Abstract
Objective:
We demonstrate the protective effects of the siRNA-mediated inhibition of the interleukin-28 receptor alpha (IL28RA) subunit on cardiomyocytes in hypoxia/reoxygenation (H/R) injury and explore the associated mechanism.
Methods:
After designing and synthesizing three pairs of siRNA that effectively reduced IL28RA gene expression in vitro (siRNA-6158, siRNA-6160, and siRNA-6162), primary neonatal rat cardiomyocytes were transfected using a liposome transfection method. Six groups were included based on the siRNA that was used and the treatment simulating reperfusion injury: control group, H/R group, H/R+negative control group, H/R+siRNA-6158 group, H/R+siRNA-6160 group, and H/R+siRNA-6162 group. Cell survival and apoptosis rates were measured along with lactate dehydrogenase levels in the cell culture supernatant. Protein levels of IL28RA, phosphatidylinositol 3-kinase, catalytic subunit gamma (PI3KCG), Bcl-2, Bax, and b-actin were also measured.
Results:
The H/R+siRNA-6158 and H/R+siRNA-6160 groups had significantly higher survival rates and increased PI3KCG-to-b-actin and Bcl-2-to-Bax ratios than the the H/R and H/R+negative control groups (p<0.05). The H/R+siRNA-6158 and H/R+siRNA-6160 groups also exhibited reduced rates of apoptosis and reduced IL28RA-to-b-actin ratios (p<0.05). No significant difference was observed among the H/R+siRNA-6162, H/R, and H/R+negative control groups.
Conclusion:
IL28RA siRNA-6158 and -6160 were able to protect cardiomyocytes from H/R injury by inhibiting apoptosis. This strategy of inhibiting IL28RA gene expression may reduce reperfusion injury in the treatment of patients with acute myocardial infarction.
Keywords: IL28RA, siRNA, transfection, hypoxia/reoxygenation injury, apoptosis
Introduction
Reperfusion (reoxygenation) injury is a common pathophysio-logic phenomenon in the clinical treatment of myocardial infarctions; it often leads to arrhythmia, infarct zone expansion, and heart failure. Hence, focus of research has been seek a more effective measure to prevent cardiac reperfusion injury (1, 2). IFN-ls are new types of interferons that include IFN-ll, IFN-l2, and IFN-l3. The functional receptor complex for the IFN-l family is a heterodimer composed of CRF2–12 (also known as IFN-l-R1/IL-28Ra or IL28RA) and CRF2-4 (also known as ILl0-R-b or ILl0RB) chains. While the interleukin-10 receptor b (IL-10RB) subunit has a role in the receptors for three different interleukins, the interleukin-28 receptor a (IL28RA) chain is unique to IFN-ls (3).
IFN-ls and their receptors (IL28RA/ILl0RB) exist in various tissues of the body, but they are especially concentrated in the heart (3). IFN-ls inhibit the proliferation of viruses such as hepatitis B/C, cytomegalovirus, and vesicular stomatitis virus (4). Clinically, IFN-ls have been used in the treatment of multiple sclerosis (5). IFN-ls have also been a part of strategies to limit tumor proliferation after a meta-analysis by Yang et al. (3) in 2010 have suggested that the expression of IL28RA in tumors is associated with tumor prognosis (6). Later studies have shown that the lower expression of IL28RA is significantly correlated with faster tumor growth and lower patient survival (7).
In 2011, Tsai et al. (8) found the same link between IL28RA and cell survival using a cardiomyopathy model by treating prepared mice with low-dose chlorpromazine. The myocardial gene expression of the anti-apoptotic Bcl-2 gene significantly dec-reased, while the expression of the IL28RA gene significantly increased, providing the first experimental evidence for IL28RA gene involvement in myocardial injury and apoptosis.
To investigate the effect of IL28RA gene in hypoxia/reoxygenation (H/R) injury in cardiomyocytes, three pairs of IL28RA siRNAs were designed and synthesized. Liposome transfection was used to transfect the siRNAs into cardiomyocytes (9). In the current study, we provide further evidence on the protective effects of IL28RA siRNAs on cardiomyocytes during reperfusion and explore a possible mechanism for the process.
