Abstract
Background: Enthusiasm for the benefits of sodium–glucose cotransporter 2 inhibitors (SGLT2i) as an adjunctive treatment in type 1 diabetes (T1D) has been offset by the possible increased risk of diabetic ketoacidosis (DKA). Since pump-treated T1D patients are susceptible to DKA due to infusion site problems, this study was undertaken to assess how treatment with SGLT2i affects patterns of early metabolic decompensation following suspension of basal insulin.
Methods: Ten T1D participants (age 19–35 years, duration 10 ± 8 years, A1c 7.4% ± 0.8%) underwent overnight pump suspension studies before and after treatment with canagliflozin (CANA). On both nights, basal insulin was suspended at 3 AM and plasma glucose (PG), β-hydroxybutyrate (BHB), free fatty acids (FFA), plasma insulin (PI), and glucagon were measured. Studies were terminated 6 h after suspension or if PG rose to >350 mg/dL or BHB >2.5 mmol/L.
Results: PI levels at the start of suspension were reduced by 30% after CANA treatment (44 ± 11 uU/mL vs. 31 ± 10 uU/mL, P < 0.01), but baseline PG, BHB, FFA, and glucagon levels were not significantly different. During the suspension, PG rose from 104 ± 10 to 301 ± 21 mg/dL before treatment, but only from 109 ± 8 to 195 ± 14 mg/dL after treatment (P = 0.002 vs. pretreatment values). On the other hand, CANA treatment did not significantly affect the magnitude of increases in FFA, BHB, and glucagon levels during the suspension study.
Conclusion: These data indicate that SGLT2i do not accelerate the rate of ketogenesis following the interruption of basal insulin infusion in T1D. Rather, the failure of patients to promptly recognize early metabolic decompensation relates to the much more gradual rise in PG levels.
Keywords: : SGLT2 inhibitor, Type 1 diabetes, Pump failure, Ketosis, Glucose levels
Introduction
Even though the Diabetes Control and Complications Trial (DCCT) demonstrated that successful intensive treatment of type 1 diabetes (T1D) could reduce the risk of long-term vascular complications more than 2 decades ago,1,2 the T1D Exchange has reported that fewer than one-third of adults in the U.S. maintain hemoglobin A1c levels <7.0% and nearly 70% of adult participants were overweight or obese.3 The limitations of insulin pump and multiple injection therapies in combination with persistent problems with diabetic ketoacidosis (DKA) and severe hypoglycemia3 have stimulated interest in using adjunctive therapies that have been approved for use in T2D in patients with T1D.
Over the past decade, a number of new drug classes have been approved for use in the treatment of T2D.4 Notably, two of these classes of medications have been associated with weight loss: glucagon-like peptide-1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 inhibitors (SGLT2i).4 SGLT2i are particularly attractive for adjunctive use in T1D, as they are orally active agents that lower plasma glucose (PG) levels by increasing urinary glucose excretion5; a mechanism that is independent of insulin.5,6 Indeed, a variety of relatively short-term clinical trials of SGLT2i in T1D have shown reductions in A1c, total daily insulin doses, and weight.7–12
Enthusiasm for use of SGLT2i in T2D has been dampened by reports suggesting an increased risk of DKA often without marked hyperglycemia in diabetic patients being treated with these agents. These reports prompted the FDA to publish a warning that T2D patients treated with insulin may be at greater risk for DKA when they are treated with an SGLT2i.13 More recently, a case series reported 13 episodes of moderate-to-severe DKA in nine patients with diabetes, in whom presenting PG levels ranged between 96 and 233 mg/dL. It is particularly notable that seven of these patients had T1D and only two had T2D.14
There are several factors that could make insulin pump-treated T1D patients who receive SGLT2i susceptible to “euglycemic” DKA during the night, including the relatively frequent occurrence of infusion site failures; increased rates of lipolysis and ketogenesis due to reductions in basal insulin infusion rates during SGLT2i treatment; and blunted increases in PG due to increased urinary losses of glucose. To address these issues, we employed an insulin interruption protocol previously used by our group to determine whether pump-treated patients receiving a rapid-acting analog (lispro) were more susceptible to DKA than patients receiving human regular insulin in the event of an infusion site failure at night.15
Research Designs and Methods
Participants were recruited from the Yale Type 1 Diabetes Program. Inclusion criteria included age 18–45 years, clinical diagnosis of T1D of at least 1 year's duration, current use of a continuous subcutaneous insulin infusion (CSII) pump for at least 3 months, A1c ≤9% (<75 mmol/mol), body weight >40 kg due to blood sampling, normal hemoglobin and estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2, and the ability to carry out daily ketone monitoring during the 7- to 14-day run-in period before the initial insulin suspension study. Exclusion criteria included history of recurrent genital mycotic infections, males who were uncircumcised (due to increased risk of genital infections), other chronic medical condition which would interfere with safety of study, use of medications (other than insulin) known to affect PG levels, and female participants who were pregnant or lactating. After a complete explanation of the study procedures, written informed consent was obtained from participants. The study was approved by the Yale University Human Investigation Committee and registered at clinicaltrials.gov (NCT02673138).
