4158/EP09155.RA. [DOI] [PubMed] [Google Scholar]
- 61.Zeharia A, Shaag A, Houtkooper RH, Hindi T, de Lonlay P, Erez G, Hubert L, Saada A, de Keyzer Y, Eshel G, Vaz FM, Pines O, Elpeleg O. Mutations in LPIN1 Cause Recurrent Acute Myoglobinuria in Childhood. 2008 doi: 10.1016/j.ajhg.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michot C, Hubert L, Brivet M, De Meirleir L, Valayannopoulos V, Müller-Felber W, Venkateswaran R, Ogier H, Desguerre I, Altuzarra C, Thompson E, Smitka M, Huebner A, Husson M, Horvath R, Chinnery P, Vaz FM, Munnich A, Elpeleg O, Delahodde A, de Keyzer Y, de Lonlay P. LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Hum Mutat. 2010;31:E1564–E1573. doi: 10.1002/humu.21282. [DOI] [PubMed] [Google Scholar]
- 63.Bergounioux J, Brassier A, Rambaud C, Bustarret O, Michot C, Hubert L, Arnoux JB, Laquerriere A, Bekri S, Galene-Gromez S, Bonnet D, Hubert P, de Lonlay P. Fatal Rhabdomyolysis in 2 Children with LPIN1 Mutations. J Pediatr. 2012;160:1052–1054. doi: 10.1016/j.jpeds.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 64.Schweitzer GG, Collier SL, Chen Z, Eaton JM, Connolly AM, Bucelli RC, Pestronk A, Harris TE, Finck BN. Rhabdomyolysis-Associated Mutations in Human LPIN1 Lead to Loss of Phosphatidic Acid Phosphohydrolase Activity. JIMD Rep. 2015;23:113–22. doi: 10.1007/8904_2015_440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson PD, Panza G, Zaleski A, Taylor B. Statin-Associated Side Effects. J Am Coll Cardiol. 2016;67:2395–2410. doi: 10.1016/j.jacc.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 66.Michot C, Mamoune A, Vamecq J, Viou MT, Hsieh LS, Testet E, Lainé J, Hubert L, Dessein AF, Fontaine M, Ottolenghi C, Fouillen L, Nadra K, Blanc E, Bastin J, Candon S, Pende M, Munnich A, Smahi A, Djouadi F, Carman GM, Romero N, de Keyzer Y, de Lonlay P. Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipin-1-deficient myoblasts. Biochim Biophys Acta - Mol Basis Dis. 2013;1832:2103–2114. doi: 10.1016/j.bbadis.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, Munnich A, Lyonnet S, Majeed HA, El-Shanti H. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–7. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma M, Ferguson PJ. Autoinflammatory bone disorders. Curr Opin Rheumatol. 2013;25:658–664. doi: 10.1097/BOR.0b013e328363eb08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Donkor J, Zhang P, Wong S, O’Loughlin L, Dewald J, Kok BPC, Brindley DN, Reue K. A Conserved Serine Residue Is Required for the Phosphatidate Phosphatase Activity but Not the Transcriptional Coactivator Functions of Lipin-1 and Lipin-2. J Biol Chem. 2009;284:29968–29978. doi: 10.1074/jbc.M109.023663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berni Canani R, Terrin G, Cardillo G, Tomaiuolo R, Castaldo G. Congenital Diarrheal Disorders: Improved Understanding of Gene Defects Is Leading to Advances in Intestinal Physiology and Clinical Management. J Pediatr Gastroenterol Nutr. 2010;50:1. doi: 10.1097/MPG.0b013e3181d135ef. [DOI] [PubMed] [Google Scholar]
- 71.Haas JT, Winter HS, Lim E, Kirby A, Blumenstiel B, DeFelice M, Gabriel S, Jalas C, Branski D, Grueter CA, Toporovski MS, Walther TC, Daly MJ, Farese RV. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest. 2012;122:4680–4. doi: 10.1172/JCI64873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV., Jr Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. [accessed January 21, 2017];Proc Natl Acad Sci U S A. 1998 95:13018–23. doi: 10.1073/pnas.95.22.13018. http://www.ncbi.nlm.nih.gov/pubmed/9789033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gluchowski NL, Chitraju C, Picoraro JA, Mejhert N, Pinto S, Xin W, Kamin DS, Winter HS, Chung WK, Walther TC, Farese RV. Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. J Lipid Res. 2017 doi: 10.1194/jlr.P075119. jlr.P075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bin Hong Y, Kang J, Kim JH, Lee J, Kwak G, Hyun YS, Nam SH, Hong HD, Choi YR, Jung SC, Koo H, Lee JE, Choi BO, Chung KW. DGAT2 Mutation in a Family with Autosomal-Dominant Early-Onset Axonal Charcot-Marie-Tooth Disease. Hum Mutat. 2016;37:473–480. doi: 10.1002/humu.22959. [DOI] [PubMed] [Google Scholar]
- 75.Yamashita A, Hayashi Y, Matsumoto N, Nemoto-Sasaki Y, Oka S, Tanikawa T, Sugiura T. Glycerophosphate/Acylglycerophosphate Acyltransferases. Biology (Basel) 2014;3:801–830. doi: 10.3390/biology3040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gimeno RE, Cao J. Thematic review series: glycerolipids. Mammalian glycerol-3-phosphate acyltransferases: new genes for an old activity. J Lipid Res. 2008;49:2079–88. doi: 10.1194/jlr.R800013-JLR200. [DOI] [PubMed] [Google Scholar]
