Abstract
Anemia is one of the most common health problems in the primary care setting. Macrocytosis in adults is defined as a red blood cell (RBC) mean corpuscular volume (MCV) >100 femtoliter (fL). Macrocytic anemias are generally classified into megaloblastic or nonmegaloblastic anemia. Megaloblastic anemia is caused by deficiency or impaired utilization of vitamin B12 and/or folate, whereas nonmegaloblastic macrocytic anemia is caused by various diseases such as myelodysplastic syndrome (MDS), liver dysfunction, alcoholism, hypothyroidism, certain drugs, and by less commonly inherited disorders of DNA synthesis. Macrocytic anemias are treated with cause‐specific therapies, and it is crucial to differentiate nonmegaloblastic from megaloblastic anemia. Because MDS and myeloid neoplasms commonly affect the elderly, primary care physicians may encounter more cases of macrocytic anemias in the near future, as the older population increases. When MDS is suspected along with leukocytopenia and/or thrombocytopenia with anemia, a hematology consultation may be appropriate.
Keywords: macrocytic anemias, megaloblastic, myelodysplastic syndrome, nonmegaloblastic, pernicious anemia
1. Introduction
Anemia is one of the most commonly diagnosed conditions by primary care physicians. In 2010, global anemia prevalence was 32.9%, that is, more than 2.2 billion people were affected.1 The World Health Organization (WHO) defines anemia as a hemoglobin (Hb) count of less than 13 g/L in men, less than 12 g/L in nonpregnant women, and less than 11 g/L in pregnant women and the elderly. The cause of anemia varies by age, sex, and geography, and iron‐deficiency anemia is the most common etiology.1 For differential diagnosis, it is useful to classify the type of anemia based on the red cell indices of Wintrobe,2 which is calculated from red blood cell count, hemoglobin concentration, and hematocrit. The mean corpuscular volume (MCV) is calculated from hematocrit (%) × 10/RBC count (106/μl), and macrocytic anemias are defined as MCV >100 fL.
Although macrocytic anemias are not frequently encountered by general physicians, a report from a family practice group found macrocytosis in 2%‐4% of patients,3 and a study of 1784 randomly selected older adults living at home found macrocytosis in 6.3% of men and 3.3% of women.4 The causes of anemia in older adults are complicated because they often have multiple comorbidities. According to the Stanford study, hematologic malignancy and iron‐deficiency anemia accounted for 22% and 12% of the older adult patients with anemia, respectively, and the etiology of anemia remained unknown in 35% of the patients.5 With the expected future increase in the older population, it is likely that the number of cases of macrocytic anemia encountered by primary care physicians will increase. Thus, this review summarizes the diagnosis and treatment of macrocytic anemias in adults.
2. Etiology
The cause of macrocytic anemia is classified into one of the following categories, megaloblastic or nonmegaloblastic. Megaloblastic anemia is caused by deficiency or impairment of utilization of vitamin B12 or folate. Nonmegaloblastic anemia may be the result of liver dysfunction, alcoholism, myelodysplastic syndrome (MDS), or hypothyroidism. Common causes of macrocytosis are different by region and setting. For example, in New York, 37% of cases diagnosed in hospitalized patients were medication related.6 Antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infections accounted for 13%.6 In Finland, the common causes of macrocytic anemias were alcoholism (65%)7 and vitamin B12 or folate deficiency (28%)8 in outpatients over 75 years of age.
3. Vitamin B12 Deficiency
Vitamin B12 deficiency is the most common cause of megaloblastic anemia. Vitamin B12 deficiency is caused by insufficient dietary intake, as in the cases of vegetarians or malnutrition, malabsorption due to the absence of intrinsic factor caused by pernicious anemia or following gastric surgery, congenital disorders, such as transcobalamin II deficiency, or exposure to nitrous oxide.
The result of one study, conducted in Japan, indicated that the most common cause of megaloblastic anemia is pernicious anemia (61%), followed by vitamin B12 deficiency due to gastrectomy (34%), vitamin B12 deficiency due to other causes (2%), and folate deficiency (2%).9 Vitamin B12 is contained in animal foods, and the daily intake is approximately 3‐30 μg. The daily required amount is approximately 1‐3 μg, and except for stomach or intestinal obstruction, or being a strict vegetarian, vitamin B12 deficiency is rare.
