Abstract
Compared with younger people, elderly people show age‐related sleep changes, including an advanced sleep phase and decreased slow‐wave sleep, which result in fragmented sleep and early awakening. Multiple etiologies contribute to insomnia in the elderly, consistent with the observation that elderly people are likely to have comorbid conditions and medications. When elderly individuals complain of insomnia, it is important to assess treatable medical conditions and medication use that may be responsible for the insomnia before the use of hypnotics is initiated. Also, screening for primary sleep disorders, such as sleep apnea syndrome, restless legs syndrome and rapid eye movement sleep behavior disorder, is essential. We review sleep disorders commonly observed in the elderly and describe their diagnosis and management.
Keywords: diagnosis, elderly, management, sleep disorders
1. Introduction
Sleep disturbances are common symptoms in adults and are related to various factors, including the use of caffeine, tobacco, and alcohol; sleep habits; and comorbid diseases. Sleep apnea syndrome (SAS), rapid eye movement (REM) sleep behavior disorder (RBD), restless legs syndrome (RLS), and psychiatric diseases such as depression and anxiety should always be screened for in subjects who present with sleep disturbances. Modern life is characterized by reduced sleep times and worsened sleep quality due to changes in modern lifestyles (working late and using the computer and internet and watching TV late at night).1 An epidemiological survey performed in Japan reported an insomnia prevalence of 21.4% when insomnia was defined to include at least one instance of difficulty initiating sleep (8.3%), maintaining sleep (15.0%), or early morning awakening (8.0%).2 More than half of older adults suffer from insomnia, and these subjects are often undertreated.3 The annual incidence of insomnia in older people is reported to be 5‐8%.4, 5, 6 In a large epidemiological study of 28 714 subjects, the prevalence of excessive daytime sleepiness, defined as a self‐reported feeling of excessive daytime sleepiness “always” or “often” among five choices, was 2.5%.7
At any age, managing insomnia is a challenging issue that may require lifestyle changes. The recognition of insomnia is especially important in the elderly due to age‐related increases in comorbid medical conditions and medication use as well as age‐related changes in sleep structure, which shorten sleep time and impair sleep quality.
Spielman et al.8 proposed the 3P model of insomnia, which includes the following components: (i) predisposing factors: genetic, physiological, or psychological predispositions that increase the risk of insomnia (gender differences, vulnerability to stress, etc.); (ii) precipitating factors: physiological, environmental, or psychological stressors that trigger the onset of insomnia (life events, acute stress, etc.); and (iii) perpetuating factors: behavioral, psychological, environmental, and physiological factors that maintain insomnia (increase in the amount of time spend in bed, taking more naps, etc.). In this model, the predisposing and precipitating factors contribute to the development of insomnia, while the additional perpetuating factors are responsible for the maintenance of insomnia.9
When daytime sleepiness or sleep problems are present in older people, it is essential to assess whether sleep duration, quality, and timing are adequate. Hypersomnia disorders such as narcolepsy and idiopathic hypersomnia, which are conditions characterized by the impairment of arousal systems, typically emerge in younger subjects and are rare in older subjects. Table 1 lists causes of chronic insomnia in older people.3 Mental disorders or medical conditions that may cause insomnia should also be checked. Loss of appetite and interest in addition to insomnia may suggest depression. In addition, delirium related to dementia, anxiety disorders, alcoholism, psychological factors, and life events (loneliness, the death of a partner/spouse or hospitalization) may also cause insomnia in the elderly. Habitual snoring and witnessed apnea during sleep are signs of obstructive sleep apnea (OSA). Greater functional impairment is more strongly associated with older subjects with insomnia comorbid with SAS than with those having neither insomnia nor SAS.10 Sleep‐initiation and/or maintenance problems that are accompanied by restlessness of the legs should prompt evaluation for RLS. RBD should be suspected when nocturnal vocalization, sleep talking, and abnormal movements or behavior related to dream content are witnessed by a bed partner. In this review, we describe sleep disturbances commonly observed in older people as well as their causes and treatment.
Table 1.
Causes of chronic insomnia in older people (modified from ref. 3)
| (1) Primary sleep disorders |
| Sleep apnea syndrome |
| Restless legs syndrome, periodic limb movement disorder |
| Rapid eye movement sleep behavior disorder |
| Circadian rhythm sleep‐wake disorders (advanced and delayed sleep‐wake phase disorder) |
| (2) Acute and chronic medical illness |
| Allergy (allergic rhinitis, hay fever); Pain (arthritis, musculoskeletal pain); Cardiovascular (heart failure, acute coronary syndrome); Pulmonary (pneumonia, chronic obstructive pulmonary disease); Metabolic (diabetes, thyroid disorders), Gastrointestinal (gastroesophageal reflux disease, constipation/diarrhea, acute colitis, gastric ulcer); Urinary (nocturia, incontinence, overactive bladder, benign prostate hypertrophy for men); Psychiatric diseases (depression, anxiety, psychosis, delirium, alcoholism); Neurological disorders (Alzheimer's disease, Parkinson's disease, cerebrovascular disease, epilepsy); Pruritus; Menopause |
| (3) Behavioral causes and psychological/physical stressors |
| Daytime napping; go to bed too early; use the bed for other activities (watching TV, reading); lack of exercise during daytime; death of a partner/spouse; loneliness; hospitalization |
| (4) Environmental causes |
| Noise, light, cold/hot temperature, humidity, uncomfortable bedding, lack of light exposure during daytime |
| (5) Medications |
| Psychostimulants; antidepressants (selective serotonin reuptake inhibitors); antihypertensives (beta‐blocker, alpha‐blocker); antiparkinsonian drugs (levodopa); bronchodilators (theophylline); steroids; antihistamines (H1 and H2 blockers); anticholinergics; alcohol; interferons |
2. Physiology of Sleep and Age‐Related Changes in Sleep
The human 24‐hour sleep/wake cycle is tightly regulated by the circadian master clock located in the suprachiasmatic nuclei of the hypothalamus; this clock is synchronized by external entrainers such as light and food. It is well known that on the day after sleep loss, more sleep is needed to compensate (homeostatic sleep pressure). Thus, the homeostatic system promotes the amount of sleep we need, whereas the circadian system optimizes the best timing to sleep.11 The suprachiasmatic nucleus regulates melatonin secretion by the pineal gland.12 Melatonin modifies circadian rhythm and signals day‐night transitions. Melatonin levels in the pineal gland are low during the daytime and increase after the onset of darkness (9‐10 pm), reaching peak levels when it is darkest (3‐5 am).13, 14 The two‐process model of sleep regulation, which has been used to explain 24‐hour sleep regulation in humans, includes the following: Process S, which is entirely determined by the temporal sequence of behavioral states; and Process C, which is totally controlled by the circadian pacemaker, irrespective of behavioral state.15
In the elderly, reduced homeostatic sleep pressure decreases the amount of slow‐wave sleep.16 Additionally, reduced circadian signals in the elderly result in reduced core body temperature and a phase advance of wake and sleep times.
