Abstract
Bone morphogenetic proteins (BMPs) and the growth factors (GDFs) play an important role in ovarian folliculogenesis and essential regulator of processes of numerous granulosa cells. BMP15 gene variations linked to various ovarian phenotypic consequences subject to the species, from infertility to improved prolificacy in sheep, primary ovarian insufficiency in women or associated with minor subfertility in mouse. To study the evolving role of BMP15 and GDF9, a phylogenetic analysis was performed. To find out the candidate gene associated with prolificacy in mammals, the nucleotide sequence of BMP15 and GDF9 genes was recognized under positive selection in various mammalian species. Maximum‐likelihood approaches used on BMP15 and GDF9 genes exhibited a robust divergence and a prompted evolution as compared to other TGFβ family members. Furthermore, among 32 mammalian species, we identified positive selection signals in the hominidae clade resulting to 132D, 147E, 163Y, 191W, and 236P codon sites of BMP15 and 162F, 188K, 206R, 240A, 244L, 246H, 248S, 251D, 253L, 254F and other codon sites of GDF9. The positively selected amino acid sites such as Alanine, Lucien, Arginine, and lysine are important for signaling. In conclusion, this study evidences that GDF9 and BMP15 genes have rapid evolution than other TGFß family members and was subjected to positive selection in the mammalian clade. Selected sites under the positive selection are of remarkable significance for the particular functioning of the protein and consequently for female fertility.
Keywords: BMP15, evolution, GDF9, mammals, maximum likelihood, positive selection
1. INTRODUCTION
Ovarian folliculogenesis is essential for mammalian female fecundity and is regulated by a composite endocrine network between the pituitary and the ovary. In this milieu, the intra‐ovarian paracrine communications are important in oocyte development and follicle evolution and maturation and provide the tolerable sensitivity to gonadotrophic inducement. The molecular machinery is necessary during oogenesis in oocyte preparation to support embryonic development (Sánchez & Smitz, 2012). However, the oocyte development within the follicular structure involves uninterrupted two‐way dialogs between the oocyte and cumulus complex, as well as the other somatic cells in the follicles, such as the granulosa and theca cells (Wigglesworth et al., 2013). The granulosa cells are important components of the follicular environment for the achievement of oocyte capability, ovulation, and fertilization as they regulate the expression of luteinizing hormone receptor (LHR), production of estradiol and progesterone, Inhibin A and B secretion, and production of several transcripts vital proteins (Ceko et al., 2014; Hatzirodos et al., 2014). The bone morphogenetic protein 15 and growth differentiation factor 9, belong to the TGF‐β superfamily, act on the granulosa cells to regulate oocyte growth and differentiation. These are expressed in all phases of follicle development in the mammalian species and are involved in steroidogenic regulation of granulosa cells (Dias, Khan, Adams, Sirard, & Singh, 2014; Peng et al., 2013). A recent phylogenetic analysis revealed that the GDF9 and BMP15 genes diverged promptly and showed fast evolution as compared to other BMPs. However, only BMP15 was acquiesced to a positive selection in the mammalian clade (Auclair et al., 2013). The candidate gene associated with prolificacy in goats, the most part nucleotide sequence of genes, including GDF9 and BMP15, were recognized in various goat breeds for their possible association to the high fertility (He, Ma, Liu, Zhang, & Li, 2010). As BMP15 and GDF9 play an important role in fertility and prolificacy, it therefore means that consideration should be made to the gene sequences (using both wet and dry lab methods), that are generally accountable for the detected phenotypic variations. A good understanding of these genes sequences will help in recognizing the modifications accountable for various factors ascribed to the gene. The objective of this study was to explore the selection signatures using maximum‐likelihood approaches on the bases of molecular genetic difference of BMP15 and GDF9 among mammalian species with a view to provide applicable genetic information for marker assisted selection in the different species.
