Abstract
8-Hydroxyquinoline (8HQ) compounds have been reported to possess diverse bioactivities. In recent years, drug repositioning has gained considerable attention in drug discovery and development. Herein, 8HQ (1) and its derivatives (2–9) bearing various substituents (amino, nitro, cyano and halogen) were investigated for their antimicrobial against 27 microorganisms (agar dilution method) and antioxidant (DPPH method) activities. The parent 8HQ (1) exerted a highly potent antimicrobial activity against Gram-positive bacteria including diploid fungi and yeast with MIC values in the range of 3.44–13.78 μM. Moreover, the halogenated 8HQ, especially 7-bromo-8HQ (4) and clioquinol (6), displayed a high antigrowth activity against Gram-negative bacteria compared with the parent compound (1). Apparently, the derivatives with a relatively high safely index, e.g., nitroxoline (2), exhibited strong antibacterial activity against Aeromonas hydrophila (MIC=5.26 μM) and selectively inhibited the growth of P. aeruginosa with the MIC value of 84.14 μM; cloxyquin (3) showed a strong activity against Listseria monocytogenes and Plesiomonas shigelloides with MIC values of 5.57 and 11.14 μM, respectively. Most compounds displayed an antioxidant activity. Specifically, 5-amino-8HQ (8) was shown to be the most potent antioxidant (IC50=8.70 μM) compared with the positive control (α-tocopherol) with IC50 of 13.47 μM. The findings reveal that 8HQ derivatives are potential candidates to be further developed as antimicrobial and antioxidant agents.
Keywords: 8-Hydroxyquinoline, Nitroxoline, Clioquinol, Cloxyquin, Antimicrobial, Antioxidant
Highlights
-
•
8-Hydroxyquinoline exerted highly potent antibacterial activity (Gram positive).
-
•
Nitroxoline exhibited strong antibacterial activity against Pseudomonas aeruginosa.
-
•
Cloxyquin displayed a high growth inhibition against Listeria monocytogenes and Plesiomonas shigelloides.
-
•
5-Amino-8-hydroxyquinoline exerted the most potent antioxidant activity (IC50=8.70 μM).
-
•
Nitroxoline and cloxyquin had a relatively high selectivity index.
1. Introduction
Nitrogen heterocycles constitute a large group of compounds with a vast array of pharmacological activities [1], [2], [3]. Specifically, quinoline is a privileged structure that is found in a variety of natural products and therapeutics. 8-Hydroxyquinoline (8HQ), a derivative of quinoline, has a strong metal chelating property [4]. The derivatives of 8HQ have been reported to have multifunctional uses as antimicrobial, antioxidant, anticancer, antiinflammatory and antineurodegenerative agents [5], [6], [7], [8]. The halogenated 8HQs, such as cloxyquin (5-chloro-8HQ), clioquinol (5-chloro-7-iodo-8HQ or CQ), 7-bromo-8HQ and iodoquinol (5,7-diiodo-8HQ), have been synthesized and are commercially available [9]. The cloxyquin was reported to show good antitubercular and antiamoebic activities [10]. CQ was also used for several years as an antidiarrheal agent to treat amoebic infection. Then, it was banned from oral consumption in the 1960s because it can cause subacute myelo-optic neuropathy (SMON) [11]. However, the neurotoxicity of CQ can be solved by the recommended dosage control and vitamin supplementation. Nitroxoline (5-nitro-8HQ or NQ) has been used for the treatment and prophylaxis of acute and recurrent urinary tract infection [7]. In addition, NQ has been approved by the Food and Drug Administration (FDA), and is widely used as an antineurodegenerative drug to treat Alzheimer's disease and cancer in humans [6]. Moreover, metal complexes in 8HQ have been reported to enhance 8HQ bioactivities [12]. The search for novel potent lead compounds and repositioning of the well-known compounds/drugs for therapeutic applications are the main challenges [13], [14], [15], [16]. In recent years, drug repositioning or repurposing has attracted pharmaceutical companies because the possibility of using the approved or investigational drug in a new therapeutic area avoids the expensive and time-consuming pharmacokinetic and toxicity tests that are required for new drug candidates [17]. Currently, diverse bioactivities of the 8HQ derivatives have not been fully explored. Therefore, 8HQ and its derivatives that bear substituents (amino, halogen, nitro) at positions 5 and/or 7 as well as a cyano group at 2-position were investigated for their antimicrobial and antioxidant activities as well as cytotoxic effect.
