Abstract
Objective: Depression and brief periods of manic symptoms are linked to a significant risk of progression to bipolar disorder (BD) in children who have a first-degree relative with BD I or II. However, little evidence exists to guide the pharmacologic management of children with these high-risk phenotypes. We propose a pharmacological treatment algorithm for high-risk youth and present results on its use in a study of children with a first-degree relative with BD.
Methods: Subjects were 40 youth (mean 12.7 years, range 9–17 years) who had (1) a first-degree relative with lifetime history of BD I or II, (2) DSM-IV-TR diagnoses of BD not otherwise specified, major depressive disorder or cyclothymic disorder, and (3) active symptoms of depression, mania, or hypomania. Participants and their families were enrolled in a randomized trial examining the effects of two psychosocial interventions on the 1-year course of mood disorder. At study intake, participants received a psychiatric evaluation and were offered medications or had existing medications optimized to decrease symptom severity. During the 1-year study, psychiatrists treated participants using a medication algorithm to treat depressive or manic symptoms as well as comorbid anxiety and/or attention-deficit/hyperactivity disorder.
Results: At study entry, 25 of 40 (62.5%) of the participants were taking at least one psychiatric medication. At 1 year, nearly an identical proportion were taking medications (22 of 35, 63%). Independent ratings indicated that in 84.7% of the study visits, physicians maintained adherence to the algorithm. No patients experienced antidepressant- or stimulant-induced mania during the study.
Conclusions: An algorithmic approach to pharmacologic interventions may aid in the management of youth (i.e., age <18) at high risk for BD. Future studies should compare outcomes in high-risk patients receiving algorithm-prescribed treatment versus those receiving treatment as usual.
Clinical Trial Registration Information: Early Family-Focused Treatment for Youth at Risk for Bipolar Disorder; www.clinicaltrials.gov/; NCT00943085.
Keywords: : bipolar disorder, early intervention, high risk, pediatric, treatment, antidepressant, mood stabilizer, antipsychotic
Introduction
Bipolar disorder (BD) affects ∼2% of children and adolescents across nations (Van Meter et al. 2011). Youth with BD have a preponderance of mixed (manic and depressive) and psychotic symptoms, more psychiatric comorbidities, poorer academic and family functioning, and an increased risk for suicide attempts compared with adults with BD (Geller et al. 2002). Given the social and individual costs associated with pediatric onset BD, early recognition and intervention of prodromal symptoms is of considerable public health importance (Van Meter et al. 2016).
Conversion rates to fully syndromal BD have been found to be highest among children or adolescents with a family history of mania who present with depression, subthreshold mania, mood lability, and anxiety (Fiedorowicz et al. 2011; Nusslock and Frank 2011). Youth diagnosed with bipolar disorder not otherwise specified (BD NOS) and who have a first- or second-degree relative with mania are at particularly high risk for conversion, with 58% of youth with a family history of BD converting to BD I or II over 4–5 years, compared with 36% of youth with BD NOS whose family history was negative for BD (Birmaher et al. 2009). More recent studies also suggest that higher rates of conversion are seen among children with anxiety/depression, mood lability, and parents with early onset BD (Hafeman et al. 2016).
While pharmacological guidelines currently exist for treating fully syndromal pediatric BD or youth with major depressive disorder (MDD), no medication guidelines have been constructed specifically for the treatment of a pediatric population at high risk for BD. Few studies have examined the pharmacological management of mood symptoms in high-risk cohorts. Thus, high-risk youth tend to be treated with a wide variety of medications and therapies, or do not receive any treatment at all (Axelson et al. 2006). Delay in treatment carries its own risk for patients, and appears associated with greater depressive morbidity and less time euthymic in later adulthood (Post et al. 2010).
We previously reported on the outcomes of a 4-month family focused therapy for high-risk youth (FFT-HR) compared with an educational control therapy on the 1-year course of mood symptoms in youth at high familial risk for BD (Miklowitz et al. 2013). During the trial, medication treatment was based on an algorithm developed by the study's pharmacotherapists. This article describes the algorithm used and the rationale for its development, as well as its use in our study of high-risk participants.
Methods
Development of the algorithm
Initial versions of the pharmacologic algorithm were derived from the literature on treatments for youth with or at high risk for BD (including youth with comorbid anxiety or attention-deficit/hyperactivity disorder (ADHD) (DelBello et al. 2001; Goldsmith et al. 2011), treatment guidelines for children and adolescents with syndromal BD (Kowatch et al. 2005), and the American Academy of Child and Adolescent Psychiatry (AACAP) guidelines for treatment of depression in youth (American Academy of Child and Adolescent Psychiatry 2007c) (see Table 1 for studies in youth at risk for BD and pediatric bipolar depression studies). When no treatment studies were available, algorithm decisions were based upon expert opinion and/or consensus among the study psychiatrists (all with expertise in the treatment of youth with BD).
Table 1.
Supporting Evidence in High-Risk Populations (Top) and Pediatric Bipolar Depression (Lower)
| Study | Diagnosis | N | Ages | Design | Length | Medication | 1° Outcome | Results |
|---|---|---|---|---|---|---|---|---|
| High-risk studies (all subjects with positive family history of bipolar disorder) | ||||||||
| Geller et al. (1998) | MDD | 30 | 6–12 | DB, PCB-C | 6 weeks | LIT vs. PCB | CGAS, 9-item K-SADS | No difference between active and PCB groups |
| Chang et al. (2003) | MDD, DYS, CYCL, ADHD | 24 | 6–18 | Open-label | 12 weeks | DVX | CGI-I | 18 of 23 (78%) responders |
| DelBello et al. (2007) | BD NOS, DYS, BD II, CYCL, MDD | 20 | 12–18 | Single-blind | 12 weeks | QUE | CGI-I | 15 of 20 (75%) responders |
| Findling et al. (2007) | BD NOS, CYCL | 56 | 5–17 | DB, PCB-C | 5 years | DVX vs. PCB | Time to study discontinuation | No difference between groups |
| Findling et al. (2008) | MDD | 9 | 7–16 | Open-label, randomized | up to 24 months | PAR vs. DVX | YMRS, CDRS, CGAS, CGI-S, CGI-I | Over 50% of subjects had manic/hypomanic symptoms or suicidality; study discontinued after nine subjects enrolled |
| Pediatric bipolar depression studiesa | ||||||||
| Chang et al. (2006) | BD I, II, NOS | 20 | 12–17 | Open-label | 8 weeks | LTG | CGI-I | 16 of 19 responders (84%) |
| Patel et al. (2006) | BD I | 27 | 12–18 | Open-label | 6 weeks | LIT | CDRS-R, CGI-BP | 13 of 27 (48%) response, 8 of 27 (30%) remission |
| DelBello et al. (2009) | BD I | 32 | 12–18 | DB, PCB-C | 8 weeks | QUE vs. PCB | CDRS-R | No difference between treatment and PCB group |
Olanzapine/fluoxetine was approved for treatment of pediatric bipolar disorder after the study and, therefore, was not included in the algorithm.
