Abstract
Peripheral ischemia as a result of occlusive vascular disease is a widespread problem in patients older than the age of 65. Angiogenic therapies that can induce microvascular growth have great potential for providing a long-lasting solution for patients with ischemia and would provide an appealing alternative to surgical and percutaneous interventions. However, many angiogenic therapies have seen poor efficacy in clinical trials, suggesting that patients with long-term peripheral ischemia have considerable therapeutic resistance to angiogenic stimuli. Glioblastoma is one of the most angiogenic tumor types, inducing robust vessel growth in the area surrounding the tumor. One major angiogenic mechanism used by the tumor cells to induce blood vessel growth is the production of exosomes and other extracellular vesicles that can carry pro-angiogenic and immunomodulatory signals. Here, we explored whether the pro-angiogenic aspects of glioblastoma-derived exosomes could be harnessed to promote angiogenesis and healing in the context of peripheral ischemic disease. We demonstrate that the exosomes derived from glioblastoma markedly enhance endothelial cell proliferation and increase endothelial tubule formation in vitro. An analysis of the microRNA expression using next generation sequencing identified that exosomes contained a high concentration of miR-221. In addition, we found that glioblastoma exosomes contained significant amounts of the proteoglycans glypican-1 and syndecan-4, which can serve as co-receptors for angiogenic factors, including fibroblast growth factor-2 (FGF-2). In a hindlimb ischemia model in mice, we found that the exosomes promoted enhanced revascularization in comparison to control alginate gels and FGF-2 treatment alone. Taken together, our results support the fact that glioblastoma-derived exosomes have powerful effects in increasing revascularization in the context of peripheral ischemia.
Keywords: : ischemia, therapeutic angiogenesis, exosomes, miR-221, glioblastoma
Introduction
Exosomes are lipid vesicles that are secreted by cells into their environment and range in size from ∼30 to 100 nm in diameter.1 These secreted extracellular vesicles are initially formed as invaginations in the endosomal membrane to create a multivesicular body.2 This process is in contrast to the origin of larger microvesicles (200–1000 nm in diameter) that are formed through membrane shedding.3 Exosomes are released from the cell through multivesicular endosomal fusion with the plasma membrane. Extracellular vesicles are found in many biofluids and these secreted vesicles are increasingly recognized as mediators of cell–cell communication, which are capable of transferring proteins, nucleic acids, and lipids between distant cells.4 In addition, these secreted vesicles have recently been identified as important diagnostic targets for cancer and cardiovascular diseases.5,6 Exosomes, in particular, are known to have high levels of tetraspanins, trafficking/export-related molecules and heat shock proteins.7–10 In addition, exosomes contain proteins, microRNA (miRNA), and, in some cases, double-stranded DNA.11,12 Exosomes also carry bioactive lipids including sphingomyelin, eicosanoids, cholesterol, and the ganglioside GM3.13 Extracellular vesicles, including exosomes, can be efficiently taken up by cells and can be used to target particular cell populations where they modify the target cells' transcriptional and protein expression profiles.14
Exosomes from many cell types are known to mediate angiogenesis.15–20 Exosomes derived from mesenchymal stem cells have also been shown to induce angiogenesis and modulate revascularization in ischemia and wound healing.19,20 Glioblastoma is one of the most angiogenic tumors, inducing an intense growth of blood vessels surrounding the tumor mass.21 The exosomes secreted by glioblastoma cells prime endothelial cells to respond to hypoxic conditions with a potent angiogenic response.22 Moreover, primary glioblastoma cells carry angiogenic miRNAs and proteins that facilitate angiogenic differentiation of endothelial cells.23 Moreover, the exosomes produced by these cells have been linked to growth-promoting signals in endothelial cells.24 In addition, glioma-derived exosomes have immunomodulatory effects that drive macrophages toward the M2-phenotype.25 The mechanism of inducing phenotype switching in macrophages may allow tumor cells to evade recognition by the immune system, and macrophages polarized toward M2 phenotype also secrete vascular endothelial growth factor (VEGF) and promote angiogenesis.26,27
Although many of the properties of glioma exosomes serve to support the growing tumor, these same activities could provide benefit in the context of peripheral vascular disease and critical limb ischemia. For instance, in chronic wounds and long-term ischemia, excessive inflammation and M1 macrophage phenotype are believed to play an important role in preventing the normal healing of the tissues.28,29 We and others have found that long-term peripheral ischemia, particularly in the context of diabetes, is a state of therapeutic resistance in which angiogenic growth factors or gene therapy fail to provide sufficient signals to induce the growth of vasculature.30–36 This concept is consistent with the results of many clinical trials on growth factor and angiogenic gene therapy that have achieved limited benefits in patients with peripheral ischemic disease.37
Cancer cells must strive to overcome homeostatic and immunologic mechanisms of resistance that could inhibit their growth and ability to induce vasculature. Thus, in the process of evolving to effectively induce angiogenesis in a resistant environment, they may have also developed mechanisms that may be inadvertently useful in addressing the issues of therapeutic resistance in intractable peripheral ischemia.
Exosomes are an emerging therapeutic strategy and have been explored as direct treatments for disease and drug carriers.14,38–40 Exosomes from mesenchymal stem cells have generated significant interest and have been used to induce angiogenesis in wound healing and ischemia.20,41 In addition, mesenchymal stem cell exosomes can improve the recovery after myocardial infarction and enhance cardiac regeneration.42,43 The use of a soluble factor derived from cells as a therapy provides practical benefits over cell implantation, both in the production of a therapy and in the ease of translation into clinical practice and delivery.
In this study, we aimed at examining the use of glioma-derived exosomes to enhance revascularization in peripheral limb ischemia. We chose glioblastomas because they are one the most highly vascularized solid tumors and produce an intense angiogenic response, and their exosomes have not been linked to tumorigenic activity. Here, we explored whether this powerful aspect of tumor biology could be harnessed to enhance angiogenesis in the context of peripheral ischemic disease.
Materials and Methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Promocell, Inc., and glioblastoma cells (A-172) were purchased from ATCC. The HUVECs were cultured in MCDB-131 medium with 7.5% fetal bovine serum (FBS), endothelial growth medium-2 (EGM-2) supplements (Lonza), l-glutamine, and antibiotics. The glioblastoma cell line was cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% FBS, l-glutamine, and antibiotics. Both cell lines were grown at 37°C with a 5% CO2 atmosphere.
