Abstract
Purpose
Our objective was to estimate the economic outcomes of using mirabegron versus antimuscarinics in the treatment of patients with overactive bladder (OAB) from a societal perspective in the UK.
Materials and Methods
A Markov model was developed using Microsoft Excel®. The time horizon and cycle length are 12 and 1 months, respectively; and the hypothetical cohort size 100 patients. Antimuscarinic comparators are fesoterodine, oxybutynin extended release (ER) and immediate release (IR), solifenacin, tolterodine ER/IR, trospium ER/IR, darifenacin and flavoxate. Model inputs included real-world treatment patterns data, healthcare resource use (e.g. clinic visits) and direct and indirect costs (e.g. drug acquisition and productivity loss). Model outputs included patient disposition, healthcare resource use, drug acquisition costs and other treatment-related costs over a 1-year time horizon. A one-way sensitivity analysis was performed to determine the key drivers of the model.
Results
In a hypothetical cohort of 100 patients, total annual costs per patient were lower with mirabegron than with all antimuscarinics (£1270.84 vs. 1321.71–1607.48). Healthcare resource use was lower with mirabegron than with all antimuscarinics (115 vs. 119–123 general practitioner visits; 173 vs. 178–185 specialist visits and 0.0042 vs. 0.0050–0.0060 surgical operations) and fewer work hours were lost (4017 vs. 5114–6990 [all per 100 patients]). Sensitivity analysis showed the model was sensitive to persistence and switching rates, although the impact on the overall results was minimal.
Conclusions
In the UK, using mirabegron to treat OAB may improve persistence and lead to reductions in switching treatment, healthcare resource utilization, productivity costs, and overall treatment costs versus antimuscarinics.
Electronic supplementary material
The online version of this article (doi:10.1007/s41669-017-0011-x) contains supplementary material, which is available to authorized users.
Keywords: Healthcare Resource, Oxybutynin, Tolterodine, Immediate Release, Solifenacin
Key Points for Decision Makers
| Treatment of overactive bladder (OAB) with mirabegron 50 mg in the UK may result in lower healthcare resource utilization, lost productivity costs, and overall treatment costs compared with antimuscarinics. |
| Further work is needed to confirm these findings in different populations to assess the effects of mirabegron on the costs of treatment in other countries. |
Introduction
Overactive bladder (OAB) is characterized by urinary urgency, usually with urinary frequency and nocturia, with or without urinary incontinence [1]. OAB affects up to 17% of adults aged ≥40 years in Europe and up to 17% of all adults in the USA [2, 3]. In Europe, a higher proportion (30–40%) of OAB is observed in adults ≥75 years of age [2].
The clinical and economic burden of OAB is significant. Symptoms of OAB have been reported to adversely affect patients’ daily activities, sleep, mental health and personal relationships [4, 5]. These symptoms also have a significant impact on productivity in the workplace and healthcare resource use [6]. In the UK, the total direct economic impact (incremental costs) of OAB on the national healthcare system is estimated to be in excess of £1 billion [6].
Following the use of conservative management strategies, treatment with pharmacotherapy can result in symptom improvement. However, bothersome on-treatment adverse events (AEs) from antimuscarinic agents (such as dry mouth and constipation) are common because of a lack of target organ specificity [7]. Side effects observed with antimuscarinics may also have deleterious effects on treatment persistence. A screening survey conducted in the USA identified 6577 patients with one or more antimuscarinic prescriptions for OAB [8]. Of the 1322 patients who reported discontinuing treatment prior to follow-up, 1177 (89%) discontinuations were primarily due to unmet treatment expectations and/or tolerability [8].
Mirabegron is a first-in-class licensed selective oral β3-adrenoceptor agonist approved in the UK for the treatment of OAB [9]. It promotes bladder relaxation of the detrusor muscle during the storage phase, improving bladder capacity [10]. Mirabegron demonstrated overall efficacy similar to that of antimuscarinic therapies in clinical trials [11] but with an improved overall tolerability profile [11, 12]. Evidence from a recent retrospective analysis of prescription claims in Canada demonstrated that, over a 12-month period, patients with OAB treated with mirabegron have improved persistence compared with those treated with antimuscarinics [13].
A prospective study of women with OAB who received treatment with fesoterodine for 8 weeks also showed that those who adhered to treatment reported significantly greater improvements in clinical symptoms at the end of the treatment period than those who were non-adherent [14]. In addition, persistence with treatment has been shown to reduce consumption of healthcare resources in OAB [15]. Given the impact of OAB on work absenteeism and productivity [6], persistence and symptom control may be expected to reduce the impact of these factors.
This study aimed to use predominantly real-world evidence to estimate the economic outcomes associated with persistence with mirabegron treatment versus antimuscarinic agents in patients with OAB in the UK.
Methods
Model Overview
This is a Microsoft® Excel-based (version 17.0, Microsoft Corporation, Redmond, WA, USA) Markov model. The time horizon is 12 months, the cycle length is 1 month and the default cohort size is 100 patients. Mirabegron 50 mg/day is compared with the following antimuscarinic treatments (all doses reported are per day): fesoterodine 4/8 mg, oxybutynin extended release (ER) 5 mg, oxybutynin immediate release (IR) 5 mg, solifenacin 5/10 mg, tolterodine ER or IR both 4 mg, trospium ER 60 mg or IR 40 mg, darifenacin 7.5 mg, and flavoxate 600 mg.
The base-case analysis provided a societal perspective of OAB management by accounting for all costs incurred by the UK NHS as well as those associated with lost productivity. Patient disposition, healthcare resource use, direct costs, indirect costs and total costs were all assessed as part of the base-case analysis.
