Abstract
Imbalance between T regulatory (Treg) and T effector (Teff) cells is likely to contribute to induction and perpetuation of liver damage in autoimmune hepatitis (AIH) and autoimmune sclerosing cholangitis (AISC) either through inability of Tregs to restrain proliferation and effector cytokine production by responders or through conversion of Tregs into T helper type 1 (Th1) or type 17 (Th17) effector lymphocytes.
We investigated the effect of Treg skewing on the phenotypic and functional properties of CD4+CD127+CD25high cells, an activated subset of Teff, in 32 patients with AIH, 20 with AISC and in 36 healthy subjects (HS).
In AIH/AISC we note a substantial increase in peripheral blood derived CD4+CD127+CD25high cells that display a Th1/Th17 phenotypic profile, as reflected by heightened IFNγ and IL-17 production, as well as by high levels of T-bet and RORC expression, and which is strongly correlated with disease activity. CD4+CD127+CD25high cells are unresponsive to low dose IL-2 and in patients have marked proliferative ability, further enhanced by stimulation with IL-7. CD4+CD127+CD25high cells obtained from CD4+ cells exposed to Treg polarizing conditions display enhanced IL-10 production, upregulate CD49b and LAG-3, markers of T regulatory 1 (Tr1) cells, and effectively suppress responder cell proliferation both in health and AIH/AISC patients through a mechanism which is dependent on IFNγ and IL-17. Suppressive function of CD4+CD127+CD25high cells is maintained upon pro-inflammatory challenge in HS but not in AIH/AISC.
Conclusion
Treg skewing confers activated Teff phenotypic and functional properties of Tr1 cells in health and in AIH/AISC, though suppressive function is lost in patients upon pro-inflammatory challenge. Protracted modulation of the inflammatory environment is required to attenuate the effector potential while boosting immunoregulatory properties in Teff.
Keywords: effector T-cells, T regulatory 1 cells, liver autoimmunity, cytokine, CD127
Introduction
Autoimmune hepatitis type 1 (AIH-1) and autoimmune sclerosing cholangitis (AISC) are forms of autoimmune liver disease in which hypergammaglobulinemia and positivity for anti-nuclear (ANA) and/or anti-smooth muscle antibody (SMA) co-exist with evidence of interface hepatitis on histology and, in case of AISC, bile duct abnormalities (1, 2).
In AIH and AISC, numerical and functional defects of CD4+CD25highFOXP3+ regulatory T cells (Tregs) are associated with overwhelming T helper type 1 (Th1) and T helper type 17 (Th17) effector responses (Teff) (3–5). Imbalance between Tregs and Teff has been found to contribute to the perpetuation of tissue injury in AIH and AISC because of inability of Tregs to control the proliferation and effector cytokine production by Teff; and because of acquisition of Teff properties by Tregs (6, 7).
A wealth of studies has provided evidence that Tregs and Teff are not terminally differentiated lymphocyte subsets, but are both susceptible to phenotypic and functional plasticity. Thus, exposure of Tregs to pro-inflammatory cytokines promotes conversion into Th17 cells (8) or acquisition of Th1 effector properties, including production of IFNγ (9). On the other hand, transdifferentiation of Th17 into IL-10 producing T regulatory 1 (Tr1) cells has been reported in the small intestine, in the setting of experimental colitis induced either by anti-CD3 monoclonal antibody treatment or infectious agents (10, 11). We previously reported that Tregs isolated from the peripheral blood of AIH patients are prone to acquire effector properties when exposed to pro-inflammatory mediators like IL-6 and IL-1β, which are abundantly produced by CD4 Teff in AIH (12). Despite retaining Treg phenotype, these cells produce IFNγ and IL-17, participating in the maintenance of the Teff pool, and contributing to the perpetuation of hepatic damage in AIH. However, we have demonstrated that in vitro blockade of IL-17 can favor de novo generation of Tregs that are phenotypically stable upon pro-inflammatory challenge both in health and in AIH patients (12).
Collectively these studies support the concept that modulation of cytokine and inflammatory environment can substantially affect the phenotypic and functional properties of both Teff and Tregs. In this context activated Teff expressing high levels of CD25 and CD127 are of particular interest. Levels of CD127, the IL-7 receptor α chain normally present on activated Teff (13), correlate negatively with the expression of FOXP3, as a result of FOXP3 binding to the CD127 promoter (14). However, though expression of CD127 normally denotes an effector phenotype, CD127+ cells can acquire functional properties of suppressive cells upon modulation of the cytokine environment (15), suggesting the possibility of deriving Tregs from Teff.
In the present study, we investigate the effect of Treg skewing on the phenotypic and functional properties of the CD4+CD127+CD25high Teff subset in patients with AIH and AISC.
Subjects and methods
Subjects
Thirty-two patients with AIH type 1 and 20 with AISC were studied. All were ANA and/or SMA positive at presentation. A liver biopsy performed at the time of or close to diagnosis showed histopathological features of interface hepatitis in all patients. AISC patients were diagnosed on the basis of bile duct changes of sclerosing cholangitis on retrograde cholangiography (2). Twenty-five patients (16 with AIH and 9 with AISC) were females. Twenty-one patients (6 with AIH and 15 with AISC) had inflammatory bowel disease (IBD), including 19 with ulcerative colitis (UC) and 2 with Crohn’s disease; all the 6 AIH patients had UC; 13 of the AISC patients had UC and 2 had Crohn’s. Thirty-two patients (20 with AIH and 12 with AISC) were studied during drug-induced remission (i.e. normal transaminase levels, [R]); 20 patients (12 with AIH and 8 with AISC) had active disease [A] at the time of study. In 6 of the [A] patients (4 with AIH and 2 with AISC), blood was obtained at disease presentation before the immunosuppression was started; the remaining 14 patients (8 with AIH and 6 with AISC) were studied during an episode of relapse while on stable maintenance treatment. Two patients in remission (both with AISC) were off immunosuppressive treatment at the time of study. Demographic and laboratory data of AIH and AISC patients are presented in Table 1a and Table 1b. Patients were treated with prednisolone (2.5–5 mg daily at remission and 1–2 mg/kg/day at relapse) either alone or in combination with azathioprine (1–2 mg/kg/day) or mycophenolate mofetil (MMF, up to 40 mg/kg/day). In AISC patients ursodeoxycholic acid (UDCA) at a dose of 15–20 mg/kg/day was added to the immunosuppressive regimen. Twenty-nine healthy subjects (HS, median age 28.9 years, range 22.6–39, 19 females) served as normal controls. The age difference between patients and HS is due to ethical constraints in obtaining blood from healthy children. The study was approved by the Ethics Committee of King’s College Hospital, London and written consent was obtained from each AIH, AISC patient or legal guardian and HS enrolled in the study.
Table 1a.
