Abstract
Reactive oxygen species (ROS) largely originating in the mitochondria play essential roles in the metabolic and (epi)genetic reprogramming of cancer cell evolution towards more aggressive phenotypes. Recent studies have indicated that the activity of superoxide dismutase (SOD2) may promote tumor progression by serving as a source of hydrogen peroxide (H2O2). H2O2 is a form of ROS that is particularly active as a redox agent affecting cell signaling due to its ability to freely diffuse out of the mitochondria and alter redox active amino acid residues on regulatory proteins. Therefore, there is likely a dichotomy whereas SOD2 can be considered a protective anti-oxidant, as well as a pro-oxidant during cancer progression, with these effects depending on the accumulation and detoxification of H2O2. Glutathione peroxidase-1 GPX1, is a selenium-dependent scavenger of H2O2 which partitions between the mitochondria and the cytosol. Epidemiologic studies indicated that allelic variations in the SOD2 and GPX1 genes alter the distribution and relative concentrations of SOD2 and GPX1 in mitochondria, thereby affecting the dynamic between the production and elimination of H2O2. Experimental and epidemiological evidence supporting a conflicting role of SOD2 in tumor biology, and epidemiological evidence that SOD2 and GPX1 can interact to affect cancer risk and progression indicated that it is the net accumulation of mitochondrial H2O2 (mtH2O2) resulting from of the balance between the activities SOD2 and anti-oxidants such as GPX1 that determines whether SOD2 prevents or promotes oncogenesis. In this review, research supporting the idea that GPX1 is a gatekeeper restraining the oncogenic power of mitochondrial ROS generated by SOD2 is presented. This article is part of a Special Issue entitled Respiratory complex I, edited by Giuseppe Gasparre, Rodrigue Rossignol and Pierre Sonveaux.
Keywords: Oxidative stress, Cancer, Manganese superoxide dismutase, Selenium, Glutathione peroxidase
1. Introduction
The adaptation of aerobic organisms to use oxygen as a bolster of catabolic reactions enabled the activation of robust mechanisms of energy production that promoted the survival and evolution of pluricellular organisms. However, the utilization of respiration as a major source of ATP came with a price. Though many factors ranging from tissue oxygenation to the mitochondrial coupling state can influence the yield of reactive oxygen species produced by the electron transport chain, it has been estimated that between 0.1–1% of the oxygen being used for mitochondrial respiration is converted to superoxide (reviewed in [1]), a reactive oxygen species (ROS) capable of oxidizing and thus inactivating some of the critical iron-sulfur cluster dependent enzymes involved in respiration itself (reviewed in [2]). Hence, primordial aerobic organisms were forced to adapt by evolving mechanisms to cope with ROS as unavoidable products of life in an oxygen rich environment. As a result cells and organisms not only adapted to coexisting with elevated ROS levels, but also “learned” to use these reactive species to execute essential tasks such as cellular signaling, adaptation to microenvironmental changes, defense against pathogens and promoting phenotypic plasticity [3–5]. As a consequence to the multitude of roles that ROS play, ROS production, diffusion and clearance must be tightly regulated in the cellular milieu so a level of target specificity can be attained. Failure to buffer ROS levels or to regulate their intrinsic unselective reactivity can promote widespread oxidative damage to biomolecules, a process that often results in aberrant activation of adaptive responses to stress, including the compensatory enhancement of resistance and cell survival pathways [6]. Extensive research now indicates that cancer is one example of a disease that is promoted by dys-regulated ROS homeostasis resulting in cellular maladaptation and disarray [6–8].
SOD2 is a mitochondrial enzyme with established roles in the metabolism of ROS formed in the mitochondrial matrix [9–11]. Changes in SOD2 levels or function by a variety of recently identified posttranslational modifications [12–14] have a direct impact on mtH2O2 generation, accumulation and clearance in the mitochondria. In fact, some posttranslational modifications that occur in cancers can promote the accumulation of mtH2O2 in those tissues. Hence, the activity of SOD2 can be a primary generator of H2O2 leaving the mitochondria to stimulate signaling pathways that likely promote more aggressive cancer phenotypes. Therefore, SOD2 should not just be considered an intrinsic antioxidant since the inability to detoxify its catalytic product, H2O2 would actually lead to oxidative stress. The challenge is to understand under what circumstances SOD2 displays a protective (antioxidant) or deleterious (pro-oxidant) role. Though this quandary is being actively pursued, mounting evidence indicates that posttranslational modifications of SOD2 that affect its activity as well as the activity of H2O2 detoxifying enzymes conspire to determine the net amount of H2O2 available for signaling. The impact of H2O2 on signal transduction in cancer has been reviewed extensively [15,16], instead this review focuses on the potential impact of antioxidants such as GPX1 in attenuating mtH2O2 derived from mitochondrial SOD2 activity.