Treatment Pathway
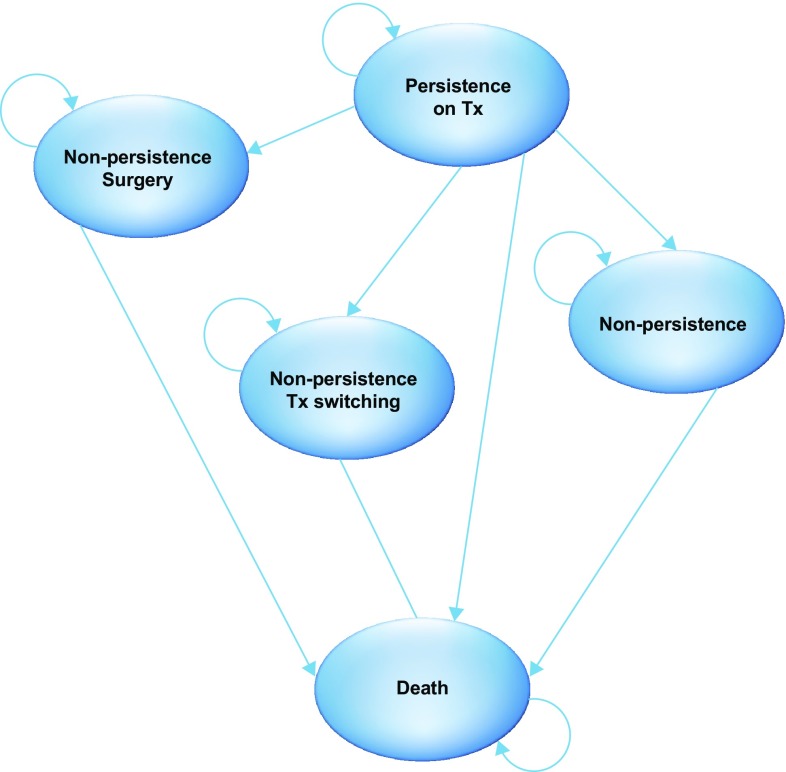
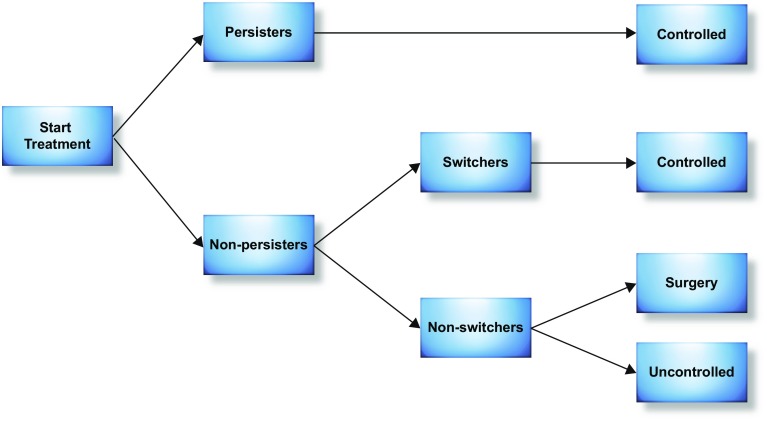
Patients enter the model in the ‘persistence on treatment’ health state (Fig. 1) and are assigned to treatment with mirabegron 50 mg (the recommended approved dose in the UK [9]) or an antimuscarinic agent (Fig. 2). At the end of each month, patients either persist with treatment or discontinue. Those who discontinue initial treatment switch to an alternative pharmacological intervention, undergo surgical operations (onabotulinumtoxinA injection or sacral nerve stimulation [SNS]) or discontinue treatment altogether. Patients can transition to other treatment states or death at each cycle. To make the analysis tractable within a 12-month time horizon, the model assumes that switching to a second-line pharmacotherapy and undergoing surgical operations both lead to symptom control (with no option for additional lines of treatment) until the end of the time horizon. Patients who discontinue treatment altogether are assumed to remain uncontrolled. Other model assumptions are reported in Table 1.
Fig. 1.
Markov model structure tx treatment
Fig. 2.
Treatment pathway
Table 1.
Model assumptions
| Non-persisters were assumed to either switch, undergo surgical operations or remain uncontrolled |
| Switchers and patients having surgical operations were assumed to have controlled symptoms |
| At treatment initiation (the start of the model), all patients were assumed to incur one visit to a GP and 1.5 visits to a specialist (urologist) [17] |
| Switchers were assumed to have one GP visit, 1.5 visits to a specialist (urologist) and one urodynamic test [17] |
| Patients who switched treatment were ascribed a drug acquisition cost, which was weighted by the market share of each treatment. The market share data were provided by Astellas [24] |
| One total probability of surgical operations was included and costs were applied [19], assuming that 50% of patients had onabotulinumtoxinA injection and 50% had SNS |
| Only non-persisters with uncontrolled symptoms were assumed to be at risk of co-morbidities (depression and UTI) [25]; the risk was applied in each cycle |
| Patients who were non-persistent and uncontrolled were assumed to have a 21.1% decrease in hours worked [25] |
| The persistence at 12 months was assumed to be the same for each treatment irrespective of the dose received [16] |
| The persistence at 12 months was assumed to be the same for trospium ER and IR formulations, and tolterodine ER and IR [16] |
ER extended release, GP general practitioner, IR immediate release, SNS sacral nerve stimulation, UTI urinary tract infection
Model Input Parameters
Clinical Inputs
We derived 12-month persistence data (Table 2) from a retrospective database study conducted in the UK [16]. In addition, the treatment switching rate (26.06%) was taken from a previously published cost-effectiveness analysis of mirabegron compared with antimuscarinics for the treatment of adults with OAB in the UK [17]. Mortality for the population with OAB was based on the UK age-standardized general population mortality rate [18] and converted to a 1-month probability (P) using the formula: P = 1 − e−rt, where r represents rate and t time.