- 77.Csaki L, Dwyer J, Fong L, Tontonoz P, Young S, Reue K. Lipins, lipinopathies, and the modulation of cellular lipid storage and signaling. Prog Lipid Res. 2013;52:305–316. doi: 10.1016/j.plipres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yen CLE, Stone SJ, Koliwad S, Harris C, Farese RV. Thematic Review Series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez-Baro MR, Coleman RA. Mitochondrial acyltransferases and glycerophospholipid metabolism. Biochim Biophys Acta - Mol Cell Biol Lipids. 2017;1862:49–55. doi: 10.1016/j.bbalip.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 80.Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol. 2002;22:8204–14. doi: 10.1128/MCB.22.23.8204-8214.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yazdi M, Ahnmark A, William-Olsson L, Snaith M, Turner N, Osla F, Wedin M, Asztély A-K, Elmgren A, Bohlooly-Y M, Schreyer S, Lindén D. The role of mitochondrial glycerol-3-phosphate acyltransferase-1 in regulating lipid and glucose homeostasis in high-fat diet fed mice. 2008 doi: 10.1016/j.bbrc.2008.02.156. [DOI] [PubMed] [Google Scholar]
- 82.Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J Biol Chem. 2005;280:25629–36. doi: 10.1074/jbc.M503181200. [DOI] [PubMed] [Google Scholar]
- 83.Wendel AA, Li LO, Li Y, Cline GW, Shulman GI, Coleman RA. Glycerol-3-Phosphate Acyltransferase 1 Deficiency in ob/ob Mice Diminishes Hepatic Steatosis but Does Not Protect Against Insulin Resistance or Obesity. Diabetes. 2010;59 doi: 10.2337/db09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewin TM, de Jong H, Schwerbrock NJM, Hammond LE, Watkins SM, Combs TP, Coleman RA. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim Biophys Acta - Mol Cell Biol Lipids. 2008;1781:352–358. doi: 10.1016/j.bbalip.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hammond LE, Albright CD, He L, Rusyn I, Watkins SM, Doughman SD, Lemasters JJ, Coleman RA. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol. 2007;82:210–219. doi: 10.1016/j.yexmp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagle CA, An J, Shiota M, Torres TP, Cline GW, Liu ZX, Wang S, Catlin RL, Shulman GI, Newgard CB, Coleman RA. Hepatic overexpression of glycerol-sn-3-phosphate acyltransferase 1 in rats causes insulin resistance. J Biol Chem. 2007;282:14807–15. doi: 10.1074/jbc.M611550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ellis JM, Paul DS, DePetrillo MA, Singh BP, Malarkey DE, Coleman RA. Mice Deficient in Glycerol-3-Phosphate Acyltransferase-1 Have a Reduced Susceptibility to Liver Cancer. Toxicol Pathol. 2012;40:513–521. doi: 10.1177/0192623311432298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Collison LW, Murphy EJ, Jolly CA. Glycerol-3-phosphate acyltransferase-1 regulates murine T-lymphocyte proliferation and cytokine production. Am J Physiol - Cell Physiol. 2008;295 doi: 10.1152/ajpcell.00371.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gulvady AA, Murphy EJ, Ciolino HP, Cabrera RM, Jolly CA. Glycerol-3-Phosphate Acyltransferase-1 Gene Ablation Results in Altered Thymocyte Lipid Content and Reduces Thymic T Cell Production in Mice. Lipids. 2013;48:3–12. doi: 10.1007/s11745-012-3741-7. [DOI] [PubMed] [Google Scholar]
- 90.Faris R, Weber MM, Seeger DR, Cavazos D, de Graffenried L, Murphy EJ, Jolly CA. Mitochondrial Glycerol-3-Phosphate Acyltransferase-Dependent Phospholipid Synthesis Modulates Phospholipid Mass and IL-2 Production in Jurkat T Cells. Lipids. 2016;51:291–301. doi: 10.1007/s11745-016-4121-5. [DOI] [PubMed] [Google Scholar]
- 91.Vergnes L, Beigneux AP, Davis R, Watkins SM, Young SG, Reue K. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J Lipid Res. 2006;47:745–54. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen YQ, Kuo MS, Li S, Bui HH, Peake DA, Sanders PE, Thibodeaux SJ, Chu S, Qian YW, Zhao Y, Bredt DS, Moller DE, Konrad RJ, Beigneux AP, Young SG, Cao G. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J Biol Chem. 2008;283:10048–57. doi: 10.1074/jbc.M708151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis R, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, Young SG. Agpat6--a novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J Lipid Res. 2006;47:734–744. doi: 10.1194/jlr.M500556-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortés VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, Agarwal AK, Horton JD, Garg A. Molecular Mechanisms of Hepatic Steatosis and Insulin Resistance in the AGPAT2-Deficient Mouse Model of Congenital Generalized Lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gale SE, Frolov A, Han X, Bickel PE, Cao L, Bowcock A, Schaffer JE, Ory DS. A regulatory role for 1-acylglycerol-3-phosphate-O-acyltransferase 2 in adipocyte differentiation. J Biol Chem. 2006;281:11082–9. doi: 10.1074/jbc.M509612200. [DOI] [PubMed] [Google Scholar]
