Abstract
Emerging research highlights the complex inter-relationships between sleep disordered breathing and cardiovascular disease, presenting clinical and research opportunities as well as challenges. Patients presenting to cardiology clinics have a high prevalence of obstructive (OSA) and central sleep apnea associated with Cheyne-Stokes Respiration (CSA-CSR). Multiple mechanisms have been identified by which sleep disturbances adversely affect cardiovascular structure and function. Epidemiological research indicates that OSA is associated with increases in the incidence and progression of coronary heart disease, heart failure, stroke and atrial fibrillation. CSA-CSR predicts incident heart failure and atrial fibrillation; among patients with heart failure, CSA-CSR strongly predicts mortality. Thus, a strong literature provides the mechanistic and empirical bases for considering OSA and CSA-CSR as potentially modifiable risk factors for cardiovascular disease. Data from small trials provide evidence that treatment of OSA with continuous positive airway pressure (CPAP) not only improves patient-reported outcomes such as sleepiness, quality of life and mood, but also improves intermediate cardiovascular endpoints such as blood pressure, cardiac ejection fraction, vascular parameters and arrhythmias. However, data from large scale randomized controlled trials do not currently support a role for positive pressure therapies for reducing cardiovascular mortality. The results of two recent large randomized controlled trials, published in 2015 and 2016, raise questions on the effectiveness of pressure therapies in reducing clinical endpoints, although one supported the beneficial effect of CPAP on quality of life, mood, and work absenteeism. This review provides a contextual framework for interpreting the results of recent studies, key clinical messages, and suggestions for future sleep and cardiovascular research, which include further consideration of individual risk factors, use of existing and new multi-modality therapies which also address adherence, and implementation of trials that are sufficiently powered to target endpoints and to support subgroup analyses. These goals may best be addressed through strengthening collaboration among cardiology, sleep medicine and clinical trial communities.
Keywords: Sleep apnea, cardiovascular diseases, randomized trials, perspectives
Introduction
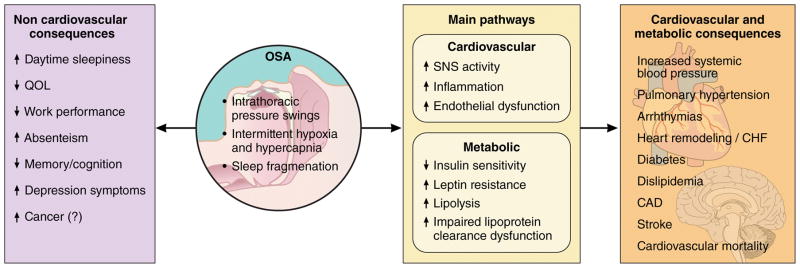
Human beings spend around one-third of their lives sleeping. Sleep is no longer considered a passive and homogenous state, but is understood to consist of cyclic periods of complex and changing brain activity, behavior and physiology.1 Over the last decades, dramatic growth in the Sleep Medicine field occurred concurrent with marked advances in clinical and basic research. Sleep disturbances, particularly obstructive sleep apnea (OSA), were identified to impact or co-vary with numerous health outcomes and physiological processes, particularly cardiovascular (CV) disease (Figure 1). Blood pressure and heart rate normally change across the sleep period, and evidence from basic, translational, and clinical research identified adverse cardiovascular responses to disturbed sleep, particularly due to OSA and its associated intermittent hypoxia and sleep fragmentation.2,3 Notably, repetitive collapse of the upper airway and impaired gas exchange with intermittent hypoxia was shown to result in sleep disruption and impaired gas exchange. The associated surges in sympathetic activity were shown to result in acute blood pressure elevations, release of inflammatory mediators, lipolysis, and worsened insulin resistance.3 Cardiac remodeling, common in patients with OSA, was attributed to exposures to hypoxemia, catecholamine excess, blood pressure elevation, and intra-thoracic pressure swings affecting pre- and after-load and left atrial and ventricular transmural pressures.
Figure 1.
Proposed consequences of obstructive sleep apnea (OSA)
The observational evidence showing that OSA occurs commonly in both the general population and in patients with CV disease, and associates with both coronary and cerebrovascular morbidity and mortality4–6 suggested the possibility of targeting OSA as a novel and modifiable CV risk factor. Recognition of the high frequency of other sleep disorders occurring in patients with CV disease, such as central sleep apnea/Cheyne-Stokes Respiration (CSA-CSR),7 insomnia,8 and short sleep duration,9 also suggested the potential benefits of more broadly considering sleep health as fundamental to CV health,10 encouraging consideration of including sleep disturbances in the top 10 potentially modifiable CV risk factors.11
Despite the high prevalence of OSA, there is considerable uncertainty about the role for systematic screening. Improvement in major cardiovascular endpoints has not been shown in clinical trials, and the U.S. Preventive Health Services Task Force12 has recommended against screening asymptomatic patients in the general population. On the other hand, patients with heart disease may present with less typical symptoms of sleep apnea, such as fatigue or insomnia rather than sleepiness, and it can therefore be difficult to differentiate symptoms of OSA vs. underlying medical conditions. Other areas of concern are: (1) limitations of animal models that may not resemble sleep disturbances in humans; and (2) potential for some analyses to be biased by residual confounding, especially regarding the influences of adiposity. However, the leading criticism is the limited evidence from rigorous and well-powered randomized control trials designed to address the impact of treatment on meaningful clinical outcomes, as noted by a recent meta-analysis.13 Compared with other research areas, the use of randomized trials in sleep is in its infancy, reflecting both the relatively recent emergence of Sleep Medicine as an academic discipline as well as the challenges in designing and implementing non-pharmacological intervention studies. The main aim of this perspective paper is to discuss the implications of several recently published trials, contextualize the results, and identify future directions. We highlight studies that address the impact of non-invasive ventilation, namely continuous positive airway pressure (CPAP) and adaptive servo ventilation (ASV), noting the current paucity of data addressing additional treatments (e.g., nocturnal oxygen supplementation, pharmacological treatments) for treatment of OSA or CSA-CSR on clinical endpoints.