Table 2.
Clinical inputs
| Variable | Value (%) | Source |
|---|---|---|
| Proportion of patients with incontinence | 60.00 | Nitti et al. [22]a |
| Percentage of patients switching treatment | 26.06 | Nazir et al. [17] |
| 12-month persistence (UK CPRD) | ||
| Mirabegron 50 mg | 37.70 | Astellas [16] |
| Fesoterodine 4/8 mg | 24.00 | Astellas [16] |
| Oxybutynin ER 5 mg | 17.20 | Astellas [16] |
| Oxybutynin IR 5 mg | 12.40 | Astellas [16] |
| Solifenacin 5/10 mg | 24.80 | Astellas [16] |
| Tolterodine ER 4 mg | 20.60 | Astellas [16] |
| Tolterodine IR 4 mg | 20.60 | Astellas [16] |
| Trospium ER 60 mg | 19.10 | Astellas [16] |
| Trospium IR 40 mg | 19.10 | Astellas [16] |
| Darifenacin 7.5 mg | 15.90 | Astellas [16] |
| Flavoxate 600 mg | 8.30 | Astellas [16] |
| General population mortality | 0.97 | ONS [18] |
CPRD Clinical Practice Research Datalink, ER extended release, IR immediate release, ONS Office for National Statistics
aA conservative estimate of 60% was applied to the model based on 65.7% of the total population who were reported to have urgency or mixed incontinence at baseline. All doses reported are the total dose per day
Healthcare Resource Use
Costs associated with resource use such as general practitioner (GP) and specialist visits were derived from NHS data [19–21]. Unit costs were calculated/estimated for one GP visit and 1.5 referrals to a urologist at treatment initiation and treatment switch (Table 3) [17]. One test by a specialist clinician (e.g. urologist, gynaecologist or other) was performed for each patient who switched to a second-line pharmacotherapy [17]. The proportion of patients with incontinence and requiring pads was estimated to be 60% based on the number of patients with wet OAB [22]. Pad use was estimated to be 2.5 pads per day for patients on treatment (i.e. persisters and switchers) and 5.5 pads per day for those off treatment (i.e. those who underwent surgical operations or discontinued all treatment) [19].
Table 3.
Resource inputs
| Parameter | Value or probability | Unit cost | Source |
|---|---|---|---|
| Drug acquisition | Cost per day (£) | ||
| First-line pharmacotherapy | – | ||
| Mirabegron 50 mg | – | 0.97 | BNF 71 [23] |
| Fesoterodine 4/8 mg | – | 0.92 | BNF 71 [23] |
| Oxybutynin ER 5 mg | – | 0.46 | BNF 71 [23] |
| Oxybutynin IR 5 mg | – | 0.07 | BNF 71 [23] |
| Solifenacin 5/10 mga | – | 1.00 | BNF 71 [23] |
| Tolterodine ER 4 mg | – | 0.92 | BNF 71 [23] |
| Tolterodine IR 4 mg | – | 0.09 | BNF 71 [23] |
| Trospium ER 60 mg | – | 0.82 | BNF 71 [23] |
| Trospium IR 40 mg | – | 0.83 | BNF 71 [23] |
| Darifenacin 7.5 mg | – | 0.91 | BNF 71 [23] |
| Flavoxate 600 mg | – | 0.39 | BNF 71 [23] |
| Second-line pharmacotherapyb | – | 0.64 | BNF 71 [23], Astellas [24] |
| Surgical operationsc | 0.01% | 1242.59 | |
| Visits/test | |||
| GP | 1 | 46.89 | Nazir et al. [19]; PSSRU [21] |
| Urologist | 1.5 | 102.16 | Nazir et al. [19]; UK Department of Health |
| Urodynamic test | 1 | 282.00 | Nazir et al. [19]; South Devon Healthcare [30] |
| Pads | Cost per pad (£) | ||
| On treatment | 2.5/day | 0.25 | Nazir et al. [19]; Incontinence Direct [31] |
| Off treatment | 5.5/day | 0.25 | Nazir et al. [19]; Incontinence Direct [31] |
| Co-morbidities | |||
| Depression | 19.0%d | 99.10 | Arlandis-Guzman et al. [25]; Irwin et al. [6] |
| UTI | 30.7%d | 2.79 | Arlandis-Guzman et al. [25]; Irwin et al. [6] |
All doses reported are the total dose per day
BNF British National Formulary, ER extended release, GP general practitioner, IR immediate release, OAB overactive bladder, UTI urinary tract infection
aWeighted average of solifenacin 5 mg (70%) and 10 mg (30%); unit costs of 30 tablet packs of £27.62 and £35.91, respectively
bWeighted cost based on market share of oral therapies for OAB
cWeighted cost based on a 50:50 ratio of onabotulinumtoxinA and sacral nerve stimulation
d6-month probability; unit costs based on 2005 monthly costs, converted to 2015 values using the UK forex exchange rate (as of October 2015)
Drug acquisition costs were calculated based on the treatment cost per pack (Table 3) [23] and were correct as of quarter 1, 2016. The cost of second-line pharmacotherapy was based on a weighted average derived from UK market share data [24].