1.1. Pro-carcinogenic effects of SOD2 and their suppression by anti-oxidants
As summarized above, H2O2 production is a means by which SOD2 could potentially impact tumor biology. For example, the increased expression of SOD2 in U87 glioma cells achieved due to transfection of an SOD2 expression construct was shown to stimulate cellular characteristics associated with transformation. These characteristics include cell migration, invasiveness, as well as the activation of signaling cascades associated with transformation and the increase in the levels of MMP-1 and MMP-9 matrix metalloproteinases required for metastasis [17]. These effects were due to the pro-oxidant activity of SOD2 which was established by the demonstration that they could be suppressed by the supplementation of the culture media with N-acetyl-1-cysteine (NAC), a precursor of glutathione that under most conditions acts as an antioxidant [17]. Over-expression of SOD2 in HT-1080 fibrosarcoma cells increased the binding of transcription factors associated with transformation to DNA and stimulated the expression of the metastasis-associated matrix metalloproteinase-1; these effects were attenuated by catalase expression [18]. A different study using a breast cancer cell line i.e. MCF7 focused on the impact of SOD2 on cellular metabolism and found that the ectopic expression of SOD2 leading to an increase in its activity stimulated glycolysis and the uncoupling of glycolysis and respiration which are hallmarks of aggressive tumors [19]. Using cells engineered to express increasing levels of SOD2, it was also demonstrated that as a consequence of increasing SOD2 levels, there was a proportional increase in the levels of H2O2 in these cells. The increase in H2O2 levels was determined to be the effector of changes in energetics using mito-catalase, a scavenger of H2O2 genetically targeted to the mitochondria by attachment of a mitochondria-targeting sequence (MTS) [19]. Collectively, these studies provide significant evidence that elevated SOD2 expression can contribute to the progression of transformation and this occurs through the accumulation of H2O2.
1.2. Human genetic data supports a role for higher SOD2 levels contributing to cancer development
For the reasons summarized above, it should not be surprising that altered SOD2 expression and activity are frequently detected in most human cancers where ROS metabolism is particularly disturbed by drastic metabolic and microenvironmental perturbations. Though both higher or lower expression levels of SOD2 have been detected in tumors compared to corresponding normal tissues [20], SOD2 is often upregulated in the more aggressive phenotypes. For example, a recent analysis examined the levels of SOD2 in human clinical samples and the data indicated that SOD2 levels increased progressively with breast cancer tumor grade when compared to either non-tumor or hyperplastic benign breast tissues, and this pattern was also evident when prostate and colon tissues representing progressive malignancy were examined for SOD2 levels [19]
Supporting evidence for a role played by excess SOD2 can be found in human genetic data. The most characterized polymorphism in the gene for SOD2 results in a SOD2 protein containing either an alanine or a valine at codon 16 of the mitochondrial targeting sequence [21], each allele occurring with similar frequencies among the Caucasian population [22]. The polymorphism occurs 9 codons upstream of the signal peptide cleavage site and the same polymorphism sometimes appears in the literature as an Ala9Val variation. Based on the contribution of valine and alanine to the protein structure, it was predicted that the valine-containing protein would form a β-sheet rather than an α-helix that would better facilitate the transport SOD2 to the mitochondria [21]. Consistent with this prediction, it was shown that the import of SOD2val into the mitochondria was less efficient than SOD2ala [23]. Moreover, it was subsequently shown that the mRNA for SOD2val exhibited reduced stability compared to SOD2ala transcripts [24]. Epidemiological studies examining whether the amino acid at codon 16 of SOD2 is associated with cancer risk have yielded mixed results, with an elevated risk of several cancer types being reported for the alanine allele, including prostate cancer [25–28]. Meta-analyses of multiple studies have also yielded mixed results with some indicating an association while others did not [29–31].