The impact of OSA treatment on cardiovascular outcomes
Due to the differences in characteristics of patients and prognosis, we divided this section into primary and secondary cardiovascular prevention through OSA treatment.
Primary CV prevention
Although clinicians hypothesized that treatment of OSA with CPAP would facilitate weight loss, a recent meta-analysis suggests the converse is true. Specifically, a meta-analysis reporting on data from more than 3,000 patients showed that abolishing OSA events per se is associated with slight weight gain rather than weight loss, regardless of baseline symptoms and treatment adherence.14 These new findings underscore the need for patients prescribed CPAP to also receive counseling or support to address chronic weight management needs. Combining weight loss and CPAP may have additive CV benefits as suggested by the results of a randomized trial evaluating the impact of CPAP, weight loss or both.15
As described below, a major recent research focus is the evaluation of the influence of CPAP on blood pressure, a key risk factor for CV disease. More than 30 randomized controlled trials examined blood pressure responses to CPAP. These studies demonstrated a modest but significant blood pressure reduction,16 especially in those with resistant hypertension.17,18 Improvements were identified in both day and nighttime blood pressure, as well as in non-dipping blood pressure profiles. Randomized controlled trials have also evaluated the impact of OSA treatment on surrogate markers of CV risk, such as endothelial dysfunction, arterial stiffness, intima-media thickness, inflammatory markers, insulin sensitivity, cardiac ejection fraction, cardiac ectopy, and others.19–23 The evidence is generally consistent with a positive effect of CPAP on vascular and metabolic functions and components of the atherosclerotic process.
The treatment of OSA may reduce the incidence of hypertension, as initially suggested by a small trial that showed normalization of blood pressure among patients with pre-hypertension and/or masked hypertension.24 This important concept was further tested in a large multicenter trial in 14 teaching hospitals in Spain.25 In this trial, 723 patients who had an apnea-hypopnea index (AHI) >20 events/hour (consistent with moderate to severe OSA) and without excessive daytime sleepiness (Epworth Sleepiness Scale score of 10 or less; scores range from 0 to 24, with >10 indicating sleepiness), were randomized to CPAP (n=357) or to a control group (n=366) and followed for a median duration of 4 years (interquartile range, 2.7–4.4). Compared to usual care, CPAP was not associated with a statistically significant reduction in the incidence of hypertension or CV events in the intention to treat analysis. Analyses stratified by CPAP adherence showed that adherent users (defined by CPAP ≥4 h/night) had a significant reduction in the combined endpoints (incidence density ratio, IDR: 0.69 CI 0.50–0.94). This finding, however, may be biased by differences in health characteristics of patients who are adherent compared to those who are non-adherent.
In summary, our current knowledge of primary prevention of CV disease with CPAP is limited to surrogate endpoints, combined endpoints and observational data.
Secondary CV prevention
There is consistent evidence from observational studies indicating that untreated moderate to severe OSA in patients with established coronary disease26–28 or heart failure29 associates with increased CV morbidity and mortality. Given the high prevalence of OSA in patients with CV disease,30 the obvious next question is to determine whether OSA treatment prevents new CV events or decreases mortality in these high-risk patients. Similar to studies of primary prevention, several trials have identified improvement in blood pressure, including 24 hour blood pressure profiles, in patients with existing CV disease and OSA. In the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) study, CPAP was compared with supplemental oxygen therapy and usual care in patients with CV disease or multiple CV risk factors, most of whom were under the care of cardiologists.31 Compared with patients in the usual care group, the CPAP group experienced a significant 2.4 mmHg reduction of 24 hour mean arterial blood pressure, with larger improvements for mean nocturnal blood pressure (by 3.5 mmHg). This level of blood pressure improvement is consistent with a significant population-based reduction in stroke.
Fewer studies, however, have directly addressed clinical endpoints. In one of these studies (RICCADSA – Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA),32 non-sleepy patients with established coronary artery disease and moderate or more severe OSA defined by an AHI ≥15 events/hour were randomized to CPAP (n=122) or usual care (n=122) in a single-center. The primary endpoint was the first event of repeat coronary revascularization, myocardial infarction, stroke, or cardiovascular mortality. Over a median follow-up of 57 months, the incidence of the primary endpoint was 18.1% in the CPAP group compared to 22.1% in the control group; this difference was not statistically significant (hazard ratio: 0.80; 95% CI 0.46–1.41). In the per protocol analysis, a large, significant CV risk reduction was reported in those who used CPAP for ≥4 hours/night (hazard ratio, 0.29; 95% confidence interval, 0.10–0.86). Although this finding is consistent with a positive effect of CPAP among those who use the therapy regularly, the per protocol analysis also is likely biased by generally more positive health behaviors in adherent compared to non-adherent patients. It is important to note that the sample size of the primary intention-to-treat study was an order of magnitude lower than most major CV trials, reducing power to detect effects that are generally considered sufficient to change clinical practice.
