Abstract
Animal models are vital tools for the preclinical development and testing of therapies aimed at providing solutions for several musculoskeletal disorders. For bone tissue engineering strategies addressing nonunion conditions, rodent models are particularly useful for studying bone healing in a controlled environment. The mouse calvarial defect model permits evaluation of drug, growth factor, or cell transplantation efficacy, together with offering the benefit of utilizing genetic models to study intramembranous bone formation within defect sites. In this study, we describe a detailed methodology for creating calvarial defects in mouse and present our results on bone morphogenetic protein-2-loaded fibrin scaffolds, thus advocating the utility of this functional orthotopic mouse model for the evaluation of therapeutic interventions (such as growth factors or cells) intended for successful bone regeneration therapies.
Keywords: : calvaria, animal model, bone regeneration, mouse/mice, calvarial defect
Introduction
Reconstruction of cranial defects to restore the calvarium following trauma or a pathological lesion requires surgical intervention with the use of autografts or synthetic bone replacement materials. Large-scale calvarial defect models could be utilized to assess bone regenerative capacities of osteoinductive or conductive biomaterials, progenitor cells, growth factors, and epigenetic drugs. The calvarial defect model offers a major advantage of studying bone healing in an orthotopic and clinically relevant setting and complements subcutaneous or intramuscular ectopic bone formation models.1–4 Most importantly, the use of the calvarial model circumvents the need for defect stabilization, which is a major challenge in long bone critical-sized nonunion fracture simulation models.5–7
Several investigations have been conducted on calvarial healing in rats with defect sizes measuring 8 mm in diameter,8–12 including a well-detailed method by Spicer et al.12 However, there is a scarcity of descriptive and methodical reports on smaller-sized calvarial defects in mice.13 Mouse models offer cost advantages and, notably, permit relatively convenient breeding methods to generate transgenic study groups of any desired genotype or phenotype. In this protocol, we explicate the creation of a 2.5 mm diameter circular defect on the surface of exposed mouse calvarium of 12–14-week-old animals using a dental trephine and dental drill. Based on key investigations by Cooper et al.13 and Cowan et al.,14 there is no spontaneous complete healing of circular defects sized 1.8 and 2 mm after 6 and 12 weeks, respectively. Therefore, we chose 2.5 mm diameter as our choice of defect size.
We tested this model using bone morphogenetic protein-2 (BMP-2) loaded on a fibrin scaffold and measured bone formation 4 weeks after surgery. We used BMP-2 because it is a potent osteoinductive morphogen that is routinely used in preclinical animal models as a positive control for in vivo bone formation. Our results from radiographic, micro-computed tomography (micro-CT), and histological analyses of vehicle and BMP-2 treatment groups verified the utility of this calvarial model for studying bone regeneration. While the calvarial defect model offers several advantages over other fracture models that require stabilization, it must be noted that this model does not fully allow for evaluation of mechanical properties of biomaterials because the bone healing site is not load bearing. In summary, this protocol provides a well-described and versatile approach to examine intramembranous bone formation in a clinically relevant bone regeneration model useful for preclinical testing of novel therapies for bone tissue engineering through a rapid and cost-effective system.
Materials Required
1. Animal species
Skeletally mature mice. (This protocol used inbred C57bl/6J mice, aged 12–14 weeks, male, Jackson Laboratories, cat. no. 000664)
2. Equipment
i. Rodent Anesthesia Inhalation System (VetEquip, cat. no. 901801).
ii. Handpiece short 2-speed motor (Mid West, cat. no. 710024D).
iii. Contra angle sheath shorty (Mid West, cat. no. 71004).
iv. Push button latch type head (Mid West, cat. no. 720485).
v. Horizontal mount manual control (DCI, cat. no. 4401).
vi. X-ray machine- Faxitron Laboratory Radiography System, Model LX-60 (Faxitron).
vii. Micro-CT scanner: SkyScan 1272 (Bruker).
viii. Sterile surgical gown, sterile gloves, mask, and sterile head cover (Kimberly-Clark Professional).