2. MATERIAL AND METHODS
2.1. Sequence analysis and data set preparation
The coding nucleotide and amino acid sequences of BMP15 and GDF9 genes used in this analyses were recovered from GenBank (www.ncbi.nlm.nih.gov/genbank), Ensembl (http://useast.ensembl.org/index.html), and UniProt (http://www.uniprot.org), and recovered sequences were aligned using ClustalOmega, executed in MEGA 6.0 program (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013), followed by manual adjustment. The phylogenetic tree of BMP15 and GDF9 genes was generated with MEGA 6.0 based on maximum‐likelihood method. The taxa clustered together in the bootstrap test 1,000 replicates based on maximum‐likelihood method selecting the topology with higher log likelihood value and the branch length measured in the number of substitutions per site. (Ahmad et al., 2017; Asif, Awais, Qadri, Ahmad, & Du, 2017). The accession numbers and identification of species used for BMP15 and GDF9 are listed in Table S1.
2.2. Codon‐based positive selection analysis
In order to recognize particular codons under positive selection of mammalian BMP15 and GDF9 sequences, the different ω ratios (dN/dS) were compared using two maximum‐likelihood approaches, the HyPhy package implemented in the DATAMONKEY Web Server (http://www.datamonkey.org/) (Poon, Frost, & Pond, 2009) and CODEML implemented in PAML version 4 (Yang, 2007) being considered in the analysis the results where ω ratios were significantly higher than 1.
The analysis involves of two main steps. In the first step, we used the maximum‐likelihood ratio test to find out positive selection, that is, manifestation of sites with ω > 1. We achieved this by comparing a (null) model that does not allow for sites with ω > 1 and a general (discrete) model that does. The likelihood log (2Δl) is compared with df = 4 with the χ2 distribution. The M7 model (null) postulates a β distribution with ω restricted in the interval (0 and 1). The M8 model (ω and beta) is an alternative model includes two parameters, so ω value obtained from the data set can be greater than one. Positive selection clues in BMP15 and GDF9 genes were identified by calculating the rates of synonymous and nonsynonymous variations at each site in alligned sequence using different likelihood tests such as fixed effect likelihood (FEL), random effect likelihood (REL), and single likelihood ancestor counting (SLAC) methods (Ahmad et al., 2017) by estimating global ω values.
The second main step is to find out amino acid subjected to positive selection when their presence is confirmed by likelihood test. It is inferred by using the Bayes theorem to estimate the posterior probabilities for each site, from the different ω classes (Bielawski & Yang, 2003). The amino acid residues with high probabilities having ω > 1 are probably found to be under selection. Amino acid locations subjected to positive selection were drawn onto the crystal structure using Phyre (http://www.sbg.bio.ic.ac.uk/phyre2/html) and Swiss model (http://swissmodel.expasy.org) online programs (Kelley & Sternberg, 2009). The level of evolutionary conservation amino acid/nucleic acid positions in protein was predicted using the bioinformatics tool, the ConSurf server (http://consurftest.tau.ac.il) based on phylogenetic relationship between sequences (Glaser et al., 2003). To further ratify codon sites under the selection pressure, aligned codon sequence of BMP15 and GDF9 was tested in the Selecton, version 2.2 (http://selecton.tau.ac.il/) that allows shifting the ω ratio between different codons within the aligned sequence and this was measured by maximum‐likelihood test through Bayesian inference method (Yang, Liao, Zhuang, & Zhang, 2012). Moreover, the selecton results are shown with color scales demonstrating various types of selection.
2.3. Protein–protein interaction network analysis
To further expose the molecular functioning mechanisms of BMP15 and GDF9, we recognized the vital genes interacted with BMP15 and GDF9 followed by protein–protein interaction linkage analysis sing STRING (version 9.1, http://www.string-db.org/) (Franceschini et al., 2012) which is web server and biological databank which comprises widely anticipated and identified interaction data. The interactions between protein encoded by the BMP15 and GDF9 were sought. The pooled score <0.4 was used as the cutoff standard. The bioinformatics databank as an open access source comprises interactions of proteins involved in various pathways. The middle nodes indicate the protein which own essential biological function and are highly connected, were identified by estimating the betweenness value and the number of line connections between proteins of each node. The network was constructed using STRING and was visualized by Cytoscape software (http://www.cytoscape.org/) (Li, Zhao, Wang, Zong, & Yang, 2017).