ADHD, attention-deficit/hyperactivity disorder; BD NOS, bipolar disorder not otherwise specified; BD I, bipolar I disorder; BD II, bipolar II disorder; CGAS, Children's Global Assessment Scale; CDRS-R, Child Depression Rating Scale, Revised; CYCL, cyclothymia; DYS, dysthymia; MDD, major depressive disorder; DVX, divalproex; FLU, fluoxetine; LIT, lithium; LTG, lamotrigine; OLZ, olanzapine; PAR, paroxetine; PCB, placebo; QUE, quetiapine; WASH-U-KSADS, Washington University Kiddie Schedule for Affective Disorders and Schizophrenia; YMRS, Young Mania Rating Scale.
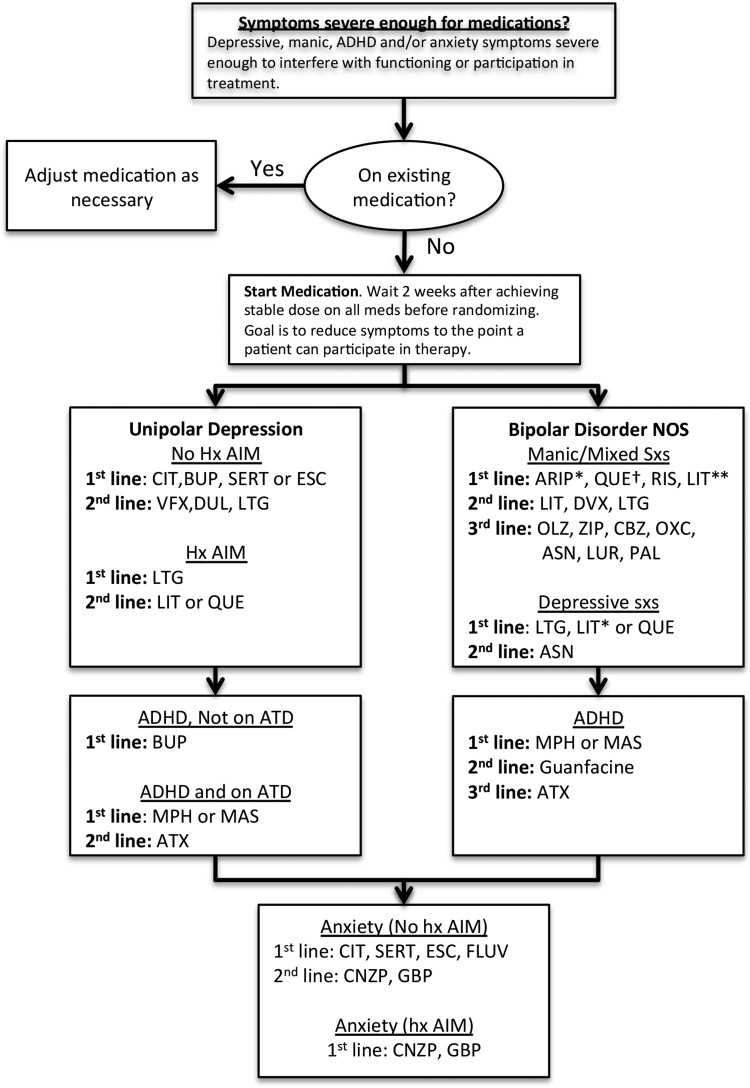
Before beginning the study, psychiatrists reviewed drafts of the study's pharmacotherapy protocol and made further modifications, to decide on first-, second-, and occasionally third-line treatments for BD NOS, MDD, and comorbid conditions. Further refinements were made to the algorithm during a 13-case open trial series (Miklowitz et al. 2011). The algorithm was finalized at the study launch meeting (see Fig. 1 for the complete medication algorithm).
FIG. 1.
Initial decision making is by mood diagnosis (unipolar versus bipolar disorder not otherwise specified), then by presenting mood symptoms, then by history of antidepressant-induced mania. Mood symptoms are treated first, followed by reassessment and treatment (if necessary) of comorbid conditions. *Especially if comborbid ADHD present. **Especially if family history of lithium response. †Check EKG if doses > 600 mg. ADHD, attention-deficit/hyperactivity disorder; ARIP, aripiprazole; ASN, asenapine; ATX, atomoxetine; BUP, bupropion; CBZ, carbamazepine; CIT, citalopram; CNZP, clonazepam; DUL, duloxetine; DVX, divalproex; ESC, escitalopram; FLUV, fluvoxamine; GBP, gabapentin; LIT, lithium; LTG, lamotrigine; LUR, lurasidone; MAS, mixed amphetamine salts; MPH, methylphenidate; OLZ, olanzapine; OXC, oxcarbazepine; PAL, paliperidone; QUE, quetiapine; RIS, risperidone; VFX, venlafaxine; ZIP, ziprasidone.
Participants
Children between ages 9 years, 0 months and 17 years, 11 months who had a first-degree relative with BD I or II were recruited at the University of Colorado, Boulder and the Stanford University School of Medicine between June 2008 and August 2010. Referrals originated from community practitioners, parent support groups, e-mail advertisements, and inpatient settings. The study was reviewed and approved by the Institutional Review Boards of each university. After a full explanation of the procedures, children and parents read and signed the approved assent and consent forms.