3. Reagents and surgical materials
ix. Analgesic: Rimadyl/Carprofen (Zoetis, NADA# 141-199); Dose: 5 mg/kg (injectable Carprofen 50 mg/mL) in drinking water; 0.025 mg/mL Final Carprofen concentration in drinking water.
x. Fibrin TISSEEL sealant 4 mL (Baxter, cat. no. 1504515).
xi. Phosphate buffered saline (Gibco, cat. no. 10010-023).
xii. BMP-2 (R&D Systems, cat. no. 355-BM).
xiii. Sterile diluent, 0.9% normal saline injection, USP (Hospira, NDC 0409-4888-02).
xiv. Antiseptic: Povidone-iodine solution (Aplicare, item no. 82–226, NDC 52380-1905-6).
xv. Ethanol (Brenntag Great Lakes, cat. no. 693509).
xvi. Isoflurane (Cardinal Health, cat. no. 4985248).
xvii. Eye ointment, Puralube® Vet Ointment (Dechra, NDC 17033-211-38).
xviii. Tuberculin syringe 0.5 cc (Becton Dickinson, cat. no. 305620).
xix. Buprenorphine hydrochloride, 0.3 mg/mL (NDC 42023-179-05).
xx. Sterile gauze sponge (Curity Covidien™, cat. no. 397110).
xxi. Scalpel, disposable No. 11 (Feather®, cat. no. 2975#11).
xxii. Sutures, 7-0 Coated VICRYL (Ethicon, cat. no. J546G).
xxiii. 2.5 mm diameter trephine (Hager & Meisinger GmbH, cat. no. 229 RAL 025).
xxiv. Acepromazine maleate injection, USP, 10 mg/mL (Phoenix™, cat. no. 382705-01, NDC 57319-604-04).
xxv. Ketamine HCl injection, USP, 100 mg/mL (Ketalar®, NDC 42023-115-10).
xxvi. Xylazine sterile solution, 20 mg/mL, AnaSed® injection (Akorn, Inc., NADA #139-236).
xxvii. Eighteen gauge needle (Becton Dickinson, cat. no. 305195).
xxviii. Twenty-five gauge needle (Becton Dickinson, cat. no. 305122).
xxix. Forceps.
xxx. Needle driver.
xxxi. Scissors.
xxxii. Electric hair trimmer (Philips).
xxxiii. Weighing Scale.
xxxiv. Carbon dioxide, USP Grade.
xxxv. Formalin (Fisher Scientific, cat. no. SF100).
Methods
Surgical procedure
All procedures on mice were conducted upon obtaining approval from Mayo Clinic's Institutional Animal Care and Use Committee and were performed in agreement with the institutional and national regulations for animal handling and use. The key steps of the surgical procedure are shown in a schematic (Fig. 1), and the detailed surgical approach is described below.
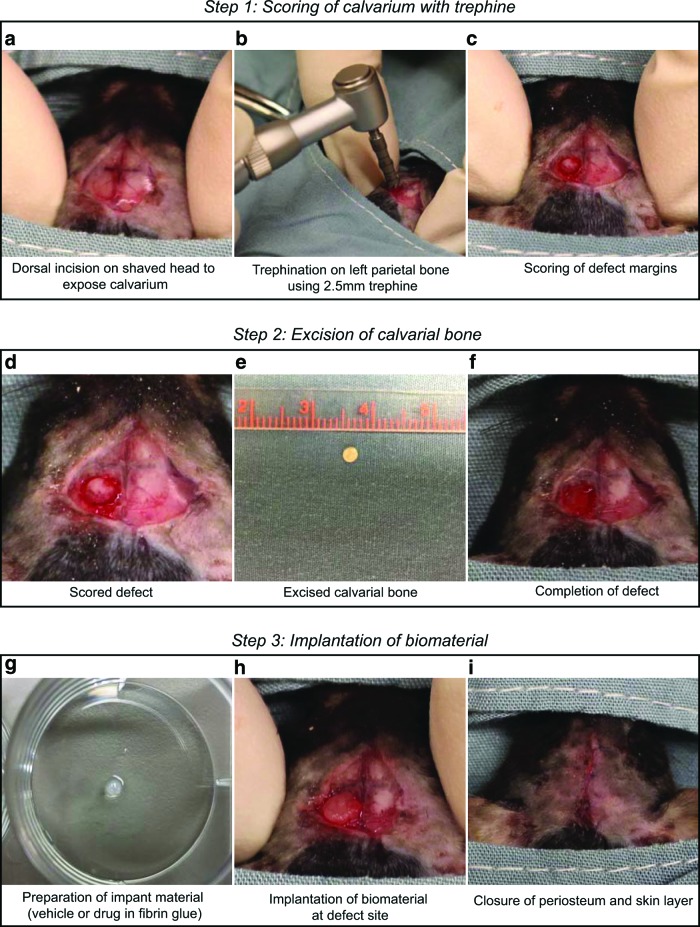
FIG. 1.
