Abstract
Mycoplasma pneumoniae (Mp) is one of the leading causes of community‐acquired pneumonia and can cause a number of extrapulmonary manifestations in the absence of pneumonia. In this regard, primary care physicians should know how to suspect, diagnose, and manage patients with Mp infection. This review gives a general overview of the basic clinical aspects of Mp infection with special reference to pneumonia, which will help further understanding of the disease.
Keywords: clinical features, general overview, Mycoplasma pneumoniae pneumonia
1. Introduction
Mycoplasma and ureaplasma species are known to occur in humans. The term “mycoplasma” (Greek; “mykes”=fungus and “plasma”=formed) emerged in the 1950s1 and replaced the older pleuropneumonia‐like organisms (PPLO) terminology. Mycoplasma pneumoniae (Mp) are 1‐2 μm long and 0.1‐0.2 μm wide, compared with a typical bacillus of 1‐4 μm in length and 0.5‐1.0 μm in width. The cell volume of Mp is less than 5% of that of a typical bacillus. Typical colonies of Mp, shown in Figure 1 like a “fried egg” on agar plates, rarely exceed 100 μm in diameter. Mp lacks a cell wall, which makes it intrinsically resistant to antimicrobials, such as beta‐lactams that work by targeting the cell wall, and thus causes a wide spectrum of clinical symptoms and disease manifestations. This article focuses on the diverse clinical aspects of Mp infection, including a review of the current literature.
Figure 1.
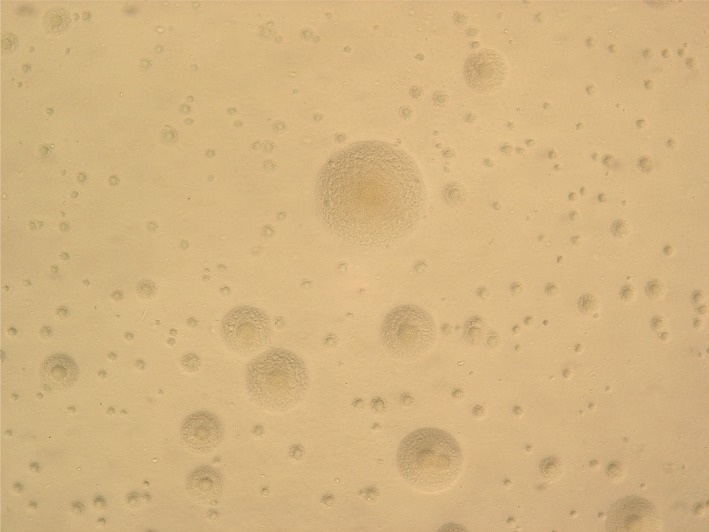
Colonies of Mycoplasma pneumoniae on an agar plate typically have a unique “fried egg” appearance
2. History of Isolation of Mycoplasma pneumoniae
In 1938, Reimann reported seven patients with an unusual form of tracheobronchopneumonia and severe constitutional symptoms.2, 3 He believed the clinical picture of this disease differed from that of diseases caused by influenza viruses or known bacteria and “primary atypical pneumonia” was suspected. In the early 1940s, Eaton et al.4, 5, 6 identified an agent that was the principal cause of primary atypical pneumonia using cotton rats, hamsters, and chick embryos, which was referred to as the “Eaton agent.” Chanock7 succeeded in culturing the Eaton agent in mammalian cell‐free medium and proposed the taxonomic designation “Mycoplasma pneumoniae” in 1963. A more detailed history is available in two different articles, “The history of Mycoplasma pneumoniae pneumonia” by Saraya8 or by Marmion.9
3. Epidemiology
Mycoplasma infections occur sporadically throughout the year, and widespread community outbreaks can occur. The Japanese Respiratory Society (JRS) reports that the frequency of Mp pneumonia among patients with community‐acquired pneumonia (CAP) is approximately 5.2%‐27.4%.10 Mp is one of the most common pathogens that cause community‐acquired pneumonia. The first and largest prospective multicenter study involving 156 Japanese medical institutions looking at atypical pneumonia in Japan was conducted between April 2005 and September 2008.11 This study examined 223 patients with Mp pneumonia who were equally distributed with regard to gender, and the mean age was 37.9±16.6 (mean±SD) years.
Importantly, macrolide‐resistant Mp was first isolated from Japanese children in 200012 and in adults in 2007, all of whom possessed a 23S‐rRNA mutation. In the 2000s, the prevalence of macrolide‐resistant Mp having either an A2063G or an A2064G mutation rapidly increased in Japan13 as well as in East Asia, with a notably high isolation rate (92%) in China (Figure 2).14
Figure 2.
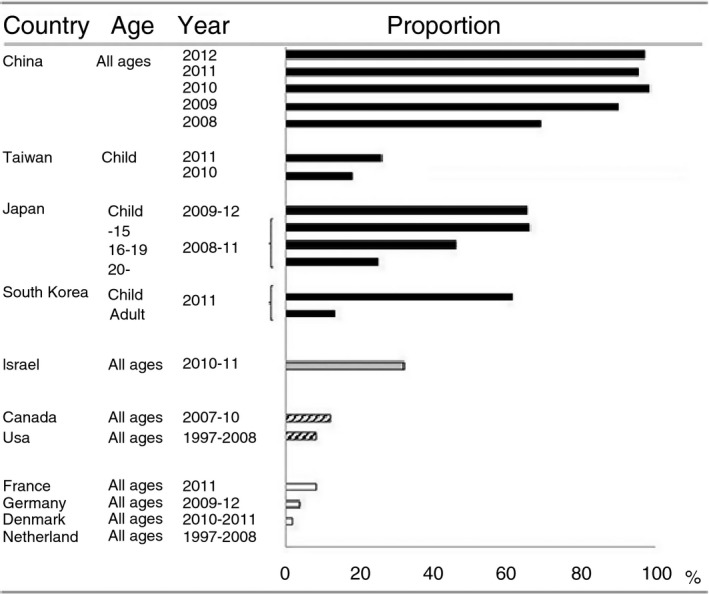
Proportions of macrolide‐resistant Mycoplasma pneumoniae in individual countries. Data are cited from Ref. 14
4. Clinical Features
Most patients with Mp infection present with minor respiratory illness, including pharyngitis and tracheobronchitis, and the infection is usually self‐limited. Only 3%‐13% of infected persons develop pneumonia. The illness spreads via droplets and tends to spread in families with an incubation period of 2‐4 weeks. The greatest risk of infection is in young persons aged 5‐20 years old. Lind et al.15 suggested that herd immunity lasts about 4 years (range: 2‐10 years) before people are again susceptible to Mp infection. Thus, only partial immunity may follow infections, and repeated infections can occur in the same person. In addition, persons with hypogammaglobulinemia have an increased susceptibility to Mp infections, and those with sickle cell disease may experience more severe illness.
