Abstract
We investigated associations of deceased donor kidney offer acceptance with likelihood of the kidney being discarded, cold ischemia time at transplant (CIT), and likelihood of the kidney being exported outside the donation service area (DSA). We used kidney offers from donors in the Scientific Registry of Transplant Recipients July 1, 2015–June 30, 2016, and a stratified logistic regression to estimate odds ratios of acceptance for candidates waitlisted in a DSA. We estimated associations between these ratios and likelihood of discard or export and CIT at transplant. Approximately 0.50 kidneys were discarded per donor; lower DSA-specific offer acceptance ratios were associated with more discards (R=−0.20; P=0.006). For a median donor, the DSA with the highest acceptance ratio would place 0.12 more kidneys per donor than the DSA with the lowest ratio. Low acceptance ratios were associated with higher CIT (R=−0.23; P<0.001). For the median donor, CIT was 2.9 hours shorter for the DSA with the highest versus lowest acceptance ratio. Low acceptance ratios were associated with more exports (R=−0.43; P<0.001); the probability was 15% higher for a median donor in the DSA with the lowest versus highest acceptance ratio. Improving lower-than-expected offer acceptance would likely reduce discards, CIT, and exports.
Keywords: Cold ischemia time, deceased donor, kidney transplant, organ offers
Introduction
In the United States, approximately 100,000 patients are on the waiting list for a deceased donor kidney transplant. Nevertheless, 20% of kidneys recovered for transplant are discarded, including 55% of kidneys with a kidney donor profile index (KDPI) over 85%, indicating a “high-risk” donor.1 The importance of decreasing the number of discarded kidneys cannot be overstated, given the long-term survival benefit of kidney transplant compared with dialysis, regardless of KDPI.2,3 For example, the Organ Procurement and Transplantation Network (OPTN) recently approved an operational rule to reduce the number of discarded kidneys with a KDPI over 85% by excluding transplants of such kidneys from evaluation of transplant program outcomes.4 However, there is substantial variability in program-specific offer acceptance of easy-to-place kidneys across transplant programs,5 which may lead to discards due to allocation inefficiency and longer cold ischemia time (CIT). Despite a desire to reduce the kidney discard rate, there has been no formal investigation of the relationship between offer acceptance and eventual kidney discard.
The effect of program-specific offer acceptance practices on allocation efficiency is difficult to evaluate due to the complicated nature of kidney allocation. Specifically, kidneys are recovered and allocated by the organ procurement organizations (OPOs) that serve each donation service area (DSA), not by individual transplant programs; i.e., kidneys are allocated at the DSA level rather than the program level. Intuitively, multiple programs must decline offers of a given kidney for it to accrue additional CIT or be discarded. Thus, the effect of individual programs and their offer acceptance behavior on allocation efficiency are difficult to isolate from each other. Specifically, multiple transplant programs are usually associated with the allocation process for each recovered kidney, and the programs responsible for eventual placement or discard depend on the decisions of other programs, which severely complicates an analysis of the association between allocation efficiency and offer acceptance of individual programs.
Previous offer acceptance research focused on demonstrating the variability in program-specific acceptance of “good” kidneys,5 the association of offer acceptance in liver transplantation with waitlist mortality,6 and the impact of Share 35 on liver offer acceptance.7 Each of these studies focused on offer acceptance up to a certain point in the allocation process, after which accepted and declined offers were ignored. However, focusing on acceptance of early offers may fail to reveal the overall effect of offer acceptance practices on allocation efficiency. For example, patients at programs with exceptionally high offer acceptance may undergo transplant before they reach the top of the waiting list. Thus, offer acceptance of, for example, the first offer may fail to identify a program with high acceptance of offers later in a match run, which could indicate a willingness to accept and transplant marginal kidneys that are at risk of discard.