Vitamin B12 binds to intrinsic factor secreted by the gastric parietal cells, and it is absorbed in the terminal ileum. Once absorbed, vitamin B12 acts as a coenzyme in the enzymatic reaction that produces methionine from homocysteine. As a result, folic acid is converted into its active form. When vitamin B12 is deficient, active folic acid is also deficient. As a result, the intracellular reaction involving the coenzyme form of folic acid is affected. Thus, not only vitamin B12 but also folate deficiencies impair DNA synthesis. Because a large amount of vitamin B12 is stored in the liver, it takes 5‐10 years for clinical problems to manifest following decreased intake or absorption of vitamin B12.10
The signs and symptoms induced by megaloblastic anemia due to vitamin B12 deficiency are fatigue, headache, palpitations, and dyspnea, and neurological symptoms such as dysesthesia and hypoesthesia may also be present. In severe cases, ataxia, decreased proprioception, and vibratory sensation, collectively known as subacute combined degeneration, may be present. Neurologic symptoms are not generally seen in folate deficiency. Vitamin B12 deficiency does not necessarily lead to anemia and macrocytosis. Other symptoms include Hunter's glossitis and gray hair.
Peripheral blood smear reveals macrocytic anemias and pancytopenia, and hypersegmented neutrophils may be present in severe cases. Megaloblastic changes in erythroblasts and giant metamyelocytes are seen in bone marrow, resulting from impaired nuclear differentiation. Biochemical analysis of blood shows increased levels of indirect bilirubin and lactate dehydrogenase (LDH), and a decreased level of haptoglobin. Vitamin B12 deficiency is treated with parenteral administration of vitamin B12, and hematological levels generally return to normal within one month. For patients with a permanent decrease in the ability to absorb dietary vitamin B12, such as associated with pernicious anemia or total gastrectomy, lifelong treatment is necessary.10 During hematopoietic recovery, an iron deficiency may develop. Although it is not an established treatment, recently it has been reported that oral treatment is effective, because 1%‐5% of vitamin B12 absorption in the terminal ileum is by passive diffusion, which does not involve intrinsic factor.10
4. Pernicious Anemia
Pernicious anemia accounts for 20%‐50% of the vitamin B12 deficiency in adults,11 and is associated with autoimmune gastritis, resulting in the destruction of gastric parietal cells and the associated lack of intrinsic factor.12 The prevalence of pernicious anemia is estimated at 10‐50 per 100 000 persons, among North Europeans and Caucasian Americans. The prevalence of pernicious anemia in Japan is rare, 1‐5 per 100 000 persons,13 compared with the West. Pernicious anemia is caused by autoimmune metaplastic atrophic gastritis (AMAG), which predominantly manifests in the stomach body and fundus. In pernicious anemia, antigastric parietal cell autoantibodies are detected specifically against the hydrogen potassium adenosine triphosphatase (H+/K+‐ATPase) proton pump. Helicobacter pylori (H. pylori) are not generally considered to be associated with AMAG. However, Hershko et al. have reported that H. pylori might serve as a trigger of AMAG and pernicious anemia, based on their observation that the prevalence of H. pylori infection was 87.5% in patients under 20 years of age.14 In addition, one theory regarding the initiating event of AMAG is molecular mimicry between H. pylori antigens and gastric H+/K+−ATPase.15
The destruction of parietal cells leads to decreased acid production and intrinsic factor secretion, and autoantibodies against intrinsic factor inhibit the absorption of vitamin B12. As a result, gastrin secretion from antral G cells increases, and hypergastrinemia induces proliferation of oxyntic mucosal cells including enterochromaffin‐like cells and parietal cells.16 The clinical manifestations are similar to other vitamin B12 deficiencies, but pernicious anemia is sometimes associated with other autoimmune diseases such as type 1 diabetes, autoimmune thyroiditis, and Addison's disease. Sensitivity and specificity of the anti‐intrinsic factor antibody test were 50%‐70%, and greater than 95%, respectively.17 Sensitivity and specificity of the antigastric parietal cell antibody test were more than 90% and 50%, respectively.18 The treatment for pernicious anemia is lifelong administration of vitamin B12. Patients with pernicious anemia are at high risk of developing gastric adenocarcinoma and carcinoid tumors.19 Significant risk factors for the development of gastric carcinoma in AMAG include the presence of pernicious anemia, severity of mucosal atrophy, intestinal metaplasia, disease duration, and over 50 years of age.16 Periodic stomach examinations are recommended for patients with pernicious anemia.