Sleep is divided into non‐rapid eye movement (NREM) and REM sleep. NREM sleep is further divided into light sleep (stages N1 and N2) and slow‐wave sleep (stage N3).6 REM sleep occurs periodically in cycles of approximately 90‐120 minutes of sleep. In polysomnographic studies, four consistent changes related to aging are observed: decreased total sleep time, sleep efficiency, and slow‐wave sleep; and increased waking after sleep onset.17 A meta‐analysis of 3577 subjects aged 5‐102 years demonstrated age‐related changes in sleep architecture (Figure 1).17 In adults, total sleep time, sleep efficiency, percentage of slow‐wave sleep, percentage of REM sleep, and REM latency all decreased with age. Sleep latency increased with age, but this change was very subtle. Only sleep efficiency continued to decrease after 60 years of age.
Figure 1.
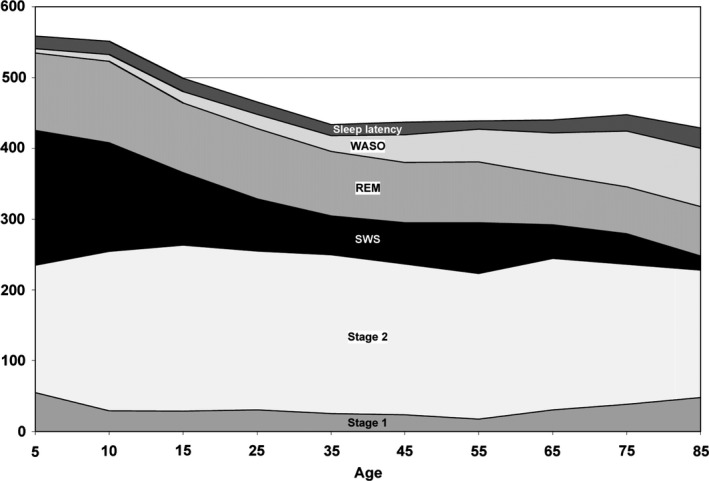
Age‐related trends for stage 1 sleep (stage N1), stage 2 sleep (stage N2), slow‐wave sleep (SWS), rapid eye movement (REM) sleep, wake after sleep onset (WASO), and sleep latency (in minutes)17
3. Diagnostic Criteria of Insomnia Disorder
Insomnia is a subjective sleep problem that is typically defined by difficulty with sleep initiation, duration, consolidation, or quality that occurs despite adequate opportunities and situations for sleep.18 In contrast, “insomnia disorder” is a syndrome characterized by insomnia complaints, as defined by the International Classification of Sleep Disorders (ICSD), 2nd or 3rd edition. In the ICSD‐2,19 primary insomnia includes various subtypes, such as psychological insomnia, idiopathic insomnia, paradoxical insomnia, and inadequate sleep hygiene. Psychological insomnia is characterized by hyperarousal and learned sleep‐preventing associations, while paradoxical insomnia is characterized by the misperception of sleep, including a propensity to underestimate the amount of actual sleep time. Secondary insomnia includes insomnia attributed to medical conditions and mental disorders such as depression, pain, or anxiety disorders (ICSD‐2). However, the various subtypes of primary sleep disorders often co‐occur, the symptoms of these disorders can overlap with those of both primary and secondary insomnia. Also, because it is often unclear whether insomnia is the result of co‐occurring comorbid disorders or conditions, in the latest ICSD, the 3rd version,20 regardless of whether the insomnia is classified as primary or secondary, all subtypes of insomnia disorders that occur at least three nights per week for at least 3 months are included as “chronic insomnia disorder” (ICSD‐3) (Table 2). According to ISCD‐3,20 when insomnia symptoms independently develop from the co‐occurring sleep disorders including RLS, SAS, and RBD, medical disorders or psychiatric disorders, or insomnia symptoms persist despite adequate treatment for the co‐occurring sleep and psychiatric disorders, a chronic insomnia disorder diagnosis would apply. Conversely, a chronic insomnia disorder diagnosis would not apply, when treatment of the co‐occurring sleep, medical, or psychiatric disorder resolves the insomnia symptoms.
Table 2.
