Abstract
Hookah (water pipe) smoking is a major new understudied epidemic affecting youth. Because burning charcoal is used to heat the tobacco product, hookah smoke delivers not only nicotine but also large amounts of charcoal combustion products, including carbon-rich nanoparticles that constitute putative coronary vasoconstrictor stimuli and carbon monoxide, a known coronary vasodilator. We used myocardial contrast echocardiography perfusion imaging with intravenous lipid shelled microbubbles in young adult hookah smokers to determine the net effect of smoking hookah on myocardial blood flow. In 9 hookah smokers (age 27 – 5 years, mean – SD), we measured myocardial blood flow velocity (β), myocardial blood volume (A), myocardial blood flow (A × β) as well as myocardial oxygen consumption (MVO2) before and immediately after 30 minutes of ad lib hookah smoking. Myocardial blood flow did not decrease with hookah smoking but rather increased acutely (88 – 10 to 120 – 19 a.u./s, mean – SE, p = 0.02), matching a mild increase in MVO2 (6.5 – 0.3 to 7.6 – 0.4 ml·minute−1, p <0.001). This was manifested primarily by increased myocardial blood flow velocity (0.7 – 0.1 to 0.9 – 0.1 second−1, p = 0.01) with unchanged myocardial blood volume (133 – 7 to 137 – 7 a.u., p = ns), the same pattern of coronary microvascular response seen with a low-dose β-adrenergic agonist. Indeed, with hookah, the increased MVO2 was accompanied by decreased heart rate variability, an indirect index of adrenergic overactivity, and eliminated by β-adrenergic blockade (i.v. propranolol). In conclusion, nanoparticle-enriched hookah smoke either is not an acute coronary vasoconstrictor stimulus or its vasoconstrictor effect is too weak to overcome the physiologic dilation of coronary microvessels matching mild cardiac β-adrenergic stimulation.
Hookah (water pipe) smoking is a major new understudied epidemic affecting more than 100 million people worldwide.1 Hookah is increasingly popular among young adults because of the combination of dried fruit and tobacco making a sweet-flavored product, unregulated expansion of hookah cafes near college campuses enabling freshman and sophomores to enjoy the bar scene before the legal alcohol drinking age, and social media claims that the smoke is detoxified as it passes through the water pipe—a spurious belief that is endorsed by most hookah smokers.2,3 The smoke contains many chemicals that could acutely alter myocardial blood flow. Nicotine delivery with either medicinal nicotine or cigarette smoking increases blood pressure (BP) and heart rate4; in young adults, the resultant increase in myocardial oxygen demand (MVO2) is matched by a proportional increase in myocardial blood flow.5 Hookah smoking also acutely increases BP and heart rate6—and thus MVO2—and decreases heart rate variability (HRV),7 suggesting sympathovagal imbalance. Because burning charcoal is used to heat the tobacco product, hookah smoke also delivers large amounts of charcoal combustion products, including carbon-rich nanoparticles that constitute putative coronary vasoconstrictor stimuli and carbon monoxide (CO), a known coronary vasodilator.8–12 Thus, the goal of this study was to determine the net effect of hookah smoking on myocardial blood flow using myocardial contrast echocardiography (MCE) perfusion imaging.
Methods
The research protocol was approved by the Institutional Review Board at Cedars-Sinai Medical Center. We recruited 28 overtly healthy habitual hookah smokers who do not smoke cigarettes. All subjects provided their written informed consent to participate and agreed to abstain from hookah smoking for 72 hours before study. Inclusion criteria were: age 18 to 39 years; no cardiopulmonary disease; no diabetes; no history of illicit substance use; BP <140/90 mm Hg; BMI <35 kg m2; heart rate at rest <100 beats/min; normal electrocardiogram (ECG) and 2D echocardiogram; and smoked hookah >12 times in the past 12 months. Exclusion criteria (in addition to not meeting inclusion criteria) were: smoked any cigarettes in the past 12 months or >100 cigarettes per lifetime; end-expiratory CO >10 ppm before study (indicating noncompliance with the requisite 72-hour period of hookah abstinence); prescription medication other than oral contraceptives; positive pregnancy test; psychiatric illness; any other condition that the physician investigators deem participation unsafe; and, before the MCE protocol, suboptimal echocardiographic images.