Diagnostic evaluation
Trained MA/MD/PhD level diagnosticians with at least 2 years of clinical experience administered the Washington University Schedule for Affective Disorders and Schizophrenia in Children (WASH-U-KSADS.) (Geller et al. 2001). Board-certified psychiatrists conducted separate evaluations with the youth and at least one parent. Final diagnoses were made based on consensus between research evaluators and study psychiatrists. Details of the diagnostic interview procedures as well as inter-rater reliability among Stanford and Colorado raters are detailed in our earlier publication from this study (Miklowitz et al. 2013).
Eligibility criteria included the following: English speaking; at least one first-degree relative met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria for BD I or II, based on the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998); the child met DSM-IV-TR criteria for a lifetime diagnosis of BD NOS, MDD, or cyclothymic disorder. To meet the study's diagnostic criteria for BD NOS, participants had to have a distinct period of abnormally elevated, expansive, or irritable mood, plus two (three, if irritable only) DSM-IV-TR symptoms of mania that caused a change in functioning, lasted ≥4 hours in a day, and occurred for a total of four or more days in the child's lifetime. If the main diagnosis was MDD, the youth must have had a full Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association 1994) major depressive episode within the previous 2 years. Cyclothymic disorder required that, in the preceding year, the child had multiple, brief (i.e., 1–2 day) periods of hypomanic, depressive or subsyndromal mixed symptoms, and no more than a 2-month period without mood symptoms, without meeting the DSM-IV criteria for MDD or BD NOS. Participants also had to have had either significant current manic or hypomanic symptoms, with the Young Mania Rating Scale (YMRS) score >11 during the prior week or the Child Depression Rating Scale, Revised (CDRS-R) score >29 over the prior 2 weeks (Young et al. 1978; Poznanski and Mokros 1995).
Baseline pharmacological evaluation and initiation of medications
All patients were evaluated by a study psychiatrist at intake and current medications (if any) reviewed with the patient and family to determine the need for medication initiation or adjustment of existing medications. The decision to initiate medications or alter existing medications was based on symptom severity and impairment in psychosocial/academic functioning. All patients were seen weekly or biweekly until they were clinically stable, and then at least monthly or more frequently when clinical crises arose.
Psychosocial treatments
Participants were randomly assigned to FFT-HR or an education control treatment (for details on randomization procedures and therapy, see Miklowitz et al. 2013).
Ongoing mood assessments
Independent evaluators (IEs) interviewed the youth and at least one parent at baseline (covering the prior 4 months) and again at 4, 8, and 12 months postrandomization. During the visits, IEs recorded complete medication regimens at the time of the assessment.
IEs also rated each week of the previous 4-month interval using the adolescent longitudinal interval follow-up evaluation (A-LIFE) and assigned psychiatric status ratings (PSRs) that reflected the severity of depression, mania, and hypomania symptoms week by week (Keller et al. 1987).
Medication treatment and physician adherence to the algorithm
Throughout the study, pharmacological treatment was monitored by a pharmacotherapy oversight committee (Dr. Chang at Stanford and Dr. Schneck at Colorado). Study psychiatrists also participated in biweekly conference calls to discuss difficult-to-treat patients, decisions to modify treatments, protocol deviations, and handling of adverse events.
Medication choice was based on careful discussion and collaborative decision making between clinicians, patients, and family members, taking into account patient/family preferences, prior response to medications, and family history. Attempts were first made to adjust any existing medications (dosage, time of administration, formulation) to decrease symptoms. New medications were chosen based on diagnosis and symptoms, in accordance with the algorithm.
Physician adherence to the algorithm was rated post-hoc for each 4-month study interval. For each adherence rating, a supervising psychiatrist (C.D.S.) considered the clinical status of the patient at the time of the mood assessment (mania, hypomania, or depression severity according to the A-LIFE), the subject's diagnosis, and compared current medications prescribed by the psychiatrist to those suggested by the algorithm. One of three adherence ratings was then assigned: nonadherent, partially adherent, or fully adherent. Nonadherence was assigned when a patient was prescribed a medication deemed inappropriate for a particular mood state (e.g., prescribing an antidepressant to a patient in a current hypomanic episode) or using a medication not included in the algorithm as monotherapy (e.g., using only a typical antipsychotic). Partial adherence was assigned if a physician used off-protocol medications in addition to protocol medications (e.g., combining a first-generation antipsychotic with a second-generation antipsychotic [SGA]), or used combinations of medications not described in the algorithm (e.g., combining two SGAs). In a previous trial, inter-rater reliability between two expert psychopharmacologists for these guideline adherence ratings was .83 (kappa) (Miklowitz et al. 2014).
Treatment of children diagnosed with MDD and no history of antidepressant-induced mania
Children with MDD and a family history of BD present a treatment dilemma. Whereas antidepressants may provide relief of depressive symptoms, such children may be at increased risk for manic switches or other treatment-emergent adverse effects (Goldsmith et al. 2011). The actual risk of antidepressant-induced mania (AIM) in offspring of bipolar parents is unknown. Rates of AIM in youth with unipolar depression appear to be relatively low (2%) in randomized clinical trials (Cheung et al. 2005). However, Findling et al. (2008) reported that among nine children (ages 7–16) diagnosed with MDD who had at least one parent with BD, five developed manic symptoms or suicidal ideation when treated with paroxetine monotherapy or with the combination of paroxetine and divalproex over a 24-month period (Table 1).
Current AACAP Practice Parameters (American Academy of Child and Adolescent Psychiatry 2007c) recommend SSRIs as first-line pharmacotherapy for youth with depressive symptoms, as SSRIs have yielded relatively good response rates in depressed youth (40%–70%) and have been generally well tolerated. We designated citalopram, escitalopram, sertraline, and bupropion as first-line treatments for youth with MDD and no history of AIM. Escitalopram was added based on expert opinion and based on the relation to its stereoisomer citalopram. Bupropion was chosen based on small, open-label trials in treating adolescent MDD with and without ADHD, and in an effort to provide a non-SSRI alternative in the depression treatment arm (Glod et al. 2003). Fluoxetine was not included out of concern for its long half-life in the event of AIM.