5. Mp Pneumonia
The clinical findings of Mp pneumonia are diverse16 (Table 1) and are characterized by the insidious onset of fever, malaise, headache, and cough. The persistent cough is the clinical hallmark of Mp infection, of which the severity and frequency increase over the next 1‐2 days, and which may become debilitating. Mp infection has similar clinical findings to those of respiratory viruses including but not limited to influenza and adenovirus. However, Mp pneumonia is different from those viral infections in that it has a more gradual onset of symptoms and that diarrhea, nausea, and vomiting are unusual. Interestingly, compared with pneumococcal pneumonia, pleuritic pain and shaking chills are relatively rare.
Table 1.
Frequencies of clinical findings in Mp pneumonia
| Findings | Frequency (%) |
|---|---|
| Symptom | |
| Cough | 93–100 |
| Malaise | 74–89 |
| Headache | 60–84 |
| Chilliness | 58–78 |
| Sore throat | 53–71 |
| Chest discomfort | 42–69 |
| Nasal symptoms | 29–69 |
| Myalgias | 45 |
| Sign | |
| Fever | 96–100 |
| Rales, wheezes | 80–84 |
| Pharyngeal erythema (without exudate) | 12–73 |
| Cervical adenopathy | 18–27 |
Table is from Ref. 15.
Goto reported that almost all patients with Mp pneumonia were classified as having mild (85.5%) or moderate (14.0%) infection based on the A‐DROP severity score (A, age; D, dehydration; R, respiration; O, orientation; P, blood pressure) by the Committee for the JRS Guidelines for the management of respiratory infections.17 The A‐DROP score classifies the infection as mild (score: 0), moderate (score: 1‐2), severe (score 3), and very severe (score 4‐5). The mean baseline body temperature of patients with Mp pneumonia was 37.7±1.0°C. This trend was similar to our single‐center retrospective study performed between January 2006 and November 2013, gathering 65 Mp pneumonia patients (Table 2).18 Among all 65 patients, hypoxemia was noted in only 12.8% (n=5), and body temperature was 37.9±1.3°C.
Table 2.
Characteristics of 65 patients with Mp pneumonia
| Total number of patients | 65 |
| Age (mean±SD) | 34.1±15.2 |
| Less than 40 years old | 72.3% (n=47) |
| Male | 32.3% (n=21) |
| Smoker | 39.1% (n=18) |
| Underlying disease | 43.5% (n=20) |
| ADROP score (≥3) | 0% (n=0) |
On physical examination, more than half of the patients with Mp pneumonia had no audible crackles and were likely to have late inspiratory crackles.19 Therefore, primary care physicians should be aware of these six factors that help discriminate Mp pneumonia from other causes of CAP using the JRS “diagnostic tests”10 (Table 4).
Table 4.
Characteristics of patients with Mp pneumonia
| Items used for diagnosis |
| Under 60 y of age |
| No or minor underlying disease |
| Stubborn cough |
| Poor chest auscultatory findings |
| No sputum or etiological agent identified by rapid diagnosis |
| A peripheral white blood cell count <10 000/μL |
6. Macrolide‐Resistant Mp Pneumonia
Macrolide‐resistant (MR) Mp emerged and became widespread in East Asia after 2000. Persistent fever for more than 48 h after initiation of a macrolide may suggest the presence of MR‐Mp. In Japan, 87% of all pediatric Mp infections are due to MR‐Mp20 while the prevalence in adult patients seems to be over 30%, mainly due to an A2063G mutation.21 Determination of MR‐Mp is difficult without application of the test for 23S rRNA mutations. However, the duration of febrile illness in patients with MR‐Mp pneumonia was significantly longer (4.1±2.3 days) than in patients with macrolide‐susceptible (MS) Mp disease (1.6±0.8 days).13 Similarly, Suzuki et al.22 reported that patients with MR‐Mp pneumonia have a longer duration of febrile illness and the number of febrile days during macrolide administration (median; 8 days, 3 days) than patients with MS‐Mp pneumonia (median; 5 days, 1 day). Another study showed that the numbers of febrile days or coughing days from the start of macrolide treatment were significantly longer in patients with MR‐Mp (mean; 4 days, 11.4 days) than in patients with MS‐Mp (mean 1.5 days, 7 days).23 This suggests that if Mp‐infected patients have persistent fever for 48 h or more after initiation of macrolide therapy, we should be aware of the possibility of MR‐Mp infection.