Purification and characterization of exosomes
The glioblastoma cells were cultured to 60% confluence, rinsed with phosphate buffered saline (PBS) and the culture media were replaced with media that were supplemented with exosome-depleted FBS, l-glutamine, and antibiotics. After 48 h, the media were collected and exosomes were isolated from the media by using the Exoquick-TC isolation kit (Systems Bioscience) and then stored at −80°C until further use. The number of exosomes was normalized by using a CD63 ELISA (Systems Bioscience). The size of exosomes was measured by using dynamic light scattering (Malvern Zetasizer Nano ZS). The instrument was calibrated by using 54-nm-diameter polystyrene particles, and the exosomes were diluted to fit the detection region of the instrument. Final results were an average of 50 size measurements. For imaging the exosomes with cryo-electron microscopy, the samples were plunge-frozen in liquid ethane on carbon film grids as previously described (R2x2 Quantifoil; Micro Tools GmbH, Jena, Germany).44 The grids were then transferred to a specimen holder (Gatan 626) under liquid nitrogen and imaged by using a transmission electron microscope (JEOL 2100 LaB6) operating at 200 keV. Grids were maintained at low temperatures during the imaging session (−172°C to −180°C). The exosomes were imaged at 20,000× magnification with a 4000 × 4000 slow-scan CCD camera (UltraScan 895; GATAN, Inc.) by using a low-dose imaging procedure. Enzyme-linked immunosorbent assay (ELISA) assays were used to quantify the presence of glypican-1 (RayBiotech) and syndecan-4 (Clontech Laboratories).
Proliferation assay
HUVECs were cultured to 70% confluence and then the media were changed to low-serum media (2% FBS) and incubated for 24 h. The cells were then passaged into a 96-well plate at 2500 cells/well with exosomes and/or FGF-2 (10 ng/mL). BrdU was added to the cells 24 h after the treatments. Then, proliferation was assessed by BrdU incorporation at 12 h thereafter by using a BrdU Assay (Cell Signaling).
In vitro tube formation assay
The differentiation of endothelial cells was measured by using an in vitro tube formation assay. Briefly, culture plates were coated with growth factor reduced Matrigel at 37°C for 1 h. In each well, 20,000 cells were seeded in the presence of different treatments with exosomes in different amounts or FGF-2 (10 ng/mL). After 16 h, the cells were imaged by using phase-contrast microscopy. Quantification of the number of tubes and tube length was performed by using MetaMorph software (Molecular Devices).
Antibody array assay for angiogenic growth factors
HUVECs were cultured to 70% confluence and then culture medium was changed with low serum (2% FBS) with or without 30 × 108 exosomes/mL. After 48 h of incubation, the condition media from the cells were collected, centrifuged for 10 min at 3000 g to get rid of cell debris and the supernatant was stored. An antibody array was used to analyze the concentration of growth factors in the culture media (Proteome Profiler Human Angiogenesis Array Kit; R&D Systems, Inc.) according to the manufacturer's instructions.
Synthesis of alginate beads and measurement of release kinetics
Alginate beads were formed by using equal volumes of 4% alginate and a 0.85% NaCl solution. Exosomes were added to the 2% alginate solution to create a concentration of 65 billion exosomes/mL to match the concentration used in the mouse hind limb ischemia model. The alginate gel was formed into beads by using a syringe pump for controlled extrusion through a 30 g needle into a 1.1% CaCl2 crosslinking solution. Crosslinking was allowed to continue for 1 h at 4°C. Alginate beads with exosomes were stored in plastic scintillation vials containing PBS with calcium and magnesium and placed on a shaker at 37°C. Samples were collected at various time points. At each point, a portion of the volume was collected and promptly replaced with an equal volume of fresh PBS. Exosome content was measured via ExoELISA-Ultra CD63 Kit (System Biosciences).
Soft agar colony formation assay
Control 3T3 fibroblasts were treated with 500 ng/mL of 1-methyl-3-nitro-1-nitrosoguanidine for 3 days and then cultured in normal media for 3 days. Next, the cells were treated with 4.4 × 108 exosomes/mL and 30 × 108 exosomes/mL for 2 weeks. Equal parts of 1% agar and 2 × DMEM media with 20% FBS were mixed for the base layer of agar to a final concentration of 0.5% agar. A 24-well plate was coated with 400 μL/well. Equal parts of 0.7% agarose and 2 × DMEM media with 20% FBS were mixed, and 3T3 cells were added to a final concentration of 2500 cells/well in 0.35% agarose. Cells were cultured for 3 weeks before imaging for colony formation.45
Mouse model of hind limb ischemia
Wild-type C57BL/6 mice were used in the hind limb ischemia studies (five mice per group). The mice were anesthetized by using isofluorane gas, and the femoral artery was exposed through an incision in the inguinal region. The artery was separated from the femoral nerve and vein, and it was then ligated in two locations by using a 6-0 silk suture. Alginate beads containing the exosomes (∼13 billion exosomes/mouse) and/or FGF-2 (1.5 μg/mouse) were implanted in a total volume of 200 μL. The incision was then closed by using degradable sutures. Relative blood flow between the ischemic and the contralateral control limb was measured at days 1, 3, 5, 7, and 14 by using laser speckle imaging as previously described.47 Briefly, the hind paws of the mouse were illuminated by a 785 nm, 50 mW laser diode (Thor Labs) and imaged by using a Zoom-7000 lens (Navitar) and a Bassler CCD camera. Relative perfusion for the hindlimb ischemia study was quantified and normalized to the contralateral limb as a control. At day 14, the mice were sacrificed and the tissues of the hind limb were harvested and frozen in liquid N2-cooled isopentane or fixed in formalin before histological processing. Immunostaining for platelet endothelial cell adhesion molecule (PECAM-1) was performed as previously described.36 All quantifications were performed on 10 fields of view of images taken at low magnification on the tissues. For the quantification of ischemic fibers, we defined ischemic fiber changes as loss of muscle fibers within the bundles. These changes appear as round holes in the muscle fibers in contrast to cracks that form as artifacts of histological processing. We had used a similar definition in previous studies and found that it correlated well with perfusion recovery in the hind limb ischemia model.31,36 All animal procedures were approved by the Institutional Animal Care and Use Committee of UT Austin and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Exosome small RNA sequencing
The glioblastoma cell lines (A-172 cells) were grown to 70% confluence. The media were then changed to exosome-depleted media, and cells were further cultured for 48 h. The media were then collected, and the exosomes were isolated from the media by using Exoquick TC (Systems Bioscience). The total RNA was isolated from the isolated exosomes by using TRIzol. The quality of the isolated RNA was assessed by using the Agilent Bioanalyzer 2100 from the Functional Genomics Laboratory in the University of California in Berkeley. An RIN score higher than nine qualified the sample for cDNA production. To construct the library, 1 μg of total RNA was used to isolate poly(A) purified mRNA. Average fragment sizes were 400 bp. Sequencing was done with an Ilumina HiSeq 2500, and each sample had 25–29 million 100 bp end reads. Read alignment was done by mapping to the mouse reference genome (UCSC version mm9) by using Tophat48, and HTSeq49 was used to sum mapped reads for gene expression levels. DEseq was used to normalize the read counts.50
Statistical analysis
All results are shown as mean ± standard error of the mean. Comparisons between only two groups were performed by using a two-tailed Student's t-test. Differences were considered significant at p < 0.05. Multiple comparisons between groups were analyzed by two-way ANOVA followed by a Tukey post hoc test. A two-tailed probability value p < 0.05 was considered statistically significant.