Treatment of children diagnosed with MDD and a history of AIM
Youth with MDD and a history of AIM were considered to be at high risk for another AIM episode and so antidepressants were avoided if at all possible. Treatment for depressed patients with a history of AIM included lamotrigine as first-line, and lithium or quetiapine as second-line treatments. These treatment strategies were largely extrapolated from treatment of children with fully syndromal bipolar depression (Table 1), adult treatment of bipolar depression, or negative pediatric studies that provided safety/tolerability data. For example, in an open-label 8-week study, Chang et al. (2006) showed efficacy of lamotrigine in the treatment of children with bipolar depression.
Second-line treatment comprised quetiapine and lithium. DelBello et al. (2007) conducted a study of 20 high-risk adolescents (ages 12–18) using quetiapine in a single-blind, 12-week prospective study (Table 1). Thirteen of the 15 subjects (87%) who completed the trial were rated as “responders” (CGI ≤2), with a mean quetiapine dose at endpoint of 460 mg/day.
Lithium was also considered second line, with one positive, open-label study in adolescents with BD I (Patel et al. 2006), but one negative study in high-risk depressed children (Geller et al. 1998) (Table 1). The AACAP guidelines for pediatric BD recommend lithium as a treatment for pediatric bipolar depression (Kowatch et al. 2005), based on evidence from adult studies.
Treatment of children diagnosed with BD NOS presenting with manic or mixed symptoms
Patients diagnosed with BD NOS were treated in the same manner as those with fully syndromal BD. This decision was due to the lack of existing empirical evidence of the treatment of youth with BD NOS, and the consideration that such youth are often as ill as those with fully syndromal BD (Birmaher et al. 2009). Selection of medications for subthreshold manic or mixed symptoms was guided by the extensive literature on treating adolescent and pediatric mania (Kowatch et al. 2005).
For children diagnosed with BD NOS and presenting with subthreshold manic or mixed symptoms, first-line treatment included aripiprazole, quetiapine, risperidone, and lithium if there was a family history of a positive lithium response (Grof et al. 2002) (Fig. 1). Quetiapine and risperidone were selected based on their inclusion in the current treatment guidelines for child and adolescent BD I, manic or mixed symptoms without psychosis (Kowatch et al. 2005). Aripiprazole was included as a first-line agent given its 2008 FDA approval for treatment of mania/mixed states in children aged 10–17. Aripiprazole was favored for high-risk children with comorbid ADHD, given the positive trial evidence in bipolar youth with ADHD (Tramontina et al. 2006).
Lithium, divalproex, and lamotrigine were designated as second-line agents. In the AACAP parameters, both lithium and divalproex were designated as first-line agents for mania or mixed states in patients with fully syndromal BD (Kowatch et al. 2005). However, divalproex monotherapy was associated with inconclusive outcomes in two studies of children with BD NOS (Table 1). Lamotrigine was selected based on open-trial data examining its efficacy in 46 pediatric patients (ages 8–18) presenting with mania or hypomania (Pavuluri et al. 2009).
Medications were relegated to third-line options for manic/mixed symptoms if they had adverse side effect profiles or limited evidence of efficacy by trial evidence or clinician experience. These included olanzapine, carbamazepine, oxcarbazepine, and ziprasidone. The inclusion of asenapine and paliperidone was a later modification based solely on clinician experience, and to allow study subjects a possible alternative if other medications failed or were deemed unacceptable.
Treatment of children diagnosed with BD NOS presenting with depressive symptoms
First-line agents for youth diagnosed with BD NOS and depressive symptoms included the same medications and rationale used for children with MDD and a history of AIM, that is, lamotrigine, lithium, or quetiapine.
Asenapine was considered the only second-line agent for the treatment of depressive symptoms in children with BD NOS. It was added to the algorithm halfway through the study, as it did not receive FDA approval for treatment of adult schizophrenia and BD until August, 2009. The selection of asenapine was based purely on clinical experience of the study psychiatrists. Because of the concerns about AIM, antidepressants were not included in the treatment arm for depressed BD NOS subjects.
Treatment of children with comorbid ADHD
Treatment of comorbid ADHD in patients at risk for BD—especially using psychostimulants—understandably raises concern for stimulant-induced mania (SIM), mood dysregulation, or both (Goldsmith et al. 2011). Treatment of ADHD was based primarily on AACAP (American Academy of Child and Adolescent Psychiatry 2007b) and the American Academy of Pediatrics guidelines (Subcommittee on Attention-Deficit/Hyperactivity Disorder Steering Committee on Quality Improvement 2011), as well as the treatment guidelines for children and adolescents with BD (Kowatch et al. 2005). Treatment of youth with stimulants may be associated with increased risk for BD (DelBello et al. 2001), although some studies suggest that stimulant exposure does not seem instrumental in the development of BD, even in patients at risk for BD or when symptoms of mania are present (Goldsmith et al. 2011).
For patients with MDD who were not treated with an antidepressant and without a history of AIM, first-line treatment was bupropion due to the potential for treating both symptoms of depression and ADHD (American Academy of Child and Adolescent Psychiatry 2007b). If patients were already taking an antidepressant, first-line treatment was stimulants (either methylphenidate or mixed amphetamine salts), and second-line treatment was atomoxetine. For patients diagnosed with BD NOS, assuming adequate stabilization of mood symptoms, first-line treatment was stimulants (methylphenidate or mixed amphetamine salts), second-line was guanfacine, and third-line was atomoxetine.
Treatment of comorbid anxiety disorder
No studies to date have examined pharmacological treatment of anxiety disorders in children at risk for BD or in those who have been diagnosed with fully syndromal BD. Pharmacological choices were largely based on AACAP practice parameters for treatment of children with anxiety disorders without BD (American Academy of Child and Adolescent Psychiatry 2007a).
First-line treatment for children with no history of AIM comprised the SSRIs citalopram, escitalopram, sertraline, or fluvoxamine. Second-line agents included clonazepam or gabapentin, and were based largely on adult data and the likely decreased risk for invoking cycling or switches. Gabapentin was chosen from its limited evidence of improvement in adults (Pande et al. 2000; Urbano et al. 2009) and from clinical experience among the study psychiatrists.