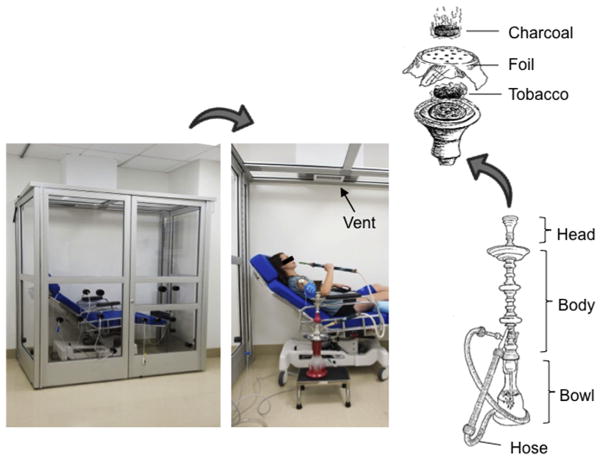
Subjects smoked hookah while seated semi-upright in a custom-built plexiglass smoking chamber, housed in the Cedars-Sinai Hypertension and Vascular Biology Clinical Research Center (Figure 1). A fan within the exhaust system continuously removed air through a vent in the ceiling. Multiple airtight rubber ports on the front and side panels allow wires and tubing to be connected to recording equipment outside the chamber. Subjects rested comfortably on a padded outpatient procedure chair while they smoked ad lib for 30 minutes.
Figure 1.
Hookah Smoking Chamber and Water pipe Schematic. The Plexiglass and aluminum smoking chamber with a procedure chair enclosed. Multiple airtight rubber ports on the front and side panels allow wires and tubing to be connected to recording equipment outside the closed chamber (Left panel). Closeup of a mock subject holding the water pipe. A fan within the exhaust system continuously pulls air out through the vent (arrow) in the ceiling (Middle panel). The water pipe schematic showing the burning charcoal used to heat the tobacco (Right panel).
The traditional water pipe (Figure 1) consisted of a chrome body fitted into a glass base with 1,360 ml of water placed into the base. Twelve grams of the most popular brand of hookah tobacco (Starbuzz Tobacco, Inc, Anaheim, California) was placed inside the pipe’s ceramic head, which was covered with a piece of aluminum foil. The aluminum foil was perforated using a “screen pincher,” which standardized the number and size of holes in the foil. The tobacco was then heated with 2 20 × 20–mm natural charcoal briquettes (Coco Nara 100% Natural Coal).
Heart rate was measured continuously before, during, and after the 30-minute hookah smoking sessions and recorded/displayed in real time using a PowerLab A–D Converter (ADInstruments, Colorado Springs, Colorado). Heart rate was measured by a cardiotachometer triggered by the R wave of the ECG and recorded at 1,000 Hz. BP was measured using a validated oscillometric sphygmomanometer (Datascope Mindray Passport V, Mindray North America, Mahwah, New Jersey).13 Respiratory excursions were monitored with a pneumobelt placed around the thorax (Pneumotrace; UFI, Morro Bay, California) to measure respiratory rate before and immediately after hookah smoking.
MCE was performed with continuous intravenous (i.v.) infusion of lipid-shelled perflutren-containing microbubbles by one experienced sonographer (XT) as previously described, using proved methods to minimize variability.14,15 Contrast-enhanced 2-dimensional transthoracic echocardiography was performed using a phased-array probe interfaced with an imaging system (iE33; Philips Medical Systems, Andover, Massachusetts). One vial (1.3 ml) of lipid-shell octafluoropropane microbubbles (Definity; Lantheus Medical Imaging, North Billerica, Massachusetts) was diluted to a total volume of 30 ml in normal saline solution and administered intravenously with an infusion pump (Medfusion 3500 Syringe Pump; Smiths Medical ASD Inc, St. Paul, Minnesota) at a rate of 1.0 ml/min. After myocardial Definity concentration reached steady state, microbubbles within the imaging sector were destroyed with a high (0.8) mechanical index ultrasound pulse, and the rate of replenishment was interrogated with low (0.1) mechanical index imaging. Only end-systolic frames were used for analysis. Gain, depth, transmit focus, and postprocessing were optimized at the beginning of the study and held constant throughout. Imaging was performed in the apical 4-chamber view, with the septum focused in the middle of the window.