Results
Characterization of size and morphology of glioblastoma exosomes
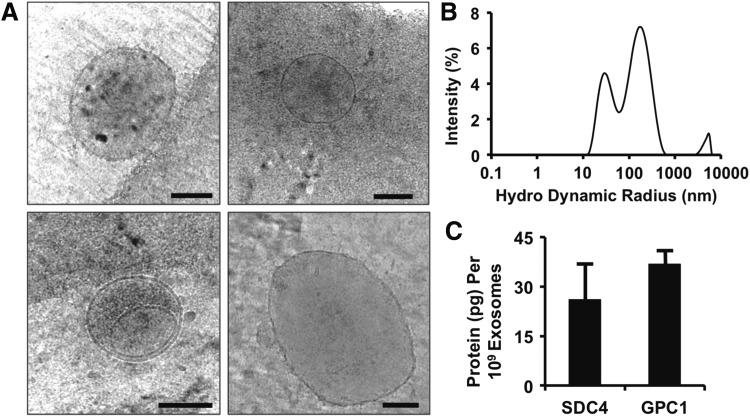
We isolated exosomes from glioblastoma cells and analyzed them by using cryo-electron microscopy. We found a heterogeneous mix of vesicles that were predominantly in the range of 30–100 nm but also included a significant portion of larger microvesicles and exosome aggregates (Fig. 1A). Analysis with dynamic light scattering (DLS) revealed a size distribution that included two peaks with maximums of 28 and 164 nm (Fig. 1B). Our group has recently shown that delivery of syndecan-4 or glypican-1 containing vesicles enhances angiogenic growth factor therapy.32,34 Consequently, we performed an ELISA for syndecan-4 and glypican-1 on the exosomes and found that there were high concentrations of both proteins in the isolated exosomes (Fig. 1C).
FIG. 1.
Characterization of exosomes isolated from glioblastoma cell line. (A) Cryo-electron microscopy of isolated exosomes and microvesicles. Scale bar =100 nm. (B) Size distribution of isolated vesicles from cultured glioblastoma cells measured by dynamic light scattering. (C) Protein concentration of syndecan-4 (SDC4) and glypican-1 (GPC1) in the exosomes.
Analysis of small RNA in glioma-derived exosomes
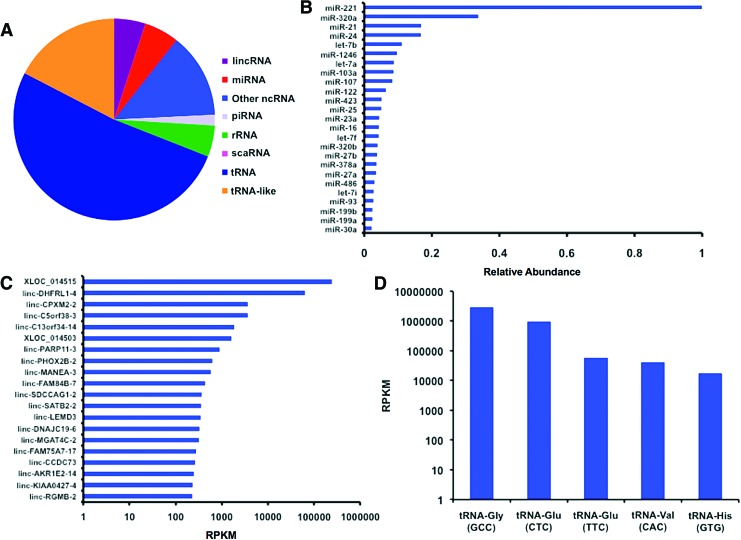
A major mechanism in the angiogenic activity of exosomes is the delivery of pro-angiogenic miRNAs.22 We used next-generation RNA sequencing on the exosome samples to examine small RNA content of the isolated exosomes (Fig. 2). Although there was significant content of miRNA in the samples, there were higher levels of transfer RNA (tRNA) and tRNA-like small RNAs (Fig. 2A). The most abundant was miR-221, which was present at threefold higher levels over other miRNAs (Fig. 2B). This miRNA is key in endothelial tip function in vascular development.51 We then looked at the angiogenic potential of these miRNA and categorized their association with cancer proliferation and metastasis (Table 1). Overall, there appeared to be angiogenic properties for many of the most abundant miRNAs present, including miR-21, miR-24, miR-1246 miR-103, and miR-107. However, there were also several miRNAs that have been associated with anti-angiogenesis in tumor and anti-growth signaling including miR-320a, let-7b, and miR-122. An analysis of the long intergenic non-coding RNA (lincRNA) revealed relatively high levels of XLOC_014515 and linc-DHFRL1-4 (mitochondrial dihydrofolate reductase; Fig. 2C). These lincRNAs were expressed at several orders of magnitude over all the other lincRNAs present. Among the tRNAs, tRNA-Gly and tRNA-Glu were the most predominant and were present at orders of magnitude over the other tRNAs (Fig. 2D).
FIG. 2.
MicroRNA content of exosomes measured by using miRNA-seq. (A) Relative composition of the small RNAs present in the isolated exosome samples. (B) Relative abundance of mature miRNAs detected in the isolated glioblastoma exosomes. All miRNA abundances were normalized to the most abundant miRNA (miR-221). (C) Expression levels of lincRNA in the glioma-derived exosomes. (D) Predominant tRNAs present in the glioma-derived exosomes. lincRNA, long intergenic non-coding RNA; miRNA, microRNA. Color images available online at www.liebertpub.com/tea
Table 1.
