Abstract
Background:
The widespread availability of the coronary artery calcium scan to diagnose coronary artery atheroma semiquantitatively and its prognostic significance has frequently resulted in a difficult therapeutic decision for physicians caring for asymptomatic patients.
Patients and Risk Factors:
Of particular concern are patients over 40 years of age and young adults characterized by multiple cardiovascular risk factors. The correct prognostic interpretation of coronary artery calcium scores and the potential benefits and risks of various therapeutic modalities need to be understood.
Conclusion:
This review describes the therapeutic choices available to endocrinologists and provides recommendations for various treatment options.
Keywords: calcium scan, heart disease, coronary angiography, stress testing, statins, LDL cholesterol
The endocrinologist is often consulted concerning a positive coronary artery calcium scan in an asymptomatic patient. This article provides a pertinent data review and suggested clinical management.
The coronary artery calcium (CAC) scan has become popular for individuals at risk for atherosclerotic cardiovascular disease. Indeed, CAC testing is increasingly being promoted to the public as a means of self-assessment of cardiovascular risk. There are several reasons for the widespread use of the coronary calcium scan, including its relatively low cost ($50 to $200 in most cities), its excellent predictive value for atherosclerotic cardiovascular disease (ASCVD) and mortality [1, 2], the noninvasive nature of the test, the low-dose radiation exposure (equivalent radiation to living in Denver for 3 months), and the fact that a physician’s prescription is usually not required. The number of asymptomatic individuals in the high and intermediate risk categories in the United States is large and includes all diabetic patients and individuals with sufficient Framingham risk factors and/or genetic causes of hyperlipidemia. These individuals are often under the care of endocrinologists in concert with primary care physicians [3].
Decisions facing clinicians after the report of a positive coronary calcium scan in asymptomatic patients are (1) which medical therapy and lifestyle modifications to recommend, (2) how aggressively to reduce plasma low-density lipoprotein cholesterol (LDL-C), and (3) whether stress testing and/or referral to a cardiologist is indicated. This review discusses the pros and cons of these choices and makes recommendations based on the current medical literature. These recommendations apply only to asymptomatic patients without clinical evidence of heart disease and are primary prevention strategies. In asymptomatic patients with no report of angina but a positive calcium scan, one possibility is that the physician will order an exercise treadmill test. Excluding false-positive results (e.g., induced by spasm of a coronary artery), a positive result indicates an obstruction of a coronary artery by at least 50%. What is the best next step? The choice is between coronary angiography with the intention to place a stent at the site of a constriction or to rapidly initiate aggressive medical therapy to reverse the atherosclerosis and the obstruction. During the reversal period, collateral channels may develop, increasing blood flow around the obstruction. Because long-term follow-up (>10 years) data from patients with stable angina demonstrate that intensive medical therapy is as beneficial as placement of a stent (which is more invasive and costly), there is minimal reason to order an initial stress test in patients with stable angina [4, 5].
Although randomized clinical trials evaluating the treatment of patients on the basis of their CAC score have not been done, extensive epidemiological and observational data are compelling and deserve serious consideration to guide physicians in making correct choices to recommend to their patients. Although the Multi-Ethnic Study of Atherosclerosis (MESA) clinical trial is the most well-described study [6], other investigators have also published their observations in populations that have received coronary calcium scores. For example, Arad et al. [7] studied 1177 high-risk, asymptomatic, middle-aged adult subjects for 3.6 years. The mean coronary artery calcium score was 764 among subjects with events, compared with a mean score of 135 among those without events. They conclude that “in asymptomatic adults, EBCT (coronary calcium) predicts coronary death and non-fatal MI.” Alternatively, in a meta-analysis of five large population studies comprised of 6739 women at low cardiovascular risk, Kavousi et al. [8] reported that a positive calcium score was present in approximately one-third of individuals and was associated with an increased risk of cardiovascular events. Keelan et al. [9] performed CAC studies in 288 patients undergoing coronary angiography. After a follow-up period of 6.9 years, 22 patients experienced clinically important cardiovascular events. They conclude that “when a stepwise multivariable model was used, only age and CAC extent predicted hard events.” Kuller et al. [10] studied 532 elderly individuals (age >80 years) in the Cardiovascular Health Study-Cognition Study. They concluded that “CAC, as a marker for atherosclerosis, is a determinant of mortality and risk of coronary heart disease and MI.” In a study of the prevalence and severity of CAC in different racial groups, Lee et al. [11] studied 999 consecutive patients comprised of non-Hispanic white and non-Hispanic black racial groups. They conclude that “despite a worse cardiovascular profile, black Americans have significantly less CAC than white Americans.” In a study of CAC and the occurrence of myocardial infarction in 1153 individuals with and without diabetes, Raggi et al. [12] concluded that diabetes causes an accelerated progression of atherosclerosis and that coronary calcium monitoring is an effective method to assess treatment efficacy. Rodrigues et al. [13] studied 621 patients with type 1 diabetes and concluded that overweight and obesity were independently associated with CAC and that only obesity was associated with CAC progression. Shaw et al. [14] studied 9715 asymptomatic individuals with a median follow-up of 14.6 years. They concluded that “the extent of CAC accurately predicts 15 year mortality in a large cohort of asymptomatic patients.” Finally, in a very large cohort of 44,052 individuals, Tota-Maharaj et al. [2] studied the predictive value of calcium scoring in both young and elderly individuals. They conclude that “the value of CAC for predicting mortality extends to both elderly patients and those less than 45 years old. Elderly persons with no CAC have a lower mortality rate than younger persons with high CAC.” The above studies are representative of many other studies that have examined the variables associated with CAC testing in asymptomatic individuals. This body of literature supports the conclusion that CAC testing is a major advance in noninvasive methodology to detect coronary artery disease and to predict future cardiovascular events and death.