Demographic, laboratory and clinical data of AIH patients
| AIH (n=32) |
Sex (F/M) |
Age at the time of study (years) |
Age at diagnosis (years) |
IBD (Disease activity) |
AST (nv < 50IU/L) |
Bilirubin (nv < 20 μmol/L) |
IgG (nv: 6.5–17 g/L) |
Autoantibody titer*
|
IS treatment |
Assay | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANA | SMA | LKM1 | ||||||||||
| [A] | 7/5 | 15.4 (10–30.2) | 12.7 (3.8–20) | 2 | 146 (51–2,462) | 26.2 (7–257) | 20.5 (8.2–44) | 20 (0–2,560) | 40 (0–1,280) | 0 | ||
| AIH-A1 | F | 10 | 9 | - | 2,462 | 243 | 20.4 | 2,560 | 1,280 | 0 | P | ctf |
| AIH-A2 | F | 11.1 | 10.4 | UC (++) | 61 | 73 | 22.17 | 20 | 0 | 0 | P+A | p,pa |
| AIH-A3 | F | 12.1 | 8.2 | - | 64 | 10 | 16.5 | 80 | 80 | 0 | P+A | p |
| AIH-A4§ | F | 13.5 | 13.5 | - | 1,061 | 127 | 44 | 0 | 10 | 0 | - | p,f |
| AIH-A5 | M | 14.9 | 8.8 | - | 91 | 15 | 16.58 | 0 | 40 | 0 | P+MMF | p |
| AIH-A6§ | F | 15.4 | 15.4 | - | 391 | 13 | 25.8 | 80 | 640 | 0 | - | p,ctf |
| AIH-A7§ | M | 15.4 | 15.4 | - | 294 | 7 | 20.6 | 0 | 320 | 0 | - | p |
| AIH-A8 | M | 15.6 | 3.8 | UC (+) | 539 | 257 | 9.55 | 0 | 0 | 0 | P | ctf |
| AIH-A9§ | F | 15.8 | 15.8 | - | 78 | 26 | 13.8 | 40 | 0 | 0 | - | p,ctf,s |
| AIH-A10 | F | 16.8 | 14.9 | - | 197 | 8 | 24 | 40 | 80 | 0 | P+A | p |
| AIH-A11 | M | 22.2 | 20 | - | 95 | 26.5 | 23.48 | 10 | 0 | 0 | P | pas,fas |
| AIH-A12 | M | 30.2 | 12 | - | 51 | 26.4 | 8.2 | 0 | 40 | 0 | P+A | pas,fas |
|
| ||||||||||||
| [R] | 9/11 | 16.5 (8.9–37.6) | 10.1 (0.4–27.8) | 4 | 26 (11–49) ¶ | 12 (3–44)† | 11.9 (6.4–29.9)‡ | 0 (0–2,560) | 0 (0–80)∫ | 0 | ||
| AIH-R1 | M | 8.9 | 0.4 | UC (+) | 21 | 12 | 17.3 | 40 | 0 | 0 | P+MMF | p,ctf |
| AIH-R2 | M | 10.4 | 7 | - | 36 | 5 | 7.71 | 0 | 0 | 0 | P | p |
| AIH-R3 | F | 10.5 | 8 | - | 28 | 6 | 13.16 | 0 | 80 | 0 | P+A | p |
| AIH-R4 | M | 11.7 | 9 | - | 22 | 4 | 14.63 | 10 | 0 | 0 | P | p,pa,s |
| AIH-R5 | F | 12.7 | 12 | - | 49 | 6 | 21 | 160 | 0 | 0 | P+A | p,s |
| AIH-R6 | M | 13 | 11 | - | 20 | 15 | 8.98 | 160 | 0 | 0 | P+A | p,ctf,s |
| AIH-R7 | M | 13.2 | 11.8 | UC (+) | 24 | 14 | 10 | 0 | 0 | 0 | P | p,ctf |
| AIH-R8 | F | 13.2 | 9.8 | - | 27 | 6 | 20.8 | 0 | 0 | 0 | P+A | p,pa |
| AIH-R9 | F | 16.2 | 10 | - | 24 | 3 | 11.48 | 0 | 0 | 0 | P+A | pas,fas |
| AIH-R10 | F | 16.5 | 10.2 | - | 33 | 6 | 12.89 | 0 | 0 | 0 | P+A | p,s |
| AIH-R11 | M | 16.5 | 9.8 | - | 38 | 16 | 12.3 | 0 | 0 | 0 | P | p,pa |
| AIH-R12 | M | 16.5 | 7.8 | - | 26 | 44 | 6.44 | 0 | 0 | 0 | P+MMF | ctf,pa |
| AIH-R13 | F | 16.8 | 15.2 | UC (+) | 26 | 11 | 20.3 | 0 | 80 | 0 | P | pas,fas |
| AIH-R14 | F | 17 | 16 | - | 11 | 15 | 11.1 | 0 | 0 | 0 | P+A | s,pas,fas |
| AIH-R15 | M | 17.1 | 8 | - | 28 | 13 | 11.06 | 0 | 0 | 0 | P+A | p |
| AIH-R16 | M | 17.8 | 13.8 | - | 19 | 12 | 6.5 | 0 | 0 | 0 | P+A | p |
| AIH-R17 | M | 18 | 14 | UC (+) | 24 | 15 | 12 | 0 | 0 | 0 | P+A | pas,fas |
| AIH-R18 | F | 26.6 | 3.6 | - | 25 | 12 | 29.9 | 2,560 | 0 | 0 | P+A | pas,fas,f |
| AIH-R19 | F | 36 | 15.2 | - | 26 | 16 | 11.5 | 0 | 0 | 0 | P+A | pas,fas |
| AIH-R20 | M | 37.6 | 27.8 | - | 26 | 30 | 11.96 | 0 | 0 | 0 | P+A | pas,fas |
Cumulative data from [A] and [R] patients presented as range (median); nv: normal value
[A]: patients with active disease
[R]: patients at remission
F: female
M: male
AIH: autoimmune hepatitis
IBD: inflammatory bowel disease. IBD disease activity was determined on the basis of clinical, histological (i.e. presence of neutrophils in the crypts) and serological (i.e. levels of fecal calprotectin) parameters. +, mild; ++, moderate. No patient had severe IBD at the time of study.
UC: ulcerative colitis
AST: aspartate amino transferase
IgG: immunoglobulin G
: Autoantibody titer shown as reciprocal
ANA: anti-nuclear antibody
SMA: smooth muscle antibody
LKM-1: liver kidney microsomal antibody type 1
IS treatment: immunosuppressive treatment
P: prednisolone
A: azathioprine
MMF: mycophenolate mofetil
p: phenotype analysis
ctf: cytokine and transcription profile analysis
s: pSTAT-5 staining before cell skewing
f: functional assay before cell skewing
pa: proliferation assay
pas: phenotype analysis after cell skewing
fas: functional assay after cell skewing
: patients studied at disease presentation before starting immunosuppression
P= 0.01,
P= 0.009,
P= 0.015,
P=0.027 when comparing AST, bilirubin, IgG levels and SMA titers between [A] and [R] patients
Table 1b.
