Abstract
Objective
Epithelial ovarian cancer continues to be the deadliest gynecologic malignancy. Patients with both diabetes mellitus and obesity have poorer outcomes, yet research correlating metabolic abnormalities, such as metabolic syndrome, to ovarian cancer risk and outcomes is lacking. This article reviews the literature regarding metabolic derangements and their relationship to epithelial ovarian cancer, with a focus on potential mechanisms behind these associations.
Methods
PubMed and Google Scholar were searched for articles in the English language regarding epithelial ovarian cancer, obesity, diabetes mellitus, and metabolic syndrome, with a focus on studies conducted since 1990.
Results
Obesity, type II diabetes mellitus, and metabolic syndrome have been associated with poor outcomes in epithelial ovarian cancer. More studies investigating the relationship between metabolic syndrome and epithelial ovarian cancer are needed. A variety of pathologic factors may contribute to cancer risk in patients with metabolic derangements, including altered adipokine and cytokine expression, altered immune responses to tumor cells, and changes in pro-tumorigenic signaling pathways.
Conclusion
More research is needed to examine the effects of metabolic syndrome on epithelial ovarian cancer risk and mortality, as well as the underlying pathophysiologies in patients with obesity, diabetes mellitus, and metabolic syndrome that may be targeted for therapeutic intervention.
Keywords: Epithelial ovarian cancer, Metabolic syndrome, Diabetes mellitus, Immune suppression, Obesity
1. Introduction
Epithelial ovarian cancer (EOC) remains the deadliest gynecologic malignancy. In 2015, there were 21,290 new cases and 14,180 deaths from this disease [1]. EOC is responsible for only 2.6% of malignancies in women, yet causes 5.1% of their cancer deaths, placing ovarian cancer as the 5th leading cause of cancer related deaths in women. Poor survival in ovarian cancer can primarily be attributed to the fact that 75% of patients present with metastatic disease beyond the pelvis [1,2]. Most prognostic factors for EOC are non-modifiable, including stage at diagnosis, age, and tumor grade. Thus, attention has become focused on modifiable risk factors for poor outcomes such as obesity, type II diabetes mellitus (DM), and other metabolic abnormalities. The prevalence of metabolic disturbances such as obesity, type II diabetes mellitus, and metabolic syndrome (MetS) has been increasing, and a growing number of studies suggest associations between each of these conditions and ovarian cancer incidence and poor outcomes [1–5].
Data from the National Health and Nutrition Examination Survey (NHANES) indicate that more than two-thirds of US adults are overweight (body mass index [BMI] ≥ 25) or obese (BMI ≥ 30); 35% are obese and 6% have a BMI > 40 [6]. Evidence for an association between obesity and an increased risk of ovarian cancer has been solidified by a meta-analysis of multiple studies (RR 1.3; 95% CI 1.1–1.5) [3]. Accompanying the rising incidence of obesity, DM is also becoming more prevalent; the World Health Organization (WHO) estimates that globally 346 million people have DM. Approximately 9% of the entire US population and 25% of adults over 65 have diabetes [7]. Among adults with DM, approximately 60% also have obesity and 80% have a BMI > 25 [8]. The link between DM and EOC incidence is debated [9]; however, DM has been clearly associated with poorer outcomes and shorter survival in EOC [4,10]. MetS is defined by the presence of three of the five following metabolic derangements in an individual: elevated waist circumference (population and country specific cutoffs), elevated triglycerides (≥150 mg/dL), reduced high-density lipoprotein cholesterol (<40 mg/dL in males, <50 mg/dL in females), hypertension (systolic ≥ 130, diastolic ≥ 85), and elevated fasting glucose (≥ 100 mg/dL) [11]. Estimates for MetS prevalence mirror that of obesity; approximately 38% of US adult females meet criteria for diagnosis of MetS [11,12]. According to NHANES data, approximately 65% of patients with obesity also have MetS compared to only 10% of people whose BMI is between 18.5 and 25 [12]. At present, potential associations between MetS and EOC are incompletely described.
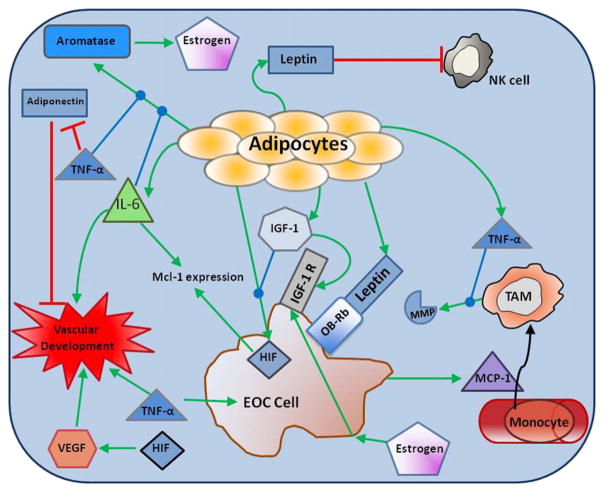
The mechanisms linking metabolic dysregulation and ovarian cancer incidence and progression are incompletely understood. This review will focus on conveying reported correlations between metabolic abnormalities (MetS, obesity, and diabetes mellitus) and epithelial ovarian cancer incidence and mortality, and potential underlying pathophysiologic mechanisms, including changes in adipose tissue, immune function, and pro-tumorigenic signaling pathways. Fig. 1 summarizes the mechanisms that we will discuss, and illustrates possible links among metabolic disturbances, immune dysfunction, and EOC.
Fig. 1.
Alterations in adipocytokine expression patterns in obesity and EOC. Green arrows indicate upregulation and red arrows indicate downregulation. Black arrow indicates cytokine recruitment of cells to the tumor microenvironment. Blue arrows with a circular end indicate that the cytokine increases the primary process indicated by the green arrow. NK cell – natural killer cell; IFN-γ – interferon gamma; IGF-1 – insulin-like growth factor 1; IGF-1 R – insulin-like growth factor 1 receptor; TNF-α – tumor necrosis factor alpha; TAM – tumor associated macrophage; HIF – hypoxia inducible factor; IL-6 – interleukin 6; VEGF – vascular endothelial growth factor; OB-Rb – Leptin receptor; EOC – epithelial ovarian cancer.
2. Methods
We utilized MEDLINE (PubMed) and Google Scholar to conduct an English language literature search for papers on metabolic abnormalities and their relation to EOC. Publications from January 1, 1990 until September 15, 2016 were considered. Keywords searched included “epithelial ovarian cancer”, “diabetes mellitus”, “metabolic syndrome”, “obesity”, “cancer incidence”, “metabolic risk factors”, and “ovarian cancer”. Additional publications were identified during review of the reference lists of the initial publications found.