Methods
Materials
LipofectamineTM2000 (Invitrogen, Carlsbad, USA), type II collagenase (Gibco, Carlsbad, USA), Dulbecco modified Eagle’s medium (DMEM), fetal bovine serum (FBS) (HyClone, Logan, USA), a lactate dehydrogenase (LDH) detection kit (Jiancheng Bio Ltd, Nanjing, China), Cell Counting Kit-8 (CCK-8), and cell proliferation–toxicity testing kit (Dojindo, Tokyo, Japan) were purchased. Apoptosis and necrosis assay kits (Cat C1056) (Beyotime, Nantong, China) were also purchased. Rabbit anti-rat monoclonal IL28RA antibody (Sigma, Darmstadt, Germany), rabbit anti-rat PI3KCG antibody, and HRP-labeled goat anti-rabbit secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Rabbit anti-rat Bax, Bcl-2, and b-actin antibodies were purchased from Cell Signaling Technology (Boston, USA). An anaerobic bag (Becton Dickinson, Inc., Franklin Lakes, USA) was also purchased.
Neonatal SD rats less than 3 days of age and weighing between 6 and 8 g were provided by Jiangsu Province Animal Center. Three pairs of rat IL28RA siRNAs (siRNA-6158, -6160, -6162) and the negative control hydroxyl fluorescein (FAM-siRNA) were designed and synthesized in Shanghai Gene Pharma Co., Ltd (Shanghai, China). Their respective nucleotide sequences are listed in Table 1.
Table 1.
Nucleotide sequence of IL28RA siRNA
| siRNA | Nucleotide sequence |
|---|---|
| siRNA-6158 | 5’-CUCUCCGGUUGGAGAAUAUTT-3’(sense) |
| 5’-AUAUUCUCCAACCGGAGAGTT-3’(anti-sense) | |
| siRNA-6160 | 5’-GGACUCCUCUUAUAAAGAUTT-3’(sense) |
| 5’-AUCUUUAUAAGAGGAGUCCTT-3’(anti-sense) | |
| siRNA-6162 | 5’-GGUCACUGGAACUCAAGUATT-3’(sense) |
| 5’-UACUUCAGUUCCAGUGACCTT-3’(anti-sense) | |
| FAM-siRNA | 5’-UUCUCCGAACGUGUCACGUTT-3’(sense) |
| 5’-ACGUGACACGUUCGGAGAATT-3’(anti-sense) |
Cardiomyocyte isolation and culture (10): The present study was approved by the Ethics Committee of The First Affiliated Hospital of Nanjing Medical University. After the rats were euthanized by CO2, the hearts were immediately removed from the chest cavity and were put into phosphate-buffered saline (PBS) at 4°C. The ventricular tissue was then cut into pieces and digested three times by a 0.1% collagenase solution on a shaking table at 37°C. After centrifuging the digested solution for 10 min at 1000 rpm at 4°C, the cells were collected in a 10-cm-diameter culture dish with DMEM and 10% FBS. After the cells were incubated under 5% CO2 at 37°C for 90 min, they were aspirated with the concentration being adjusted to 3´105/mL and then seeded in 96-and 6-well plates, which were randomly allocated for examination on the third day, and transferred to 96-or 6-well plates at a density of 3´105 cells/mL.
The cardiomyocytes were divided into six groups: H/R+negative control group, H/R+siRNA-6158 group, H/R+siRNA-6160 group, H/R+siRNA-6162 group, control group, and H/R group.
Transfection of cardiomyocytes with siRNA
Primary cardiomyocytes were cultured for 3 days and replaced with DMEM without serum or double antibiotics. The three pairs of siRNAs were transfected into primary neonatal rat cardiomyocytes at 80 nmoL/L FAM-siRNA.
After Lipofectamine 2000 dissolved into DMEM medium was mixed with siRNA, the Lipofectamine–siRNA mixture was added into corresponding culture plate wells and gently mixed. Six hours later, each well was replaced with DMEM with 10% FBS and penicillin/streptomycin. Meanwhile, the transfection efficiency was measured using a fluorescence microscope. Triplicate wells were established for each group.