1. Risk Factor Assessment and CAC Scoring
Prior to the availability of calcium scoring, combining cardiovascular risk factors was (and remains) a popular approach to determining an individual’s overall risk of future cardiovascular events. There are several approaches that combine several risk categories to attain an overall risk score for an individual patient (e.g., Framingham, American Heart Association, Reynolds, and MESA). One of the most popular is the Framingham Risk Score, in which several risk categories are entered into an online risk calculator and a 10-year cardiovascular risk score is generated [15]. The main differences between the various risk scores available on the internet are the specific risk categories entered and the weights given to each category of risk. In addition, these risk scores have each undergone several revisions in attempts to improve their prognostic accuracy. The major difficulty of using all risk scores is the fact that the information entered into the risk score database reflects only one point in time, making it difficult to predict the risk in an individual whose cardiovascular risks may have changed in the past [16]. In addition, the model used to generate the risk predictions are usually based on one specific population. For example, the Framingham Risk Score is based on the white population in Framingham, MA, and may not be applicable to other racial groups.
The score assigned to a CAC scan summarizes the degree and extent of calcium contained in the four major coronary arteries and thus defines the magnitude of cardiac atherosclerosis present. The most commonly used scoring system is the Agatston score [17], which involves identifying “regions of interest” that contain calcium within coronary arteries and determining the area of all lesions >1 mm2 in total area as well as the maximum calcific density for all lesions >130 Hounsfield Units. In spite of its limitations, the Agatston score has a long track record of proven utility in predicting new ASCVD events and remains the most widely used CAC scoring method for clinical application [18].
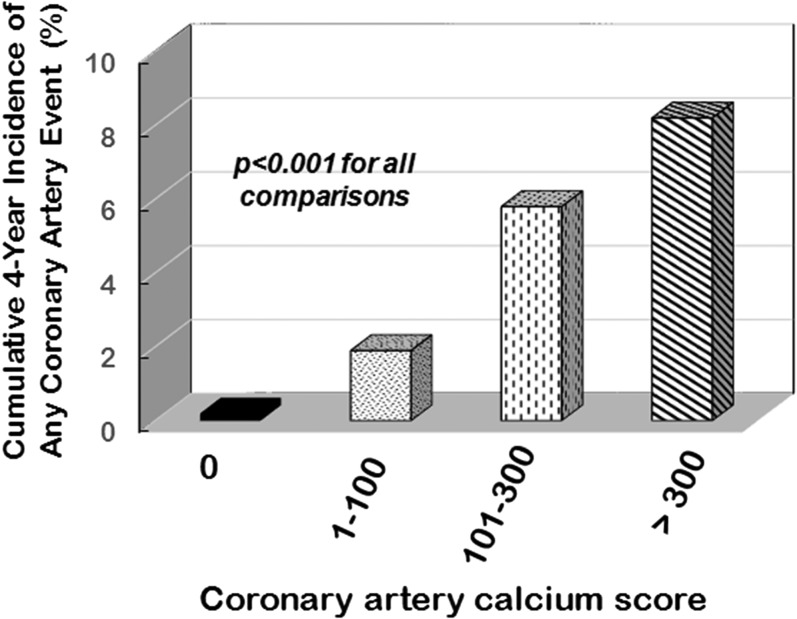
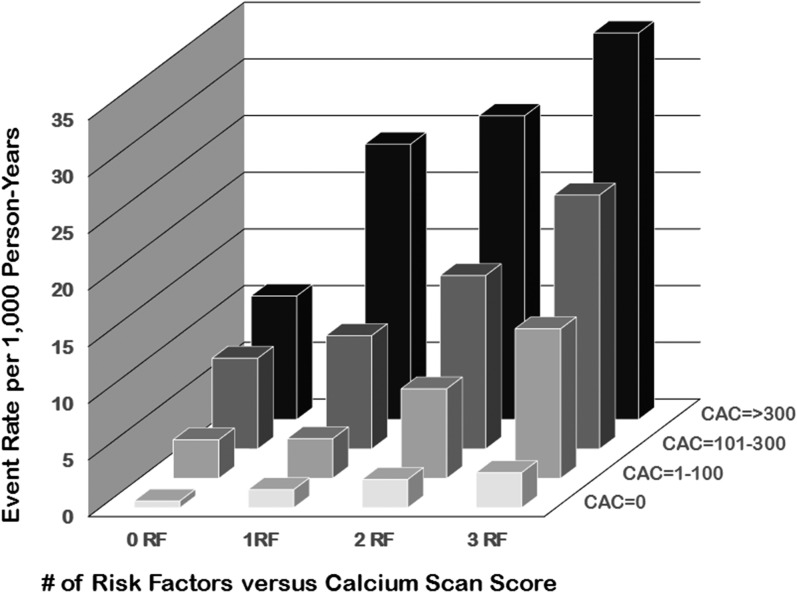
Although coronary angiography has often been considered the “gold standard” approach to defining the severity of atherosclerosis, recent studies have shown that CAC scores are better predictors of future cardiovascular events in asymptomatic individuals [19–22]. For example, in the MESA study, 6722 men and women were followed for a median of 3.8 years (Fig. 1) [23]. As shown in Fig. 2, when combined with traditional risk factors, the baseline CAC score predicted future cardiovascular events with improved accuracy. When the Framingham Risk score and the CAC score are combined, an improved prognostic outcome can be calculated [1]. Easy access to this improved, validated tool for cardiovascular event prediction is available at https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx [24]. The presence of calcium in coronary arteries is very strong evidence of the presence of atherosclerotic plaque [19]. The calcium should be considered a sign of atheroma presence and not necessarily a threat for future cardiovascular events. In fact, the majority of infarction-associated arterial thromboses are due to rupture of noncalcified, nonobstructive plaques [26].
Figure 1.
Cumulative incidence of any coronary event over 4 to 5 years of follow-up after receiving a CAC score evaluation among 6722 multiethnic men and women without preexisting cardiovascular disease in the MESA study. Adapted from Detrano et al. [23].
Figure 2.