Life‐threatening illness is extremely rare, and there are no apparent risk factors for fulminant Mp pneumonia.24
7. Extrapulmonary Manifestations
Our preliminary data showed the typical diverse symptoms of Mp pneumonia (Figure 3). About 70% of patients experienced fever and cough (typically dry cough). Interestingly, 7% of patients had dermatologic involvement. Importantly, extrapulmonary manifestations due to Mp infection sometimes occur in the absence of Mp pneumonia or even in the absence of respiratory symptoms.25 As seen in Figure 4, Mp infections cause various extrapulmonary complications, such as neurologic, cardiovascular, dermatological, digestive, hematological, and musculoskeletal manifestations, as well as others. Neurologic complications include encephalitis, meningoencephalitis, aseptic meningitis, myelitis, polyradiculitis, and psychosis. Central nervous system manifestations occur in less than 1 per 1000 cases and are most often noted in hospitalized patients. Guillain‐Barré syndrome and peripheral neuropathy have also both been reported. The pathogenesis of CNS involvement is unknown, but direct invasion of organisms, generation of toxins, and autoimmunity (production of autoantibodies) are possible mechanisms.26 Cardiovascular manifestations include pericarditis, endocarditis, and myocarditis. The signs and symptoms are arrhythmia, congestive failure, chest pain, and electrocardiographic abnormalities, particularly conduction defects. In patients with pericarditis, Mp is frequently isolated from the pericardial fluid and/or tissue.25 Regarding skin conditions, Stevens‐Johnson syndrome and erythema multiforme are associated with Mp infection. Hepatic involvement has also been reported, characterized by liver dysfunction 7‐10 days after the onset of fever. However, our preliminary data included both children (n=72) and adults (n=54) with Mp pneumonia who rarely showed derangement of serum alanine aminotransferase. On the other hand, the frequency of elevation of aspartate aminotransferase was significantly higher in children than in adults, which might reflect mild rhabdomyolysis as a result of musculoskeletal involvement. Among hematological manifestations, autoimmune hemolytic anemia is the most common indirect extrapulmonary involvement. Other diseases, such as hemophagocytic syndrome, are recognized as cytokine‐storm disorders, and a hypercoagulable state can present as disseminated intravascular coagulation. Musculoskeletal diseases such as arthritis and rhabdomyolysis are rare but can be seen. Other diseases (mucositis, otitis media, and glomerulonephritis) are also sporadically recognized.
Figure 3.
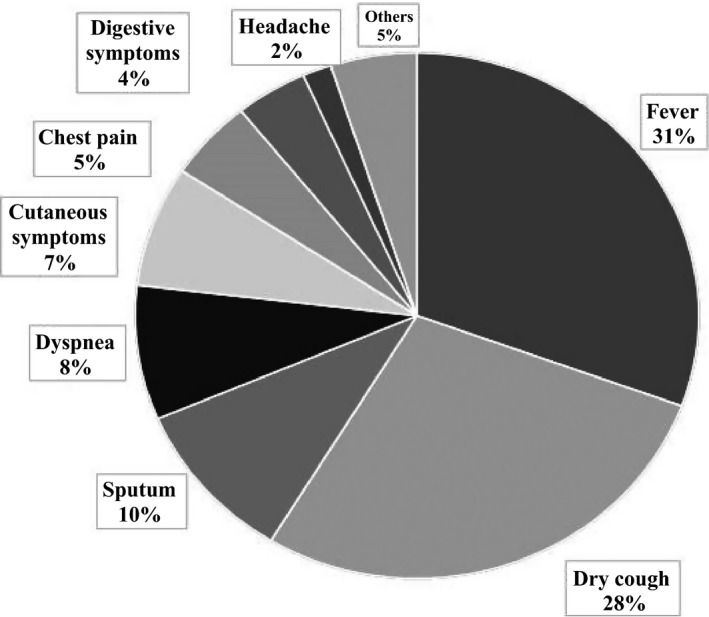
Symptoms of 54 patients with Mycoplasma pneumoniae pneumonia treated at Kyorin University
Figure 4.
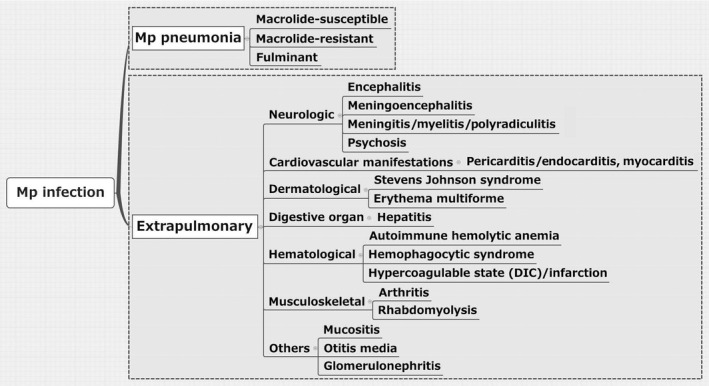
Classification of Mycoplasma pneumoniae infection with or without extrapulmonary involvement. DIC, disseminated intravascular coagulation
8. Pathogenesis of Mp Pneumonia
The severity of Mp respiratory disease appears to be related to the degree to which the host immune response reacts to the infection. This was confirmed by our “mice models” for Mp pneumonia sensitized with various conditions.14, 27, 28, 29 Mp attaches to bronchial epithelial cells, mediated by a surface adhesion molecule, the P1 protein.
Several virulence mechanisms of Mp have been recognized, including (i) lipoproteins/lipopeptides, (ii) community‐acquired respiratory distress syndrome (CARDS) toxin, and (iii) other factors (hemolysin, hydrogen peroxide, and superoxide radicals) (Figure 5). Mp has lipoproteins/lipopeptides with potent inflammatory properties, which are recognized by host immune systems (ie, bronchial epithelial cells and alveolar macrophages) via Toll‐like receptors TLR1, TLR2, and TLR6.14, 27, 30 Recently, Shimizu et al.31 reported that TLR4 and autophagy played important roles in the induction of the TLR2‐independent pathway. Recently, CARDS toxin was recognized by Kannan et al.,32 which is involved in the pathophysiology of Mp disease. The toxin binds to human surfactant protein A and annexin A2 on airway epithelial cells and is internalized, leading to a range of pathogenetic events.33 Other factors are well known for their harmful effects via oxidative stress.