- 96.Cautivo KM, Lizama CO, Tapia PJ, Agarwal AK, Garg A, Horton JD, Cortés VA. AGPAT2 is essential for postnatal development and maintenance of white and brown adipose tissue. Mol Metab. 2016;5:491–505. doi: 10.1016/j.molmet.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vogel P, Read R, Hansen G, Wingert J, DaCosta CM, Buhring LM, Shadoan M. Pathology of Congenital Generalized Lipodystrophy in Agpat2−/−Mice. Vet Pathol. 2011;48:642–654. doi: 10.1177/0300985810383870. [DOI] [PubMed] [Google Scholar]
- 98.Agarwal AK, Tunison K, Dalal JS, Yen CLE, Farese RV, Horton JD, Garg A. Mogat1 deletion does not ameliorate hepatic steatosis in lipodystrophic (Agpat2−/−) or obese (ob/ob) mice. J Lipid Res. 2016;57:616–30. doi: 10.1194/jlr.M065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agarwal AK, Sukumaran S, Cortés VA, Tunison K, Mizrachi D, Sankella S, Gerard RD, Horton JD, Garg A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(−/−) gene lipodystrophic mice. J Biol Chem. 2011;286:37676–91. doi: 10.1074/jbc.M111.250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cortes VA, Cautivo KM, Rong S, Garg A, Horton JD, Agarwal AK. Leptin ameliorates insulin resistance and hepatic steatosis in Agpat2−/− lipodystrophic mice independent of hepatocyte leptin receptors. J Lipid Res. 2014;55:276–288. doi: 10.1194/jlr.M045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reue K, Péterfy M, Phan J, Xu P. Lipodystrophy in the fld mouse results from mutation of a newgene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 102.Langner CA, Birkenmeier EH, Ben-Zeev O, Schotz MC, Sweet HO, Davisson MT, Gordon JI. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. [accessed April 25, 2017];J Biol Chem. 1989 264:7994–8003. http://www.ncbi.nlm.nih.gov/pubmed/2722772. [PubMed] [Google Scholar]
- 103.Langner C, Birkenmeier E, Roth K, Bronson R, Gordon J. Characterization of the peripheral neuropathy in neonatal and adult mice that are homozygous for the fatty liver dystrophy (fld) mutation. [accessed April 24, 2017];J Biol Chem. 1991 266:11955–64. http://www.ncbi.nlm.nih.gov/pubmed/2050689. [PubMed] [Google Scholar]
- 104.Reue K, Xu P, Wang XP, Slavin BG. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. [accessed February 1, 2017];J Lipid Res. 2000 41:1067–76. http://www.ncbi.nlm.nih.gov/pubmed/10884287. [PubMed] [Google Scholar]
- 105.Phan J, Peterfy M, Reue K. Lipin Expression Preceding Peroxisome Proliferator-activated Receptor- Is Critical for Adipogenesis in Vivo and in Vitro. J Biol Chem. 2004;279:29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 106.Zhang P, Takeuchi K, Csaki LS, Reue K. Lipin-1 Phosphatidic Phosphatase Activity Modulates Phosphatidate Levels to Promote Peroxisome Proliferator-activated Receptor (PPAR) Gene Expression during Adipogenesis. J Biol Chem. 2012;287:3485–3494. doi: 10.1074/jbc.M111.296681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Phan J, Reue K. Lipin, a lipodystrophy and obesity gene. Cell Metab. 2005;1:73–83. doi: 10.1016/j.cmet.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 108.Peterfy M, Phan J, Reue K. Alternatively Spliced Lipin Isoforms Exhibit Distinct Expression Pattern, Subcellular Localization, and Role in Adipogenesis. J Biol Chem. 2005;280:32883–32889. doi: 10.1074/jbc.M503885200. [DOI] [PubMed] [Google Scholar]
- 109.Verheijen M, Chrast R, Burrola P, Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 2003;17:2450–2464. doi: 10.1101/gad.1116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mitra MS, Chen Z, Ren H, Harris TE, Chambers KT, Hall AM, Nadra K, Klein S, Chrast R, Su X, Morris AJ, Finck BN. Mice with an adipocyte-specific lipin 1 separation-of-function allele reveal unexpected roles for phosphatidic acid in metabolic regulation. Proc Natl Acad Sci. 2013;110:642–647. doi: 10.1073/pnas.1213493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu M, Yin H, Mitra MS, Liang X, Ajmo JM, Nadra K, Chrast R, Finck BN, You M. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology. 2013;58:1953–1963. doi: 10.1002/hep.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schweitzer GG, Chen Z, Gan C, McCommis KS, Soufi N, Chrast R, Mitra MS, Yang K, Gross RW, Finck BN. Liver-specific loss of lipin-1-mediated phosphatidic acid phosphatase activity does not mitigate intrahepatic TG accumulation in mice. J Lipid Res. 2015;56:848–58. doi: 10.1194/jlr.M055962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, Kim C, Jogasuria A, Han Y, Hu X, Wu J, Shen H, Chrast R, Finck BN, You M. Myeloid Cell-Specific Lipin-1 Deficiency Stimulates Endocrine Adiponectin-FGF15 Axis and Ameliorates Ethanol-Induced Liver Injury in Mice. Sci Rep. 2016;6:34117. doi: 10.1038/srep34117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Temprano A, Sembongi H, Han GS, Sebastián D, Capellades J, Moreno C, Guardiola J, Wabitsch M, Richart C, Yanes O, Zorzano A, Carman GM, Siniossoglou S, Miranda M. Redundant roles of the phosphatidate phosphatase family in triacylglycerol synthesis in human adipocytes. Diabetologia. 