In the much-awaited Sleep Apnea Cardiovascular Endpoints (SAVE) trial,33 a multicenter, parallel-group, open-label trial, with blinded end-point assessment, 2717 patients (~1700 patients from China) with a history of coronary artery disease or cerebrovascular disease and untreated moderate-to-severe OSA were randomly assigned to receive CPAP plus usual care (n=1346) or usual care alone (n=1341). Of note, very sleepy patients (Epworth Sleepiness Scale score >15) and patients with severe hypoxemia (oxyhemoglobin saturation <80% for >10% of sleep study time) were excluded, thus limiting the sample to a potentially lower risk group. The primary outcome was a composite of death from cardiovascular causes, myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack. In a mean follow-up of 3.7 years, CPAP use did not reduce the primary endpoint (hazard ratio with CPAP: 1.10; 95% CI 0.91 to 1.32). The lack of an effect on clinical outcomes was observed in multiple prespecified subgroup analyses. As observed in previous studies, CPAP use resulted in significant beneficial effects on quality of life, mood, daytime sleepiness, and work productivity. To estimate the effect in patients who were adherent to CPAP therapy (defined as an average of ≥4 hours/night) over the first 2 years, a pre-specified propensity score matching strategy was used to compare 561 adherent patients to a comparable group of 561 participants from the usual care group. A total of 86 primary end-points were observed in the CPAP group (15.3%) versus 98 (17.5%) in the usual-care group (hazard ratio, 0.80; 95%CI, 0.60 to 1.07; P = 0.13). Individuals who were adherent to CPAP therapy had a lower risk of stroke than those in the usual-care group (hazard ratio, 0.56; 95% CI, 0.32 to 1.00; P = 0.05), as well as a lower risk of a composite (not pre-specified) end point of cerebral events (hazard ratio, 0.52; 95% CI, 0.30 to 0.90; P=0.02). Although these findings were from secondary analyses and not adjusted for multiple testing, the larger effect size seen for cerebrovascular disease is consistent with observational data showing that OSA appears to be more strongly linked to cerebrovascular than to coronary artery disease.34
A number of explanations have been proposed to help interpret the SAVE findings, including the relatively low treatment adherence levels. The nature of the “dose-response” association between CPAP adherence and change in CV disease risk is not well established, and it is plausible that low adherence to CPAP may have contributed to the null result, particularly if accompanied by non-usage during the early morning when rapid eye movement (REM) sleep predominates. REM–related apneas are typically long and are associated with deep oxyhemoglobin desaturations and high sympathetic tone; OSA occurring in REM sleep is specifically associated with incident or recent onset hypertension.35,36 Alternatively, it is possible that cohort studies overestimated the direct effects of OSA on CV risk due to residual confounding by unadjusted risks such as from visceral or ectopic fat. Visceral obesity is strongly associated with increased CV risk,37 decreased upper-airway size due to increased tongue volume,38 and an increase in the severity of OSA.38,39 Finally, OSA treatment may not confer significant CV benefit among patients who already have existing CV disease and are under guideline-based CV disease treatment regimens (that include therapies aimed at managing several of the intermediate mechanisms implicated in OSA: control of blood pressure, glucose and dyslipidemia).
The finding for a potential benefit in patients who are strongly adherent to CPAP therapy, however, underscores the need for future trials that incorporate strategies to improve CPAP use and/or incorporate new or additive alternative treatments to OSA. Emerging data also suggest that oral appliances (mandibular advancement devices) lead to blood pressure improvements comparable to that observed with CPAP, suggesting a potential role for this therapy in cardiovascular endpoint studies.40
Treatment of central apnea in heart failure patients with Central Sleep Apnea-Cheyne Stokes Respiration (CSA-CSR)
CSA-CSR is a distinct disorder characterized by an oscillatory pattern of ventilation in which central apneas and hypopneas alternate with periods of hyperventilation, typically recognized as a waxing and waning pattern of breathing.41 Central sleep apnea occurs in the absence of significant upper airway obstruction, commonly reflecting exaggerated respiratory chemosensitivity associated with cardiac dysfunction and pulmonary congestion.42 CSA-CSR is associated with sleep fragmentation and sympathetic nervous system activation that chronically could be deleterious to the failing heart. Indeed, previous investigations suggest that CSA-CSR is a strong independent marker of mortality in patients with heart failure.43 Thus, suppression of central sleep apneas is suggested as a physiologically appropriate target for the treatment of patients with heart failure. This treatment can be considered at two levels:42 The first approach is to aggressively treat the heart failure, a major contributor if not the cause of CSA-CSR. Optimizing heart failure with guideline-based medications, as well as coronary revascularization and cardiac resynchronization therapy in selected patients, may improve CSA-CSR. However, these approaches result in variable and frequently incomplete improvement in CSA-CSR. The second approach is directed at improving ventilation. Several strategies have been considered. Pharmacological respiratory stimulants (such as theophylline and acetazolamide) were tested in small trials.44–46 Although significant reductions in respiratory event frequency and improved oxygenation were observed, concern over adverse effects, including cardiac arrhythmias, has limited enthusiasm for these interventions. Nocturnal supplemental oxygen therapy has been proposed as a physiologically sound intervention for decreasing the severity of CSA-CSR by effects on peripheral chemoreceptors (dampening “over-shoot” ventilation) and countering acute adverse effects of hypoxia on myocardial function.47 However, high levels of oxygen can increase systemic vascular resistance, and trials are needed to evaluate the role of this treatment in heart failure and CSA-CSR. Recent research identified the phenomenon of rostral redistribution of peripheral fluid to the lungs and upper airway during sleep.48 These observations led to recommendations to consider use of elastic stockings and exercise for reducing peripheral edema, thus reducing both obstructive and central apneas. This idea is supported by a population study reporting that increased walking and living in “walkable” neighborhoods associates with lower levels of sleep apnea,49 although the impact of interventions needs to be rigorously evaluated.