3. Obesity and epithelial ovarian cancer
Multiple studies have examined the relationship between obesity and EOC. The Million Women Study based in the United Kingdom followed 1.2 million women for an average of 5.4 years for cancer incidence and 7.0 years for cancer mortality. This study found women with a BMI > 25 have a higher incidence of epithelial ovarian cancer compared to their normal weight counterparts (floating absolute risk (FAR) 1.13 (1.02–1.25) for BMI 27.5–29.5 (n = 349) vs. 22.5–24.9 (n = 631); FAR 1.12 (1.02–1.23) for BMI ≥ 30 (n = 438) vs. 22.5–24.9 (n = 631)). Women who were mildly overweight (BMI 25–27.4) did not have an apparent increase in risk for EOC [13]. There was an increased risk of EOC for each incremental increase in BMI (FAR 1.14 for every 10 unit increase in BMI, 95% CI 1.03–1.27). A meta-analysis by Olsen et al. also demonstrated an increased EOC risk in patients with obesity (BMI > 30, pooled effect risk 1.30, 95% CI 1.12–1.50) [3]. The same trend, although to a lesser degree, was also seen in patients who are overweight (BMI 25–29.99, OR 1.16; 95% CI 1.01–1.32). In one multivariate analysis (n = 100,418), the quartile with the largest waist-to-hip ratio (>0.89) was associated with increased risk of having epithelial ovarian cancer (age adjusted RR 1.54; CI 1.05–2.40) [14]. It has been postulated that waist-to-hip ratio might give a more accurate estimate of true visceral adiposity and thus obesity related cancer risk than BMI; however, too few studies report this measure to allow for a comprehensive analysis of this association [15].
Interestingly, the time at which obesity develops during a woman’s life may impact her ovarian cancer risk. Multiple studies have demonstrated that an elevated BMI in adolescence/early adulthood increases the subsequent risk for epithelial ovarian cancer [3,14,16,17]. The Iowa Women’s Health Study found that BMI > 30 at age 18 was positively associated with ovarian cancer incidence (RR 2.15; CI 1.05–4.40) when compared to a BMI < 25 at age 18 [14]. A prospective study that followed 1.1 million Norwegian women for an average of 25 years demonstrated that women with an elevated BMI in adolescence were more likely to develop EOC in adulthood (RR 1.43 for 75th–84th percentile vs. 25th–74th, 95% CI 1.00–2.04; RR 1.56 for >85th percentile vs. 25th–74th; 95% CI 1.04–2.32). However, this study found no association between adult (age 20–74 in this study) BMI and ovarian cancer risk [16]. A recent observational study demonstrated that an increased cancer risk occurs in postmenopausal women who had both greater duration and severity of obesity [18], although it has been postulated that obesity confers greater ovarian cancer risk to premenopausal women than post-menopausal [13]. However, most studies lack sufficient numbers of pre-menopausal women to validate this theory. A meta-analysis by Liu et al. demonstrated an increased risk of EOC in premenopausal women who were overweight (RR 1.31; 95% CI 1.04–1.65) or obese (RR 1.5, 95% CI 1.12–2.00), but not in postmenopausal women (overweight, pooled RR = 0.97, 95% CI 0.76–1.24; obese, pooled RR = 0.93, 95% CI 0.61–1.42) [19]. These results are similar to those seen in an earlier meta-analysis [3].
A meta-analysis by Protani et al. indicated that in addition to increasing ovarian cancer risk, obesity is also associated with a decrease in both overall survival and ovarian-cancer specific survival [2]. The Million Women Study also found an increased cancer specific mortality for ovarian cancer patients with obesity (FAR 1.16, 95% CI 1.04–1.30 for BMI ≥ 30 (n = 326) vs. BMI 22.5–24.9 (n = 439)). There was an increasing risk of cancer-related mortality for each 10 unit incremental increase in BMI (FAR 1.17, 95% CI 1.03–1.33). A separate study analyzed 12,390 women with EOC and found a considerable overall survival (OS) disadvantage in women with obesity (BMI: 30–34.9, pooled hazard ratio (pHR) 1.10, 95% CI 0.99–1.23; BMI ≥ 35, pHR 1.12, 95% CI 1.01–1.25) with similar results in regards to progression free survival (PFS) and ovarian cancer-specific survival [20]. Treatment approaches may also differ in EOC patients with obesity. These women may receive sub-optimal doses of chemotherapeutic agents due to toxicity concerns and dose capping practices [21]. During the time frame for most of these studies, standard clinical practice utilized a body surface area (BSA) cap in chemotherapy dosing; although current clinical practice has changed to no longer have a BSA cap. Table 1 summarizes the key studies reviewed regarding obesity and EOC.
Table 1.
| Reference and important notes | Sample Size | Findings in Overweight | Findings in Obese | Trend per increase in BMI |
|---|---|---|---|---|
| Protani et al. [2] | Meta-analysis of 14 studies | N/a | Poorer survival in obese (pooled HR 1.17; 95% CI 1.03–1.34) | N/a |
| Olsen et al. [3] | Meta-analysis of 28 studies | Increased EOC incidence: Pooled Effect Estimate 1.16 (1.01–1.32) | Increased EOC incidence: Pooled Effect Estimate 1.30 (1.12–1.50) | N/a |
| Reeves et al. [13] “Million Women Study” prospective cohort out of UK *BMI based on self reported ht and wt |
1.2 million in cohort 2406 EOC cases observed 1651 EOC deaths observed |
Increased EOC Incidence: FAR 1.13 (1.02–1.25) BMI 27.5–29.5 (n=349) vs. 22.5–24.9 (n=631) No Evidence of association in mildly overweight (BMI 25–27.4) Mortality: No evidence for correlation |
Increased EOC Incidence: FAR 1.12 (1.02–1.23) BMI ≥30 (n=438) vs. 22.5–24.9 (n=631) Poorer survival and increased mortality: FAR 1.16 (1.04–1.30) (n=326) |
Incidence: FAR 1.14 (1.03–1.27) for every 10 unit increase in BMI Mortality: FAR 1.17 (1.03–1.33) for every 10 unit increase in BMI |
| Engeland et al. [16] *BMI based on measured ht and wt |
1.1 million in cohort 7720 EOC cases observed | No evidence of association between overweight and EOC incidence | Increased incidence of EOC if BMI high (75th–84th %tile): RR 1.43 (1.00–2.04) or very high (≥ 85th %tile) RR 1.56 (1.04–2.32) in adolescence Increased risk for EOC if BMI ≥30.0 in twenties. RR 1.45 (1.02–2.04) No evidence of association between adult obesity and EOC |
N/a |
| Anderson et al. [14] “Iowa Women’s Health Study” Prospective cohort of women 55–69 followed for 15 years in Iowa, USA *BMI based on reported Ht and Wt *Reference group included underweight women (BMI <18.5) |
41,836 in cohort 223 EOC cases observed | No association with EOC incidence demonstrated | Increased EOC incidence RR 2.15 (1.05–4.40) BMI ≥ 30 vs. BMI <25 at age 18 Current BMI not associated with EOC risk | Linear dose response not found |
| Nagle et al. [20] | Meta-analysis of 21 studies with 12390 EOC cases | No significant association with OS demonstrated | OS disadvantage for women with BMI 30–34.9, pHR 1.10 (0.99–1.23) | Decreased OS pHR 1.03 (1.00–1.06) for every 5 unit increase in BMI |
| Liu et al. [19] | Meta-analysis of 26 studies with 12963 EOC cases | Increased risk for EOC, RR 1.07 (1.02–1.12) | Increased risk for EOC, RR 1.28 (1.16–1.41) | N/a |
FAR=floating absolute risk; RR=relative risk.