Demographic, laboratory and clinical data of AISC patients
| AISC (n=20) | Sex (F/M) | Age at the time of study (years) | Age at diagnosis (years) | IBD (Disease activity) | AST (nv < 50IU/L) | Bilirubin (nv < 20 μmol/L) | IgG (nv: 6.5–17 g/L) | Autoantibody titer*
|
IS treatment | Assay | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANA | SMA | LKM1 | ||||||||||
| [A] | 5/3 | 15 (10.3–18.4) | 11 (3.6–14.2) | 5 | 102 (51–534) | 18 (3–47) | 17.3 (11.6–25.6) | 20 (0–160) | 0 (0–80) | 0 | ||
| AISC-A1§ | M | 10.3 | 10.3 | - | 534 | 47 | 25.6 | 10 | 80 | 0 | - | p,f |
| AISC-A2 | M | 12.1 | 10 | UC (+) | 93 | 26.4 | 11.99 | 0 | 0 | 0 | P+A | p,ctf, f |
| AISC-A3§ | M | 13.8 | 13.8 | UC (+) | 51 | 3 | 20.4 | 40 | 0 | 0 | - | p,f |
| AISC-A4 | F | 14.4 | 11.8 | - | 112 | 7 | 11.66 | 40 | 0 | 0 | P+A | p,ctf |
| AISC-A5 | F | 15.6 | 14.2 | - | 82 | 24 | 25 | 0 | 0 | 0 | P+A | p,s |
| AISC-A6 | F | 16.4 | 3.6 | UC (+) | 125 | 12 | 17.1 | 0 | 40 | 0 | P+MMF | p |
| AISC-A7 | F | 16.7 | 11.8 | CD (+) | 58 | 26.2 | 17.6 | 160 | 0 | 0 | P+A | f |
| AISC-A8 | F | 18.4 | 10 | UC (++) | 119 | 7 | 15.7 | 40 | 0 | 0 | P+A | pas,fas |
|
| ||||||||||||
| [R] | 4/8 | 15.9 (8.2–26) | 12 (5.8–16) | 10 | 28 (16–47) ¶ | 8 (4–21)† | 16.9 (9.8–27.1) | 10 (0–160) | 0 (0–80) | 0 | ||
| AISC-R1 | M | 8.2 | 7 | UC (+) | 20 | 13 | 18.5 | 20 | 0 | 0 | P+A | ctf,pa |
| AISC-R2 | M | 8.2 | 7.1 | - | 30 | 8 | 22.93 | 160 | 0 | 0 | P | p,ctf |
| AISC-R3 | F | 9.5 | 6.6 | - | 27 | 4 | 17.06 | 0 | 40 | 0 | P+A | p,ctf |
| AISC-R4 | F | 12.7 | 12 | UC (++) | 41 | 5 | 27.14 | 0 | 80 | 0 | P+A | p,ctf,s |
| AISC-R5 | F | 14.3 | 7.7 | UC (+) | 25 | 7 | 19.6 | 20 | 0 | 0 | P+A | pas,fas |
| AISC-R6 | M | 15.7 | 12 | UC (+) | 21 | 4 | 12.4 | 0 | 0 | 0 | P | f,s |
| AISC-R7 | M | 16.1 | 5.8 | UC (+) | 47 | 14 | 12.99 | 160 | 0 | 0 | P+A | p,ctf,s |
| AISC-R8 | F | 16.7 | 16 | UC (+) | 33 | 5 | 11.29 | 0 | 0 | 0 | P | p,ctf |
| AISC-R9 | M | 17.2 | 14.6 | UC (+) | 27 | 15 | 16.9 | 0 | 0 | 0 | - | pas,fas |
| AISC-R10 | M | 18.3 | 14.8 | UC (+) | 16 | 21 | 9.8 | 80 | 0 | 0 | P+MMF | pas,fas |
| AISC-R11 | M | 25.4 | 14 | UC (++) | 45 | 8 | 19.1 | 0 | 0 | na | - | pas,fas |
| AISC-R12 | M | 26 | 14 | CD (+) | 41 | 9 | 12.21 | 80 | 0 | 0 | P | p,ctf,f |
Cumulative data from [A] and [R] patients presented as range (median); nv: normal value
[A]: patients with active disease
[R]: patients at remission
F: female
M: male
AISC: autoimmune sclerosing cholangitis
IBD: inflammatory bowel disease. IBD disease activity was determined on the basis of clinical, histological (i.e. presence of neutrophils in the crypts) and serological (i.e. levels of fecal calprotectin) parameters. +, mild; ++, moderate. No patient had severe IBD at the time of study.
UC: ulcerative colitis
CD: Crohn’s disease
AST: aspartate amino transferase
IgG: immunoglobulin G
: Autoantibody titer shown as reciprocal
ANA: anti-nuclear antibody
SMA: smooth muscle antibody
LKM-1: liver kidney microsomal antibody type 1
IS treatment: immunosuppressive treatment
P: prednisolone
A: azathioprine
MMF: mycophenolate mofetil
p: phenotype analysis
ctf: cytokine and transcription profile analysis
s: pSTAT-5 staining before cell skewing
f: functional assay before cell skewing
pa: proliferation assay
pas: phenotype analysis after cell skewing
fas: functional assay after cell skewing
: patients studied at disease presentation before starting immunosuppression
P= 0.049,
P= 0.049 when comparing AST and bilirubin levels between [A] and [R] patients
Cell separation
Peripheral blood mononuclear cells (PBMCs) were obtained as previously described (3). Mononuclear cell viability, determined by Trypan blue exclusion, exceeded 98%.
Flow cytometry
The frequency and phenotypic properties of CD4+CD127+CD25high cells were determined by flow cytometry. PBMCs were stained with allophycocyanin (APC)-cychrome (Cy)-7-conjugated anti-CD4, fluorescein isothyiocyanate (FITC)-conjugated or phycoerythrin (PE)-Cy7-conjugated anti-CD25, and FITC or PE-conjugated anti-CD127 (all from BD Bioscience Discovery Labware, Oxford, UK); and with PE-conjugated CD49b and Alexa-Fluor 647-conjugated LAG-3 (both from Biolegend, London). Cells were incubated at 4°C in the dark for 30 minutes, washed with phosphate buffered saline (PBS)/1% fetal calf serum (FCS), resuspended and analyzed by flow cytometry on a Becton Dickinson fluorescent activated cell sorter (FACSCanto II or LSR II, Becton Dickinson Immunocytochemistry Systems, San José, CA); FACSDiva or FlowJo 2 (TreeStar Inc) software were used for analysis. A minimum of 2–5×104 gated events was acquired for each sample.