Image analysis was performed offline by a single experienced operator (SS) using an established method.16 Briefly, a large region of interest was placed over the mid-ventricular septum of 10 to 15 consecutive end-systolic images beginning with the immediate postdestruction frame. Plots of time versus background-subtracted acoustic intensity were then generated and fit to the exponential function y = A (1–eβt), where y is the acoustic intensity at time (t), A is the plateau where acoustic intensity represents myocardial blood volume, and β is the mean microbubble (i.e., blood flow) velocity through the microcirculation (referred to as myocardial blood flow velocity and expressed in acoustic intensity units per second). Data are reported as the mean of 3 separate plots of microbubble destruction, refill time versus acoustic intensity, each satisfying all the following criteria: (1) adequate destruction of microbubbles after high mechanical index pulse by visual inspection; (2) focus of the septum in the middle of the window throughout the entire destruction-refill observation period; and (3) a consistent imaging plane. Myocardial blood flow was calculated as the product of myocardial blood volume (A) and blood flow velocity (β). In our laboratory, the coefficient of variation is 3% for myocardial A, and 13% for both myocardial β and myocardial perfusion (A·β), documenting test–retest reproducibility.14
To evaluate left ventricular (LV) systolic function and to estimate cardiac work as a reflection of MVO2, we performed contrast-enhanced imaging in both the parasternal and apical widows. Apical 4-chamber and 2-chamber images were acquired, with the endocardial surface traced manually at end diastole and end systole for measurement of LV end-diastolic and end-systolic volumes, respectively, using the modified Simpson method.17 All data were reported as the average of at least 3 cardiac cycles acquired with the breath held at end expiration. Stroke volume was calculated as end-diastolic volume subtracted by the end-systolic volume, and cardiac output was calculated as the heart rate multiplied by the stroke volume. Ejection fraction was calculated as stroke volume divided by end-diastolic volume. LV end-systolic single point elastance was calculated as end-systolic pressure (0.9 × systolic BP) divided by end-systolic volume. MVO2 was calculated as the rate pressure product by the validated equation: (7.2 × 10−4) × (heart rate × systolic BP) + 1.4218 and also reflected in total myocardial work: (heart rate × systolic BP × stroke volume).
To parse sympathetic versus parasympathetic mediation of the increase in MVO2 evoked by hookah, we used 2 complementary approaches: (1) HRV19 and, in a subset of subjects, and (2) i.v. propranolol at a dose (0.2 mg/kg) that provides near-maximal steady-state β-adrenergic blockade for ≥60 minutes.20 The HRV was derived from the continuous ECG according to recommended standards19 using LabChart software (LabChart 7.0, ADInstruments). Mean values were calculated for 5 consecutive minutes before and immediately after hookah smoking. Time and frequency domain measurements were calculated by established standards,19 which state that vagal activity is the major contributor to high frequency component (HF 0.15 to 0.40 Hz) and that disagreement exists with respect to the low frequency component (LF 0.04 to 0.15 Hz), with some studies suggesting that LF, when normalized for total power, is a quantitative marker of sympathetic control of heart rate, whereas others view LF as reflecting both sympathetic and vagal activity. Thus, ratio of LF to HF power is considered to reflect sympathovagal balance.19
Expired CO and plasma nicotine concentrations were measured immediately before and after 30 minutes of hookah smoking to characterize the smoking exposure. End-tidal CO was measured with a CO meter (Micro Smokerlyzer; Bedfont Scientific Ltd.; Kent, United Kingdom). Plasma nicotine levels were assayed in the Clinical Pharmacology Laboratory at San Francisco General Hospital by gas chromatography with nitrogen-phosphorus detection, using 5-methylnicotine and 1-methyl-5-(2-pyridyl)-pyrrolidin-2-one (“ortho-cotinine”) as internal standards. This method has been modified for simultaneous extraction of nicotine with determination using capillary gas chromatography. The limits of quantitation are 1 ng/ml for nicotine.21
We used paired t tests for comparisons between data obtained before and after 30 minutes of hookah smoking. Data were considered statistically different when p <0.05; there was no adjustment for testing of multiple variables. Results are reported as mean ± SE, unless otherwise specified.
Results
Of 55 potential subjects who were screened for participation, 27 were excluded for the following reasons: suboptimal echocardiographic image quality for MCE (n = 10); exhaled CO >10 ppm on screening (n = 2); a history of cigarette smoking (n = 8); and a medical history of chronic systemic illness including asthma or diabetes (n = 7). The characteristics of the remaining 28 study subjects are presented in Table 1. This racially diverse group of young adults, including 8 women and 20 men, had a mean age of 27 years (range, 19 to 39 years). They smoked hookah on average twice weekly, with each session lasting on average 102 minutes (range, 60 to 240 minutes).
Table 1.
Subject characteristics (n = 28)
| Variable | N or mean ± SD |
|---|---|
| Female/Male | 8/20 |
| Age (years) | 27 ± 5 |
| Body Mass Index (kg·m2) | 25.0 ± 4.0 |
| Non-Hispanic White | 9 |
| Non-Hispanic Black | 14 |
| Hispanic | 1 |
| Asian | 1 |
| Native Hawaiian/Pacific Islander | 1 |
| Middle-Eastern Origin | 2 |
| Level of Education Attained | |
| High school | 6 |
| College | 20 |
| Graduate | 2 |
| Hookah Smoking History | |
| Number of Hookah Sessions per week | 2 ± 2 |
| Session Duration (minutes) | 102 ± 60 |
| Age of Hookah Smoking Onset (years) | |
| ≤ 17 | 5 |
| 18–24 | 19 |
| 25–29 | 3 |
| 30–39 | 1 |
Data reported as number (N) or mean ± SD.