The surgical procedure to create a calvarial defect in mice. Schematic showing steps in creating the defect by trephination. Step 1 (a–c) shows incision of the skin and scoring of the defect. Step 2 (d–f) describes the creation and completion of the defect, and Step 3 (g–i) shows implantation of biomaterial and closure of the incision. Color images available online at www.liebertpub.com/tec
Preoperative procedures
Administration of analgesics (24 h before surgery)
Between 24 and 48 h before surgery, add Carprofen (Rimadyl®) to drinking water at a final concentration of 0.025 mg/mL to provide moderate pain relief.
Sterilization
1. Sterilize surgical instruments by autoclaving followed by drying in an oven. Clean, wipe down, and sterilize procedure table using 70% ethanol.
Biomaterial preparation
Note: In this study, we used fibrin sealant (TISSEEL) as the biomaterial of choice to load our drug of interest, BMP-2. Alternatively, other implant materials can be used depending on the nature and objective of the study.
2. Following the manufacturer's instructions provided with the TISSEEL Kit, prepare the individual components for creating the fibrin sealant. Reconstitute freeze-dried sealer protein concentrate with fibrinolysis inhibitor solution; reconstitute thrombin with calcium chloride to prepare thrombin solution. Ensure that solution components are not exposed to temperatures above 37°C. Do not refrigerate or freeze solutions after reconstitution.
3. Prepare test drug or vehicle to a final volume of 5 μL. In our case, we reconstituted BMP-2 in 4 mM HCl at a concentration of 240 μg/mL. Aliquots of this solution (300 ng BMP-2 in 1.25 μL) were buffered to pH ∼7.0 by adding PBS (3.75 μL) before use. Thus, a total amount of 300 ng BMP-2 was delivered to the defect site. We note that osteogenic differentiation of mesenchymal progenitors requires relatively high doses of BMP-2 both in vitro15 and in vivo16,17 based on previous reports and unpublished data from our group. The 300 ng dose we selected in our study for the mouse calvarial drill hole model is in the same range as reported for similar sized defects in rat calvaria (doses ranging from 300 to 600 ng per 5 mm defect in rat).
4. Mix drug or vehicle with 5 μL of thrombin solution. To this, add 5 μL of sealer protein solution to form a fibrin gel encapsulating the drug or vehicle. Clotting takes ∼20–30 s. At this time, the biomaterial is ready for implantation.
Recommended: Prepare drugs and implant material fresh before implantation to ensure maximum drug efficacy.
Administration of anesthesia and analgesics (on the day of surgery)
5. Weigh the animal.
6. Before surgery (>1 h), administer buprenorphine subcutaneously at a dose of 0.2 mg/kg to mitigate pain.
7. Place the animal inside the rodent anesthesia inhalation chamber and administer anesthesia using 2% isoflurane and 2.0 L per min (LPM) of oxygen.
8. Administer intraperitoneal injection of a triple drug anesthetic cocktail at final concentrations of 10 mg/mL ketamine, 1 mg/mL xylazine, and 0.3 mg/mL acepromazine in a final dose volume of 100 μL/10 g body weight of the animal. Instructions for preparing 10 mL of anesthesia cocktail are provided in Table 1.
9. Transfer animal to sterile preoperative preparation bench for shaving.
Table 1.