5. Folate Deficiency
Folic acid is contained in green vegetables and animal products, such as liver. The recommended dietary allowance of folic acid for adults is 240 μg a day, and an intake of around 400 μg each day is necessary for pregnant or lactating women. Folate deficiency may increase the risk of a congenital neural tube stenosis during pregnancy. Folic acid is absorbed in the upper jejunum by both passive diffusion and active uptake. Folate deficiency is caused by nutritional deficiency (eg, poor diet, alcoholism), malabsorption (eg, celiac disease, inflammatory bowel disease), increased requirements (eg, pregnancy, lactation, chronic hemolysis), or medication (eg, methotrexate, trimethoprim, phenytoin). Because serum folate levels fluctuate with dietary intake, measurement of RBC folate levels, which reflect folate stores in tissue, has been considered more reliable.20 Patients are usually treated with oral folic acid if the cause of folate deficiency is nutritional deficiency or increased nutritional requirements.
6. Myelodysplastic Syndrome
MDS is defined as a clonal hematopoietic stem cell disorder characterized by cytopenia, myelodysplasia, ineffective hematopoiesis, and increased risk of progression to acute myeloid leukemia (AML).21 The prevalence in Japan is approximately 3 per 100 000 persons, and is increasing. In a study of 124 patients aged 75 years or older, with an elevated MCV (>95 fL), six patients were diagnosed with MDS.8 MDS is caused by a stepwise acquisition of oncogenic mutations. Clonal chromosomal abnormalities are observed in 30 to 50% of patients with MDS and gene mutations are also present. With the advent of next‐generation sequencing, recurrent somatic mutations in genes involved in epigenetic regulation (TET2, ASXL1, EZH2, DNMT3A, IDH1/2), RNA splicing (SF3B1, SRSF2, U2AF1, ZRSR2), DNA damage response (TP53), transcriptional regulation (RUNX1, BCOR, ETV6), and signal transduction (CBL, NRAS, JAK2) have been identified in patients with MDS.22 Patients with MDS may present with anemia, bleeding due to thrombocytopenia, and infection or fever due to neutropenia. Peripheral blood examination reveals cytopenia resulting from ineffective hematopoiesis. MDS is classified according to the WHO classification system based on a combination of morphology, immunophenotype, genetics, and clinical features. Treatments are selected based on the MDS subtype and patient age. Patients who are under 55 years of age with severe bone marrow failure or high risk of developing AML may receive allogeneic hematopoietic stem cell transplantation. However, patients over 65 years of age or patients with low‐risk diseases are generally treated with supportive care, such as transfusions and the use of antibiotics for bacterial infections.
7. Alcoholism
Alcoholism is a well‐known cause of macrocytic anemias. Chronic consumption of more than 80 grams of alcohol per day has adverse effects on the hematologic system.23 Even before anemia develops, approximately 90% of alcoholics have macrocytosis (MCV between 100 and 110 fL).24 Diagnosing alcoholism is often difficult, but the Michigan Alcoholism Screening test and γ‐glutamyltransferase levels are found to be the two most sensitive tests for detecting alcoholism in patients with macrocytosis.25 In patients with elevated MCV, it may be valuable to perform the above tests, taking into consideration the possibility of alcoholism. Abstinence from alcohol rapidly returns elevated MCV26 to normal levels.
8. Hypothyroidism
Anemia associated with hypothyroidism is usually normocytic or macrocytic. Because thyroid hormone stimulates the production of erythropoietin and affects hematopoiesis, a reduction in thyroid hormone production may cause anemia.