In total, we tested acute effects of hookah smoking on: (1) myocardial perfusion imaging by MCE and 2-dimensional echocardiographic indexes of LV systolic function in the 9 subjects with optimal echocardiographic image quality and (2) HRV—because of its inherently large intersubject variability— in 21 subjects, of whom, 4 returned on a separate day to repeat the protocol after β-adrenergic blockade with i.v. propranolol. Two subjects participated in both MCE and HRV protocols. Heart rate and BP were measured in all subjects.
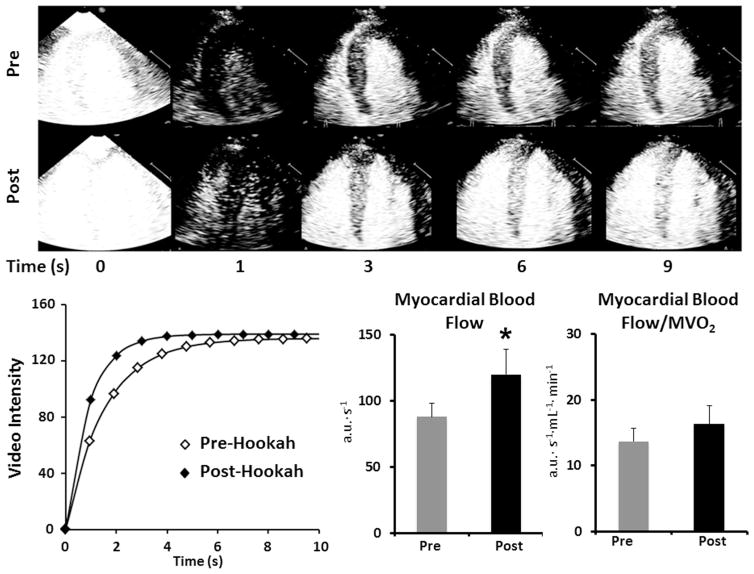
The effect of hookah smoking on myocardial blood flow is presented in Table 2 and Figure 2. In these subjects, myocardial blood flow velocity increased, whereas myocardial blood volume remained unchanged. As a result, myocardial blood flow (and conductance) increased by 36%. The ratio of myocardial perfusion to MVO2 was unaffected by hookah smoking, indicating proportionate increases in oxygen delivery and demand. Hookah smoking had no effect on several systolic phase indexes, including LV ejection fraction, LV end-systolic elastance, or LV stroke work, whereas LV total work increased modestly (Table 2).
Table 2.
Myocardial responses to hookah smoking
| Pre-Hookah | Post-Hookah | p-value | |
|---|---|---|---|
| Myocardial Contrast Echocardiography | |||
| Myocardial A, a.u. | 133 ± 7 | 137 ± 7 | 0.45 |
| Myocardial β, s−1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.01 |
| Myocardial blood flow (A·β), a.u.·s−1 | 88 ± 10 | 120 ± 19 | 0.02 |
| Myocardial conductance, a.u.·mmHg−1 | 1.1 ± 0.2 | 1.5 ± 0.3 | 0.04 |
| Myocardial blood flow/MVO2, a.u.· s−1·mL−1· min−1 | 14 ± 2 | 16 ± 3 | 0.18 |
| Conductance/MVO2, a.u.·mmHg−1·ml·min−1 | 0.2 ± 0.03 | 0.2 ± 0.04 | 0.28 |
| Indices of LV work and Oxygen Demand | |||
| LV Stroke volume, mL | 66 ± 5 | 66 ± 4 | 0.93 |
| LV End-systolic volume, mL | 41 ± 4 | 39 ± 2 | 0.55 |
| LV End-diastolic volume, mL | 107 ± 9 | 105 ± 6 | 0.75 |
| LV Ejection Fraction, % | 62 ± 1 | 62 ± 1 | 0.74 |
| LV end-systolic elastance, mmHg·mL−1 | 2.7 ± 0.3 | 2.8 ± 0.2 | 0.56 |
| LV stroke work (×10−3), mmHg·mL−1 | 2.9 ± 0.5 | 2.6 ± 0.3 | 0.45 |
| LV total work, mmHg· mL−1·beats.min−1 | 166.2 ± 22 | 202.3 ± 23 | 0.02 |
| MVO2, ml·min−1 | 6.48 ± 0.24 | 7.60 ± 0.38 | 0.001 |
| Hemodynamics | |||
| Heart Rate. beats·min−1 | 62 ± 2 | 71 ± 4 | 0.01 |
| Systolic Blood Pressure, mmHg | 114 ± 4 | 122 ± 5 | 0.01 |
| Diastolic Blood Pressure, mmHg | 62 ± 3 | 66 ± 5 | 0.07 |
| Mean Arterial Pressure, mmHg | 79 ± 3 | 84 ± 5 | 0.01 |
| Cardiac Output, L·min−1 | 4.0 + 0.3 | 4.6 + 0.3 | 0.02 |
| Systemic Vascular Resistance, dyn·s/cm5 | 1679 ± 172 | 1503 ± 128 | 0.17 |
Data are mean ± SEM for 9 subjects.
a.u. = arbitrary units for acoustic intensity; LV = left ventricle; MVO2 = myocardial oxygen consumption.