Anesthesia Cocktail Preparation Chart
| Components | Stock concentration (mg/mL) | Dose (mg/kg) | Final cocktail concentration (mg/mL) | For preparing 10 mL of anesthesia cocktail (mL) |
|---|---|---|---|---|
| Ketamine | 100 | 100 | 10 | 1 |
| Xylazine | 20 | 10 | 1 | 0.5 |
| Acepromazine | 10 | 3 | 0.3 | 0.3 |
| Sterile saline (0.9%) | — | — | — | 8.2 |
Animal preparation
10. Using an electric shaver, remove hair starting from the region between the eyes to the posterior end of the skull.
11. Disinfect shaved region by gentle swabbing with sterile cotton gauze dipped in povidone iodine solution followed by 70% alcohol swabs and sterile normal saline swabs, avoiding contact with the eyes.
12. Apply lubricating eye ointment to prevent dryness of eyes during surgery.
13. Transfer the animal to a heating pad overlaid with sterile drapes. Using sterile keyhole drapes cover the body of the animal to expose only the field of surgical operation.
Operative procedure
14. Using a sterile scalpel, make a deep longitudinal skin incision of about 1.5 cm on the scalp of the mouse starting from the region just behind the eyes down to the midsagittal area of the skull. Pull the skin laterally to scrape off the underlying fascia and to expose the calvarium. Gently push the periosteum layer away from the skull surface (Fig. 1, Step 1a).
15. Manually retract the incised skin and soft tissue using thumb and forefinger (or use a small retractor) and expose the calvarium (Fig. 1, Step 1a). Take care to drip sterile saline at the site where the defect was created to maintain temperature equilibrium before and during drilling.
16. Using a dental trephine attached to a low-speed dental drill (∼1500 rpm) controlled by a manual foot pedal, score the left parietal bone of the calvarium making sure not to drill too deeply (Fig. 1, Step 1b).
Note: The calvarium is not a flat surface but is slightly dome shaped. To efficiently create a clear-cut marking on the skull, use steady precession of the trephine, while taking care not to penetrate the bone tissue.
17. Create the defect margin with the trephine by gently applying pressure (Fig. 1, Step 1c).
18. Withdraw the trephine intermittently to gauge the defect margin and depth of trephination.
19. Upon visualizing the thinning of the calvarium at the defect margin, run a blunt forceps around the defect and finish the defect creation by gently scooping out the trephined bone (Fig. 1, Step 2d, 2e), while taking care not to damage the underlying dura mater.
20. After completing the defect, wash the surgical site with sterile saline (Fig. 1, Step 2f).
21. Place the biomaterial or scaffold into the defect site without applying pressure on the underlying brain (Fig. 1, Step 3g, 3h).
22. Using a needle driver and 7-0 VICRYL® absorbable sutures, close the periosteum and skin tissue with a simple interrupted suture pattern (Fig. 1, Step 3i).
Postoperative care
23. After the surgery, transfer the animal to a clean heating pad to allow recovery from anesthesia. Monitor the animal continuously for 2 h to observe any signs of labored respiration.
24. Once the observation is complete, and the animal starts to show signs of being responsive, transfer the animal to a clean cage, allowing free access to food and water.
25. Subcutaneously administer analgesic (buprenorphine) at 8, 24, and 36 h postsurgery. Carprofen (in drinking water) is provided to the animals for 7 days after surgery.
Euthanasia and tissue harvest
26. Sacrifice mice through carbon dioxide (CO2) inhalation at preferred time points (either 4 or 8 weeks postsurgery). Place the mice inside the euthanizing chamber and turn on CO2 at the rate of 2.0 LPM until mice stop moving and then increase the flow rate to 10.0 LPM until mice stop breathing. Confirm euthanasia by observation followed by secondary euthanasia (e.g., heart puncture or cervical dislocation).
27. Document the weight of the animal at sacrifice.
28. Harvest the head of the animal.
29. Fix the head in 10% formalin solution for 48 h.
30. Transfer to 70% ethanol until further processing for X-rays, micro-CT, and histology.
Methods of Analysis
Micro-CT
31. To evaluate new bone formation at the defect site, perform micro-CT using established parameter settings for bone tissue. In this protocol, 70 kV as source voltage with exposure time of 2375 ms and image pixel size of 16.259 μm was used based on previously established settings for mouse skull analysis reported by our group.18,19 Micro-CT was performed 4 weeks after surgery.
