With the recent increase in the aging population, the number of cancer patients is also increasing. Because of this increase in the number of cancer patients, even primary care physicians who are not specialized in cancer treatment will presumably have more opportunities to examine patients undergoing treatment with anticancer medications. Recently, oral multikinase inhibitors, which can cause various skin toxicities, such as acneiform eruption and hand‐foot syndrome, are increasingly used for anticancer drug therapy. Therefore, management of skin toxicity is very important for treatment continuation. In terms of primary care, it also seems necessary to differentiate rashes observed in cancer patients from skin toxicities due to anticancer medications. A 29‐year‐old man with appendiceal cancer and peritoneal dissemination received regorafenib as third‐line chemotherapy.1 Fourteen days after the first regorafenib course, numerous erythematous and coalescing papules were observed over the patient's entire body, except for the face. The rash was mainly edematous erythema and was especially prominent on the extremities. It manifested after the start of regorafenib administration, while he neither received other possibly responsible agents nor had an infection or other relevant conditions. Skin biopsy revealed no specific findings except lymphocyte infiltration into the epidermis, which was largely consistent with the features of erythema multiforme (Figure 1). Based on the clinical course and the skin biopsy results, the patient was diagnosed with grade 3 erythema multiforme due to regorafenib (Figure 1). Neither mucosal lesions nor hand‐foot skin reaction was observed. Regorafenib administration was stopped, and the patient was treated with antihistamines and steroids, which relieved the rash. Subsequently, regorafenib was resumed at a reduced dose. Although no relapse of the rash was noted, disease progression was observed after the second regorafenib course. Because the patient's systemic condition worsened, the treatment was changed to the best supportive care.
Figure 1.
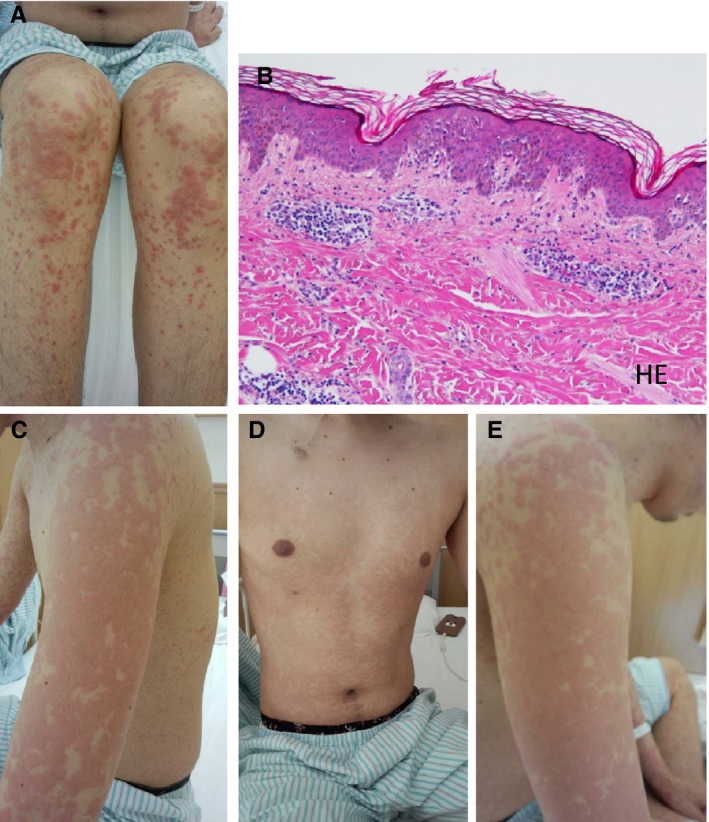
Macroscopic skin findings and skin biopsy findings. (A) Macroscopic skin findings on the lower extremities: numerous erythematous and coalescing papules were observed. (B) Skin biopsy findings of an erythematous skin lesion: lymphocyte infiltration was observed within the epidermis. Hematoxylin and eosin staining, magnification 100×. (C) Left upper extremity; (D) Chest/abdominal region; (E) Right upper extremity. As with the lower extremities, numerous erythematous and coalescing papules were observed on each region
The incidence of grade 3 or more severe rash associated with regorafenib has been reported to be 6%.1 Our literature search on PubMed (excluding meeting minutes) revealed that grade 3 or more severe erythema multiforme associated with regorafenib has been reported in two patients, both of whom were Japanese.2, 3 However, we presume that the actual incidence is higher, considering that some cases may not have been reported. According to the package insert of regorafenib, the incidence of erythema multiforme as an adverse event is 0.6%.
This case shows that careful prophylaxis and treatment are necessary in the administration of regorafenib because of the potential for development of Stevens‐Johnson syndrome with the risk of multiple organ failure.
Conflict of Interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Matsunaga M, Ushijima T, Fukahori M, Tanikawa K, Miwa K. Erythema multiforme induced by regorafenib. J Gen Fam Med. 2017;18:90–91. https://doi.org/10.1002/jgf2.29
References
- 1. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- 2. Mii Y, Fukuoka E, Murata K, et al. A case of erythema multiforme induced by regorafenib therapy for metastatic colon cancer. Gan To Kagaku Ryoho. 2014;41:1841–3. Japanese. [PubMed] [Google Scholar]
- 3. Mihara Y, Yamaguchi K, Nakama T, et al. Stevens‐Johnson syndrome induced by regorafenib in a patient with progressive recurrent rectal carcinoma. Gan To Kagaku Ryoho. 2015;42:233–6. Japanese. [PubMed] [Google Scholar]