Relative Abundance of Mature microRNA in Glioblastoma Exosomes
| Abundance | miRNA | Function |
|---|---|---|
| 1.000 | miR-221 | Plays a role in new vessel formation,51,61 upregulates proliferation56 and migration62 |
| 0.337 | miR-320a | Inhibits cell proliferation, migration, and anti-angiogenesis in tumors63,64 |
| 0.168 | miR-21 | Enhances angiogenesis,65,66 anti-oncogenic,67 promotes metastasis68 and proliferation69 |
| 0.168 | miR-24 | Downregulates cardiac tissue angiogenesis,70 upregulates tumor angiogenesis and apoptosis,71 and upregulates proliferation/metastasis72 |
| 0.111 | let-7b | Linked to reduced tumor angiogenesis,73 inhibits glioblastoma cell migration74 |
| 0.097 | miR-1246 | Linked to increased angiogenesis,75 promotes growth and metastasis76 |
| 0.088 | let-7a | Inhibits glioma malignancy77 |
| 0.087 | miR-103 | Upregulates VEGF, angiogenesis,78 and metastasis79 and downregulates proliferation80 |
| 0.083 | miR-107 | Upregulates angiogenesis poststroke,81 downregulates tumor angiogenesis82 |
| 0.064 | miR-122 | Inhibits tumor proliferation,83 inhibits metastasis84 |
| 0.052 | miR-423 | Upregulates proliferation85 |
| 0.050 | miR-25 | Associated with glioma progression86 |
| 0.044 | miR-23a | Downregulates angiogenesis,87 increases glioma progression88 |
| 0.043 | miR-16 | Suppresses angiogenesis,89 leads to apoptosis,90 and inhibits metastasis91 |
| 0.042 | let-7f | Inhibits tumor proliferation,92 inhibits metastasis93 |
| 0.039 | miR-320b | Downregulates proliferation94 |
| 0.038 | miR-27b | Downregulates VEGF-C,95 upregulates tumor proliferation96 and metastasis97 |
| 0.036 | miR-378a | Upregulates angiogenesis and tumor proliferation98 |
| 0.035 | miR-27a | Promotes proliferation and migration99 |
| 0.030 | miR-486 | Promotes angiogenesis and proliferation100 |
| 0.028 | let-7i | Leads to cell death101 |
| 0.027 | miR-93 | Induces angiogenesis, proliferation, and migration102 |
| 0.024 | miR-199a | Downregulates angiogenesis103 and proliferation104 |
| 0.024 | miR-199b | Promotes angiogenesis,105 promotes cell proliferation and migration106 |
| 0.021 | miR-30a | Promotes angiogenesis107 and migration,108 can lead to apoptosis109 |
miRNA, microRNA; VEGF, vascular endothelial growth factor; VEGF-C, vascular endothelial growth factor-C.
Glioblastoma exosomes enhance proliferation and tube formation in endothelial cells
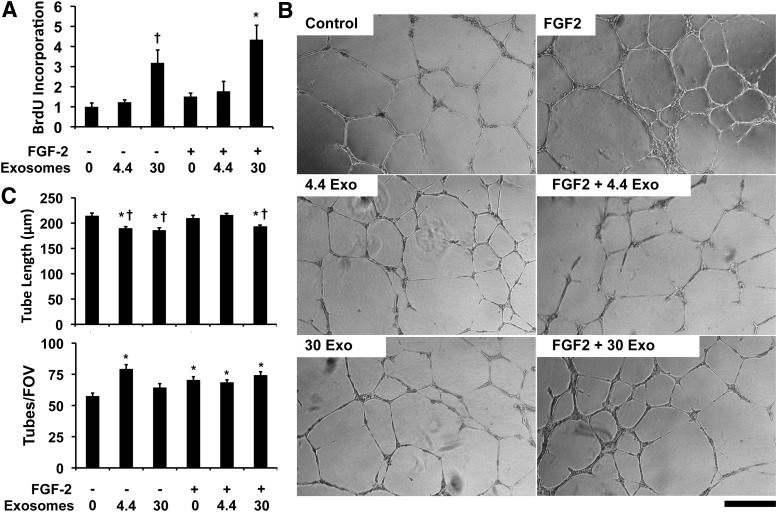
We next examined whether exosomes could alter the behavior of endothelial cells in culture. As our previous work has shown that proteoliposomes with syndecan-4 or glypican-1 can increase the activity of FGF-2,30–34,36 we treated the endothelial cells with exosomes in combination with FGF-2. We found that high levels of exosomes had synergistic activity with FGF-2 in increasing endothelial cell proliferation (Fig. 3A). We next tested the effect of exosomes in altering tube formation in endothelial cells. The exosomes increased the number of tubes formed at lower concentrations (Fig. 3B, C). In addition, the exosomes reduced the tube length of the network formed both with and without FGF-2 treatment. To assess whether exosomes induced the expression of angiogenic growth factors in endothelial cells, we treated HUVECs with exosomes at the higher concentration used in the tube formation assay. We collected conditioned media from the cells and assayed the concentration of 55 growth factors by using an antibody array. We found that there was increased interleukin-8 (IL-8) and angiopoietin-2 (Ang-2) with exosome treatment but there were no other significant increases in angiogenesis-related growth factors in the conditioned media of exosome-treated endothelial cells versus control cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). IL-8 has been linked to proangiogenic activity52,53 and Ang-2 regulated angiogenesis in a context-dependent manner.54 To assess the potential tumorigenic properties of the exosomes, we treated NIH 3T3 fibroblasts for 2 weeks with glioblastoma exosomes at the high concentration used in the angiogenesis study. We then performed an anchorage-independent transformation assay (soft-agar assay) and found that there was no colony formation with the exosome treatment (Supplementary Fig. S2).
FIG. 3.
Glioblastoma exosomes increase endothelial cell proliferation and reduce tube length in tube formation assay. (A) Proliferation of endothelial cells measured by BrdU incorporation after 24 h of treatment with FGF-2 or FGF-2 and exosomes. *Statistically significant different from groups treated with low concentrations of exosomes with and without FGF-2 (p < 0.05). †Statistically significant difference from the control or FGF-2-treated cells (p < 0.05). (B) Endothelial cells were grown on Matrigel and treated with exosomes (108/mL) and/or 10 ng/mL FGF-2. After 16 h, the formation of tubes was assessed by phase-contrast microscopy. (C) Quantification of tube length and tubes per field of view. *Statistically significant difference from control group (p < 0.05). †Statistically significant difference from FGF-2 and FGF-2 with low exosome dose groups (p < 0.05). Scale bar = 200 μm. FGF-2, fibroblast growth factor-2.