Patients with obesity may also be at risk for different histologic subtypes of EOC than their normal weight counterparts. Studies have shown an association between obesity and endometrioid ovarian tumors [22]. Studies examining the association between histology and outcomes in patients with obesity have shown increased mortality with low grade serous (pHR 1.12 per 5 kg/m2), endometrioid (pHR 1.08 per 5 kg/m2), and to some extent, high grade serous (pHR 1.05 per 5 kg/m2) tumors [20].
4. Diabetes mellitus and epithelial ovarian cancer
Conflicting data exist regarding the correlation between DM and EOC incidence. This could be due to numerous confounding factors that arise when trying to correlate DM and EOC; these include duration of disease, degree of glycemic control, type of diabetic therapy, and the presence of chronic complications seen in diabetics. A meta-analysis of 19 observational studies demonstrated an increased ovarian cancer incidence in diabetic women (RR 1.17, 95% CI 1.02–1.33) [23]. A stronger association was found in studies that controlled for age, BMI, smoking, and alcohol use (RR 1.55, 95% CI 1.11–2.19) [23]. Vrachnis et al. demonstrated an increased risk for ovarian cancer in premenopausal, but not post-menopausal, diabetic women [9]. Conversely, Chen et al. followed a cohort of Taiwanese women (n = 319,310 with diabetes, n = 319,308 without DM) for nine years and failed to show an increased incidence of EOC (adjusted HR = 1.06, 95% CI 0.92–1.42) [24]. Metformin usage is another confounding variable when assessing the correlation of diabetes to ovarian cancer. Some reports suggest that metformin reduces cancer mortality [25], whereas others have reported no survival difference among patients taking metformin [4].
Diabetes is an independent risk factor for mortality in patients with EOC. In one study of ovarian cancer (n = 642) diabetic patients were found to have a two and a half year lifespan reduction compared to non-diabetics [10]. This was partially attributed to co-morbidities frequently present in diabetics; however, a multivariable analysis confirmed that the presence of diabetes was an independent risk factor for death with regard to overall survival (HR 2.04, 95% CI 1.31–3.17, p = 0.002) [10]. A retrospective cohort study (n = 367) mirrored these findings, with diabetic patients exhibiting both poorer PFS (10.3 vs. 16.3 months) and OS (26.1 vs. 42.2 months) [4]. It has been postulated that survival differences may be partially attributable to different treatment approaches in diabetic patients due to comorbidities and dose limiting chemotherapy toxicities such as neuropathy. However, in one single institution study diabetic patients were not less likely to receive optimal surgical cytoreduction or to undergo complete surgical staging and they were equally as likely to undergo neoadjuvant treatment than non-diabetics [10]. Furthermore, the frequency with which diabetics receive intraperitoneal (IP) chemotherapy was equivalent to their non-diabetic counterparts in a single-institution study [4]. Diabetic patients may experience dose-limiting toxicities during chemotherapy that could contribute to their poorer survival, most commonly development or worsening of neuropathy. The incidence of neuropathy in diabetic patients is approximately 30%, with an increased risk with increasing age, duration of disease, and poorer glycemic control [26, 27]. Bakhru et al. demonstrated that diabetics with EOC were more likely to be older (59.9 vs. 54.7 years old in non-diabetics) and postmenopausal [10], conflicting with findings from Shah et al., which found no difference in the age of diabetic vs. non-diabetic patients with EOC (64.6 vs. 63.2) [4]. Findings by Shah et al. suggest that in those with early stage disease, diabetic patients have a worse prognosis. Interestingly, this study also demonstrated that diabetics were less likely to have either stage I or IV disease than their non-diabetic counterparts (p = 0.039) [4].
With regard to the combined effect of diabetes and obesity on EOC, one study divided diabetic patients into cohorts by BMI (BMI < 30 vs. BMI ≥ 30) and demonstrated a trend toward improved survival in diabetic patients with BMI < 30; however this did not reach statistical significance [4]. Given that patients with DM and obesity both experience poor outcomes, it is plausible that possessing both risk factors would act synergistically to predispose patients to even worse outcomes. However, diabetics often have a higher BMI than their non-diabetic counterparts; therefore it is difficult to determine which risk factor acts as the driving factor for malignant development and poor outcomes. Diabetic patients could have worse outcomes because they are obese; alternatively patients with obesity could have poor outcomes because they are predisposed to other metabolic derangements like diabetes. Unfortunately, these relationships are not clearly defined by current data [4,10]. Table 2 summarizes key studies investigating the relationship between DM, MetS, and EOC.
Table 2.
| Reference | Sample Size | Key Findings | Effect of Metformin |
|---|---|---|---|
| Shah et al. [4] | 367 cases of EOC, 62 (17%) also had DM | Decreased PFS (10.3 vs. 16.3 months, p=0.024) and OS (26.1 vs. 42.2 months, p=0.005) | Metformin did not increase PFS (p=0.62) or OS (p=0.7) in diabetic patients |
| Bakhru et al. [10] | 642 cases of EOC, 72 (11%) also had DM | Median survival 4 years for diabetics, vs. 6 for non-diabetic patients with EOC Decreased OS in EOC patients with DM, HR 2.04 (1.31–3.17), p=0.002 | Not analyzed |
| Romero et al. [25] | 341 cases of EOC, 44 (13%) also had DM | Diabetic patients taking metformin had increased PFS, decreased hazard for disease recurrence. Trend for increased platinum sensitivity in diabetics taking metformin, although not statistically significant | Metformin use increased PFS (51% vs. 8% at 5 years for diabetics taking metformin vs. those not) Metformin did not improve OS |
| Esposito et al. [28] | Meta-analysis of 654 EOC cases, 2 studies | EOC incidence increased in patients with MetS RR 1.26 (1.0–1.59), p=0.054 | N/a |
| Bjørge et al. [29] | Prospective cohort study following 290,000 women, 644 EOC cases | No evidence for association between MetS and EOC risk Increased mortality risk if MetS developed before age 50 RR 1.52 (1.00–2.30) |
N/a |
HR=hazard ratio, PFS=progression free survival, OS=overall survival, RR=relative risk.
5. Metabolic syndrome and epithelial ovarian cancer
Although there are a multitude of studies examining potential links between obesity, diabetes, and EOC, few of these examine the complete spectrum of associated metabolic abnormalities and their role in carcinogenesis. Patients with DM often meet diagnostic criteria for MetS; however, this may not be clearly documented in their medical records. A national report released in 2014 by the Center for Disease Control (CDC) on diabetes revealed that 65% of adults with diabetes had LDL cholesterol >100 or used cholesterol lowering medications, and 71% of these adults also had blood pressure >140/90 or used blood pressure lowering medications [7]. Although this report did not provide the percentage of adults with DM who would also meet diagnostic criteria for MetS, calculations based on their data suggest the number would exceed 36%.