Chronic insomnia disorder (ref. 20)
| Diagnostic criteria |
|---|
| Criteria A‐F must be met |
| A. The patient reports, or the patient's parent or caregiver observes, one or more of the following: |
| 1. Difficulty initiating sleep. |
| 2. Difficulty maintaining sleep. |
| 3. Waking up earlier than desired. |
| 4. Resistance to going to bed on appropriate schedule. |
| 5. Difficulty sleeping without parent or caregiver intervention. |
| B. The patient reports, or the patient's parent or caregiver observes, one or more of the following related to the nighttime sleep difficulty: |
| 1. Fatigue/malaise. |
| 2. Attention, concentration, or memory impairment. |
| 3. Impaired social, family, occupational, or academic performance. |
| 4. Mood disturbance/irritability. |
| 5. Daytime sleepiness. |
| 6. Behavioral problems (eg, hyperactivity, impulsivity, aggression). |
| 7. Reduced motivation/energy/initiative. |
| 8. Proneness for errors/accidents. |
| 9. Concerns about or dissatisfaction with sleep. |
| C. The reported sleep/wake complaints cannot be explained purely by inadequate opportunity (ie, enough time is allotted for sleep) or inadequate circumstances (ie, the environment is safe, dark, quiet, and comfortable) for sleep. |
| D. The sleep disturbance and associated daytime symptoms occur at least three times per week. |
| E. The sleep disturbance and associated daytime symptoms have been present for at least 3 mo |
| F. The sleep/wake difficulty is not better explained by another sleep disorder. |
These criteria clearly state that insomnia sufferers experience daytime dysfunction such as fatigue and daytime sleepiness. Nonrestorative sleep remains a factor in the International Classification of Disease 10th edition (ICD‐10), which requires a duration of one month for an insomnia diagnosis, but was eliminated from the Diagnostic and Statistical Manual, version 5 (DSM‐V), which requires a duration of at least 3 months for a diagnosis of insomnia. Therefore, depending on the insomnia criteria used, the prevalence of insomnia can vary widely: The reported prevalence of insomnia among adults was 15% and 4% according to the ICSD‐2 and ICD‐10, respectively.6
4. Insomnia in the Elderly
In elderly individuals, sleep‐maintenance insomnia and early awakening are more common complaints than sleep‐onset insomnia; this is likely due to the age‐related changes in sleep architecture and circadian rhythm described above.21 Unruh et al.22 administered unattended home polysomnography (PSG) and sleep questionnaires to 5407 community‐dwelling adults aged 45‐99 years (mean age, 63 years) who were members of the Sleep Heart Health Study Cohort. The results showed that in both men and women, older age was associated with shorter sleep duration, reduced sleep efficiency, and increased arousal. In men but not women, older age was independently associated with increased light stages of sleep (Stage 1 and Stage 2), while older women had more trouble falling asleep and tended to have more problems with waking up during the night and waking up too early. In a cross‐sectional study of 13 563 adults aged 47‐69 years who were participants in a prospective population‐based study of cardiovascular disease, the prevalence of sleep complaints involving difficulty falling asleep, difficulty staying asleep, and nonrestorative sleep was 22%, 39%, and 35%, respectively.23 Increasing age was associated with difficulty staying asleep but not with difficulty falling asleep or nonrestorative sleep. In a cross‐sectional, multicenter study of 2862 community‐dwelling men at least 65 years of age, greater sleep fragmentation (sleep efficiency <80% and/or waking after sleep onset) and hypoxia (>1% of sleep time with O2 saturation <90%) were associated with poorer physical function, such as decreased grip strength and gait speed.24
Falls and fractures in older people are significant problems that affect their quality of life. Stone et al.25 investigated the risk of falls in 2978 community‐dwelling women 70 years and older using actigraphy. The authors showed that short nighttime sleep duration (<5 hours; odds ratio, 1.52; 95% CI, 1.03‐2.24) and increased sleep fragmentation increased the risk of falls (odds ratio, 1.36; 95% CI, 1.07‐174) in older women, independent of benzodiazepine use, and other risk factors for falls. Although the results remain controversial,26 Morimoto et al.27 demonstrated that obstructive, central, and mixed SAS were associated with an increased risk of cardiovascular‐related and all‐cause mortality. Elderly participants with persistent insomnia have also been reported to have an increased risk for developing depression.28 Despite the fact that sleep becomes increasingly fragmented with age, several studies have shown that older people are less likely to complain of sleep problems than younger people, possibly because of age‐related adjustments of sleep status in older adults or because older adults tend to be more tolerant of sleep deprivation.6
5. Differential Diagnosis of Insomnia
When making a diagnosis of insomnia, sleep habits (sleep/wake schedule, sleep time, and naps) should be assessed. A sleep diary is a useful tool to assess sleep patterns and usual sleep times and thus also in diagnosing sleep problems. A flowchart for the screening of sleep problems is illustrated in Figure 2.29 When patients have sleep problems, changes in environmental conditions, physical/psychosocial stressors, medications, and chronic and acute medical illnesses should be screened first. Then, accompanying symptoms or a detailed sleep history may reveal psychiatric diseases such as depression, SAS, RLS, or parasomnia. Additionally, when patients present with abnormal nocturnal behaviors and are unaware of them, epilepsy, night delirium, or metabolic disorders such as hypoglycemia due to insulinoma should be ruled out (Table 3).30 NREM parasomnia is common in younger subjects, whereas REM parasomnia, such as RBD, is common in subjects aged 50 years or older. The RBD screening questionnaire (RBDSQ) is a widely used,31 including in Japan,32 and useful way of screening for RBD (a cutoff RBDSQ score ≥5 suggests probable RBD).
Figure 2.
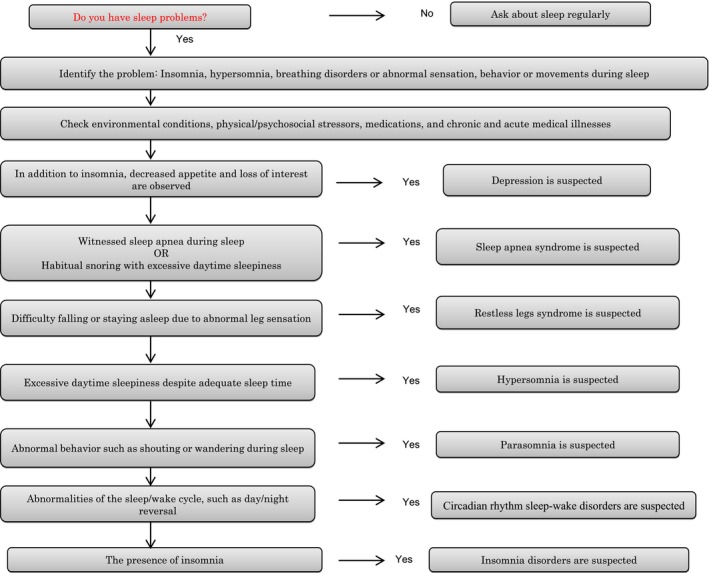
Flowchart of screening for sleep disturbances (modified from ref. 29)
Table 3.