32. Reconstruction was done using the manufacturer's software (DataViewer, NRecon, CTVox, and CTAn) as previously reported18 and corrected for beam hardening, misalignment, as well as ring artifact correction. Postprocessing threshold for bone analyses was defined using CTAn software using lower and upper gray threshold values set to 70 and 255, respectively, based on previously optimized settings for mouse skulls.18,19
2D X-ray radiography
33. Radiography on stored specimens is performed by transferring the specimen from ethanol storage and positioning the skulls on the X-ray imaging platform with the calvarium facing up.
34. Adjust the platform to the desired distance from the irradiation source.
35. After ensuring complete closure of the X-ray chamber, expose the specimen at 25 kV for 10 s. In this method, no digital gain settings were applied.
36. Return the specimen to 70% ethanol storage and proceed for histological processing.
Histological analysis
37. Retrieve samples from 70% ethanol and proceed with decalcification following the recommended methods described for paraffin embedding and sectioning5 or process undecalcified samples for the resin/methyl methacrylate method,20 depending on the histological staining required.
38. Recommended staining for studying bone tissue formation is Gomori's trichrome staining,21 which measures collagen, and the von Kossa/McNeal method,5,22 which stains mineralized tissue. In this study, bone formation was quantified by performing histomorphometric analyses on stained sections using OsteoMeasure software.
Statistics
Statistics was performed with GraphPad Prism 7 software and data represented as mean ± standard deviation with N = 3 animals per group. Student's t-test was performed to determine if groups were significantly different from each other, and a p-value of 0.05 was considered significant (*p < 0.05; **p < 0.001).
Results and Discussion
The procedure described in this study allows evaluation of bone tissue regeneration using several research strategies, including novel osteogenic drug testing, biomaterial function and compatibility, in vivo efficacy of transplanted cells, as well as to understand the basic structural and biological characteristics of bone development. In our studies, we adopted mouse models because they are less expensive than rat models and easier to breed. More importantly, mouse models permit bone healing studies using genetic models that are broadly available, while rat models are more challenging to acquire or generate.
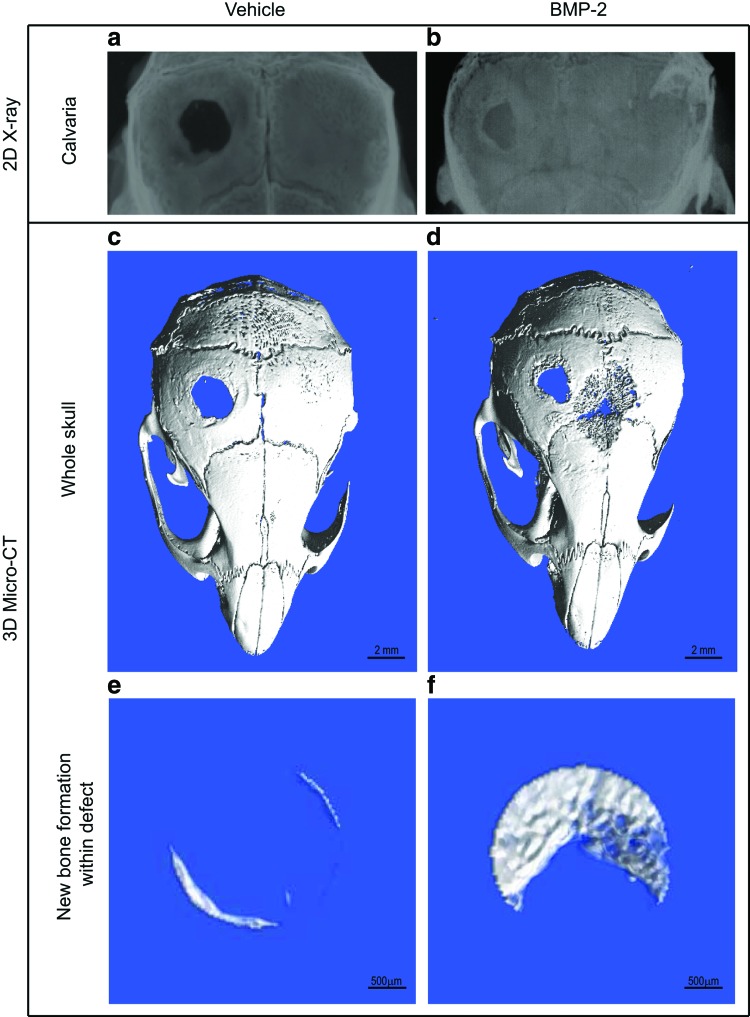
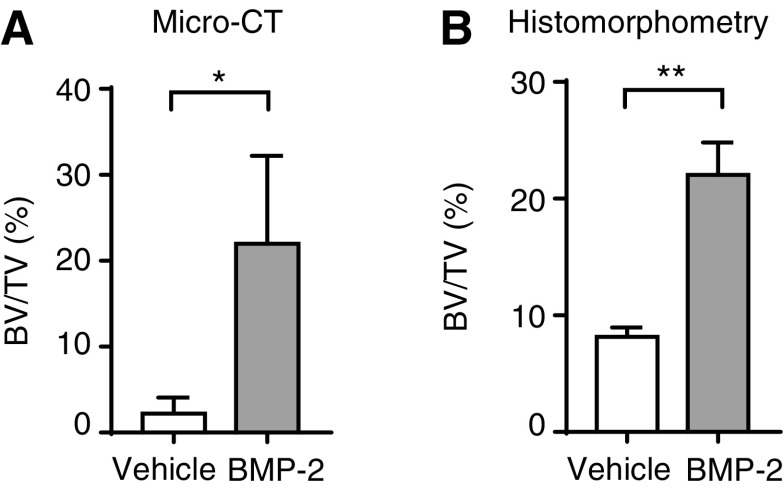