Rather than narrowly focus on acceptance of the first offer, we evaluated the association between the aggregated offer acceptance behavior of programs within a DSA (called DSA-specific offer acceptance) and metrics of allocation efficiency for kidneys recovered in the DSA. Since kidneys are recovered and allocated by the OPOs that serve each DSA, DSA-specific offer acceptance provided better alignment with the kidney recovery and allocation process. Additionally, each recovered kidney has a single DSA-specific offer acceptance ratio that characterizes the offer acceptance practices of the local programs, which are given substantial priority in the kidney allocation system. The specific metrics of allocation efficiency were the likelihood of the kidney being discarded, CIT at transplant, and the likelihood of the kidney being exported (i.e., transplanted in a DSA different from the recovery DSA).
Materials and Methods
Donor and candidate characteristics were retrieved from the November 2016 Scientific Registry of Transplant Recipients (SRTR) standard analytic file. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the US, submitted by the members of OPTN, and has been described elsewhere.8 The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors.
Kidney Allocation and Match Runs
In the United States, deceased donor kidneys are allocated through a complicated system of rules that depend on donor quality, candidate health, calculated panel-reactive antibodies (cPRA), and time spent on dialysis or the waiting list. With notable exceptions for candidates with extremely high cPRA or zero-HLA mismatches, deceased donor kidneys are typically offered first to candidates listed in the donor’s DSA, with priority given to candidates with the longest time on dialysis or on the waiting list; kidneys with KDPI 85% or above are typically offered to candidates listed in the same OPTN region rather than DSA. The OPOs that serve each DSA simultaneously offer a deceased donor kidney to multiple candidates, but the offer can only be accepted once every candidate with higher allocation priority formally declines it. Programs have 1 hour to accept the offer before the next candidate can formally accept it. Further information on kidney match runs is provided in the Supplementary Materials.
Kidney Offer Acceptance Model
Discrete-time survival models estimated the probability of acceptance separately for pediatric and adult offers. The time-scale was the number of previous offers, and was estimated by a generalized linear model with a logit link and a semi-parametric baseline hazard function (i.e., the effect of the number of previous offers), which ensured a non-zero probability of acceptance for each offer. The survival model for adult offers was stratified across donor quality, measured by the kidney donor risk index (KDRI). The offer acceptance model adjusted for donor and candidate characteristics including donor quality, candidate health, and donor-candidate interactions. The model was estimated with offers from match runs that ended in acceptance for kidneys recovered between July 1, 2015, and June 30, 2016. The Supplemental Materials provide a thorough description of the offer acceptance model.
The offer acceptance model implicitly assumed that offers within a match run are independent. This assumption is likely false as numerous anecdotes describe programs declining every offer associated with a donor after receiving the initial offer. Programs that receive offers of kidneys from unacceptable donors will likely have lower offer acceptance ratios than they would if they had never received the offers. However, programs have the ability not to receive offers from donors with certain characteristics, and failure to properly screen offers may slow kidney allocation, increase CIT, and eventually lead to discard. Thus, this is a potential mechanism through which low offer acceptance could lead to discarded organs, and we did not want the model to remove the effect.
Estimation of DSA-specific Offer Acceptance Ratios
Due to the extent of kidney offer data (over 1.5 million offers during a year), DSA-specific offer acceptance ratios were estimated after fitting the initial offer acceptance model. Specifically, the ratios were estimated by a random effect for the waitlisting DSA in a generalized linear mixed model (GLMM) with a logit link. The GLMM accounted for donor and candidate risk factors through an offset term equal to the linear predictors of the initial offer acceptance model. The Supplemental Materials provide a detailed description of the model fitting process, including the estimation of offer acceptance ratios.
The characteristics of accepted and declined offers were summarized across important donor and candidate factors. Means and standard deviations described continuous variables, while percentages described categorical variables.