Figure 2.
Effect of hookah smoking on myocardial blood flow by MCE. Illustrative MCE images of the left ventricle at various time intervals after the destructive pulse sequence (denoted as T0) in one subject. The rate of bubble replenishment in the myocardium was faster after hookah smoking (Top panel). Time-video intensity plot before and after hookah smoking in the same subject. The reappearance rate (myocardial blood flow velocity, β) was faster after hookah smoking, whereas the peak video intensity (myocardial blood volume, A) was unchanged (Bottom left panel). Summary data for myocardial blood flow (A × β) and myocardial blood flow normalized to myocardial oxygen consumption (MVO2) before and after hookah smoking (Bottom right bar graphs). Data are mean ± SE for 9 subjects, *p <0.05.
In the 21 subjects in whom we measured HRV before and immediately after smoking hookah, respiratory rate was unchanged (17 ± 1 to 18 ± 1 breaths·minute−1, p = 0.07), whereas there were significant increases in heart rate, systolic, and diastolic BP (Table 3). When HRV was expressed as absolute values, neither LF power nor HF power changed after hookah. However, when normalized to total power, low frequency normalized units (LFnu) (i.e., sympathetic activity) increased by 26% and high frequency normalized units (HFnu) (i.e., vagal activity) decreased by 35%. The LF/HF ratio increased by 112%. Multiple time-domain indexes were unaffected (Table 3).
Table 3.
Acute effects of hookah smoking on heart rate variability
| Pre-Hookah | Post-Hookah | P Value | |
|---|---|---|---|
| Heart Rate and Blood Pressure | |||
| Heart Rate, beats·min−1 | 60 ± 2 | 75 ± 2 | < 0.001 |
| Systolic blood pressure, mmHg | 116 ± 2 | 125 ± 3 | < 0.001 |
| Diastolic blood pressure, mmHg | 71 ± 3 | 80 ± 3 | < 0.001 |
| Mean arterial pressure, mmHg | 84 ± 2 | 92 ± 2 | < 0.001 |
| Heart Rate Variability | |||
| Frequency Domain | |||
| VLF power, ms2 | 3560.9 ± 993.6 | 3340.9 ± 831.1 | 0.822 |
| LF Power | |||
| Ms2 | 3120.5 ± 858.5 | 3032.7 ± 831.4 | 0.929 |
| Nu | 41.5 ± 3.8 | 54.6 ± 4.1 | 0.021 |
| HF Power | |||
| Ms2 | 2501.8 ± 487.9 | 3166.4 ± 1485.6 | 0.66 |
| Nu | 50.6 ± 3.5 | 33.6 ± 3.4 | 0.002 |
| LF/HF | 1.09 ± 0.2 | 2.31 ± 0.5 | 0.033 |
| Time Domain | |||
| NN50 count | 115.2 ± 11.2 | 106.9 ± 32.6 | 0.803 |
| SDNN, ms | 88.6 ± 9.9 | 99.9 ± 15.0 | 0.376 |
| SDSD, ms | 82.1 ± 9.3 | 86.8 ± 17.3 | 0.735 |
| r-MSSD, ms | 80.5 ± 8.7 | 86.6 ± 17.3 | 0.676 |
| Total Number of Heart Beats | 298 ± 11 | 371 ± 10 | <0.001 |
Data reported as mean ± SE for 21 subjects.
HF = high frequency; LF = low frequency; ms = milliseconds; NN50 = normal-to-normal intervals >50 ms; r-MSSD = root mean squared of successive differences; SDNN = standard deviation of the normal-to-normal interval; SDSD = standard deviation of successive differences; VLF = very low frequency.
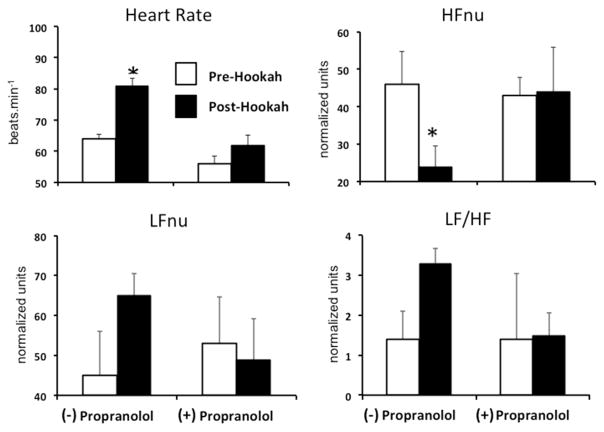
Because of the large interindividual variability in the HRV responses to hookah smoking and inconsistent changes across indexes, we performed additional β-blocking studies in a small subset of subjects to parse sympathetic from parasympathetic mediation. Intravenous propranolol eliminated the hookah-induced increases in MVO2 and its component increases in heart rate and systolic BP (Supplementary Table 1). In this subset of subjects, before propranolol, hookah induced the same directional changes in LFnu, HFnu, and LF/HF ratio as in the entire sample, but only the decrease in HFnu was statistically significant and it also was eliminated by propranolol (Supplementary Table 1 and Figure 3).