2. Materials and methods
2.1. Compounds and chemical reagents
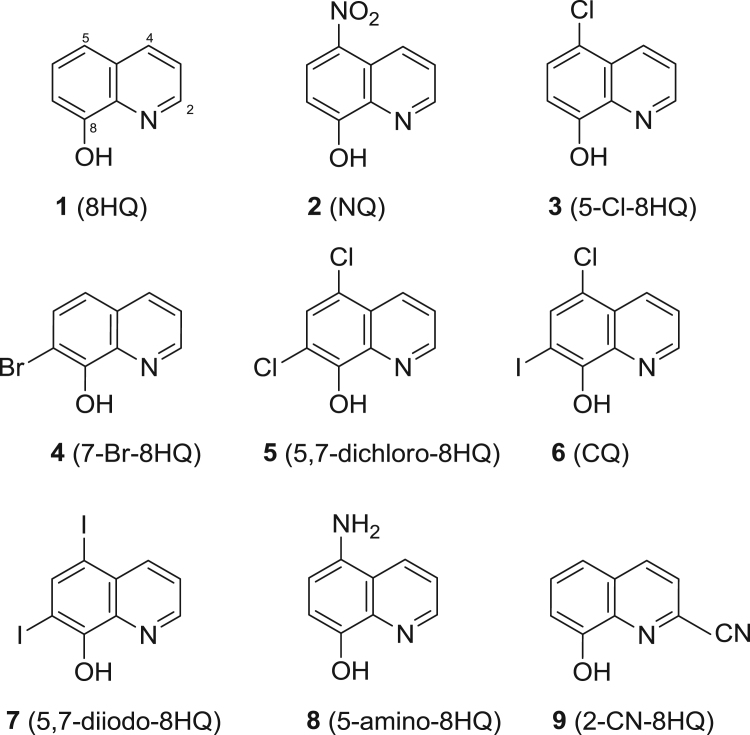
Nine tested compounds (1–9, Fig. 1) are commercially available. Specifically, 8HQ (1), NQ (2), cloxyquin (3), 7-bromo-8HQ (4), CQ (6), iodoquinol (7), and 5-amino-8HQ (8) were purchased from Sigma, USA. Compounds 5,7-dichloro-8HQ (5) and 8HQ-2-carbonitrile (2-CN-8HQ, 9) were purchased from Acros Organics.
Fig. 1.
Chemical structures of 8HQ and its derivatives (1–9).
α-Tocopherol (vitamin E), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were supplied by Sigma, USA. Dimethyl sulfoxide (DMSO) was purchased from Merck, Germany.
2.2. Antimicrobial activity assay
Antimicrobial activity of 8HQ and its derivatives (1–9) was performed using the agar dilution method, as previously described [2]. Briefly, the tested compound was dissolved in DMSO and, then, was mixed with the Müeller Hinton (MH) broth. The compound solution was two-fold diluted, and 1 mL of each dilution was mixed into the MH agar to obtain the final concentration range of 0.25–256 μg/mL. DMSO (0.5%) was added into the MH agar and was used as a reagent control. The microorganisms were cultured in the MH broth at 37 °C overnight and were diluted with a normal saline solution until the cell density was 0.5 McFarland standard (1.5×108 CFU/mL). The microorganisms were inoculated onto the agar plates and were incubated at 37 °C for 24–48 h. A minimum inhibitory concentration (MIC) of the compounds was determined. Twenty-seven strains of the tested microorganisms were Gram-negative bacteria: Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Serratia marcescens ATCC 8100, Salmonella Typhimurium ATCC 13311, Salmonella Choleraesuis ATCC 10708, Salmonella Enteritidis, Shigella dysenteriae, Morganella morganii, Citrobacter freundii, Plesiomonas shigelloides, Aeromonas hydrophila, Pseudomonas aeruginosa ATCC 27853, Pseudomonas stutzeri ATCC 17587, Shewanella putrefaciens ATCC 8071, Achromobacter xylosoxidans ATCC 27061; Gram-positive bacteria: Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 29213, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 10240, Enterococcus faecalis ATCC 29212, Enterococcus faecalis ATCC 33186, Corynebacterium diphtheriae NCTC 10356, Bacillus subtilis ATCC 6633, Listeria monocytogenes, Bacillus cereus; and diploid fungi and yeast: Candida albicans ATCC 90028 and Saccharomyces cerevisiae ATCC 2601.
2.3. Antioxidant activity assay
Compounds (1–9) were determined for their antioxidant properties using the DPPH assay [18]. DPPH (a stable purple color radical) reacts with an antioxidant to form a light-yellow diphenylpicrylhydrazine, which is the reduced product that can be detected using a spectrophotometer. The assay was initiated by adding a 1 mL solution of DPPH in methanol (0.1 mM) to a sample solution (0.45 mL, 1 mg/mL dissolved in DMSO). The reaction mixture was incubated for 30 min in a dark room. The absorbance at 517 nm was measured using a UV–visible spectrophotometer (UV-1610, Shimadzu), and the percentage of radical scavenging activity (RSA) was calculated using the following equation:
where Abs.control is the absorbance of the control reaction, and Abs.sample is the absorbance of the tested compound.
2.4. Cytotoxicity assay
Cytotoxicity of compounds (1–9) was performed using a normal embryonic lung cell line (MRC-5) [12]. Briefly, the MRC-5 cells were grown in a DMEM medium that was supplemented with 100 U/mL of penicillin-streptomycin and 10% FBS. Then, the cell lines were seeded in a 96-well plate at a density of 5,000-20,000 cells/well and were incubated at 37 °C under a humidified atmosphere (95% air and 5% CO2) for 24 h. The cells were treated with the tested compounds at different concentrations and, then, incubated for 48 h. Cell viability was measured by staining with MTT. The MTT solution (10 µL/100 µL medium) was added to all assay wells and was incubated for 4 hours. After the incubation, DMSO was added to dissolve the resulting formazan using sonication. The plates were read on a microplate reader using a test wavelength of 550 nm and a reference wavelength of 650 nm. Cytotoxic activity of the tested compound was expressed as the compound concentration that inhibited the cell growth by 50% (IC50). A tested compound with IC50 greater than 50 μg/mL is considered to be non-cytotoxic. Doxorubicin was used as a positive control, while DMSO was used as a negative control.
