Abstract
The opioid agonists endomorphins (Tyr–Pro–Trp–Phe–NH2; EM1 and Tyr–Pro–Phe–Phe–NH2; EM2) and morphiceptin (Tyr–Pro–Phe–Pro–NH2) exhibit an extremely high selectivity for μ-opioid receptor. Here a series of novel EM2 and morphiceptin analogues containing in place of the proline at position 2 the S and R residues of β-homologues of proline (HPro), of 2-pyrrolidinemethanesulphonic acid (HPrs) and of 3-pyrrolidinesulphonic acid (βPrs) have been synthesized and their binding affinity and functional activity have been investigated. The highest μ-receptor affinity is shown by [(S)βPrs2]EM2 analogue (6e) which represents the first example of a β-sulphonamido analogue in the field of opioid peptides.
Keywords: Endomorphins, Opioid peptides, Peptide synthesis, β-Sulphonamido peptides, Unusual amino acids
1. Introduction
Due to their role in the modulation and perception of pain the opioid receptors continue to be an extremely important target in medicinal chemistry. Three major subtypes (μ, κ, δ) of this G-protein coupled family of receptors have been defined and it is well established that the μ group represents the major target of the analgesics. Although several endogenous peptide ligands of opioid receptors are known most of them, including enkephalins, does not show significant μ-selective agonistic activity. A notable exception is represented by morphiceptin, a tetrapeptide amide (Tyr–Pro–Phe–Pro–NH2) isolated from an enzymatic digest of bovine β-casein [1–3] and by the structurally related opioid ligands endomorphin-1 (EM1: Tyr–Pro–Trp–Phe–NH2) and endomorphin-2 (EM2: Tyr–Pro–Phe–Phe–NH2) which exhibit high μ-opioid receptor selectivity and agonist potency [4].
As it is well established [5,6] and clearly discussed by Goodman and Schiller [7], in the structure of natural opioid peptides two biological relevant fragments can be identified, namely the N-terminal message sequence, containing the two pharmacophoric aromatic residues of Tyr and Phe, and the remaining C-terminal fragment which represents the address sequence. EM2 and morphiceptin have a different C-terminal address sequence [3] which is –Phe–NH2 for EM2 and –Pro–NH2 for morphiceptin, but an identical Tyr–Pro–Phe– N-terminal message sequence. This feature, common to EMs and morphiceptin, is different from that found in enkephalins and other endogenous opioid peptides such as endorphins and dynorphins. These latter ligands possess the tetrapeptidic fragment Tyr–Gly–Gly–Phe– as a characteristic N-terminal message sequence. Thus, a topochemical characteristic which differentiates the N-terminal sequences present in the two classes of peptides is the type of the spacer group separating the Tyr and Phe aromatic residues. It thus can be inferred that different spacers, namely the Pro residue at position 2 in morphiceptin [8,9], and in EMs [10–12], as well as the Gly–Gly dipeptide fragment in enkephalins, fulfill the stereochemical requirements needed for a correct interaction of the N-terminal phaarmacophore aromatic side chains with the involved receptor area.
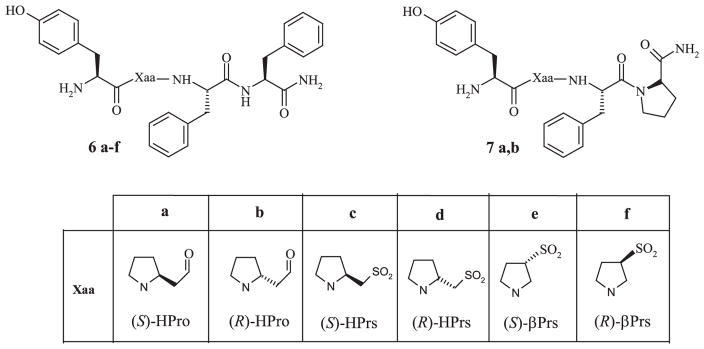
On the basis of the above considerations and in order to obtain additional information on the biochemical consequences of the structural modifications performed at the level of the Tyr1/Phe3 spacer residue we synthesized and biologically evaluated a new group of analogues of EM2 and morphiceptin reported in Fig. 1.
Fig. 1.
Schematic representation of the reported EM2 (6a–f) and morphiceptin (7a,b) analogues.
In the first group of analogues (6a,b and 7a,b) the (S) and (R) β-homologues of proline, namely (S)-homoproline [(S)-HPro–OH] and (R)-homoproline [(R)-HPro–OH], respectively, replace the native Pro residue at position 2. In the remaining four EM2 analogues (6c,d and 6e,f) two different types of Pro β-homologues have been inserted as spacer groups and in both of them the –SO2–NH– sulphonamido group replaces the –CO–NH– peptide bond. This bioisosteric replacement, initially introduced in 1989 by Lucente and coworkers [13,14] and then extensively investigated [15–18], generates a metabolically stable junction associated with significant changes in backbone conformational properties, polarity and H-bonding capacity [19]. Although the potentiality of sulfonamidopeptides is now well established [20,21] and applied to different classes of bioactive peptides [22–24] and enzyme inhibitors [25], analogues of EMs containing the sulfonamide junction have not been described. The here reported compounds 6c and 6d contain the (R) and (S) residues of the 2-pyrrolidinemethanesulphonic acid [(S)-HPrs–OH and (R)-HPrs–OH] and are then β-sulphonamido analogues of 6a and 6b, respectively. To a second and new type of β-sulphonamido peptides belong the analogues 6e and 6f. These are characterized by the presence of the 3-pyrrolidinesulphonic acid [(S) or (R) βPrs–OH] residue. This cyclic β-amino sulfonic acid, synthesized for the first time for the present research, is the sulfonyl analogue of β-proline (3-pyrrolidinecarboxylic acid; β-Pro–OH), a well known β-amino carboxylic acid, previously incorporated into the EM1 molecule to give the highly active Tyr–(R)-β-Pro–Trp–Phe–NH2 tetrapeptide [26].
2. Chemistry
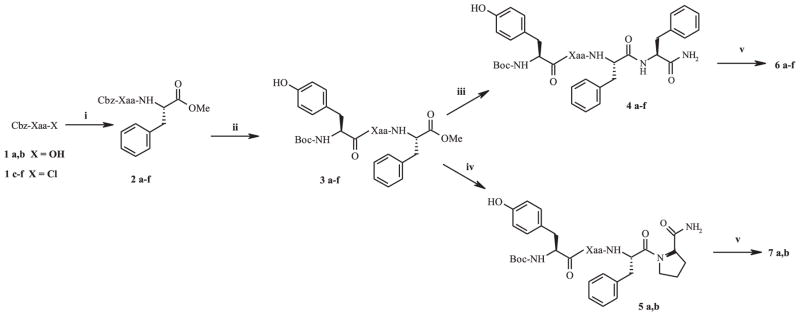
The synthesis of peptides 6a–f and 7a,b (Scheme 1) was performed in solution using the carbodiimide method for coupling steps.
Scheme 1.