Differential diagnosis of abnormal nocturnal behavior: sleep disorders versus disturbance of consciousness (ref. 30)
| Sleep disorders | Disturbance of consciousness | ||||
|---|---|---|---|---|---|
| REM parasomnia | Non‐REM parasomnia | ||||
| REM sleep behavior disorder | Arousal disorder (sleepwalking and sleep terrors) | Epilepsy | Night delirium | Hypoglycemia due to insulinoma | |
| Peak onset age | >50 y of age | Child | Child/elderly | Elderly | Any age (middle age) |
| Time of day of the occurrence of abnormal behavior | Early morning | First half of the night | Anytime | Night (anytime) | Early morning |
| Type of movement | Complex | Complex | Stereotyped | Complex | Complex, stereotyped |
| Walking | ±/+ | + | + | + | + |
| Urinary incontinence | − | − | ±/+ | ±/+ | + |
| Nightmares | + | ± | − | − | − |
| Dream recall | + | − | − | − | − |
| Arousal in response to external stimuli | Quick to awaken | Difficult to arouse | Difficult to arouse | Difficult to arouse | Difficult to arouse |
| Electroencephalographic findings | REM sleep without atonia | Epileptic discharge | Slow dominant rhythm | Slow dominant rhythm | |
| Relevant sleep stage | REM sleep |
Non‐REM sleep Stage N3 |
Non‐REM sleep | ||
6. Management of Insomnia
There are both nonpharmacological approaches and pharmacological treatments for insomnia. Sleep hygiene education should be the first approach because the development of good sleep habits benefits all insomnia sufferers. As mentioned previously, when other sleep disorders or mental/physical illnesses related to insomnia are suspected, prompt screening for each condition is needed. In parallel, treatment for insomnia is recommended; it is not necessary to wait for a response to treatment for comorbid conditions. However, the use of benzodiazepines should be avoided when severe SAS is strongly suspected, as these drugs aggravate sleep apnea. Also, antidepressants such as selective serotonin reuptake inhibitors can worsen or trigger RLS or RBD. Good sleep hygiene includes regular exercise and meals, the avoidance of stimulants such as caffeine and tobacco, and the creation of a comfortable sleep environment (Table 4).33 Table 5 lists nonpharmacological treatments for insomnia in the elderly, including stimulus control, sleep restriction, and cognitive behavioral therapy for insomnia (CBT‐I). CBT‐I has been shown to be useful in elderly subjects,34 although in Japan, CBT‐I is available in a limited number of facilities. Insomnia can co‐occur with medical diseases, which sometimes result in secondary insomnia, and in these cases, it is important to treat the primary disease. However, all subtypes of insomnia disorders are included in the ICSD‐3 classification of chronic insomnia disorders, without any differentiation between secondary insomnia and primary insomnia, suggesting that treatment of insomnia should be initiated along with treatment for comorbid diseases, rather than waiting to determine the effect that treating the primary disease has on the insomnia. If left untreated, insomnia is known to have a negative impact on the quality of life of sufferers.
Table 4.
Sleep hygiene (modified from ref. 33)
| Tips | Contents |
|---|---|
| (1) Regular exercise | Take regular exercise. Adequate aerobic exercise improves the ability to fall asleep. Exercise in the early morning and early evening promotes deep sleep and improves sleep quality; however, exercise just before bedtime should be avoided. |
| (2) Bedroom environment | Keep bedroom dark and quiet. Noises and dim light can interrupt sleep. Maintain a comfortable bedroom temperature (below 24 degrees Celsius [75 degrees Fahrenheit]). During the summer season, consider using an air conditioner. |
| (3) Regular meals | Keep regular eating patterns, 3 times/day. When you feel hungry, eat a light snack (cheese, milk, nuts, or carbohydrates) but avoid heavy meals before bedtime. |
| (4) Limit fluid intake before bedtime | Limit fluid intake before bedtime to reduce the frequency of urination during sleep. In cases of cerebral infarction or angina pectoris, follow the instructions of your primary physician. |
| (5) Avoid caffeine | Caffeine intake before bedtime may result in sleep‐initiation and maintenance problems. Limit caffeinated foods and beverages (Green tea, tea, coffee, cola, and chocolate) to the equivalent of three cups of coffee and ingest them no later than 4 h before bedtime. |
| (6) Avoid alcohol | Limit alcoholic beverages, which may promote sleep initiation but cause fragmented and unrefreshing sleep. |
| (7) Avoid smoking | Avoid smoking in the evening. Nicotine acts as a stimulant, interfering with sleep. |
Table 5.
Nonpharmacological treatments for insomnia in the elderly (modified from ref. 33)
|
Stimulus control Ask patients to go to bed only when sleepy. The bed should be used for sleep only. If the patient is unable to fall asleep for 20 min, suggest they get up and go to another room to do something. The patient can go back to the bedroom when sleepy. The patient should get up at the same time every morning, irrespective of how much sleep the patient had during the night. Avoid naps. |
|
Sleep restriction Patients should be instructed to sleep for the average total sleep time estimated from a 2‐week sleep diary (minimum 4.5 h). Sleep efficiency, as defined by total sleep time/time spent in bed, should be evaluated regularly. When sleep efficiency improves, the time spent in bed can be increased. When sleep efficiency is >90%, the patient go to bed 15 min earlier. |
|
Sleep hygiene (see Table 4) For good sleep habits, several tips are recommended: regular exercise and meals, the avoidance of stimulants such as caffeine and tobacco, and the creation of a comfortable sleep environment. |
|
Cognitive behavioral treatment Identify incorrect thoughts, beliefs, or knowledge about sleep and correct knowledge, emotions, and behaviors related to sleep. |
|
Bright light therapy When the patients have advances in sleep phases or early awakenings, light exposure or bright light therapy in the evening is recommended. |
When nonpharmacological strategies do not work well, pharmacological treatments should be considered. Hypnotic drugs should be used in the lowest effective dose, and short‐term use is recommended. The hypnotics available in Japan are listed in Table 6. For sleep‐onset insomnia, ultra‐short‐acting or short‐acting types are used, while for frequent arousal during sleep or early morning awakening, intermediate types or long‐acting types may be chosen. However, attention should be paid to potential next‐day hangover effects when prescribing intermediate or long‐acting hypnotics, especially in the elderly. Both benzodiazepine and nonbenzodiazepine drugs (eszopiclone, zopiclone, and zolpidem) bind to GABAA receptors in the brain, exerting hypnotic effects. Benzodiazepine hypnotics bind equivalently to all GABAA receptors (α1‐, α2‐, α3‐, and α5‐containing subtypes). In contrast, nonbenzodiazepine hypnotics (zolpidem, zopiclone, and eszopiclone) preferentially bind α1‐containing subtypes, and the shorter half‐life of the effects of binding to these receptors lowers the risk of fall and residual sleepiness.35 In a recent randomized placebo‐controlled study that was conducted over 12 months of observation and included 89 patients with primary insomnia, 10 mg/day zolpidem significantly increased sleep efficiency and reduced sleep latency and waking after sleep onset. There were also no differences in efficacy between women and men, and rebound insomnia and dose escalation were also absent.36 Eszopiclone has been reported to be efficacious in treating patients with insomnia with comorbid diseases such as depression37 and Parkinson's disease.38 Compared to nonbenzodiazepine drugs, benzodiazepine drugs have more anxiolytic effects, which are dependent on the integrity of the α2 subunit, but postwithdrawal rebound, tolerance, and dependence are more important issues.39 Although the association between the use of hypnotics and the subsequent risk of dementia is inconclusive, several studies have demonstrated an association between the use of benzodiazepines and subsequent dementia. In prospective studies that included older people, long‐term use of benzodiazepines was related to an increased risk of dementia (odds ratio 1.6‐3.5).40, 41 In addition, over 8 years of follow‐up of 8204 people aged 65 or greater, Shash et al.42 showed that the use of long‐half‐life benzodiazepine was associated with an increased risk of dementia (adjusted hazard ratio=1.62; 95% CI, 1.11‐2.37). However, because untreated insomnia can impair daytime performance and cognitive function, when the use of benzodiazepines is considered, the balance of risks and benefits should be considered, and the lowest effective dose for short‐term use is recommended.