2016;59:1985–1994. doi: 10.1007/s00125-016-4018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang P, Verity MA, Reue K. Lipin-1 Regulates Autophagy Clearance and Intersects with Statin Drug Effects in Skeletal Muscle. Cell Metab. 2014;20:267–279. doi: 10.1016/j.cmet.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang W, Zhu J, Zhuang X, Zhang X, Luo T, Esser KA, Ren H. Lipin1 Regulates Skeletal Muscle Differentiation through Extracellular Signal-regulated Kinase (ERK) Activation and Cyclin D Complex-regulated Cell Cycle Withdrawal. J Biol Chem. 2015;290:23646–23655. doi: 10.1074/jbc.M115.686519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P, Reue K. Lipin-1 flexes its muscle in autophagy. Cell Cycle. 2014;13:3789–90. doi: 10.4161/15384101.2014.988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dwyer JR, Donkor J, Zhang P, Csaki LS, Vergnes L, Lee JM, Dewald J, Brindley DN, Atti E, Tetradis S, Yoshinaga Y, De Jong PJ, Fong LG, Young SG, Reue K. Mouse lipin-1 and lipin-2 cooperate to maintain glycerolipid homeostasis in liver and aging cerebellum. Proc Natl Acad Sci. 2012;109:E2486–E2495. doi: 10.1073/pnas.1205221109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lordén G, Sanjuán-García I, de Pablo N, Meana C, Alvarez-Miguel I, Pérez-García MT, Pelegrín P, Balsinde J, Balboa MA. Lipin-2 regulates NLRP3 inflammasome by affecting P2X7 receptor activation. J Exp Med. 2016 doi: 10.1084/jem.20161452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 121.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Burri BJ, Hamilton RL, Abumrad NA, Farese RV. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–9. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 122.Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1−/− mice. J Lipid Res. 2010;51:1770–1780. doi: 10.1194/jlr.M002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV. Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–55. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV. Dissociation of Obesity and Impaired Glucose Disposal in Mice Overexpressing Acyl Coenzyme A:Diacylglycerol Acyltransferase 1 in White Adipose Tissue. Diabetes. 2002;51 doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 125.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1679–89. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–81. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chandak PG, Obrowsky S, Radovic B, Doddapattar P, Aflaki E, Kratzer A, Doshi LS, Povoden S, Ahammer H, Hoefler G, Levak-Frank S, Kratky D. Lack of acyl-CoA:diacylglycerol acyltransferase 1 reduces intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E knockout mice. Biochim Biophys Acta - Mol Cell Biol Lipids. 2011;1811:1011–1020. doi: 10.1016/j.bbalip.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–76. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 129.Liu L, Jiang Q, Wang X, Zhang Y, Lin RCY, Lam SM, Shui G, Zhou L, Li P, Wang Y, Cui X, Gao M, Zhang L, Lv Y, Xu G, Liu G, Zhao D, Yang H. Adipose-Specific Knockout of Seipin/Bscl2 Results in Progressive Lipodystrophy. Diabetes. 2014;63:2320–2331. doi: 10.2337/db13-0729. [DOI] [PubMed] [Google Scholar]
- 130.Hishikawa D, Hashidate T, Shimizu T, Shindou H. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J Lipid Res. 2014;55:799–807. doi: 10.1194/jlr.R046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Csaki LS, Reue K. Lipins: Multifunctional Lipid Metabolism Proteins. Annu Rev Nutr. 2010;30:257–272. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jerkins AA, Liu WR, Lee S, Sul HS. Characterization of the murine mitochondrial glycerol-3-phosphate acyltransferase promoter. J Biol Chem. 1995;270:1416–21. doi: 10.1074/JBC.270.3.1416. [DOI] [PubMed] [Google Scholar]
- 133.Aneja KK, Guha P, Shilpi RY, Chakraborty S, Schramm LM, Haldar D. The presence of distal and proximal promoters for rat mitochondrial glycerol-3-phosphate acyltransferase. Arch Biochem Biophys. 2008;470:35–43. doi: 10.1016/j.abb.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guha P, Aneja KK, Shilpi RY, Haldar D. Transcriptional regulation of mitochondrial glycerophosphate acyltransferase is mediated by distal promoter via ChREBP and SREBP- 1. Arch Biochem Biophys. 2009;490:85–95. doi: 10.1016/j.abb.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin J, Iakova P, Breaux M, Sullivan E, Jawanmardi N, Chen D, Jiang Y, Medrano EM, Timchenko NA. Increased Expression of Enzymes of Triglyceride Synthesis Is Essential for the Development of Hepatic Steatosis. Cell Rep. 2013;3:831–843. doi: 10.1016/j.celrep.