Results
Sample composition
The mean age ± SD of the 40 participants was 12.7 ± 2.9 years (Table 2); 17 (42.5%) were female. Participants were most frequently diagnosed with BD-NOS (n = 20, 50%) or MDD (n = 17, 42.5%); 3 (7.5%) had cyclothymic disorder. Ten subjects (25%) had comorbid ADHD, 2 subjects (5%) had an anxiety disorder, and 14 (35%) had both anxiety and ADHD. Although there was variability in clinical status in the month before randomization, no participant met criteria for a DSM-IV-TR manic or mixed episode. One patient did not have PSR and was dropped from further analyses.
Table 2.
Demographics, Illness History, and Medication Variables at Study Entry for 40 Subjects
| Variable | Subjects (N) |
|---|---|
| Age, years, mean ± SD | 12.7 ± 2.88 |
| Female sex, n (%) | 17 (42.5) |
| Race, non-white | 4 (10.0) |
| Psychotherapy n (%) | |
| FFT-HR | 21 (52.5) |
| Education control | 19 (47.5) |
| Primary diagnosis, n (%) | |
| Major depression | 17 (42.5) |
| BD NOS | 20 (50.0) |
| Cyclothymia | 3 (7.5) |
| Comorbid disorders, any, n (%) | 26 (65.0) |
| Anxiety | 2 (5.0) |
| MDD | 2 (5.0) |
| BD NOS | 0 (0) |
| Cyclothymia | 0 (0) |
| ADHD | 10 (25.0) |
| MDD | 4 (10.0) |
| BD NOS | 6 (15.0) |
| Cyclothymia | 0 (0) |
| ADHD + Anxiety | 14 (35.0) |
| MDD | 4 (10.0) |
| BD NOS | 8 (20.0) |
| Cyclothymia | 2 (5.0) |
| Index mood episode, n (%)a | |
| Depression | 21 (52.5) |
| Hypomania | 3 (7.5) |
| Mixed, subsyndromal | 8 (20.0) |
| Remitted | 6 (15.0) |
| Missing | 1 (2.5) |
| Any psychiatric medication at study entry, n (%) | 25 (62.5) |
| Mood stabilizers | 9 (22.5) |
| Atypical antipsychotics | 17 (42.5) |
| Antidepressants | 7 (17.5) |
| Psychostimulants | 12 (30.0) |
| Antianxiety medication | 2 (5.0) |
ADHD, attention-deficit/hyperactivity disorder; BD NOS, bipolar disorder not otherwise specified; FFT-HR, family-focused therapy for high-risk youth; MDD, major depressive disorder.
Based on weekly adolescent longitudinal interval follow-up evaluation scores, for the 4 weeks before random assignment. Youth with syndromal mood episodes had at least 2 weeks (for depression) or 1 week for hypomania. Youth in remission had four continuous weeks with minimal mood symptoms.
Medications during the course of the study
At study start, 25 of 40 patients (62.5%) were taking psychiatric medications. During the course of the study, a total of 30 patients (75%) received medication sessions from study psychiatrists (mean 6.27 ± 3.34 visits, range 2–14). In these 30 patients, there were a total of 87 visits, where medication data were recorded.
Of the 15 patients who did not take medications at study start, 7 patients began taking medications at some point during the study, whereas 8 patients (20%) remained off medications for the entire study (Table 3 lists medication use by diagnostic group). Three patients began the study while taking medications, but had them discontinued during the study. Of the 25 patients taking medications at study intake, study psychiatrists modified the regimens of 22 (88%) during the 12-month study. At the beginning of the study, 25 of the 40 participants (62.5%) were taking at least one psychiatric medication. At 1 year, nearly an identical percentage of patients were taking medications (22 of 35, or 63%; 5 had no medication follow-up data).
Table 3.
Medications and Medication Combinations Used During the 12 Months of the Study, by Diagnostic Group
| Medication(s) | BD NOS (n = 20) | MDD (n = 17) | Cyclothymia (n = 3) | Total (n = 40) |
|---|---|---|---|---|
| Never on medication | 4 | 3 | 1 | 8 |
| Antidepressant only | 0 | 4 | 0 | 4 |
| Stimulant only | 2 | 1 | 1 | 4 |
| Antidepressant + other medication* | 4 | 7 | 0 | 11 |
| One SGA | 12 | 5 | 1 | 18 |
| Two SGAs | 4 | 2 | 1 | 7 |
| One mood stabilizer | 7 | 6 | 0 | 13 |
| Two mood stabilizers | 0 | 0 | 1 | 1 |
| Mood stabilizer + SGA | 6 | 4 | 0 | 10 |
| Three or more medications | 6 | 5 | 0 | 11 |
| No. of medication changes, mean ± SD | 2.27 ± 1.6 | 2.27 +/1.9 | — |
Antidepressants included sertraline, citalopram, escitalopram, or bupropion. Stimulants included methylphenidate or mixed amphetamine salts. SGAs included aripiprazole, quetiapine, risperidone, and ziprasidone. Mood stabilizers included carbamazepine, divalproex, lamotrigine, and lithium. Anxiolytics included clonazepam or gabapentin.
Includes, mood stabilizers, SGAs, stimulants.
BD NOS, bipolar disorder not otherwise specified; MDD, major depressive disorder; SGA, second-generation antipsychotic.
The number of medication changes did not vary by diagnostic group (Table 3). Patients with BD NOS had a mean of 2.27 ± 1.6 medication changes during the study, whereas patients with MDD had a mean of 2.27 ± 1.9 changes. Four patients with MDD took only antidepressants during the study and none experienced AIM. Two patients with BD NOS, one with MDD, and one with cyclothymia took only psychostimulants and had no instances of SIM. Changes in prescriptions for mood stabilizers, SGAs, psychostimulants, or antidepressants over the study year were unrelated to the assigned psychotherapy condition (FFT-HR vs. education control; all ps > 0.10).
Combination strategies were frequently employed. Medication combination strategies were used at 55 of the 87 visits (63.2%), in which medications could be tabulated. Combination strategies often included the use of a mood stabilizer and a SGA, two SGAs, or antidepressants combined with mood stabilizers and/or SGAs (Table 4). By drug class, antipsychotics were the most prescribed medication (77.0% of visits), followed by stimulants (47.1%), mood stabilizers (44.8%), antidepressants (27.6%), and finally anxiolytics (4%).