In our protocol, BMP-2 was used as the drug of choice to validate the usefulness of the mouse calvarial defect model, because BMP-2 is a potent bone anabolic agent that is likely to accelerate bone healing. Analysis by 2D radiography (X-rays) and 3D micro-CT (n = 3 mice per group) at 4 weeks posttreatment showed that BMP-2 induced significantly higher new bone formation within the defect site (Fig. 2B, D, and F), compared to vehicle-treated groups (Fig. 2A, C, and E). We performed X-rays because this method permits cost-effective imaging of mineralized tissues at a gross anatomical level of resolution and permits rapid prescreening of calvaria before high resolution analysis using micro-CT, which requires labor-intensive scanning and reconstruction analysis. Micro-CT analysis of whole skulls revealed new bone formation from within the inner edges of the defect (Fig. 2C, D). New bone formed within the defect (region of interest) was quantified and expressed as bone volume over total volume (BV/TV%) and was found to be significantly higher with BMP-2 compared to control (Figs. 2E, F and 4A). BMP-2 administered through fibrin sealant promoted fairly even bone formation based on gross skull observation and micro-CT measurements. No heterotopic ossification was observed during sample harvest.
FIG. 2.
Analysis of new bone formation within defect site at 4 weeks postsurgery. The 2.5 mm diameter calvarial defect was created in the left parietal bone and the defect treated with vehicle or BMP-2 loaded in fibrin scaffolds. Four weeks posttreatment, X-ray analyses (a, b) of mouse skulls showed new bone filling the defect site with BMP-2 treatment (b) compared to little or no new bone formation with vehicle (a). Micro-CT reconstruction of the calvaria showed significantly higher bone formation with BMP-2 (d, f) compared to the vehicle-treated group (c, e). Scale bar: 2 mm (c, d); 500 μm (e, f). BMP-2, bone morphogenetic protein-2. Color images available online at www.liebertpub.com/tec
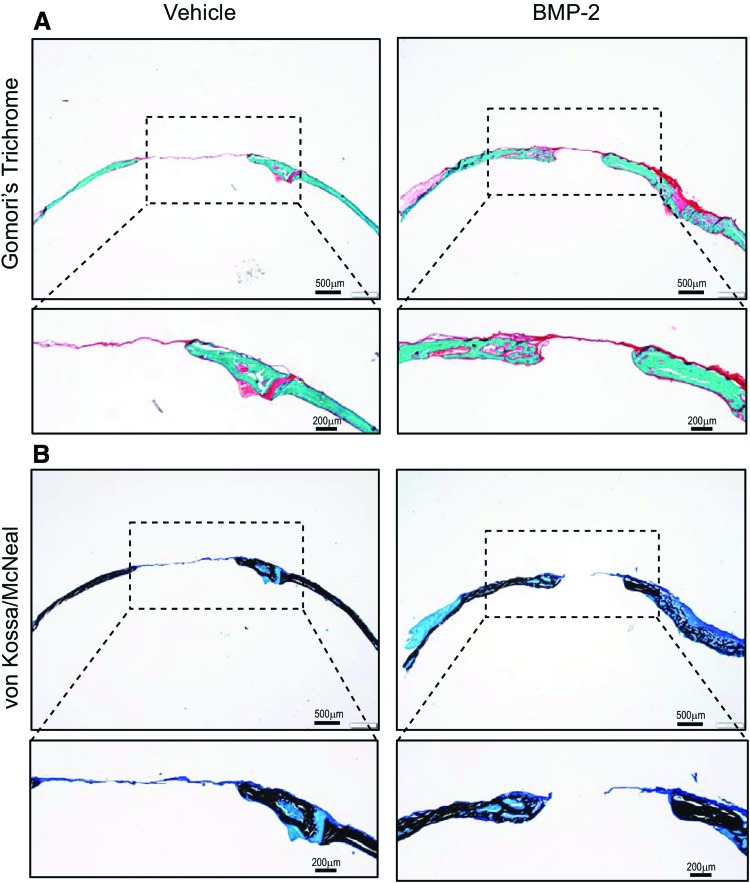
Histological analysis of coronal sections of calvaria was performed by Gomori's trichrome staining and the von Kossa staining method to identify, respectively, collagen and mineralized tissue formation (Fig. 3). Staining results revealed newly formed bone tissue connecting the inner edges of the defect. As anticipated, the defect gap was wider with the vehicle-treated group compared to BMP-2-treated group (Fig. 3A). Promotion of new bone formation by BMP-2 is evident by the bridging of the defect region with new bone tissue and the significantly reduced defect cavity region. Accordingly, mineralization was