Synthesis of pseudopeptides 6a–f and 7a,b. Reagents: (i) for 2a,b: Phe–OMe.HCl, EDC, HOBt, TEA, DCM; for 2c–f: Phe–OMe.HCl, TEA, DCM; (ii) a: H2, Pd/C, MeOH, TFA; b: Boc–Tyr–OH, EDC, HOBt, TEA, DCM; (iii) a: 1 M NaOH, MeOH; b: Phe-NH2HCl, EDC, HOBt, TEA, DCM; (iv) a: 1 M NaOH, MeOH; b: Pro-NH2HCl, EDC, HOBt, TEA, DCM; (v) TFA/H2O 95:5. Structures of Xaa residues are reported in Fig. 1.
Only in the case of the synthesis of the sulphonamido junction couplings were performed by using sulfonyl chlorides. The N-protected pseudoamino acids 1a–f were coupled with H–Phe–OMe.HCl and the resulting dipeptides 2a–f were subjected to catalytic hydrogenation. Successive coupling with Boc–Tyr–OH gave the tripeptides 3a–f which were O-deprotected by alkaline hydrolysis and successively coupled with H–Phe–NH2 or H–Pro–NH2 leading to the tetrapeptides 4a–f and 5a,b, respectively. Treatment with TFA 95% afforded the required final free peptides 6a–f and 7a,b as trifluoroacetate salts (final products were characterized by mass spectra analysis, see Table 1).
Table 1.
Sequence and mass spectra analysis of the reported analogues 6a–f and 7a,b.
| Peptide | Sequencea | M(H+) obsd (MW calcd) |
|---|---|---|
| 6a | H–Tyr–(S)-HPro–Phe–Phe–NH2 | 586.3792 (585.2951) |
| 6b | H–Tyr–(R)-HPro–Phe–Phe–NH2 | 586.2837 (585.2951) |
| 6c | H–Tyr–(S)-HPrs–Phe–Phe–NH2 | 622.3729 (621.2621) |
| 6d | H–Tyr–(R)-HPrs–Phe–Phe–NH2 | 622.4567 (621.2621) |
| 6e | H–Tyr–(S)-βPrs–Phe–Phe–NH2 | 608.5681 (607.7216) |
| 6f | H–Tyr–(R)-βPrs–Phe–Phe–NH2 | 608.6743 (607.7216) |
| 7a | H–Tyr–(S)-HPro–Phe–Pro–NH2 | 536.4761 (535.2795) |
| 7b | H–Tyr–(R)-HPro–Phe–Pro–NH2 | 536.3954 (535.2795) |
Modification are shown in bold letters.
The N-Cbz (S) and (R) homoprolines 1a,b, necessary for the synthesis of peptides 6a,b and 7a,b, were prepared according to literature [27] through the diazomethyl ketone route, starting from the appropriate N-Cbz derivative.
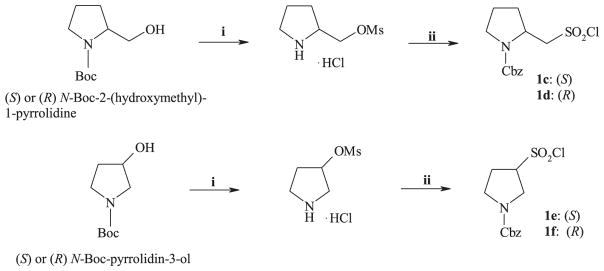
The (S) and (R) pyrrolidinesulfonyl chlorides 1c–f, required for the preparation of peptides 6c–f, were synthesized starting from the corresponding amino alcohol according to Scheme 2 [28–30].
Scheme 2.
Synthesis of sulfonyl chlorides 1c–f. Reagents: i) a: MsCl, TEA, DCM; b: HCl, dioxane; ii) a: Na2SO3, H2O; b: Cbz–Cl, aq. NaOH; c: COCl2/toluene, DMF, DCM.
3. Biological evaluation and conclusions
The binding affinities for opioid receptors and functional bioactivities, exhibited by the EM2 and the morphiceptin analogues 6a–f and 7a,b are summarized in Table 2.
Table 2.
Binding affinity and in vitro activity for compounds 6 a–f and 7 a,b.
| Compound | Receptor affinitya,b (nM)
|
Selectivity
|
Bioassayb (nM)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
MVD (IC50) | GPI (IC50) | |||||
| EM2c | 8360 ± 1314 | 8.23 ± 0.48 | – | 1016 | 510 ± 35d | 15 ± 2d | ||||
| Morphyceptinc | > 10000 | 135 ± 18.4 | – | > 74.1 | – | – | ||||
| 6a | 4900 ± 540 | 58 ± 5 | 990 ± 12 | 85 | 1300 ± 300 | 260 ± 32 | ||||
| 6b | > 10000 | 293 ± 27 | 10000 ± 1200 | > 34 | 1900 ± 230 | 420 ± 91 | ||||
| 6c | 8600 ± 940 | 100 ± 8 | 4800 ± 367 | 86 | 1100 ± 260 | 730 ± 64 | ||||
| 6d | 7800 ± 850 | 510 ± 48 | 6300 ± 540 | 15.3 | 36% at 1 μM | 2700 ± 220 | ||||
| 6e | > 10000 | 19 ± 3 | 3400 ± 330 | > 532.9 | 530 ± 91 | 260 ± 56 | ||||
| 6f | 7100 ± 782 | 63 ± 5 | 7100 ± 628 | 113.1 | 390 ± 56 | 220 ± 41 | ||||
| 7a | > 10000 | 6100 ± 553 | 4400 ± 356 | > 6 | 33.7% at 1 μM | 1100 ± 115 | ||||
| 7b | > 10000 | 30 ± 4 | 10000 ± 982 | > 333 | 96 ± 12 | 17 ± 3 | ||||
All compounds show very low k- and δ-opioid receptor affinities (micromolar range) and high δ/μ selectivity. Results concerning the four EM2 derivatives containing β-homologues of proline, both of β-carboxylic and β-sulfonic type (namely 6a,b and 6c,d respectively), confirm the strong influence of the stereochemistry at position 2 on the binding affinity. In this group of four analogues only the [(S) HPro2]-EM2 (6a), containing the (S)-β-homoproline residue, maintains good μ-opioid receptor affinity ( ) whereas the corresponding [(R)HPro2]-EM2 (6b) is sensibly less potent ( ). The same trend is found in the case of the two derivatives containing the β-homo-sulfonyl residue HPrs where the [(R)HPrs2]-EM2 (6d) is 5-fold less active then [(S)HPrs2]-EM2 (6c). These results are in agreement with those previously reported for β-amino carboxylic acid containing EM1 analogues [33] where the [(S) HPro2]-EM1 has been found to be about 33-fold more potent than the [(R)HPro2]-EM1. Although with an opposite trend, a high influence on the affinity is also shown by the stereochemistry at position 2 of the two morphiceptin analogues 7a and 7b containing enantiomeric β-homologues of proline. Here it has been found that the values for [(S)HPro2]-morphiceptin (7a) and [(R)HPro2]-morphiceptin (7b) are and , respectively. Thus, when these data are compared to those shown by the above cited affinities of 6a and 6b, it can be seen that the absolute configuration of the HPro residue at position 2 exerts an opposite effect on binding of the morphiceptin and EM2 analogues and leads to the [(R)HPro2]-morphiceptin (7b) endowed with the highest potency in the GPI assays (IC50 =17) as well as high δ/μ binding selectivity. This result is not in disagreement with previous studies which highlight the role of the spacer residue structure at position 2 in giving correct spatial orientation to the aromatic residues of the ligand [6,34] and confirms the observation that each class of opioid peptides shows distinct chiral requirements for the spacers between the biologically important Tyr and Phe residues [7].