9. Drugs
Many drugs cause megaloblastic anemia by impairing the cellular availability or the utilization of folic acid or vitamin B12. This could be caused by interference with absorption, plasma transport, or delivery of folate or vitamin B12, competition for reducing enzymes, end‐product inhibition of co‐factor‐mediated reactions, or physical destruction of the vitamins.27 Common drugs that cause macrocytosis are hydroxyurea, methotrexate, zidovudine, azathioprine, antiretroviral agents, valproic acid, and phenytoin (Table 1).28
Table 1.
Drug‐induced macrocytosis
| Antineoplastic | Antibacterial |
| Azathioprine | Sulfamethoxazole‐trimethoprim |
| Capecitabine | |
| Cladribine | Antimalarial |
| Cyclophosphamide | Pyrimethamine |
| Cytosine arabinoside | |
| Hydroxyurea | Anticonvulsant |
| Imatinib | Phenytoin |
| Methotrexate | Primidone |
| Sunitinib | Valproic acid |
| 5‐Fluorouracil | |
| 6‐Mercaptopurine | Anti‐inflammatory |
| Sulfasalazine | |
| Antiviral | |
| d4T | Antidiabetic |
| Lamivudine | Metformin |
| Valacyclovir | |
| Zidovudine | Diuretic |
| Triamterene |
10. Differential Diagnosis of Macrocytic Anemias
Once macrocytosis has been identified, differential diagnosis should begin with determining the serum levels of vitamin B12 and folate and examining the peripheral blood smear (Figure 1). Macrocytic cells are red blood cells larger than the nucleus of a small lymphocyte. Although the presence of macro‐ovalocytes, anisocytosis, and hypersegmented neutrophils suggest megaloblastic anemia associated with vitamin B12 or folate deficiency, these morphological abnormalities may also be seen in MDS or drug‐induced disorders of DNA synthesis. Less commonly, macrocytic anemia with the similar morphology results from inherited disorders of DNA synthesis such as Lesch‐Nyhan syndrome and transcobalamin deficiency.
Figure 1.
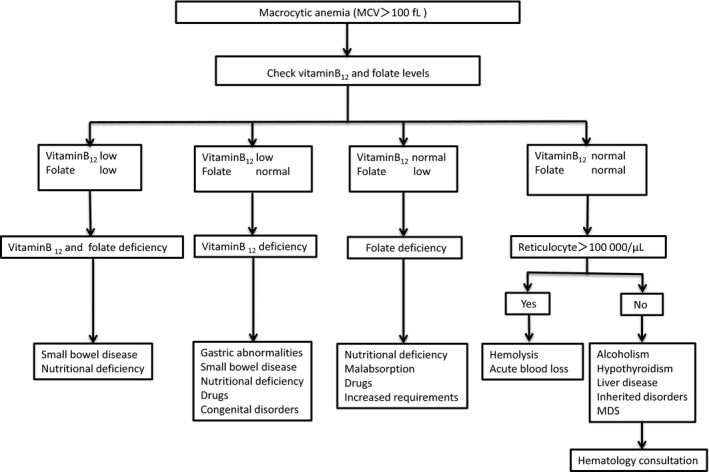
Flowchart for the differential diagnosis of macrocytic anemias (MDS, myelodysplastic syndrome)
If serum vitamin B12 levels are <200 pg/mL, vitamin B12 deficiency is very likely.29 If serum vitamin B12 levels are >300 pg/mL, vitamin B12 deficiency is unlikely.12 However, it should be noted that recent studies show normal values or falsely high values of vitamin B12 in many patients with pernicious anemia when the current immunoenzymatic methods are used.30 If serum vitamin B12 levels are between 200 and 300 pg/mL, metabolite testing should be reserved for those patients most likely to have a vitamin B12 deficiency; however, methylmalonic acid (MMA) measurement is not covered by insurance in Japan. Serum concentrations of homocysteine as well as serum concentrations of MMA are elevated in vitamin B12 deficiency, whereas only homocysteine is elevated in folate deficiency.31 If the serum folate level is >4 ng/mL, folate deficiency can be effectively ruled out, and a serum folate level <2 ng/mL is diagnostic for folate deficiency. Combined vitamin B12 and folate deficiency may be caused by tropical sprue or gluten‐sensitive enteropathy.