Association between Offer Acceptance and Metrics of Allocation Efficiency
Unadjusted and adjusted analyses estimated the association of DSA-specific offer acceptance ratios with the average number of discarded kidneys per donor, CIT at transplant, and proportions of exports of kidneys recovered in the DSA. The unadjusted association was estimated with a Pearson correlation between each metric of allocation efficiency and the natural-log of the DSA-specific offer acceptance ratio. The natural-log adjusts for the skewed nature of the ratio. The adjusted association between offer acceptance and eventual kidney discard was estimated by an ordinal logistic regression (0–2 kidneys discarded per recovered donor). A “recovered donor” is a donor from whom at least one organ was recovered. This is the definition used for the regulatory evaluation of OPOs, but it differs from the definition of offers in the match run data, which is offers of kidneys eventually accepted and transplanted. The adjusted association of offer acceptance with CIT at transplant and likelihood of kidney export was estimated by, respectively, a linear and logistic regression. Generalized estimating equations with an exchangeable working correlation structure accounted for potential correlation between kidneys from the same donor, and 95% confidence intervals were estimated with robust standard errors.9 Each regression adjusted for potentially important donor factors: KDRI, an indicator for missing KDRI, age, blood type, donation after circulatory death (DCD), controlled DCD, and US Public Health Service (PHS) increased infectious risk. B-splines with 5 degrees of freedom accounted for the potentially non-linear effects of KDRI and donor age. Finally, as large disparities in kidney supply across DSAs may affect kidney placement,10 a penalized spline with 5 degrees of freedom adjusted for the ratio of recovered donors to new kidney waitlist registrations within a DSA.
All analyses were completed in R v3.2.2. B-spline basis was generated with the “splines” package and generalized estimating equations were estimated with the “gee” package.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Results
Descriptive Statistics of Accepted and Declined Offers
Accepted offers were associated with younger candidates, lower estimated posttransplant survival, and lower candidate body mass index (Table 1). Kidneys with KDPI < 35% had higher acceptance rates than kidneys with KDPI > 85%. DCD kidneys were slightly more likely to be declined, and kidneys with PHS increased infectious risk were slightly more likely to be accepted. Offers with fewer HLA mismatches were significantly more likely to be accepted, while higher offer numbers were associated with substantially lower acceptance.
Table 1.
Characteristics of Declined and Accepted Offers
| Characteristics | Declined Offers | Accepted Offers |
|---|---|---|
| n | 1,512,496 | 11,922 |
| Candidate age at offer, years, mean (SD) | 55 (13) | 51 (15) |
| Candidate EPTS, mean (SD) | 2.16 (0.71) | 2.01 (0.80) |
| KDPI | ||
| < 35% | 15.7 | 37.4 |
| 35–85% | 61.4 | 53.7 |
| > 85% | 22.9 | 8.9 |
| DCD donor | 21.7 | 20.1 |
| PHS increased infectious risk donor | 21.1 | 22.1 |
| Candidate BMI, kg/m2 | ||
| < 18.5 | 1.5 | 3.6 |
| 18.5–25 | 20.3 | 24.9 |
| 25–30 | 33.6 | 32.6 |
| 30–35 | 27.7 | 24.1 |
| > 35 | 16.8 | 14.6 |
| HLA mismatches | ||
| 0 | 0.2 | 4.8 |
| 1 | 0.3 | 1.5 |
| 2 | 2.3 | 5.4 |
| 3 | 10.1 | 14.9 |
| 4 | 25.9 | 28.0 |
| 5 | 38.2 | 31.1 |
| 6 | 23.0 | 14.4 |
| Offer number | ||
| 1–10 | 2.8 | 62.8 |
| 11–100 | 11.2 | 23.1 |
| > 100 | 86.0 | 14.1 |
BMI, body mass index; DCD, donation after circulatory death; EPTS, estimated posttransplant survival; KDPI, kidney donor profile index; PHS, Public Health Service; SD, standard deviation.
Note: Unless otherwise indicated, values are percentages.
Association between DSA-Specific Offer Acceptance and Kidney Discard
Low offer acceptance within a DSA was significantly associated with more discarded kidneys per donor recovered in the DSA (Figure 1). Approximately 0.50 kidneys were discarded across the United States for each donor with a recovered organ. The Pearson correlation between DSA-specific offer acceptance ratio and kidneys discarded per donor was approximately −0.20. For the median donor, the DSA with the highest offer acceptance was expected to discard 0.30 kidneys per recovered donor, while the DSA with the lowest offer acceptance was expected to discard 0.42 kidneys per recovered donor. Therefore, for a median donor, the DSA with the highest offer acceptance was expected to place 0.12 more kidneys per donor than the DSA with the lowest offer acceptance (P = 0.006).