Study Design
The study utilized an open-label design in which each participant was studied under two conditions: suspension of CSII during treatment with insulin alone (control) versus suspension of CSII during treatment with insulin and canagliflozin (CANA). CANA was chosen because the largest trials of this class of medication in T1D had been conducted with CANA at the time of study initiation.11 Primary outcomes of interest were the incremental changes from baseline in PG and β-hydroxybutyrate (BHB) levels at the end of insulin suspension and secondary outcomes included the incremental changes from baseline in free fatty acids (FFA) and glucagon levels at the end of the insulin suspension.
Study Procedures
Preparation for suspension studies
At enrollment, participants were instructed to monitor urinary ketones first thing in the morning while they were fasting, and before bedtime in the evening during a 1- to 2-week run-in period. If urinary ketones were trace or greater, participants were then advised to measure blood BHB levels by a Precision Xtra Meter (Abbott Diabetes Care, Inc., Alameda, CA).
On the evenings of planned suspension studies, participants arrived at the Yale New Haven Hospital Research Unit between 5 and 6 PM, at which time intravenous catheters were inserted for blood sampling. On both study days, identical, self-selected meals were provided at 6 PM. Carbohydrate intake for dinner averaged 70 ± 22 g (range 38–104). Participants were allowed to calculate bolus doses to mimic routine care. No snacks were allowed, except for treatment of hypoglycemia and no supplemental insulin was given after 10 PM to avoid having insulin on board at start of suspension, which was at 3 AM.
Suspension protocol
The suspension protocol utilized in this study both before and after CANA therapy was identical to that previously described by our group.15 Briefly, to proceed with interruption phase of the study scheduled to commence at 3 AM, PG levels had to be <150 mg/dL and BHB levels had to be <0.6 mmol/L. PG and BHB were monitored at the bedside every 30 min, measured by a YSI 2300 Glucose Analyzer (YSI Life Sciences, Yellow Springs, OH) and Precision Xtra Meter, respectively. The insulin interruption phase of the study was terminated after 6 h, or earlier if bedside BHB was ≥2.5 mmol/L or PG was >350 mg/dL (19.4 mmol/L).
Blood samples were obtained for laboratory measurement of PG, BHB, FFA, and glucagon levels just before and every 30 min following interruption of the insulin infusion. Plasma insulin (PI) samples were obtained just before the interruption of basal insulin. A correction dose of rapid-acting insulin analog was given at the end of each study. Before discharge home, bedside BHB levels had to be <1.0 mmol/L and PG >90 mg/dL.
Outpatient CANA dose titration phase
The day after the control suspension visit, participants initiated treatment with CANA 100 mg once daily. With initiation of CANA therapy, basal and bolus insulin doses were reduced by ∼20% in all subjects. Participants were in daily phone contact with the study team for the first week after starting CANA to allow for titration of insulin doses and elicit if there were any side effects from the medication. After one week at the 100 mg dose, CANA was increased to a dose of 300 mg once daily. Participants were then contacted every 2–3 days until their second inpatient suspension study; total outpatient treatment period was 3–4 weeks.