Establishment of the cardiomyocyte reperfusion injury model (10)
After transfecting the monolayer of cardiomyocytes for 3 days, the transfection solution was replaced with low-glucose DMEM (1000 mg/L glucose), and the cells underwent H/R treatment. First, the cells were first placed in a sterile and sealed anaerobic bag containing 5% CO2 and 95% N2 at 37°C for 6 h. Then, hypoxic cardiomyocytes were removed from the bag, and the culture medium was changed with high-glucose (4500 mg/L glucose) DMEM containing 10% FBS and placed in an incubator with 21% O2 and 5% CO2 for conventional culture for 2 h at 37°C.
Measurement of cardiomyocyte beat frequency
After H/R treatment, the field of view was randomly selected under an inverted microscope to observe the beats per minute of each set of three wells, with the mean value being calculated.
Detection of cardiomyocyte viability using the CCK-8 method
After 10 µL CCK-8 solution was added into each well, cell culture plates were placed into the incubator for 1 h at 37°C. The absorbance was then measured at 450 nm using a plate reader. The following formula was used: cell survival rate (%)=[(experimental absorbance -blank absorbance)/(normal absorbance -blank absorbance)] ´ 100%. Blank control wells were treated in the same way as the experimental wells, wherein only the cell culture medium containing the CCK-8 solution was added without adding cells.
LDH activity assay in the cardiomyocyte culture supernatant
LDH was measured after reperfusion. LDH activity in the culture medium of each group was determined with LDH detection kits. In total, 20 µL cardiomyocyte culture supernatant was taken out from the cell culture medium of each group. The absorbance was measured at 450 nm using a plate reader. The following formula was used: LDH activity (U/L)=[(measured OD -control OD)/(standard OD -blank OD)] ´ standard concentration (0.2 mmoL/L) ´ 1000. No sample or enzyme-labelled reagents were included in the blank control well.
Measurement of the cardiomyocyte apoptosis rate (11)
The Hoechst 33342 and propidium iodide (PI) double-staining method was used to analyze cardiomyocyte apoptosis rates. Cardiomyocytes were seeded in 12-well plates. After H/R treatment, 500 µL Hoechst 33342 staining solution was added into each well. After incubating cardiomyocytes on ice for 30 min, 5 µL PI staining solution was added to each well. Cardiomyocytes were incubated on ice for another 10 min and washed with PBS for three times. They were then observed under an inverted mic-roscope and photographed.
Measurement of protein expression in cultured cardiomyocytes by western blotting
Cultured cardiomyocytes were lysed with RIPA lysis buffer for determining the total protein content. Then, 36 µg total extracted proteins was separated by electrophoresis on 10% SDS-PAGE gels and then transferred to PVDF membranes. After being blocked with 5% skim milk overnight at 4°C, the PVDF membranes were incubated with primary antibodies against PI3KCG (1:1000), IL28RA (1:1000), Bax (1:1000), Bcl-2 (1:1000), and b-actin (1:1000) overnight at 4°C. Then, they were incubated with the corresponding HRP-labeled secondary antibodies (1:5000) for 2 h at 4°C. Blots were developed with an ECL detection reagent.
Statistical analysis
Data were analyzed using SPSS 20.0. Measurement data are represented by mean±standard deviation (). A normality test was performed to applied parametric or nonparametric statistical test analyzing continuous variables. If continuous variables are normally distributed, a parametric test was performed. Dunnett’s multiple comparison test was performed for comparing the experi-mental group with the normal group and Tukey’s or Duncan’s test was performed for simultaneously comparing the groups. Measurement data between two groups were compared using the t-test; measurement data among multiple groups were compared using one-way ANOVA. P<0.05 indicated a significant difference.