The lack of consistency in detecting an association between the SOD2 codon 16 polymorphism and cancer risk may be due to the influence of modifiers such as the dietary intake of anti-oxidants. Examining the association between SOD2ala and the risk of prostate cancer, a 10-fold excess in the risk of aggressive prostate cancer among men who expressed SOD2ala comparing the lowest quartile of total antioxidant consumption and the highest, with those consuming the lowest levels of dietary antioxidants being at greatest risk was reported. [26]. This relationship was not evident for those SOD2val-expressing individuals. Consistent with these results, there was a 3-fold increase risk of aggressive prostate cancer for SOD2ala men with low carotenoid status [P = 0.02, confidence interval 1.37–7.02] [27]. It was proposed that increased SOD2ala mRNA stability and mitochondrial transport can be protective when antioxidant activity is high and the enzymatic product H2O2, can be reduced to water [26]. Low levels of dietary antioxidant intake or anti-oxidant proteins with less efficiency could facilitate the propagation of ROS production thereby promoting the development of cancers. These epidemiological data may shed light on some of the conflicting reports of the benefits of SOD2 being that the same protein may be beneficial under conditions of high anti-oxidant intake, and detrimental when the anti-oxidant resources needed to further detoxify H2O2 are insufficient to maintain H2O2 levels below a critical level.
1.3. Glutathione peroxidase 1 (GPX1)
In 2000, the effects of over-expressing an H2O2 detoxifying enzyme, glutathione peroxidase 1 (GPX1), on SOD2-induced phenotypes associated with carcinogenesis were published [32]. In this study, over-expression of SOD2 achieved by the transfection of an expression construct into a derivative clone of the human glioma U118-9 cell line caused drastic changes to doubling time, plating efficiency and tumori-genicity. All of these phenotypic changes were prevented or reversed by co-expression of GPX1, an enzyme that reduces H2O2 to water with reducing equivalents obtained from GSH [32].
GPX1 is one member of a family of proteins that contain the amino acid selenocysteine, an amino acid similar to cysteine but containing an atom of selenium at the position where sulfur resides in cysteines [33]. In this class of selenoproteins, selenocysteine is inserted co-translationally in response to one or more in-frame UGA codons in the selenoprotein mRNA directed to encode selenocysteine due to the presence of a Selenium Insertion Sequence (SECIS) element in the 3' untranslated region of the mRNA [34,35]. Selenocysteine in proteins has a lower pKa in comparison with cysteine, which makes it more efficient in oxidoreductase reactions and a much more efficient anti-oxidant in proteins that include that function [36]. In addition to its role as a protective anti-oxidant, GPX1 has broader roles in modifying the activity of proteins and pathways that are influenced by ROS (see reference [37] for a comprehensive review).
In addition to being located in the cytoplasm, GPX1GPx-1 is also located in the mitochondria where it can detoxify H2O2 generated from the dismutation of superoxide by SOD2 [38]. The GPX1 gene is polymorphic and the best characterized of these genetic variations is one in the coding region at codon 198, resulting in either a leucine (leu) or a pro-line (pro); with the leu-encoding allele being associated with increased risk of cancers of the lung, breast, bladder, liver as well as lymphoma (reviewed in [39]). The GPX1leu allele is the most frequently associated with cancer and encodes a protein that is less responsive to selenium compared to the same protein with a proline at that position [40,41]. This genetic variation influences the distribution of GPX1 between the cytoplasm and the mitochondria. Using cultured cells that exclusively express GPX1 containing either a leu or pro at position 198, it was shown that the GPX1leu protein was located more in the cytoplasm than the mitochondria as compared to the same protein encoded by the pro allele at that position [42]. Moreover, the location of GPX1 in the cell was shown to be important. By attaching a mitochondrial leader sequence to target GPX1 to that organelle, it was demonstrated that both the primary sequence and the cellular location impacted the redox millieu of those cells, energy metabolism and the levels of signaling molecules associated with cancer risk [42].