In addition to the analogues 6a–d and 7a,b containing the β-homoproline residues HPro and HPrs, the EM2 analogues 6e,f contain at position 2 the enantiomers of the β-proline sulfonyl residue βPrs. These are characterized by relevant structural differences as compared with the β-homoproline (HPro and HPrs) containing residues 6a–d and 7a,b. In fact, in addition to the presence of the SO2–NH junction, replacing the usual CO–NH peptide bond, in the βPrs residues the acylating group is directly bound to a carbon atom of the 5-membered ring. Conversely, in 6a–d and 7a,b a –CH2– bridge is inserted between the pyrrolidine ring and the acylating group. This feature may greatly change the conformational preferences of the entire molecule, leading to systems with higher flexibility and conformational freedom. As shown in Table 2 the μ-opioid receptor affinity ( ) of the tetrapeptide amide 6e, containing the (S)-βPrs sulfonyl residue, is the highest among the here studied ligands. The epimeric analogue [(R)-βPrs2]-EM2 (6f), in which the stereochemistry of the residue at position 2 has been changed, shows a sensible decrease of the binding affinity ( ). Thus, as previously found for the EM1 analogues containing the βPro residue (i.e. the carboxylic analogue of βPrs) [26], the absolute configuration at position 2 highly influences the binding. However, an opposite spatial orientation of the acylating group at position 2 characterizes the most active analogues in the two cases. In fact, when the β-carboxylic residue is involved, the [(R)-βPro2]-EM1 [26] was found to be more active than the corresponding [(S)-βPrs2]-EM1 ( versus ) [26].
Table 2 summarizes, in addition to the affinity data, the functional activity on μ- and δ-opioid receptors of the here studied analogues. The highest potency as μ-agonist is that of the analogue 7b which, in the GPI functional assay, shows a value (IC50 = 17) practical equal to that of the EM2 (IC50 = 15) although with a sensibly lower selectivity as indicated by the MVD/GPI IC50 ratio which is 34 versus 5.7, respectively. Notable is in this case the strong influence of the absolute configuration of the residue at position 2 as shown by the binding and activity values (see Table 2) found for 7b, the epimeric analogue of 7a. Although to a lower degree, a strong effect of the chirality at position 2 is here observed in the case of 6a and 6b and was previously reported for the couple of EM1 analogues containing (R) and (S) HPro residues [33]. The two groups of peptides 6c,d and 6e,f contain a sulphonamido junction replacing the native Pro2-Phe3 peptide bond. In this case, structurally characterized by a CH2 group inserted between the SO2–NH bond and the pyrrolidine ring, a clear μ-receptor preference is shown by the analogue 6c, possessing the (S)βPrs residue at position 2 (IC50, GPI = 730 and 2700 nM, for 6c and 6d, respectively). This behavior is analogous to that exhibited by 6a and 6b containing the (S)HPro and (R)HPro residue, respectively. In the models 6e and 6f the SO2–NH junction is directly bound to the five-membered ring and this strongly limits the backbone flexibility as compared with 6c and 6d. In this case both the epimers [(S)βPrs2] EM2 6e and [(R)βPrs2]EM2 6f show unexpectedly low GPI and MVD potencies while it is still retained significant receptor affinity for both μ- and δ-opioid receptor types. This suggests that this couple of analogues may have a mixed agonist/antagonist property for both receptor types.
To the best of our knowledge the here reported βPrs containing tetrapeptides 6e,f are the first examples of analogues containing a β-sulphonamido replacement in the field of opioid peptides. The high μ-receptor affinity shown by the tetrapeptide [(S)-βPrs2]EM2 (6e) suggests further study of this type of derivatives and underlines, at the same time, the highly different interactions which β-sulphonamido pseudopeptides, as compared with the β-carboxyamido counterparts, may establish with the receptors.
4. Experimental protocols
4.1. General
IR spectra were recorded in 1% CHCl3 solution employing a Perkin-Elmer FT-IR Spectrum 1000 spectrophotometer. [α]D was measured at 20 °C with a Schmidt–Haensch polarimeter at a 1% concentration in CHCl3 (unless otherwise specified) with a 1 cm cell. 1H NMR spectra were determined in CDCl3 solution with a Bruker AM 400 spectrometer and chemical shifts were indirectly referred to TMS. The mass spectra were performed on a Q-TOFMICRO spectrometer (Micromass, now Waters, Manchester, UK) equipped with an ESI source, in the positive ion mode and data were analyzed using the MassLynx software (Waters). Thin-layer and preparative layer chromatographies were performed on silica gel Merck 60 F254 plates. The drying agent was sodium sulfate. Elemental analyses for C, H and N (where necessary a sample was further purified by preparative TLC) were performed in the laboratories of the Servizio Microanalisi del CNR, Area della Ricerca di Roma, Montelibretti, Italy, and were within 0.4% of the theoretical values.
4.1.1. Preparation of sulfonic acids. General procedure
An HCl saturated solution of the Boc-mesylates (1.00 mmol) in dioxane (30 mL) was allowed to stand at room temperature for 30 min. The precipitate was filtered and crystallized from ethanol-ethyl ether. A solution of the recovered precipitate and Na2SO3 (2.6 mmol) in water (5 mL) was stirred for 24 h at room temperature, then passed firstly on Amberlite IR-120 (H+ form) and then on Dowex 11 (acetate form) columns. Evaporation of the eluate under vacuum gave a crude residue which was crystallized from water–ethanol.
4.1.1.1. (S)-3-pyrrolidinesulfonic acid
From N-Boc-(S)-3-pyrrolidinemethansulfonate [30] (4.90 g, 18.48 mmol). White solid (78%). 1H NMR (D2O) δ: 2.12 (m, 2H, Pyr γCH2), 3.06–3.28 (two m, 3H, Pyr βCH and δCH2), 4.02 (m, 2H, Pyr αCH2). Anal. Calcd for C4H9NO3S: C 31.78, H 6.00, N 9.26; found C 31.85, H 6.23, N 9.21.
4.1.1.2. (R)-3-pyrrolidinesulfonic acid
From N-Boc-(R)-3-pyrrolidinemethansulfonate [30] (3.83 g, 14.43 mmol). White solid (86%). 1H NMR (D2O) δ: 2.12 (m, 2H, Pyr γCH2), 3.06–3.28 (two m, 3H, Pyr βCH and δCH2), 4.02 (m, 2H, Pyr αCH2). Anal. Calcd for C4H9NO3S: C 31.78, H 6.00, N 9.26; found C 31.91, H 6.20, N 9.33.