Table 6.
Pharmacological treatment for insomnia: primary hypnotic drugs available in Japan
| Class | Type | Generic name | Dose (mg) | Half‐life (h) |
|---|---|---|---|---|
| Benzodiazepine | Ultra‐short‐acting | Triazolam | 0.125–0.25 | 2–4 |
| Short‐acting | Etizolam | 0.5–1 | 6 | |
| Brotizolam | 0.25 | 7 | ||
| Rilmazafone | 1–2 | 10 | ||
| Lormetazepam | 1–2 | 10 | ||
| Intermediate‐acting | Flunitrazepam | 0.5–2 | 24 | |
| Estazolam | 1–4 | 24 | ||
| Nitrazepam | 5–10 | 28 | ||
| Long‐acting | Quazepam | 15–30 | 36 | |
| Flurazepam | 10–30 | 65 | ||
| Nonbenzodiazepine | Ultra‐short‐acting | Zolpidem | 5–10 | 2 |
| Zopiclone | 7.5–10 | 4 | ||
| Eszopiclone | 1–3 (Elderly 1–2) |
Adults 5 Elderly 7 |
||
| Melatonin receptor agonist | Ramelteon | 8 | 1–2 | |
| Dual orexin receptor antagonist | Suvorexant | 20 (Elderly 15) | 12 |
In a single‐center study in Japan that evaluated fall rates among 1469 older inpatients taking different types of hypnotics, eszopiclone had the lowest fall rate (1.36%), followed by zolpidem (2.32%), zopiclone (3.85%), brotizolam (4.14%), triazolam (12.0%), and estazolam (13.1%).43 However, in this study, confounding factors, such as diabetes, depression, and total sleep time, were not evaluated. Although melatonin receptor agonists (ramelteon) and orexin receptor antagonists (such as suvorexant) were not included in this study, they should have a lower risk of falls because their mechanisms differ from GABAA stimulation.
Ramelteon is a selective agonist of MT1 and MT2 melatonin receptors located in the suprachiasmatic nuclei in the hypothalamus11. Activation of MT1 receptors decreases the alerting signal in the evening, and activation of MT2 receptors can shift the phase of the circadian system. Compared with benzodiazepine hypnotics, ramelteon is associated with lower risk of falling, withdrawal, and tolerance. Ramelteon has been shown to be effective in treating sleep‐onset insomnia and delayed sleep phase syndrome. Its half‐life is short (0.83‐1.90 hours); however, its effects in reducing wakefulness last up to 6 hours.11, 44 Ramelteon has been reported to be useful in improving sleep‐onset latency in OSA patients treated with auto‐titrating positive airway pressure therapy.45 Additionally, the administration of 8 mg/day ramelteon for 7 days to elderly patients admitted for acute care protected against the occurrence of delirium in a multicenter, rater‐blinded, randomized, placebo‐controlled setting.46
Suvorexant, a dual orexin receptor antagonist, is a new class of hypnotic that works by selectively blocking the binding of the wakefulness‐promoting neuropeptides orexin A and B to OX1 and OX2 receptors, which suppresses wakefulness.47 Suvorexant has a half‐life of approximately 12 hours and is useful in subjects with sleep‐onset as well as sleep‐maintenance insomnia. There was no evidence of withdrawal or rebound effects upon discontinuation after up to 1 year of treatment. Based on the drug's mechanism of action, a benign side effect profile has been suggested.48 The administration of 30‐40 mg/day suvorexant (up to twice the maximum recommended dose for treating insomnia in the USA and Japan) in patients with mild‐to‐moderate chronic obstructive pulmonary disease49 or mild‐to‐moderate OSA50 did not have a detrimental effect on respiratory status as measured by SpO2 or the apnea hypopnea index (AHI).
7. Primary Sleep Disorders and Its Management
7.1. Rapid eye movement sleep behavior disorder
Rapid eye movement sleep behavior disorder is a parasomnia that occurs during REM sleep and is characterized by dream‐enacting behavior, which is the acting out of dream content, including talking, shouting, punching, and kicking; REM sleep without atonia (RWA), loss of normal skeletal muscle atonia during REM sleep, as documented on polysomnography; as well as altered dream mentation—nightmares of being chased or attacked by unfamiliar people or animals are common.51 Abnormal nocturnal behaviors due to RBD are often associated with injury of the patients and their bed partners during sleep. Diagnosis of RBD requires repeated episodes of sleep‐related vocalization and/or complex motor behaviors; these behaviors are documented by PSG during REM sleep or presumed to occur during REM sleep based on clinical history, and the presence of RWA on PSG (ICSD‐3).20 Upon awakening, individuals with RBD are typically alert and can recall the contents of their dreams. However, approximately 8% of idiopathic RBD subjects did not recall unpleasant dreams, and 46% were unaware of their abnormal night behavior.52 Additionally, in patients with RBD, the details of dreams can be almost forgotten by noon the following day.51 The prevalence of RBD has been estimated as 0.5% of the general population.53 In a large, random‐sample survey of 19 961 members of the general population of Finland, Germany, Italy, Portugal, Spain, and the United Kingdom who were 15 years and older, 1.6% of the respondents reported violent behavior during sleep; 78.7% of these individuals reported vivid dreams, and 31.4% hurt themselves or someone else.54 A study of 349 elderly inpatients and outpatients at the University of Hong Kong identified 2 (0.57%) with RBD based on polysomnographic findings.55 In a recent study of 488 subjects aged 60‐97 years (without Parkinson's disease) representative of the general elderly community, the prevalence of probable RBD was 4.6% and 7.7% according to the RBD screening questionnaire and Innsbruck RBD‐Inventory, respectively.56 RBD is more common in males and usually occurs after 50 years of age. Early‐onset RBD (onset before 50 years of age) has been associated with increased gender parity, an increased proportion of idiopathic RBD and an increased occurrence of narcolepsy and antidepressant use.57
It is becoming increasingly clear that 50% of idiopathic patients with RBD convert to a parkinsonian disorder within a decade and that 81%‐90% patients with RBD eventually develop a neurodegenerative disorder.58 Older patients with RBD should be regularly evaluated for the presence of subtle parkinsonism and cognitive and olfactory impairment.