Figure 1.
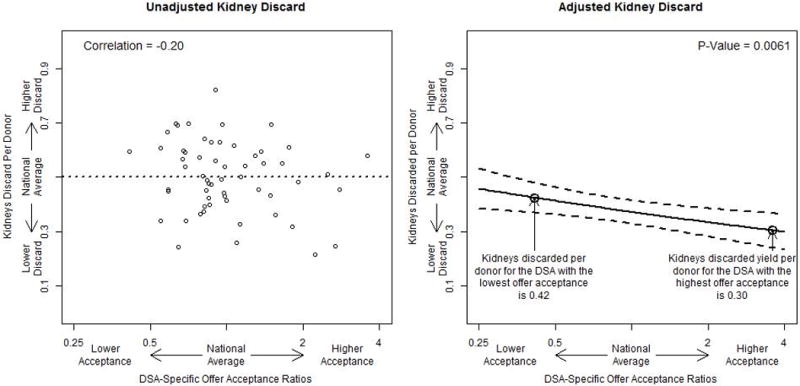
The association between DSA-specific offer acceptance ratios and the number of kidneys discarded per recovered donor. The “national average” is the average discard rate per donor across each DSA. The adjusted analysis presents the expected discard rate for a median donor across the spectrum of DSA-specific offer acceptance ratios. DSA, donation service area.
Association between DSA-Specific Offer Acceptance and CIT at Transplant
Low offer acceptance within a DSA was significantly associated with higher CIT at transplant for kidneys recovered in the DSA (Figure 2). The average CIT at transplant was approximately 17 hours and the average across DSAs ranged from less than 14 hours to longer than 24 hours. The Pearson correlation between DSA-specific offer acceptance ratio and CIT at transplant was approximately −0.23. For the median donor, the DSA with the highest offer acceptance was expected to have 15.4 hours of CIT at transplant, while the DSA with the lowest offer acceptance was expected to have 18.3 hours of CIT at transplant. Therefore, for the median donor, the difference in CIT at transplant for kidneys recovered in a DSA was 2.9 hours lower for the DSA with the highest offer acceptance than for the DSA with the lowest offer acceptance (P < 0.001).
Figure 2.
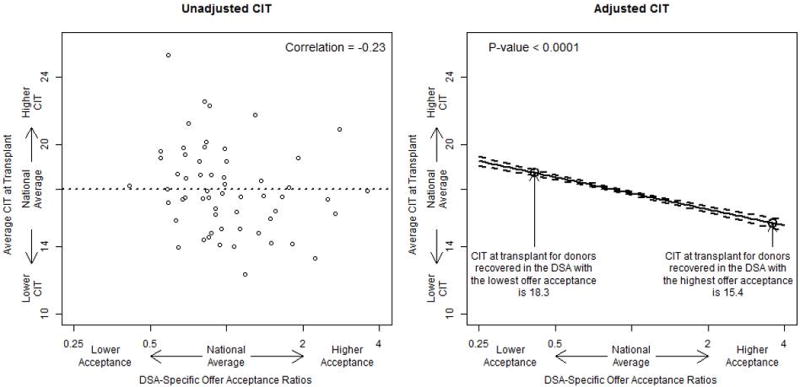
The association between DSA-specific offer acceptance ratios and the average CIT at transplant in hours. The “national average” is the average CIT at transplant for donors recovered in each DSA. The adjusted analysis presents the expected CIT at transplant for a median donor across the spectrum of DSA-specific offer acceptance ratios. CIT, cold ischemia time; DSA, donation service area.
Association between DSA-Specific Offer Acceptance and Proportion of Exported Kidneys
Low offer acceptance within a DSA was significantly associated with a higher proportion of exported kidneys (Figure 3). The average DSA exported slightly over 30% of kidneys recovered within it, and the average across DSAs ranged from about 15% to nearly 60% of recovered kidneys. The Pearson correlation between DSA-specific offer acceptance ratio and the proportion of exported kidneys was −0.43. For the median donor, the DSA with the highest offer acceptance ratio was expected to export 27% of recovered kidneys, while the DSA with the lowest offer acceptance ratio was expected to export 42% of recovered kidneys. Therefore, for the median donor, the difference in the proportion of exported kidneys was approximately 15% lower for the DSA with the highest offer acceptance than for the DSA with the lowest offer acceptance (P < 0.001).