confirmed by von Kossa/McNeal staining which demonstrated the presence of new bone tissue (stained black) within the defect region (Fig. 3B). Vehicle-treated defects (negative control) resulted in significantly minimal formation at the margins of the defect. Histomorphometric analysis of stained tissue sections revealed significantly higher bone volume (BV/TV%) with BMP-2 treatment, thus corroborating micro-CT measurements (Fig. 4B). The repair site resembled intramembranous bone, consistent with the known developmental origin of calvaria that form by intramembranous ossification. Membranous bone formation permits a direct in vivo assessment of the efficacy of bone-anabolic compounds from fibroblastic mesenchymal calvarial progenitor cells unencumbered by the complexities of assessing pro-anabolic effects with bone that forms through a cartilage intermediate that needs to be removed before osteoblast-mediated new bone formation. Together, our data confirm the usefulness of the mouse calvarial defect model for bone tissue healing and skeletal engineering studies.
FIG. 3.
Histological analysis of bone formation within calvarial defect. (A) Gomori's trichrome staining showing new bone tissue forming within the defect from the inner edges. Vehicle group shows larger defect gap compared to BMP-2-treated group. Collagen is stained green. Dotted box highlights new bone formation. Significantly higher bone formation was seen with BMP-2 treatment as indicated by the bridging of the defect gap with new bone tissue. (B) von Kossa/McNeal staining showing correspondingly higher mineralized bone tissue within defect with BMP-2 treatment compared to vehicle-treated group. Mineralized bone tissue is stained black. Dotted box highlights new bone formation. Color images available online at www.liebertpub.com/tec
FIG. 4.
Quantification of new bone formation. (A) Bar graph showing quantification of bone formation by micro-CT analysis. Bone volume density (BV/TV) was significantly higher with BMP-2 treatment; *p = 0.0302. (B) Bar graph showing bone volume density (BV/TV) derived by histomorphometric analysis. Significantly higher bone formation was seen with BMP-2 treatment; **p = 0.0011. BV/TV, bone volume over total volume.
Conclusion
This article describes a straightforward, practical, and reproducible model useful for studying bone regeneration in mice. This orthotopic model permits assessment of bone parameters in an in vivo setting in a reliable manner and offers notable advantages of investigating transgenic mouse models in bone and mineral research, as well as for skeletal tissue engineering applications.