Patients who were uncontrolled were considered to be at risk of two co-morbidities: depression and urinary tract infection (UTI). The 6-month probabilities of depression and UTI were 18.8 and 30.7%, respectively [25], and the monthly probability of surgical operations was 0.01% [17].
The costs for all resources were based on 2015 values, or inflated to 2015 values using the consumer price index (CPI) where applicable. The costs of depression and UTI sourced from Irwin et al. [6] were converted from € to British ₤ using the UK Forex exchange rate (as of December 2015).
Loss of Productivity
The model accounts for loss of work productivity only in patients who are non-persistent with treatment, have uncontrolled symptoms and are in employment. The base-case analysis assumed that 34.7% of the patients were employed for an average of 40 h per week [25, 26], had uncontrolled OAB and had a 21.1% reduction in the hours worked [25]. The average hourly wage of £12.62 (2011 value) was inflated to £13.94 (2015 value) using the CPI [27].
Sensitivity Analysis
A one-way univariate deterministic sensitivity analysis (DSA) was performed to assess the uncertainty of chosen parameters [28]. Here, a standard 20% variation in the following model parameters was tested individually and the effects on the incremental costs determined: persistence at 12 months, percentage of patients switching and probability of surgical operations. Mirabegron 50 mg was compared with solifenacin 5/10 mg and oxybutynin IR 5 mg. The results of the DSA were presented as a tornado diagram, with the parameters that had the greatest impact at the top.
Results
Patient Disposition
After 12 months, more patients persisted on treatment with mirabegron 50 mg than with all antimuscarinics (39 vs. 9–26%, respectively [values for all antimuscarinics presented as a range]) (Fig. 3). This resulted in fewer patients either switching treatment (16 vs. 19–23%, respectively) or becoming non-persistent uncontrolled patients (44 vs. 54–66%, respectively).
Fig. 3.
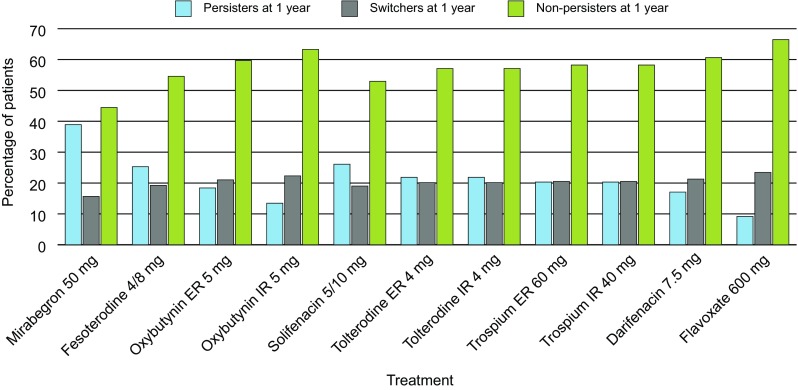
Summary of patient disposition after 12 months for each treatment. Total disposition may not sum to 100% because of rounding. All doses reported are the total dose per day. ER extended release, IR immediate release
Healthcare Resources
Resource use was lower with mirabegron 50 mg than with all antimuscarinics, including GP visits (115 vs. 119–123, respectively, per 100 patients), specialist visits (173 vs. 178–185, respectively, per 100 patients) and number of surgical operations (0.0042 vs. 0.0050–0.0060, respectively, per 100 patients) (Table 4). Total pad use per patient over 12 months was also lower with mirabegron 50 mg than with all antimuscarinics (71,807 vs. 76,531–84,602, respectively, per 100 patients) (Fig. 4).
Table 4.
Healthcare resource use over 12 months
| Result parameter | Mirabegron 50 mg | Fesoterodine 4/8 mg | Oxybutynin ER 5 mg | Oxybutynin IR 5 mg | Solifenacin 5/10 mg | Tolterodine ER 4 mg | Tolterodine IR 4 mg | Trospium ER 60 mg | Trospium IR 40 mg | Darifenacin 7.5 mg | Flavoxate 600 mg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GP visits | 115 | 119 | 121 | 122 | 119 | 120 | 120 | 120 | 120 | 121 | 123 |
| Specialist visits | 173 | 178 | 181 | 183 | 178 | 180 | 180 | 180 | 180 | 182 | 185 |
| Surgical operations | 0.0042 | 0.0051 | 0.0055 | 0.0058 | 0.0050 | 0.0053 | 0.0053 | 0.0054 | 0.0054 | 0.0056 | 0.0060 |
| Total pad use | 71,807 | 76,853 | 79,798 | 82,194 | 76,531 | 78,274 | 78,274 | 78,932 | 78,932 | 80,414 | 84,602 |
| On treatment | 40,123 | 35,918 | 33,464 | 31,467 | 36,187 | 34,734 | 34,734 | 34,186 | 34,186 | 32,950 | 29,461 |
| Off treatment | 31,684 | 40,934 | 46,334 | 50,727 | 40,334 | 43,540 | 43,540 | 44,747 | 44,747 | 47,464 | 55,142 |
All doses reported are the total dose per day
ER extended release, GP general practitioner, IR immediate release
Fig. 4.