When vitamin B12 or folate deficiency is ruled out, a reticulocyte count should be obtained. Reticulocytes are immature nonnucleated erythrocytes. In general, approximately 1% of red blood cells are counted as reticulocytes. If the absolute reticulocyte count is more than 100 000/μl, acute blood loss or hemolysis is suspected. High levels of indirect bilirubin and LDH, and decreased levels of haptoglobin suggest the presence of hemolytic anemia. If hemolysis is not present, acute blood loss should be included in the differential diagnosis. The absence of reticulocytosis suggests the possibility of alcoholism, liver dysfunction, hypothyroidism, or MDS. Macrocytic target cells are commonly seen on peripheral blood smears in liver disease, and hypolobulated or hypogranular neutrophils, large and/or abnormally granulated platelets, and monocytosis may be seen with MDS.
Extremely high MCV (>130 fL) narrows the differential diagnosis, to include ART treatment for HIV infection, use of hydroxyurea, and vitamin B12 or folate deficiency.32 The MCV values may be falsely elevated when significant reticulocytosis is present, because the reticulocyte volume is high.33 Moreover, hyperglycemia, marked leukocytosis, and cold agglutinins cause spurious macrocytosis.20
Of importance is the indication for bone marrow examinations. This is recommended for patients with abnormal cells in blood circulation or patients who do not respond to treatments, such as vitamin replacement.
11. Conclusions
Primary care physicians may encounter more cases of macrocytic anemias in the near future than they have over the past several decades, as the older population increases, because macrocytic anemias commonly appear in elderly patients. Although macrocytic anemias have many different etiologies, MDS is one of the leading causes of macrocytic anemias in the elderly, and clonal hematopoiesis with somatic mutations, similar to those of MDS patients, has been confirmed in 10% of persons over 65 years of age without apparent hematological disorders.34 When the cause of anemia cannot be determined, in spite of the effort with noninvasive diagnostic tests and procedures, a consultation with a hematologist is recommended.
Conflict of Interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Acknowledgements
We would like to thank Drs. Shigeharu Ueki, Tomoo Saga, Ayumi Omokawa, and Yuki Moritoki for their careful and critical reading of this manuscript. This work was supported in part by grants from the Ministry of Education, Science, Sports, and Culture of Japan (MH, 15K08639) and the Idiopathic Disorders of Hematopoietic Organs Research Committee of the Ministry of Health, Labour, and Welfare of Japan.
Nagao T, Hirokawa M. Diagnosis and treatment of macrocytic anemias in adults. J Gen Fam Med. 2017;18:200–204. https://doi.org/10.1002/jgf2.31
References
- 1. Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2000. Blood 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wintrobe MM. Morphology, intrinsic metabolism, function, laboratory evaluation, Wintrobe MM(ed): Clinical Hematology. Philadelphia: Lea & Febiger, 1974; pp. 80–134. [Google Scholar]
- 3. Davenport J. Macrocytic anemia. Am Fam Physician 1996;53:155–62. [PubMed] [Google Scholar]
- 4. Inelmen EM, D'Alessio M, Gatto MR, et al. Descriptive analysis of the prevalence of anemia in a randomly selected sample of elderly people living at home: some results of an Italian multicentric study. Aging (Milano). 1994;6:81. [DOI] [PubMed] [Google Scholar]
- 5. Price EA, Mehra R, Holmes TH, Schrier SL. Anemia in older persons: etiology and evaluation. Blood Cells Mol Dis 2011;46:159. [DOI] [PubMed] [Google Scholar]
- 6. Savage DG, Ogundipe A, Allen RH, Stabler SP, Lindenbaum J. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci 2000;319:343–52. [DOI] [PubMed] [Google Scholar]
- 7. Seppä K, Heinilä K, Sillanaukee P, Saarni M. Evaluation of macrocytosis by general practitioners. J Stud Alcohol 1996;57:97–100. [DOI] [PubMed] [Google Scholar]