Results
Comparison of the beat frequency and survival rates in cardiomyocytes during reperfusion
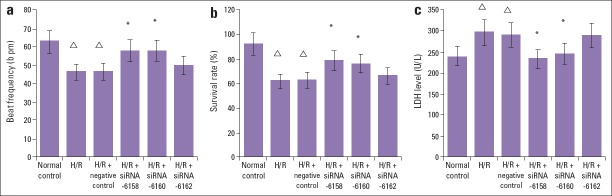
After H/R, the beat frequency and survival rate of cardiomyocytes were significantly lower than those of the control group (p<0.05). Compared with the H/R group, the cell viability in the siRNA-6158 group and siRNA-6160 group significantly increased (p<0.05). The survival rate in the H/R+siRNA-6162 group and H/R+negative control group showed no significant changes (p>0.05), and there were no significant difference (p>0.05) between H/R+siRNA-6158 group or H/R+siRNA-6160 group (Table 2, Fig. 1a, Fig. 1b).
Table 2.
Comparison of cardiomyocyte beat frequency, survival rate, and supernatant LDH level among the six groups (X̄ ± S, n=6)
| Beat frequency, bpm | Survival rate, % | LDH level, U/L | |
|---|---|---|---|
| Normal control group | 62.57±5.71 | 92.11±8.07 | 239.52±21.17 |
| H/R group | 46.29±3.48△ | 61.39±5.83△ | 295.99±27.71△ |
| H/R+negative control group | 46.71±3.51△ | 62.6±5.89△ | 289.38±26.59△ |
| H/R+siRNA-6158 group | 57.86±4.79* | 78.71±6.57* | 232.65±22.34* |
| H/R+siRNA-6160 group- | 57.71±4.82* | 76.32±6.48* | 244.71±23.32* |
| H/R+siRNA-6162 group | 49.86±3.61 | 65.99±5.95 | 288.99±25.48 |
P<0.05 compared with H/R group and H/R+negative control group;
P<0.05 compared with the control group. H/R - hypoxia/reoxygenation; LDH - lactate dehydrogenase
Figure 1.
Comparison results of cardiomyocytes beat frequency, survival rate and supernatant LDH level among the six groups (±s, n=6). (a) Compared with H/R and H/R+negative control group, the beat frequency in the H/R+siRNA-6518 group and H/R+siRNA-6160 group were significantly increased (P<0.05). Compared with normal control group, the beat frequency in the H/R and H/R+negative control group were significantly decreased (P<0.05). No significant difference was detected in the beat frequency between H/R+ siRNA-6162 transfection group, and H/R group, or H/R+negative control group (P>0.05). *P<0.05, compared with H/R group and H/R+negative control transfection group; P<0.05, compared with normal control group. (b) Compared with H/R and H/R+negative control group, the survival rate in the H/R+siRNA-6518 group and H/R+siRNA-6160 group were significantly increased (P<0.05). Compared with normal control group, the survival rate activity in the H/R and H/R+negative control group were significantly decreased (P<0.05). No significant difference was detected in the survival rate between H/R+ siRNA-6162 transfection group, and H/R group, or H/R+negative control group (P>0.05). *P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group. (c) Compared with H/R and H/R+negative control group, the LDH activity in the H/R+siRNA-6518 group and H/R+siRNA-6160 group were significantly decreased (P<0.05). Compared with normal control group, the LDH activity in the H/R and H/R+negative control group were significantly increased (P<0.05). No significant difference was detected in the beat frequency, survival rate and supernatant LDH level between H/R+ siRNA-6162 transfection group, and H/R group, or H/R+negative control group (P>0.05). *P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group
Comparison of the LDH level during H/R
The LDH level in the supernatant in the H/R group was significantly higher than that in the control group (p<0.05). The LDH levels in the supernatant in the H/R+siRNA-6158 and H/R+siRNA-6160 groups was significantly lower than those in the H/R and H/R+negative control groups (p<0.05). No significant difference was detected in the LDH level between the H/R+siRNA-6162 group, and H/R group, or H/R+negative control group (p>0.05) (Table 2, Fig. 1c).