Metabolic syndrome has been correlated with an increased risk for endometrial, pancreatic, postmenopausal breast, and colorectal cancers in women [28]. One meta-analysis demonstrated a borderline correlation between MetS and EOC (RR 1.26, p = 0.054); however, the data included only 654 cases of ovarian cancer from two European cohort studies [28]. To our knowledge, only one prospective cohort study has been performed to exclusively evaluate the relationship between MetS and EOC incidence and mortality [29]. In this study, Bjørge et al. failed to demonstrate a relationship between MetS and EOC incidence; however, the authors did find an increased risk of ovarian cancer mortality in women under 50 with MetS (RR 1.52, 95% CI 1.00–2.30). Additionally, this study showed that increased levels of cholesterol were associated with an increased risk of mucinous tumors (RR 1.52, 95% CI 1.01–2.29), and hypertension with an increased risk of endometrioid tumors (RR 1.79 95% CI 1.12–2.86) [29]. One limitation of this study is its lack of racial diversity, as all women were from Austria, Norway and Sweden. Although this study did not report racial demographics, it is probable that women of African, Hispanic, and Asian descent were vastly under-represented. As African American and Mexican American females are one and a half times more likely to have MetS than non-Hispanic Caucasian females [12], it is important to conduct similar studies on women from diverse ethnic groups, as findings may vary. However, the probable racial homogeneity in the Bjørge et al. study cohort strengthens the authors’ findings with respect to this particular demographic group.
6. Mechanisms linking metabolic abnormalities and epithelial ovarian cancer
The mechanisms by which obesity, diabetes, and other metabolic derangements contribute to increased cancer risk and mortality are multi-faceted and incompletely understood. Excess adipose tissue causes dysregulation of adipokine and cytokine levels [30]; the resulting adipocytokine expression patterns alter tissue immune responses and aid tumor evasion of immune responses [31]. Additionally, excess adiposity results in altered endocrine function that can cause major changes in pro-tumorigenic signal transduction pathways [9]. The key mechanisms displayed in Fig. 1 linking metabolic disturbance and immune dysfunction to EOC will be discussed.
6.1. Cytokines and adipokines in epithelial ovarian cancer
A plethora of adipocytokines have been shown to be increased in obesity including IL-5, IL-6, IL-10, IL-12, IL-13, leptin, C reactive protein (CRP), IFNγ, monocyte chemotactic protein-1 (MCP-1), and TNF-α compared to non-obese patients. Others, such as adiponectin, are decreased in patients with obesity [32,33]. Adipocytes produce IL-6, TNF-α, leptin, while adipose tissue resident immune cells are responsible for the production of IL-10, IL-13, MCP-1,TNF-α and IFNγ [34–36]. Elevated numbers of immune cells are present in adipose tissue in individuals with obesity, particularly macrophages, which are responsible for the production of many cytokines that are elevated in obesity [36]. Alterations in these cytokines further promote neoplasia and alter immune recognition of abnormal cells. We discuss several key cytokines thought to be involved in cancer risk in obesity, diabetes, and metabolic syndrome. Table 3 provides a summary of these adipokines and cytokines and their role in EOC development and progression.
Table 3.
Adipokines and cytokines implicated in EOC development and poor outcomes.
| Cytokine | Change under condition of metabolic abnormalities | Mechanism contributing to EOC development and progression |
|---|---|---|
| IL-6 Source: Adipocytes [73], macrophages [73], tumor cells |
Increased in obesity and DM [74] Reduced in primates [75] and postmenopausal women under calorie restriction Increased by higher levels of ROS [76] |
Promotes angiogenesis Induces aromatase, increasing estrogen levels Promotes expression of anti-apoptotic proteins, including Mcl-1 Chemotherapy resistance Main regulatory cytokine of hepatic CRP synthesis Predicts response to bevacizumab therapy |
| TNFα Source: Macrophages [77], ovarian tumor cells, adipocytes |
Increased in obesity and DM [74] | Induces MMP production by macrophages, which leads to increased tumor invasiveness [77] Promotes angiogenesis Acts as autocrine and paracrine growth factor for ovarian tumor cells Decreases adiponectin production Induces expression of aromatase in adipose tissue |
| Leptin Source: Adipocytes |
Increased in obesity | Reduces anti-tumor cytotoxicity, perforin production, and IFN-γ secretion by NK cells Suppresses Treg differentiation Increases monocyte secretion of IL-6 and TNFα Apoptotic resistance Increased proliferation in ovarian cancer cells Increases cyclin D1 expression Decreased PFS in EOC tumors with increased leptin receptor (Ob-R) expression Facilitates cell migration |
| Adiponectin Source: Adipocytes |
Decreased in obesity Negatively correlated with waist-hip ratio and visceral fat content Decreased in DM |
Inhibits TNF-α induced NF-κB signaling Negative regulator of angiogenesis |
| MCP-1 Source: Ovarian tumor cells [77] |
Induced by HIF, which is elevated in obesity | Recruits circulating monocytes to the tumor microenvironment Tumor derived MCP-1 significantly correlates with TAM density in ovarian tumors [77] May play a role in angiogenesis regulation [77] |
| CRP Source: Liver |
Increased in obesity and DM [74] | Elevated CRP associated with increased risk of ovarian cancer |
6.1.1. Interleukin 6 (IL-6)
In adipose tissue, IL-6 and TNF-α act in concert to induce aromatase activity leading to increased synthesis of estrogen. Additionally, IL-6 promotes angiogenesis, stimulates cell growth, and inhibits apoptosis [37,38]. Specifically, IL-6 has been reported to induce expression of Mcl-1, a member of the Bcl-2 family of anti-apoptotic proteins. Mcl-1 expression has been associated with advanced stage, high tumor grade, and poor survival in EOC [37]. IL-6 has been associated with chemoresistance in ovarian tumors [39,40]. Increased levels of IL-6 have been demonstrated to predict responses to bevacizumab in EOC, further demonstrating the role of this cytokine in EOC outcomes [41].
6.1.2. Tumor necrosis factor α (TNF-α), monocyte chemotactic protein-1 (MCP-1), and C reactive protein (CRP)
TNF-α, MCP-1, and CRP are additional inflammatory molecules that have been widely linked to cancer progression in a variety of tumor types. TNF-α is classically thought of as a product of macrophages; however, multiple cancers including ovarian tumors are also capable of producing TNF-α [42]. A positive correlation has been demonstrated between TNF-α levels and tumor grade in EOC [43]. Furthermore, increased levels of TNF-α in ascitic fluid of EOC patients have been correlated with poorer OS (HR = 2.8, 95% CI 1.1–7.0) and PFS (HR = 2.9, 95%CI1.2–6.7) [44]. TNF-α also contributes to insulin resistance, mediates transcription of proteins involved in inflammation, increases cell survival and proliferation, and prevents apoptosis via activation of NF-kappaβ and MAPK signaling pathways [42,45]. MCP-1, also known as CCL2, is involved in recruitment of circulating monocytes to the tumor, where the monocytes become macrophages. MCP-1 is frequently overexpressed in ovarian tumors [46]. Furthermore, hypoxia inducible factor 1α (HIF-1α), which is elevated in obesity, further increases levels of MCP-1 [47]. CRP is often used as a marker of inflammation and has been found to be elevated in 60% of women with obesity [45]. Elevated CRP is associated with an increased risk of developing ovarian cancer [45]. Thus, each of these factors has shown some association with increased ovarian cancer risk or decreased survival.