Table 4.
Frequency and Type of Monotherapy or Combination Therapy Prescribed for 40 High-Risk Youths Over 12 Months (n = 87 Visits)
| Visits where prescribed, n (%) | |
|---|---|
| Medication monotherapy/combinations | |
| Antidepressant only | 7 (8.0) |
| Stimulant only | 14 (16.1) |
| Mood stabilizer or SGA monotherapya | 11 (12.6) |
| SGA + either SGA or mood stabilizera | 17 (19.5) |
| Stimulant + either SGA or mood stabilizer | 14 (16.1) |
| Antidepressant + either SGA or mood stabilizer | 9 (10.3) |
| Stimulant + ATD + either mood stabilizer or SGA | 10 (11.5) |
| Other combinationb | 5 (5.7) |
| Medication | |
| Antipsychotics | 67 (77.0) |
| Aripiprazole | 37 (42.5) |
| Quetiapine | 14 (16.1) |
| Risperidone | 9 (10.3) |
| Typical | 4 (4.6) |
| Ziprasidone | 3 (3.4) |
| Stimulants | 41 (47.1) |
| Mood stabilizers | 39 (44.8) |
| Lithium | 15 (17.2) |
| Lamotrigine | 12 (13.8) |
| Divalproex | 9 (10.3) |
| Carbamazepine | 3 (3.4) |
| Antidepressants | 24 (27.6) |
| Anxiolytics | 4 (4.6) |
Stimulants included methylphenidate or mixed amphetamine salts. Antidepressants included sertraline, citalopram, escitalopram, or bupropion. Anxiolytics included clonazepam or gabapentin.
Includes patients taking a typical antipsychotic.
Includes patients taking anxiolytic combination therapies; one patient was prescribed off-protocol topiramate.
ATD, antidepressant; SGA, second-generation antipsychotic.
Treatment of comorbid conditions
Patients with comorbid ADHD were commonly treated with medications. Thirteen of 24 patients (54.2%) diagnosed with ADHD were treated with stimulants (7 with BD NOS, 5 with MDD, and 1 with cyclothymia). In patients with comorbid anxiety disorders, 5 of 16 patients (31%) were treated with antidepressants (2 with MDD, 1 with BD NOS, and 2 with cyclothymia). There were no instances of stimulant-induced or antidepressant-induced manias in any of the diagnostic groups.
Physician adherence to the algorithm
Physician adherence to the treatment algorithm was high. Of 118 pharmacotherapy visits (including visits in which patients were not taking medications), 100 (84.7%) were rated as highly adherent, 16 (13.6%) as partially adherent, and 2 (1.7%) as nonadherent. Partial adherence was most commonly assigned when two SGAs or two mood stabilizers were combined. Other cases of partial adherence included continued use of an antidepressant in combination with a mood stabilizer for a patient with continuing hypomanic symptoms, and in another use of a first-generation antipsychotic in combination with protocol medications. Nonadherence included one patient with BD NOS for whom the physician prescribed antidepressant monotherapy and one in whom a first-generation antipsychotic was used as monotherapy. Physicians did not differ in guideline adherence when treating patients in FFT-HR versus the education control.
Discussion
This article describes the development and implementation of a medication algorithm to treat children and adolescents who are at high risk for bipolar illness. Little empirical evidence exists to guide the treatment of high-risk youth or adults. Our medication algorithm used in this study is an attempt to operationalize treatment decisions based on diagnosis and current mood state and derived from the extant literature and expert opinion. It was intended as an initial effort to shape the development of future clinical guidelines.
Physicians maintained a high degree of concordance with the treatment algorithms (84.7%). The apparent adaptability and acceptability of the algorithm by clinicians may be the result of the several medication choices that were built in to each level. Giving patients and physicians a choice between several SSRIs and bupropion, for example, provided flexibility and an ability to accommodate patient, family, or physician opinion. When partial adherence to the algorithm was observed, the most common reason given was, patients wanting to continue medications prescribed before study entry, but that were not part of the algorithm (e.g., use of topiramate for mood symptoms, or using low-dose SGAs for sleep in combination with more robust dosing of another antipsychotic). It remains to be seen whether a similar degree of guideline adherence would be maintained by physicians working outside the context of a research trial. For example, we would expect lower rates of adherence in community settings, where patients may be more diagnostically complex and/or less reliable in taking medications or keeping appointments. Regardless, the high adherence rate may indicate that the algorithm was well understood by physicians and reasonable in its choices of medications. Of note, we did not measure patient adherence to the medication regimens, and so are unable to gauge how truly acceptable such an algorithm was on the patient level.
Physicians employed combination medication strategies frequently. Two or more medications were used in at least 63% of visits, where often two SGAs, two mood stabilizers or an antidepressant and a mood stabilizer, were combined. The frequency of combination medication use was similar to adults with fully syndromal BD, in which 68% of patients take two or more medications (Goldberg et al. 2009). Prescribers favored SGAs (77%) over mood stabilizers (44.8%). Not surprisingly, stimulants were the second most commonly prescribed class of medications, given that 60% of subjects had comorbid ADHD. Finally, pharmacological management was not necessarily simpler in subjects diagnosed with MDD, as there was an equal number of medication changes as the BD NOS group. Thus, youth at high risk for BD may require combination therapy and frequent monitoring similar to youth with fully syndromal BD.
No instances of medication-induced mania were observed in the four study patients with MDD who were on antidepressant monotherapy or the four patients receiving stimulant monotherapy (two with BD NOS, one with MDD, and one with cyclothymia). We recognize that 12 months may not be long enough to observe antidepressant- or stimulant-induced mood cycling. In addition, physicians may have factored in variables other than a history of AIM in deciding whether to recommend antidepressant monotherapy. Moreover, the presence of adjunctive mood stabilizers or SGAs may have been protective against switches. Nonetheless, the absence of AIM or similar events is encouraging and further supports the use of this algorithm in vulnerable children. Further study in this important area is presently ongoing in high-risk youth (www.clinicaltrials.gov NCT02553161).