Comparison of the apoptosis rate during H/R
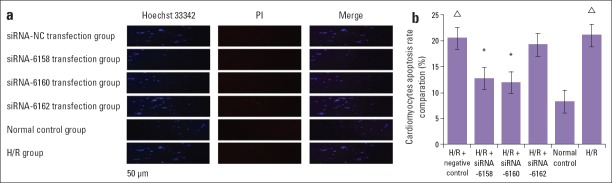
Cardiomyocytes in the control group presented a uniformly dispersed blue fluorescence after they were double stained by Hoechst 33342 and PI. The apoptosis rate was only 8.32%±0.2%. Compared with the control group, cardiomyocytes in the H/R and H/R+negative control groups had a non-uniform fluorescence, and they were highly concentrated with a strong blue and red fluorescence. The apoptosis rates in the H/R and H/R+negative control groups were 21.11%±1.1% and 20.56%±1.0%, respectively, which were significantly higher than that in the control group (p<0.05). siRNA transfection decreased the apoptosis rates to only 12.76%±1.1% and 11.97%±1.0% in the H/R+siRNA-6158 and H/R+siRNA-6160 groups, respectively, which were significantly lower than those in the H/R and H/R+negative control groups (p<0.05). The strong blue and red fluorescence signals were significantly decreased in H/R+siRNA-6158 and H/R+siRNA-6160 groups compared with those in in the H/R and H/R+negative control groups. No significant difference was detected in the apoptosis rate among the H/R+siRNA-6162 group (19.23%±1.1%), H/R group, or H/R+negative control group (p>0.05) (Fig. 2a, Fig. 2b).
Figure 2.
The cardiomyocytes apoptosis rate comparation by using the Hoechst 33342 and PI double staining method (x200). (a) Blue color of Hoechst 33342 staining detects presence of nucleus. The strong red color of PI detects presence of necrosis. The cardiomyocytes nucleus in normal control group showed the normally blue and weak red color. In the H/R group and H/R+negative control group, the cardiomyocytes nucleus showed densely thick blue color representing apoptosis. In the H/R+siRNA-6518 group and H/R+siRNA-6160 group, the densely thick blue color percentage was distinctly reduced than that in the H/R group and H/R+negative control group. No visual difference was observed between H/R+ siRNA-6162 transfection group, and H/R group, or H/R+negative control group. (b) Compared with H/R and H/R+negative control group, the apoptosis rate in the H/R+siRNA-6518 group and H/R+siRNA-6160 group were significantly decreased (P<0.05). Compared with normal control group, the apoptosis rate in the H/R and H/R+negative control group were significantly increased (P<0.05). *P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group. H/R+NC transfection group-H/R+negative control transfection group
Detection of protein expression by western blotting
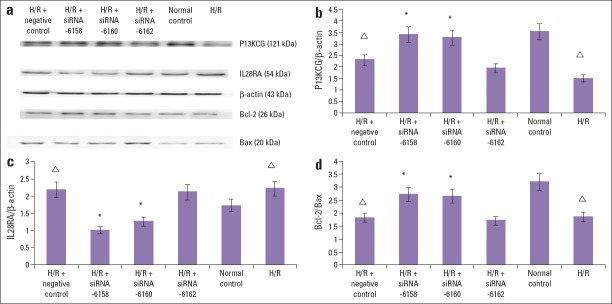
The IL28RA/b-actin, PI3KCG/b-actin, and Bcl-2/Bax ratios in the control group were 1.72±0.03, 3.54±0.06, and 3.20±0.05, res-pectively. Compared with control group, in the H/R group and H/R+negative control group, the IL28RA/b-actin protein ratio was significantly increased (2.21±0.02 and 2.18±0.03, respectively) and the PI3KCG/b-actin ratio (1.49±0.03 and 2.31±0.04, respectively) and Bcl-2/Bax ratio (1.85±0.02 and 1.81±0.02, respectively) significantly decreased (p<0.05). Compared with the H/R group and H/R+negative control group, in the H/R+siRNA-6158 group and H/R+siRNA-6160 group, the IL28RA/b-actin protein ratio significantly decreased (1.01±0.008 and 1.25±0.007, respectively) and the PI3KCG/b-actin ratio (3.42±0.08 and 3.28±0.07, respectively) and Bcl-2/Bax ratio (2.71±0.03 and 2.66±0.04, respectively) significantly increased (p<0.05). Compared with the H/R group and H/R+negative control group, the IL28RA/b-actin, PI3KCG/b-actin, Bcl-2/Bax ratios in the H/R+siRNA-6162 group did not significantly change (2.11±0.03, 1.94±0.06, and 1.71±0.04, res-pectively) (p>0.05). The results suggested that siRNA-6158 and siRNA-6160 transfection significantly inhibited IL28RA protein expression and promoted PI3KCG expression, thereby preventing apoptosis. It was speculated that the protective effects of IL28RA siRNA on H/R injury in cardiomyocytes were associated with apoptosis inhibition through PI3K/Akt signal pathway activation (Fig. 3a–d).