3. RESULTS
The average ω ratio (dN/dS) across the sites and lineage are <1 for BMP15 and GDF9 (Table 1). However, these proteins subjected to positive selection and might have conserved amino acid exposed to purifying selection and have ω less than one. The level of evolutionary conservation amino acid/nucleic acid positions in protein was predicted using the ConSurf server (http://consurftest.tau.ac.il) based on phylogenetic relationship between sequences and Selecton version 2.2 that implements the mechanistic empirical combination (MEC) model for estimating adaptive selection pressure at different codons. A huge number of conserved amino acids would mask the positive selection signals, and we found positive selection on variable amino acids which were exposed or buried residues according to the neural network algorithm BMP15 and GDF9.
Table 1.
Log likelihood values and test statistics for PAML site models of positive selection
| Gene | No. of residues | No. of species | Model | Parameter estimates | lnL M7 | lnL M8 | 2ΔlnL | PAML | FEL | REL | SLAC | % of sites |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMP15 | 1234 | 37 | M7 | p = .60414, q = 0.76298 | −19,737.9 | −19,329.1 | 17.69** | 40, 49, 75, 90, 109, 133, 176, 191, 199, | 17, 126, 144, 148, | 45, 95, 126, 144, | 45, 384, 386 | 0.53 |
| M8 | P0 = 0.94697, p = .66983, q = 0.97138 | 200, 203, 258, 289, | 248, 363 | 363, 384 | ||||||||
| P1 = 0.05303, ω1 = 1.56019 | 297, 315, 323, 365 | |||||||||||
| GDF9 | 1371 | 32 | M7 | p = .48463, q = 0.91798 | −13,717.5 | −13,716.2 | 2.59* | 30, 186, 245, 254, | 21, 30, 34, 139, 186 | 21, 30, 186, 299, | 21, 30, 186, 299, | 0.78 |
| M8 | P0 = 0. 99219, p = .49979, q = 0. 97580 | 292, 302, 304 | 299, 304, 339 | 304, 339 | 304 | |||||||
| P1 = 0.00781, ω1 = 1.93407 |
The proportion of sites under positive selection (p1), or under selective constraint (p0), and parameters p and q for the beta distribution. Parameters indicating positive selection are in bold. p: significant at 5% level; p: significant at 1% level. Sites potentially under positive selection identified under model M8 are listed according to the human sequence numbering. Positively selected sites with posterior probability 0.9 are italicized, 0.8–0.9 in bold, and 0.5–0.7 in plain text. The test statistic 2Δl is compared to a χ2 distribution with 2 df, critical values 5.99, 9.21, and 13.82 at 5%, 1%, and 0.1% significance, respectively.
**Significant at 1% level; *Significant at 5% level.
As a refined selection test, M8 was compared with M7. M8 was significant and fit the data more significantly than M7. We found positive selection for BMP15 and GDF9 proteins under model M8. The proportion of sites under M8 are 0.53% with ω = 1.56 for BMP15 and 0.78% with ω = 1.93 for GDF9. The proportion of positively selected sites under M8 is clear statistical evidence positive Darwinian selection (Table 1). There were 17 codon sites of BMP15 and 7 sites of GDF9 under positive selection in various likelihood approaches. Evolutionary signal of positive selection was inferred by computing global ω values using FEL, REL, and SLAC tests. The results revealed that there was robust sign of positive selection in BMP15 and GDF9 genes in mammals. The FEL and REL detected six sites and SLAC identified three sites under the positive selection for BMP15 at various codon positions, respectively (Table 1), while FEL, REL, and SLAC analyses detected eight, six, and five sites under positive selection, respectively (Table 1). REL detected positive sites at 95% confidence interval. REL analysis detected sites under positive selection which used Bayes factor, and the values > 20. p Values < .05 were measured as significant for other analysis and all identified sites were considerably different and having p‐values < .05.