1.4. Human data indicates and interaction between SOD2 and GPx-1
Based on the information summarized above, it seems likely that the detrimental consequences of H2O2 production that arises from elevated SOD2 expression could be diminished by the H2O2 scavenging activity of GPX1 in human tissues. Epidemiological data has supported such relationship in several cases. Cox et al. initially reported the lack of a statistically significant association between the GPX1leu and breast cancer risk using data obtained from the Nurse's Health Study [43]. However, in a follow-up study using a case-control design that compared the genotypes of 1262 women diagnosed with breast cancer with the genotypes obtained from 1533 disease free women, they reported a significant risk for breast cancer (OR 1.87, 95% CI 1.09–3.19) among participants of the same cohort when the data was stratified by SOD2 alleles; women who were homozygous for the GPX1leu and SOD2ala were at increased risk of breast cancer with an odds ratio of 1.87 [95% confidence level, 1.09–3.19] [44]. GPX1 may be a particularly important H2O2-detoxifying enzyme because of its cellular location in the mitochondria and cytoplasm, as well as the nucleus [32,45,46]. Additional support for the interaction between these enzymes comes from human data indicating that polymorphisms in the gene for the selenium transport protein selenoprotein P (SELENOP or SEPP1), that result in less SELENOP in the plasma and reduced levels of GPX1, are associated with a significant risk of aggressive prostate cancer only in SOD2ala/ala men [47].
Hence, based on available data it is proposed that the elevated levels of SOD2 resulting in excess H2O2 contribute to the progression of the transformed phenotype unless the H2O2 is removed by the activity of a ROS detoxifying enzyme like GPX1. It is therefore noteworthy that the SOD2ala is not uncommon, with 25% of people being homozygotes [26,43].
2. Conclusion
Elevated levels of ROS are associated with tumorigenesis and participate in the process of tumor progression toward phenotypes that are more aggressive, challenging to treat and prone to recur as metastatic disease. In this regard, several studies have indicated that there is an association between SOD2 upregulation in cancer, increased H2O2 and more aggressive phenotypes, establishing SOD2 as a source of H2O2 production which may promote tumor progression. In most cases, reversing SOD2-driven H2O2 production results in reduced invasiveness, aggressiveness and either a delay or prevention of further transformation. Along these lines, the role of scavengers of H2O2 either chemical, dietary or enzymatic (and particularly GPX1) in attenuating the pro-carcinogenic effect of SOD2 upregulation in cancer cells is supported by several studies in many types of tumors as summarized above. Hence, a partnership between SOD2 and GPX1 is presumed where the mitochondrial resident protein SOD2 dismutates superoxide to H2O2 which requires further detoxification to water by GPX1. Epidemiological studies indicating the association between these two enzymes includes genetic data indicating that specific polymorphisms in these proteins can interact to impact the risk of cancers, as summarized in Fig. 1. Future studies should include the examination of both the levels and genotypes of these proteins in order to grasp the importance of how altered redox states may affect the physiology of both normal and cancer cells.
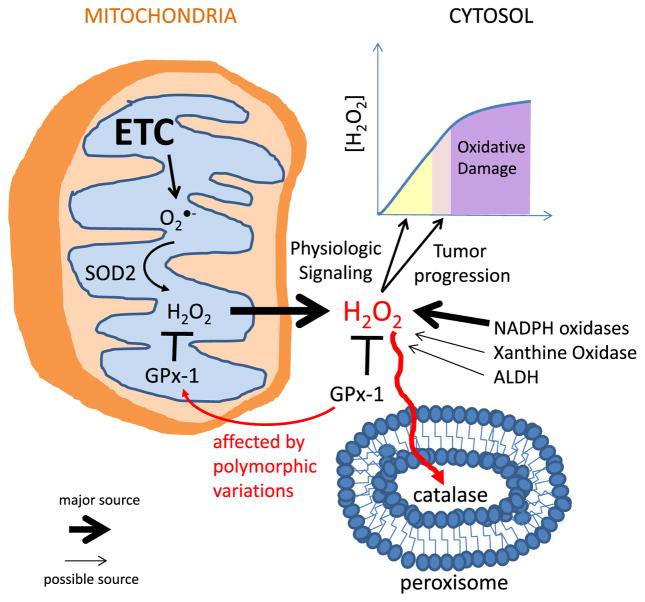
Fig. 1.