It is notable that 8 of 40 patients (20%; 4 with BD NOS, 3 with MDD, and 1 with cyclothymia) did not take medications during the entirety of the study. This raises the question whether or not psychotherapy may be sufficient to treat children at risk for BD, and would avoid the potential complications of medication therapies. Larger, randomized studies will be needed to better determine the optimal treatment of high-risk youth.
We did not evaluate whether use of this algorithm reduced future risk of bipolar conversion in high-risk patients. Conversion risk reduction might occur by avoiding medications that could potentially accelerate conversion (antidepressants or stimulants, for example), or by using mood stabilizers early in the development of mood symptoms. Clinicians and parents were understandably most concerned about treating the acute symptoms of depression, hypomania, irritability, inattention, or suicidality, as opposed to actively trying to mitigate future risk of conversion by potentially avoiding particular medications. Of note, however, guideline-driven or algorithmic-driven care has been shown to improve patient outcomes in some populations and settings. For example, Katon et al. (1995) found that patients who received algorithm-driven care for depression had higher response rates than patients who received usual care (74% vs. 44%).
Limitations of this study include the nonrandomized nature of medication management, the lack of a comparator medication strategy, and the potential confounding of medications with psychosocial treatment effects. It is possible that some high-risk youth, such as those with cyclothymia or milder forms of BD-NOS, can be stabilized without pharmacological intervention. We did not have the necessary sample sizes to determine whether features of participants' family history of BD–such as the parents' early illness onset or adolescence or whether parents responded to specific classes of medications–were important predictors of effective medication strategies for the high-risk offspring. It is also unclear whether creating a medication decision tree based on categorical diagnoses (i.e., MDD vs. BD NOS vs. cyclothymia) is more effective than one based on dimensional ratings of symptoms (such as the presence of psychosis, insomnia, or suicidal ideation).
Future research should evaluate the relationship between pharmacological strategies and the trajectory of common and specific symptom domains, such as sleep disruption, anxiety, or mood instability. Controlled studies of comparative medications in this population are clearly needed. Other future studies might establish whether algorithm-driven pharmacological treatment hastens and sustain remission from mood episodes in high-risk youth, and whether these acute effects translate into preventing or delaying the onset of the full bipolar syndrome.
Acknowledgments
L. Miriam Dickinson, PhD served as the statistical expert for this research. The authors thank David Axelson, MD at Nationwide Children's Hospital, Columbus, Ohio and Judy Garber, PhD, of Vanderbilt University, for their consultation on study design; Julia Maximon, MD, Kathleen Kovner-Kline, MD, Kimberly Kelsay, MD, and Marianne Wamboldt, MD, of the University of Colorado School of Medicine, Department of Psychiatry for serving as study physicians; Elizabeth L. George, PhD, Aimee Sullivan, PhD, Dawn Taylor, PhD, Victoria E. Cosgrove, PhD, Dana Elkun, MA, Christopher Hawkey, PhD, Jedediah Bopp, PhD, Jessica Lunsford-Avery, PhD, and Zachary Millman of the University of Colorado–Boulder for serving as study therapists and research assistants; Meghan E. Howe, MA for serving as study therapist, and Tenah Acquaye, Erica Marie Sanders, and Erica Weitz of the Stanford University School of Medicine for serving as research assistants.
Conclusions and Clinical Significance
Little evidence exists to guide the treatment of youth who are at high risk for bipolar disorder, that is, for youth with depressive or hypomanic symptoms and at least one first- or second-degree relative with bipolar I or II disorder. We created a pharmacologic treatment algorithm for high risk youth in an attempt to operationalize treatment decisions based on diagnosis (i.e. major depressive disorder or unspecified bipolar disorder) and current mood state. In a study of 40 youth at high risk for bipolar disorder, physicians were able to adhere well to the algorithm. This article is an initial effort to shape the development of future guidelines.
Disclosures
Dr. Schneck has received research support from the National Institute of Mental Health and the Crown Family Foundation. Dr. Chang is an unpaid consultant for GlaxoSmithKline, Lilly, and Bristol Myers Squibb. He is on the data safety monitoring board for Sunovion. In the past 3 years, he has received research support from GlaxoSmithKline and Merck, and has been a consultant for Actavis and Janssen. Dr. Singh receives research support from the National Institute of Mental Health, the Office of Research in Women's Health, Brain and Behavior Research Foundation, Janssen, Neuronetics, and the Stanford Child Health Research Institute. Dr. Delbello has received research support from Otsuka, Lundbeck, Purdue, Sunovion, Pfizer, Johnson and Johnson, Supernus, Amarex, and AssureRx. She has also served as a consultant or on an advisory board, or received honoraria from Pfizer, Lundbeck, Sunovion, Supernus, Takeda, Johnson and Johnson, Neuronetics, and Akili. Dr. Miklowitz has received research support from the NIMH, American Foundation of Suicide Prevention, Brain and Behavior Research Foundation, Carl and Roberta Deutsch Foundation, Kayne Family Foundation, Knapp Family Foundation, Attias Family Foundation, Danny Alberts Foundation, and Max Grey Foundation; and book royalties from John Wiley and Sons and Guilford Press.