Local delivery of glioblastoma exosomes enhances therapeutic angiogenesis in the ischemic hind limb of mice
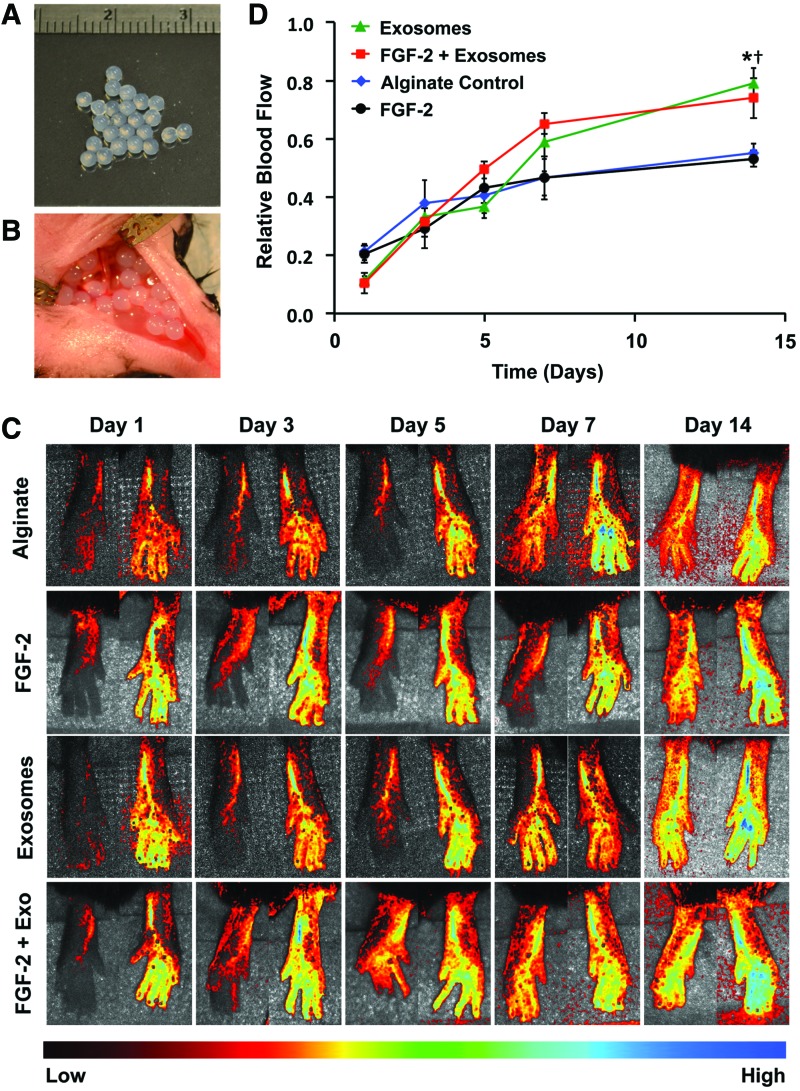
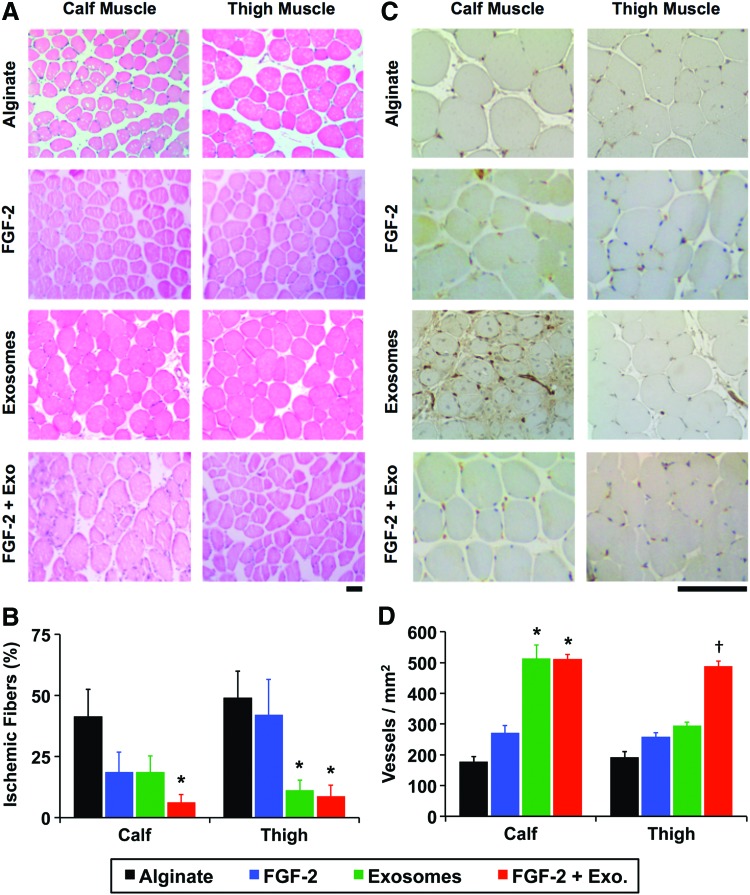
The delivery of FGF-2 enhances revascularization in some animal models of ischemia.32,34 Our group has recently shown that delivering proteoliposomes that contain syndecan-4 or glypican-1 in combination with growth factors markedly improves revascularization in healthy and diabetic animals.32,34 We encapsulated exosomes in alginate gels and found that they were nearly completely released over 7 days (Supplementary Fig. S3). We created ischemia in the hind limb of mice by ligating the femoral artery and implanted alginate beads with exosomes or a combination of exosomes and FGF-2 (Fig. 4A, B). Exosomes enhanced the recovery of perfusion in the hind limbs, with the exosomes alone or exosomes with FGF-2, restoring ∼80% of the perfusion relative to the control limb after 14 days (Fig. 4C, D). We have performed nontreated controls in previous studies and found that they did not have a significant difference with alginate-treated mice, using the identical formulation of alginate used in this study, and found there is around 50% recovery of perfusion after 14 days.31,32,36 In addition, we found a reduction in the ischemic muscle fiber changes on histological analysis of the ischemic limbs in the groups treated with exosomes or exosomes and FGF-2 (Fig. 5A, B). We immunostained for the vessels within the muscles and found increased capillary density in the leg muscles in the animals treated with exosomes or exosomes and FGF-2, consistent with the increased perfusion observed by laser speckle imaging (Fig. 5C, D).
FIG. 4.