4.1.2. Preparation of sulfonyl chlorides. General procedure
To a solution of the appropriate sulfonic acid (1.00 mmol) in H2O (10 mL), adjusted to pH 8.5 with NaOH, Cbz-chloride (1.1 mmol) was added in five portions at room temperature and under vigorous stirring, maintaining the pH at 8–8.5 by small amounts of 1 N NaOH. After additional 2 h from the last addition, water was added (10 mL) and the aqueous phase was washed with Et2O (2 × 10 mL), evaporated under reduced pressure and coevaporated with toluene. The crude sodium sulfonate salt, white solid, was dried overnight under high vacuum on P2O5. To a suspension of the dried residue in DCM (30 mL) a solution of phosgene in toluene (20% p/p, 4.0 mL) and DMF (0.6 mL) in DCM (30 mL) was added under N2. If the reaction was not complete (TLC) after 1 h, an additional amount (1–2 mL) of the latter solution was added. After stirring for 2 h at room temperature, concentration of the mixture and purification on silica gel column (CH2Cl2) gave the products which were stored under argon and used as such.
4.1.2.1. Cbz–(R)-2-pyrrolidinemethanesulfonyl chloride [Cbz–(R)-HPrs-Cl] (1d)
From (R)-2-pyrrolidinemethanesulfonic acid [29] (2.05 g, 12.42 mmol). Colourless oil (56%). IR ν: 3020, 2957, 2873, 1701, 1414 cm−1; 1H NMR δ: 1.81–2.34 (m, 4H, Pyr β and γCH2),3.16 (m,2H, CH2SO2), 3.25 (m,1H, Pyr αCH), 3.96–4.25 (m, 2H, Pyr δCH2), 5.21 (s, 2H, Cbz CH2), 7.21–7.53 (m, 5H, Ar). Anal. Calcd for C13H16ClNO4S: C 49.13, H 5.07, N 4.41; found C 49.01, H 5.18, N 4.56.
4.1.2.2. Cbz-(S)-3-pyrrolidinesulfonyl chloride [Cbz–(S)-βPrs-Cl] (1e)
From (S)-3-pyrrolidinesulfonic acid (1.93 g, 12.77 mmol). Colourless oil (62%). IR ν: 3018, 2955, 2871, 1769, 1414 cm−1; 1H NMR δ: 1.96–2.43 (m, 4H, Pyr γ and δCH2), 3.02 (m, 1H, Pyr βCH), 3.4 (m, 2H, Pyr αCH2), 5.19 (s, 2H, Cbz CH2), 7.20–7.54 (m, 5H, Ar). Anal. Calcd for C12H14ClNO4S: C 47.45, H 4.65, N 4.61; found C 47.38, H 4.72, N 4.57.
4.1.2.3. Cbz-(R)-3-pyrrolidinesulfonyl chloride [Cbz–(R)-βPrs-Cl] (1f)
From (R)-3-pyrrolidinesulfonic acid (1.45 g, 9.60 mmol). Colourless oil (48%). IR ν: 3018, 2955, 2871,1769, 1414 cm−1; 1H NMR δ: 1.96–2.43 (m, 4H, Pyr γ and δCH2), 3.02 (m, 1H, Pyr βCH), 3.4 (m, 2H, Pyr αCH2), 5.19 (s, 2H, Cbz CH2), 7.20–7.54 (m, 5H, Ar). Anal. Calcd for C12H14ClNO4S: C 47.45, H 4.65, N 4.61; found C 47.29, H 4.86, N 4.45.
4.1.3. Preparation of peptidosulfonamides. General procedure
To an ice-cooled mixture containing the appropriate Cbz–sulfonyl chloride (1.0 mmol) and Phe–OMe.HCl (2.0 mmol) in anhydrous DCM (10 mL) a solution of TEA (2.0 mmol) in DCM (4.0 mL) was added dropwise under stirring. The reaction was stirred overnight at room temperature, then was diluted with DCM (25 mL) and consecutively washed with 1 N HCl (2 × 20 mL), sat. NaHCO3 (2 × 20 mL) and brine (20 mL). The organic phase was dried and evaporated. The crude products were purified on silica gel flash chromatography (CHCl3) and were obtained as pale yellow oil which solidified on standing.
4.1.3.1. Cbz–(R)-HPrs–Phe–OMe (2d)
From 1d (1.750 g, 5.50 mmol). Pale yellow solid (48%); [α]D + 7° (1, H2O); IR ν: 3430, 3008, 1747, 1668, 1430 cm−1; 1H NMR δ: 1.87–2.40 (m, 4H, HPrs β and γCH2), 2.94 and 3.12 (dd, 2H, A and B of an ABX, J = 7.9, 5.5 and 13.5 Hz, Phe βCH2), 3.26 (m, 2H, HPrs δCH2), 3.54 (m, 2H, CH2SO2), 3.76 (s, 3H, OCH3), 4.18 (m,1H, HPrs αCH), 4.22 (m,1H, Phe αCH), 5.21 (s, 2H, Cbz CH2), 5.84 (br d, 1H, Phe NH), 7.04–7.12 (m, 10H, Ar). Anal. Calcd for C23H28N2O6S: C 59.98, H 6.13, N 6.08; found C 60.12, H 6.27, N 5.96.
4.1.3.2. Cbz–(S)-βPrs–Phe–OMe (2e)
From 1e (0.827 g, 2.72 mmol). Pale yellow solid (46%); [α]D − 3° (1, H2O); IR ν:3689, 3012, 1699, 1426 cm−1; 1H NMR δ: 2.02 (m, 2H, βPrs γCH2), 2.90–3.05 (m, 5H, Phe βCH2, βPrs βCH and δCH2), 3.80 (s, 3H, OCH3), 4.31 (br d, 2H, βPrs αCH2), 4.90–5.12 (m, 3H, Phe αCH and Cbz CH2), 5.10 (s, 1H, Phe NH), 7.01–7.23 (m, 10H, Ar). Anal. Calcd for C22H26N2O6S: C 59.18, H 5.87, N 6.27; found C 59.33, H 6.07, N 6.12.
4.1.3.3. Cbz–(R)-βPrs–Phe–OMe (2f)
From 1f (0.975 g, 3.21 mmol). Colourless oil (52%); [α]D + 2° (1, H2O); IR ν:3691, 3020, 1687, 1432 cm−1; 1H NMR δ: 1.98 (m, 2H, βPrs γCH2), 2.93–3.12 (m, 5H, Phe βCH2, βPrs βCH and δCH2), 3.87 (s, 3H, OCH3), 4.26 (br d, 2H, βPrs αCH2), 4.92–5.15 (m, 3H, Phe αCH and Cbz CH2), 5.11 (s,1H, Phe NH), 7.08–7.21 (m, 10H, Ar). Anal. Calcd for C22H26N2O6S: C 59.18, H 5.87, N 6.27; found C 59.14, H 6.02, N 6.19.
4.1.4. Carbodiimide coupling. General procedure
To an ice-cooled mixture containing the C-protected amino acid or peptide salt (1.0 mmol), the required N-protected amino acid (1.0 mmol), HOBt (1.2 mmol) and TEA (2.2 mmol) in anhydrous DCM (6.0 mL), EDC (1.2 mmol) was added and the reaction mixture was allowed to warm slowly to room temperature overnight. The mixture was then diluted with DCM (20 mL) and washed with 1 M KHSO4 (2 × 15 mL), saturated aqueous NaHCO3 (2 × 15 mL) and brine (15 mL). The organic phase was dried and evaporated under reduced pressure.