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114 doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 137.Pellon-Maison M, Montanaro MA, Lacunza E, Garcia-Fabiani MB, Soler-Gerino MC, Cattaneo ER, Quiroga IY, Abba MC, Coleman RA, Gonzalez-Baro MR. Glycerol-3-Phosphate Acyltranferase-2 Behaves as a Cancer Testis Gene and Promotes Growth and Tumorigenicity of the Breast Cancer MDA-MB-231 Cell Line. PLoS One. 2014;9:e100896. doi: 10.1371/journal.pone.0100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ehara T, Kamei Y, Takahashi M, Yuan X, Kanai S, Tamura E, Tanaka M, Yamazaki T, Miura S, Ezaki O, Suganami T, Okano M, Ogawa Y. Role of DNA Methylation in the Regulation of Lipogenic Glycerol-3-Phosphate Acyltransferase 1 Gene Expression in the Mouse Neonatal Liver. Diabetes. 2012;61 doi: 10.2337/db11-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bronnikov GE, Aboulaich N, Vener AV, Strålfors P. Acute effects of insulin on the activity of mitochondrial GPAT1 in primary adipocytes. Biochem Biophys Res Commun. 2008;367:201–207. doi: 10.1016/j.bbrc.2007.12.127. [DOI] [PubMed] [Google Scholar]
- 140.Shan D, Li J, Wu L, Li D, Hurov J, Tobin JF, Gimeno RE, Cao J. GPAT3 and GPAT4 are regulated by insulin-stimulated phosphorylation and play distinct roles in adipogenesis. J Lipid Res. 2010;51:1971–81. doi: 10.1194/jlr.M006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Subauste AR, Elliott B, Das AK, Burant CF. A role for 1-acylglycerol-3-phosphate-O-acyltransferase-1 in myoblast differentiation. Differentiation. 2010;80:140–146. doi: 10.1016/j.diff.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu B, Jiang YJ, Man MQ, Brown B, Elias PM, Feingold KR. Expression and regulation of 1-acyl-sn-glycerol- 3-phosphate acyltransferases in the epidermis. J Lipid Res. 2005;46:2448–57. doi: 10.1194/jlr.M500258-JLR200. [DOI] [PubMed] [Google Scholar]
- 143.Yuki K, Shindou H, Hishikawa D, Shimizu T. Characterization of mouse lysophosphatidic acid acyltransferase 3: an enzyme with dual functions in the testis. J Lipid Res. 2009;50:860–9. doi: 10.1194/jlr.M800468-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang P, O’Loughlin L, Brindley DN, Reue K. Regulation of lipin-1 gene expression by glucocorticoids during adipogenesis. J Lipid Res. 2008;49:1519–28. doi: 10.1194/jlr.M800061-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manmontri B, Sariahmetoglu M, Donkor J, Bou Khalil M, Sundaram M, Yao Z, Reue K, Lehner R, Brindley DN. Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically. J Lipid Res. 2008;49:1056–67. doi: 10.1194/jlr.M800013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kok BPC, Dyck JRB, Harris TE, Brindley DN. Differential regulation of the expressions of the PGC-1α splice variants, lipins, and PPARα in heart compared to liver. J Lipid Res. 2013;54:1662–77. doi: 10.1194/jlr.M036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ishimoto K, Nakamura H, Tachibana K, Yamasaki D, Ota A, Hirano K, Tanaka T, Hamakubo T, Sakai J, Kodama T, Doi T. Sterol-mediated regulation of human lipin 1 gene expression in hepatoblastoma cells. J Biol Chem. 2009;284:22195–205. doi: 10.1074/jbc.M109.028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mylonis I, Sembongi H, Befani C, Liakos P, Siniossoglou S, Simos G. Hypoxia causes triglyceride accumulation by HIF-1-mediated stimulation of lipin 1 expression. J Cell Sci. 2012;125 doi: 10.1242/jcs.106682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim DK, Kim JR, Koh M, Kim YD, Lee JM, Chanda D, Park SB, Min JJ, Lee CH, Park TS, Choi HS. Estrogen-related receptor γ (ERRγ) is a novel transcriptional regulator of phosphatidic acid phosphatase, LIPIN1, and inhibits hepatic insulin signaling. J Biol Chem. 2011;286:38035–42. doi: 10.1074/jbc.M111.250613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitra MS, Schilling JD, Wang X, Jay PY, Huss JM, Su X, Finck BN. Cardiac lipin 1 expression is regulated by the peroxisome proliferator activated receptor γ coactivator 1α/estrogen related receptor axis. J Mol Cell Cardiol. 2011;51:120–128. doi: 10.1016/j.yjmcc.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Z, Gropler MC, Mitra MS, Finck BN. Complex interplay between the lipin 1 and the hepatocyte nuclear factor 4 α (HNF4α) pathways to regulate liver lipid metabolism. PLoS One. 2012;7:e51320. doi: 10.1371/journal.pone.0051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirotsu Y, Hataya N, Katsuoka F, Yamamoto M. NF-E2-related factor 1 (Nrf1) serves as a novel regulator of hepatic lipid metabolism through regulation of the Lipin1 and PGC- 1β genes. Mol Cell Biol. 2012;32:2760–70. doi: 10.1128/MCB.06706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu M, Wang F, Li X, Rogers CQ, Liang X, Finck BN, Mitra MS, Zhang R, Mitchell DA, You M. Regulation of hepatic lipin-1 by ethanol: Role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology. 