Hazard avoidance, including removing potentially dangerous objects from the bedroom or placing a mattress on the floor, is important to prevent sleep‐related injury in patients with RBD. Clonazepam is effective in approximately 90% of patients with RBD, but for elderly patients, the potential side effects of daytime sleepiness and dizziness should be noted. When patients with dream‐enacting behavior also have a history of habitual snoring or witnessed sleep apnea, SAS should be screened for because clonazepam may worsen sleep apnea in patients with comorbid SAS. Additionally, it is important to note that severe SAS can mimic RBD—such cases are called pseudo‐RBD—and that the abnormal behavior related to SAS can be effectively treated with continuous positive airway pressure (CPAP) therapy.59 In the USA and Europe, 3‐12 mg melatonin at bedtime has had clinical efficacy in treating RBD with a reduced risk of fall.60 The efficacy of the herbal medicine Yi‐Gan San has also been reported in Japan.61
7.2. Restless legs syndrome
Restless legs syndrome is classified as a sleep‐related movement disorder20 that is typified by an urge to move the legs and abnormal leg sensation, resulting in sleep‐initiation and/or sleep‐maintenance problems.62 RLS is also known as Willis–Ekbom disease. The RLS diagnostic criteria proposed by the International RLS study group (IRLSSG) include four essential features63: (i) an urge to move the legs; (ii) the symptoms (the urge to move the legs and any accompanying unpleasant sensations) begin or worsen during periods of inactivity; (iii) the symptoms are partially or totally relieved by movement; and (iv) the symptoms occur only in the evening or at night or are worse during these times. The latest criteria of the IRLSSG added as a fifth criterion that other conditions or diseases that mimic RLS be ruled out (Table 7).62 Clinical features that support a RLS diagnosis include family history of RLS (more than 50% of patients with RLS have a family history of RLS), a favorable response to dopaminergic treatment, the presence of periodic limb movements during sleep or resting wakefulness, and the lack of profound daytime sleepiness. Despite significant sleep loss, patients with RLS rarely report daytime sleepiness.64 Using proton magnetic resonance spectroscopy, Allen et al.65 found increased thalamic glutamate levels in patients with RLS that were correlated with wake times during the sleep period, supporting the concept that the hyperarousal state that characterizes RLS is mediated by a nondopaminergic system. When RLS affects older people who are cognitively impaired, the assessment of symptoms and clinical diagnosis are challenging. Table 8 shows diagnostic criteria for probable RLS in the cognitively impaired elderly.63 An urge to move the legs is suggested when patients rub their legs or exhibit excessive motor activity. Supporting criteria such as dopaminergic responsiveness, significant sleep‐onset problems, and family history of RLS may also be useful.
Table 7.
Essential and supportive diagnostic criteria for RLS (ref. 62)
| Essential diagnostic criteria of RLS | |
| 1 | An urge to move the legs usually but not always accompanied by, or felt to be caused by, uncomfortable and unpleasant sensations in the legs. |
| 2 | The urge to move the legs and any accompanying unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting. |
| 3 | The urge to move the legs and any accompanying unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues. |
| 4 | The urge to move the legs and any accompanying unpleasant sensations during rest or inactivity only occur or are worse in the evening or night than during the day. |
| 5 | The occurrence of the above features is not solely accounted for as symptoms primary to another medical or a behavioral condition (eg, myalgia, venous stasis, leg edema, arthritis, leg cramps, positional discomfort, habitual foot tapping). |
| Clinical features supporting the diagnosis of RLS | |
| The following features, although not essential for diagnosis, are closely associated with RLS/Willis–Ekbom disease (WED) and should be noted when present: | |
| 1 | Periodic limb movements (PLM): presence of periodic leg movements in sleep (PLMS) or resting wake (PLMW) at rates or intensity greater than expected for age or medical/medication status. |
| 2 | Dopaminergic treatment response: reduction in symptoms, at least initially with dopaminergic treatment. |
| 3 | Family history of RLS/WED among first‐degree relatives. |
| 4 | Lack of profound daytime sleepiness. |
Table 8.
Essential and supportive criteria for the diagnosis of probable RLS in the cognitively impaired elderly (ref. 64)
| Essential diagnostic criteria of RLS in the cognitively impaired elderly | |
| 1 | Signs of leg discomfort such as rubbing or kneading the legs and groaning while holding the lower extremities are present. |
| 2 | Excessive motor activity in the lower extremities, such as pacing, fidgeting, repetitive kicking, tossing and turning in bed, slapping the legs on the mattress, cycling movements of the lower limbs, repetitive foot tapping, rubbing the feet together, and the inability to remain seated, are present. |
| 3 | Signs of leg discomfort are exclusively present or worsen during periods of rest or inactivity. |
| 4 | Signs of leg discomfort are diminished with activity. |
| 5 | Criteria 1 and 2 occur only in the evening or at night or are worse at those times than during the day. |
| Supportive diagnostic criteria of RLS in the cognitively impaired elderly | |
| a | Dopaminergic responsiveness |
| b | Patient's past history—as reported by a family member, caregiver, or friend—is suggestive of RLS |
| c | A first‐degree, biologic relative (sibling, child, or parent) has RLS |
| d | Observed periodic limb movements while awake or during sleep |
| e | Periodic limb movements of sleep recorded by polysomnography or actigraphy |
| f | Significant sleep‐onset problems |
| g | Better quality sleep in the day than at night |
| h | The use of restraints at night (for institutionalized patients) |
| i | Low serum ferritin level |
| j | End‐stage renal disease |
| k | Diabetes |
| l | Clinical, electromyographic, or nerve‐conduction evidence of peripheral neuropathy or radiculopathy |
Iron replacement therapy should be considered for RLS treatment when serum ferritin levels are lower than 50 μg/L, even if patients do not have iron deficiency anemia. Low‐dose dopamine agonists, such as pramipexole and the rotigotine patch, are effective. Additionally, alpha‐2 delta ligands, such as gabapentin, pregabalin, and gabapentin enacarbil, are effective. Opioids may be alternatively used in severe cases.