The importance of both the calcium score and the number of risk factors in determining the prognosis of individuals. The composite scores may be easily obtained by using the MESA risk calculator as described in the text. Adapted from Silverman et al. [25]. The risk factors considered to construct the bar graphs were: current smoking, hypertension, diabetes, and a family history of coronary heart disease. Definitions of these risk factors are provided in Silverman et al. [25].
2. Stress Testing
Stress testing is often recommended to exclude potentially dangerous coronary artery disease. This approach involves stressing the cardiovascular system either with standardized exercise or stimulating the cardiovascular system with medication. The primary purpose is to identify coronary artery obstructive lesions with greater than 50% luminal obstruction. However, stress testing in asymptomatic individuals has low sensitivity and specificity (45% to 60%) [27]. The cost of the test can range between $300 and $700 depending on the institution and whether radioactive isotopes are used. Of note is that the risk to benefit ratio for adverse events increases in low-risk individuals [27].
In many locations, stress testing is performed after referring the asymptomatic patient to a cardiologist. After a positive stress test, the next step is usually coronary angiography to identify obstructive lesions. A recent review of coronary angiography recommends caution in the use of this test because (1) the resolution of coronary angiography is low; (2) the obtained images are two dimensional, making it difficult to define the shape of the vessel; and (3) the assessment of obstruction does not include the presence of previously developed collateral vessels, which may provide adequate blood flow past the obstruction [28]. According to the US Preventive Services Task Force: “The primary tangible harm of screening exercise tolerance testing is the potential for medical complications related to cardiac catheterization done to further evaluate a positive result. Coronary angiography is generally considered a safe procedure. Of all persons undergoing outpatient coronary angiography, however, an estimated 0.08% will die as a result of the procedure and 1.8% will experience a complication. Complications of coronary angiography include myocardial infarction, stroke, arrhythmia, dissection of the aorta and coronary artery, retroperitoneal bleeding, femoral artery aneurysm, renal dysfunction, and systemic infection” [29]. In addition, the charges for this test are between $5000 and $10,000 at most locations, and the patient’s copay for the procedure may approach 50% [30].
The greatest objection to stress testing and coronary angiography is the fact that the identification of an obstructive lesion does not usually identify future causative sites of myocardial infarctions. Because myocardial infarctions almost always result from unstable atherosclerotic plaques that rupture into the coronary artery lumen, coronary angiography identification of an obstructive lesion does not usually identify alternative causative sites of future myocardial infarctions [26]. Unstable plaques may be far removed from the lumen-narrowing lesions observed during coronary angiography. Thus, in asymptomatic individuals who have no anginal symptoms during their normal daily activities, the benefit from ordering stress testing or coronary angiography is questionable. For the above reasons, stress testing in asymptomatic individuals has not been shown to reduce future cardiovascular events [29]. Stress testing guidelines generally discourage using this approach in asymptomatic individuals.