Figure 5.
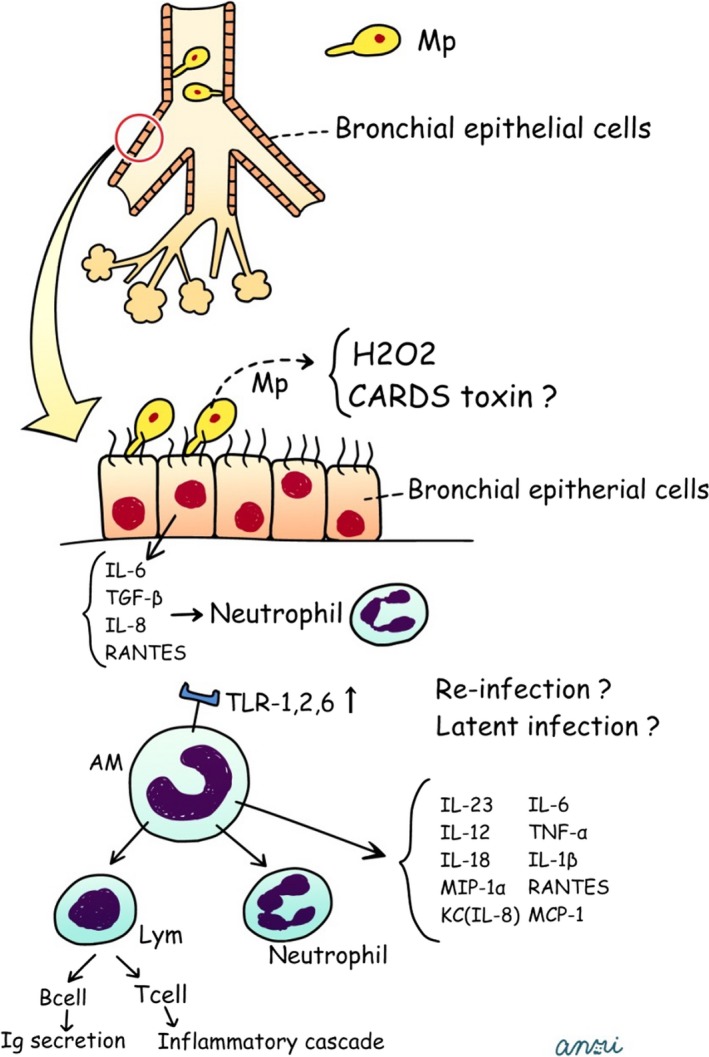
Postulated schema for generating process in human Mycoplasma pneumoniae pneumonia. CARDS, community‐acquired respiratory distress syndrome; TNF, tumor necrosis factor; RANTES, regulated on activation, normal T cell expressed and secreted; MCP‐1, monocyte chemotactic protein‐1. Figure cited from Ref. 14
9. Association of Mycoplasma pneumoniae with Bronchial Asthma
Several reports have described that Mp infection may be associated with pediatric asthma exacerbation34 more often than in adults.35, 36 Yano et al.37 reported that mycoplasmal respiratory infections on the airway involved a complex interplay of airway inflammation via Mp‐specific IgE‐mediated hypersensitivity, resulted in the initial asthmatic symptoms. Kraft et al.38 stated that Mp can be detected even in the airways of chronic, stable adult asthma patients. Thus, latent Mp infection and the asymptomatic carrier state may play a role in the pathogenesis of chronic asthma or asthma exacerbation. However, our data suggest that asthma exacerbation is not associated with Mp infection but rather with viral infection (ie, human rhinovirus, human metapneumovirus, and respiratory syncytial virus) in up to approximately 50% of hospitalized asthmatic patients.39
10. Human Pathology and Bronchoalveolar Lavage Fluid Findings
The pathological findings of Mp pneumonia have scarcely been reported. However, several reports have discussed different tissue‐based diagnostic techniques, such as transbronchial lung biopsy,40, 41, 42 video‐assisted thoracoscopic surgery,43 open lung biopsy,44, 45, 46, 47, 48 and autopsy.49, 50, 51, 52, 53, 54, 55 According to those previous reports, the characteristic pathological feature of Mp pneumonia is a marked plasma cell‐rich lymphocytic infiltration in the peribronchovascular areas (PBVAs) with accumulations of macrophages, neutrophils, and lymphocytes in the alveolar spaces.
Interestingly, bronchoalveolar lavage fluid analysis of Mp pneumonia has scarcely been reported until recently.56, 57 Those few studies demonstrated that PMNs and lymphocytes increased more than other cell types. The elevation of CD4‐to‐CD8 ratios in the bronchoalveolar lavage fluid (BALF) was noted and ranged from 2.156 to 3.5±2.157 at various sampling times.
11. Diagnostic Methods
Although the presence of cold agglutinins is not specific, a titer of 1:64 or more is suggestive of Mp infection. Culture is the gold standard method for diagnosis of Mp infection; however, it requires 1‐2 weeks for definitive results and is rarely carried out. Serological methods such as particle agglutination (PA), complement fixation (CF), and rapid diagnosis such as ImmunoCard® (IC) (mycoplasma immunoglobulin M [IgM]) are commercially available. The loop‐mediated isothermal amplification (LAMP) method and a rapid antigen test (Ribotest; Asahi Kasei Pharma Corp, Japan)58, 59 using throat swabs are also commercially available.