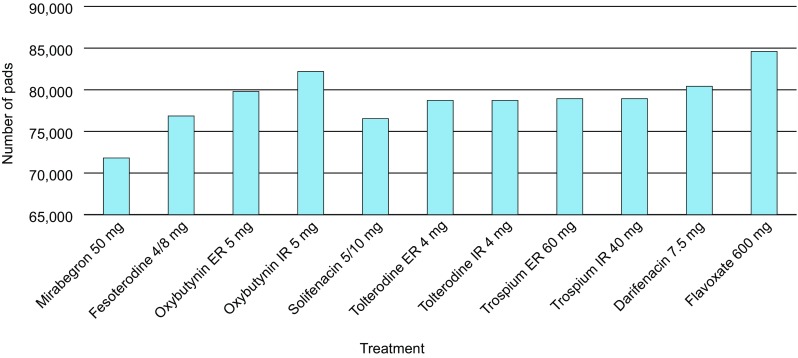
Total pad use per 100 patients, after 12 months for each treatment. All doses reported are the total dose per day. ER extended release, IR immediate release
Direct Costs
Mirabegron 50 mg had a higher medication cost than all antimuscarinics (£24,744 vs. 4599–22,634 per 100 patients) (Table 5). However, resource use costs, including those for the co-morbidities of depression and UTI, GP visits, specialist visits, surgical operations, urodynamic tests and pads, were lower with mirabegron than with all antimuscarinics (Table 5).
Table 5.
Total direct costs over 12 months for each treatment
| Cost (£) | Mirabegron 50 mg | Fesoterodine 4/8 mg | Oxybutynin ER 5 mg | Oxybutynin IR 5 mg | Solifenacin 5/10 mg | Tolterodine ER 4 mg | Tolterodine IR 4 mg | Trospium ER 60 mg | Trospium IR 40 mg | Darifenacin 7.5 mg | Flavoxate 600 mg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All medication | 24,744 | 20,810 | 11,124 | 4599 | 22,634 | 20,004 | 4677 | 17,875 | 17,907 | 18,609 | 9104 |
| First line | 22,586 | 18,022 | 7969 | 1145 | 19,886 | 17,038 | 1712 | 14,827 | 14,859 | 15,377 | 5349 |
| Second line | 2158 | 2788 | 3155 | 3455 | 2748 | 2965 | 2965 | 3047 | 3047 | 3232 | 3755 |
| Co-morbidities | 1121 | 1448 | 1639 | 1795 | 1427 | 1540 | 1540 | 1583 | 1583 | 1679 | 1951 |
| GP visits | 5408 | 5577 | 5664 | 5727 | 5567 | 5620 | 5620 | 5639 | 5639 | 5680 | 5782 |
| Specialist visits | 17,674 | 18,226 | 18,509 | 18,715 | 18,193 | 18,367 | 18,367 | 18,429 | 18,429 | 18,565 | 18,897 |
| Surgical operations | 5.19 | 6.30 | 6.83 | 7.18 | 6.24 | 6.57 | 6.57 | 6.68 | 6.68 | 6.93 | 7.45 |
| Urodynamic tests | 4326 | 5342 | 5863 | 6243 | 5281 | 5601 | 5601 | 5716 | 5716 | 5965 | 6577 |
| Total pad use | 17,824 | 19,076 | 19,807 | 20,402 | 18,996 | 19,429 | 19,429 | 19,592 | 19,592 | 19,960 | 20,999 |
| On treatment | 9959 | 8915 | 8306 | 7811 | 8982 | 8621 | 8621 | 8485 | 8485 | 8179 | 7313 |
| Off treatment | 7864 | 10,161 | 11,501 | 12,591 | 10,014 | 10,807 | 10,807 | 11,107 | 11,107 | 11,781 | 13,687 |
All doses reported are the total dose per day
ER extended release, GP general practitioner, IR immediate release
Indirect Costs
The number of work hours lost (4017 vs. 5114–6990 per 100 patients) and the cost due to work hours lost (£55,983 vs. 71,284–97,430 per 100 patients) were both lower with mirabegron 50 mg than with all antimuscarinics (Fig. 5).
Fig. 5.
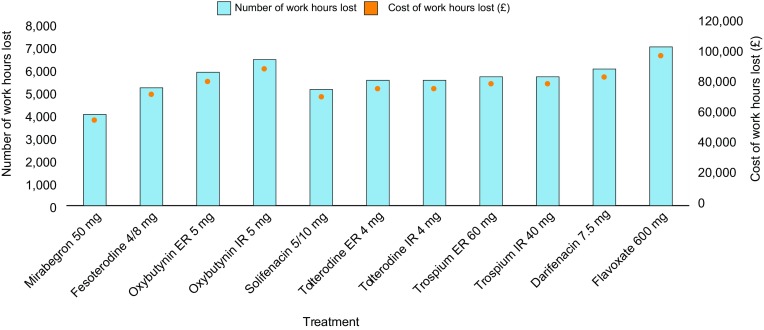
Total work hours lost over 12 months and associated costs for each treatment per 100 patients. All doses reported are the total dose per day. ER extended release, IR immediate release
Total Costs
Total costs were lower for mirabegron 50 mg than for all antimuscarinics (£127,084 vs. 132,171–160,748, respectively, per 100 patients) (Fig. 6). This translated into lower respective total costs per patient (£1271 vs. 1322–1607) and per patient per day (£3.48 vs. 3.62–4.40).
Fig. 6.