Positive airway pressure therapy for CSA-CSR gained interest over the last two decades. The seminal study, the Canadian Continuous Positive Airway Pressure (CANPAP) for Patients with Central Sleep Apnea and Heart Failure trial,50 randomized 258 stable patients with heart failure (mean ejection fraction of 24.5%) and central sleep apnea to receive CPAP (128 patients) or no CPAP (130 patients). The mean follow-up was two years. As early as three months after randomization, CPAP, as compared to usual care, reduced norepinephrine levels and improved cardiac ejection fraction and the distance walked in six minutes, with effects occurring in parallel with the attenuation of the central sleep apneic events. However, CPAP did not affect survival. Post-hoc analysis51 identified that CPAP did not fully suppress central events in a significant proportion of patients. Analyses stratified by degree of central event suppression identified that the subgroup that experienced improvement in central events had both a greater increase in left ventricular ejection fraction at 3 months and a significantly better transplant-free survival than control subjects. Although the bases for differential responses to CPAP among patients with CSA-CSR are not well understood, the CANPAP findings reinforced the concept that new therapies are necessary for fully suppressing CSA-CSR.
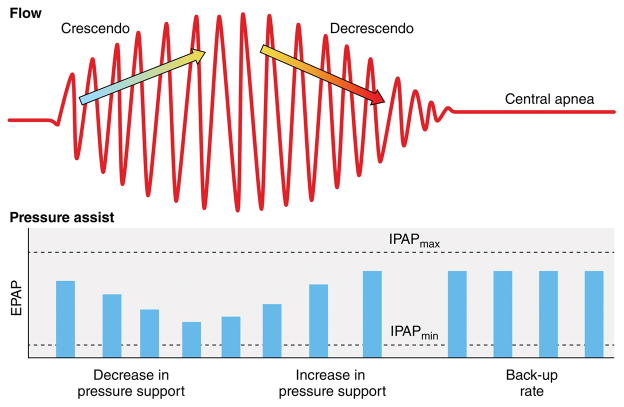
An attractive alternative to CPAP is adaptive servo-ventilation (ASV), described for the first time in 2001.52 ASV is a noninvasive ventilator therapy that alleviates central sleep apnea by delivering servo-controlled inspiratory pressure support on top of expiratory positive airway pressure, thus adjusting pressure support in response to breath by breath changes in ventilation.53 Figure 2 summarizes the principle of ASV. Preliminary evidence suggested that ASV is more effective than oxygen, CPAP47 and bi-level positive airway pressure (BIPAP) in treating CSA-CSR.54 Moreover, ASV was able to improve surrogate markers of cardiovascular risk, such as sympathetic activation,55 NT-pro brain natriuretic peptide (BNP)56 and left ventricular ejection fraction.56,57 These promising results stimulated interest in testing the ability of ASV to prevent fatal and non-fatal events in patients with heart failure and CSA-CSR. The first major, multinational trial to test this hypothesis, the Adaptive Servo Ventilation in Patients with Heart Failure (SERVE-HF) trial,58 enrolled 1325 patients with a left ventricular ejection fraction of 45% or less, New York Heart Association (NYHA) class III or IV heart failure or NYHA class II heart failure with at least one heart failure–related hospitalization within the 24 months before randomization, and stable, guideline-based medical treatment. Patients were randomized to usual care plus ASV or usual care alone. The primary end-point in the time-to event analysis was the first event of death from any cause, lifesaving cardiovascular intervention (cardiac transplantation, implantation of a ventricular assist device, resuscitation after sudden cardiac arrest, or appropriate lifesaving shock), or unplanned hospitalization for worsening heart failure. At a mean follow-up of 31 months, no differences in the composite endpoint were observed in the two groups. However, an unexpected 34% increase in all cause and cardiovascular death occurred in the ASV group. The bases for the early and sustained increase in cardiovascular mortality seen in the ASV group remain to be elucidated. Several commentaries have been published;59,60 suggested explanations include inadequate average treatment adherence; negative effects of the specific pressure therapy used, particularly in a setting of markedly impaired left ventricular function; adverse effects associated with potential induction of alkalosis; and loss of a postulated beneficial effect of CSR.
Figure 2. Adaptive servo ventilation (ASV).
The air flow tracing depicts a classical crescendo and decrescendo pattern of Cheyne-Stokes Respiration, followed by an ensuing central apnea. The servo-controlled automatic adjustment of the inspiratory positive airway pressure (IPAP) level is inversely related to the changes in peak flow over a moving time window. Specifically, during the crescendo pattern of peak flow rates the IPAP level decreases in order to dampen the rise in inspiratory peak flow rate. Conversely, during the decrescendo pattern of peak flow rates the IPAP level increases in order to dampen the fall in inspiratory peak flow rate. Therefore, the servo system dampens the inherent oscillatory behavior of the patient’s breathing pattern and smooths respiration. During a central apnea, however, the device backup rate kicks in and ventilates the patient. Modified with permission from: Antonescu-Turcu A, Parthasarathy S. CPAP and bi-level PAP therapy: new and established roles. Respir Care. 2010;55:1216–1229.