Figure 3.
Effect of hookah smoking on heart rate variability before and after β-adrenergic blockade (i.v. propranolol). Panels illustrate heart rate, normalized high frequency power (HFnu), normalized low frequency power (LFnu), and the LF/HF ratio before (white bars) and immediately after (black bars) 30 minutes of hookah smoking with and without β blockade. Summary data for 4 subjects reported as mean ± SE, *p <0.05. HFnu = high frequency normalized units; LFnu = low frequency normalized units.
After a single 30 minute session of smoking hookah in the smoking chamber, plasma nicotine increased from 0.9 ± 0.1 to 7.1 ± 1.5 ng/ml (before vs after hookah, p = 0.002) and expired CO increased from 3 ± 0.4 to 25 ± 2 ppm (before vs after hookah, p <0.001).
Discussion
Contrary to the social media buzz promoting the safety of hookah smoking, there is concern in the tobacco research community about hookah’s putative health risks, including acute cardiovascular toxicity.6,22 In healthy cigarette smokers and with medicinal nicotine, myocardial blood flow increases acutely with nicotine delivery as a physiological response to increased myocardial work and increased MVO2.4,5 For hookah smoking, we hypothesized that myocardial blood flow would decrease acutely because the charcoal combustion produces a large amount of nanoparticles that could trigger vasoconstriction in the coronary microcirculation. We tested this hypothesis using MCE perfusion imaging, a powerful clinical research tool that previously has not been applied to tobacco disease–related research. In contrast to what was hypothesized, we found that myocardial blood flow increases rather than decreases with hookah smoking—at least in a controlled laboratory setting with young adults who regularly smoke hookah but do not smoke cigarettes. Thus, the response is similar to that observed in healthy, young cigarette smokers.
The hookah-induced increase in myocardial blood flow most likely is a physiological response to increased myocardial work and oxygen demand because (1) it was proportional to the increased MVO2 and (2) the specific pattern of microvascular response—increased myocardial blood flow velocity with unchanged myocardial blood volume—is the same (although half the magnitude) as seen by MCE whenMVO2 is increased by low-dose i.v. dobutamine in both dogs23 and young men.14 Although CO is a direct coronary vasodilator,12 the mechanism of the increase in myocardial blood flow with acute CO inhalation is mainly capillary recruitment,24 causing increased myocardial blood volume with unchanged myocardial blood flow velocity—the opposite pattern of microvascular response to what we saw with hookah smoking.
Although myocardial blood volume was unaffected by hookah, it decreases sharply in young adults challenged with a low-dose intranasal cocaine—a sympathomimetic that increases α- and β-adrenergic drive and impairs endothelial-dependent vasodilation.14 Our rationale for hypothesizing that the response to hookah smoking might be similar to the cocaine response is that acute inhalation of smoke nanoparticles, small enough to penetrate the lung parenchyma and enter the systemic circulation, would activate lung afferents that trigger reflex α-adrenergic coronary vasoconstriction and generate reactive oxygen species that impair endothelial-dependent coronary vasodilation.8 Because mainstream hookah smoke delivers a much larger exposure to both fine particulates <2.5 μm (particulate matter [PM2.5]) and ultrafine particles <0.1 μm (PM0.1) than either carbon fuel emissions in air pollution or cigarette smoke,9,11,25,26 we interpret our MCE data to suggest that the nanoparticle-enriched hookah smoke either does not constitute an acute coronary vasoconstrictor stimulus or that nanoparticle-induced vasoconstriction is too weak to overcome physiological coronary vasodilation in the setting of increased MVO2.