4.1.4.1. Cbz–(R)-HPro–Phe–OMe (2b)
From 1b [27] (0.650 g, 2.47 mmol) and H–Phe–OMe. HCl (0.532 g, 2.47 mmol). Pale yellow oil (81%); [α]D + 10°; IR ν:3689, 3425, 3029, 3009,1740,1676 cm−1; 1H NMR δ: 2.06–2.10 (m, 4H, HPro β and γCH2), 2.90–2.97 (dd, 2H, A and B of an ABX, J = 8.0, 5.5 and 13.5 Hz, Phe βCH2), 3.18 (m, 2H, CH2CO), 3.55 (m, 2H, HPro δCH2), 3.74 (s, 3H, OCH3), 4.13 (br, 1H, HPro αCH), 4.92 (m, 1H, Phe αCH), 5.10 (m, 2H, Cbz CH2), 5.89 (br, 1H, Phe NH), 6.98–7.57 (m, 10H, Ar). Anal. Calcd for C24H28N2O5: C 67.91, H 6.65, N, 6.60; found: C 67.82, H 6.58, N 6.49.
4.1.4.2. Boc–Tyr–(S)-HPro–Phe–OMe (3a)
Hydrogenolysis of 2a [28] (1.37 g, 3.23 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 92%) which was coupled with Boc–Tyr–OH (0.838 g, 2.98 mmol). Purified on SiO2 (DCM/EtOAc 1:1). White solid (63%); [α]D + 46°; IR ν:3689, 3432, 3032, 1743, 1670, 1629 cm−1; 1H NMR δ: 1.30 [s, 9H, C (CH3)3], 1.20–1.54 (m, 4H, HPro β and γCH2), 2.39–3.45 (four m, 8H, Tyr βCH2, Phe βCH2, HPro δCH2 and CH2CO), 3.73 (s, 3H, OCH3), 4.20 (br s, 1H, HPro αCH), 4.51 (m, 1H, Phe αCH), 4.93 (m, 1H, Tyr αCH), 5.23 (m, 1H, Tyr NH), 6.80–7.20 (m, 10H, Ar), 7.54 (s, 1H, Phe NH), 9.83 (m, 1H, OH). Anal. Calcd for C30H39N3O7: C 65.08, H 7.10, N 7.59; found: C 65.22, H 6.97, N 7.71.
4.1.4.3. Boc–Tyr–(R)-HPro–Phe–OMe (3b)
Hydrogenolysis of 2b (0.806 g, 1.90 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 88%) which was coupled with Boc–Tyr–OH (0.470 g, 1.67 mmol). Purified on SiO2 (DCM/EtOAc 1:1). Colourless oil (76%); [α]D + 2°; IR ν: 3639, 3429, 3012, 2835, 1742, 1697, 1631 cm−1; 1H NMR δ: 1.32 [s, 9H, C(CH3)3], 1.51–1.74 (m, 4H, HPro β and γCH2), 2.40–3.42 (four m, 8H, Tyr βCH2, Phe βCH2, HPro δCH2 and CH2CO), 3.70 (s, 3H, OCH3), 4.22 (br s, 1H, HPro αCH), 4.50 (br s, 1H, Phe αCH), 4.91 (m, 1H, Tyr αCH), 5.24 (m, 1H, Tyr NH), 6.81–7.23 (m, 10H, Ar), 7.52 (m, 1H, Phe NH), 9.80 (s, 1H, OH). Anal. Calcd for C30H39N3O7: C 65.08, H 7.10, N 7.59; found: C 65.14, H 7.02, N 7.67.
4.1.4.4. Boc–Tyr–(S)-HPrs–Phe–OMe (3c)
Hydrogenolysis of 2c [28] (0.161 g, 0.35 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 78%) which was coupled with Boc–Tyr–OH (0.075 g, 0.27 mmol). Purified on SiO2 (DCM/MeOH 99:1). Pale yellow solid (90%); [α]D − 5°; IR ν: 3433, 3027, 1704, 1671 cm−1; 1H NMR δ: 1.34 [s, 9H, C (CH3)3], 1.81 (m, 2H, HPrs γCH2), 2.10–2.81 (two m, 4H, Tyr βCH2 and HPrs βCH2), 2.84–3.33 (m, 6H, Phe βCH2, HPrs δCH2 and CH2SO2), 3.72 (s, 3H, OCH3), 4.27 (br d, 1H, HPrs αCH), 4.47 (m, 1H, Phe αCH), 4.78 (m, 1H, Tyr αCH), 5.45 (m, 1H, Tyr NH), 6.32 (m, 1H, Phe NH), 6.89–7.21 (m, 10H, Ar), 9.01 (s, 1H, OH). Anal. Calcd for C29H39N3O8S: C 59.07, H 6.67, N 7.13; found: C 59.25, H 6.58, N 7.01.
4.1.4.5. Boc–Tyr–(R)-HPrs–Phe–OMe (3d)
Hydrogenolysis of 2d (0.850 g, 1.84 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 90%) which was coupled with Boc–Tyr–OH (0.467 g, 1.66 mmol). Purified on SiO2 (DCM/MeOH 99:1). Pale yellow solid (70%); [α]D + 6°; IR ν: 3432, 3032, 3028, 1702, 1671 cm−1; 1H NMR δ: 1.34 [s, 9H, C (CH3)3], 1.83 (m, 2H, HPrs γCH2), 2.12–2.80 (two m, 4H, Tyr βCH2, HPrs βCH2), 2.85–3.37 (m, 6H, Phe βCH2, HPrs δCH2 and CH2SO2), 3.75 (s, 3H, OCH3), 4.29 (br s, 1H, HPrs αCH), 4.54 (m, 1H, Phe αCH), 4.80 (m, 1H, Tyr αCH), 5.48 (m, 1H, Tyr NH), 6.38 (m, 1H, Phe NH), 6.90–7.24 (m, 10H, Ar), 9.07 (s, 1H, OH). Anal. Calcd for C29H39N3O8S: C 59.07, H 6.67, N 7.13; found: C 59.13, H 6.42, N 6.98.
4.1.4.6. Boc–Tyr–(S)-βPrs–Phe–OMe (3e)
Hydrogenolysis of 2e (0.161 g, 0.36 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 85%) which was coupled with Boc–Tyr–OH (0.086 g, 0.31 mmol). Purified on SiO2 (DCM/MeOH 95:5). Colourless oil (80%); [α]D − 5°; IR ν:3689, 3432, 3032, 3011, 1744 cm−1; 1H NMR δ: 1.49 [s, 9H, C (CH3)3], 2.47 (m, 2H, βPrs γCH2), 2.70–3.51 (m, 6H, Phe βCH2, βPrs α and δCH2), 3.81 (s, 3H, OCH3), 4.31 (br d, 1H, βPrs βCH), 4.41–4.72 (m, 2H, Phe and Tyr αCH), 5.22 (s, 1H, Phe NH), 5.52 (m, 1H, Tyr NH), 7.02–7.24 (m, 10H, Ar), 9.01 (s, 1H, OH). Anal. Calcd for C28H37N3O8S: C 58.42, H 6.48, N 7.30; found: C 58.63, H 6.39, N 7.57.