3. Results
3.1. Biological activities
3.1.1. Antimicrobial activity
All the tested compounds, 8HQ and its derivatives (1–9), were evaluated for their antimicrobial activities against twenty-seven strains of microorganisms using the agar dilution method. The results showed that these compounds exhibited the antimicrobial potency against both Gram-positive and Gram-negative bacteria (Table 1). 8HQ (1) showed a great inhibitory activity to Gram-positive bacteria such as S. aureus, S. epidermidis, M. luteus, E. faecalis, C. diphtheriae, B. cereus, B. subtilis and L. monocytogenes with the MICs in the range of 3.44-13.78 μM. The activity against Gram-negative bacteria, such as E. coli, K. pneumoniae, S. marcescens, Salmonella spp., S. dysenteriae, M. morganii, C. freundii, P. shigelloides, A. hydrophila, P. stutzeri, S. putrefaciens and A. xylosoxidans, were found to be 13.78–881.79 μM, except for P. aeruginosa ATCC 27853 (MIC >1763.57 μM). Among the Gram-negative bacteria, the highest activity of compound 1 was noted for P. shigelloides with the lowest MIC value of 13.78 μM, followed by A. hydrophila (MIC =110.22 μM). Additionally, the diploid fungi and yeast (C. albicans ATCC 90028 and S. cerevisiae ATCC 2601) were inhibited by the compound 1 with MICs of 13.78 μM. The 8HQ derivatives (2–9) have a wide range of MIC values that depend on the activity of each compound and bacterial group. The antimicrobial activity of NQ (2) against both the Gram-positive and Gram-negative bacteria was observed with MIC in the range of 5.26–84.14 μM. Particularly, NQ is the only compound that displayed the activity against P. aeruginosa ATCC 27853 with a MIC value of 84.14 μM, while the other derivatives (3–9), including 8HQ, exhibited antigrowth activity against this microorganism with the MIC value greater than 644.92 μM. The halogenated 8HQ (3–6) inhibited Gram-positive bacteria with the MIC range of 5.57–89.09 μM. Whereas, compound 7 showed a weaker antimicrobial activity (MIC ≥644.92 μM) against most of the bacterial strains, except for M. luteus ATCC 10240 and B. subtilis ATCC 6633 (MIC =322.45 and 80.61 μM, respectively). In addition, 2-CN-8HQ (9) exerted the antimicrobial activity with the MIC value higher than 1504.38 μM against most of the tested microorganisms. The derivatives 2–6 exhibited activity against diploid fungi and yeasts with MIC values of 26.19–178.17 μM, while compounds 7–9 had the MIC values ≥644.92 μM.
Table 1.
Antimicrobial activity (MIC) of 8HQ and its derivatives (1–9).
| Bacterial strain | MIC (μM)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 8HQ (1) | NQ (2) | Cloxyquin (3) | 7-bromo-8HQ (4) | 5,7-dichloro-8HQ (5) | CQ (6) | Iodoquinol (7) | 5-amino-8HQ (8) | 2-CN-8HQ (9) | |
| S. aureus ATCC 25923 | 6.89 | 42.07 | 89.09 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | >1504.38 |
| S. aureus ATCC 29213 | 6.89 | 42.07 | 89.09 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | >1504.38 |
| S. epidermidis ATCC 12228 | 6.89 | 42.07 | 22.27 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | 1504.38 |
| M. luteus ATCC 10240 | 13.78 | 10.52 | 22.27 | 35.71 | 37.37 | 26.19 | 322.45 | 274.57 | 1504.38 |
| E. faecalis ATCC 29212 | 13.78 | 42.07 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | >1504.38 |
| E. faecalis ATCC 33186 | 13.78 | 42.07 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | >1504.38 |
| C. diphtheriae NCTC 10356 | 13.78 | 42.07 | 22.27 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | >1504.38 |
| B. subtilis ATCC 6633 | 6.89 | 42.07 | 22.27 | 35.71 | 37.37 | 26.19 | 80.61 | 274.57 | >1504.38 |
| B. cereus | 13.78 | 21.03 | 44.54 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | >1504.38 |
| L. monocytogenes | 3.44 | 42.07 | 5.57 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | 1504.38 |
| E. coli ATCC 25922 | 220.45 | 21.03 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | 1504.38 |
| K. pneumoniae ATCC 700603 | 881.79 | 84.14 | 712.69 | 71.41 | 1195.98 | >837.97 | >644.92 | 1098.29 | >1504.38 |
| S. marcescens ATCC 8100 | 220.45 | 21.03 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | >1504.38 |
| S. Typhimurium ATCC 13311 | 220.45 | 21.03 | 178.17 | 35.71 | 37.37 | 837.97 | >644.92 | 1098.29 | >1504.38 |
| S. Choleraesuis ATCC 10708 | 881.79 | 84.14 | 712.69 | 71.41 | 1195.98 | >837.97 | >644.92 | 1098.29 | >1504.38 |
| S. Enteritidis | 881.79 | 21.03 | 178.17 | 35.71 | 37.37 | 104.75 | >644.92 | 1098.29 | >1504.38 |
| S. dysenteriae | 220.45 | 21.03 | 178.17 | 35.71 | 37.37 | 837.97 | 644.92 | 1098.29 | 1504.38 |
| M. morganii | 881.79 | 42.07 | 178.17 | 35.71 | 149.50 | 837.97 | >644.92 | 1098.29 | >1504.38 |
| C. freundii | 220.45 | 21.03 | 178.17 | 35.71 | 37.37 | 418.99 | >644.92 | 1098.29 | >1504.38 |
| P. shigelloides | 13.78 | 42.07 | 11.14 | 35.71 | 37.37 | 26.19 | 644.92 | 1098.29 | 1504.38 |
| A. hydrophila | 110.22 | 5.26 | 44.54 | 35.71 | 37.37 | 26.19 | 644.92 | 274.57 | 1504.38 |
| P. aeruginosa ATCC 27853 | >1763.57 | 84.14 | >1425.39 | >1142.60 | >1195.98 | >837.97 | >644.92 | 1098.29 | >1504.38 |
| P. stutzeri ATCC 17587 | 220.45 | 10.52 | 178.17 | 35.71 | 37.37 | 104.75 | >644.92 | 1098.29 | >1504.38 |
| S. putrefaciens ATCC 8071 | 220.45 | 10.52 | 178.17 | 35.71 | 37.37 | 26.19 | 644.92 | 1098.29 | 1504.38 |
| A. xylosoxidans ATCC 27061 | 55.11 | 21.03 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 549.14 | >1504.38 |
| C. albicans ATCC 90028 | 13.78 | 42.07 | 178.17 | 35.71 | 37.37 | 26.19 | 644.92 | 1098.29 | 1504.38 |
| S. cerevisiae ATCC 2601 | 13.78 | 42.07 | 89.09 | 35.71 | 37.37 | 26.19 | >644.92 | 1098.29 | >1504.38 |
Ampicillin (26.93 μM) was used as the control system of antimicrobial activity. It showed 100% inhibition against S. aureus ATCC 25923, S. aureus ATCC 29213, S. epidermidis ATCC 12228, M. luteus ATCC 10240, E. faecalis ATCC 29212, E. faecalis ATCC 33186, C. diphtheriae NCTC 10356, B. subtilis ATCC 6633, L. monocytogenes, S. Typhimurium ATCC 13311, S. Enteritidis, P. stutzeri ATCC 17587, S. putrefaciens ATCC 8071 and P. shigelloides.