7.3. Sleep apnea syndrome
Among the forms of SAS, OSA is more common in the general population, including the elderly. When central sleep apnea or Cheyne‐Stokes respiration is identified, screening for cardiac and cerebrovascular disease should be performed. OSA is associated with metabolic syndrome, diabetes, hypertension, and cardiovascular events.66, 67 Patients with moderate‐to‐severe OSA experience an independently increased risk for all‐cause mortality and stroke;68, 69 however, adequate use of long‐term CPAP therapy improves cardiovascular outcomes.70 In a study of 5615 community‐dwelling men and women between 40‐98 years of age, sleep apnea was found to be common in subjects aged 60 years or older (approximately 50% had an AHI of 5‐14, and approximately 20% had an AHI≥15).71 In this study, breathing pauses and obesity were insensitive detectors of sleep apnea in older subjects, likely due to the increased upper airway collapsibility in older subjects compared to younger subjects.6 In a study of 99 older adults and 100 controls, the presence of insomnia symptoms and SAS (AHI≥15) was associated with lower daytime functioning and longer psychomotor reaction times.10
Oral appliances and CPAP therapy are used for mild and severe OSA, respectively. For overweight to obese subjects, diet and regular exercise should be recommended.
8. Conclusion
Here, we reviewed and discussed the differential diagnosis, clinical diagnosis, and management of insomnia in elderly subjects. In elderly subjects, the effects on sleep of many comorbid medical conditions, concomitant medication use, and age‐related physiological changes in sleep architecture and circadian rhythm should be considered. Comprehensive assessment and management of insomnia along with treatment of comorbid medical conditions may improve the patient's quality of sleep as well as of daytime life.
Conflict of Interest
The authors declare no conflict of interests regarding this article.
Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: Diagnosis and management. J Gen Fam Med. 2017;18:61–71. https://doi.org/10.1002/jgf2.27
References
- 1. Bixler E. Sleep and society: an epidemiological perspective. Sleep Med. 2009;10(Suppl 1):S3–6. [DOI] [PubMed] [Google Scholar]
- 2. Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23:41–7. [PubMed] [Google Scholar]
- 3. Kamel NS, Gammack JK. Insomnia in the elderly: cause, approach, and treatment. Am J Med. 2006;119:463–9. [DOI] [PubMed] [Google Scholar]
- 4. Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–72. [PubMed] [Google Scholar]
- 5. Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22(Suppl 2):S373–8. [PubMed] [Google Scholar]
- 6. Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30:591–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaneita Y, Ohida T, Uchiyama M, et al. Excessive daytime sleepiness among the Japanese general population. J Epidemiol. 2005;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 9. Buysse DJ, Germain A, Hall M, Monk TH, Nofzinger EA. A neurobiological model of insomnia. Drug Discov Today Dis Models. 2011;8:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gooneratne NS, Gehrman PR, Nkwuo JE, et al. Consequences of comorbid insomnia symptoms and sleep‐related breathing disorder in elderly subjects. Arch Intern Med. 2006;166:1732–8. [DOI] [PubMed] [Google Scholar]
- 11. Neubauer DN. A review of ramelteon in the treatment of sleep disorders. Neuropsychiatr Dis Treat. 2008;4:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benarroch EE. Suprachiasmatic nucleus and melatonin: reciprocal interactions and clinical correlations. Neurology. 2008;71:594–8. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki K, Miyamoto M, Miyamoto T, Sakuta H, Hirata K. The impact of sleep disturbances on neuroendocrine and autonomic functions. Nihon Rinsho. 2012;70:1169–76. [PubMed] [Google Scholar]
- 14. Guadiola‐Lemaitre B, Quera‐Salva MA. Melatonin and the regulation of sleep and circadian rhythms In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine, 5th ed St. Louis: Saunders, 2011; p. 420–30. [Google Scholar]
- 15. Beersma DG, Gordijn MC. Circadian control of the sleep‐wake cycle. Physiol Behav. 2007;90:190–5. [DOI] [PubMed] [Google Scholar]
- 16. Ancoli‐Israel S, Shochat T. Insomnia in older adults In: Principles and practice of sleep medicine, 5th ed Kryger M, Roth T, Dement W, editors. Philadelphia: Saunders, 2010, p. 1544–50. [Google Scholar]
- 17. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta‐analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. [DOI] [PubMed] [Google Scholar]
- 18. Vaughn BV, D'Cruz O. Cardinal manifestations of sleep disorders In: Principles and practice of sleep medicine, 5th ed Kryger MH, Roth T, Dement WC, editors. St. Louis: Saunders, 2011; p. 647–58. [Google Scholar]
- 19. American Academy of Sleep Medicine . International classification of sleep disorders: diagnostic and coding manual, 2nd ed Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 20. American Academy of Sleep Medicine . International classification of sleep disorders, 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 21. Suzuki K, Miyamoto M, Hirata K. Neurological common diseases in the super‐elder society. Topics: V. Dizziness, faintness, numbness and insomnia: 3. Characteristics and treatment of sleep disorders in the elderly. Nihon Naika Gakkai Zasshi. 2014;103:1885–95. [DOI] [PubMed] [Google Scholar]
- 22. Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56:1218–27. [DOI] [PubMed] [Google Scholar]
- 23. Phillips B, Mannino D. Correlates of sleep complaints in adults: the ARIC study. J Clin Sleep Med. 2005;1:277–83. [PubMed] [Google Scholar]
- 24. Dam TT, Ewing S, Ancoli‐Israel S, et al. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stone KL, Ancoli‐Israel S, Blackwell T, et al. Actigraphy‐measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–75. [DOI] [PubMed] [Google Scholar]
- 26. Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28:965–71. [DOI] [PubMed] [Google Scholar]
- 27. Morimoto S, Takahashi T, Okaishi K, et al. Sleep apnoea syndrome as a risk for mortality in elderly inpatients. J Int Med Res. 2012;40:601–11. [DOI] [PubMed] [Google Scholar]
- 28. Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. [DOI] [PubMed] [Google Scholar]
- 29. Tagaya H, Shimizu T. Screening guideline for sleep disorders in general healthcare facilities. Sleep Med Jpn. 2008;2:267–70. [Google Scholar]
- 30. Suzuki K, Kawasaki A, Miyamoto M, et al. Insulinoma masquerading as rapid eye movement sleep behavior disorder: case series and literature review. Medicine (Baltimore). 2015;94:e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stiasny‐Kolster K, Mayer G, Schafer S, Moller JC, Heinzel‐Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov Disord. 2007;22:2386–93. [DOI] [PubMed] [Google Scholar]