Intracellular staining: the frequency of cells positive for FOXP3, T-bet and RORC was determined by intracellular staining after cell fixation and permeabilization with Cytofix/Cytoperm (BD Bioscience) and counterstaining with FITC or APC-conjugated anti-FOXP3 (clone PCH101), peridinin chlorophyll protein (PerCP)-Cy5-conjugated anti-T-bet or PE-conjugated anti-RORC monoclonal antibodies (all from eBioscience, Hatfield, UK). The frequency of IFNγ, IL-17 and IL-10 producing cells was assessed after exposure to phorbol 12-myristate 13-acetate (PMA) (10 ng/ml)/Ionomycin (500 ng/ml) (both from Sigma Aldrich Company Ltd., Gillingham, UK), incubation with Brefeldin A (10 μg/ml, Sigma Aldrich) for 5 hours and counterstaining with AlexaFluor 488, PE or APC-conjugated anti-IL-17 (eBioscience), anti-IFNγ (IQ Products, Groningen, The Netherlands) and anti-IL-10 (BD Bioscience) monoclonal antibodies. To assess the expression of the phospho signal transducer and activator of transcription 5 (pSTAT-5), cells were initially stained with fluorochrome-conjugated anti-CD4, CD25 and CD127 monoclonal antibodies and then treated with intracellular IC fixation buffer (eBioscience). After incubation at room temperature for 30 minute and centrifugation at 600×g for 5 minutes, ice-cold 100% methanol was added. After incubation at 4ºC for 30 minutes and washing, cells were stained with PE-conjugated pSTAT-5 (Y694) monoclonal antibodies (eBioscience). Flow cytometry was performed as indicated above.
Cell purification
CD4+, CD4+CD25high and CD4+CD25− cells to be used in cell polarization experiments (CD4+) and suppression assays (CD4+CD25high and CD4+CD25−) were purified from PBMCs using immunomagnetic beads (Dynal Invitrogen, Oslo, Norway) as previously described (3, 16). CD4+CD25high cells were further purified according to the expression of CD127. Briefly, CD4+CD25high cells were incubated with PE-conjugated anti-CD127 for 30 minutes, then with microbeads conjugated with monoclonal anti-PE antibodies (Miltenyi Biotec, Bergisch-Gladbach, Germany) for 15 minutes at 4ºC. The CD4+CD127+CD25high cell population was purified by positive selection using MS columns (Miltenyi Biotec) according to the manufacturer’s instructions. CD4+CD127−CD25high cells were obtained using the same protocol and used as control. The purity of CD4+ and CD4+CD25− cells exceeded 95%; that of CD4+CD127+CD25high and CD4+CD127−CD25high cells was consistently higher than 92% and 95%.
Cell polarization
Purified CD4+ cells were exposed to Treg polarizing conditions, consisting of recombinant human (rh) TGF-β at 10 ng/ml, IL-2 at 100U/ml and anti-CD3/anti-CD28 T-cell expander (ratio bead/cell 1:2; Dynal Invitrogen). All recombinant cytokines were from R&D Systems. CD4+CD127+CD25high cell phenotype, pSTAT-5 expression and cytokine profile were analyzed by flow cytometry at baseline and following 4 days in culture in the presence of Treg polarizing conditions. Suppressive function of CD4+CD127+CD25high and CD4+CD127−CD25high cells, isolated by immunomagnetic beads after polarization, was also tested (see below).
Suppression assay
CD4+CD127+CD25high cells, isolated from PBMCs or after CD4 cell polarization, were added at 1:8 ratio to CD4+CD25− target cells (16, 17). Parallel cultures of CD4+CD25− cells on their own were set up under identical conditions. When suppressive function was assessed after Treg skewing, CD4+CD127−CD25high cells were tested as controls. Cells were co-cultured at 37ºC and 5% CO2 for 5 days in the presence of anti-CD3/anti-CD28 T-cell expander (Dynal Invitrogen) (ratio bead/cell 1:2) and IL-2 (30 U/ml). All experiments were performed in duplicate. For the last 18 hours cells were pulsed with 0.25 μCi/well 3H-thymidine and harvested using a multi-channel harvester. The percentage inhibition was calculated using the formula: [1-count per minute (cpm) in the presence of CD4+CD127+CD25high or CD4+CD127−CD25high cells/cpm in the absence of CD4+CD127+CD25high or CD4+CD127−CD25high cells]. As the extent of inhibition of cell proliferation measured by either 3H-thymidine incorporation or carboxy fluorescein succinimidyl ester (CFSE) staining in our previous studies is comparable (6), the 3H-thymidine incorporation method was selected as it requires fewer lymphocytes and it is therefore suitable when dealing with low yield cell samples.
Neutralization and challenge assay
To assess whether the suppressive function of CD4+CD127+CD25high cells, purified after Treg skewing, is related to and/or influenced by the release of cytokines, purified mouse monoclonal anti-human IFNγ (clone 45–15, Miltenyi Biotec), IL-17 (clone 41809 R&D Systems) and IL-10 (clone JES-9D7 Miltenyi Biotec) neutralizing antibodies were added at a final concentration of 10 μg/ml to purified CD4+CD127+CD25high cells. The suppressive function of CD4+CD127+CD25high cells purified after Treg skewing was also assessed in the presence of cytokine challenge, i.e. IL-6 (50 ng/ml) and IL-1β (10 ng/ml; both from R&D Systems). After 12–24 hours, pre-treated/challenged CD4+CD127+CD25high cells were added to autologous CD4+CD25− responder cells. Following a 5-day co-culture, cells were tested for their proliferative response.
IL-2 and IL-7 stimulation
Responsiveness of CD4+CD127+CD25high cells to low dose IL-2 was measured by pSTAT-5 expression after 20 minutes stimulation in the presence of 10 ng/ml IL-2. Proliferative response of CD4+CD127+CD25high cells to IL-7 (10 ng/mL, R&D Systems) was assessed after 48 hours by 3H-thymidine incorporation (see above). Control cultures, to which IL-2 or IL-7 was not added, were also included.
Statistical analysis
The normality of variable distribution was assessed by the Kolmogorov-Smirnov goodness-of-fit-test; once the hypothesis of normality was accepted (P>0.05), comparisons were performed by paired or unpaired Student t test as appropriate. A one-way analysis of variance, followed by Tukey’s multiple comparisons test, was used to compare means of multiple samples. Results are expressed as mean ± standard error of the mean (SEM), unless otherwise stated, and P values <0.05 were considered significant. Data were analyzed using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA) and SPSS software (IBM; Hampshire, UK).
Results
Increase in CD4+CD127+CD25high cells in AIH and AISC
We determined the frequency of CD4 cells that are positive for CD127 and concomitantly express high levels of CD25. CD4+CD127+CD25high cell frequency was assessed in the peripheral blood of 32 patients, 18 [R] (AIH: R1–8, R10–11, R15–16 in Table 1a; AISC: R2–4, R7–8, R12 in Table 1b), 14 [A]) (AIH: A2–7, A9–10; AISC: A1–6) and 14 HS. Cells were initially gated on CD4 lymphocytes and then the proportion of CD127+CD25high within these cells was measured (Figure 1A). The proportion of CD4+CD127+CD25high cells was higher in AIH/AISC (both [A] and [R] patients) than in HS (Figure 1B). Amongst [A] patients no difference was noted between those studied at disease presentation, before immunosuppression was instituted, or during an episode of relapse (Figure 1C). The frequency of CD4+CD127+CD25high cells was similar in AIH and AISC patients (Figure 1D) and in AIH/AISC patients with and without IBD (Figure 1D), suggesting that concomitant presence of bile duct damage or gut inflammation is not associated with heightened activation of CD4 T cells in the circulation. Percentage of CD4+CD127+CD25high cells was correlated with levels of AST, total bilirubin, IgG, and SMA autoantibody titer (Figure 1E). We also noted a trend towards significance when correlating the frequency of CD4+CD127+CD25high cells with the number of relapse episodes (Figure 1E). Amongst treated AIH/AISC patients, we observed that those treated with a combination of prednisolone and MMF had the lowest proportion of CD4+CD127+CD25high cells (Figure 1F), indicating a differential impact of immunosuppressive drug regimens on this cell subset.