MIC is the lowest concentration that inhibits the growth of microorganisms.
3.1.2. Antioxidant activity
The radical scavenging activity (RSA) of compounds (1–9) was performed using the DPPH assay. The results (Table 2) demonstrated that 5-amino-8HQ (8) exhibited the highest percentage of RSA with an IC50 value of 8.71 μM, while the parent compound 8HQ (1) had the IC50 value of 614.77 μM. The halogenated 8HQ (3–6) displayed the antioxidant activity with the IC50 in the range of 335.90–1050.67 μM, whereas 5,7-diiodo-8HQ (7) was shown to be an inactive antioxidant. In addition, inactive antioxidants were noted for the 5-nitro (NQ, 2) and 2-cyano (9) derivatives of 8HQ.
Table 2.
RSA (IC50) of 8HQ and its derivatives (1–9).
| Compound | IC50 (μM) |
|---|---|
| 8HQ (1) | 614.77 |
| NQ (2) | –a |
| Cloxyquin (3) | 1050.67 |
| 7-Br-8HQ (4) | 597.46 |
| 5,7-dichloro-8HQ (5) | 335.90 |
| CQ (6) | 366.02 |
| 5,7-diiodo-8HQ (7) | –a |
| 5-amino-8HQ (8) | 8.71 |
| 2-CN-8HQ (9) | –a |
| α-tocopherolb | 13.47 |
Compounds that exhibited <50% inhibition at 300 μg/mL were denoted as inactive antioxidants.
α-tocopherol was used as the positive control.
3.1.3. Cytotoxicity
The cytotoxic activity of compounds (1–9, Table 3) was tested against normal cells (MRC-5) using the MTT assay. It was found that compounds 7 and 9 were non-cytotoxic toward the normal cells. Cytotoxic effect was observed for the compounds 2, 3, and 6 (IC50 76.17–88.14 µM), compounds 4, 5, and 8 (IC50 22.09–27.98 µM), and 8HQ (1) with IC50 value of 6.27 µM. Selectivity index (SI) of potent antimicrobials (1–3 and 6) was determined. The result (Table 4) showed that these compounds had SI values in the range of 0.91–16.67.
Table 3.
Cytotoxicity (IC50) of compound 1-9 against MRC-5 cell line.
| Compound | IC50 (µM) |
|---|---|
| 8HQ (1)a | 6.27±0.58 |
| NQ (2) | 88.14±2.11 |
| Cloxyquin (3) | 81.74±0.96 |
| 7-Br-8HQ (4) | 22.09±1.30 |
| 5,7-dichloro-8HQ (5) | 27.98±0.87 |
| CQ (6) | 76.17±0.23 |
| 5,7-diiodo-8HQ (7) | –b |
| 5-amino-8HQ (8) | 22.14±1.02 |
| 2-CN-8HQ (9) | –b |
| Doxorubicinc | 0.62±0.03 |
See reference [12].
Compounds that exhibited <50% inhibition of cell growth at 50 µg/mL were denoted as non-cytotoxic.
Doxorubicin was used as the positive control.
Table 4.
Selectivity index (SI) of compounds 1–3 and 6.