4.1.4.7. Boc–Tyr–(R)-βPrs–Phe–OMe (3f)
Hydrogenolysis of 2f (0.270 g, 0.60 mmol) in MeOH on 10% Pd/C in the presence of TFA afforded the corresponding trifluoroacetate salt (white foam, 92%) which was coupled with Boc–Tyr–OH (0.156 g, 0.55 mmol). Purified on SiO2 (DCM/MeOH 95:5). Colourless oil (78%); [α]D − 5°; IR ν:3689, 3432, 3033, 3011, 1744 cm−1; 1H NMR δ: 1.53 [s, 9H, C (CH3)3], 2.49 (m, 2H, βPrs γCH2), 2.68–3.52 (m, 6H, Phe βCH2, βPrs α and δCH2), 3.79 (s, 3H, OCH3), 4.33 (br d, 1H, βPrs βCH), 4.40–4.68 (m, 2H, Phe and Tyr αCH), 5.20 (s, 1H, Phe NH), 5.53 (m, 1H, Tyr NH), 7.00–7.23 (m, 10H, Ar), 9.02 (s, 1H, OH). Anal. Calcd for C28H37N3O8S: C 58.42, H 6.48, N 7.30; found: C 58.59, H 6.72, N 7.47.
4.1.4.8. Boc–Tyr–(S)-HPro–Phe–Phe–NH2 (4a)
Alkaline hydrolysis of 3a (0.372 g, 0.69 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (white solid, 72%) which was coupled with H–Phe–NH2. HCl (0.100 g, 0.50 mmol). Triturated with hexane. White solid (64%); [α]D − 12°; IR ν:3684, 3295, 3029, 2975, 1643 cm−1; 1H NMR (d6-DMSO) δ: 1.37 [s, 9H, C(CH3)3], 1.75–1.80 (m, 4H, HPro β and γCH2), 2.61–2.95 (m, 8H, Tyr βCH2, two Phe βCH2 and CH2CO), 3.12 (br s, 2H, HPro δCH2), 3.73 (br s, 1H, HPro αCH), 3.91 (m, 2H, two Phe αCH), 4.28 (m, 1H, Tyr αCH), 6.71 (m, 1H, Tyr NH), 6.90–7.22 (m, 14H, Ar), 8.01–8.22 (m, 4H, NH2 and two Phe NH), 9.21 (m, 1H, OH). Anal. Calcd for C38H47N5O7: C 66.55, H 6.91, N 10.21; found: C 66.27, H 7.03, N 10.35.
4.1.4.9. Boc–Tyr–(R)-HPro–Phe–Phe–NH2 (4b)
Alkaline hydrolysis of 3b (0.448 g, 0.81 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (white solid, 87%) which was coupled with H–Phe–NH2. HCl (0.141 g, 0.70 mmol). Triturated with hexane. White solid (66%); [α]D − 9°; IR ν:3690, 3336, 3034, 2989, 1682 cm−1; 1H NMR (d6-DMSO) δ: 1.39 [s, 9H, C(CH3)3], 1.72–1.81 (m, 4H, HPro β and γCH2), 2.59–2.91 (m, 8H, Tyr βCH2, two Phe βCH2 and CH2CO), 3.14 (br s, 2H, HPro δCH2), 3.70 (br s, 1H, HPro αCH), 3.89 (m, 2H, two Phe αCH), 4.33 (m, 1H, Tyr αCH), 6.68 (m, 1H, Tyr NH), 6.92–7.29 (m, 14H, Ar), 8.09–8.25 (m, 4H, NH2 and two Phe NH), 9.26 (m, 1H, OH). Anal. Calcd for C38H47N5O7: C 66.55, H 6.91, N 10.21; found: C 66.33, H 6.76, N 10.29.
4.1.4.10. Boc–Tyr–(S)-HPrs–Phe–Phe–NH2 (4c)
Alkaline hydrolysis of 3c (0.271 g, 0.46 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (white solid, 84%) which was coupled with H–Phe–NH2. HCl (0.077 g, 0.38 mmol). Triturated with hexane. White solid (58%); [α]D − 36°; IR ν:3691, 3372, 3012, 2931, 1685 cm−1; 1H NMR (d6-DMSO) δ: 1.42 [s, 9H, C(CH3)3], 1.80–2.00 (m, 4H, HPrs β and γCH2), 2.70–3.49 (four m, 8H, Tyr βCH2, two Phe βCH2 and HPrs δCH2), 3.96 (m, 2H, CH2SO2), 3.91–4.32 (four m, 4H, two Phe, HPrs and Tyr αCH), 5.22 (br s, 1H, Tyr NH), 5.90–6.22 (m, 2H, two Phe NH), 6.72–7.64 (m, 14H, Ar), 9.79 (m, 1H, OH). Anal. Calcd for C37H47N5O8S: C 61.56, H 6.56, N 9.70; found: C 61.37, H 6.76, N 9.98.
4.1.4.11. Boc–Tyr–(R)-HPrs–Phe–Phe–NH2 (4d)
Alkaline hydrolysis of 3d (0.200 g, 0.34 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (white solid, 87%) which was coupled with H–Phe–NH.2 HCl (0.059 g, 0.29 mmol). Triturated with hexane. White solid (64%); [α]D − 38°; IR ν:3689, 3367, 3021, 2930, 1676 cm−1; 1H NMR (d6-DMSO) δ: 1.42 [s, 9H, C(CH3)3], 1.84–2.03 (m, 4H, HPrs β and γCH2), 2.67–3.45 (four m, 8H, Tyr βCH2, two Phe βCH2 and HPrs δCH2), 3.89 (m, 2H, CH2SO2), 3.89–4.28 (four m, 4H, two Phe, HPrs and Tyr αCH), 5.20 (br s, 1H, Tyr NH), 5.87–6.19 (m, 2H, two Phe NH), 6.78–7.59 (m, 14H, Ar), 9.84 (m, 1H, OH). Anal. Calcd for C37H47N5O8S: C 61.56, H 6.56, N 9.70; found: C 61.28, H 6.34, N 9.56.
4.1.4.12. Boc–Tyr–(S)-βPrs–Phe–Phe–NH2 (4e)
Alkaline hydrolysis of 3e (0.109 g, 0.19 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (pale yellow solid, 89%) which was coupled with H–Phe–NH2. HCl (0.034 g, 0.17 mmol). Triturated with hexane. White solid (66%); [α]D − 10°; IR ν:3689, 3032, 2337, 1683 cm−1; 1H NMR (d6-DMSO) δ: 1.36 [s, 9H, C(CH3)3], 2.20–2.79 (m, 8H, Tyr βCH2, two Phe βCH2 and βPrs γCH2), 3.10–3.29 (m, 4H, βPrs α and δCH2), 3.51–4.72 (four m, 4H, βPrs βCH, two Phe and Tyr αCH), 6.32–6.54 (m, 2H, Tyr NH and NHSO2), 6.73–7.68 (m, 14H, Ar), 7.51–8.00 (m, 3H, Phe NH and NH2), 8.90 (br m, 1H, OH). Anal. Calcd for C36H45N5O8S: C 61.09, H 6.41, N 9.89; found: C 61.17, H 6.29, N 9.48.