Figure 1. Frequency of CD4+CD127+CD25high cells is higher in AIH and AISC than health and is directly correlated with markers of disease activity.
Frequency of CD4+CD127+CD25high cells was determined by flow cytometry. Patients’ numbers in brackets refer to Table 1a for AIH and to Table 1b for AISC. (A) Cells were initially gated on CD4 lymphocytes and the proportion of CD127+CD25high cells quantified afterwards. Representative flow cytometry plots from one AIH patient studied during active [A] disease (A5), one AIH patient studied at remission [R] (R2) and one healthy subject (HS). (B) Mean+SEM frequency of CD4+CD127+CD25high cells in 14 [A] (AIH: A2–7, A9–10; AISC: A1–6), 18 [R] (AIH: R1–8, R10–11, R15–16; AISC: R2–4, R7–8, R12) patients and in 14 HS. (C) Mean+SEM frequency of CD4+CD127+CD25high cells in [A] patients studied at diagnosis (n=6) (AIH: A4, A6, A7, A9; AISC: A1, A3) or during an episode of relapse (n=8) (AIH: A2–3, A5, A10; AISC: A2, A4–6) (D) Mean+SEM frequency of CD4+CD127+CD25high cells in patients with AISC (n=12) (A1–6, R2–4, R7–8, R12) and AIH (n=20) (AIH: A2–7, A9–10, R1–8, R10–11, R15–16) and in patients with (n=10) (AIH: A2, R1, R7; AISC: A2–3, A6, R4, R7–8, R12) and without (n=22) (AIH: A3–7, A9–10, R2–6, R8, R10–11, R15–16; AISC: A1, A4–5, R2–3) IBD. (E) Correlation analysis between the frequency of CD4+CD127+CD25high cells and levels (log) of AST, total bilirubin, IgG, SMA autoantibody titer and number of relapses. (F) Mean+SEM frequency of CD4+CD127+CD25high cells in patients treated with prednisolone alone (n=7) (AIH: R2, R4, R7, R11; AISC: R2, R8, R12), prednisolone and azathioprine (n=16) (AIH: A2–3, A10, R3, R5–6, R8, R10, R15–16; AISC: A2, A4–5, R3–4, R7) or prednisolone and MMF (n=3) (AIH: A5, R1; AISC: A6).
Collectively these data show that the proportion of activated CD4+CD127+CD25high cells is elevated in AIH and AISC, especially during active disease; that it correlates with indices of disease activity and is lowered when MMF is included in the immunosuppressive drug regimen.
CD4+CD127+CD25high cells from AIH and AISC patients display a mixed Th1/Th17 phenotype
Analysis of the functional phenotype performed in 17 patients, 6 [A] (AIH: A1, A6, A8–9 in Table 1a; AISC: A2, A4 in Table 1b) 11 [R] (AIH: R1, R6–7, R12; AISC: R21–24, R27–28, R32) and in 10 HS revealed that, compared to controls, CD4+CD127+CD25high cells from AIH and AISC patients contain higher proportions of IFNγ+ and IL-17+ lymphocytes (Figure 2A) and of T-bet+ cells (Figure 2B), but similar frequencies of RORC+ and FOXP3+ lymphocytes (Figure 2B). Since CD4+CD127+CD25high cells, in contrast to conventional CD4+CD127−CD25high Tregs (7), are unresponsive to low dose IL-2, as expected we found no pSTAT-5 upregulation in both patients (AIH: A9, R4–6, R10, R14 in Table 1a; AISC: A5, R4 and R6–7 in Table 1b) and HS (Figure 2C). The proliferative response of CD4+CD127+CD25high cells after exposure to anti-CD3/anti-CD28 beads is higher in patients (AIH: A2, R4, R8, R11–12 in Table 1a; AISC: R1 in Table 1b) than HS (Figure 2D) and is further enhanced by stimulation with IL-7 in the former but not in the latter (Figure 2D).
Figure 2. In AILD CD4+CD127+CD25high cells display a mixed Th1/Th17 phenotypic profile.
Cytokine and transcription factor profile was determined by flow cytometry in AIH/AISC patients and HS. Patients’ numbers in brackets refer to Table 1a for AIH and to Table 1b for AISC. Frequency of CD4+CD127+CD25high cells positive for (A) IFNγ and IL-17 and for (B) T-bet, RORC and FOXP3 in patients (n=17) (AIH: A1, A6, A8–9, R1, R6–7, R12; AISC: A2, A4, R1–4, R7–8, R12) and in HS (n=10). (C) Mean fluorescence intensity (MFI) of pSTAT-5 in CD4+CD127+CD25high cells before and after exposure to low dose IL-2 in 10 patients, including 2 [A] (AIH: A9; AISC: A5) and 8 [R] (AIH: R4–6, R10, R14; AISC: R4, R6–7) and in 8 HS. (D) Counts per minute (cpm) of CD4+CD127+CD25high cells exposed to anti-CD3/anti-CD28 beads in the absence or presence of IL-7 in patients (n=6) (AIH: A2, R4, R8, R11–12; AISC: R1) and HS (n=6). Results are presented as mean+SEM.
Assessment of suppressive function indicated that CD4+CD127+CD25high cells from both patients (AIH: A4, R18 in Table 1a; AISC: A1–3, A7, R6, R12 in Table 1b) and HS are unable to restrain CD4+CD25− target cell proliferation (HS: 32,252±2,462 vs 32,776±2,431; patients: 18,991±2,297 vs 19,466±1,929). No differences in cytokine and transcription factor profiles, pSTAT-5 upregulation, proliferative response and suppressive function were noted between CD4+CD127+CD25high cells from AIH and AISC patients.
These data show that in AIH and AISC, CD4+CD127+CD25high cells display a mixed Th1/Th17 phenotypic profile, do not have suppressive function and are unresponsive to low dose IL-2.