3. Reversibility of Atherosclerosis
New information concerning the pathogenesis of atherosclerosis has become available during the last decade [31]. Both hyperlipidemia and inflammation play critical roles with other cardiovascular risk factors, such as hypertension and diabetes, increasing cardiovascular risk [32]. Risk factors damage the arterial endothelium, which then permits increased transmigration of LDL particles. Oxidized LDL particles attract increased numbers of inflammatory cells, which promote atherosclerotic plaque formation [33].
On the positive side, there is also ample evidence that atherosclerosis is a reversible disease. Several controlled trials have demonstrated that reversibility occurs at an LDL-C of approximately 65 mg/dL, assuming other risk factors are also addressed [34–39]. Statins, which are the mainstay of antiatherosclerosis therapy, have both LDL-C lowering and anti-inflammatory activity [40]. These beneficial effects of statin therapy on atherosclerotic plaque composition can be observed within 1 month of starting statin therapy and are consistent with converting an unstable plaque to a stable plaque [41]. These observations provide a strong impetus for physicians to aggressively address risk factors, contributing to an initial positive calcium score.
4. Aggressive Medical Therapy
Most major professional organizations recommend an LDL-C <70 mg/dL for patients at increased ASCVD risk. The LDL-C principle, which was recently reviewed [42], states that the lower the LDL-C concentration, the lower the incidence of a cardiovascular atherosclerotic events (there is no lower threshold for a beneficial effect). This principle suggests that LDL-C level <70 mg/dL should be the goal for everyone with an elevated risk for cardiovascular disease and that an even lower LDL-C level should be the goal for individuals with multiple risk factors [42].
Achieving an LDL-C goal of <70 mg/dL is possible in almost all individuals without a genetic cause of severe hyperlipidemia. Moreover, no unexpected side effects have been observed at LDL-C levels <50 mg/dL [43]. Because most individuals were born with an LDL-C of between 50 and 70 mg/dL, these levels should not be considered abnormal [44]. In addition, the normal LDL-C range is 50 to 70 mg/dL for native hunter-gatherers, free-living primates, and other wild mammals (none of which develop atherosclerosis) [45]. Because all cells in the body have the capacity to synthesize cholesterol, additional cholesterol from circulating LDL-C is not required in adults for health, and the recent availability of high-potency statins, ezetimibe, and PCSK9 inhibitors make the goal of <70 mg/dL practical [46, 47].
5. Medical Therapy vs Percutaneous Intervention
A clinical trial comparing medical therapy alone vs percutaneous coronary intervention (PCI) alone in asymptomatic individuals with a positive calcium scan score would provide important information concerning the relative value of each therapy. Unfortunately, there are no published clinical trials addressing this issue. Alternatively, clinical trials are available that compare medical therapy vs PCI plus medical therapy in individuals with stable angina. These individuals usually do not have symptoms of angina at rest, although they may have angina with exercise. The conclusion of a meta-analysis examining these studies concluded no benefit of PCI beyond that achieved with optimal medical treatment except relief of angina, particularly for single-vessel disease [4, 48, 49]. In addition, the longest observational study available (15 years) demonstrated no difference in mortality between PCI and medical therapy in these patients [5]. Furthermore, what formerly was considered “optimal medical therapy” no longer meets current treatment guidelines because improved medications to treat atherosclerosis are now available [42].
6. Antihyperlipidemic Therapy
Assuming the LDL-C goal for asymptomatic individuals with a positive calcium score is <70 mg/dL, how should this be achieved? Most outcome trials include a statin, preferably a high-potency statin. Both atorvastatin and rosuvastatin are now generic, and one of these medications should be prescribed first. Because side effects of statins are dose related, the lowest possible dose should be used. This is particularly true because the lowest usual dose of a potent statin (10 mg) achieves the greatest percentage reduction in LDL-C (~75%) of the total response. Doubling the 10-mg dose usually results in only a 6% reduction in LDL concentration [50]. The reason for this reduced effect is that statins increase PCSK9 protein as the statin dose increases, which impairs their ability to progressively lower LDL-C [51]. Statins also indirectly increase the intestinal absorption of cholesterol by reducing the intrahepatic content of cholesterol [52]. For this reason, ezetimibe works well with statins by blocking cholesterol absorption from the gut with minimal side effects [53]. Based on these data, our initial treatment recommendation is to prescribe 10 mg of ezetimibe and 10 mg of either atorvastatin or rosuvastatin. This combination is well tolerated by almost all patients and results in a very major reduction (~50%) of LDL-c within 6 weeks [54].