If either CF titers are higher than 1:64 or PA titers are higher than 1:320, a diagnosis of Mp infection can be made. In previous reports, the results of the IC Mycoplasma test were not concordant with that of the PA test, especially in adults,60 which requires a fourfold rise in titers for a diagnosis to be made in adults (Table 3).
Table 3.
Diagnostic methods for Mp pneumonia
| Diagnostic method | Diagnostic criteria | |
|---|---|---|
| Antibody levels | ||
| PA | Single | ≥1:320 titer |
| Pair | ×4 | |
| CF | Single | ≥1:64 titer |
| Pair | ×4 | |
| Culture | ||
| ImmunoCard® | ||
| PCR | ||
| LAMP | ||
| Rapid antigen test (Ribotest) | ||
Although a few laboratories can use PCR‐based diagnostics, the sensitivity and specificity for Mp infection are 40.7%‐66.7% and 88.8%‐98.5%, respectively, with the serological diagnosis used as the reference standard.14, 61 The LAMP assay, a novel nucleic acid amplification method for the rapid detection of Mp by throat swab, was recently developed and became commercially available in 2010 in Japan with the same sensitivity and specificity for detecting Mp as PCR.62 When the results of serological tests were considered as the standard, the sensitivity, specificity, and positive and negative predictive values of the LAMP assay were 94.8%, 91.9%, 91.1%, and 95.2%, respectively.63 The median duration of pharyngeal carriage, as measured by the LAMP assay, was 9.5 days. When the LAMP assay is compared to a validated real‐time PCR test, the sensitivity of the LAMP assay was 88.5% tested on extracted nucleic acid and 82.1% evaluated on unextracted clinical specimens.64 In this regard, the LAMP assay will enable rapid (within a few hours), low‐cost detection of Mp infection. Optimal sample site varies in terms of pathogens or age. Sputum may be superior to nasopharyngeal swabs or oropharyngeal swabs in detection of Mp by nucleic acid amplification method from adults patients.65
Ribotest Mp (Asahi Kasei Pharma Corp), a rapid antigen kit for the detection of the Mp ribosomal protein L7/L12 using an immunochromatographic assay, became available in Japan in 2013. Another study showed the threshold of Mp for this test was 1.1×104 cfu/mL.58 However, the diagnostic sensitivity of the Ribotest compared with PCR was not high, at approximately 60%.59 From this perspective, the diagnosis of Mp pneumonia should be based on the following six factors proposed by the JRS17: (i) <60 years of age; (ii) absence of or only minor underlying disease; (iii) stubborn cough; (iv) abnormal findings on chest auscultation; (v) absence of sputum or presence of an identifiable etiological agent by rapid diagnostic testing; and (vi) peripheral white blood cell count <10 000/μL (Table 4). If four or more of the proposed factors are present, Mp pneumonia can be easily discriminated from the other pathogens with high sensitivity (88.7%) and moderate specificity (77.5%).66
12. Radiological Features
In our unpublished data, a prominent feature of chest X‐ray, both in children and adults with Mp pneumonia, was consolidation followed by ground glass opacities, which was predominantly seen in the middle to lower lung fields.
Regarding thoracic computed tomography of Mp pneumonia, only several small case series have been reported so far. The radiological findings are diverse; however, consolidation (Figure 6A), ground glass opacities (Figure 6B), and bronchovascular bundle thickening (Figure 6C) with peribronchovascular (Figure 6B,C) or centrilobular nodules (Figure 6D) are prominent features of Mp pneumonia.18, 67, 68 In differentiating from Streptococcus pneumoniae pneumonia, the presence of centrilobular nodules indicates Mp pneumonia.18
Figure 6.
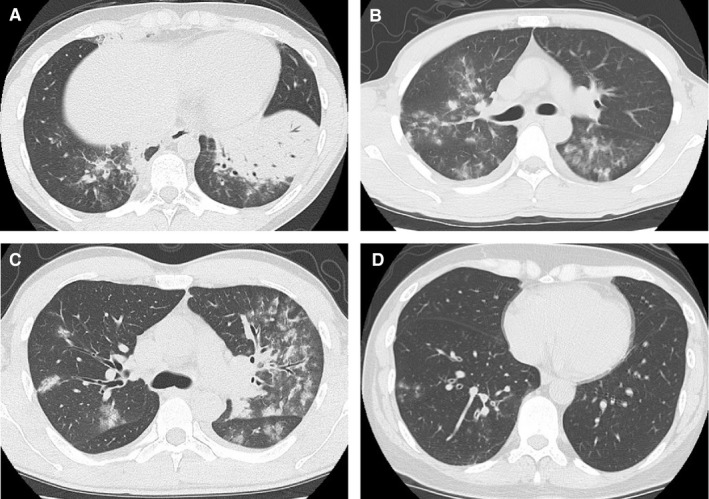
Thoracic computed tomography demonstrates massive consolidation in the left lower lung lobe with air bronchograms (A), GGO in the peribronchovascular area (B, C) with thickening of bronchovascular bundles (C). (D) shows centrilobular nodules with bronchial thickening. GGO, ground glass opacity
13. Treatment
Macrolides have direct effects on neutrophil function and production of cytokines involved in inflammation cascades. They have an oral bioavailability of 50% and tissue concentrations of up to 300‐fold the serum concentration in the epithelial lining fluid, alveolar macrophages, and neutrophils.69 For Mp infections, 14‐ or 15‐membered ring macrolides are usually considered the first‐line agents, which are well known for anti‐inflammatory, immunomodulative effects.