With hookah, the increased MVO2 was (1) caused by cardiac β-adrenergic stimulation because it was eliminated by i.v. propranolol, and (2) mild—less than half the magnitude seen when young adults are challenged with low-dose i.v. dobutamine—5 μg/kg/min, which is ~1/10 the maximal dose of the β agonist used in clinical stress testing.14 Although low-dose dobutamine increases multiple echocardiographic LV ejection phase indexes,14 hookah smoking had no detectable effect. The increase in MVO2 with hookah is explained by the effect of nicotine, which is consistent with previous research showing effects of cigarette smoking and medicinal nicotine.4 The increase in plasma nicotine is on average less with hookah compared with cigarette smoking,9 but the nicotine dose–response is such that even small increases in plasma nicotine stimulate catecholamine release27 and increase in BP and heart rate.7
Our HRV data differ from those of a previous study by Cobb et al,7 who found that hookah smoking acutely increased absolute LF power with unchanged absolute HF power, suggesting that the observed sympathovagal imbalance was caused by either sympathetic activation or baroreflex impairment. In contrast, using a different brand of hookah tobacco that produced a similar nicotine boost but larger CO boost than in the study by Cobb et al,7 we found (1) no increase in LF power, until we normalized the data for total power and (2) a decrease in both absolute and normalized HF power, implicating a robust vagolytic action of hookah. That i.v. propranolol abolished this presumed vagolytic action proves β-adrenergic mediation and shows the limitation of HRV for parsing sympathetic versus vagolytic actions of hookah.
Our study has several limitations. The present findings cannot be generalized directly to the natural setting of hookah cafés or to hookah smokers who use other tobacco-related products. With our single-subject smoking chamber, the hookah “dose” may have underestimated the exposure when smoking with friends in a hookah café because of the: (1) shorter smoking session (30 minutes vs 102 minutes for their typical session outside the laboratory); (2) constant recirculation of air through the chamber, which removes side-stream smoke; and (3) no secondhand smoke due to lack of companion smokers. In social settings, users often share a single hookah and do not smoke continuously. The mean CO boost of 25 ppm in our study is within the range of the 25 to 38 ppm boost in other studies of ad lib hookah smoking in both an inpatient clinical research center9 and actual hookah cafés.28 We could not measure mainstream nanoparticle size and concentration, which requires specialized technology. However, CO and PM2.5 concentrations are highly correlated,29 as both are largely charcoal combustion products; our subjects’ measured CO boost suggests a PM2.5 exposure of ~1,200 μg per m3, which is 60 times higher than with air pollution in South Central Los Angeles.25 Charcoal combustion results in a mainstream hookah smoke that is highly enriched with PM0.1 (mean particle size of 0.04 μm).10 Inhaled carbon-rich PM0.1, which is postulated to be more toxic than PM2.5, is absorbed into the systemic circulation within 60 seconds and reaches a peak concentration by 5 to 10 minutes,30 which is well within the time frame of our hookah protocol.
With cigarette smoking in young adults, myocardial blood flow (measured by positron emission tomography) also increases acutely in proportion to increased MVO2, but maximal dipyridamole-induced vasodilator capacity is significantly decreased.5 Thus, cigarette smoking is considered to decrease myocardial blood flow reserve. Additional research will be needed to compare effects of hookah with those of cigarettes on myocardial blood flow reserve and endothelial-dependent coronary vasodilation. We studied 1 hookah pipe and 1 brand of hookah tobacco. Because hookah products are unregulated, many unmeasured contaminants may contribute to the net effect of hookah smoking on cardiovascular function, and our findings cannot be extrapolated to other brands. However, we studied the most popular brand of hookah tobacco and charcoal briquettes used by our subjects. Because ours were acute studies of young adults, the chronic effect of hookah smoking on the coronary circulation remains an open question. Despite these limitations, our study shows that hookah smoking does not acutely impair myocardial perfusion in young adults who only smoke hookah, but rather increases myocardial perfusion, similar to that observed with cigarette smoking.
Supplementary Material
Acknowledgments
This study was funded by an Exploratory/Developmental research grant TRDRP #22XT-0017 to Dr. Victor from the Tobacco-Related Disease Research Program, Oakland, California. Doctoral candidate Rezk-Hanna was supported by a TRDRP Dissertation Research Award (#23DT-0102).
The authors thank Dr. Peyton Jacob III, PhD, Lisa Yu, BS, and Trisha Mao, BS for supervising the nicotine analyses in the Clinical Pharmacology Laboratory at the San Francisco General Hospital, University of California, San Francisco.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.amjcard.2016.03.007.