During the outpatient dose titration phase, urine ketones were measured daily before breakfast, before bed, and if PG >200 mg/dL (11 mmol/L). If urine ketones were trace or larger, participants were advised to check BHB by a Precision Xtra Meter to further quantitate the degree of ketonemia.
Suspension while on SGLT2i therapy
After being treated with CANA for ∼3–4 weeks, participants were admitted for a second inpatient suspension visit. Study procedures replicated the first visit, except that participants took their 300 mg dose of CANA at 8 AM on the day of admission.
Analytical methods
Laboratory BHB levels were measured by UV/Vis colorimetric spectrophotometer (Wako Autokit3-HB), PI levels were measured by radioimmunoassay (EMD Millipore), PG levels were measured by YSI 2300 Glucose Analyzer (YSI Life Sciences, Yellow Springs, OH), FFA by UV/Vis colorimetric spectrophotometer (Wako NEFA-HR2), glucagon by radioimmunoassay (EMD Millipore), and A1c by the DCA Vantage (Siemens Medical Solutions Diagnostics).
Statistical methods
BHB concentrations were used to compare differences in metabolic decompensation between the two treatment conditions: control versus CANA. Studies in which there was premature termination of the suspension phase, the last level before resuming basal insulin was carried forward. Incremental increases were calculated for PG, BHB, FFA, and glucagon levels to correct for any baseline differences between the two studies. Statistical comparisons between control and CANA visits used paired t-tests for normally distributed data and Wilcoxon matched pairs signed-rank tests for non-normally distributed data. Data are presented as mean ± SE. Calculations were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA).
Results
Participants
Twelve participants were recruited into the study. However, one participant withdrew secondary to hypoglycemic seizure before any study procedures and another was excluded due to an A1c >9% at the enrollment visit. The 10 participants (2 males) who completed the study were aged 23 ± 5 years (range 19–35 years), had diabetes duration of 10 ± 8 years, and a mean A1c level of 7.4% ± 0.8% (57.0 ± 8.7 mmol/mol) ranging between 6.3% and 8.8% (45.0–73.0 mmol/mol).
Outpatient insulin dose titration
As shown in Table 1, at the end of the 3–4 weeks of CANA treatment, total daily insulin doses decreased by 16%, which was due to reductions in basal and bolus doses of insulin. Despite the reduction in total daily insulin doses, A1c fell slightly versus baseline values. Individual participant values are shown in Supplementary Table S1; Supplementary data are available at http://online.liebertpub.com/suppl/10.1089/dia.2017.0267.
Table 1.
Changes in Insulin Doses Pre- and PostCanagliflozin Treatment
| TDD (units) | Basal insulin (units) | Overnight basala(units) | |
|---|---|---|---|
| Baseline | 50.9 ± 4.2 | 30.8 ± 2.5 | 6.4 ± 0.5 |
| Posttreatment | 42.8 ± 3.5 | 26.1 ± 0.7 | 5.3 ± 0.6 |
| Change | 8.1 | 4.7 | 1.1 |
| % Change | 16 | 15 | 17 |
Data are presented as mean ± SE.
Overnight basal insulin from 12a to 6a.
TDD, total daily dose.
Insulin suspension studies
None of the participants needed to have their visits rescheduled, as they all met the criteria to carry out the suspension on both occasions; namely, baseline PG <150 mg/dL and bedside BHB level <0.6 mmol/L.