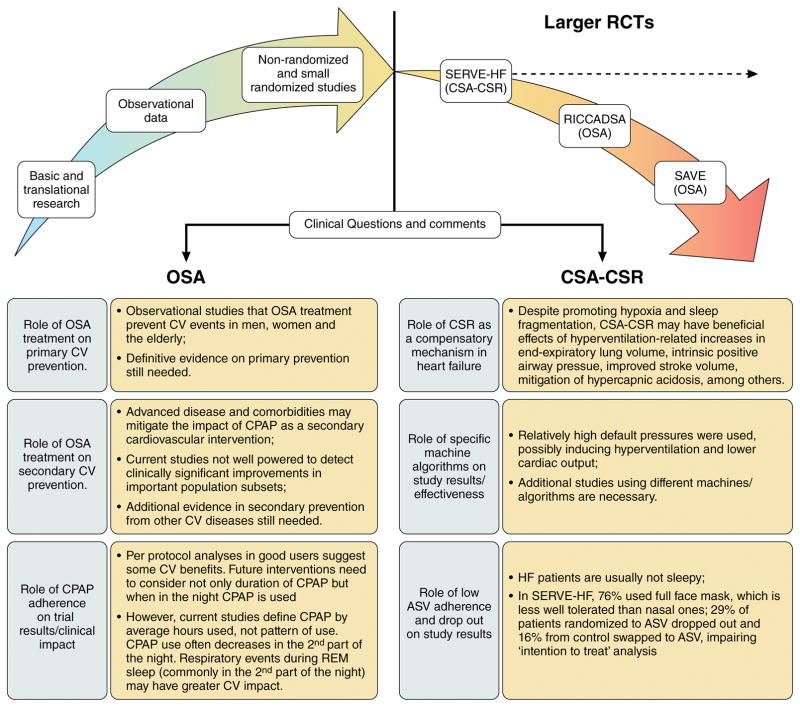
Figure 3 summarizes the key points discussed so far, highlighting potential scenarios for the role of OSA and CSA-CSR on cardiovascular disease.
Figure 3. The ‘Crossroads’ of Obstructive Sleep Apnea (OSA) and Central Sleep Apnea associated with Cheyne-Stokes Respiration (CSA-CSR) on cardiovascular (CV) diseases.
Consistent evidence provides biological plausibility for supporting OSA as a potential CV risk factor and the detrimental effects of CSA-CSR in patients with heart failure (HF) but recent larger randomized trials (RCTs) results have not matched expectations (dotted line). All quoted studies (SERVE-HF, RICCADSA and SAVE) had neutral results on the primary endpoint. However, in the SERVE-HF trial, all-cause mortality and cardiovascular mortality (secondary endpoints) were significantly higher in the adaptive servo-ventilation group than in the control group. The potential reasons for these results are discussed in the lower panel.
Non-Cardiovascular Outcomes
Patient-reported outcomes and health related quality of life are important components of disease management to improve patient well-being, and also are important endpoints in clinical trials. Cardiovascular disease is associated with reduced quality of life,61 as is even a mild degree of OSA.62 There is growing interest in OSA treatment as a means for improving quality of life and well-being in patients with heart disease. Several randomized clinical trials evaluated the impact of CPAP treatment on symptoms, health related quality of life, and mood as secondary outcomes.63–65 Despite low to modest levels of CPAP adherence, these trials showed that, compared to control conditions, CPAP resulted in improved physical functioning, vitality, and mood, as well as less sleepiness and pain, and fewer missed work days.34,66,67 The results of these trials, that ranged in duration from 3 months to over 3 years,34,67 support the beneficial effects of CPAP on outcomes of importance to patients, providers and health care systems.
Perspectives and Needs
Table 1 summarizes the key messages to our patients, challenges and a proposed research agenda. There is a need for continuing high quality basic and translational research to understand the mechanisms by which OSA and CSA-CSR contribute to CV diseases, further strengthening collaborations among cardiologists, sleep medicine clinicians, and clinical trialists. Improvements in experimental designs using animal models may help better inform translational approaches to human sleep apnea. There is also a need to better understand the variability in responses to treatment, which is likely manifold, including differences in baseline characteristics of patients in regards to severity and duration of sleep apnea, age, etiologies of cardiovascular diseases, and other risk factors; and differences in residual apneic activity and sleep with treatment.
Table.
Take-Home Messages and Proposed Research Agenda
| Scenario | What can we tell our patients? | Challenges and Future Research Agenda |
|---|---|---|
| Obstructive Sleep Apnea (OSA) | ||
| Primary prevention |
|
|
| Secondary prevention |
|
|
| Central Sleep Apnea with Cheyne-Stokes Respiration (CSA-CSR) | ||
| Secondary prevention |
|
|
Data from two ongoing randomized clinical trials may clarify some unanswered questions. For instance, the ISAACC trial68 (NCT01335087) is a multicenter study from Spain developed to evaluate the impact of OSA and its treatment on outcomes in 1864 patients with acute coronary syndrome. The primary aim is to determine if CPAP treatment will reduce the rate of composite cardiovascular events in patients with acute coronary syndrome and co-occurring OSA. The ADVENT trial (Effect of Adapto-Servo-Ventilation on Survival and Hospitalizations; NCT01128816)69 is a multicenter study designed to assess the effects of ASV on survival and frequency of hospital admissions in patients with heart failure and OSA or CSA-CSR. The estimated enrollment is 860 patients. The primary composite outcome will be death or first cardiovascular hospital admission or new onset atrial fibrillation/flutter requiring anti-coagulation but not hospitalization or delivery of an appropriate shock from an implanted cardiac defibrillation not resulting in hospitalization.