Unfortunately, some patients are intolerant of statins or do not achieve LDL-C goals despite the addition of ezetimibe to the statin. Such patients may be ideal candidates for a PCSK9 inhibitor, but this will likely require preauthorization and a higher-tier copay level. In patients with a genetic cause of hypercholesterolemia, the combination of all three classes of drugs will usually succeed in dramatically reducing the LDL-C concentration.
7. American College of Cardiology/American Heart Association Guidelines
The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines have given CAC testing a class IIB rating, which means that there are no randomized control studies addressing CAC scoring and that conclusions may be based on one nonrandomized controlled study. In evaluating this rating, several issues should be considered. First, CAC testing was also discussed in the 2010 ACC/AHA guidelines, and these recommendations state that “measurement of CAC is reasonable for cardiovascular risk assessment in asymptomatic adults at intermediate risk (10% to 20% 10-year risk)” [55]. Second, the one cited study involved 6814 white, black, Hispanic, and Chinese men and women aged 45 to 84 years with no clinical cardiovascular disease who participated in the MESA [56]. This multicenter landmark study, from which many additional publications were generated, provides convincing data on the importance of calcium scoring to assess future cardiovascular risk [1, 57, 58]. Third, many additional studies have been published since the 2013 guidelines were published, validating the use of CAC testing in different populations (see specific studies cited above). Fourth, a randomized controlled study comparing CAC testing with placebo will never be successfully completed because of prohibitive ethical issues, the expense and long duration required (>10 years), and the necessity to maintain individuals at risk in an untreated state. Other frequently used tests that provide insight into the cardiovascular condition of an individual (e.g., the electrocardiogram, cardiac stress testing, ankle-brachial index, and coronary angiography) have also never been validated with a randomized controlled trial [59]. Fifth, a recent review of 19 different professional organizations that have also issued treatment guidelines for cardiovascular disease demonstrates very little agreement on diagnostic testing and treatment [60]. Guidelines published by the SHAPE (Screening for Heart Attack Prevention and Education) organization are in agreement with the recommendations in this article [61].
8. Recommendations
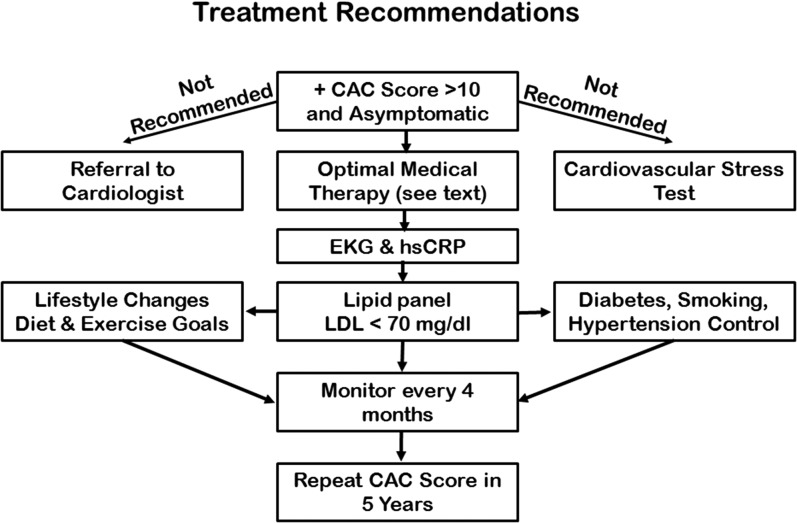
Considering the above cited data, our recommendations for asymptomatic individuals at rest with a positive CAC score are to immediately initiate lifestyle plus aggressive LDL-lowering therapy. Reassessment of the LDL concentration within 3 months with adjustment of the medication is critical to reversing atherosclerosis (Fig. 3). Referral for stress testing and cardiology consultation should be delayed until evidence of unstable angina exists. No immediate or long-term benefit to such individuals from either stress testing or coronary angiography has been documented in the literature. In addition, cardiac catheterization entails a major increase in cost to the insuring entity and to the patient as well as an increased risk for adverse events. Appropriate exercise, diet, and medications should be considered lifelong strategies to counteract the previous lifetime exposure to atherosclerotic risk factors [62].
Figure 3.
Treatment recommendations for an asymptomatic patient presenting with a positive calcium scan. The algorithm is based on aggressively addressing the four main cardiovascular risk factors, including abnormal lipids, hypertension, diabetes, and smoking through lifestyle improvement and medication prescription and adherence. EKG, electrocardiogram; hsCRP, high-sensitivity C-reactive protein.