Figure 3.
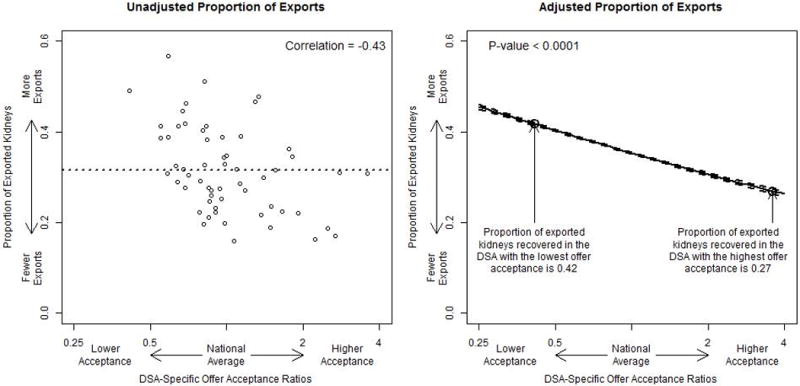
The association between DSA-specific offer acceptance ratios and the proportion of exported kidneys. The “national average” is the average proportion of exported kidneys across each DSA. The adjusted analysis presents the expected proportion of exported kidneys for a median donor across the spectrum of DSA-specific offer acceptance ratios. DSA, donation service area.
Discussion
Low offer acceptance in a DSA was associated with increased likelihood of kidneys being discarded, higher CIT at transplant, and increased likelihood of kidneys being exported, for kidneys recovered in the DSA. This is the first study to confirm the expectation of previous investigations of kidney offer acceptance.5 Given the large variability in program-specific offer acceptance even for high-quality kidneys,5 efforts to improve offer acceptance may help increase access to kidney transplant by reducing overall discards and improve transplant recipient outcomes by reducing CIT at transplant.
The offer acceptance practices of kidney transplant programs likely have the most important, and modifiable, effect on allocation efficiency. However, the complexity of the kidney allocation system severely impedes the ability to evaluate the effect of program-level offer acceptance practices on allocation efficiency. An alternative approach could, for example, investigate the association of eventual discard or CIT of a kidney with program-specific acceptance of the first offer. This approach may fail to reveal the entire effect of a given program’s practices on the allocation system because aggressive programs may perform transplants in its patients before they reach the top of the waiting list. Separately, the variability of program-specific offer acceptance practices in a DSA may modify the effect of DSA-specific offer acceptance (or, equivalently, DSA-specific offer acceptance ratio). For example, a DSA with several extremely conservative programs but one aggressive program may be less burdensome on the allocation process than a DSA with only moderately conservative programs. Thus, further investigation of the relationships between DSA-specific offer acceptance, program-specific offer acceptance, and allocation efficiency could help identify the programs with the largest impact on allocation efficiency.
The effect of offer acceptance on allocation efficiency may depend on the quality of the donor. A preliminary analysis of KDPI subgroups (Supplementary Materials; Table S1) suggests a complicated interaction between offer acceptance, donor quality, and allocation efficiency. For example, DSA-specific offer acceptance of low-KDPI kidneys had the largest absolute impact on CIT at transplant, despite a non-significant association with the likelihood of kidney discard or export. In contrast, DSA-specific offer acceptance of high-KDPI kidneys showed a significant association with likelihood of kidney export but no significant association with CIT at transplant or likelihood of kidney discard. Donor quality may therefore modify the relationship between offer acceptance and metrics of allocation efficiency. Interventions to improve offer acceptance may help kidneys across the entire spectrum of donor quality, although the components of allocation efficiency may change with donor quality. Regardless, further research is warranted regarding the relationship between offer acceptance, allocation efficiency, and potential efficacy of interventions across donor quality.