Acknowledgments
The authors acknowledge the funding support from NIH (RO1 AR049069 to A.J.v.W. and F32 AR066508 to A.D.), Robert and Arlene Kogod Center on Aging Career Development Award (to R.M.S.), Mayo Clinic Center of Regenerative Medicine, and generous philanthropic support of William and Karen Eby. The authors thank the members of our laboratory, including Bashar Hasan, Christopher Paradise, Janet Denbeigh, Roman Thaler, Eric Lewallen, Catalina Galeano-Garces, and Farzaneh Khani, for stimulating discussions. The authors also acknowledge the support and assistance of Jim Herrick, Bob Brown, Brian Waletzki, and Lee Miller with micro-CT and histology from the Biomaterials and Histomorphometry Core Laboratory at Mayo Clinic, Rochester.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bhakta G., et al. The influence of collagen and hyaluronan matrices on the delivery and bioactivity of bone morphogenetic protein-2 and ectopic bone formation. Acta Biomater 9, 9098, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Bhakta G., et al. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 33, 6113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samsonraj R.M., et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells 33, 1878, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J., and Glimcher M.J. Characterization of matrix-induced osteogenesis in rat calvarial bone defects: I. Differences in the cellular response to demineralized bone matrix implanted in calvarial defects and in subcutaneous sites. Calcif Tissue Int 65, 156, 1999 [DOI] [PubMed] [Google Scholar]
- 5.McGee-Lawrence M.E., and Razidlo D.F. Induction of fully stabilized cortical bone defects to study intramembranous bone regeneration. In: Westendorf J.J., and van Wijnen A.J., eds. Osteoporosis and Osteoarthritis. New York, NY: Springer, 2015, pp. 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai B., et al. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials 31, 7960, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Helledie T., et al. Heparan sulfate enhances the self-renewal and therapeutic potential of mesenchymal stem cells from human adult bone marrow. Stem Cells Dev 21, 1897, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X., et al. Enhanced healing of rat calvarial defects with MSCs loaded on BMP-2 releasing chitosan/alginate/hydroxyapatite scaffolds. PLoS One 9, e104061, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schantz J.T., et al. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo. Tissue Eng 9 Suppl 1, S127, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Suenaga H., et al. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci 26, 254, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodruff M.A., et al. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J Mol Histol 38, 425, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Spicer P.P., et al. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protocols 7, 1918, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper G.M., et al. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast Reconstr Surg 125, 1685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan C.M., et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22, 560, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Lee M.H., et al. Transient upregulation of CBFA1 in response to bone morphogenetic protein-2 and transforming growth factor beta1 in C2C12 myogenic cells coincides with suppression of the myogenic phenotype but is not sufficient for osteoblast differentiation. J Cell Biochem 73, 114–25, 1999 [PubMed] [Google Scholar]
- 16.Aghaloo T., et al. The effect of NELL1 and bone morphogenetic protein-2 on calvarial bone regeneration. J Oral Maxillofac Surg 68, 300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugamori Y., et al. Peptide drugs accelerate BMP-2-induced calvarial bone regeneration and stimulate osteoblast differentiation through mTORC1 signaling. Bioessays 38, 717, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudakovic A., et al. Epigenetic control of skeletal development by the histone methyltransferase Ezh2. J Biol Chem 290, 27604, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee-Lawrence M.E., et al. Runx2 is required for early stages of endochondral bone formation but delays final stages of bone repair in Axin2-deficient mice. Bone 66, 277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An Y.M., Moreira P.L., Kang Q.K., Gruber H.E. Principles of embedding and common protocols. In: An Y.H., and Martin K.L. eds. Handbook of Histology Methods for Bone and Cartilage. Totowa, NJ: Humana Press Inc., 2003, pp. 185–206 [Google Scholar]
- 21.Villanueva A.R., Mehr L. Modifications of the Goldner and Gomori one-step trichrome stains for plastic-embedded thin sections of bone. Am J Med Technol 43, 536, 1977 [PubMed] [Google Scholar]
- 22.Bouaicha S., et al. Histological remodelling of demineralised bone matrix allograft in posterolateral fusion of the spine—an ex vivo study. BMC Surg 13, 58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]