References
- American Academy of Child and Adolescent Psychiatry: Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 46:267–283, 2007a [DOI] [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 46:894–921, 2007b [DOI] [PubMed] [Google Scholar]
- American Academy of Child and Adolescent Psychiatry: Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 46:1503–1526, 2007c [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M: Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 63:1139–1148, 2006 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Lyengar S, Kim E, Yen S: Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry 166:795–804, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Saxena K, Howe M: An open-label study of lamotrigine adjunct or monotherapy for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry 45:298–304, 2006 [DOI] [PubMed] [Google Scholar]
- Chang KD, Dienes K, Blasey C, Adleman N, Ketter T, Steiner H: Divalproex monotherapy in the treatment of bipolar offspring with mood and behavioral disorders and at least mild affective symptoms. J Clin Psychiatry 64:936–942, 2003 [DOI] [PubMed] [Google Scholar]
- Cheung AH, Emslie GJ, Mayes TL: Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry 46:735–754, 2005 [DOI] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Whitsel RM, Stanford KE, Strakowski SM: A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry 68:789–795, 2007 [DOI] [PubMed] [Google Scholar]
- DelBello MP, Chang K, Welge JA, Adler CM, Rana M, Howe M, Bryan H, Vogel D, Sampang S, Delgado SV, Sorter M, Strakowski SM: A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord 11:483–493, 2009 [DOI] [PubMed] [Google Scholar]
- DelBello MP, Soutullo CA, Hendricks W, Niemeier RT, McElroy SL, Strakowski SM: Prior stimulant treatment in adolescents with bipolar disorder: Association with age at onset. Bipolar Disord 3:53–57, 2001 [DOI] [PubMed] [Google Scholar]
- Fiedorowicz JG, Endicott J, Leon AC, Solomon DA, Keller MB, Coryell WH: Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 168:40–48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Frazier TW, Youngstrom EA, McNamara NK, Stansbrey RJ, Gracious BL, Reed MD, Demeter CA, Calabrese JR: Double-blind, placebo-controlled trial of divalproex monotherapy in the treatment of symptomatic youth at high risk for developing bipolar disorder. J Clin Psychiatry 68:781–788, 2007 [DOI] [PubMed] [Google Scholar]
- Findling RL, Lingler J, Rowles BM, McNamara NK, Calabrese JR: A pilot pharmacotherapy trial for depressed youths at high genetic risk for bipolarity. J Child Adolesc Psychopharmacol 18:615–621, 2008 [DOI] [PubMed] [Google Scholar]
- Geller B, Cooper TB, Zimerman B, Frazier J, Williams M, Heath J, Warner K: Lithium for prepubertal depressed children with family history predictors of future bipolarity: A double-blind, placebo-controlled study. J Affect Disord 51:165–175, 1998 [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C: Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry 40:450–455, 2001 [DOI] [PubMed] [Google Scholar]
- Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B: Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 159:927–933, 2002 [DOI] [PubMed] [Google Scholar]
- Glod CA, Lynch A, Flynn E, Berkowitz C, Baldessarini RJ: Open trial of bupropion SR in adolescent major depression. J Child Adolesc Psychiatr Nurs 16:123–130, 2003 [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Brooks JO, 3rd, Kurita K, Hoblyn JC, Ghaemi SN, Perlis RH, Miklowitz DJ, Ketter TA, Sachs GS, Thase ME: Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: Findings from the STEP-BD. J Clin Psychiatry 70:155–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M, Singh M, Chang K: Antidepressants and psychostimulants in pediatric populations: Isthere an association with mania? Paediatr Drugs 13:225–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O'Donovan C, Alda M: Is response to prophylactic lithium a familial trait? J Clin Psychiatry 63:942–947, 2002 [DOI] [PubMed] [Google Scholar]
- Hafeman DM, Merranko J, Axelson D, Goldstein BI, Goldstein T, Monk K, Hickey MB, Sakolsky D, Diler R, Lyengar S, Brent D, Kupfer K, Birmaher B: Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry 173:695–704, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, Robinson P, Russo J: Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA 273:1026–1031, 1995 [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC: The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 44:540–548, 1987 [DOI] [PubMed] [Google Scholar]
- Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M; Child Psychiatric Workdgroup on Bipolar Disorders. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 44:213–235, 2005 [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Chang KD, Taylor DO, George EL, Singh MK, Schneck CD, Dickinson LM, Howe ME, Garber J: Early psychosocial intervention for youth at risk for bipolar I or II disorder: A one-year treatment development trial. Bipolar Disord 13:67–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, George EL, Taylor DO, Sugar CA, Birmaher B, Kowatch RA, DelBello MP, Axelson DA: Pharmacotherapy and family-focused treatment for adolescents with bipolar I and II disorders: A 2-year randomized trial. Am J Psychiatry 171:658–667, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Schneck CD, Singh MK, Taylor DO, George EL, Cosgrove VE, Howe ME, Dickinson LM, Garber J, Chang KD: Early intervention for symptomatic youth at risk for bipolar disorder: A randomized trial of family-focused therapy. J Am Acad Child Adolesc Psychiatry 52:121–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Frank E: Subthreshold bipolarity: Diagnostic issues and challenges. Bipolar Disord 13:587–603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, Taylor CB, Dager SR, Shiovitz T: Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 20:467–471, 2000 [DOI] [PubMed] [Google Scholar]
- Patel NC, DelBello MP, Bryan HS, Adler CM, Kowatch RA, Stanford K, Strakowski SM: Open-label lithium for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry 45:289–297, 2006 [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Henry DB, Moss M, Mohammed T, Carbray JA, Sweeney JA: Effectiveness of lamotrigine in maintaining symptom control in pediatric bipolar disorder. J Child Adolesc Psychopharmacol 19:75–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Leverich GS, Kupka RW, Keck PE, Altshuler LL, Frye MA, Luckenbaught DA, Rowe M, Grunze H, Suppes T, Nolen WA: Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry 71:864–872, 2010 [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB: Children's Depression Rating Scale, Revised (CDRS-R) Manual. Los Angeles, CA, Western Psychological Services, 1995 [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20:22–33, 1998 [PubMed] [Google Scholar]
- Subcommittee on Attention-Deficit/Hyperactivity D, Steering Committee on Quality Improvement and Management. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontina S, Zeni CP, Ketzer CR, Pheula GF, Narvaez J, Rohde LA: Aripiprazole in children and adolescents with bipolar disorder comorbid with attention-deficit/hyperactivity disorder: A pilot randomized clinical trial. J Clin Psychiatry 70:756–764, 2006 [DOI] [PubMed] [Google Scholar]
- Urbano MR, Spiegel DR, Laguerta N, Shrader CJ, Rowe DF, Hategan LF: Gabapentin and tiagabine for social anxiety: A randomized, double-blind, crossover study of 8 adults. Prim Care Companion J Clin Psychiatry 11:123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter AR, Burke C, Youngstrom EA, Faedda GL, Correll CU: The bipolar prodrome: Meta-analysis of symptom prevalence prior to initial or recurrent mood episodes. J Am Acad Child Adolesc Psychiatry 55:543–555, 2016 [DOI] [PubMed] [Google Scholar]
- Van Meter AR, Moreira AL, Youngstrom EA: Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry 72:1250–1256, 2011 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry 133:429–435, 1978 [DOI] [PubMed] [Google Scholar]