Priority areas for future trials
There is a critical need for well powered and rigorous trials that address the impact of OSA treatment on the secondary prevention of atrial fibrillation and in prevention and treatment of preserved ejection fraction heart failure and stroke. Lessons from prior trials can inform these studies. In particular, new studies may benefit from careful identification of sub-groups most likely to respond to the intervention, larger sample size (allowing detection of clinically relevant effects across stratum), and incorporation of methods for improving treatment adherence over long periods of observation, such as addition of motivational education to CPAP interventions, a strategy that recently was shown to significantly improved CPAP adherence in patients with OSA and CVD.70 It should be emphasized that the largest trial of sleep apnea and CV disease (SAVE) is one-tenth the size of many primary and secondary multi-national CV disease trials. The Cardiology community has long recognized that the relatively modest rates of clinical events, even in high risk patients, requires very large samples to detect clinically relevant reductions of event rates by 15% to 25%; this scale of investigation has not yet been attempted in Sleep Medicine but clearly is needed. Whether composite outcomes are most appropriate should be weighed against data suggesting heterogeneity in the strength of associations between sleep apnea and different CV disease outcomes.
Development of better diagnostic/prognostic tools, new treatments and personalized medicine for OSA and CSA
The most common diagnostic metric used for characterizing sleep apnea severity, the apnea hypopnea index (AHI), does not strongly predict adverse health outcomes or response to treatment. The apparent variation in susceptibility to sleepiness, cognitive deficits and cardiovascular disease for any given AHI level suggests a need to develop improved measurements of disease and to identify important disease modifying factors. In the beginning of the era of the precision medicine, biomarkers of cardiovascular risk may help to select individuals either at greatest risk for sleep apnea-related physiological stress, and/or those most likely to respond to specific, and possibly alternative, treatments. New measures of sleep apnea pathophysiology, such as quantitative measures of ventilatory drive or airway collapsibility, may help to better define patient subgroups.
There is a strong need for developing new and effective treatments for OSA and CSA-CSR, with deliberate tailoring of such treatments to the diverse needs and pathophysiology of specific subgroups of patients, such as those with underlying cardiovascular disease or heart failure, or those identified through use of biomarkers of susceptibility. Patients with sleep apnea are heterogeneous with respect to disease etiology. Physiological studies show that patients with sleep apnea have variable combinations of abnormalities in airway anatomy, neuromuscular responsiveness, respiratory chemosensitivity, and loop gain,71 with each of these components potentially responsive to different single or combinations of therapeutic interventions. Although poor adherence to CPAP is often considered a “failure” of the patient to comply with therapy, it is likely that a proportion of the poor adherence observed clinically reflects the usage patterns in patients who experience suboptimal responses to a fixed (CPAP) or variable (APAP, ASV) pressure treatments and require alternative treatments. For example, a small proportion of patients may develop central apneas when exposed to positive pressure therapy, a disorder termed “complex sleep apnea.” The emergence of new therapies, such as hypoglossal nerve stimulation for treatment of OSA72 and an implantable device which transvenously stimulates the phrenic nerve causing diaphragmatic contraction for central sleep apnea73 hold promise as physiologically-grounded treatments for selected patients. Older treatments, such as nocturnal oxygen supplementation, also may have a role in treating selected patients, such as those with augmented loop gain and central sleep apnea, although these need to be rigorously evaluated with randomized controlled trials. Finally, a “stepped” approach to treatment, that considers the many co-morbidities of patients with heart disease, and integrates behavioral, pharmacological and device-based treatments provides a future paradigm for providing the patient with both sleep apnea and cardiovascular disease individually optimized treatment.
Creating Partnerships and Cross Fertilization
There is increasing interest in developing integrated models of care among specialists and between specialists and primary care practitioners. There are fertile opportunities for Sleep Medicine and Cardiology to develop co-management practices given the high co-aggregation of sleep disordered breathing and cardiovascular disease and the potential for bi-directional associations. Similar synergies are possible in advancing a research agenda to generate better evidence to guide treatment of sleep disordered breathing. The practice of Cardiovascular Medicine has benefited from large, rigorous multi-national randomized controlled trials that evaluated a wide range of behavioral, pharmacological and device-based interventions. With a growing set of available interventions for treating sleep apnea, the Sleep Medicine field is poised to partner with the Cardiology community.
Supplementary Material
Acknowledgments
Funding Sources:
Dr. Drager is supported by a research fellowship grant (2012/02953-2) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Dr Lorenzi-Filho is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Dr. Redline is supported by a research grant from National Institutes of Health (1R35HL135818).
Footnotes
Disclosure Statement:
Dr. Drager reports receiving equipment from Philips Respironics for performing investigator initiated studies.
Dr. McEvoy reports receiving research grants from Philips Respironics, Fisher & Paykel and the National Health and Medical Research Council (NHMRC) of Australia; research equipment grants from ResMed, Somnomed and Airliquide; and speakers fees for Philips Respironics.
Dr. Ferran reports receiving research grants from ResMed and from the Instituto de Salud Carlos III to study the impact of OSA in patients with resistant hypertension.
Dr. Lorenzi-Filho reports no conflicts.
Dr. Redline reports receiving research grant support from Jazz Pharma and Beckman Coulter.