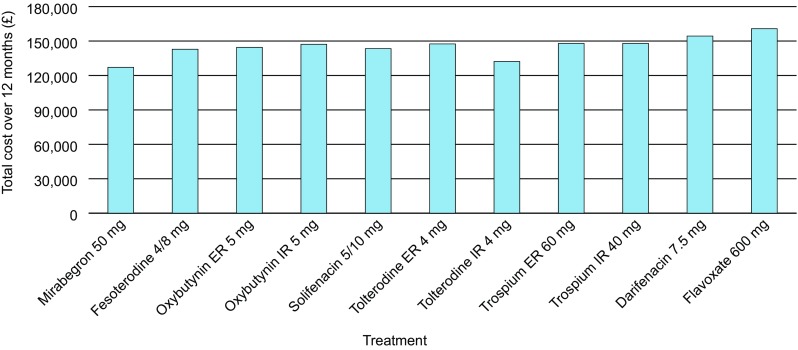
Total costs (including indirect costs) over 12 months for each treatment per 100 patients. All doses reported are the total dose per day. ER extended release, IR immediate release
Sensitivity Analysis
For both comparisons, the model was most sensitive to ± 20% changes in persistence rates for either treatment and the percentage of patients switching (Fig. 7). The incremental cost savings per patient per day were −£0.45 in the base-case analysis of mirabegron 50 mg versus solifenacin 5/10 mg and −£0.55 versus oxybutynin IR 5 mg. The overall impact was similar when the incremental cost savings per additional patient persisting on treatment were assessed (Fig. S1 in the Electronic Supplementary Material).
Fig. 7.
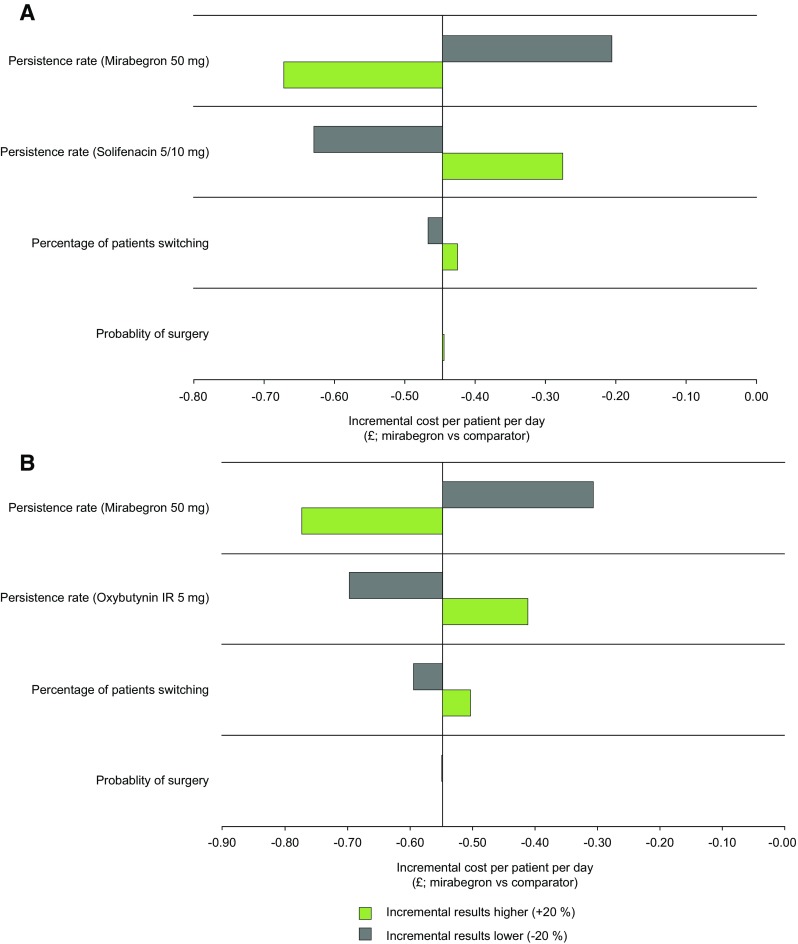
Results of the deterministic sensitivity analysis for mirabegron 50 mg versus a solifenacin 5/10 mg and b oxybutynin IR 5 mg (incremental cost per patient per day). All doses reported are the total dose per day. IR immediate release
Discussion
To our knowledge, this model is the first to estimate the economic impact of using mirabegron 50 mg versus antimuscarinic agents for the treatment of OAB from a societal perspective in the UK, largely employing real-world data (specifically persistence rates).
According to the model, after 12 months, more patients receiving mirabegron 50 mg versus all antimuscarinics persisted with treatment, fewer patients switched treatment and fewer work hours were lost. Although mirabegron 50 mg had higher medication costs over 12 months than did antimuscarinics, these costs were offset by savings attributed to other direct and indirect costs. This was partly attributable to a lower proportion of non-persisters and switchers, who had higher demand for healthcare resources and higher pad use than patients who persisted with treatment. The results suggest that more patients in the UK who receive mirabegron will persist with first-line pharmacological treatment versus antimuscarinics over 12 months, resulting in more patients with likely controlled symptoms. Administration of mirabegron as first-line pharmacotherapy for OAB could have beneficial effects in terms of lower resource use and overall treatment, containment of healthcare costs and societal benefit. Furthermore, while lower healthcare resource use has been cited as a benefit of increased treatment persistence [15], better persistence rates could also be expected to improve patient quality of life (QoL), likely because of increased symptom control and an increased ability to continue daily activities [15]. This model did not include the likely improvements in QoL for those who persist with treatment.
While similar efficacy is observed between mirabegron and antimuscarinics [11], real-world evidence indicates that a higher proportion of patients are likely to be non-persistent with treatment with antimuscarinics versus mirabegron 50 mg [13]. Potential reasons for treatment discontinuation with antimuscarinics include bothersome AEs such as dry mouth and constipation, the most common AEs in these patients [7]. A recent systematic literature review and network meta-analysis of mirabegron 50 mg versus antimuscarinics in 27,309 patients with OAB reported a similar incidence of dry mouth for mirabegron 50 mg and placebo but a lower incidence for mirabegron 50 mg versus all antimuscarinics assessed [11]. These findings may be reflected in the present study, as healthcare resource use was lower for mirabegron 50 mg than for all antimuscarinics, with fewer associated costs. However, it should be noted that treatment discontinuation can be attributed to many factors, and a greater overall focus should be applied to tailoring OAB treatment to the individual.