6.1.3. Leptin
Leptin is a hormone that is produced by immature adipocytes and is classically elevated with obesity [47]. Beyond its role in appetite regulation, leptin has been correlated with poor outcomes in EOC. High levels of leptin, increased leptin receptor expression by tumor cells, and a high leptin to adiponectin ratio are all correlated with worse outcomes in EOC [48,49]. Elevated leptin concentrations have been shown in the ascites and serum of ovarian cancer patients with obesity [48]. One report demonstrated that EOC tumor cells overexpressed the leptin receptor in 60% of ovarian tumor samples, which was associated with a significantly decreased PFS [50]. Leptin has been shown to directly stimulate ovarian cancer cell growth as a result of signaling via the PI3K/AKT cascade [37, 50]. Furthermore, leptin was shown to enhance expression of cyclin D1 and Mcl-1 in an ovarian cancer cell line; both molecules are important regulators of cellular proliferation and apoptosis inhibition, respectively [37]. Moreover, leptin contributes to metastatic spread of EOC by aiding cell migration and tissue invasion by binding to OB-Rb. This increase in metastatic potential mediated by leptin has been shown to be independent of the p-53 status of the tumor [48]. This effect is mediated via JAK/STAT3, MAPK, AKT, mTOR, RhoA/ROCK, and MYPT1 signaling pathways, which are involved in cell growth and migration [48]. Leptin further promotes tumor growth by suppression of immune responses, as it inhibits cytotoxicity of natural killer (NK) cells and decreases NK IFN-γ production [51].
6.1.4. Adiponectin
Adiponectin is secreted from mature or differentiated adipocytes and has many beneficial effects on metabolism, including insulin-sensitization, and anti-angiogenic and anti-inflammatory properties. Contrary to its counterpart, leptin, adiponectin is reduced in obesity and has anti-neoplastic properties [52]. Much of its anti-proliferative effects can be attributed to decreasing bioavailability of proinflammatory factors that play important roles in metabolic syndrome-cancer link. Furthermore, adiponectin has been shown to inhibit tumor growth in animals [15]. In one study, patients in the lowest tertile of leptin to adiponectin (L:A) ratios had longer disease-specific survival in EOC (57 months) compared to median L:A ratio (49 months) or high L:A ratio (37 months, p = 0.02) [49]. These findings suggest that increased levels of leptin and decreased levels of adiponectin may contribute to the increased EOC incidence and mortality seen in obesity.
6.2. Immune cells and epithelial ovarian cancer
The tumor microenvironment contains many non-malignant cells. These include a wide variety of leukocytes, which can either promote or inhibit disease progression and metastasis, depending on the cell type. Because obesity and metabolic syndrome are associated with marked changes in cytokine and adipokine expression patterns, it is possible that immune responses to ovarian tumors are altered as a result. These changes could extend to decreased cytolytic activity of effector cells, or altered secretion of growth factors and pro-angiogenic factors, both of which could lead to enhanced promotion of tumor growth [53]. Table 4 summarizes these key immune cells and their role in EOC.
Table 4.
Immune cells and their relationship to obesity and EOC.
| Immune cell | Subpopulations | Effects on ovarian cancer | Effects of cytokines/signaling molecules |
|---|---|---|---|
| Dendritic cell | Plasmacytoid and myeloid (classified according to their lineage) | EOC cells secrete IL-10, which promotes differentiation of dendritic cells to a subtype with less effective T-cell activation properties Suppress effector function of T cells by engagement of PD-L1 |
Recruited to tumor microenvironment by tumor stroma-derived factor 1 (SDF-1) |
| Macrophage *Monocytes recruited to tumor sites by MCP-1 where they mature into macrophages |
M1 “classically activated” TAM | Suppress cancer progression, cytotoxic to tumor cells Release ROS, nitrogen intermediates, inflammatory cytokines (IL1b, IL6, IL12, IL23, TNF) |
Induced by IFNγ |
|
M2 “alternatively activated” TAM |
Promote tumor growth, angiogenesis, metastasis Suppress immune responses Tissue repair |
Induced by TGF-β, IL-4, IL-10, IL-13 CSF-1 considered to induce differentiation to the M2 phenotype Produces CCL22, recruiting T-reg cells to the tumor site | |
| NK cell | CD16+ CD56dim – peripheral location CD16− CD56bright – primarily located in lymphoid tissue |
Higher NK activity in peripheral blood associated with higher PFS Increased NK cells in peritoneal/pleural fluids associated with poor prognosis |
MUC16 (protein source of CA-125) inhibits activity of NK cells |
| B cells | Stimulate angiogenesis in tumors [78] Higher tumor infiltration with B cells associated with poor outcomes |
||
| T cells | CD4+ | Helper T cells that produce IL-17 may have a role in tumor eradication | Function suppressed by interaction with PD-L1 |
| CD8+ | Presence of TILs positively associated with survival in large meta-analysis Higher number of CD8 T cells associated with improved survival (n = 117, median survival 55 vs. 26 months) |
Function suppressed by interaction with PD-L1 Cytotoxic activity inhibited by TGF-β |
|
| Treg cells | Associated with poor patient survival Produce IL-10 and TGF-β that suppress T-cell proliferation and inhibit immune responses Decrease cytotoxic activity of CD8+ T cells |
Recruited to tumor site by CCL22 |
6.2.1. Natural killer cells
Natural killer (NK) cells play a critical cytotoxic role in the innate immune system, analogous to CD8+ T cells in the adaptive immune system. Ovarian cancer patients with higher levels of active NK cells at the time of surgery have been shown to have longer PFS; in contrast, increased numbers of NK cells in peritoneal and pleural fluids have been associated with worse overall prognosis [31]. At this time, the effect of obesity on NK responses to ovarian tumors is unclear. However, adults with obesity are known to have elevated circulating levels of leptin. One prior report illustrated that NK cells incubated in the presence of leptin have reduced cytotoxicity against tumor cell lines and decreased IFN-γ production [51]. This finding suggests that protective NK responses may be diminished in ovarian cancer patients who are obese.