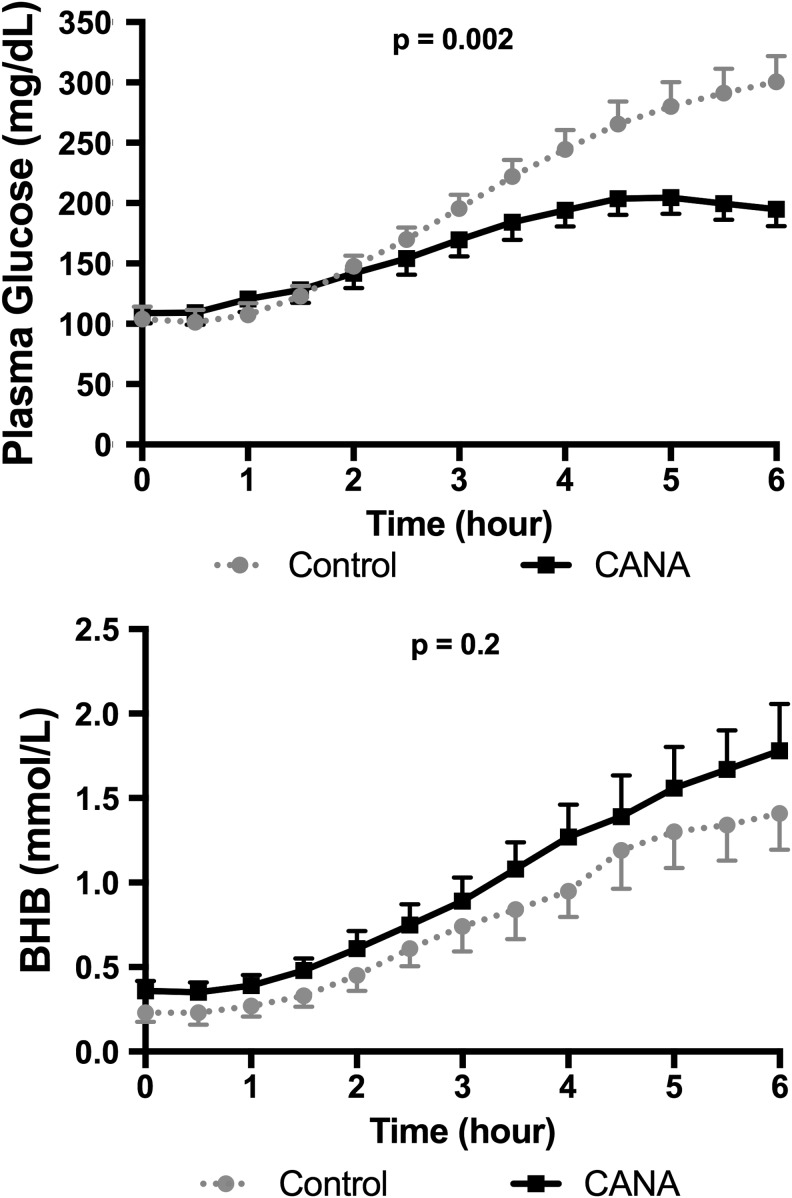
As expected from the decreases in insulin doses during CANA treatment, baseline PI levels before suspension of basal insulin were 30% lower during the CANA study than during the control study (31 ± 10 uU/mL vs. 44 ± 11 uU/mL, P < 0.006); whereas, baseline PG levels were similar at the start of the suspension on both visits (Table 2). As shown in Figure 1, the rise in PG levels between the two studies began to diverge during the third hour of insulin suspension. Consequently, PG at the end of suspension in the control study was 301 ± 21 mg/dL, which was in marked contrast to the mean PG of 195 ± 14 mg/dL during the CANA study (P = 0.002). Compared with the pretreatment control study, the peak increment in PG from baseline during treatment with CANA was reduced by 50% (P = 0.002, Table 2).
Table 2.
Data from Suspension Sessions
| During suspension | Control | CANA | P |
|---|---|---|---|
| Plasma glucose (mg/dL) | |||
| Start of suspension | 104 ± 10 | 109 ± 8 | 0.86 |
| Increment plasma glucose | 197 ± 24 | 99 ± 13 | 0.002 |
| BHB (mmol/L) | |||
| Start of suspension | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.078 |
| Increment BHB | 1.2 ± 0.2 | 1.5 ± 0.2 | 0.2 |
| Free fatty acids (mmol/L) | |||
| Start of suspension | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.065 |
| Increment FFA | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.28 |
| Glucagon (pg/mL) | |||
| Start of suspension | 88 ± 9 | 75 ± 9 | 0.2 |
| Increment glucagon | 14 ± 5 | 36 ± 15 | 0.07 |
Data are presented as mean ± SE.
BHB, β-hydroxybutyrate; CANA, canagliflozin; FFA, free fatty acids.