| Compound | Antimicrobial activity |
Cytotoxicity | SIa | |
|---|---|---|---|---|
| Microorganism | MIC (µM) | MRC-5 | ||
| IC50 (µM) | ||||
| 8HQ (1) | S. aureus ATCC 25923 | 6.89 | 6.27 | 0.91 |
| S. aureus ATCC 29213 | ||||
| S. epidermidis ATCC 12228 | ||||
| L. monocytogenes | 3.44 | 6.27 | 1.82 | |
| NQ (2) |
A. hydrophila |
5.26 |
88.14 |
16.76 |
| M. luteus ATCC 10240 | 10.52 | 88.14 | 8.38 | |
| P. stuzeri ATCC 17587 | ||||
| S. putrefaciens ATCC 8071 | ||||
| B. cereus | 21.03 | 88.14 | 4.19 | |
| E. coli ATCC 25922 | ||||
| S. marcescens ATCC 8100 | ||||
| S. Typhimurium ATCC 13311 | ||||
| S. Enteritidis | ||||
| S. dysenteriae | ||||
| C. freundii | ||||
| A. xylosoxidans ATCC 27061 | ||||
| cloxyquin (3) |
L. monocytogenes |
5.57 |
81.74 |
14.67 |
| P. shigelloides | 11.4 | 81.74 | 7.34 | |
| CQ (6) | All Gram-positive bacteria |
26.19 |
76.17 |
2.91 |
| E. coli ATCC 25922, | 26.19 | 76.17 | 2.91 | |
| S. marcescens ATCC 8100 | ||||
| P. shigelloides | ||||
| A. hydrophila | ||||
| S. putrefaciens ATCC 8071 | ||||
| A. xylosoxidans | ||||
| C. albicans ATCC 90028 | ||||
| S. cerevisiae ATCC 2601 | ||||
SI = IC50 of MRC-5 cell/MIC of microorganism.
4. Discussion
8HQ and its derivatives have been reported to be antimicrobial agents against many parasites, viruses, fungi and bacteria including Mycobacterium tuberculosis [2], [5], [19]. Moreover, CQ was used as the antineurodegenerative drug for Alzheimer's disease. Other 8HQ derivatives exhibited anticancer and anti-inflammatory activities [5], [11]. Previously, the antimicrobial activity of 8HQ and its transition metal complexes against many bacterial strains was reported [2]. In this study, 8HQ was diluted until a 1.72 μM concentration was reached. The result showed that 8HQ exerted a great antimicrobial activity (MIC =3.44–13.78 μM) against most Gram-positive bacteria and diploid fungi. Resistant pathogens have been found among both the Gram-positive and Gram-negative bacteria. Particularly, S. aureus is the most common cause of nosocomial infection [20], for example, in patients with severe burns [21], [22]. However, most Gram-negative bacteria, except for P. shigelloides (MIC =13.78 μM), were inhibited by 8HQ with a higher MIC range of 110.22–881.79 μM. Apparently, 8HQ displayed a higher activity against Gram-positive than Gram-negative bacteria. This occurs possibly because the cell wall of Gram-negative bacteria has high lipophilicity. Thus, the compounds with a more hydrophobic effect are required to enhance absorption at the site of action and to exert a higher activity. Therefore, most of the 8HQ derivatives with lipophilic substituents at 5-position (NO2, Cl), 7-position (Br) and 5,7-positions (dichloro) exhibited an improved activity against Gram-negative bacteria compared with the parent un-substituted 8HQ (1). 7-Bromo-8HQ (4) exhibited a higher antibacterial activity against Gram-negative bacteria than cloxyquin (3), except for P. shigelloides. In addition, substitution of the chloro group at the 7-position of compound 3 gave compound 5 (5,7-dichloro-8HQ) with a higher activity compared with compound 3 against most of the tested microorganisms. Substitution of 7-bromo and 5,7-dichloro groups on the 8HQ core structure afforded compounds 4 and 5 with an almost comparable activity. When the 7-position of compound 3 was substituted with iodo group, CQ (6, 5-chloro-7-iodo-8HQ) was obtained with a lower antibacterial activity against certain microorganisms such as S. Enteritidis, S. dysenteriae, M. morganii, C. freundii and P. stutzeri ATCC 17587 compared with compound 5 (5,7-dichloro-8HQ). It was found that 5,7-diiodo-8HQ (7) displayed a weak activity against Gram-negative bacteria with MIC values ≥644.92 μM. It should be noted that the derivatives of 8HQ, which displayed a higher potency against Gram-negative bacteria, required lipophilic halogen substituents at 5-position and/or 7-position. Specifically, the 5-position was substituted with a small halogen atom (Cl) but not a large atom (I), and 7-position can be either chloro, bromo or iodo groups. This could be due to the size of chloro group at the 5-position of 8HQ being appropriate for the compound to interact with the lipophilic area at the site of action. Thus far, CQ (6) and iodoquinol (7) are the known drugs with antiamoebic activities and are commonly used to treat intestinal infection [10]. Notably, NQ (2) with a strong electron withdrawing effect of NO2 group at the 5-position of 8HQ exerted the most potent activity against almost all of the Gram-negative bacteria (MIC =5.26–84.14 μM). Such electronic effects of the NO2 and halogen groups may enhance the chelating effect of 8HQ in exerting antibacterial activity [23]. However, the electron donating effect of polar NH2 group at the 5-position (8) exhibited a weaker activity (MIC =1098.29 μM) against most of the Gram-negative bacteria compared with the nitro and halogen derivatives of 8HQ (2–6). In case of the CN group substituted at the 2-position of 8HQ, compound 9 displayed a weak activity (MIC ≥1504.38 μM) against all of the tested microorganisms. This