9. Conclusions
Physicians will be faced with increasing numbers of asymptomatic individuals who have had CAC testing [3, 63, 64]. When this score is positive, patients are often concerned about their risk of having a cardiovascular event. In general, the higher the Agatston score, the greater the risk. Combining CAC scores with the Framingham risk score will further refine risk predictions. Once risk is defined, physicians should emphasize to patients that atherosclerosis is a reversible disease, provided that aggressive risk factor management is undertaken. Physicians should review the lifestyle of patients and the various cardiovascular risk factors that are amenable to change. Smoking, hypertension, and hyperglycemia should all be aggressively treated if not at goal. Based on the LDL-C principle, we recommend an LDL-C goal of <70 mg/dL, with a goal of <50 mg/dL for individuals with a high CAC score (>400). These goals are achievable in almost all individuals with the availability of high-potency statins, ezetimibe, and PCSK9 inhibitors. Inflammation, if present, should be addressed by statin and ezetimibe therapy with the goal of a high-sensitivity C-reactive protein of <2.0 mg/L. A healthy diet with reduced saturated fat will provide further support for reducing LDL-C [65]. A list of the most common frequently asked questions by physicians and/or patients with short answers and cited literature is provided in Table 1.
Table 1.
Frequently Asked Questions for Treatment of an Asymptomatic (No Ischemic Symptoms) Individual With a Positive Coronary Artery Calcium Scan
| Question | Answer | Reference |
|---|---|---|
| What CAC score is considered positive? | Traditionally a score >10, but recent data suggest that a score of 1–10 indicates increased risk. | 17, 66 |
| What is the earliest age at which a CAC scan should be recommended? | With no major risk factors: 50 y of age for female patients, 40 y of age for male patients. With risk factors (e.g., diabetes), a decade earlier for preventive treatment. | 42, 67 |
| Do higher scores indicate greater CVD risk? | Yes. Scores (risk) are usually divided 10–100, 101–200, 201–300, >300. | 23 |
| Is cardiac stress testing recommended? | No. Cardiac stress testing offers no direct patient benefit in the asymptomatic patient. | 29 |
| Is a cardiology consult recommended? | No. Cardiology consult offers no direct patient benefit of invasive procedures in the asymptomatic patient. | 28, 29 |
| What medical treatment is recommended? | Aggressive lifestyle modifications, multiple risk factor control, and Rx to decrease LDL-C. | 42 |
| What is the best risk factor outcomes predictor? | CAC score combined with Framingham risk factor assessment. | 1 |
| Where can I obtain this risk factor calculator at no cost? | https://www.mesa-hlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx | 1 |
| Is coronary calcium beneficial or detrimental? | Beneficial. It is only a marker for atherosclerosis; it may stabilize plaques. | 31 |
| What medications work well with statin therapy? | Ezetimibe is complementary and works at different cellular sites than statins. | 53, 68 |
| What dose of a statin is most effective with the fewest side effects? | The lowest dose (10 mg) achieves ~75% of the maximal therapeutic effect with minimal side effects. | 50 |
| What should be the optimal LDL goal? | Less than 70 mg/dL; preferably 50 mg/dL if any risk factors still exist. | 42 |
| Is there a proportional LDL-lowering dose response with higher statin doses? | No. Statins increase PCSK9 protein, which negates the benefits of statins on LDL by decreasing hepatic LDL receptors. | 51 |
| What are the benefits of obtaining/using a CAC score? | (1) Improved prognostic score, (2) improved adherence to therapy, (3) prevention of unnecessary medical (statin) therapy. | 19, 21 |
| What are the drawbacks of coronary angiography? | Cost = $5000–$10,000; death = 0.08%; complications = 1.8% | 29 |
Acknowledgments
Acknowledgments
This work was supported by New Mexico Clinical and Translational Science Center (NCATS) Grant NCATS 8UL1TR000449 (M.R.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACC
- American College of Cardiology
- AHA
- American Heart Association
- ASCVD
- atherosclerotic cardiovascular disease
- CAC
- coronary artery calcium
- LDL
- low-density lipoprotein
- LDL-C
- low-density lipoprotein cholesterol
- MESA
- Multi-Ethnic Study of Atherosclerosis
- PCI
- percutaneous coronary intervention.