6.2.2. Macrophages
Tumor-associated macrophages (TAMs) are one of the most abundant non-malignant cell types found in the tumor microenvironment. TAMs are implicated in tumor invasion via secretion of matrix metallo-proteinases (MMPs) [46], and can contribute to immunosuppression by secretion of CCL22, which recruits immunomodulating regulatory T cells to the tumor site [46,54]. The activation state of macrophages is altered in obesity, with polarization shifted toward alternatively activated (M2-polarized) from classically activated (M1-polarized) populations [32]. M1 macrophages are involved in secretion of pro-inflammatory cytokines and participate in Th1 driven responses to infection and cancer [46]. M2 macrophages are conversely activated by Th2 cytokines (IL-4, IL-10, and IL-13), have poor antigen presenting capabilities, and promote tissue remodeling and angiogenesis by releasing a variety of growth factors including epidermal growth factor (EGF), fibroblast growth factor (FGF), and VEGF [53]. High numbers of M2 macrophages in ovarian tumors have been associated with reduced PFS and OS [46]. One murine study demonstrated similar numbers of M2 polarized macrophages among obese and normal-weight mice; however, tumor-associated M1 macrophages were decreased in obese mice, resulting in an overall 1.5 to 4.2 fold decrease in the M1:M2 ratio in obese mice models [30]. This is notable because a higher M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients, possibly due to M1 macrophages keeping tumor progression and metastasis in check [53].
6.2.3. B cells
Tumor associated B-cells have also been shown to play a role in ovarian cancer progression. B-cells have been shown to promote vascular development in several cancers via STAT3 associated signaling [55]. B cell infiltration of ovarian tumors correlates with poorer survival, and B cells have been shown to be present in higher numbers in stage IV vs. stage III disease [55,56]. In terms of obesity-related changes in the B cell response, one study reported that obese mice had an approximately threefold increase in B cell numbers in ovarian tumors [30], a finding that could provide a partial explanation for the poor outcomes observed in patients with obesity.
6.2.4. T cells
The presence or absence of T cells infiltrating the tumor in ovarian cancer patients has a significant impact on survival, both in optimally and suboptimally debulked patients, and these findings have been demonstrated in multiple studies [57–59]. Protective TILs limit tumor growth by secreting anti-angiogenic cytokines such as IFN-γ, which has been shown to be increased in ovarian tumors with TILs present [57,58]. However, these studies omitted information on BMI status, the presence or absence of DM, and the MetS status of patients; thus further studies are needed to delineate how obesity and other metabolic disturbances influence the presence or absence of TILs, and their functional state [57–59].
One subset of T cells, called regulatory T cells (Treg), promotes a state of immune suppression that supports tumor growth [31]. Tregs can secrete cytokines such as TGF-β and IL-10 that mediate an inhibitory effect on global immune function, and can stifle the cytotoxic activities of intratumoral CD8+ T cells [59]. Tregs have been shown to negatively impact survival in ovarian cancer patients [54,60]. Zhang et al. demonstrated an increased number of Treg cells in EOC tissue compared to benign ovarian tissues; increasing Treg numbers was also correlated with advanced tumor stage [60]. However, the effect of obesity and metabolic abnormalities on Treg numbers or function has not yet been evaluated in epithelial ovarian cancer patients.
6.3. Signal transduction pathways, hormones, and epithelial ovarian cancer
6.3.1. Hypoxia inducible factor (HIF)
Hypoxia inducible factor (HIF) is a transcription factor that is induced in conditions of decreased oxygen availability. Increasing adiposity and growth of individual adipocytes leads to an increased distance between cells and their vascular supply, culminating in relative hypoxia. This relative hypoxia triggers upregulation of HIF-1α which increases expression of IL-6, CXCR4, and other inflammatory cytokines. These cy-tokines attract macrophages into adipose tissue where they propagate inflammation by releasing inflammatory factors such as TNF-α and MCP-1 [47]. In EOC, CXCR4 upregulation leads to increased recruitment of tumor associated macrophages (TAMs), which are associated with poor prognosis and produce vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and other pro-angiogen-ic growth factors [61]. Furthermore, rapidly growing tumors also express increased levels of HIF-1, particularly in areas that are relatively distant to the vasculature. Additionally, cytokines including TNF-α and IL-1β and hormones such as insulin, IGF-1, and IGF-2 demonstrate the ability to activate HIF-1 under normal oxygen conditions [42]. Thus HIF contributes a significant survival and growth advantage to dysplastic cells by increasing expression of growth factors including erythropoietin, VEGF, VEGF receptors, glucose transporters, and numerous glycolytic enzymes, particularly in the face of metabolic derangement [42]. Hypoxia has been shown to increase expression of leptin, IL-6, and VEGF; and decrease adiponectin [33]. Table 5 reviews the key signal transduction pathways implicated in EOC development and decreased survival in obesity.
Table 5.
Signal transduction pathways and hormones in EOC.
| Growth factor | Changes under conditions of metabolic abnormality | Mechanism contributing to EOC development and progression |
|---|---|---|
| HIF | Increased in obesity | Upregulates expression of IL-6, TNF, and MCP-1 Promotes angiogenesis Upregulates pro-fibrotic pathway leading to altered extracellular matrix |
| VEGF Insulin/IGF-1 |
Increased in obesity Increased in obesity Increased in diabetes mellitus Decreases in primates under conditions of caloric restriction [75] |
Promotes vascular growth Increases HIF-1α Increases tumor growth by stimulating mitogenic pathways and inhibiting apoptosis Reduces production of sex hormone binding globulin (SHBG), increasing bioavailability of estrogen |
| Estrogen | Increased in obesity | Induces expression of the IGF-1 receptor Acts as a mitogen for EOC cells |
6.3.2. Vascular endothelial growth factor (VEGF)
VEGF is a well known promoter of angiogenesis and is presumed to be one of the links between obesity and carcinogenesis. Women who are obese or overweight have increased circulating levels of VEGF and other angiogenic growth factors [30,62]. Animal studies provide further evidence for the relationship between obesity and tumor growth. One murine study showed enhanced vascularity in metastatic tumors in obese mice relative to control mice [30]. This study presented evidence that obesity promotes metastasis of ovarian cancer cells due to diet-induced changes in cellular adhesion [30]. Interestingly, VEGF production is higher in omental fat than fat elsewhere in the body, and serum VEGF levels correlate with visceral, but not subcutaneous, fat content [62]. Elevations in VEGF and other angiogenic growth factors such as angiopoietin-2 appear to have clinical significance in regard to ovarian cancer treatment. Specifically, in a study that compared standard chemotherapy vs. standard chemotherapy plus the monoclonal antibody targeting VEGF, bevacizumab, an increased PFS was seen in patients with lower BMIs treated with bevacizumab but not in those with a high BMI [62]. Elevated levels of angiogenic growth factors negatively affect treatment outcomes in patients with obesity and these patients may not glean the same benefits of anti-angiogenic therapy as their normal weight counterparts, suggesting that in these individuals, weight loss should be combined with angiogenic therapy in order to achieve maximum benefit.
6.3.3. Insulin-like growth factor 1 (IGF-1)
IGF-1 is a growth factor that is secreted by the liver and is commonly associated with obesity and hyperinsulinism [47]. Hyperinsulinism decreases hepatic secretion of IGF binding protein (IGFBP), further increasing levels of free IGF-1 [9,52,63]. Conversely, starvation and calorie restriction are associated with lower levels of IGF-1 and downstream signaling [64]. IGF-1 promotes neoplasia by stimulating cellular differentiation and angiogenesis [9]. IGF-1 has been confirmed to enhance growth in multiple malignant cell lines, including ovarian cancer cells [65]. Furthermore, higher degrees of IGF signaling in EOC have been inversely associated with survival (median OS 33 months vs. 63 months) [66]. The associated increase in IGF-1 in obesity and DM is another potential explanation of increased EOC incidence and poor outcomes.