Schematic Representation of a possible mechanism of mtH2O2 processing enacted by the interaction of SOD2 and GPx-1. Catalase which is often referred to as a major H2O2 scavenger throughout the cells is actually confined to peroxisomes and more likely acts as a buffer preventing oxidative damage when H2O2 are high enough to force H2O2 diffusion into peroxisomes. The Figure also depicts some alternative mechanisms that often contribute to the elevation of H2O2 production rates in most cells. ETC stands for Electron Transport Chain.
Acknowledgments
This work was supported by grants from the U.S. Department of Defense 67263-RT-REP to M.G.B., National Institutes of Health Grants 1RO1HL125356 (M.G.B., Co-PI) and RO1CA127943, R21CA182103 to A.M.D. and a UIC Cancer Center Pilot Grant to A.M.D. and M.B. and a Research Supplement to Promote Diversity in Health-Related Research RO1CA127943S1 to A.M.D.
Footnotes
This article is part of a Special Issue entitled Respiratory complex I, edited by Giuseppe Gasparre, Rodrigue Rossignol and Pierre Sonveaux.
Conflict of interest
The authors have no conflicts to declare.
Transparency Document
The Transparency document associated with this article can be found, in online version.
References
- 1.Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, Vercesi AE. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal. 2013;18:2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- 2.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 3.Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cell. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirche TO, Gaut JP, Heinecke JW, Belaaouaj A. Myeloperoxidase plays critical roles in killing Klebsiella pneumoniae and inactivating neutrophil elastase: effects on host defense. J Immunol. 2005;174:1557–1565. doi: 10.4049/jimmunol.174.3.1557. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haigis MC, Deng CX, Finley LW, Kim HS, Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 2012;72:2468–2472. doi: 10.1158/0008-5472.CAN-11-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 10.Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, Clair DS, Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822:794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He C, Hart PC, Germain D, Bonini MG. SOD2 and the mitochondrial UPR: partners regulating cellular phenotypic transitions. Trends Biochem Sci. 2016;41:568–577. doi: 10.1016/j.tibs.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou X, Santa-Maria CA, O'Brien J, Gius D, Zhu Y. Manganese superoxide dismutase acetylation and dysregulation, due to loss of SIRT3 activity, promote a luminal B-like breast carcinogenic-permissive phenotype. Antioxid Redox Signal. 2016;25:326–336. doi: 10.1089/ars.2016.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C, Qin L, Shi Y, Candas D, Fan M, Lu CL, Vaughan AT, Shen R, Wu LS, Liu R, Li RF, Murley JS, Woloschak G, Grdina DJ, Li JJ. CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic Biol Med. 2015;81:77–87. doi: 10.1016/j.freeradbiomed.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 16.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Wang H, Huang C, Lin J, Zhu G, Hu R, Feng H. Hydrogen peroxide contributes to the manganese superoxide dismutase promotion of migration and invasion in glioma cells. Free Radic Res. 2011;45:1154–1161. doi: 10.3109/10715762.2011.604321. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KK, Ranganathan AC, Mansouri J, Rodriguez AM, Providence KM, Rutter JL, Pumiglia K, Bennett JA, Melendez JA. Elevated sod2 activity augments matrix metalloproteinase expression: evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin Cancer Res. 2003;9:424–432. [PubMed] [Google Scholar]
- 19.Hart PC, Mao M, de Abreu AL, Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A, Diamond AM, Minshall RD, Consolaro ME, Santos JH, Bonini MG. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhar SK, Clair DKS. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. 2012;52:2209–2222. doi: 10.1016/j.freeradbiomed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun. 1996;226:561–565. doi: 10.1006/bbrc.1996.1394. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosone CB, Freudenheim JL, Thompson PA, Bowman E, Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T, Shields PG. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–606. [PubMed] [Google Scholar]