The primary strengths of this model are that it utilizes real-world data related to treatment of OAB and healthcare resource use in the UK. Therefore, the total costs derived for mirabegron versus antimuscarinics are based on effectiveness rather than efficacy. However, the findings that suggest a benefit of mirabegron (if used as first-line pharmacotherapy for OAB) depend on the several key assumptions made in the model, such as persistence at 12 months being independent of the treatment dose received and persistence not varying between trospium ER and IR and tolterodine ER and IR. Furthermore, all patients who switch treatment or undergo surgical operations are assumed to have controlled symptoms, and this would be variable. Structural edits to the model would be required to account for these factors if additional sensitivity analyses were to be performed. As OAB is a chronic condition, provision of a longer time horizon would be beneficial to accurately estimate the economic benefits of long-term treatment. Another limitation is that no direct clinical data for estimates of efficacy, tolerability or health-related QoL were included; therefore, some patient perspectives were not captured. With respect to the treatment comparators, the model includes the most common treatment options for OAB in the UK according to the results of a retrospective analysis of a clinical practice database [16]; this included flavoxate, which is not recommended for use in women with urinary incontinence in the UK [29].
Although the model does not account for persistence in different subgroups of patients, results were largely consistent with the total study population, and few differences in persistence between subgroups were observed in a Canadian study of persistence with mirabegron 50 mg versus antimuscarinics over 12 months (the only notable difference was that patients were more likely to persist as the number of co-existing prescribed medications increased [up to more than eight]) [13]. Whether differences between subgroups would be observed if the model were updated to allow these kinds of analyses is unclear. The persistence data used in the model were taken from a large non-interventional study conducted in the UK [16]; patient demographics in this study were generally well-balanced across treatment groups, although significantly more patients receiving mirabegron were female, treatment-experienced and receiving co-existing medications compared with those receiving the primary comparator treatment (tolterodine ER).
Conclusions
This model suggests that treatment of OAB in the UK with mirabegron 50 mg will result in more patients persisting with treatment and lower total costs of treatment versus antimuscarinics. Further work is needed to confirm these findings in different populations to assess the effect of mirabegron on persistence and overall treatment costs in other countries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (EPS 3433 kb) Supplementary Fig. 1 Results of the deterministic sensitivity analysis (a) for mirabegron 50 mg versus solifenacin 5/10 mg; and (b) for mirabegron 50 mg versus oxybutynin IR 5 mg (cost per additional patient persisting on treatment). All doses reported are the total dose per day. IR immediate release
Acknowledgements
PAREXEL Access Consulting developed the model, funded by Astellas Pharma Europe Ltd. Medical writing support was provided by David Griffiths of Bioscript Medical, funded by Astellas Pharma Global Development.
Author contributions
Conception and design: JN, MB, CMc, SB, ZH. Acquisition of data: JN, MB, CMc. Analysis and interpretation of the data: JN, MB, CMc, FF, SB, ZH, AW. Drafting of the manuscript: JN, MB, CMc, FF, SB, ZH, AW. Critical revision of the manuscript for important intellectual content: JN, MB, CMc, FF, SB, ZH, AW. Statistical analysis and modelling: JN, MB, CMc. Obtaining funding: JN, SB, ZH.
Compliance with Ethical Standards
Conflict of interest
AW received research grants from Astellas Pharma Europe Ltd, Pfizer Inc. and the Canadian Urological Association. He also received consulting fees or honoraria from SCA, Astellas Pharma Europe Ltd, Pfizer Inc. and Duchesnay Inc. MB and CMc received consulting fees from Astellas Pharma Europe Ltd for developing the model as part of the PAREXEL team. FF received grant funding from Astellas Pharma Europe Ltd. JN, SB and ZH are employees of Astellas Pharma Europe Ltd. No research involved human participants and/or animals, and no informed consent was required.
Data availability
The datasets generated and/or analysed during the current study are not publically available because the model is proprietary. However, all appropriate data generated or analysed during this study are included in this published article and its supplementary information files.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s41669-017-0011-x) contains supplementary material, which is available to authorized users.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61(1):37–49. doi: 10.1016/S0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87(9):760–766. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 4.Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care. 2000;6(11 Suppl):S574–S579. [PubMed] [Google Scholar]
- 5.Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6(11 Suppl):S580–S590. [PubMed] [Google Scholar]
- 6.Irwin DE, Mungapen L, Milsom I, Kopp Z, Reeves P, Kelleher C. The economic impact of overactive bladder syndrome in six Western countries. BJU Int. 2009;103(2):202–209. doi: 10.1111/j.1464-410X.2008.08036.x. [DOI] [PubMed] [Google Scholar]
- 7.Lucas MG, Bedretdinova D, Berghmans, LC, Bosch JLHR, Burkhard FC, Cruz F, et al. Guidelines on urinary incontinence. http://uroweb.org/wp-content/uploads/20-Urinary-IncontinenceLR1.pdf/. Accessed 16 May 2016.
- 8.Benner JS, Nichol MB, Rovner ES, Jumadilova Z, Alvir J, Hussein M. Patient-reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105(9):1276–1282. doi: 10.1111/j.1464-410X.2009.09036.x. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Betmiga EPAR Product Information. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002388/human_med_001605.jsp&mid=WC0b01ac058001d124/. Accessed 17 Aug 2016.