6.3.4. Estrogen
Furthermore, increased adipose tissue and metabolic abnormalities induce significant alterations in sex hormone levels, including estrogen, progesterone, and androgens [47]. Higher androgen levels lead to increased estrogen by conversion of testosterone to estrogen by aromatase. Estrogen acts as a mitogen in ovarian epithelial cells, and increased estrogen and estrogen signaling have been correlated with EOC development [67]. Premenopausal women predominately derive estrogen from aromatase in ovarian follicles; however, in postmenopausal women aromatase is primarily located in adipose tissue. After menopause, circulating estradiol is positively correlated to BMI [63]. Postmenopausal women who are obese have a 33% relative increase in unconjugated estradiol compared to non-obese women [68]. This trend correlates with a 12.8% increase in unconjugated estradiol levels for every 5 unit increase in BMI. Inflammatory cytokines elevated in obesity induce aromatase expression in human adipose tissue, further contributing to elevated estrogen levels [47]. Hyperestrogenism is further promoted by hyperinsulinemia, IGF-1 overexpression, and the consequential decrease in sex-hormone binding protein [9,52]. Although the role of estrogen exposure is more apparent in uterine carcinogenesis, mouse models provide evidence that estrogen contributes to the development of ovarian cancer as well [67]. Several phase II clinical trials have examined the effects of anti-estrogen therapy for recurrent and platinum resistant ovarian cancer, with generally poor response rates, but low rates of adverse events [69]. Anti-estrogen therapies have some benefit in recurrent, platinum resistant EOC, particularly for tumors that express high levels of the estrogen receptor expression. Given the increase in estrogen levels in obesity, it is plausible these patients may receive more benefit of anti-estrogen therapies. To our knowledge, no randomized controlled trial has evaluated initial therapy with anti-estrogen agents such as letrozole, tamoxifen, or anastrazole.
7. Discussion
Multiple studies have linked obesity, diabetes mellitus, and metabolic syndrome with an increased risk for developing ovarian cancer and/or heightened mortality. Fortunately, many risk factors for obesity, diabetes, and metabolic syndrome are modifiable by diet and exercise. There are several potential dietary interventions that show promise to reduce cancer risk in patients with obesity or prolonging survival once neoplasia develops. Calorie restriction has been shown to mitigate risk factors for ovarian neoplasia by reducing IGF-1 and leptin levels [65]. In one animal study a calorie restricted diet was associated with a lower incidence of EOC and lower mortality risk [70].
Further research is needed to evaluate the underlying biology of metabolically deranged-driven cancers. Although chemotherapeutic dose capping in patients with obesity, and dose-limiting toxicities in diabetic patients likely contribute to negative outcomes in these individuals, there are multiple underlying pathological changes in obesity, DM, and MetS that could also impact outcomes and EOC incidence. A limitation of the field is that we do not yet understand the associations between obesity, DM, MetS and disease-specific mortality for ovarian cancer, because most studies do not distinguish between all-cause and disease-specific mortality.
As described in this review, multiple obesity-associated soluble factors such as Mcl-1, TNF-α, and leptin have been linked to worse outcomes in EOC. Changes in the composition of tumor-infiltrating immune cells have also shown correlations with outcomes, as increased M1 macrophages may confer a positive prognosis whereas increased tumor associated B cells may be a negative indicator. Current data suggest these changes in tumor associated immune cell populations may be related to obesity and metabolic dysregulation. Lastly, increased signaling via IGF-1, VEGF, and HIF may lead to both increased risk of carcinogenesis and poor outcomes in epithelial ovarian cancer; all of these have been demonstrated to be increased in obesity.
Despite the fact that these associations have been described, much work remains to determine the causal relationship between obesity, diabetes, and MetS on adipokines, cytokines, growth factors, and immune/inflammatory responses. Further studies are need to better understand the clinical efficacy and feasibility of targeting these proteins or cell types to improve outcomes in ovarian cancer patients with obesity, diabetes mellitus, and metabolic syndrome.
HIGHLIGHTS.
Metabolic abnormalities are associated with poor outcomes in ovarian cancer.
The underlying mechanisms for reported negative outcomes are unclear at present.
Cytokines, adipokines, immune cells, and signaling pathways may be involved.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5:901–910. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 3.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007;43:690–709. doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Shah MM, Erickson BK, Matin T, McGwin G, Jr, Martin JY, Daily LB, et al. Diabetes mellitus and ovarian cancer: more complex than just increasing risk. Gynecol Oncol. 2014;135:273–277. doi: 10.1016/j.ygyno.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer - viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Vol. 2014 U.S. Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- 8.Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32:1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 9.Vrachnis N, Iavazzo C, Iliodromiti Z, Sifakis S, Alexandrou A, Siristatidis C, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Arch Gynecol Obstet. 2016;293:239–246. doi: 10.1007/s00404-015-3858-z. [DOI] [PubMed] [Google Scholar]
- 10.Bakhru A, Buckanovich RJ, Griggs JJ. The impact of diabetes on survival in women with ovarian cancer. Gynecol Oncol. 2011;121:106–111. doi: 10.1016/j.ygyno.2010.12.329. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 12.Ford E. Prevalence of the Metabolic Syndrome Defined by the International Diabetes Federation Among Adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 13.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JP, Ross JA, Folsom AR. Anthropometric variables, physical activity, and incidence of ovarian cancer: The Iowa Women’s Health Study. Cancer. 2004;100:1515–1521. doi: 10.1002/cncr.20146. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 16.Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244–1248. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 17.Lubin F. Body mass index at age 18 years and during adult life and ovarian cancer risk. Am J Epidemiol. 2003;157:113–120. doi: 10.1093/aje/kwf184. [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women’s health initiative: a longitudinal study from the United States. PLoS Med. 2016;13:e1002081. doi: 10.1371/journal.pmed.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Zhang TT, Zhao JJ, Qi SF, Du P, Liu DW, et al. The association between overweight, obesity and ovarian cancer: a meta-analysis. Jpn J Clin Oncol. 2015;45:1107–1115. doi: 10.1093/jjco/hyv150. [DOI] [PubMed] [Google Scholar]
- 20.Nagle CM, Dixon SC, Jensen A, KSK, Modugno F, deFazio A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113:817–826. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz NS, Wright AA. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol. 2015;138:201–206. doi: 10.1016/j.ygyno.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gates MA, Rosner BA, Hecht JL, Tworoger SS. Risk factors for epithelial ovarian cancer by histologic subtype. Am J Epidemiol. 2010;171:45–53. doi: 10.1093/aje/kwp314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Jeon I, Kim JW, Song YS, Yoon JM, Park SM. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2013;23:402–412. doi: 10.1097/IGC.0b013e31828189b2. [DOI] [PubMed] [Google Scholar]