Although JRS guidelines do not refer to the treatment regimen for Mp pneumonia10 (Table 5), the Japanese Society of Mycoplasmology70 recommends the first‐line therapy (oral clarithromycin [CAM] 200 mg bid for 7‐10 days or azithromycin [AZM] 2 g once only or AZM 500 mg/d for three days or erythromycin [EM] 800 mg‐1200 mg/d for 7‐10 days) and second‐line oral therapy (minomycin [MINO] 200 mg/d or fluoroquinolones for 7‐10 days) as an outpatient. In the inpatient setting, the Society recommends intravenous treatment with MINO 200 mg/d or EM 600 mg‐1500 mg/d for 7‐10 days or AZM 500 mg once only as first‐line therapy, and intravenous fluoroquinolones as second‐line therapy. Systemic steroid treatment as additive therapy may improve the outcome of severe Mp pneumonia, but the effects are controversial.
Table 5.
Recommended treatment for Mp pneumonia based on different individual guidelines
| JRS | IDSA/ATS | BTS | |
|---|---|---|---|
| First line | No description |
Macrolides Tetracycline |
Clarithromycin |
| Alternative | No description | Fluoroquinolone |
Doxycycline Fluoroquinolone |
JRS, Japanese Respiratory Society; IDSA, Infectious Diseases Society of America; ATS, American Thoracic Society; BTS, British Thoracic Society.
If the fever persists for 48‐72 h after the initiation of macrolides, oral or intravenous MINO 200 mg/d is recommended as MR‐Mp therapy, both in outpatient and inpatient settings. The recommended treatment for Mp pneumonia by Infectious Diseases Society of America/American Thoracic Society70 or British Thoracic Society guidelines71 is shown in Table 5. These treatments are intended for broad‐spectrum initial coverage and do not intend to differentiate the atypical pathogens from other CAP pathogens at the time of initial treatment.
Mp pneumonia can manifest diverse clinical effects, and the diagnosis can sometimes be challenging for primary care physicians. However, an understanding and recognition of the wide variety of clinical manifestations of Mp infection can lead to more accurate diagnosis and effective treatment.
Conflict of Interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Acknowledgement
I am very grateful to Satoshi Kurata (Kyorin University School of Medicine, Department of Infectious diseases) who kindly provided a picture of Mp colonies.
Saraya T. Mycoplasma pneumoniae infection: Basics. J Gen Fam Med. 2017;18:118–125. https://doi.org/10.1002/jgf2.15
References
- 1. Edward DG, Freundt EA. The classification and nomenclature of organisms of the pleuropneumonia group. J Gen Microbiol. 1956;14:197–207. [DOI] [PubMed] [Google Scholar]
- 2. Reinmann H. An acute infection of the respiratory tract with atypical pneumonia: a disease entity probably by a filtrable virus. JAMA. 1938;111:2377–84. [PubMed] [Google Scholar]
- 3. Reimann HA. Landmark article Dec 24 1938: an acute infection of the respiratory tract with atypical pneumonia. A disease entity probably caused by a filtrable virus. By Hobart A. Reimann. JAMA. 1984;251:936–44. [PubMed] [Google Scholar]
- 4. Eaton MD, Meiklejohn G, van Herick W. Studies on the etiology of primary atypical pneumonia : a filterable agent transmissible to cotton rats, hamsters, and chick embryos. J Exp Med. 1944;79:649–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eaton MD, Meiklejohn G, van Herick W, Corey M. Studies on the etiology of primary atypical pneumonia : II. Properties of the virus isolated and propagated in chick embryos. J Exp Med. 1945;82:317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eaton MD, van Herick W, Meiklejohn G. Studies on the etiology of primary atypical pneumonia: III. Specific neutralization of the virus by human serum. J Exp Med. 1945;82:329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chanock RM. Mycoplasma pneumoniae: proposed nomenclature for atypical pneumonia organism (Eaton agent). Science. 1963;140:662. [DOI] [PubMed] [Google Scholar]
- 8. Saraya T. The history of Mycoplasma pneumoniae pneumonia. Front Microbiol. 2016;7:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marmion BP. Eaton agent–science and scientific acceptance: a historical commentary. Rev Infect Dis. 1990;12:338–53. [DOI] [PubMed] [Google Scholar]
- 10. The JRS guidelines for the management of community acquired pneumonia in adults. Nihon Kokyuki Gakkai Zasshi. 2007;(suppl):2–85. [PubMed] [Google Scholar]
- 11. Goto H. Multicenter surveillance of adult atypical pneumonia in Japan: its clinical features, and efficacy and safety of clarithromycin. J Infect Chemother. 2011;17:97–104. [DOI] [PubMed] [Google Scholar]
- 12. Okazaki N, Narita M, Yamada S, et al. Characteristics of macrolide‐resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol. 2001;45:617–20. [DOI] [PubMed] [Google Scholar]
- 13. Morozumi M, Takahashi T, Ubukata K. Macrolide‐resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community‐acquired pneumonia. J Infect Chemother. 2010;16:78–86. [DOI] [PubMed] [Google Scholar]
- 14. Saraya T, Kurai D, Nakagaki K, et al. Novel aspects on the pathogenesis of Mycoplasma pneumoniae pneumonia and therapeutic implications. Front Microbiol. 2014;5:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lind K, Benzon MW, Jensen JS, Clyde WA Jr. A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50‐year period 1946‐1995. Eur J Epidemiol. 1997;13:581–6. [DOI] [PubMed] [Google Scholar]
- 16. Clyde WA Jr. Clinical overview of typical Mycoplasma pneumoniae infections. Clin Infect Dis. 1993;17(suppl 1):S32–6. [PubMed] [Google Scholar]