Treg skewing confers CD4+CD127+CD25high cells a Tr1-like phenotype
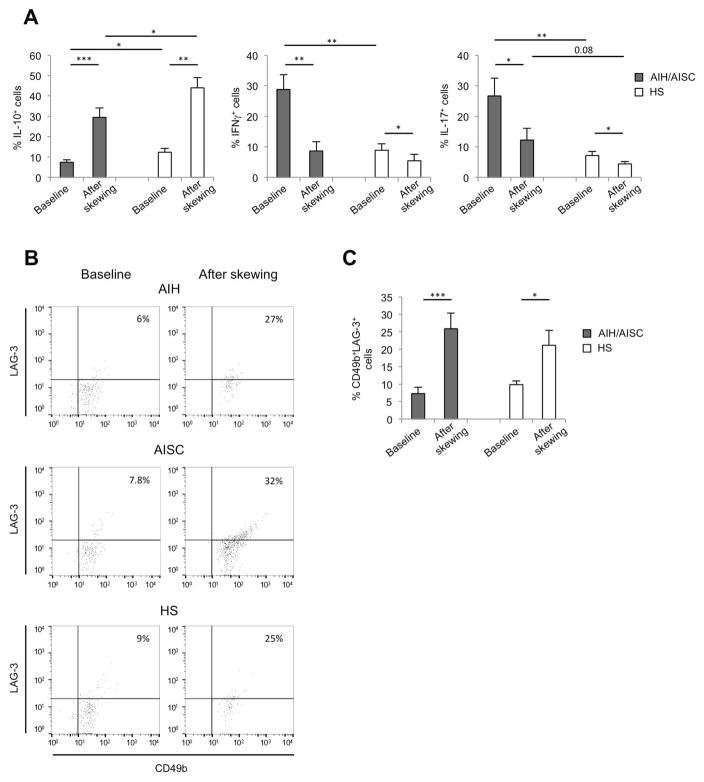
In order to evaluate the effect of cytokine modulation on their phenotypic and functional properties, CD4+CD127+CD25high cells were purified after polarization of CD4 cells under Treg skewing conditions, namely stimulation with anti-CD3/anti-CD28 in the presence of TGF-β and high concentration IL-2. This was tested in 14 patients, 3 [A] (AIH: A11–12 in Table 1a; AISC: A8 in Table 1b) and 11 [R] (AIH: R9, R13–14, R17–20 in Table 1a; AISC: R5, R9–11 in Table 1b) and in 9 HS. Following 4 to 5 days polarization, we noted an increase in the proportion of IL-10 producing lymphocytes within CD4+CD127+CD25high cells in HS and AIH/AISC patients (Figure 3A). Frequencies of IL-10+ cells within the CD4+CD127+CD25high subset were higher in HS than AIH/AISC at baseline and after skewing (Figure 3A). There was a concomitant decrease in the proportion of CD4+CD127+CD25high cells expressing IFNγ and this decrease was particularly marked in patients, who had a baseline level of IFNγ+ lymphocytes significantly higher than HS (Figure 3A). Treg skewing conditions reduced also the frequency of CD4+CD127+CD25high cells producing IL-17 in HS and in AIH/AISC patients (Figure 3A). Levels of IL-17 producing cells within CD4+CD127+CD25high lymphocytes were higher in AIH/AISC than in health at baseline and after skewing (Figure 3A). When compared to AIH, CD4+CD127+CD25high cells from AISC patients displayed higher frequencies of IFNγ+ lymphocytes at baseline (18.5±1.9 vs 37.1±6.5, P=0.04) and lower proportions of IL-17+ cells after skewing (16.7±6 vs 7.7±4.3, P=0.05).
Figure 3. Treg skewing confers CD4+CD127+CD25high cells phenotypic properties of Tr1 cells in both AILD patients and health.
CD4+CD127+CD25high cells were purified after polarization of CD4 cells under Treg skewing conditions (anti-CD3/anti-CD28, TGF-β, high concentration IL-2). Patients’ numbers in brackets refer to Table 1a for AIH and to Table 1b for AISC. (A) Frequency of IL-10, IFNγ and IL-17 producing lymphocytes among CD4+CD127+CD25high cells at baseline and after Treg skewing in 14 patients, 3 [A] (AIH: A11–12; AISC: A8) and 11 [R] (AIH: R13–14, R17–20: AISC: R5, R9–11) and in 9 HS. (B) Flow cytometry plots showing the frequency of CD49b+LAG-3+ cells within CD4+CD127+CD25high cells at baseline and after Treg skewing in one representative AIH patient (R19), one representative AISC patient (R5) and in one HS. (C) Cumulative data (mean+SEM) showing the frequency of CD49b+LAG-3+ cells within CD4+CD127+CD25high cells from 14 patients, 3 [A] (AIH: A11–12; AISC: A8) and 11 [R] (AIH: R13–14, R17–20: AISC: R5, R9–11) and in 9 HS.
Given the increase in cells producing IL-10, we sought to determine the expression of CD49b and LAG-3, markers defining Tr1 cells in mice and humans (18). As shown in Figure 3B and 3C, at baseline the proportion of CD49b+LAG-3+ cells within the CD4+CD127+CD25high subset was similar in patients and HS. Upon exposure to Treg skewing conditions, there was an increase in CD49b+LAG-3+ cells in both groups, this increase being particularly evident in the former. No difference in the proportion of CD49b+LAG-3+ cells within the CD4+CD127+CD25high subset was noted between AIH and AISC patients at baseline and after exposure to Treg skewing conditions.
These data indicate that exposure to Treg skewing conditions modulates CD4+CD127+CD25high cells, which acquire a Tr1-like phenotype by expressing higher levels of IL-10 and heightened CD49b and LAG-3 expression.
Treg skewing endows CD4+CD127+CD25high cells with suppressor properties
We determined the effect of Treg skewing on CD4+CD127+CD25high cell functional properties in 14 patients, 3 [A] (AIH: A11–12 in Table 1a; AISC: A8 in Table 1b) and 11 [R] (AIH: R9, R13–14, R17–20 in Table 1a; AISC: R5, R9–11 in Table 1b) and in 9 HS. These AIH/AISC patients and HS were the same in whom the effect of Treg skewing on CD4+CD127+CD25high cell phenotype was investigated. CD4+CD127+CD25high and CD4+CD127−CD25high cells were isolated after CD4 cell polarization in the presence of Treg skewing conditions and tested for their responsiveness to low dose IL-2.
Upon stimulation with low dose IL-2, upregulation of pSTAT-5 expression among CD4+CD127+CD25high cells was present both in HS and patients, while among CD4+CD127−CD25high cells was noted only in HS (Figure 4A). Levels of pSTAT-5 before and after exposure to low dose IL-2 were lower in patients than in HS, both within the CD4+CD127+CD25high and the CD4+CD127−CD25high subsets (Figure 4A).
Figure 4. Treg skewing confers IL-2 responsiveness and suppressor ability to CD4+CD127+CD25high cells both in AILD and health.