2012;55:437–446. doi: 10.1002/hep.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yin H, Hu M, Zhang R, Shen Z, Flatow L, You M. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J Biol Chem. 2012;287:9817–26. doi: 10.1074/jbc.M111.333534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wei Y, Yang CR, Wei YP, Ge ZJ, Zhao ZA, Zhang B, Hou Y, Schatten H, Sun QY. Enriched environment-induced maternal weight loss reprograms metabolic gene expression in mouse offspring. J Biol Chem. 2015;290:4604–19. doi: 10.1074/jbc.M114.605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huffman TA, Mothe-Satney I, Lawrence JC. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci U S A. 2002;99:1047–52. doi: 10.1073/pnas.022634399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harris TE, Huffman TA, Chi A, Shabanowitz J, Hunt DF, Kumar A, Lawrence JC. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282:277–86. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 159.Eaton JM, Mullins GR, Brindley DN, Harris TE. Phosphorylation of lipin 1 and charge on the phosphatidic acid head group control its phosphatidic acid phosphatase activity and membrane association. J Biol Chem. 2013;288:9933–45. doi: 10.1074/jbc.M112.441493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eaton JM, Takkellapati S, Lawrence RT, McQueeney KE, Boroda S, Mullins GR, Sherwood SG, Finck BN, Villén J, Harris TE. Lipin 2 binds phosphatidic acid by the electrostatic hydrogen bond switch mechanism independent of phosphorylation. J Biol Chem. 2014;289:18055–66. doi: 10.1074/jbc.M114.547604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim Y, Gentry MS, Harris TE, Wiley SE, Lawrence JC, Dixon JE. A conserved phosphatase cascade that regulates nuclear membrane biogenesis. Proc Natl Acad Sci U S A. 2007;104:6596–601. doi: 10.1073/PNAS.0702099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Han S, Bahmanyar S, Zhang P, Grishin N, Oegema K, Crooke R, Graham M, Reue K, Dixon JE, Goodman JM. Nuclear envelope phosphatase 1-regulatory subunit 1 (formerly TMEM188) is the metazoan Spo7p ortholog and functions in the lipin activation pathway. J Biol Chem. 2012;287:3123–37. doi: 10.1074/jbc.M111.324350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu GH, Gerace L. Sumoylation Regulates Nuclear Localization of Lipin-1α in Neuronal Cells. PLoS One. 2009;4:e7031. doi: 10.1371/journal.pone.0007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shimizu K, Fukushima H, Ogura K, Lien EC, Nihira NT, Zhang J, North BJ, Guo A, Nagashima K, Nakagawa T, Hoshikawa S, Watahiki A, Okabe K, Yamada A, Toker A, Asara JM, Fukumoto S, Nakayama KI, Nakayama K, Inuzuka H, Wei W. The SCFβ-TRCP E3 ubiquitin ligase complex targets Lipin1 for ubiquitination and degradation to promote hepatic lipogenesis. Sci Signal. 2017;10 doi: 10.1126/scisignal.aah4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hsieh LS, Su WM, Han GS, Carman GM. Phosphorylation regulates the ubiquitin-independent degradation of yeast Pah1 phosphatidate phosphatase by the 20S proteasome. J Biol Chem. 2015;290:11467–78. doi: 10.1074/jbc.M115.648659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ludwig E, Mahley R, Palaoglu E, Özbayrakçý S, Balestra M, Borecki I, Innerarity T, Farese R. DGAT1 promoter polymorphism associated with alterations in body mass index, high density lipoprotein levels and blood pressure in Turkish women. Clin Genet. 2002;62:68–73. doi: 10.1034/j.1399-0004.2002.620109.x. [DOI] [PubMed] [Google Scholar]
- 167.Ranganathan G, Unal R, Pokrovskaya I, Yao-Borengasser A, Phanavanh B, Lecka-Czernik B, Rasouli N, Kern PA. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J Lipid Res. 2006;47:2444–50. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Payne VA, Au WS, Gray SL, Nora ED, Rahman SM, Sanders R, Hadaschik D, Friedman JE, O’rahilly S, Rochford JJ. Sequential regulation of diacylglycerol acyltransferase 2 expression by CAAT/enhancer-binding protein beta (C/EBPbeta) and C/EBPalpha during adipogenesis. J Biol Chem. 2007;282:21005–14. doi: 10.1074/jbc.M702871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun. 2002;298:317–323. doi: 10.1016/S0006-291X(02)02466-X. [DOI] [PubMed] [Google Scholar]
- 170.Stals HK, Top W, Declercq PE. Regulation of triacylglycerol synthesis in permeabilized rat hepatocytes Role of fatty acid concentration and diacylglycerol acyltransferase. FEBS Lett. 1994;343:99–102. doi: 10.1016/0014-5793(94)80615-2. [DOI] [PubMed] [Google Scholar]