- 17. Committee for the Japanese Respiratory Society Guidelines for the Management of Respiratory Infections . Guidelines for the management of community acquired pneumonia in adults, revised edition. Respirology. 2006;11(suppl 3):S79–133. [DOI] [PubMed] [Google Scholar]
- 18. Saraya T, Ohkuma K, Tsukahara Y, et al. Relationships among clinical features, HRCT findings, and a visual scoring system in patients with Mycoplasma Pneumoniae pneumonia. Am J Respir Crit Care Med. 2015;191:A1781. [Google Scholar]
- 19. Norisue Y, Tokuda Y, Koizumi M, Kishaba T, Miyagi S. Phasic characteristics of inspiratory crackles of bacterial and atypical pneumonia. Postgrad Med J. 2008;84:432–6. [DOI] [PubMed] [Google Scholar]
- 20. Okada T, Morozumi M, Tajima T, et al. Rapid effectiveness of minocycline or doxycycline against macrolide‐resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–9. [DOI] [PubMed] [Google Scholar]
- 21. Miyashita N, Kawai Y, Akaike H, et al. Macrolide‐resistant Mycoplasma pneumoniae in adolescents with community‐acquired pneumonia. BMC Infect Dis. 2012;12:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki S, Yamazaki T, Narita M, et al. Clinical evaluation of macrolide‐resistant Mycoplasma pneumoniae . Antimicrob Agents Chemother. 2006;50:709–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsubara K, Morozumi M, Okada T, et al. A comparative clinical study of macrolide‐sensitive and macrolide‐resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother. 2009;15:380–3. [DOI] [PubMed] [Google Scholar]
- 24. Izumikawa K, Izumikawa K, Takazono T, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother. 2014;20:181–5. [DOI] [PubMed] [Google Scholar]
- 25. Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010;16:162–9. [DOI] [PubMed] [Google Scholar]
- 26. Fernald GW. Immunologic mechanisms suggested in the association of M. pneumoniae infection and extrapulmonary disease: a review. Yale J Biol Med. 1983;56:475–9. [PMC free article] [PubMed] [Google Scholar]
- 27. Saraya T, Nakata K, Nakagaki K, et al. Identification of a mechanism for lung inflammation caused by Mycoplasma pneumoniae using a novel mouse model. Results Immunol. 2011;1:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirao S, Wada H, Nakagaki K, et al. Inflammation provoked by Mycoplasma pneumoniae extract: implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol Med Microbiol. 2011;62:182–9. [DOI] [PubMed] [Google Scholar]
- 29. Kurai D, Nakagaki K, Wada H, et al. Mycoplasma pneumoniae extract induces an IL‐17‐associated inflammatory reaction in murine lung: implication for mycoplasmal pneumonia. Inflammation. 2013;36:285–93. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu T, Kida Y, Kuwano K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF‐kappa B through TLR1, TLR2, and TLR6. J Immunol. 2005;175:4641–6. [DOI] [PubMed] [Google Scholar]
- 31. Shimizu T, Kimura Y, Kida Y, et al. Cytadherence of Mycoplasma pneumoniae induces inflammatory responses through autophagy and toll‐like receptor 4. Infect Immun. 2014;82:3076–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kannan TR, Provenzano D, Wright JR, Baseman JB. Identification and characterization of human surfactant protein A binding protein of Mycoplasma pneumoniae . Infect Immun. 2005;73:2828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Becker A, Kannan TR, Taylor AB, et al. Structure of CARDS toxin, a unique ADP‐ribosylating and vacuolating cytotoxin from Mycoplasma pneumoniae . Proc Natl Acad Sci USA. 2015;112:5165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biscardi S, Lorrot M, Marc E, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–6. [DOI] [PubMed] [Google Scholar]
- 35. Nisar N, Guleria R, Kumar S, Chand Chawla T, Ranjan Biswas N. Mycoplasma pneumoniae and its role in asthma. Postgrad Med J. 2007;83:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurai D, Saraya T, Ishii H, Takizawa H. Virus‐induced exacerbations in asthma and COPD. Front Microbiol. 2013;4:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–53. [DOI] [PubMed] [Google Scholar]
- 38. Kraft M, Cassell GH, Henson JE, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. [DOI] [PubMed] [Google Scholar]
- 39. Saraya T, Kurai D, Ishii H, et al. Epidemiology of virus‐induced asthma exacerbations: with special reference to the role of human rhinovirus. Front Microbiol. 2014;5:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ganick DJ, Wolfson J, Gilbert EF, Joo P. Mycoplasma infection in the immunosuppressed leukemic patient. Arch Pathol Lab Med. 1980;104:535–6. [PubMed] [Google Scholar]
- 41. Nakajima M, Kubota Y, Miyashita N, et al. An adult case of pneumonia due to Mycoplasma pneumoniae and Chlamydia psittaci . Kansenshogaku Zasshi. 1996;70:87–92. [DOI] [PubMed] [Google Scholar]
- 42. Ohmichi M, Miyazaki M, Ohchi T, et al. Fulminant Mycoplasma pneumoniae pneumonia resulting in respiratory failure and a prolonged pulmonary lesion. Nihon Kokyuki Gakkai Zasshi. 1998;36:374–80. [PubMed] [Google Scholar]
- 43. Chan ED, Kalayanamit T, Lynch DA, et al. Mycoplasma pneumoniae‐associated bronchiolitis causing severe restrictive lung disease in adults: report of three cases and literature review. Chest. 1999;115:1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coultas DB, Samet JM, Butler C. Bronchiolitis obliterans due to Mycoplasma pneumoniae . West J Med. 1986;144:471–4. [PMC free article] [PubMed] [Google Scholar]