References
- 1.Pelayo R, Dement WC. History of sleep physiology and medicine. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 6. 2017. pp. 3–14. [Google Scholar]
- 2.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 3.Drager LF, Polotsky VY, O’Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309:H1101–H1111. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 8.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. 2011;124:2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 10.Somers VK. Sleep--a new cardiovascular frontier. N Engl J Med. 2005;353:2070–2073. doi: 10.1056/NEJMe058229. Erratum in: N Engl J Med, 2005, 353: 2523. [DOI] [PubMed] [Google Scholar]
- 11.Redline S, Foody J. Sleep disturbances: time to join the top10 potentially modifiable cardiovascular risk factors? Circulation. 2011;124:2049–2051. doi: 10.1161/CIRCULATIONAHA.111.062190. [DOI] [PubMed] [Google Scholar]
- 12.Jonas DE, Amick HR, Feltner C, Weber RP, Arvanitis M, Stine A, Lux L, Harris RP. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:415–433. doi: 10.1001/jama.2016.19635. Erratum in: JAMA, 2017, 317: 1278. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA. 2017;318:156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Benseñor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–264. doi: 10.1136/thoraxjnl-2014-205361. [DOI] [PubMed] [Google Scholar]
- 15.Chirinos JA, Gurubhagavatula I, Teff K, Rader DJ, Wadden TA, Townsend R, Foster GD, Maislin G, Saif H, Broderick P, Chittams J, Hanlon AL, Pack AI. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med. 2014;370:2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Cao Q, Guo Z, Dai Q. Continuous Positive Airway Pressure in Patients With Obstructive Sleep Apnea and Resistant Hypertension: A Meta-Analysis of Randomized Controlled Trials. J Clin Hypertens (Greenwich) 2016;18:153–158. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatureto-Borges F, Lorenzi-Filho G, Drager LF. Effectiveness of continuous positive airway pressure in lowering blood pressure in patients with obstructive sleep apnea: a critical review of the literature. Integr Blood Press Control. 2016;9:43–47. doi: 10.2147/IBPC.S70402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology. 2015;20:889–895. doi: 10.1111/resp.12573. [DOI] [PubMed] [Google Scholar]
- 20.Vlachantoni IT, Dikaiakou E, Antonopoulos CN, Stefanadis C, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep Med Rev. 2013;17:19–28. doi: 10.1016/j.smrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yu W, Gao M, Zhang F, Gu C, Yu Y, Wei Y. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.002454. pii: e002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drager LF, Pedrosa RP, Diniz PM, Diegues-Silva L, Marcondes B, Couto RB, Giorgi DM, Krieger EM, Lorenzi-Filho G. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 25.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, Martínez-Alonso M, Carmona C, Barceló A, Chiner E, Masa JF, Gonzalez M, Marín JM, Garcia-Rio F, Diaz de Atauri J, Terán J, Mayos M, de la Peña M, Monasterio C, del Campo F, Montserrat JM Spanish Sleep And Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 26.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, Loo G, Koo CY, Gao XF, Chandra S, Yang XX, Furlan SF, Ge Z, Mundhekar A, Zhang WW, Uchôa CH, Kharwar RB, Chan PF, Chen SL, Chan MY, Richards AM, Tan HC, Ong TH, Roldan G, Tai BC, Drager LF, Zhang JJ. Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation. 2016;133:2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 28.Uchôa CH, de Danzi-Soares NJ, Nunes FS, de Souza AA, Nerbass FB, Pedrosa RP, César LA, Lorenzi-Filho G, Drager LF. Impact of OSA on cardiovascular events after coronary artery bypass surgery. Chest. 2015;147:1352–1360. doi: 10.1378/chest.14-2152. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, Ruttanaumpawan P, Tomlinson G, Bradley TD. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 30.Costa LE, Uchôa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101:1288–1292. doi: 10.1136/heartjnl-2014-307276. [DOI] [PubMed] [Google Scholar]
- 31.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS SAVE Investigators and Coordinators. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 34.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, Peppard PE. Obstructive sleep apnea during REM sleep and hypertension. Results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appleton S, Vakulin A, Martin S, Lang C, Wittert G, Taylor A, McEvoy RD, Antic N, Catcheside P, Adams R. Hypertension is associated with undiagnosed obstructive sleep apnea during rapid eye movement (REM) sleep. Chest. 2016;150:495–505. doi: 10.1016/j.chest.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Despres J-P. Body Fat Distribution and Risk of Cardiovascular Disease - An Update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 38.Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kritikou I, Basta M, Tappouni R, Pejovic S, Fernandez-Mendoza J, Nazir R, Shaffer ML, Liao D, Bixler EO, Chrousos GP, Vgontzas AN. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41:601–609. doi: 10.1183/09031936.00183411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA. 2015;314:2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- 41.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 42.Naughton MT, Kee K. Sleep apnoea in heart failure: To treat or not to treat? Respirology. 2017;22:217–229. doi: 10.1111/resp.12964. [DOI] [PubMed] [Google Scholar]
- 43.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 44.Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335:562–567. doi: 10.1056/NEJM199608223350805. [DOI] [PubMed] [Google Scholar]