References and Notes
- 1.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, Bild DE, Shea S, Liu K, Watson KE, Folsom AR, Khera A, Ayers C, Mahabadi AA, Lehmann N, Jöckel KH, Moebus S, Carr JJ, Erbel R, Burke GL. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tota-Maharaj R, Blaha MJ, McEvoy JW, Blumenthal RS, Muse ED, Budoff MJ, Shaw LJ, Berman DS, Rana JS, Rumberger J, Callister T, Rivera J, Agatston A, Nasir K. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33(23):2955–2962. [DOI] [PubMed] [Google Scholar]
- 3.Eaton RP, Burge MR, Comerci G, Cavanaugh B, Ramo B, Schade DS. Abnormal coronary artery calcium scans in asymptomatic patients. Am J Med. 2017;130(4):394–397. [DOI] [PubMed] [Google Scholar]
- 4.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS; COURAGE Trial Research Group . Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. [DOI] [PubMed] [Google Scholar]
- 5.Sedlis SP, Hartigan PM, Teo KK, Maron DJ, Spertus JA, Mancini GBJ, Kostuk W, Chaitman BR, Berman D, Lorin JD, Dada M, Weintraub WS, Boden WE; COURAGE Trial Investigators . Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med. 2015;373(20):1937–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 7.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD. Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol. 2000;36(4):1253–1260. [DOI] [PubMed] [Google Scholar]
- 8.Kavousi M, Desai CS, Ayers C, Blumenthal RS, Budoff MJ, Mahabadi A-A, Ikram MA, van der Lugt A, Hofman A, Erbel R, Khera A, Geisel MH, Jöckel K-H, Lehmann N, Hoffmann U, O’Donnell CJ, Massaro JM, Liu K, Möhlenkamp S, Ning H, Franco OS, Greenland P.. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316(20):2126–2134. [DOI] [PubMed] [Google Scholar]
- 9.Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy PF II, Peyser PA, Schwartz RS. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104(4):412–417. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Lopez OL, Mackey RH, Rosano C, Edmundowicz D, Becker JT, Newman AB. Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. J Am Coll Cardiol. 2016;67(9):1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41(1):39–44. [DOI] [PubMed] [Google Scholar]
- 12.Raggi P, Cooil B, Ratti C, Callister TQ, Budoff M. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension. 2005;46(1):238–243. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther. 2011;13(10):991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw LJ, Giambrone AE, Blaha MJ, Knapper JT, Berman DS, Bellam N, Quyyumi A, Budoff MJ, Callister TQ, Min JK. Long-term prognosis after coronary artery calcification testing. Ann Intern Med. 2015;163(1):14–21. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94(1):20–24. [DOI] [PubMed] [Google Scholar]
- 16.Berry JD, Lloyd-Jones DM, Garside DB, Greenland P. Framingham risk score and prediction of coronary heart disease death in young men. Am Heart J. 2007;154(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 18.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O’Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht HS. Coronary artery calcium scanning: past, present, and future [published correction appears in JACC: Cardiovascular Imaging. 2015;8(8):992]. JACC Cardiovasc Imaging. 2015;8(5):579–596. [DOI] [PubMed] [Google Scholar]
- 20.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78(5 Pt 1):1157–1166. [DOI] [PubMed] [Google Scholar]
- 21.Hecht HS, Narula J. Coronary artery calcium scanning in asymptomatic patients with diabetes mellitus: a paradigm shift. J Diabetes. 2012;4(4):342–350. [DOI] [PubMed] [Google Scholar]
- 22.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–133. [DOI] [PubMed] [Google Scholar]
- 23.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 24.MESA-The Multi-Ethnic Study of Atherosclerosis Investigators. MESA 10-Year CHD Risk with Coronary Artery Calcification. https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed 1 May 2017.
- 25.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blankstein R, Sibley CT. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2014;35(33):2232–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312(17):1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV; American College of Cardiology Foundation . 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons [published correction appears in Circulation. 2014;129]. Circulation. 2012;126(25):3097–3137. [DOI] [PubMed] [Google Scholar]
- 28.Topol EJ, Nissen SE. Our preoccupation with coronary luminology: the dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92(8):2333–2342. [DOI] [PubMed] [Google Scholar]
- 29.Fowler-Brown A, Pignone M, Pletcher M, Tice JA, Sutton SF, Lohr KN; U.S. Preventive Services Task Force . Exercise tolerance testing to screen for coronary heart disease: a systematic review for the technical support for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):W9–W24. [DOI] [PubMed] [Google Scholar]
- 30.Costhelper.com. https://www.google.com/?gws_rd=ssl#q=costhelper&*&spf=69. Accessed 20 March 2017.