- 32. Miyamoto T, Miyamoto M, Iwanami M, et al. The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version. Sleep Med. 2009;10:1151–4. [DOI] [PubMed] [Google Scholar]
- 33. Edinger JD, Means MK, Carney CE, Manber R. Psychological and behavioral treatments for insomnia II: implementation and specific populations In: Principles and practice of sleep medicine, 5th ed Kryger M, Roth T, Dement W, editors. Philadelphia: Saunders, 2010; p. 884–904. [Google Scholar]
- 34. Montgomery P, Dennis J. A systematic review of non‐pharmacological therapies for sleep problems in later life. Sleep Med Rev. 2004;8:47–62. [DOI] [PubMed] [Google Scholar]
- 35. Monti JM, Monti D. Overview of currently available benzodiazepine and nonbenzodiazepine hypnotics In: Clinical pharmacology of sleep. Pandi‐Perumal SR, Monti JM, editors. Basel, Boston, Berlin: Birkhäuser Verlag, 2006; p. 207–24. [Google Scholar]
- 36. Roehrs TA, Roth T. Gender differences in the efficacy and safety of chronic nightly zolpidem. J Clin Sleep Med. 2016;12:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCall WV, Blocker JN, D'Agostino R Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health‐related quality of life, objective and self‐reported sleep, and depression. J Clin Sleep Med. 2010;6:322–9. [PMC free article] [PubMed] [Google Scholar]
- 38. Menza M, Dobkin RD, Marin H, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Mov Disord. 2010;25:1708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner J, Wagner ML. Non‐benzodiazepines for the treatment of insomnia. Sleep Med Rev. 2000;4:551–81. [DOI] [PubMed] [Google Scholar]
- 40. Billioti de Gage S, Begaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gallacher J, Elwood P, Pickering J, Bayer A, Fish M, Ben‐Shlomo Y. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). J Epidemiol Community Health. 2012;66:869–73. [DOI] [PubMed] [Google Scholar]
- 42. Shash D, Kurth T, Bertrand M, et al. Benzodiazepine, psychotropic medication, and dementia: a population‐based cohort study. Alzheimers Dement. 2016;12:604–13. [DOI] [PubMed] [Google Scholar]
- 43. Oda S, Inoue T. Fall rate research of Z‐drug and benzodiazepine. J New Rem & Clin. 2015;64:1468–73. [Google Scholar]
- 44. Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neurosci Ther. 2009;15:32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gooneratne NS, Gehrman P, Gurubhagavatula I, Al‐Shehabi E, Marie E, Schwab R. Effectiveness of ramelteon for insomnia symptoms in older adults with obstructive sleep apnea: a randomized placebo‐controlled pilot study. J Clin Sleep Med. 2010;6:572–80. [PMC free article] [PubMed] [Google Scholar]
- 46. Hatta K, Kishi Y, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo‐controlled trial. JAMA Psychiatry. 2014;71:397–403. [DOI] [PubMed] [Google Scholar]
- 47. Rhyne DN, Anderson SL. Suvorexant in insomnia: efficacy, safety and place in therapy. Ther Adv Drug Saf. 2015;6:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asnis GM, Thomas M, Henderson MA. Pharmacotherapy treatment options for insomnia: a primer for clinicians. Int J Mol Sci. 2016;17:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun H, Palcza J, Rosenberg R, et al. Effects of suvorexant, an orexin receptor antagonist, on breathing during sleep in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109:416–26. [DOI] [PubMed] [Google Scholar]
- 50. Sun H, Palcza J, Card D, et al. Effects of suvorexant, an orexin receptor antagonist, on respiration during sleep in patients with obstructive sleep apnea. J Clin Sleep Med. 2015;12:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder‐neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iranzo A, Santamaria J, Tolosa E. The clinical and pathophysiological relevance of REM sleep behavior disorder in neurodegenerative diseases. Sleep Med Rev. 2009;13:385–401. [DOI] [PubMed] [Google Scholar]
- 53. Ohayon MM, Caulet M, Priest RG. Violent behavior during sleep. J Clin Psychiatry. 1997;58:369–76; quiz 377. [PubMed] [Google Scholar]
- 54. Ohayon MM, Schenck CH. Violent behavior during sleep: prevalence, comorbidity and consequences. Sleep Med. 2010;11:941–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chiu HF, Wing YK, Chung DW, Ho CK. REM sleep behaviour disorder in the elderly. Int J Geriatr Psychiatry. 1997;12:888–91. [PubMed] [Google Scholar]
- 56. Mahlknecht P, Seppi K, Frauscher B, et al. Probable RBD and association with neurodegenerative disease markers: a population‐based study. Mov Disord. 2015;30:1417–21. [DOI] [PubMed] [Google Scholar]
- 57. Ju YE. Rapid eye movement sleep behavior disorder in adults younger than 50 years of age. Sleep Med. 2013;14:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Howell MJ, Schenck CH. Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol. 2015;72:707–12. [DOI] [PubMed] [Google Scholar]
- 59. Iranzo A, Santamaria J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–6. [DOI] [PubMed] [Google Scholar]
- 60. Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. 2010;6:85–95. [PMC free article] [PubMed] [Google Scholar]
- 61. Shinno H, Kamei M, Nakamura Y, Inami Y, Horiguchi J. Successful treatment with Yi‐Gan San for rapid eye movement sleep behavior disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1749–51. [DOI] [PubMed] [Google Scholar]
- 62. Allen RP, Picchietti DL, Garcia‐Borreguero D, et al. Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15:860–73. [DOI] [PubMed] [Google Scholar]
- 63. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. [DOI] [PubMed] [Google Scholar]
- 64. Allen RP, Stillman P, Myers AJ. Physician‐diagnosed restless legs syndrome in a large sample of primary medical care patients in western Europe: prevalence and characteristics. Sleep Med. 2010;11:31–7. [DOI] [PubMed] [Google Scholar]
- 65. Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80:2028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jean‐Louis G, Brown CD, Zizi F, et al. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Rev Cardiovasc Ther. 2010;8:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kawada T, Otsuka T, Nakamura T, Kon Y. Relationship between sleep‐disordered breathing and metabolic syndrome after adjustment with cardiovascular risk factors. Diabetes Metab Syndr. 2016;10:92–5. [DOI] [PubMed] [Google Scholar]
- 68. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20‐year follow‐up for all‐cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 70. Doherty LS, Kiely JL, Swan V, McNicholas WT. Long‐term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–84. [DOI] [PubMed] [Google Scholar]
- 71. Young T, Shahar E, Nieto FJ, et al. Predictors of sleep‐disordered breathing in community‐dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. [DOI] [PubMed] [Google Scholar]