Glioblastoma exosomes enhance therapeutic angiogenesis with FGF-2 in hind limb ischemia. (A) Glioblastoma exosomes were encapsulated in alginate beads. (B) Ischemia was induced in mice through femoral artery ligation, and the alginate beads were implanted at the time of surgery. (C) Laser speckle contrast imaging of blood perfusion in the feet of the mice over time. Mice were given alginate beads with either FGF-2 or FGF-2 with glioblastoma exosomes. (D) Analysis of perfusion of the feet after induction of hind limb ischemia. Relative blood flow was defined as the ratio between the ischemic limb and the control limb. *Statistically significant difference between FGF-2 with exosomes and alginate or FGF-2 groups (p < 0.05). †Statistically significant difference between exosome treatment group and alginate or FGF-2 treatment groups (p < 0.05). Color images available online at www.liebertpub.com/tea
FIG. 5.
Exosomes reduce muscle damage and enhance vascularity of the ischemic hind limb in mice. (A) Histological sections from the hind limb of mice with femoral artery ligation. Scale bar = 50 μm. (B) The number of muscle fibers with degradation was reduced in the calf muscle with FGF-2 and exosome treatment in comparison to FGF-2 alone. *Statistically significant difference from alginate group (p < 0.05). (C) Immunohistochemical staining for PECAM-1 (blood vessels) in the muscles of the limb. Scale bar =50 μm. (D) Quantification of vessel density within the muscles. *Statistically significant difference from alginate or FGF-2-alone group (p < 0.05). †Statistically significant differences in comparison to all other groups (p < 0.05). PECAM-1, platelet endothelial cell adhesion molecule. Color images available online at www.liebertpub.com/tea
Discussion
Exosomes are emerging therapeutics with applications ranging from cancer to cardiovascular disease. Several groups have shown that there are therapeutic benefits from treatment using mesenchymal stem cell-derived exosomes and, indeed, these effects may underlie the majority of positive results for cell therapy in clinical trials. Tumors require an ever-increasing vascular supply to maintain their rapid growth within the native tissue. In particular, glioblastomas show intense angiogenesis that maintains their growth within the brain.21 Here, we aimed at testing whether the powerful pro-angiogenic properties of tumor exosomes could be harnessed to treat ischemia in peripheral vascular disease. In the past, angiogenic therapies did not perform well in clinical trials.37 Thus, a robust and inhibition-resistant angiogenic stimulus from multiple sources within tumor exosomes may provide benefit where a single growth factor or gene would not. Our studies show that exosomes from glioblastoma can effectively induce angiogenesis in the context of peripheral ischemia and contain proteoglycans and small RNAs that promote revascularization.
Our analysis of the content of glioblastoma revealed that the most abundant miRNA found in our isolated glioblastoma exosomes was miR-221, which has been shown to play an important role in the formation of new blood vessels.51 This miRNA has been linked specifically to angiogenesis in glioblastoma55 and increased glioblastoma cell proliferation.56 Of the 25 most common miRNAs present in the isolated exosomes, nine have been shown to promote angiogenesis whereas only four have been shown to inhibit angiogenic pathways (Table 1). From our studies and those of others, there is strong evidence that glioblastoma-derived exosomes provide strong angiogenic signaling. However, a potential concern with using exosomes derived from cancer cells is that they may provide signals that are either tumorigenic or may induce the growth or metastasis of an occult tumor in a patient being treated for ischemia. This concern has arisen in growth factor therapies where an existing cancer is a strong contraindication for therapy with platelet-derived growth factor-BB (PDGF-BB) for diabetic ulcers. Extracellular vesicles from glioblastoma cells have been shown to carry proteins that have both oncogenic and tumor-suppressive activities, including epidermal growth factor (EGF) receptors, platelet-derived growth factor receptor alpha (PDGFR-A), and phosphatase and tensin homolog (PTEN).23,57–59 Thus, although it is well supported that glioblastoma exosomes enhance angiogenesis, it is less clear what role they play in tumor progression and metastasis. In our study, there were miRNAs in the exosomes that both support and inhibit tumor proliferation and metastasis (Table 1). Thus, it is unclear what the ultimate response of an existing cancer would be to the exosome therapy. Notably, none of the miRNAs that were most abundant in the exosomes are currently known to be linked to tumorigenesis. Interestingly, there was greater abundance of tRNA in the glioma-derived exosomes than miRNAs and, in particular, high levels of tRNA-Gly and tRNA-Glu. A recent study demonstrated that increased levels of fragments of tRNA-Val and tRNA-Gly were found in brain ischemia, mouse hind limb ischemia, and a cellular hypoxia model.60 In contrast to our study, tRNA-Val was the predominant tRNA and these fragments inhibited endothelial cell proliferation, migration, and tube formation.60 Thus, it is possible that the high levels of tRNA-Gly/Glu in the exosomes may also play a role in the pro-angiogenic effects observed in inducing revascularization in ischemia.
Our group has recently shown that proteoliposomes carrying proteoglycans, including both syndecan-4 and glypican-1, are highly effective in enhancing growth factor therapies.30–34,36 This is particularly important for inducing therapeutic angiogenesis in disease states such as diabetes, where there is a loss in these critical growth factor receptors.32 Our study supports the fact that these proteins are found in a significant concentration in the exosomes, although at a lower concentration than used in the proteoliposomes used in our prior studies. Our results suggest that exosomes act in mechanisms in addition to direct stimulation of endothelial cells. Our results from the tube formation assay show an increase in tubes with exosome treatment but a reduction in tube length. This may suggest that exosomes induce very dense vascular network formation. In our study, the exosomes provided intense growth signals to endothelial cells and a moderate effect on in vitro tube formation. However, the in vivo effect of exosomes on blood vessel growth and recovery of perfusion was very strong, suggesting that the exosomes act on other cells in addition to endothelial cells. Analysis of the blood vessel formation in mice suggested that this result was primarily from capillary growth in calf muscles rather than arteriogenesis. This is consistent with our findings in the tube formation assay that showed small capillary-like branching in response to exosome treatment.
In summary, we have shown that glioblastoma exosomes have potential as therapeutics for ischemia. Our studies support the fact that the exosomes strongly induce angiogenesis in vivo and contain a host of pro-angiogenic signals that act through multiple mechanisms. This would likely be highly beneficial as treatments for severe ischemia given the presence of therapeutic resistance in long-term disease that may prevent efficacy of single-growth factors or genes. Thus, exosomes as therapeutics may be applicable to many disease states and conditions that would benefit from enhanced capillary formation.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support through the Welch Foundation (F-1836) and the NIH Director's New Innovator Grant (1DP2 OD008716-01) to A.B.B.