can be attributed to the electron withdrawing substituent (CN) at the 2-position that deactivated the chelating effect of the N atom in the quinoline ring. Considering the activity against Gram-positive bacteria, most of the 8HQ derivatives displayed a weaker activity than the 8HQ, particularly, compounds 7 and 9. NQ (2) and halogenated-8HQ (4–6) exhibited a comparable activity (MIC =26.19–42.07 μM) against most of the Gram-positive bacteria except for M. luteus (MIC =10.52 μM) and B. cereus (MIC =21.03 μM), while cloxyquin (3) displayed an activity with the MIC range of 5.57–89.09 μM. It is presumed that the most potent activity of the un-substituted 8HQ (1) resulted from its lower lipophilicity, compared with the 8HQ derivatives that bear halogen and nitro substituents. Thus, lower lipophilicity enhances better absorption to the Gram-positive bacteria that contain hydrophilic polysaccharides and charged amino acids in the peptidoglycan [24]. CQ (6) is a well-known commercial drug for treating human cancer. However, a recent study has revealed that NQ (2) exerts a higher anticancer potency than CQ [9]. The present study shows that NQ (2) possesses higher antibacterial activity than CQ (6) against some Gram-positive bacteria (M. luteus and B. cereus), and Gram-negative bacteria (E. coli ATCC 25922, K. pneumoniae ATCC 7 00603, S. marcescens ATCC 8100, S. Typhimurium ATCC 13311, S. Choleraesuis ATCC 10708, S. Enteritidis, S. dysenteriae, M. morganii, C. freundii, A. hydrophila, P. aeruginosa ATCC 27853, P. stutzeri ATCC 17587, S. putrefaciens ATCC 8071, and A. xylosoxidans ATCC 27061). Interestingly, NQ (2) was the only compound that selectively inhibited P. aeruginosa ATCC 27853 with the MIC value of 84.14 μM, while the other derivatives including the parent 8HQ displayed MIC values that were greater than 644.92 μM. Currently, P. aeruginosa, which is the common causative agent of nosocomial infection, has been reported to generate multi-drug resistance because of its biofilm synthesis [25]. Furthermore, Sobke et al. also reported the same result, specifically, that NQ can be used as anti-biofilm agent for P. aeruginosa with the MIC range of 84.14–168.28 μM [26]. NQ (2) and 7-bromo-8HQ (4) inhibited K. pneumoniae ATCC 700603 and S. Choleraesuis ATCC 10708 with the MIC values of 84.14 and 71.41 μM, respectively, whereas the other derivatives (5–9) showed their MICs >644.92 μM. Moreover, K. pneumoniae was found to be a causative agent of pneumonia, and was also found to be a pathogen of septicemia in patients [27]. Recently, it has been reported worldwide that the spread of K. pneumoniae carbapenemase (KPC) emerged in many countries [28]. In addition, S. Typhimurium, and S. Enteritidis were inhibited by NQ (2), 7-bromo-8HQ (4) and 5,7-dichloro-8HQ (5) with MICs of 21.03, 35.71 and 37.37 μM, respectively. Salmonella species are well-known human food-borne pathogen of salmonellosis [29]. Clearly, S. Enteritidis, which is the most leading cause of salmonellosis, was inhibited by compounds 2, 4 and 5 with the lower MICs when compared with S. Choleraesuis. In addition, L. monocytogenes is an important food-borne pathogen that can contaminate both raw and processed food products. Furthermore, L. monocytogenes is a board-range causative agent of septicemia, meningitis, encephalitis and pneumonia [30]. However, this study showed that L. monocytogenes was completely inhibited by the parent 8HQ (1) and cloxyquin (3) at MICs as low as 3.44 and 5.57 μM, respectively. However, compound 3 had a higher selectivity index (SI =14.67, Table 4) than the 8HQ (1, SI =1.82) against L. monocytogenes. Additionally, compound 3 showed a high growth inhibition against P. shigelloides with the MIC value of 11.14 μM. Among the Gram-negative bacteria, the NQ (2) displayed the most potent activity (MIC =5.26 μM) and had the highest SI value (16.76) against A. hydrophila that can cause an opportunistic infection in humans. Moreover, NQ (2) also exerted the lowest cytotoxicity (IC50=88.14±2.11 μM) compared with the other compounds (1, 3–6 and 8). A relatively low cytotoxicity was noted for compounds 3 (IC50=81.74±0.96 μM) and 6 (IC50=76.17±0.23 μM). Previously, 8HQ and its copper-complexes were reported to inhibit Gram-positive bacteria, such as S. aureus, E. faecalis and L. monocytogenes, and Gram-negative bacteria such as E. coli, K. pneumoniae and P. aeruginosa [2]. CQ was shown to display activity against Gram-positive bacteria (S. aureus and B. subtilis) and against Gram-negative bacteria such as S. marcescens, P. aeruginosa and E. coli [31]. NQ was documented to inhibit E. coli with the MIC value of 42.07 μM [32]. As a result, the 8HQ derivatives with a relatively high safety index [i.e., the NQ (2, SI =16.76) and cloxyquin (3, SI =14.67)] can be selected as effective compounds to inhibit pathogens both in Gram-positive (L. monocytogenes) and Gram-negative (A. hydrophila and P. shigelloides) bacteria, whereas CQ (6) should be selected to inhibit Gram-positive bacteria. Additionally, the non-cytotoxic compounds (7 and 9) displayed a weak antimicrobial activity.