Figure 3.
The protection effects on the H/R cardiomyocytes injury intracellular mechanisms of IL28RA siRNA. (a) In the H/R procedure, the IL28RA-specific siRNA-6158, siRNA-6160 mediated PI3KCG and Bcl-2 protein increase, and IL28RA and Bax protein decrease. The b-Actin was analyzed as loading control. The siRNA-6162 has no significant effect on the PI3KCG, Bcl-2, IL28RA or Bax expression. (b) The effect of IL28RA siRNA on the PI3KCG/b-Actin protein ratio in H/R induced cardiomyocytes injury. The IL28RA siRNA-6158, siRNA-6160 mediated PI3KCG protein increase. The siRNA-6162 could not mediate this change. Data are presented as the mean±SD. (n=5). *P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group. (c) The effect of IL28RA siRNA on the IL28RA/b-Actin protein ratio in H/R induced cardiomyocytes injury. The IL28RA siRNA-6158, siRNA-6160 mediated IL28RA protein decrease. The siRNA-6162 could not mediate this change. Data are presented as the mean±SD. (n=5). *P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group. (d) The effect of IL28RA siRNA on the Bcl-2/Bax protein ratio in H/R induced cardiomyocytes injury. The IL28RA siRNA-6158, siRNA-6160 mediated Bcl-2/Bax protein ratio increase. The siRNA-6162 could not mediate this change. Data are presented as the mean±SD. (n=5).
*P<0.05, compared with H/R group and H/R+negative control transfection group; ΔP<0.05, compared with normal control group
Discussion
In this experiment, we tested three different pairs of IL28RA siRNA for their ability to mitigate cardiomyocytes apoptosis. After liposomal transfection and H/R treatment, we found two out of the three, siRNA-6158 and siRNA-6160, to be protective. The current results show that compared with the control group, the survival rate in the H/R and H/R+negative control groups significantly decreased (p<0.05) and the LDH level and apoptosis rate significantly increased (p<0.05), indicating an effective myocardial H/R model. In the preliminary function tests, we found that transfection, the beat frequency and survival rate were significantly higher in the H/R+siRNA-6158 and H/R+siRNA-6160 groups than in the H/R group; the LDH level was also significantly lower than that in the H/R group (p<0.05).
Meanwhile, IL28RA protein expression was significantly increased after H/R injury (p<0.05). After transfection with siRNA-6158 and siRNA-6160, however, IL28RA protein expression was significantly reduced (p<0.05) and the PI3KCG protein level and Bcl-2/Bax ratio significantly increased (p<0.05). These results indicate that reduced IL28RA protein expression decreased the cardiomyocyte apoptosis rate. The reduced cardiomyocytes apoptosis rate may be associated with the activation of the PI3K/AKT signaling pathway, thus promoting the downstream anti-apoptotic Bcl-2 and inhibiting pro-apoptotic Bax protein. Bcl-2 and Bax have direct and indirect relationships with the PI3K/Akt signaling pathway, which is an important signaling transduction pathway linking extracellular signal with cellular responses (12, 13). Akt and its downstream cascade are further activated by PI3K phosphorylation, which plays an anti-apoptotic role by regulating the expression of Bcl-2 family proteins (13). The lack of the protective effects of siRNA-6162 on H/R injury in cardiomyocytes may be due to the poor interference of IL28RA. As a negative regulatory gene in cell proliferation, IL28RA siRNA-6158 and -6160 can reduce IL28RA protein expression, thus protecting cardiomyocytes from H/R injury.