Disclosure Statement
The authors have filed a patent on the technology described in this work.
References
- 1.Raposo G., and Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200, 373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denzer K., Kleijmeer M.J., Heijnen H.F., Stoorvogel W., and Geuze H.J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113 Pt 19, 3365, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Cocucci E., and Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25, 364, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Verma M., Lam T.K., Hebert E., and Divi R.L. Extracellular vesicles: potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin Pathol 15, 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melo S.A., et al. . Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoefer I.E., et al. . Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J 36, 2635, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Baietti M.F., et al. . Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14, 677, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Andreu Z., and Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol 5, 442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauro B.J., et al. . Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics 12, 587, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastpar R., et al. . Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 65, 5238, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camacho L., Guerrero P., and Marchetti D. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One 8, e73790, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakur B.K., et al. . Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24, 766, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Record M., Carayon K., Poirot M., and Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841, 108, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Ohno S., et al. . Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21, 185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribeiro M.F., Zhu H., Millard R.W., and Fan G.C. Exosomes function in pro- and anti-angiogenesis. Curr Angiogenes 2, 54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan G.C. Hypoxic exosomes promote angiogenesis. Blood 124, 3669, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Hood J.L., et al. . Paracrine induction of endothelium by tumor exosomes. Lab Invest 89, 1317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi H., et al. . High-grade ovarian cancer secreting effective exosomes in tumor angiogenesis. Int J Clin Exp Pathol 8, 5062, 2015 [PMC free article] [PubMed] [Google Scholar]
- 19.Hu G.W., et al. . Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther 6, 10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shabbir A., Cox A., Rodriguez-Menocal L., Salgado M. and Van Badiavas, E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cell Dev 24, 1635, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain R.K., et al. . Angiogenesis in brain tumours. Nat Rev Neurosci 8, 610, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kucharzewska P., et al. . Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 110, 7312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skog J., et al. . Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10, 1470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S., Sun J., and Lan Q. Glioblastoma microvesicles promote endothelial cell proliferation through Akt/beta-catenin pathway. Int J Clin Exp Pathol 7, 4857, 2014 [PMC free article] [PubMed] [Google Scholar]
- 25.de Vrij J., et al. . Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer 137, 1630, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Jetten N., et al. . Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17, 109, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Zajac E., et al. . Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 122, 4054, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama K., et al. . Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 170, 1178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khanna S., et al. . Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5, e9539, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S., Majid M., and Baker A.B. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomater 42, 56, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Das S., et al. . Syndecan-4 enhances therapeutic angiogenesis after hind limb ischemia in mice with type 2 diabetes. Adv Healthc Mater 5, 1008, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S., Singh G., and Baker A.B. Overcoming disease-induced growth factor resistance in therapeutic angiogenesis using recombinant co-receptors delivered by a liposomal system. Biomaterials 35, 196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das S., et al. . Syndesome Therapeutics for Enhancing Diabetic Wound Healing. Adv Healthc Mater 5, 2248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang E., Albadawi H., Watkins M.T., Edelman E.R., and Baker A.B. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc Natl Acad Sci U S A 109, 1679, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi R., et al. . An antiangiogenic isoform of VEGF-A contributes to impaired vascularization in peripheral artery disease. Nat Med 20, 1464, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monteforte A.J., et al. . Glypican-1 nanoliposomes for potentiating growth factor activity in therapeutic angiogenesis. Biomaterials 94, 45, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annex B.H. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol 10, 387, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Johnsen K.B., et al. . A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 1846, 75, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Tang X.J., et al. . Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget 6, 44179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosca A.M., Rayia D.M., and Tutuianu R. Emerging role of stem cells—derived exosomes as valuable tools for cardiovascular therapy. Curr Stem Cell Res Ther 12, 134, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Lai R.C., et al. . Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4, 214, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., et al. . Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192, 61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicencio J.M., et al. . Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65, 1525, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Sherman M.B., et al. . Removal of divalent cations induces structural transitions in red clover necrotic mosaic virus, revealing a potential mechanism for RNA release. J Virol 80, 10395, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borowicz S., et al. . The soft agar colony formation assay. J Vis Exp e51998, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Removed
- 47.Voyvodic P.L., et al. . Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem 289, 9547, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trapnell C., Pachter L., and Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anders S., Pyl P.T., and Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anders S., and Huber W. Differential expression analysis for sequence count data. Genome Biol 11, R106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicoli S., Knyphausen C.-P., Zhu L.J., Lakshmanan A., and Lawson N.D. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell 22, 418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ning Y., et al. . Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 128, 2038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li A., Dubey S., Varney M.L., Dave B.J., and Singh R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170, 3369, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Felcht M., et al. . Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest 122, 1991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang F., et al. . MiR-221/222 promote human glioma cell invasion and angiogenesis by targeting TIMP2. Tumor Biol 36, 3763, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Novakova J., Slaby O., Vyzula R., and Michalek J. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Communications 386, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Al-Nedawi K., Meehan B., Kerbel R.S., Allison A.C., and Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 106, 3794, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bronisz A., et al. . Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res 74, 738, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Putz U., et al. . The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal 5, ra70, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Li Q., et al. . tRNA-derived small non-coding RNAs in response to ischemia inhibit angiogenesis. Sci Rep 6, 20850, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poliseno L., et al. . MicroRNAs modulate the angiogenic properties of HLTVECs. Blood 108, 3068, 2006 [DOI] [PubMed] [Google Scholar]