Expression of pSTAT-5 and suppressive function of CD4+CD127+CD25high and CD4+CD127−CD25high cells after Treg skewing was determined in AIH/AISC patients and HS. Patients’ numbers in brackets refer to Table 1a for AIH and to Table 1b for AISC. (A) pSTAT-5 MFI was determined in the absence and presence of low dose IL-2 in CD4+CD127+CD25high and CD4+CD127−CD25high cells obtained after Treg skewing in patients (n=14), 3 [A] (AIH: A11–12; AISC: A8) and 11 [R] (AIH: R13–14, R17–20: AISC: R5, R9–11), and HS (n=9). Representative pSTAT-5 histograms from one AIH patient (R20), one AISC patient (R5) and one HS are shown. Cumulative data (mean+SEM) are also shown. (B) Suppressive function of CD4+CD127+CD25high and CD4+CD127−CD25high cells purified after Treg skewing in the same patients and controls. Suppressive function was measured as ability to control proliferation of CD4+CD25− responder cells. Mean+SEM percentage inhibition of CD4+CD25− cell proliferation in the presence of CD4+CD127+CD25high cells untreated or pre-treated with anti-IL-10, anti-IFNγ, anti-IL-17 neutralizing antibodies or IL-6+IL-1β (pro-inflammatory challenge) compared to CD4+CD127−CD25high cells as control.
Analysis of suppressor function revealed that CD4+CD127+CD25high cells from AIH and AISC patients acquired ability to suppress CD4+CD25− target cell proliferation more efficiently than their CD127− counterpart (Figure 4B). Pre-treatment with anti-IL-10 neutralizing antibodies decreased CD4+CD127+CD25high cell suppression ability in HS, but not in patients (Figure 4B). Of note, pre-treatment with anti-IFNγ neutralizing antibodies tended to reduce CD4+CD127+CD25high cell ability to suppress in HS and completely abrogated it in AIH and AISC (Figure 4B). Exposure to anti-IL-17 neutralizing antibodies decreased suppressive function of CD4+CD127+CD25high cells in patients, but not in HS.
To establish whether the suppression properties of CD4+CD127+CD25high cells after Treg skewing were stably maintained, cells were challenged for 24 hours in the presence of IL-6 and IL-1β, pro-inflammatory cytokines abundantly expressed by effector CD4 cells in AILD (12), before reassessing their suppressive function. While CD4+CD127+CD25high cells from HS maintained their suppressor ability also after challenge, in AIH and AISC they completely lost their ability to control CD4+CD25− cell proliferation, suggesting the need of maintaining a steady anti-inflammatory environment in order for patient-derived CD4+CD127+CD25high cells to retain their ability to suppress (Figure 4B). The proliferative response of CD4+CD25− cells in the presence of CD4+CD127+CD25high or CD4+CD127−CD25high cells after Treg skewing is shown in Supplementary Figure 1.
No difference in pSTAT-5 expression and in the suppression ability of CD4+CD127+CD25high cells after skewing was noted between AIH and AISC patients. Collectively these data show that upon Treg skewing CD4+CD127+CD25high cells from AIH/AISC patients acquire responsiveness to low dose IL-2, as well as ability to suppress target cells. This property appears to be associated with secretion of IFNγ and be completely abrogated upon pro-inflammatory challenge.
Discussion
The present study shows that CD4+CD127+CD25high cells, an activated Teff subset, are markedly elevated in number and strikingly correlate with disease activity in patients with AIH and AISC, but are capable to acquire regulatory phenotype and function under Treg skewing conditions.
The damaging ability of CD4+CD127+CD25high cells probably derives from their mixed Th1/Th17 profile, characterized by high IFNγ and IL-17 production and positivity for T-bet and RORC transcription factors. It is not surprising that their elevation correlates with markers of disease activity, such as transaminase, bilirubin and IgG levels suggesting a direct damaging role. The observed tendency of CD4+CD127+CD25high cell frequency to correlate with the number of relapse episodes suggests a possible predictive value of the elevation of this cell subset.
Interestingly, their number is selectively reduced by the addition of MMF to prednisolone, indicating that MMF immunosuppressive activity is at least in part exerted by control over these effector cells. In this context, we have previously observed that in AIH and AISC treatment with MMF leads to a decrease in the proportion of circulating effector cells in vivo (19) and a decrease in the number of proliferating effectors exposed to mycophenolic acid in vitro (20).
In the absence of cytokine conditioning, CD4+CD127+CD25high cells display an effector phenotype, being unable to suppress in co-culture experiments, being poorly responsive to low dose IL-2, and showing enhanced proliferative response upon IL-7 stimulation. Exposure to Treg conditioning has a major impact on these cells both in health and, more markedly, in patients. Thus, though maintaining their effector signature, i.e. expression of IFNγ and IL-17, CD4+CD127+CD25high cells exposed to Treg polarizing conditions express more IL-10, drastically less IFNγ and, to some extent, IL-17, and acquire Tr1-like phenotypic properties, i.e. co-expression of CD49b and LAG-3 (18). In the experimental setting, trans-differentiation of Th17 cells into IL-10 producing Tr1 cells in the small intestine of mice undergoing experimental colitis has been interpreted as a homeostatic mechanism to control Th17 effector pathogenic potential (10, 11). Acquisition of Treg phenotypic and functional properties by Teff has also been reported in humans, where conversion of activated CD127+ effectors into Treg-like cells expressing IFNγ was observed, supporting the high plasticity of Teff upon stimulation (21); those cells, however, remained CD25− rather than being CD25high as in the present study.
Acquisition of regulatory properties by human Teff would not only reduce tissue inflammation, but would also aid at re-establishing immune tolerance through the expansion of the Treg pool. Tr1 cells are key to the control of inflammation during its chronic phase (22, 23). Thus, in the setting of liver autoimmunity, the expansion of the Tr1-like pool of effectors upon Treg skewing might aid achieving and maintaining immune homeostasis during the inflammatory process, including its chronic phase. Our data reiterate the importance of re-establishing a regulatory cell pool, which, in the case of AIH and AISC, includes conventional Tregs and cells endowed with Tr1 properties. The pivotal role of regulatory cells in maintaining immune homeostasis in the context of liver autoimmunity has been emphasized in previous studies showing Treg defects in patients with (24) and animal models of primary biliary cholangitis (25, 26) as well as in individuals suffering from primary sclerosing cholangitis (27, 28).
In AIH and AISC Treg skewing leads CD4+CD127+CD25high cells to acquire properties partly distinct from those of conventional CD4+CD127−CD25high Treg cells. When compared to conventional Tregs, CD127+ cells have enhanced suppressive function, particularly in patients, and gain the ability to respond to low dose IL-2 by upregulating pSTAT-5. Low dose IL-2, therefore, might aid in maintaining a modulatory environment by promoting production of IL-10 by conventional Tregs, as previously shown (7), as well as by promoting regulatory properties in Teff. That CD127+ cells may display suppressor potential has been previously documented in humans (15, 29), where they can alternate suppression with secretion of pro-inflammatory IL-17, depending on the stimulation signals.
Our data show that the mechanism of suppression of CD4+CD127+CD25high cells upon Treg skewing differs between patients and HS. While in HS it appears to be predominantly modulated by IL-10 and IFNγ, in patients it depends on both IFNγ and IL-17, suggesting that CD4+CD127+CD25high cells exert Treg properties also through production of pro-inflammatory cytokines. IFNγ and IL-17 have been shown to be necessary to the development of antigen-specific Tr1 cells in the context of experimental autoimmune encephalomyelitis, insulin dependent type 1 diabetes and collagen induced arthritis, indicating that these cytokines, classically associated with an effector and pro-inflammatory phenotype, play also a role in the induction of regulatory cell subsets (30). In the setting of AIH and AISC IFNγ and IL-17 might contribute to the suppressor ability of CD4+CD127+CD25high cells by either blocking directly responder cell effector function or by indirectly favoring the generation of a ‘modulatory’ environment through IL-10 secretion.