4.1.4.13. Boc–Tyr–(R)-βPrs–Phe–Phe–NH2 (4f)
Alkaline hydrolysis of 3f (0.196 g, 0.34 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (pale yellow solid, 89%) which was coupled with H–Phe–NH2. HCl (0.061 g, 0.30 mmol). Purified on SiO2 (DCM/MeOH 95:5). White foam (48%); [α]D − 10°; IR ν:3691, 3029, 2330, 1687 cm−1; 1H NMR δ: 1.34 [s, 9H, C(CH3)3], 2.23–2.84 (m, 8H, Tyr βCH2, two Phe βCH2 and βPrs γCH2), 3.12–3.33 (m, 4H, βPrs α and δCH2), 3.49–4.76 (four m, 4H, two Phe and Tyr αCH, βPrs βCH), 6.30–6.48 (m, 2H, Tyr NH and NHSO2), 6.70–7.66 (m, 14H, Ar), 7.48–7.95 (m, 3H, Phe NH and NH2), 8.92 (br m, 1H, OH). Anal. Calcd for C36H45N5O8S: C 61.09, H 6.41, N 9.89; found: C 61.25, H 6.18, N 9.67.
4.1.4.14. Boc–Tyr–(S)-HPro–Phe–Pro–NH2 (5a)
Alkaline hydrolysis of 3a (0.112 g, 0.19 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (pale yellow solid, 89%) which was coupled with H–Pro–NH.2 HCl (0.026 g, 0.17 mmol). Purified on SiO2 (DCM/MeOH 95:5). White solid (56%); [α]D − 12°; IR ν:3687,3295, 2975, 2345, 1643 cm−1; 1H NMR (d6-DMSO) δ: 1.32 [s, 9H, C(CH3)3], 1.52–2.00 (m, 4H, HPro and Pro γCH2), 2.70–3.02 (m, 6H, Tyr and Phe βCH2, CH2CO), 3.11 (m, 4H, HPro and Pro δCH2), 4.12–4.81 (four m, 4H, Phe, HPro, Pro and Tyr αCH), 6.63 (m, 1H, Tyr NH), 6.65–7.28 (m, 11H, Ar, Phe NH and Pro–NH2), 9.25 (br m, 1H, OH). Anal. Calcd for C34H45N5O7: C 64.23, H 7.13, N 11.02; found: C 64.17, H 6.98, N 11.23.
4.1.4.15. Boc–Tyr–(R)-HPro–Phe–Pro–NH2 (5b)
Alkaline hydrolysis of 3a (0.134 g, 0.26 mmol) in MeOH with aq. NaOH 2.0 N afforded the corresponding acid (pale yellow solid, 83%) which was coupled with H–Pro–NH.2 HCl (0.032 g, 0.21 mmol). Purified on SiO2 (DCM/MeOH 95:5). White solid (51%); [α]D − 9°; IR ν:3690, 3336, 2989, 2345, 1682 cm−1; 1H NMR (d6-DMSO) δ: 1.30 [s, 9H, C (CH3)3], 1.49–1.98 (m, 4H, HPro and Pro γCH2), 2.75–3.10 (m, 6H, Tyr and Phe βCH2, CH2CO), 3.09 (m, 4H, HPro and Pro δCH2), 4.10–4.75 (four m, 4H, Phe, HPro, Pro and Tyr αCH), 6.59 (m, 1H, Tyr NH), 6.73–7.32 (m, 11H, Ar, Phe NH and Pro–NH2), 9.28 (br m, 1H, OH). Anal. Calcd for C34H45N5O7: C 64.23, H 7.13, N 11.02; found: C 64.39, H 6.93, N 11.18.
4.1.5. Deprotection of Boc-peptides. General procedure
The Boc-protected peptides were dissolved in 95% trifluoroacetic acid (2.0 mL). After 2 h at room temperature, the solution was collected, the crude peptides were precipitated from the solution with peroxide-free dry diethyl ether at 0 °C and centrifuged. After several washing with ether, the precipitated peptides were dissolved in a solution of water with 1% TFA and then lyophilized to give compounds 6a–f and 7a,b.
4.2. In vitro assays
All radioligands were purchased from NEN (Boston, MA). Radioligands binding analysis was carried out using crude membrane preparations from HN9.10 cells that have been transfected with the human κ-, δ- or the μ-opioid receptor cDNA, and in each transfected cells line expressed constitutively a stable level of these receptors after clonal selection [35]. The membranes were resuspended in ice-cold Tris-buffer (50 mM, pH 7.4) containing 0.5% bovine serum albumin (BSA), and the following protease inhibitors: 30 μM bestatin, 10 μM captopril, 50 μg/mL bacitracin, 100 μM phenyl-methylsulfonyl-fluoride (PMSF). Radioligand competition analysis was carried out using membranes prepared from each of the cell lines that expressed either κ-, δ- or μ-opioid receptors. [3H]U69,593 (1.6 nM) was used to label the κ-opioid receptors, 1 nM [3H]DAMGO was used to label the μ-opioid receptors and 1 nM ([3H]DPDPE was used to label the δ-opioid receptors in the respective cell membranes preparations. For each competition assay, 10 concentrations (10−13 M to 10−4 M, in duplicate) of the examined substrate were each incubated with membranes (ranging between 15 and 25 μg) and radioligand in a total volume of 0.5 mL for 3 h at 25 °C in a shaking water bath and terminated by rapid filtration through Whatman GF/B filters (presoaked in polyethyleneimine) and washed with 2 × 4 mL of ice-cold 50 mM Tris. Non-specific binding of the radioligand was defined as the amount of radioactivity bound to the cell membranes in the presence of 10 μM naloxone. Radioactivity was determined by liquid scintillation counting. Data were fitted by non-linear least-squares analysis using GraphPad Prism. All analyses were based on 3 independent experiments [36]. The in vitro tissue bioassays (MVD and GPI/LMMP) were performed as described previously [37]. IC50 values represent means of no less than four experiments. IC50 values, relative potency estimates, and their associated standard errors were determined by fitting the data to the Hill equation by a computerized non-linear least-squares method.
Acknowledgments
This research was supported in part by grants from the U.S. Public Health Service, National Institutes of Health, DR 06784 and DA 13449 (VJH).