FIG. 1.
Plasma glucose and BHB levels during the control and CANA treatment studies. Changes in plasma glucose (top) and β-hydroxybutyrate (bottom) are shown during suspension. Control condition is depicted as dotted line with circles, CANA condition as solid line with squares. BHB, β-hydroxybutyrate; CANA, canagliflozin.
Despite the lower baseline concentrations of PI during the CANA study, baseline BHB and FFA levels were only mildly increased above what was seen during the control study (Table 2). Even more important, the rise in BHB levels were similar during both interruption studies, as were the increases in FFA levels (Table 2). Plasma glucagon levels at the start of the suspension were slightly higher during the control study (88 ± 9 pg/mL) versus the CANA study (75 ± 9 pg/mL), but these differences were not significant (P = 0.2). Conversely, the incremental increase in glucagon levels following suspension tended to be greater during CANA treatment (control: 14 ± 5 pg/mL vs. CANA: 36 ± 15 pg/mL), but these differences were also not statistically significant (P = 0.07).
A total of five suspension studies were terminated per protocol (PG >350 mg/dL and/or bedside BHB >2.5 mmol/L) before completion of the planned 6-h suspension. Two were due to hyperglycemia (both occurred during control visits) and the other three (one control and two on SGLT2i) were due to BHB >2.5 mmol/L. Table 3 shows the individual data points for the five studies that were terminated before the planned 6-h suspension.
Table 3.
Studies with Early Termination
| Visit | Duration of suspension (h) | BHB (mmol/L) | PG (mg/dL) | |
|---|---|---|---|---|
| Termination secondary to PG | ||||
| Participant 1 | Control | 5.0 | 1.0 | 355 |
| Participant 8 | Control | 5.5 | 1.5 | 364 |
| Termination secondary to BHB | ||||
| Participant 3 | Control | 4.5 | 2.8 | 292 |
| Participant 3 | CANA | 4.5 | 2.8 | 196 |
| Participant 4 | CANA | 5.0 | 2.5 | 148 |
PG, plasma glucose.
Discussion
The recent reports of DKA in patients with T1D being treated with SGLT2i prompted us to carry out a study whose primary aim was to discover whether delayed recognition of early metabolic decompensation in T1D was due to vulnerability to accelerated lipolysis and ketogenesis secondary to reductions in basal insulin doses during SGLT2i treatment. Alternatively, early metabolic decompensation might be more difficult to recognize because of a blunted rise in PG levels due to excessive urinary glucose losses with these agents or a combination of both of these factors. We further hypothesized that such vulnerability would be most readily observed in insulin pump-treated patients who suffer an infusion site failure during sleep at night.
The observations that baseline levels of FFA and BHB levels and the rise in both of these substrates were similar during both insulin suspension studies suggest that T1D patients are not susceptible to accelerated lipolysis and ketogenesis, despite the reduction in overnight basal insulin doses during SGLT2i treatment. This conclusion is supported by the modest differences in plasma glucagon between the two studies at baseline and following interruption of the basal insulin infusion.
Data from the control study before initiation of CANA therapy demonstrated that PG levels markedly increased from ∼100 to >300 mg/dL within 5–6 h with suspension of basal insulin; whereas, PG levels increased from ∼100 to only about 200 mg/dL during CANA treatment. Over this same period of time, mean BHB levels increased by only 1.0 mmol/L before and during treatment with CANA. Thus, the results of our study suggest that the failure to recognize early metabolic decompensation due to infusion site failures in T1D patients being treated with SGLT2i more likely relates to the much more gradual rate of increase in PG levels rather than to increased rates of lipolysis and ketogenesis.
The major limitation of the study is its relatively small sample size. However, the PG and BHB responses to the interruption of basal insulin during the control experiments in this study were nearly identical to those observed in our prior insulin interruption study in a larger group of T1D patricipants.15 In that study, the insulin interruption also had to be prematurely terminated due to elevated ketones in 1/3 of participitants. In addition, the blunted rise in PG during CANA treatment was very consistent between participants. The failure to observe statistically significant differences in the incremental rise of plasma glucagon levels between the two studies should be interpreted with particular caution because of the small sample size and because it has been suggested that inhibition of SGLT2 expressed in pancreatic alpha cells can promote glucagon secretion.16 Whether rates of lipolysis and ketogenesis are accelerated by SGLT2i during intercurrent illnesses or stress also remain to be determined.