- 31.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- 33.Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21, Suppl 1):II27–II33. [DOI] [PubMed] [Google Scholar]
- 34.Gao W-Q, Feng Q-Z, Li Y-F, Li Y-X, Huang Y, Chen Y-M, Yang B, Lu C-Y. Systematic study of the effects of lowering low-density lipoprotein-cholesterol on regression of coronary atherosclerotic plaques using intravascular ultrasound. BMC Cardiovasc Disord. 2014;14:60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M; JAPAN-ACS Investigators . Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54(4):293–302. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, Nissen SE. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–2087. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, Nissen SE. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007;297(5):499–508. [DOI] [PubMed] [Google Scholar]
- 38.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif J-C, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM; ASTEROID Investigators . Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–1565. [DOI] [PubMed] [Google Scholar]
- 39.Stegman B, Puri R, Cho L, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, Uno K, Kataoka Y, Nissen SE, Nicholls SJ. High-intensity statin therapy alters the natural history of diabetic coronary atherosclerosis: insights from SATURN. Diabetes Care. 2014;37(11):3114–3120. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM Jr, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Obata JE, Kitta Y, Takano H, Kobayashi T, Fujioka D, Saito Y, Kodama Y, Kawabata K, Mende A, Yano T, Hirano M, Sano K, Nakamura K, Kugiyama K. Rapid stabilization of vulnerable carotid plaque within 1 month of pitavastatin treatment in patients with acute coronary syndrome. J Cardiovasc Pharmacol. 2008;51(4):365–371. [DOI] [PubMed] [Google Scholar]
- 42.Schade DS, Cavanaugh B, Ramo B, Eaton RP. The application of the LDL principle. World J Cardiovasc Dis. 2016;6(5):109–125. [Google Scholar]
- 43.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Biervliet JP, Rosseneu M, Bury J, Caster H, Stul MS, Lamote R. Apolipoprotein and lipid composition of plasma lipoproteins in neonates during the first month of life. Pediatr Res. 1986;20(4):324–328. [DOI] [PubMed] [Google Scholar]
- 45.O’Keefe JH Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43(11):2142–2146. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP; Ezetimibe Study Group . Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. [DOI] [PubMed] [Google Scholar]
- 47.Maron DJ, Hartigan PM, Neff DR, Weintraub WS, Boden WE, COURAGE Trial Investigators . Impact of adding ezetimibe to statin to achieve low-density lipoprotein cholesterol goal (from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation [COURAGE] trial). Am J Cardiol. 2013;111(11):1557–1562. [DOI] [PubMed] [Google Scholar]
- 48.Parisi AF, Folland ED, Hartigan P; Veterans Affairs ACME Investigators . A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med. 1992;326(1):10–16. [DOI] [PubMed] [Google Scholar]
- 49.Katritsis DG, Karvouni E, Ioannidis JPA. Meta-analysis comparing drug-eluting stents with bare metal stents. Am J Cardiol. 2005;95(5):640–643. [DOI] [PubMed] [Google Scholar]
- 50.Olsson AG, Pears J, McKellar J, Mizan J, Raza A. Effect of rosuvastatin on low-density lipoprotein cholesterol in patients with hypercholesterolemia. Am J Cardiol. 2001;88(5):504–508. [DOI] [PubMed] [Google Scholar]
- 51.Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51(9):2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33(11):976–982. [DOI] [PubMed] [Google Scholar]
- 53.Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106(15):1943–1948. [DOI] [PubMed] [Google Scholar]
- 54.Nawrocki JW, Weiss SR, Davidson MH, Sprecher DL, Schwartz SL, Lupien P-J, Jones PH, Haber HE, Black DM. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol. 1995;15(5):678–682. [DOI] [PubMed] [Google Scholar]
- 55.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK, Smith SC Jr, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Nishimura R, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW; American College of Cardiology Foundation; American Heart Association . 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–e103. [DOI] [PubMed] [Google Scholar]
- 56.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313–1320. [DOI] [PubMed] [Google Scholar]
- 57.Whelton SP, Silverman MG, McEvoy JVV, Budoff MJ, Blankstein R, Eng J, Blumenthal RS, Szklo M, Nasir K, Blaha MJet al. Predictors of long-term healthy arterial aging: coronary artery calcium non development in the MESA study. JACC Cardiovasc Imaging. 2015;8(12):1393–1400. [DOI] [PubMed] [Google Scholar]
- 58.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013;61(12):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hecht HS. The deadly double standard (the saga of screening for subclinical atherosclerosis). Am J Cardiol. 2008;101(12):1805–1807. [DOI] [PubMed] [Google Scholar]
- 60.Khanji MY, Bicalho VV, van Waardhuizen CN, Ferket BS, Petersen SE, Hunink MG. Cardiovascular risk assessment: a systematic review of guidelines. Ann Intern Med. 2016;165(10):713–722. [DOI] [PubMed] [Google Scholar]
- 61.Falk E, Shah PK. The SHAPE guideline: ahead of its time or just in time? Curr Atheroscler Rep. 2011;13(5):345–352. [DOI] [PubMed] [Google Scholar]
- 62.Leening MJG, Berry JD, Allen NB. Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease. JAMA. 2016;315(14):1449–1450. [DOI] [PubMed] [Google Scholar]
- 63.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, Miedema MD, Sibley CT, Shaw LJ, Blumenthal RS, Budoff MJ, Krumholz HM. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines. J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 64.Burge MR, Eaton RP, Schade DS. The role of a coronary artery calcium scan in type 1 diabetes. Diabetes Technol Ther. 2016;18(9):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337(21):1491–1499. [DOI] [PubMed] [Google Scholar]
- 66.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. [DOI] [PubMed] [Google Scholar]
- 67.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund J-Y, Jacobson A, Sun W, Lachin JM, Nathan DM; for the DCCT/EDIC Research Group. The effect of intensive glycemic treatment on coronary artery calcification in type diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications (DCCT/EDIC) Study. Diabetes. 2006;55:3556–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia L, Betters JL, Yu L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu Rev Physiol. 2011;73:239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]