While kidney discard and allocation efficiency are important issues, significant variability in program-specific offer acceptance may affect access to kidney transplant and patient outcomes.5 For example, liver transplant programs with below average acceptance of the first offer were associated with significantly higher waitlist mortality.6 Similarly, programs with high offer acceptance may provide better access to kidney transplant than programs with lower offer acceptance, possibly leading to better outcomes for waitlisted candidates. Additionally, we found that low offer acceptance was associated with high CIT. This relationship may indicate that DSAs with low acceptance may have worse outcomes for recovered and transplanted kidneys because higher CIT is associated with worse posttransplant outcomes.11,12 Thus, further investigation is warranted regarding the relationships between DSA-specific offer acceptance, program-specific offer acceptance, and candidate outcomes.
Public reporting is a potential avenue toward improving kidney offer acceptance. For example, SRTR could integrate program-specific offer acceptance ratios for all donors and important subgroups into the program-specific reports, which are published on a public website twice a year. Additionally, programs could be provided with private detailed reports including two- and one-sided CUSUM charts,13 offer acceptance ratios for donors with characteristics that can hinder placement (e.g., DCD), and/or a detailed list of declined offers with the largest expected probability of acceptance and the eventual candidate(s) who accepted the kidney(s). Both approaches provide decision makers at kidney transplant programs with the information necessary to identify potential shortcomings and opportunities for improvement in program-specific offer acceptance practices. Public reporting for OPOs may provide another avenue toward reducing kidney discard. For example, SRTR could provide private reports to OPOs regarding program-specific offer acceptance of kidneys with, for example, over 100 offers. Thus, when a kidney becomes difficult to place, the OPO could, within the confines of OPTN policy, begin to expedite the process by offering the kidney to the programs most likely to accept it. A similar approach helps guide “rescue allocation” in Eurotransplant by offering kidneys at risk of discard to programs most likely to accept and transplant them.14 While offer acceptance will not be integrated into regulatory evaluations, the impact of public reporting on offer acceptance should be monitored due to potential unintended consequences, which may have occurred after implementation of regulatory evaluations for posttransplant outcomes.15–17
Deceased donor kidney supply varies substantially across DSAs,1 and transplant programs that use suboptimal organs tend to have lower deceased donor supply relative to demand.18 Medically appropriate offer acceptance decisions for waitlisted candidates could therefore differ in high-supply versus low-supply DSAs. These geographic disparities in the availability of deceased donor kidneys could therefore justify relatively low offer acceptance behavior. However, the association between DSA-specific offer acceptance and metrics of allocation efficiency was present after an adjustment for supply and demand. Thus, improvements in offer acceptance behavior could increase transplants regardless of supply or demand in the local DSA.
Our analysis of offer acceptance, CIT, and kidney placement is subject to potential limitations. First, the offer acceptance model could only evaluate offers of eventually accepted kidneys to ensure that programs definitively rejected the offers (see the Supplemental Materials for further discussion). It is plausible, but not certain, that programs in DSAs with relatively high discard rates would have received offer acceptance ratios in our analysis that were higher than the offer acceptance ratios corresponding to all offers regardless of eventual placement. This scenario would suggest that our analysis may have underestimated the strength of the association between offer acceptance and kidney placement. Additionally, despite adjusting for important donor factors in kidney placement, the analysis remains subject to potential confounding from unmeasured or poorly collected risk factors, e.g., cardiovascular risk factors for waitlisted candidates. Lastly, registry data cannot evaluate the daily practices of kidney transplant programs and, therefore, cannot assess the specific reasons for high or low offer acceptance.
In summary, we identified a significant association between offer acceptance practices in a DSA and kidney discard. Further efforts to improve offer acceptance practices may help eliminate unnecessary discards, reduce CIT, and thereby improve access to and outcomes of kidney transplant in the United States.
Supplementary Material
Acknowledgments
This work was conducted under the auspices of the Minneapolis Medical Research Foundation, contractor for the Scientific Registry of Transplant Recipients, as a deliverable under contract number HHSH250201500009C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The views expressed herein are those of the authors and not necessarily those of the US Government. AKI was partially supported by R01 HS 24527.