Current protocols for the early detection of infusion site failures in pump-treated T1D patients have focused on recognition of persistent elevations in PG levels that fail to respond to administration of correction boluses of insulin rather than depending on measurements of blood BHB or urine ketone levels. The effectiveness of this approach is illustrated by data from the T1D Exchange indicating that patients with insulin pump have a lower risk of DKA than patients on insulin injection treatment.3 Consequently, the most important finding of this study is that clinicians and patients cannot depend on a “glucocentric” approach to the early detection of infusion site failure in T1D patients on SGLT2i treatment. Rather, DKA prevention protocols for insulin pump-treated T1D patients being treated with SGLT2i need to include the prerequisite for routine measurement of blood or urine ketones with additional monitoring at lower PG levels and if there are any signs or symptoms of nausea, vomiting, or dehydration.14
Supplementary Material
Acknowledgments
The authors thank the participants and their families, the healthcare professionals, and staff of the Yale Children's Diabetes Clinic, the Yale Center for Clinical Investigation, and the dedicated nursing staff of the Hospital Research Unit, whose support and participation made this project possible. Funding: This publication was made possible through the support of grants from the Endocrine Fellows Foundation, Juvenile Diabetes Research Foundation (5-ECR-2014-112-A-N), and the National Institutes of Health (K12-DK-094714, UL1TR000142, P30 DK45735).
Authors' Contributions
N.S.P. research data and wrote the article. M.A.V, E.C., and S.A.W. researched data and contributed to the discussion. W.V.T and J.L.S. researched data, contributed to the discussion, and reviewed and edited the article. N.S.P. is the guarantor of this work, and as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Author Disclosure Statement
W.V.T. is a consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Medtronic Diabetes, NovoNordisk, and Sanofi. S.A.W. is a consultant and speaker for Insulet and receives research grant support from Medronic (to Yale). E.C. is a speaker for NovoNordisk and on advisory board of Novo, Mannkind, Adocia, and Lexicon. J.L.S. serves on the advisory board of Bigfoot Biomedical and Insulet and is a consultant for Medtronic Diabetes. No other potential conflicts of interest relevant to this article were reported. No sponsor had any role in the study design, data collection, data analysis, data interpretation, or writing of the report.
References
- 1.The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group: Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 3.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association: 8. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017;40(Suppl 1):S64–S74 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E, Solini A: SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8:495–502 [DOI] [PubMed] [Google Scholar]
- 6.Jurczak MJ, Lee HY, Birkenfeld AL, et al. : SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 2011;60:890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkins BA, Cherney DZ, Partridge H, et al. : Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: Results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 8.Pieber TR, Famulla S, Eilbracht J, et al. : Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab 2015;17:928–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamez HE, Tamez AL, Garza LA, et al. : Dapagliflozin as an adjunct therapy to insulin in the treatment of patients with type 1 diabetes mellitus. J Diabetes Metab Disord 2015;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry RR, Rosenstock J, Edelman S, et al. : Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care 2015;38:412–419 [DOI] [PubMed] [Google Scholar]
- 11.Henry RR, Thakkar P, Tong C, et al. : Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care 2015;38:2258–2265 [DOI] [PubMed] [Google Scholar]
- 12.Rodbard HW, Peters AL, Slee A, et al. : The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care 2017;40:171–180 [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration: FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. June 19, 2015. www.fda.gov/Drugs/DrugSafety/ucm446845.htm
- 14.Peters AL, Buschur EO, Buse JB, et al. : Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attia N, Jones TW, Holcombe J, et al. : Comparison of human regular and lispro insulins after interruption of continuous subcutaneous insulin infusion and in the treatment of acutely decompensated IDDM. Diabetes Care 1998;21:817–821 [DOI] [PubMed] [Google Scholar]
- 16.Taylor SI, Blau JE, Rother KI: SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015;100:2849–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.