- 62.He S., et al. . Downregulation of miR-221 inhibits cell migration and invasion through targeting methyl-CpG binding domain protein 2 in human oral squamous cell carcinoma cells. BioMed Res Int 2015, 751672, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., et al. . Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74, 139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X.H., et al. . MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol 36, 181, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Hermansen S.K., Nielsen B.S., Aaberg-Jessen C., and Kristensen B.W. miR-21 is linked to glioma angiogenesis: a co-localization study. J Histochem Cytochem 64, 138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., et al. . STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett 370, 125, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Kasinski A.L., and Slack F.J. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 11, 849, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh M., et al. . STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget 6, 27461, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z., Yang H., and Ren L. MiR-21 promoted proliferation and migration in hepatocellular carcinoma through negative regulation of Navigator-3. Biochem Biophys Res Commun 464, 1228, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Fiedler J., et al. . MicroRNA-24 regulates vascularity after myocardial infarction. Circulation 124, 720-U178, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Liu R., et al. . The miR-24-Bim pathway promotes tumor growth and angiogenesis in pancreatic carcinoma. Oncotarget 6, 43831, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H., et al. . Onco-miR-24 regulates cell growth and apoptosis by targeting BCL2 L11 in gastric cancer. Protein Cell 7, 141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jusufovic E., et al. . let-7b and miR-126 are down-regulated in tumor tissue and correlate with microvessel density and survival outcomes in non-small-cell lung cancer. PLoS One 7, e45577, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Y., et al. . MicroRNAs let-7b/i suppress human glioma cell invasion and migration by targeting IKBKE directly. Biochem Biophys Res Commun 458, 307, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Yamada N., et al. . Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim Biophys Acta Gene Regul Mech 1839, 1256, 2014 [DOI] [PubMed] [Google Scholar]
- 76.Wang S., et al. . MicroRNA-1246 promotes growth and metastasis of colorectal cancer cells involving CCNG2 reduction. Mol Med Rep 13, 273, 2016 [DOI] [PubMed] [Google Scholar]
- 77.Wang X.-R., et al. . Overexpressed let-7a inhibits glioma cell malignancy by directly targeting K-ras, independently of PTEN. Neuro Oncol 15, 1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z., et al. . Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest 123, 1057, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martello G., et al. . A microRNA targeting dicer for metastasis control. Cell 141, 1195-U1176, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Fu X., et al. . MicroRNA-103 suppresses tumor cell proliferation by targeting PDCD10 in prostate cancer. Prostate 76, 543, 2016 [DOI] [PubMed] [Google Scholar]
- 81.Li Y., et al. . MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep 5, 13316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L., et al. . Upregulation of miR-107 inhibits glioma angiogenesis and VEGF expression. Cell Mol Neurobiol 36, 113, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang G.Z., Zhao Y., and Zheng Y.R. miR-122/Wnt/beta-catenin regulatory circuitry sustains glioma progression. Tumor Biol 35, 8565, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Bai S., et al. . MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem 284, 32015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li H.T., Zhang H., Chen Y., Liu X.F., and Qian J. MiR-423-3p enhances cell growth through inhibition of p21Cip1/Waf1 in colorectal cancer. Cell Physiol Biochem 37, 1044, 2015 [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., et al. . miR-25 promotes glioma cell proliferation by targeting CDKN1C. Biomed Pharmacother 71, 7, 2015 [DOI] [PubMed] [Google Scholar]
- 87.Kwok H.-H., Chan L.-S., Poon P.-Y., Yue P.Y.-K., and Wong R.N.-S. Ginsenoside-Rg(1) induces angiogenesis by the inverse regulation of MET tyrosine kinase receptor expression through miR-23a. Toxicol Appl Pharmacol 287, 276, 2015 [DOI] [PubMed] [Google Scholar]
- 88.Lian S., et al. . Anti-miRNA-23a oligonucleotide suppresses glioma cells growth by targeting apoptotic protease activating factor-1. Curr Pharm Des 19, 6382, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Lee J.K., et al. . Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One 8, 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salerno E., et al. . Correcting miR-15a/16 genetic defect in New Zealand Black mouse model of CLL enhances drug sensitivity. Mol Cancer Ther 8, 2684, 2009 [DOI] [PubMed] [Google Scholar]
- 91.Wu W.-L., Wang W.-Y., Yao W.-Q., and Li G.-D. Suppressive effects of microRNA-16 on the proliferation, invasion and metastasis of hepatocellular carcinoma cells. Int J Mol Med 36, 1713, 2015 [DOI] [PubMed] [Google Scholar]
- 92.Yan S., et al. . Let-7f inhibits glioma cell proliferation, migration, and invasion by targeting periostin. J Cell Biochem 116, 1680, 2015 [DOI] [PubMed] [Google Scholar]
- 93.Liang S., et al. . MicroRNA Let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One 6, e18409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H.T., et al. . miR-320b suppresses cell proliferation by targeting c-Myc in human colorectal cancer cells. BMC Cancer 15, 9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu H.-T., et al. . MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget 6, 37467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen L., et al. . Expression and function of miR-27b in human glioma. Oncol Rep 26, 1617, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Zhang S., et al. . Elevation of miR-27b by HPV16 E7 inhibits PPAR gamma expression and promotes proliferation and invasion in cervical carcinoma cells. Int J Oncol 47, 1759, 2015 [DOI] [PubMed] [Google Scholar]
- 98.Lee D.Y., Deng Z., Wang C.-H., and Yang B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A 104, 20350, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma Y., Yu S., Zhao W., Lu Z. and Chen, J. miR-27a regulates the growth, colony formation and migration of pancreatic cancer cells by targeting Sprouty2. Cancer Lett 298, 150, 2010 [DOI] [PubMed] [Google Scholar]
- 100.Shi X.-F., et al. . MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem Biophys Res Commun 470, 670, 2016 [DOI] [PubMed] [Google Scholar]
- 101.Wu K., Yang Y., Zhao J., and Zhao S. BAG3-mediated miRNA let-7 g and let-7i inhibit proliferation and enhance apoptosis of human esophageal carcinoma cells by targeting the drug transporter ABCC10. Cancer Lett 371, 125, 2016 [DOI] [PubMed] [Google Scholar]
- 102.Fang L., et al. . MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle 11, 4352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu G.T., et al. . CCL5 promotes vascular endothelial growth factor expression and induces angiogenesis by down-regulating miR-199a in human chondrosarcoma cells. Cancer Lett 357, 476, 2015 [DOI] [PubMed] [Google Scholar]
- 104.Zeng J., et al. . MicroRNA-199a-5p regulates the proliferation of pulmonary microvascular endothelial cells in hepatopulmonary syndrome. Cell Physiol Biochem 37, 1289, 2015 [DOI] [PubMed] [Google Scholar]
- 105.Chen T., et al. . MicroRNA-199b modulates vascular cell fate during iPS cell differentiation by targeting the Notch Ligand Jagged1 and enhancing VEGF signaling. Stem Cells 33, 1405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeng H., et al. . Increased expression of microRNA-199b-5p associates with poor prognosis through promoting cell proliferation, invasion and migration abilities of human osteosarcoma. Pathol Oncol Res 22, 253, 2016 [DOI] [PubMed] [Google Scholar]
- 107.Jiang Q., et al. . miR-30a regulates endothelial tip cell formation and arteriolar branching. Hypertension 62, 592, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Dobson J.R., et al. . hsa-mir-30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell Int 14, 14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He R., et al. . MiR-30a-5p suppresses cell growth and enhances apoptosis of hepatocellular carcinoma cells via targeting AEG-1. Int J Clin Exp Pathol 8, 15632, 2015 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.