- 171.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tsai J, Qiu W, Kohen-Avramoglu R, Adeli K. MEK–ERK Inhibition Corrects the Defect in VLDL Assembly in HepG2 Cells. Arterioscler Thromb Vasc Biol. 2006;27 doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 173.Wakimoto K, Chiba H, Michibata H, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y. A novel diacylglycerol acyltransferase (DGAT2) is decreased in human psoriatic skin and increased in diabetic mice. Biochem Biophys Res Commun. 2003;310:296–302. doi: 10.1016/j.bbrc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 174.Haagsman HP, De Haas CGM, Geelen MJH, Van Goldes LMG. Regulation of Triacylglycerol Synthesis in the Liver MODULATION OF DIACYLGLYCEROL ACYLTRANSFERASE ACTIVITY IN VITRO*. J Biol Chem VoL. 1982;257:10593–10598. [PubMed] [Google Scholar]
- 175.Sooranna SR, Saggerson ED. A decrease in diacylglycerol acyltransferase after treatment of rat adipocytes with adrenaline. FEBS Lett. 1978;95:85–87. doi: 10.1016/0014-5793(78)80057-X. [DOI] [PubMed] [Google Scholar]
- 176.Le MTN, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, Lodish HF. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol. 2009;29:5290–305. doi: 10.1128/MCB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu YH, Zhang Y, Oelkers P, Sturley SL, Rader DJ, Ginsberg HN. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. J Biol Chem. 2002;277:50876–84. doi: 10.1074/jbc.M207353200. [DOI] [PubMed] [Google Scholar]
- 178.Yu J, Li Y, Zou F, Xu S, Liu P. Phosphorylation and function of DGAT1 in skeletal muscle cells. Biophys Reports. 2015;1:41–50. doi: 10.1007/s41048-015-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Choi K, Kim H, Kang H, Lee SY, Lee SJ, Back SH, Lee SH, Kim MS, Lee JE, Park JY, Kim J, Kim S, Song JH, Choi Y, Lee S, Lee HJ, Kim JH, Cho S. Regulation of diacylglycerol acyltransferase 2 protein stability by gp78-associated endoplasmic-reticulum-associated degradation. FEBS J. 2014;281:3048–3060. doi: 10.1111/febs.12841. [DOI] [PubMed] [Google Scholar]
- 180.Yu JH, Lee YJ, Kim HJ, Choi H, Choi Y, Seok JW, Kim J. Monoacylglycerol O-acyltransferase 1 is regulated by peroxisome proliferator-activated receptor γ in human hepatocytes and increases lipid accumulation. 2015 doi: 10.1016/j.bbrc.2015.03.095. [DOI] [PubMed] [Google Scholar]
- 181.Hulo N, Bairoch A, Bulliard V, Cerutti L, De Castro E, Langendijk-Genevaux PS, Pagni M, Sigrist CJA. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem. 2009;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- 183.Lewin Tal M, Wang Ping, Coleman* RA. Analysis of Amino Acid Motifs Diagnostic for the sn-Glycerol-3-phosphate Acyltransferase Reaction†. 1999 doi: 10.1021/BI982805D. [DOI] [PubMed] [Google Scholar]
- 184.Heath RJ, Rock CO. A conserved histidine is essential for glycerolipid acyltransferase catalysis. [accessed June 14, 2017];J Bacteriol. 1998 180:1425–30. doi: 10.1128/jb.180.6.1425-1430.1998. http://www.ncbi.nlm.nih.gov/pubmed/9515909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Turnbull AP, Rafferty JB, Sedelnikova SE, Slabas AR, Schierer TP, Kroon JT, Simon JW, Fawcett T, Nishida I, Murata N, Rice DW. Analysis of the structure, substrate specificity, and mechanism of squash glycerol-3-phosphate (1)-acyltransferase. [accessed February 24, 2017];Structure. 2001 9:347–53. doi: 10.1016/s0969-2126(01)00595-0. http://www.ncbi.nlm.nih.gov/pubmed/11377195. [DOI] [PubMed] [Google Scholar]
- 186.Slabas AR, Kroon JTM, Scheirer TP, Gilroy JS, Hayman M, Rice DW, Turnbull AP, Rafferty JB, Fawcett T, Simon WJ. Squash glycerol-3-phosphate (1)-acyltransferase. Alteration of substrate selectivity and identification of arginine and lysine residues important in catalytic activity. J Biol Chem. 2002;277:43918–23. doi: 10.1074/jbc.M206429200. [DOI] [PubMed] [Google Scholar]
- 187.Tamada T, Feese MD, Ferri SR, Kato Y, Yajima R, Toguri T, Kuroki R. Substrate recognition and selectivity of plant glycerol-3-phosphate acyltransferases (GPATs) from Cucurbita moscata and Spinacea oleracea. [accessed April 24, 2017];Acta Crystallogr D Biol Crystallogr. 2004 60:13–21. doi: 10.1107/s0907444903020778. http://www.ncbi.nlm.nih.gov/pubmed/14684887. [DOI] [PubMed] [Google Scholar]
- 188.Albesa-Jové D, Svetlíková Z, Tersa M, Sancho-Vaello E, Carreras-González A, Bonnet P, Arrasate P, Eguskiza A, Angala SK, Cifuente JO, Korduláková J, Jackson M, Mikušová K, Guerin ME. Structural basis for selective recognition of acyl chains by the membrane-associated acyltransferase PatA. Nat Commun. 2016;7:10906. doi: 10.1038/ncomms10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dovala D, Rath CM, Hu Q, Sawyer WS, Shia S, Elling RA, Knapp MS, Metzger LE. Structure-guided enzymology of the lipid A acyltransferase LpxM reveals a dual activity mechanism. Proc Natl Acad Sci U S A. 2016;113:E6064–E6071. doi: 10.1073/pnas.1610746113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stone SJ, Levin MC, Farese RV. Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J Biol Chem. 2006;281:40273–82. doi: 10.1074/jbc.M607986200. [DOI] [PubMed] [Google Scholar]