- 8. Mahmoud MY, Lugon M, Anderson CC. Unexplained macrocytosis in elderly patient. Age Ageing 1996;25:310–2. [DOI] [PubMed] [Google Scholar]
- 9. Komine M. Int J Hematol 2000;71(suppl 1):8. [Google Scholar]
- 10. Shipton MJ, Thachil J. Vitamin B12 deficiency‐A21st century perspective. Clin Med (Lond). 2015;15:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loukili NH, Noel E, Blaison G, et al. Update of pernicious anemia. A retrospective study of 49 cases. Rev Med Interne 2004;25:556–61. [DOI] [PubMed] [Google Scholar]
- 12. Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med 2013;368:149–60. [DOI] [PubMed] [Google Scholar]
- 13. Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr 2004;24:299–326. [DOI] [PubMed] [Google Scholar]
- 14. Hershko C, Ronson A, Souroujon M, et al. Variable hematologic presentation of autoimmune gastritis: age‐related progression from iron deficiency to cobalamin depletion. Blood 2006;107:1673. [DOI] [PubMed] [Google Scholar]
- 15. Amedai A, Bergman MP, Appelmelk BJ, et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+‐adenosine triphosphatase in human gastric autoimmunity. J Exp Med 2003;198:1147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JY, Lam‐Himlin D, Vemulapalli R. Review of autoimmune metaplastic atrophic gastritis. Gastrointest Endosc 2013;77:284–92. [DOI] [PubMed] [Google Scholar]
- 17. Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood 2008;112:2214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004;171:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirokawa M. Recent progress of diagnosis and treatment for immune‐mediated hematological diseases. Topics: III. Diagnosis and treatment: 3. Pernicious anemia. Nihon Naika Gakkai Zasshi. 2014;103:1609–12. [DOI] [PubMed] [Google Scholar]
- 20. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician 2009;79:203–8. [PubMed] [Google Scholar]
- 21. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 22. Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: contemporary review and how we treat. Am J Hematol 2016;91:76–89. [DOI] [PubMed] [Google Scholar]
- 23. Hoffbrand V, Provan D. ABC of clinical haematology. Macrocytic anaemias. BMJ 1997;314:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seppä K, Sillanaukee P, Saarni M. Blood count and hematologic morphology in nonanemic macrocytosis: differences between alcohol abuse and pernicious anemia. Alcohol 1993;10:343–7. [DOI] [PubMed] [Google Scholar]
- 25. Seppä K, Laippala P, Saarni M. Macrocytosis as a consequence of alcohol abuse among patients in general practice. Alcohol Clin Exp Res 1991;15:871–6. [DOI] [PubMed] [Google Scholar]
- 26. Maruyama S, Hirayama C, Yamamoto S, et al. Red blood cell status in alcoholic and non‐alcoholic liver disease. J Lab Clin Med 2001;138:332–7. [DOI] [PubMed] [Google Scholar]
- 27. Hesdorffer CS, Longo DL. Drug‐induced megaloblastic anemia. N Engl J Med 2015;373:1649–58. [DOI] [PubMed] [Google Scholar]
- 28. Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matchar DB, McCrory DC, Millington DS, Feussner JR. Performance of the serum cobalamin assay for diagnosis of cobalamin deficiency. Am J Med Sci 1994;308:276. [DOI] [PubMed] [Google Scholar]
- 30. Carmel R, Agrawal YP. Failures of cobalamin assays in pernicious anemia. N Engl J Med 2012;367:385–6. [DOI] [PubMed] [Google Scholar]
- 31. Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med 1999;159:1289–98. [DOI] [PubMed] [Google Scholar]
- 32. Planche V, Georgin‐Lavialle S, Avillach P, et al. Etiologies and diagnostic work‐up of extreme macrocytosis defined by an erythrocyte mean corpuscular volume over 130°fL: a study of 109 patients. Am J Hematol 2014;89:665–6. [DOI] [PubMed] [Google Scholar]
- 33. Green R, Dwyre DM. Evaluation of macrocytic anemias. Semin Hematol 2015;52:279–86. [DOI] [PubMed] [Google Scholar]
- 34. Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood‐cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