We found IL28RA siRNA-6158 and -6160 to protect cardiomyocytes from H/R injury by inhibiting apoptosis. Inhibiting IL28RA gene expression may be an important new strategy for treating myocardial injury. Myocardial ischemia/reperfusion (I/R) injury frequently occurs during the course of AMI treatment, and the inhibition of cardiomyocyte apoptosis has become the primary means of facilitating post-AMI cardiac repair (14). Though the use of siRNA is relatively new, it is commonly used. In RNA interference, siRNA binds to a complementary sequence of mRNA, preventing it from being translated into a protein. siRNA can have profound effects on gene expression due to its high specificity and efficiency. It can silence a gene, essentially producing a knockout model (15). siRNA has been shown to reduce myocardial injury through various mechanisms (16). For example, in 2014, Lin et al. (17) found that transfecting cells with MMP-2 siRNA protected against the myocardial I/R injury.
IFN-ls have been shown to limit tumor proliferation by binding to the receptor complexes cytokine R (IL28RA/ILl0RB), inducing receptor heterodimerization, and blocking the activation of the JAK-STAT signaling pathway. This blocks downstream pathways, such as the PI3K/AKT and MAPK signaling pathways, and inhibits cell proliferation and induces apoptosis (3). IL-28RA is essential to the preferred activation of Statl, Stat2, Stat3, and Stat5, as well as the activity of anti-virus and anti-proliferation, when tyrosine phosphorylation of residues 343 and 517 answered to IFN-l1 (6). Decreased IL28RA protein expression may promote the activation of the JAK-STAT, PI3K/Akt, and MAPK signaling pathways, thus inhibiting cardiomyocyte apoptosis (18–20). Further mechanistic studies are required in the future.
Study limitations
One limitation of this study is the use of neonatal myocytes over adult cardiomyocytes. Neonatal cardiomyocytes differ in their sensitivity from adult cardiomyocytes; therefore, results may not be directly translated to myocardial I/R injury in adult cardiomyocytes. Hence, a corresponding in vivo study is necessary to remedy this. It is speculated that the PI3-kinase/Akt signaling pathway mediates the protective effect of IL28RA suppression with siRNA. Although this pathway plays a role in various forms of cardioprotection, there is no direct proof that it is involved in the present setting. Further experiments would be desirable to test whether PI3-kinase/Akt inhibition abolishes the observed effects.
Conclusion
The present results demonstrated that IL28RA activation plays a fundamental role in post-hypoxic cardiomyocyte apoptosis. Meanwhile, it was shown that the inhibition of IL28RA activation by siRNAs could attenuate post-hypoxic cardiomyocyte apoptosis by activating the downstream PI3K/AKT signal pathway. In summary, IL28RA siRNA-6158 and-6160 can protect cardiomyocytes from H/R injury. The IL28RA gene is expected to be a potential new target in the clinical diagnosis and treatment of AMI. In this study, we found two IL28RA siRNAs with better IL28RA gene expression-suppressing effects. It will lay the foundation for further in vivo studies of the role of the IL28RA gene in AMI.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr. Yanyan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University to Dr. Yanyan Li (2013-2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents (2014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Y.L.; Design – Y.L., G.G.; Supervision – Y.L.; Funding – Y.L.; Materials – Y.L.; Data collection &/or processing – G.G., X.Y., H.G.; Analysis &/or interpretation – G.G.; Literature search – G.G., X.L.; Writing – G.G., Y.L., L.W.; Critical review – Y.L., Z.Y.