- 191.Liu Q, Siloto RMP, Snyder CL, Weselake RJ. Functional and topological analysis of yeast acyl-CoA:diacylglycerol acyltransferase 2, an endoplasmic reticulum enzyme essential for triacylglycerol biosynthesis. J Biol Chem. 2011;286:13115–26. doi: 10.1074/jbc.M110.204412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Holmes RS. Comparative genomics and proteomics of vertebrate diacylglycerol acyltransferase (DGAT), acyl CoA wax alcohol acyltransferase (AWAT) and monoacylglycerol acyltransferase (MGAT) Comp Biochem Physiol Part D Genomics Proteomics. 2010;5:45–54. doi: 10.1016/j.cbd.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 193.Turchetto-Zolet AC, Maraschin FS, de Morais GL, Cagliari A, Andrade CMB, Margis-Pinheiro M, Margis R. Evolutionary view of acyl-CoA diacylglycerol acyltransferase (DGAT), a key enzyme in neutral lipid biosynthesis. BMC Evol Biol. 2011;11:263. doi: 10.1186/1471-2148-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McFie PJ, Stone SL, Banman SL, Stone SJ. Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the n terminus in dimer/tetramer formation. J Biol Chem. 2010;285:37377–87. doi: 10.1074/jbc.M110.163691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Das A, Davis MA, Rudel LL. Identification of putative active site residues of ACAT enzymes. J Lipid Res. 2008;49:1770–81. doi: 10.1194/jlr.M800131-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wurie HR, Buckett L, Zammit VA. Evidence that diacylglycerol acyltransferase 1 (DGAT1) has dual membrane topology in the endoplasmic reticulum of HepG2 cells. J Biol Chem. 2011;286:36238–47. doi: 10.1074/jbc.M111.251900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ohshiro T, Tomoda H. Acyltransferase inhibitors: a patent review (2010–present) Expert Opin Ther Pat. 2015;25:145–158. doi: 10.1517/13543776.2014.989833. [DOI] [PubMed] [Google Scholar]
- 198.Wydysh EA, Medghalchi SM, Vadlamudi A, Townsend CA. Design and Synthesis of Small Molecule Glycerol 3-Phosphate Acyltransferase Inhibitors. J Med Chem. 2009;52:3317–3327. doi: 10.1021/jm900251a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kuhajda FP, Aja S, Tu Y, Han WF, Medghalchi SM, El Meskini R, Landree LE, Peterson JM, Daniels K, Wong K, Wydysh EA, Townsend CA, Ronnett GV. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am J Physiol - Regul Integr Comp Physiol. 2011;301 doi: 10.1152/ajpregu.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. Troxerutin improves hepatic lipid homeostasis by restoring NAD+-depletion-mediated dysfunction of lipin 1 signaling in high-fat diet-treated mice. Biochem Pharmacol. 2014;91:74–86. doi: 10.1016/j.bcp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 201.Meyers C, Gaudet D, Tremblay K, Amer A, Chen J, Aimin F. The DGAT1 Inhibitor LCQ908 Decreases Triglyceride Levels in Patients with the Familial Chylomicronemia Syndrome. J Clin Lipidol. 2012;6:266–267. doi: 10.1016/j.jacl.2012.04.034. [DOI] [Google Scholar]
- 202.McCoull W, Addie MS, Birch AM, Birtles S, Buckett LK, Butlin RJ, Bowker SS, Boyd S, Chapman S, Davies RDM, Donald CS, Green CP, Jenner C, Kemmitt PD, Leach AG, Moody GC, Morentin Gutierrez P, Newcombe NJ, Nowak T, Packer MJ, Plowright AT, Revill J, Schofield P, Sheldon C, Stokes S, Turnbull AV, Wang SJY, Whalley DP, Matthew Wood J. Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. 2012 doi: 10.1016/j.bmcl.2012.04.117. [DOI] [PubMed] [Google Scholar]
- 203.Denison H, Nilsson C, Löfgren L, Himmelmann A, Mårtensson G, Knutsson M, AL-Shurbaji A, Tornqvist H, Eriksson JW. Diacylglycerol acyltransferase 1 inhibition with AZD7687 alters lipid handling and hormone secretion in the gut with intolerable side effects: a randomized clinical trial. Diabetes, Obes Metab. 2014;16:334–343. doi: 10.1111/dom.12221. [DOI] [PubMed] [Google Scholar]
- 204.Kim MO, Lee SU, Lee HJ, Choi K, Kim H, Lee S, Oh SJ, Kim S, Kang JS, Lee HS, Kwak YS, Cho S. Identification and Validation of a Selective Small Molecule Inhibitor Targeting the Diacylglycerol Acyltransferase 2 Activity. Biol Pharm Bull. 2013;36:1167–1173. doi: 10.1248/bpb.b13-00152. [DOI] [PubMed] [Google Scholar]
- 205.Take K, Mochida T, Maki T, Satomi Y, Hirayama M, Nakakariya M, Amano N, Adachi R, Sato K, Kitazaki T, Takekawa S. Pharmacological Inhibition of Monoacylglycerol O-Acyltransferase 2 Improves Hyperlipidemia, Obesity, and Diabetes by Change in Intestinal Fat Utilization. PLoS One. 2016;11:e0150976. doi: 10.1371/journal.pone.0150976. [DOI] [PMC free article] [PubMed] [Google Scholar]