The authors thank Scientific Registry of Transplant Recipients colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
Footnotes
The authors have no financial or other conflicts of interest pertinent to this manuscript.
Author Contributions: research idea and study design: AW, NS, BLK, AKI, JJS; data acquisition: AW; data analysis/interpretation: AW, NS, JJS; statistical analysis: AW; supervision or mentorship: NS, BLK, AKI, JJS.
Supplemental Material: Supplemental Methods
Match Run Data
Offer Acceptance Model
Variable Selection
KDPI Subgroup Analysis
References
- 1.Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR 2014 Annual Data Report: Kidney. Am J Transplant. 2016;16(Suppl 2):11–46. [Google Scholar]
- 2.Gill J, Dong J, Rose C, Gill JS. The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int. 2016;89:1331–1336. doi: 10.1016/j.kint.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Massie AB, Luo X, Chow EKH, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14:2310–2316. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 4.OPTN/UNOS Membership and Professional Standards Committee. Transplant Program Performance Measures Review (Outcomes Measures) 2016 Available at: https://optn.transplant.hrsa.gov/media/1248/performance_metrics_concept_paper.pdf Accessed June 2, 2017.
- 5.Wolfe RA, LaPorte FB, Rodgers AM, Roys EC, Fant G, Leichtman AB. Developing organ offer and acceptance measures: when ‘good’ organs are turned down. Am J Transplant. 2007;7(Suppl 1):1404–1411. doi: 10.1111/j.1600-6143.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DS, French B, Lewis JD, Scott FI, Mamtani R, Gilroy R, et al. Liver transplant center variability in accepting organ offers and its impact on patient survival. J Hepatol. 2016;64:843–851. doi: 10.1016/j.jhep.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg DS, Levine M, Karp S, Gilroy R, Abt PL. Share 35 changes in center-level liver acceptance practices. Liver Transpl. 2017;23:604–613. doi: 10.1002/lt.24749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, et al. Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States. Transplantation Rev. 2013;27:50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd. Oxford University Press; 2002. [Google Scholar]
- 10.Davis AE, Mehrota S, Ladner DP, Kilambi V, Friedewald JJ. Changes in geographic disparity in kidney transplantation since the final rule. Transplantation. 2014;98:931–936. doi: 10.1097/TP.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 11.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibric DM, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation. 2002;74:1377–1381. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 13.Snyder JJ, Salkowski N, Zaun D, Leppke SN, Leighton T, Israni AK, et al. New quality monitoring tools provided by the Scientific Registry of Transplant Recipients: CUSUM. Am J Transplant. 2014;14:515–523. doi: 10.1111/ajt.12628. [DOI] [PubMed] [Google Scholar]
- 14.Vinkers MT, Smits JM, Tieken IC, de Boer J, Ysebaert D, Rahmel AO. Kidney donation and transplantation in Eurotransplant 2006-2007: minimizing discard rates by using a rescue allocation policy. Prog Transplant. 2009;19:365–370. doi: 10.1177/152692480901900414. [DOI] [PubMed] [Google Scholar]
- 15.Schold JD, Buccini LD, Poggio ED, Flechner SM, Goldfarb DA. Association of candidate removals from the kidney transplant waiting list and center performance oversight. Am J Transplant. 2016;16:1276–1284. doi: 10.1111/ajt.13594. [DOI] [PubMed] [Google Scholar]
- 16.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM, et al. The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant. 2013;13:67–75. doi: 10.1111/j.1600-6143.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton TE. Regulatory oversight in transplantation: are the patients really better off? Curr Opin Organ Transplant. 2013;18:203–209. doi: 10.1097/MOT.0b013e32835f3fb4. [DOI] [PubMed] [Google Scholar]
- 18.Garonzik-Wang JM, James NT, Weatherspoon KC, Deshpande NA, Berger JA, Hall EC, et al. The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant. 2012;12:400–408. doi: 10.1111/j.1600-6143.2011.03789.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