The antioxidant properties of 8HQ and its derivatives resulting from their metal chelating ability have been reported [5]. 8HQ was shown to be a strong iron chelator with an antioxidant activity [33]. Recently, Kharadi [31] reported the antioxidant activity of CQ using the ferric-reducing antioxidant power (FRAP) method. In this study, the antioxidant activity of the parent 8HQ (1) and its derivatives (2–9) were evaluated via the DPPH assay using α-tocopherol as a positive control. It was found that 8HQ (1) exhibited the activity with the IC50 value of 614.77 μM. Previously, 8HQ was reported to display the SOD (superoxide dismutase) activity with the IC50 of 91.83 μM [34]. Interestingly, 5-amino-8HQ (8) was shown to be the most potent antioxidant with the IC50 value of 8.71 μM compared with the positive control (IC50=13.47 μM). In addition, compound 8 displayed cytotoxic effect against normal cells with a higher IC50 value (22.14±1.02 μM) compared with its antioxidant activity (IC50=8.71 μM). This indicated that the antioxidant compound 8 had a safety index with SI value of 2.54. Among the halogenated 8HQ, compound 5 was the best antioxidant (IC50=335.90 μM), whereas the IC50 values of other derivatives (3, 4 and 6) were 1050.67, 597.46 and 366.02 μM, respectively. However, NQ (2) and 2-CN-8HQ (9) were found to be inactive antioxidants. The antioxidant activity of compounds (1–9) may be attributed to the chelating property and electronic effects of substituent groups on the 8HQ scaffold. Specifically, compound 8 with an electron donating group (NH2) at the 5-position of 8HQ can stabilize the phenoxyl radical (derived from the phenolic moiety) in enhancing the radical scavenging activity. However, the total loss of antioxidant activity was noted for compound 2 (NQ) with a strong electron withdrawing group (NO2) at the 5-position of 8HQ. Together, the 8HQ derivative with an electron withdrawing group (NO2) as noted for NQ (2) exerted better antimicrobial activity against Gram-negative bacteria compared with the 8HQ (1) but had no antioxidant activity. In addition, the absence of antioxidant activity was observed for derivative (9) that contained an electron withdrawing group (CN) at the 2-position of 8HQ. Compound 8 with an electron donating effect (NH2) at the 5-position of 8HQ exhibited the most potent antioxidant effect.
Currently, there are many drug-resistant strains that are generated in Gram-positive bacteria, such as methicillin-resistant S. aureus (MRSA), vancomycin-resistant Enterococci (VRE) and penicillin-resistant S. pneumoniae (PRSP), and in Gram-negative bacilli, such as extended spectrum β-lactamase (ESBL), AmpC β-lactamase and carbapenemase-producing Enterobacteriaceae (CPE). Therefore, it is urgent to discover new and potent antimicrobial drugs because of the increase in the number of multi-drug resistant strains. This finding reveals that 8HQ derivatives, especially NQ (2) and the halogenated 8HQ (3–6), are possible candidates to be further developed as antimicrobial agents to treat these multi-drug resistant bacteria. In addition, 5-amino-8HQ (8) is a promising compound with a highly potent antioxidant activity. The property and position of substituents on the 8HQ pharmacophore provide insight into further development of antimicrobial agents.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgements
This work is supported by the Office of the Higher Education Commission, Mahidol University under the National Research Universities Initiative (2558 B.E.) and Annual Government Grant under Mahidol University (2556-2558 B.E.).
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.03.014.
Contributor Information
Supaluk Prachayasittikul, Email: supaluk@swu.ac.th.
Virapong Prachayasittikul, Email: virapong.pra@mahidol.ac.th.
Appendix A. Transparency document
Transparency document
.
Transparency document
.
References
- 1.Pinkaew R., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Synthesis and cytotoxicity of novel 4-(4-(substituted)-1H-1,2,3-triazol-1-yl)-N-phenethylbenzenesulfonamides. Med. Chem. Res. 2014;23:1768–1780. [Google Scholar]
- 2.Srisung S., Suksrichavalit T., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Antimicrobial activity of 8-hydroxyquinoline and transition metal complexes. Int. J. Pharmacol. 2013;9(2):170–175. [Google Scholar]
- 3.Tanzer J.M., Slee A.M., Kamay B., Scheer E. Activity of three 8-hydroxyquinoline derivatives against in vitro dental plaque. Antimicrob. Agents Chemother. 1978;13(6):1044–1045. doi: 10.1128/aac.13.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kos J., Zadrazilova I., Nevin E., Soral M., Gonec T., Kollar P. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as potential antimycobacterial agents. Bioorganic Med. Chem. 2015;23(15):4188–4196. doi: 10.1016/j.bmc.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Prachayasittikul V., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug. Des. Dev. Ther. 2013;7:1157–1178. doi: 10.2147/DDDT.S49763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazovic J., Guo L., Nakashima J., Mirsadraei L., Yong W., Kim H.J. Nitroxoline induces apoptosis and slows glioma growth in vivo. Neuro-Oncol. 2015;17(1):53–62. doi: 10.1093/neuonc/nou139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naber K.G., Niggemann H., Stein G. Review of the literature and individual patients’ data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect. Dis. 2014;14:628. doi: 10.1186/s12879-014-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan-On W., Huyen N.T.B., Songtawee N., Suwanjang W., Prachayasittikul S., Prachayasittikul V. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Drug Des. Dev. Ther. 2015;9:2033–2047. doi: 10.2147/DDDT.S79313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H., Taggart J.E., Zhang X., Benbrook D.M., Lind S.E., Ding W.Q. Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline) Cancer Lett. 2011;312(1):11–17. doi: 10.1016/j.canlet.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hongmanee P., Rukseree K., Buabut B., Somsri B., Palittapongarnpim P. In vitro activities of cloxyquin (5-chloroquinolin-8-ol) against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007;51(3):1105–1106. doi: 10.1128/AAC.01310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bareggi S.R., Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci. Ther. 2012;18(1):41–46. doi: 10.1111/j.1755-5949.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prachayasittikul V., Pingaew R., Nantasenamat C., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Investigation of aromatase inhibitory activity of metal complexes of 8-hydroxyquinoline and uracil derivatives. Drug Des. Dev. Ther. 2014;8:1089–1096. doi: 10.2147/DDDT.S67300. Epub 2014/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barot K., Jain S., Kremer L., Singh S., Ghate M. Recent advances and therapeutic journey of coumarins: current status and perspectives. Med. Chem. Res. 2015;24(7):2771–2798. [Google Scholar]