- 45. Rollins S, Colby T, Clayton F. Open lung biopsy in Mycoplasma pneumoniae pneumonia. Arch Pathol Lab Med. 1986;110:34–41. [PubMed] [Google Scholar]
- 46. Llibre JM, Urban A, Garcia E, Carrasco MA, Murcia C. Bronchiolitis obliterans organizing pneumonia associated with acute Mycoplasma pneumoniae infection. Clin Infect Dis. 1997;25:1340–2. [DOI] [PubMed] [Google Scholar]
- 47. Ebnother M, Schoenenberger RA, Perruchoud AP, Soler M, Gudat F, Dalquen P. Severe bronchiolitis in acute Mycoplasma pneumoniae infection. Virchows Arch. 2001;439:818–22. [DOI] [PubMed] [Google Scholar]
- 48. Wachowski O, Demirakca S, Muller KM, Scheurlen W. Mycoplasma pneumoniae associated organising pneumonia in a 10 year old boy. Arch Dis Child. 2003;88:270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parker F Jr, Jolliffe LS, Finland M. Primary atypical pneumonia; report of eight cases with autopsies. Arch Pathol (Chic). 1947;44:581–608. [PubMed] [Google Scholar]
- 50. Maisel JC, Babbitt LH, John TJ. Fatal Mycoplasma pneumoniae infection with isolation of organisms from lung. JAMA. 1967;202:287–90. [PubMed] [Google Scholar]
- 51. Benisch BM, Fayemi A, Gerber MA, Axelrod J. Mycoplasmal pneumonia in a patient with rheumatic heart disease. Am J Clin Pathol. 1972;58:343–8. [DOI] [PubMed] [Google Scholar]
- 52. Meyers BR, Hirschman SZ. Fatal infections associated with Mycoplasma pneumoniae: discussion of three cases with necropsy findings. Mt Sinai J Med. 1972;39:258–64. [PubMed] [Google Scholar]
- 53. Halal F, Brochu P, Delage G, Lamarre A, Rivard G. Severe disseminated lung disease and bronchiectasis probably due to Mycoplasma pneumoniae . Can Med Assoc J. 1977;117:1055–6. [PMC free article] [PubMed] [Google Scholar]
- 54. Kaufman JM, Cuvelier CA, Van der Straeten M. Mycoplasma pneumonia with fulminant evolution into diffuse interstitial fibrosis. Thorax. 1980;35:140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Koletsky R, Weinstein A. Fulminant Mycoplasma pneumoniae infection. Report of a fatal case, and a review of the literature. Am Rev Respir Dis. 1980;122:491–6. [DOI] [PubMed] [Google Scholar]
- 56. Hayashi S, Ichikawa Y, Fujino K, et al. Analysis of lymphocyte subsets in peripheral blood and bronchoalveolar lavage fluid in patients with pneumonia due to Mycoplasma pneumoniae . Nihon Kyobu Shikkan Gakkai Zasshi. 1986;24:162–7. [PubMed] [Google Scholar]
- 57. Hayashi Y, Asano T, Ito G, et al. Study of cell populations of bronchoalveolar lavage fluid in patients with pneumonia due to Chlamydia psittaci and Mycoplasma pneumoniae . Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:569–74. [PubMed] [Google Scholar]
- 58. Itoh H. An immunochromatographic device for rapid detection of ribosomal protein L7/L12 as a diagnostic test for Mycoplasma pneumonia. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2014;25:17. [PubMed] [Google Scholar]
- 59. Miyashita N, Kawai Y, Tanaka T, et al. Diagnostic sensitivity of a rapid antigen test for the detection of Mycoplasma pneumoniae: comparison with real‐time PCR. J Infect Chemother. 2015;21:473–5. [DOI] [PubMed] [Google Scholar]
- 60. Fuse ET, Genma H, Sato M, et al. Evaluation of ImmnoCard mycoplasma for diagnosis of Mycoplasma pneumoniae infection. Nihon Kokyuki Gakkai Zasshi. 2007;45:936–42. [PubMed] [Google Scholar]
- 61. Martinez MA, Ruiz M, Zunino E, Luchsinger V, Avendano LF. Detection of Mycoplasma pneumoniae in adult community‐acquired pneumonia by PCR and serology. J Med Microbiol. 2008;57(Pt 12):1491–5. [DOI] [PubMed] [Google Scholar]
- 62. Yoshino M, Annaka T, Kojima T, Ikedo M. Sensitive and rapid detection of Mycoplasma pneumoniae by loop‐mediated isothermal amplification. Kansenshogaku Zasshi. 2008;82:168–76. [DOI] [PubMed] [Google Scholar]
- 63. Gotoh K, Nishimura N, Takeuchi S, et al. Assessment of the loop‐mediated isothermal amplification assay for rapid diagnosis of Mycoplasma pneumoniae in pediatric community‐acquired pneumonia. Jpn J Infect Dis. 2013;66:539–42. [DOI] [PubMed] [Google Scholar]
- 64. Petrone BL, Wolff BJ, DeLaney AA, Diaz MH, Winchell JM. Isothermal detection of Mycoplasma pneumoniae directly from respiratory clinical specimens. J Clin Microbiol. 2015;53:2970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loens K, Van Heirstraeten L, Malhotra‐Kumar S, Goossens H, Ieven M. Optimal sampling sites and methods for detection of pathogens possibly causing community‐acquired lower respiratory tract infections. J Clin Microbiol. 2009;47:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yin YD, Zhao F, Ren LL, et al. Evaluation of the Japanese Respiratory Society guidelines for the identification of Mycoplasma pneumoniae pneumonia. Respirology. 2012;17:1131–6. [DOI] [PubMed] [Google Scholar]
- 67. Nambu A, Saito A, Araki T, et al. Chlamydia pneumoniae: comparison with findings of Mycoplasma pneumoniae and Streptococcus pneumoniae at thin‐section CT. Radiology. 2006;238:330–8. [DOI] [PubMed] [Google Scholar]
- 68. Miyashita N, Sugiu T, Kawai Y, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging. 2009;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Emmet OM, Restrepo MI, Martin‐Loeches I. Update on the combination effect of macrolide antibiotics in community‐acquired pneumonia. Respir Investig. 2015;53:201–9. [DOI] [PubMed] [Google Scholar]
- 70. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(suppl 3):iii1–55. [DOI] [PubMed] [Google Scholar]