Importantly, in contrast to what observed in HS, CD4+CD127+CD25high cells from AIH and AISC patients do not retain suppressive properties in the presence of an inflammatory challenge. This emphasizes the need for maintaining a stable control of the inflammatory milieu in order for these cells to exert their suppressive function. Due to the higher plasticity of conventional and induced Tregs in the autoimmune setting, it is crucial to employ therapeutic strategies that create favorable conditions for both Tregs/Tr1 and Teff.
As the present cohort consists mainly of patients with juvenile onset AIH or AISC, with an unusually equal sex distribution and a relatively high incidence of inflammatory bowel disease, probably reflecting our active screening policy (2), our findings cannot be immediately applied to adult AIH and sclerosing cholangitis. Though in a previous study we found that age does not influence effector and regulatory T cell immune responses (6), investigation of a larger number of adults and children with AIH/AISC is needed to address the impact of age and gender on T cell immunity and to elucidate further the immunophenotypic differences we have observed between AIH and AISC in this study (baseline levels of IFNγ and post-skewing levels of IL-17 produced by CD4+CD127+CD25high cells) and in a previous report (levels of CD39 in Th17 cells) (19). In summary, we provide evidence that in AIH and AISC activated CD4+CD127+CD25high Teff are likely to play a major damaging role, but following Treg conditioning they acquire immune modulatory Tr1-like properties. However, stability of the cytokine milieu is key to the maintenance of an effective T regulatory pool, not only through the expansion of already existing Tregs, but also through the skewing of Teff into Treg/Tr1 cells. Successful control of T cell plasticity would help re-shaping Teff/Treg interactions in autoimmunity and would contribute to generating stable and long lasting tolerance.
Supplementary Material
Acknowledgments
Financial support: R Liberal: King’s Health Partners Research and Development Challenge Fund, King’s College London, UK. CR Grant and J Graham: Alex P Mowat PhD Studentship, King’s College Hospital Charity, UK. M Yuksel: Entry Level Fellowship from the European Association for the Study of the Liver. MS Longhi: the Roger Dobson Fund, King’s College Hospital Charity, UK, Clinician Scientist Fellowship from the MRC, UK; and National Institute of Health grant R01 DK108894.
Abbreviations
- AIH
autoimmune hepatitis
- AISC
autoimmune sclerosing cholangitis
- ANA
anti-nuclear antibody
- AST
aspartate aminotransferase
- SMA
smooth muscle antibody
- Treg
regulatory T cell
- Tr1
T regulatory 1
- Teff
effector T cell
References
- 1.Mieli-Vergani G, Vergani D. Autoimmune hepatitis. Nat Rev Gastroenterol Hepatol. 2011;8:320–329. doi: 10.1038/nrgastro.2011.69. [DOI] [PubMed] [Google Scholar]
- 2.Gregorio GV, Portmann B, Karani J, Harrison P, Donaldson PT, Vergani D, Mieli-Vergani G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: a 16-year prospective study. Hepatology. 2001;33:544–553. doi: 10.1053/jhep.2001.22131. [DOI] [PubMed] [Google Scholar]
- 3.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, et al. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, Qiu D, et al. Interleukin-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin-6 expression. PLoS One. 2011;6:e18909. doi: 10.1371/journal.pone.0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, Mieli-Vergani G, et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–1015. doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberal R, Grant CR, Holder BS, Cardone J, Martinez-Llordella M, Ma Y, Heneghan MA, et al. In autoimmune hepatitis type 1 or the autoimmune hepatitis-sclerosing cholangitis variant defective regulatory T-cell responsiveness to IL-2 results in low IL-10 production and impaired suppression. Hepatology. 2015;62:863–875. doi: 10.1002/hep.27884. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 9.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W, Jr, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longhi MS, Liberal R, Holder B, Robson SC, Ma Y, Mieli-Vergani G, Vergani D. Inhibition of interleukin-17 promotes differentiation of CD25(−) cells into stable T regulatory cells in patients with autoimmune hepatitis. Gastroenterology. 2012;142:1526–1535. e1526. doi: 10.1053/j.gastro.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Caro V, D’Anneo A, Phillips B, Engman C, Harnaha J, Lakomy R, Styche A, et al. Interleukin-7 matures suppressive CD127(+) forkhead box P3 (FoxP3)(+) T cells into CD127(−) CD25(high) FoxP3(+) regulatory T cells. Clin Exp Immunol. 2011;165:60–76. doi: 10.1111/j.1365-2249.2011.04334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, Mieli-Vergani G, et al. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 19.Liberal R, Grant CR, Ma Y, Csizmadia E, Jiang ZG, Heneghan MA, Yee EU, et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun. 2016;72:102–112. doi: 10.1016/j.jaut.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant CR, Holder BS, Liberal R, Heneghan MA, Ma Y, Mieli-Vergani G, Vergani D, et al. Immunosuppressive drugs affect ifngamma and pd1 kinetics in patients with newly diagnosed autoimmune hepatitis. Clin Exp Immunol. 2017 doi: 10.1111/cei.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venigalla RK, Guttikonda PJ, Eckstein V, Ho AD, Sertel S, Lorenz HM, Tretter T. Identification of a human Th1-like IFNgamma-secreting Treg subtype deriving from effector T cells. J Autoimmun. 2012;39:377–387. doi: 10.1016/j.jaut.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 23.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol. 2014;380:39–68. doi: 10.1007/978-3-662-43492-5_3. [DOI] [PubMed] [Google Scholar]
- 24.Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Yang W, Yang JB, Jia YJ, Tang W, Gershwin ME, Ridgway WM, et al. Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J Autoimmun. 2015;59:26–37. doi: 10.1016/j.jaut.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Sharma R, Ju ST, He XS, Tao Y, Tsuneyama K, Tian Z, et al. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2009;49:545–552. doi: 10.1002/hep.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sebode M, Peiseler M, Franke B, Schwinge D, Schoknecht T, Wortmann F, Quaas A, et al. Reduced FOXP3(+) regulatory T cells in patients with primary sclerosing cholangitis are associated with IL2RA gene polymorphisms. J Hepatol. 2014;60:1010–1016. doi: 10.1016/j.jhep.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Schwinge D, von Haxthausen F, Quaas A, Carambia A, Otto B, Glaser F, Hoh B, et al. Dysfunction of hepatic regulatory T cells in experimental sclerosing cholangitis is related with IL-12 signaling. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Baecher-Allan CM, Costantino CM, Cvetanovich GL, Ashley CW, Beriou G, Dominguez-Villar M, Hafler DA. CD2 costimulation reveals defective activity by human CD4+CD25(hi) regulatory cells in patients with multiple sclerosis. J Immunol. 2011;186:3317–3326. doi: 10.4049/jimmunol.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.