- 45.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 46.Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675–1680. doi: 10.1016/j.amjcard.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 47.Bordier P. Sleep apnoea in patients with heart failure: part II: therapy. Arch Cardiovasc Dis. 2009;102:711–720. doi: 10.1016/j.acvd.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 48.White LH, Bradley TD. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591:1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billings ME, Johnson DA, Simonelli G, Moore K, Patel SR, Diez Roux AV, Redline S. Neighborhood Walking Environment and Activity Level Are Associated With OSA: The Multi-Ethnic Study of Atherosclerosis. Chest. 2016;150:1042–1049. doi: 10.1016/j.chest.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradley TD, Logan AG, Kimoff RJ, Sériès F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 51.Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115:3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 52.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 53.Javaheri S, Brown LK, Randerath WJ. Positive airway pressure therapy with adaptive servoventilation: part 1: operational algorithms. Chest. 2014;146:514–523. doi: 10.1378/chest.13-1776. [DOI] [PubMed] [Google Scholar]
- 54.Arzt M, Wensel R, Montalvan S, Schichtl T, Schroll S, Budweiser S, Blumberg FC, Riegger GA, Pfeifer M. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–66. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 55.D’Elia E, Vanoli E, La Rovere MT, Fanfulla F, Maggioni A, Casali V, Damiano S, Specchia G, Mortara A. Adaptive servo ventilation reduces central sleep apnea in chronic heart failure patients: beneficial effects on autonomic modulation of heart rate. J Cardiovasc Med (Hagerstown) 2013;14:296–300. doi: 10.2459/JCM.0b013e32835364b2. [DOI] [PubMed] [Google Scholar]
- 56.Oldenburg O, Schmidt A, Lamp B, Bitter T, Muntean BG, Langer C, Horstkotte D. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–586. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Hastings PC, Vazir A, Meadows GE, Dayer M, Poole-Wilson PA, McIntyre HF, Morrell MJ, Cowie MR, Simonds AK. Adaptive servo-ventilation in heart failure patients with sleep apnea: a real world study. Int J Cardiol. 2010;139:17–24. doi: 10.1016/j.ijcard.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Cowie MR, Woehrle H, Wegscheider K, Angermann C, d’Ortho MP, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Javaheri S, Brown LK, Randerath W, Khayat R. SERVE-HF: More Questions Than Answers. Chest. 2016;149:900–904. doi: 10.1016/j.chest.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Bradley TD, Floras JS ADVENT-HF Investigators. The SERVE-HF Trial. Can Respir J. 2015;22:313. doi: 10.1155/2015/751615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C, Ford ES, Mokdad AH, Balluz LS, Brown DW, Giles WH. Clustering of cardiovascular disease risk factors and health-related quality of life among US adults. Value Health. 2008;11:689–699. doi: 10.1111/j.1524-4733.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 62.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2:477–491. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 63.McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, Nunn AJ, Stradling JR, Riha RL, Morrell MJ PREDICT Investigators. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804–812. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 64.Campos-Rodriguez F, Queipo-Corona C, Carmona-Bernal C, Jurado-Gamez B, Cordero-Guevara J, Reyes-Nuñez N, Troncoso-Acevedo F, Abad-Fernandez A, Teran-Santos J, Caballero-Rodriguez J, Martin-Romero M, Encabo-Motiño A, Sacristan-Bou L, Navarro-Esteva J, Somoza-Gonzalez M, Masa JF, Sanchez-Quiroga MA, Jara-Chinarro B, Orosa-Bertol B, Martinez-Garcia MA Spanish SleepNetwork. Continuous Positive Airway Pressure Improves Quality of Life in Women with Obstructive Sleep Apnea. A Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:1286–1294. doi: 10.1164/rccm.201602-0265OC. [DOI] [PubMed] [Google Scholar]
- 65.Engleman HM, Cheshire KE, Deary IJ, Douglas NJ. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax. 1993;48:911–914. doi: 10.1136/thx.48.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao YY, Wang R, Gleason KJ, Lewis EF, Quan SF, Toth CM, Morrical M, Rueschman M, Weng J, Ware JH, Mittleman MA, Redline S BestAIR Investigators. Effect of Continuous Positive Airway Pressure Treatment on Health-Related Quality of Life and Sleepiness in High Cardiovascular Risk Individuals With Sleep Apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep. 2017:40. doi: 10.1093/sleep/zsx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis EF, Wang R, Punjabi N, Gottlieb DJ, Quan SF, Bhatt DL, Patel SR, Mehra R, Blumenthal RS, Weng J, Rueschman M, Redline S. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59–67. doi: 10.1016/j.ahj.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esquinas C, Sánchez-de-la Torre M, Aldomá A, Florés M, Martínez M, Barceló A, Barbé F Spanish Sleep Network. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: the ISAACC Trial. Clin Cardiol. 2013;36:495–501. doi: 10.1002/clc.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyons OD, Floras JS, Logan AG, Beanlands R, Cantolla JD, Fitzpatrick M, Fleetham J, John Kimoff R, Leung RS, Lorenzi Filho G, Mayer P, Mielniczuk L, Morrison DL, Ryan CM, Series F, Tomlinson GA, Woo A, Arzt M, Parthasarathy S, Redolfi S, Kasai T, Parati G, Delgado DH, Bradley TD ADVENT-HF Investigators. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19:579–587. doi: 10.1002/ejhf.790. [DOI] [PubMed] [Google Scholar]
- 70.Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, Gleason KJ, Rueschman M, Dorsey C, Patel SR, Ware JH, Mittleman MA, Redline S. Motivational Enhancement for Increasing Adherence to CPAP: A Randomized Controlled Trial. Chest. 2016;150:337–345. doi: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strollo PJ, Jr, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, Hanson RD, Padhya TA, Steward DL, Gillespie MB, Woodson BT, Van de Heyning PH, Goetting MG, Vanderveken OM, Feldman N, Knaack L, Strohl KP STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 73.Costanzo MR, Ponikowski P, Javaheri S, Augostini R, Goldberg L, Holcomb R, Kao A, Khayat RN, Oldenburg O, Stellbrink C, Abraham WT remedé System Pivotal Trial Study Group. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388:974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.