References
- 1.Maziak W, Taleb ZB, Bahelah R, Islam F, Jaber R, Auf R, Salloum R. The global epidemiology of waterpipe smoking. Tob Control. 2014;1:i3–i12. doi: 10.1136/tobaccocontrol-2014-051903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrock SM, Gordon T, Zelikoff JT, Weitzman M. Hookah use among adolescents in the United States: results of a national survey. Nicotine Tob Res. 2014;16:231–237. doi: 10.1093/ntr/ntt160. [DOI] [PubMed] [Google Scholar]
- 3.Rezk-Hanna M, Macabasco-O’Connell A, Woo M. Hookah smoking among young adults in southern California. Nurs Res. 2014;63:300–306. doi: 10.1097/NNR.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL, Hansson A, Jacob P., III Cardiovascular effects of nasal and transdermal nicotine and cigarette smoking. Hypertension. 2002;39:1107–1112. doi: 10.1161/01.hyp.0000018825.76673.ea. [DOI] [PubMed] [Google Scholar]
- 5.Czernin J, Sun K, Brunken R, Bottcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891–2897. doi: 10.1161/01.cir.91.12.2891. [DOI] [PubMed] [Google Scholar]
- 6.Hakim F, Hellou E, Goldbart A, Katz R, Bentur Y, Bentur L. The acute effects of water-pipe smoking on the cardiorespiratory system. Chest. 2011;139:775–781. doi: 10.1378/chest.10-1833. [DOI] [PubMed] [Google Scholar]
- 7.Cobb CO, Sahmarani K, Eissenberg T, Shihadeh A. Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a “healthy” tobacco-free alternative. Toxicol Lett. 2012;215:70–75. doi: 10.1016/j.toxlet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 9.Jacob P, III, Abu Raddaha AH, Dempsey D, Havel C, Peng M, Yu L, Benowitz NL. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol Biomarkers Prev. 2011;20:2345–2353. doi: 10.1158/1055-9965.EPI-11-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monn C, Kindler P, Meile A, Brandli O. Ultrafine particle emissions from waterpipes. Tob Control. 2007;16:390–393. doi: 10.1136/tc.2007.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, Weitzman M, Vilcassim R, Wilson J, Legrand N, Saunders E, Travers M, Chen LC, Peltier R, Gordon T. Air quality in New York City hookah bars. Tob Control. 2014;24:e193–e198. doi: 10.1136/tobaccocontrol-2014-051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayres SM, Giannelli S, Jr, Mueller H. Myocardial and systemic responses to carboxyhemoglobin. Ann N Y Acad Sci. 1970;174:268–293. doi: 10.1111/j.1749-6632.1970.tb49795.x. [DOI] [PubMed] [Google Scholar]
- 13.Anwar YA, Tendler BE, McCabe EJ, Mansoor GA, White WB. Evaluation of the datascope accutorr plus according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1997;2:105–110. [PubMed] [Google Scholar]
- 14.Gurudevan SV, Nelson MD, Rader F, Tang X, Lewis J, Johannes J, Belcik JT, Elashoff RM, Lindner JR, Victor RG. Cocaine-induced vasoconstriction in the human coronary microcirculation: new evidence from myocardial contrast echocardiography. Circulation. 2013;128:598–604. doi: 10.1161/CIRCULATIONAHA.113.002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang MX, Mulvana H, Gauthier T, Lim AK, Cosgrove DO, Eckersley RJ, Stride E. Quantitative contrast-enhanced ultrasound imaging: a review of sources of variability. Interface Focus. 2011;1:520–539. doi: 10.1098/rsfs.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Rooke GA, Feigl EO. Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res. 1982;50:273–286. doi: 10.1161/01.res.50.2.273. [DOI] [PubMed] [Google Scholar]
- 19.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Vongpatanasin W, Taylor JA, Victor RG. Effects of cocaine on heart rate variability in healthy subjects. Am J Cardiol. 2004;93:385–388. doi: 10.1016/j.amjcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Jacob P, III, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3′,3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 22.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tob Control. 2015;24(Suppl 1):i31–i43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le DE, Bin JP, Coggins MP, Wei K, Lindner JR, Kaul S. Relation between myocardial oxygen consumption and myocardial blood volume: a study using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2002;15:857–863. doi: 10.1067/mje.2002.121275. [DOI] [PubMed] [Google Scholar]
- 24.Kleinert HD, Scales JL, Weiss HR. Effects of carbon monoxide or low oxygen gas mixture inhalation on regional oxygenation, blood flow, and small vessel blood content of the rabbit heart. Pflugers Arch. 1980;383:105–111. doi: 10.1007/BF00581870. [DOI] [PubMed] [Google Scholar]
- 25.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maziak W, Rastam S, Ibrahim I, Ward KD, Eissenberg T. Waterpipe-associated particulate matter emissions. Nicotine Tob Res. 2008;10:519–523. doi: 10.1080/14622200801901989. [DOI] [PubMed] [Google Scholar]
- 27.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 28.Barnett TE, Curbow BA, Soule EK, Jr, Tomar SL, Thombs DL. Carbon monoxide levels among patrons of hookah cafes. Am J Prev Med. 2011;40:324–328. doi: 10.1016/j.amepre.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Torrey CM, Moon KA, Williams DA, Green T, Cohen JE, Navas-Acien A, Breysse PN. Waterpipe cafes in Baltimore, Maryland: carbon monoxide, particulate matter, and nicotine exposure. J Expo Sci Environ Epidemiol. 2015;25:405–410. doi: 10.1038/jes.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.