Biochemist, MD. Meral Egüz’s collections
References
- 1.Pu J, Yuan A, Shan P, Gao E, Wang X, Wang Y, et al. Cardiomyocyte-expressed farnesoid-X-receptor is a novel apoptosis mediator and contributes to myocardial ischaemia/reperfusion injury. Eur Heart J. 2013;34:1834–45. doi: 10.1093/eurheartj/ehs011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–61. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Luo Y, Wei J, He S. Integrative genomic analyses on IL28RA, the common receptor of interferon-lambda1, -lambda2 and -lambda3. Int J Mol Med. 2010;25:807–12. [PubMed] [Google Scholar]
- 4.Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142:978–88. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez de Lapuente A, Alloza I, Goertsches R, Zettl UK, Urcelay E, Arroyo R, et al. Analysis of the IL28RA locus as genetic risk factor for multiple sclerosis. J Neuroimmunol. 2012;245:98–101. doi: 10.1016/j.jneuroim.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1:similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–74. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Wei J, He S. Integrative genomic analyses on interferon-lambdas and their roles in cancer prediction. Int J Mol Med. 2010;25:299–304. [PubMed] [Google Scholar]
- 8.Tsai CT, Ikematsu K, Sakai S, Matsuo A, Nakasono I. Expression of Bcl2l1, Clcf1, IL-28ra and Pias1 in the mouse heart after single and repeated administration of chlorpromazine. Leg Med (Tokyo) 2011;13:221–5. doi: 10.1016/j.legalmed.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Liang GY, Liu DX, Tang Q, Zhang J, Cai QY, et al. Effect of Si-RNA-silenced HIF-1alpha gene on myocardial ischemia-reperfusion-induced insulin resistance. Int J Clin Exp Med. 2015;8:15514–20. [PMC free article] [PubMed] [Google Scholar]
- 10.Li YY, Zhang H, Lu XZ. Lentiviral vector PLV-PI3KCG gene transfer inhibits hypoxic cardiomyocytes apoptosis. Int J Clin Exp Med. 2015;8:20208–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Jin HJ, Xie XL, Ye JM, Li CG. TanshinoneIIA and Cryptotanshinone Protect against Hypoxia-Induced Mitochondrial Apoptosis in H9c2 Cells. PLoS One. 2013;8:e51720. doi: 10.1371/journal.pone.0051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3'-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30:905–18. [PubMed] [Google Scholar]
- 14.Paul A, Binsalamah ZM, Khan AA, Abbasia S, Elias CB, Shum-Tim D, et al. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials. 2011;32:8304–18. doi: 10.1016/j.biomaterials.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Caplen NJ. Gene therapy progress and prospects. Downregulating gene expression:the impact of RNA interference. Gene Ther. 2004;11:1241–8. doi: 10.1038/sj.gt.3302324. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Lian D, Wong A, Bygrave M, Ichim TE, Khoshniat M, et al. Novel small interfering RNA-containing solution protecting donor organs in heart transplantation. Circulation. 2009;120:1099–107. doi: 10.1161/CIRCULATIONAHA.108.787390. [DOI] [PubMed] [Google Scholar]
- 17.Lin HB, Cadete VJ, Sra B, Sawicka J, Chen Z, Bekar LK, et al. Inhibition of MMP-2 expression with siRNA increases baseline cardiomyocyte contractility and protects against simulated ischemic reperfusion injury. Biomed Res Int. 2014;2014:810371. doi: 10.1155/2014/810371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen K, Li G, Geng F, Zhang Z, Li J, Yang M, et al. Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K-Akt signaling in diabetic rats. Apoptosis. 2014;19:946–57. doi: 10.1007/s10495-014-0977-0. [DOI] [PubMed] [Google Scholar]
- 19.Jian J, Xuan F, Qin F, Huang R. Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. Drug Des Devel Ther. 2015;9:5933–45. doi: 10.2147/DDDT.S92549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuan F, Jian J, Lin X, Huang J, Jiao Y, Huang W, et al. 17-Methoxyl-7-hydroxy-benzene-furanchalcone ameliorates myocardial ischemia/reperfusion injury in rat by inhibiting apoptosis and autophagy via the PI3K-Akt signal pathway. Cardiovasc Toxicol. 2017;17:79–87. doi: 10.1007/s12012-016-9358-y. [DOI] [PubMed] [Google Scholar]