- 23.Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 24.Sutton A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, Pessayre D, Degoul F. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics. 2005;15:311–319. doi: 10.1097/01213011-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kang D, Lee KM, Park SK, Berndt SI, Peters U, Reding D, Chatterjee N, Welch R, Chanock S, Huang WY, Hayes RB. Functional variant of manganese superoxide dismutase (SOD2 V16A) polymorphism is associated with prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer study. Cancer Epidemiol Bio-markers Prev. 2007;16:1581–1586. doi: 10.1158/1055-9965.EPI-07-0160. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Kantoff PW, Giovannucci E, Leitzmann MF, Gaziano JM, Stampfer MJ, Ma J. Manganese superoxide dismutase polymorphism, prediagnostic antioxidant status, and risk of clinical significant prostate cancer. Cancer Res. 2005;65:2498–2504. doi: 10.1158/0008-5472.CAN-04-3535. [DOI] [PubMed] [Google Scholar]
- 27.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Wu K, Erdman JW, Jr, Giovannucci E. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels and prostate cancer risk. Carcinogenesis. 2008;29:2335–2340. doi: 10.1093/carcin/bgn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutton A, Nahon P, Pessayre D, Rufat P, Poire A, Ziol M, Vidaud D, Barget N, Ganne-Carrie N, Charnaux N, Trinchet JC, Gattegno L, Beaugrand M. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66:2844–2852. doi: 10.1158/0008-5472.CAN-05-2566. [DOI] [PubMed] [Google Scholar]
- 29.Kang SW. Superoxide dismutase 2 gene and cancer risk: evidence from an updated meta-analysis. Int J Clin Exp Med. 2015;8:14647–14655. [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Huang HQ, Zhang GS. Association between SOD2 C47T polymorphism and lung cancer susceptibility: a meta-analysis. Tumour Biol. 2014;35:955–959. doi: 10.1007/s13277-013-1127-y. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Chen C, Xiong H, Fan J, Li Y, Lin H, Xu R, Huang G, Xu B. No association between SOD2 Val16Ala polymorphism and breast cancer susceptibility: a meta-analysis based on 9,710 cases and 11,041 controls. Breast Cancer Res Treat. 2010;122:509–514. doi: 10.1007/s10549-009-0725-2. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 33.Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry MJ, Banu L, Chen Y, Mandel SJ, Kiefer JD, Harney JW, Larsen PR. Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- 35.Caban K, Copeland PR. Size matters: a view of selenocysteine incorporation from the ribosome. Cell Mol Life Sci. 2006;63:73–81. doi: 10.1007/s00018-005-5402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hondal RJ, Marino SM, Gladyshev VN. Selenocysteine in thiol/disulfide-like exchange reactions. Antioxid Redox Signal. 2013;18:1675–1689. doi: 10.1089/ars.2012.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhuo P, Diamond AM. Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta. 2009;115:227–242. doi: 10.1016/j.bbagen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- 41.Zhuo P, Goldberg M, Herman L, Lee BS, Wang H, Brown RL, Foster CB, Peters U, Diamond AM. Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res. 2009;69:8183–8190. doi: 10.1158/0008-5472.CAN-09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bera S, Weinberg F, Ekoue DN, Ansenberger-Fricano K, Mao M, Bonini MG, Diamond AM. Natural allelic variations in glutathione peroxidase-1 affect its subcellular localization and function. Cancer Res. 2014;74:5118–5126. doi: 10.1158/0008-5472.CAN-14-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox DG, Hankinson SE, Kraft P, Hunter DJ. No association between GPX1 Pro198Leu and breast cancer risk. Cancer Epidemiol Biomark Prev. 2004;13:1821–1822. [PubMed] [Google Scholar]
- 44.Cox DG, Tamimi RM, Hunter DJ. Gene × Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer. 2006;6:217. doi: 10.1186/1471-2407-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utsunomiya H, Komatsu N, Yoshimura S, Tsutsumi Y, Watanabe K. Exact ultra-structural localization of glutathione peroxidase in normal rat hepatocytes: advantages of microwave fixation. J Histochem Cytochem. 1991;39:1167–1174. doi: 10.1177/39.9.1918936. [DOI] [PubMed] [Google Scholar]
- 46.Asayama K, Yokota S, Dobashi K, Hayashibe H, Kawaoi A, Nakazawa S. Purification and immunoelectron microscopic localization of cellular glutathione peroxidase in rat hepatocytes: quantitative analysis by postembedding method. Histochemistry. 1994;102:213–219. doi: 10.1007/BF00268898. [DOI] [PubMed] [Google Scholar]
- 47.Cooper ML, Adami HO, Gronberg H, Wiklund F, Green FR, Rayman MP. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008;68:10171–10177. doi: 10.1158/0008-5472.CAN-08-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]