- 10.Sacco E, Bientinesi R. Mirabegron: a review of recent data and its prospects in the management of overactive bladder. Ther Adv Urol. 2012;4(6):315–324. doi: 10.1177/1756287212457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65(4):755–765. doi: 10.1016/j.eururo.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Nitti VW, Chapple CR, Walters C, Blauwet MB, Herschorn S, Milsom I, et al. Safety and tolerability of the beta3-adrenoceptor agonist mirabegron, for the treatment of overactive bladder: results of a prospective pooled analysis of three 12-week randomised Phase III trials and of a 1-year randomised Phase III trial. Int J Clin Pract. 2014;68(8):972–985. doi: 10.1111/ijcp.12433. [DOI] [PubMed] [Google Scholar]
- 13.Wagg A, Franks B, Ramos B, Berner T. Persistence and adherence with the new beta-3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J. 2015;9(9–10):343–350. doi: 10.5489/cuaj.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andy UU, Arya LA, Smith AL, Propert KJ, Bogner HR, Colavita K, et al. Is self-reported adherence associated with clinical outcomes in women treated with anticholinergic medication for overactive bladder? Neurourol Urodyn. 2015;35(6):738–742. doi: 10.1002/nau.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TH, Lee KS. Persistence and compliance with medication management in the treatment of overactive bladder. Investig Clin Urol. 2016;57(2):84–93. doi: 10.4111/icu.2016.57.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astellas. Mirabegron vs antimuscarinics in OAB: retrospective analysis of a UK general practice prescription database. Data on file. 2016. [DOI] [PubMed]
- 17.Nazir J, Maman K, Neine ME, Briquet B, Odeyemi IA, Hakimi Z, et al. Cost-effectiveness of mirabegron compared with antimuscarinic agents for the treatment of adults with overactive bladder in the United Kingdom. Value Health. 2015;18(6):783–790. doi: 10.1016/j.jval.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Office for National Statistics. Mortality statistics: Deaths registered in the UK by areas of usual residence. http://www.ons.gov.uk/ons/rel/vsob1/deaths-registered-area-usual-residence/2014/index.html/. Accessed 17 May 2016.
- 19.Nazir J, Posnett J, Walker A, Odeyemi IA, Hakimi Z, Garnham A. Economic evaluation of pharmacological treatments for overactive bladder from the perspective of the UK National Health Service. J Med Econ. 2015;18(5):390–397. doi: 10.3111/13696998.2014.995300. [DOI] [PubMed] [Google Scholar]
- 20.National Health Service. NHS reference costs 2014 to 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015/. Accessed 1 July 2016.
- 21.Personal Social Services Research Unit (PSSRU). Unit costs of health and social care 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/. Accessed 1 July 2016.
- 22.Nitti VW, Khullar V, van Kerrebroeck P, Herschorn S, Cambronero J, Angulo JC, et al. Mirabegron for the treatment of overactive bladder: a prespecified pooled efficacy analysis and pooled safety analysis of three randomised, double-blind, placebo-controlled, phase III studies. Int J Clin Pract. 2013;67(7):619–632. doi: 10.1111/ijcp.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.British Medical Association, Royal Pharmaceutical Society. British National Formulary 71st edition. Joint Formulary Committee. London: British Medical Association; 2016.
- 24.Astellas. Mirabegron. IMS Country Retail Sales Audit from March 2016. UK Market Shares for HEOR. Data on file. 2016.
- 25.Arlandis-Guzman S, Errando-Smet C, Trocio J, Arumi D, Rejas J. Cost-effectiveness analysis of antimuscarinics in the treatment of patients with overactive bladder in Spain: a decision-tree model. BMC Urol. 2011;11:9. doi: 10.1186/1471-2490-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astellas. Mirabegron. Mirabegron cost-effectiveness model for the UK. Technical report. Data on file. 2016.
- 27.Office for National Statistics. Earnings (inflation adjusted) have risen by 62% since 1986. http://webarchive.nationalarchives.gov.uk/20160105160709/http:/www.ons.gov.uk/ons/rel/lmac/earnings-in-the-uk-over-the-past-25-years/2012/sty-pay-over-25-years.html/. Accessed 19 Jan 2017
- 28.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, et al. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence. Urinary incontinence in women: management. Clinical guideline. London: NICE; 11 September 2013. https://www.nice.org.uk/guidance/cg171/resources/urinary-incontinence-in-women-management-35109747194821/. Accessed 1 July 2016.
- 30.NHS South Devonshire. Private patient and overseas visitor price list, effective from 1st of April-31st March 2016. http://www.torbayandsouthdevon.nhs.uk/uploads/23968.pdf. Accessed 18 May 2016.
- 31.Incontinence Direct UK: LILIFORM Large Shaped Pads. http://www.incontinencedirect.co.uk/ladies-incontinence-products/disposable-products/LILFORM-Large-Shaped-Pads/. Accessed 1 July 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (EPS 3433 kb) Supplementary Fig. 1 Results of the deterministic sensitivity analysis (a) for mirabegron 50 mg versus solifenacin 5/10 mg; and (b) for mirabegron 50 mg versus oxybutynin IR 5 mg (cost per additional patient persisting on treatment). All doses reported are the total dose per day. IR immediate release
Data Availability Statement
The datasets generated and/or analysed during the current study are not publically available because the model is proprietary. However, all appropriate data generated or analysed during this study are included in this published article and its supplementary information files.