- 24.Chen HF, Chang YH, Ko MC, Li CY. A large scale population-based cohort study on the risk of ovarian neoplasm in patients with type 2 diabetes mellitus. Gynecol Oncol. 2014;134:576–580. doi: 10.1016/j.ygyno.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol. 2012;119:61–67. doi: 10.1097/AOG.0b013e3182393ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katon JG, Reiber GE, Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2013;36:1604–1606. doi: 10.2337/dc12-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvotelli L, Stoico V, Perrone F, Cacciatori V, Negri C, Brangani C, et al. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: the Verona Diabetic Foot Screening Program. J Diabetes Complicat. 2015;29:1066–1070. doi: 10.1016/j.jdiacomp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Esposito KCP, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorge T, Lukanova A, Tretli S, Manjer J, Ulmer H, Stocks T, et al. Metabolic risk factors and ovarian cancer in the metabolic syndrome and cancer project. Int J Epidemiol. 2011;40:1667–1677. doi: 10.1093/ije/dyr130. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Metzinger MN, Lewellen KA, Cripps SN, Carey KD, Harper EI, et al. Obesity contributes to ovarian cancer metastatic success through increased lipogenesis, enhanced vascularity, and decreased infiltration of M1 macrophages. Cancer Res. 2015;75:5046–5057. doi: 10.1158/0008-5472.CAN-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy. 2011;3:539–556. doi: 10.2217/imt.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10:e0121971. doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Chang YC, Lan MS, Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- 38.Wu SBC, Whitaker RS, Berchuck A, Wiener JR, Weinberg JB, Bast RC. Tumor necrosis factor a as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor a expression. Cancer Res. 1993;53:1939–1944. [PubMed] [Google Scholar]
- 39.Gastl G, Plante M. Bioactive interleukin-6 levels in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. In: Bartlett JMS, editor. Ovarian Cancer: Methods and Protocols. Humana Press; Totowa, NJ: 2001. pp. 121–123. [DOI] [PubMed] [Google Scholar]
- 40.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martínez-Maza O. Serum in-terleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164:1038–1043. doi: 10.1016/0002-9378(91)90582-c. [DOI] [PubMed] [Google Scholar]
- 41.Secord AATD, Liu Y, Starr MD, Brady JC, Lankes HA, Hurwitz H, Mannel RS, Tewari KS, O’Malley DM, Gray HJ, Bakkum-Gamez JN, Fujiwara K, Boente M, Deng W, Burger RA, Birrer MJ, Nixon AB. Prognostic and predictive blood-based biomarkers (BMs) in patients (pts) with advanced epithelial ovarian cancer (EOC) treated with carboplatin–paclitaxel (CP) ± bevacizumab (BEV): results from GOG-0218. Annu Meet Am Soc Clin Oncol. 2016 [Google Scholar]
- 42.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 43.GWHS, MSN, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer: potential role in disease progression. J Clin Invest. 1993;91:2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolomeyevskaya N, Eng KH, Khan AN, Grzankowski KS, Singel KL, Moysich K, et al. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-alpha and interleukin-6 in predicting reduced progression-free survival in epithelial ovarian cancer. Gynecol Oncol. 2015;138:352–357. doi: 10.1016/j.ygyno.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun SBWC, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci. 2011;7:1003–1015. doi: 10.7150/ijbs.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colvin EK. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi: 10.3389/fonc.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato S, A-CL, Trigo C, Delpiano A, Sanhueza C, Garcia K, Ibanez C, Hormazabal K, Diaz D, Branes J, Castellon E, Bravo E, Owen G, Cuello M. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: an explanation for poor outcomes in obese women. Oncotarget. 2015;6:21100–21119. doi: 10.18632/oncotarget.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz ES, Karlan BY, Li AJ. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol Oncol. 2013;129:353–357. doi: 10.1016/j.ygyno.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Uddin S, Bu R, Ahmed M, Abubaker J, Al-Dayel F, Bavi P, et al. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol Cancer. 2009;8:74. doi: 10.1186/1476-4598-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naylor C, Petri WA., Jr Leptin regulation of immune responses. Trends Mol Med. 2016;22:88–98. doi: 10.1016/j.molmed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 52.Mendonca FM, de Sousa FR, Barbosa AL, Martins SC, Araujo RL, Soares R, et al. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182–189. doi: 10.1016/j.metabol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. Journal of Ovarian Research. 2014;7 doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 55.Yang C, Lee H, Jove V, Deng J, Zhang W, Liu X, et al. Prognostic significance of B-cells and pSTAT3 in patients with ovarian cancer. PLoS One. 2013;8:e54029. doi: 10.1371/journal.pone.0054029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol. 2006;125:451–458. [PubMed] [Google Scholar]
- 57.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 59.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, Ke X, Zeng S, Wu M, Lou J, Wu L, et al. Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment. Cell Mol Immunol. 2015;12:580–591. doi: 10.1038/cmi.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen JM, Coleman RL, Sood AK. Targeting the tumour microenvironment in ovarian cancer. Eur J Cancer. 2016;56:131–143. doi: 10.1016/j.ejca.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slaughter KN, Thai T, Penaroza S, Benbrook DM, Thavathiru E, Ding K, et al. Measurements of adiposity as clinical biomarkers for first-line bevacizumab-based chemotherapy in epithelial ovarian cancer. Gynecol Oncol. 2014;133:11–15. doi: 10.1016/j.ygyno.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 63.Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–1522. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 65.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 66.Spentzos D, Cannistra SA, Grall F, Levine DA, Pillay K, Libermann TA, et al. IGF axis gene expression patterns are prognostic of survival in epithelial ovarian cancer. Endocr Relat Cancer. 2007;14:781–790. doi: 10.1677/ERC-06-0073. [DOI] [PubMed] [Google Scholar]
- 67.Laws MJ, Kannan A, Pawar S, Haschek WM, Bagchi MK, Bagchi IC. Dysregulated estrogen receptor signaling in the hypothalamic-pituitary-ovarian axis leads to ovarian epithelial tumorigenesis in mice. PLoS Genet. 2014;10:e1004230. doi: 10.1371/journal.pgen.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schairer C, Fuhrman BJ, Boyd-Morin J, Genkinger JM, Gail MH, Hoover RN, et al. Quantifying the role of circulating unconjugated estradiol in mediating the body mass index-breast cancer association. Cancer Epidemiol Biomark Prev. 2016;25:105–113. doi: 10.1158/1055-9965.EPI-15-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papadimitriou CA, Markaki S, Siapkaras J, Vlachos G, Efstathiou E, Grimani I, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology. 2004;66:112–117. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 70.Carver DK, Barnes HJ, Anderson KE, Petitte JN, Whitaker R, Berchuck A, et al. Reduction of ovarian and oviductal cancers in calorie-restricted laying chickens. Cancer Prev Res (Phila) 2011;4:562–567. doi: 10.1158/1940-6207.CAPR-10-0294. [DOI] [PubMed] [Google Scholar]