Abbreviations
- HPr–OH
homoproline (2-pyrrolidineacetic acid)
- HPrs-OH
2-pyrrolidinemethanesulphonic acid
- βPro-OH
β-proline (3-pyrrolidinecarboxylic acid)
- βPrs-OH
3-pyrrolidinesulphonic acid
- DMSO
dimethylsulfoxide
- Cbz
benzyloxycarbonyl
- Boc
tert-butyloxycarbonyl
- DCM
dichloromethane
- HOBt
hydroxybenzotriazole
- TEA
triethylamine
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
References
- 1.Chang KJ, Killian A, Hazum E, Cuatrecasas P, Chang JK. Science. 1981;212:75–77. doi: 10.1126/science.6259732. [DOI] [PubMed] [Google Scholar]
- 2.Chang KJ, Wei ET, Chang JK, Pharmacol J. Exp Ther. 1983;227:403–408. [PubMed] [Google Scholar]
- 3.Chang KJ, Su IF, Brent DA, Chang JK. J Biol Chem. 1985;260:9706–9712. [PubMed] [Google Scholar]
- 4.Zadina JE, Hackler L, Ge LJ, Kastin AJ. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 5.Schwyzer R, Ann NY. Acad Sci. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 6.Leitgeb B. Chem Biodiversity. 2007;4:2703–2724. doi: 10.1002/cbdv.200790221. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki T, Ro S, Goodman M, Chung NN, Schiller PW. J Med Chem. 1993;36:708–719. doi: 10.1021/jm00058a007. [DOI] [PubMed] [Google Scholar]
- 8.Grieco P, Giusti L, Carotenuto A, Campiglia P, Calderone V, Lama T, Gomez-Monterrey I, Tartaro G, Mazzoni MR, Novellino E. J Med Chem. 2005;48:3153–3163. doi: 10.1021/jm040867y. [DOI] [PubMed] [Google Scholar]
- 9.Keller M, Boissard C, Patiny L, Chung NN, Lemieux C, Mutter M, Schiller PW. J Med Chem. 2001;44:3896–3903. doi: 10.1021/jm000332e. [DOI] [PubMed] [Google Scholar]
- 10.Doi M, Asano A, Komura E, Ueda Y. Biochem Biophys Res Commun. 2002;297:138–142. doi: 10.1016/s0006-291x(02)02087-9. [DOI] [PubMed] [Google Scholar]
- 11.Perlikowska R, Katarzyna G, Fichna J, Toth G, Walkowiak B, do-Rego J-C, Janecka A. Bioorg Med Chem. 2009;17:3789–3794. doi: 10.1016/j.bmc.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Torino D, Mollica A, Pinnen F, Lucente G, Feliciani F, Davis P, Lai J, Ma SW, Porreca F, Hruby VJ. Bioorg Med Chem Lett. 2009;19:4115–4118. doi: 10.1016/j.bmcl.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcagni A, Gavuzzo E, Lucente G, Mazza F, Pochetti G, Rossi D. Int J Pept Protein Res. 1989;34:319–324. doi: 10.1111/j.1399-3011.1989.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 14.Calcagni A, Gavuzzo E, Lucente G, Mazza F, Pinnen F, Pochetti G, Rossi D. Int J Pept Protein Res. 1989;34:471–479. doi: 10.1111/j.1399-3011.1989.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 15.Moree WJ, van der Marel GA, Liskamp RMJ. Tetrahedron Lett. 1991;32:409–412. [Google Scholar]
- 16.de Bont DBA, Moree WJ, Liskamp RMJ. Bioorg Med Chem. 1996;4:667–672. doi: 10.1016/0968-0896(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 17.Gennari C, Salom B, Potenza D, Longari C, Fioravanti E, Carugo O, Sardone N. Chem Eur J. 1996;2:644–655. [Google Scholar]
- 18.Brouwer AJ, Liskamp RMJ. J Org Chem. 2004;69:3662–3668. doi: 10.1021/jo0358325. [DOI] [PubMed] [Google Scholar]
- 19.Gennari C, Gude M, Potenza D, Piarulli U. Chem Eur J. 1998;4:1924–1931. [Google Scholar]
- 20.Obreza A, Gobec S. Curr Med Chem. 2004;11:3263–3278. doi: 10.2174/0929867043363659. [DOI] [PubMed] [Google Scholar]
- 21.Papandrea G, Ponticelli F. Synth Commun. 2008;38:858–865. [Google Scholar]
- 22.de Bont DBA, Dijkstra GDH, den Hartog JAJ, Liskamp RMJ. Bioorg Med Chem Lett. 1996;6:3035–3040. [Google Scholar]
- 23.Wels B, Kruijtzer JAW, Garner KM, Adan RAH, Liskamp RMJ. Bioorg Med Chem. 2005;15:287–290. doi: 10.1016/j.bmcl.2004.10.082. [DOI] [PubMed] [Google Scholar]
- 24.Giordano C, Lucente G, Masi A, Paglialunga Paradisi M, Sansone A, Spisani S. Bioorg Med Chem. 2006;14:2642–2652. doi: 10.1016/j.bmc.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Humlijan J, Kotnik M, Boniface A, Solmajer T, Urleb U, Blanot D, Gobec S. Tetrahedron. 2006;62:10980–10988. [Google Scholar]
- 26.Cardillo G, Gentilucci L, Melchiorre P, Spampinato S. Bioorg Med Chem Lett. 2000;10:2755–2758. doi: 10.1016/s0960-894x(00)00562-x. [DOI] [PubMed] [Google Scholar]
- 27.Cassal JM, Furst A, Meier W. Helv Chim Acta. 1976;59:1917–1924. doi: 10.1002/hlca.19760590404. [DOI] [PubMed] [Google Scholar]
- 28.Giordano C, Masi A, Pizzini A, Sansone A, Consalvi V, Chiaraluce R, Lucente G. Eur J Med Chem. 2009;44:179–189. doi: 10.1016/j.ejmech.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Braghiroli D, Avallone R, Di Bella M. Tetrahedron: Asymmetry. 1997;8:2209–2213. [Google Scholar]
- 30.Benard C, Mohammad R, Saraswat N, Shan R, Maiti SN, Wuts PGM, Stier M, Lints T, Bradow J, Schwarz JB. Synth Commun. 2008;38:517–524. [Google Scholar]
- 31.Gao Y, Liu X, Liu W, Qi Y, Liu X, Zhou Y, Wang R. Bioorg Med Chem Lett. 2006;16:3688–3692. doi: 10.1016/j.bmcl.2006.04.063. [DOI] [PubMed] [Google Scholar]
- 32.Biondi B, Giannini E, Negri L, Melchiorri P, Lattanti R, Rosso F, Ciocca L, Rocchi R. Int J Pept Res Ther. 2006;12:145–151. [Google Scholar]
- 33.Cardillo G, Gentilucci L, Qasem AR, Sgarzi F, Spampanato S. J Med Chem. 2002;45:2571–2578. doi: 10.1021/jm011059z. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Q-y, Chen Q, Yang D-j, Feng Y, Long Y, Wang P, Wang R. Life Sci. 2005;77:1155–1165. doi: 10.1016/j.lfs.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Lai J, Ma SW, Zhu RH, Rothman RB, Lentes KU, Porreca F. NeuroReport. 1994;5:2161–2164. doi: 10.1097/00001756-199410270-00043. [DOI] [PubMed] [Google Scholar]
- 36.Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, Menzaghi F, Wisniewski K, Stalewski J, Sueiras-Diaz J, Galyean R, Schteingart C, Junien J, Trojnar J, Rivière PJM. Eur J Pharmacol. 2008;583:62–72. doi: 10.1016/j.ejphar.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Kramer TH, Davis P, Hruby VJ, Burks TF, Porreca F. J Pharmacol Exp Ther. 1993;266:577–584. [PubMed] [Google Scholar]