- 14.Li E., Subramanian J., Anderson S., Thomas D., McKinley J., Jacobs I.A. Development of biosimilars in an era of oncologic drug shortages. Drug Des. Dev. Ther. 2015;9:3247–3255. doi: 10.2147/DDDT.S75219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchardt R.A., Rolston K.V. Antibiotic shortages: effective alternatives in the face of a growing problem. J. Am. Acad. Phys. Assist. 2013;26(2):13. doi: 10.1097/01720610-201302000-00004. 18. [DOI] [PubMed] [Google Scholar]
- 16.Chong C.R., Sullivan D.J. New uses for old drugs. Nature. 2007;448(7154):645–646. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 17.Anighoro A., Bajorath J., Rastelli G. Polypharmacology: challenges and opportunities in drug discovery. J. Med. Chem. 2014;57(19):7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 18.Cherdtrakulkiat R., Boonpangrak S., Pingaew R., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Bioactive triterpenoids, antimicrobial, antioxidant and cytotoxic activities of Eclipta porostrata Linn. J. Appl. Pharm. Sci. 2015;5(3):46–50. [Google Scholar]
- 19.Lam K.H., Gambari R., Lee K.K., Chen Y.X., Kok S.H., Wong R.S. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg Med. Chem. Lett. 2014;24(1):367–370. doi: 10.1016/j.bmcl.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 20.Diekema D.J., Pfaller M.A., Schmitz F.J., Smayevsky J., Bell J., Jones R.N. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001;32(Suppl. 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 21.Ressner R.A., Murray C.K., Griffith M.E., Rasnake M.S., Hospenthal D.R., Wolf S.E. Outcomes of bacteremia in burn patients involved in combat operations overseas. J. Am. Coll. Surg. 2008;206(3):439–444. doi: 10.1016/j.jamcollsurg.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Lawung R., Treeratanapiboon L., Prachayasittikul S., Prachayasittikul V. 3-(1-Adamantylthio)-4-phenylpyridine as a potential therapeutic for methicillin-resistant Staphylococcus aureus. Lett. Drug Des. Discov. 2010;7:674–678. [Google Scholar]
- 23.Warner V.D., Musto J.D., Sane J.N., Kim K.H., Grunewald G.L. Quantitative structure-activity relationships for 5-substituted 8-hydroxyquinolines as inhibitors of dental plaque. J. Med. Chem. 1977;20(1):92–96. doi: 10.1021/jm00211a019. [DOI] [PubMed] [Google Scholar]
- 24.R.V. Goering, H.M. Dockrell, M. Zuckerman, P.L. Chiodini, I.M. Roitt, The bacteria. Mims's Medical Microbiology, 5th edition. Elsevier, China, 2013, pp. 7–26
- 25.Taylor P.K., Yeung A.T.Y., Hancock R.E.W. Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014;191:121–130. doi: 10.1016/j.jbiotec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Sobke A., Klinger M., Hermann B., Sachse S., Nietzsche S., Makarewicz O. The urinary antibiotic 5-nitro-8-hydroxyquinoline (Nitroxoline) reduces the formation and induces the dispersal of Pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob. Agents Chemother. 2012;56(11):6021–6025. doi: 10.1128/AAC.01484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S., Abulhamd A. Phenotypic and molecular characterization of nosocomial K. pneumoniae isolates by ribotyping. Adv. Med. Sci. 2015;60(1):69–75. doi: 10.1016/j.advms.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Lerner A., Adler A., Abu-Hanna J., Cohen Percia S., Kazma Matalon M., Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin. Microbiol. Infect. 2015;21(5) doi: 10.1016/j.cmi.2014.12.015. 470.e1-470.e7. [DOI] [PubMed] [Google Scholar]
- 29.Campos J., Pichel M., Vaz T.M.I., Tavechio A.T., Fernandes S.A., Muñoz N. Building PulseNet Latin America and Caribbean Salmonella regional database: first conclusions of genetic subtypes of S. Typhi, S. Typhimurium and S. Enteritidis circulating in six countries of the region. Food Res. Int. 2012;45(2):1030–1036. [Google Scholar]
- 30.Roed C., Engsig F.N., Omland L.H., Skinhoj P., Obel N. Long-term mortality in patients diagnosed with Listeria monocytogenes meningitis: a Danish nationwide cohort study. J. Infect. 2012;64(1):34–40. doi: 10.1016/j.jinf.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Kharadi G.J. Effect of substituent of terpyridines on the in vitro antioxidant, antitubercular, biocidal and fluorescence studies of copper(II) complexes with clioquinol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;117:662–668. doi: 10.1016/j.saa.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 32.Murugasu-Oei B., Dick T. In vitro activity of the chelating agents nitroxoline and oxine against Mycobacterium bovis BCG. Int. J. Antimicrob. Agents. 2001;18(6):579–582. doi: 10.1016/s0924-8579(01)00437-x. [DOI] [PubMed] [Google Scholar]
- 33.Zheng H., Weiner L.M., Bar-Am O., Epsztejn S., Cabantchik Z.I., Warshawsky A. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer's, Parkinson's, and other neurodegenerative diseases. Bioorganic Med. Chem. 2005;13(3):773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Prachayasittikul S., Worachartcheewan A., Pingaew R., Suksrichavalit T., Isarankura-Na-Ayudhya C., Ruchirawat S. Metal complexes of uracil derivatives with cytotoxicity and superoxide scavenging activity. Lett. Drug Des. Discov. 2012;9(3):282–287. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document
Transparency document