3.1. Positive selection on amino acid positions
Indicating the positions of amino acids evolutionary conservation is important for maintaining the protein structure and function. Therefore, detection of selected sites may enlighten the selection forces and detects the functionally significant sites for bone morphogenetic protein interaction. To detect such sites, we utilized the Bayes method to estimate the posterior probabilities for each site. The sites with more probabilities are expected to be positively selected with ω > 1. Using Using BEB analysis for 391 amino acids of BMP15, seventeen were found under positive selection but no site could be identified at 99% or 95% posterior probability. GDF9 had 453 amino acid sites, and only seven amino acids showed positive selection (Table 2; Figure 1a,b). Regarding PAML false positive results, we also performed positive selection test in the selecton server (http://selecton.tau.ac.il/) that uses the Mechanistic Empirical Combination (MEC) model for estimating the selection pressure at particular codons. The MEC model takes into account the variances between amino acid substitution rates. Adaptive selection pressure was found at various codons in BMP15 (Figure 2) and GDF9 (Figure 3), identified under positive selection.
Table 2.
Positively selected sites under different PAML site models using bayes empirical bayes analysis
| Gene | Model | Codon | Amino acid | Posterior probability | Post mean ± SE for ω |
|---|---|---|---|---|---|
| BMP15 | M8: selection, beta+ ω | 40 | V | 0.846 | 1.407 ± 0.22 |
| 49 | I | 0.678 | 1.303 ± 0.29 | ||
| 75 | Q | 0.755 | 1.350 ± 0.27 | ||
| 90 | R | 0.923 | 1.455 ± 0.16 | ||
| 109 | A | 0.824 | 1.393 ± 0.24 | ||
| 133 | V | 0.888 | 1.434 ± 0.19 | ||
| 176 | H | 0.55 | 1.218 ± 0.32 | ||
| 191 | P | 0.703 | 1.315 ± 0.29 | ||
| 199 | G | 0.567 | 1.224 ± 0.32 | ||
| 200 | R | 0.651 | 1.281 ± 0.31 | ||
| 203 | P | 0.607 | 1.249 ± 0.32 | ||
| 258 | W | 0.572 | 1.227 ± 0.32 | ||
| 289 | K | 0.609 | 1.253 ± 0.32 | ||
| 297 | K | 0.765 | 1.358 ± 0.26 | ||
| 315 | A | 0.727 | 1.331 ± 0.28 | ||
| 323 | R | 0.775 | 1.362 ± 0.26 | ||
| 365 | V | 0.578 | 1.238 ± 0.31 | ||
| GDF‐9 | M8: selection, beta+ ω | 30 | G | 0.774 | 1.576 ± 0.39 |
| 186 | F | 0.581 | 1.270 ± 0.34 | ||
| 245 | L | 0.814 | 1.185 ± 0.34 | ||
| 254 | L | 0.722 | 1.242 ± 0.34 | ||
| 292 | G | 0.824 | 1.203 ± 0.34 | ||
| 302 | Y | 0.531 | 1.350 ± 0.36 | ||
| 304 | V | 0.958a | 1.271 ± 0.33 |
Posterior probabilities >90%.
Figure 1.
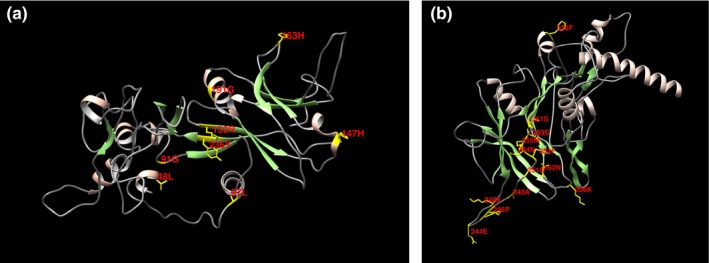
(a) Location of positively selected amino acid sites identified BMP15 gene. The crystal structure of human BMP as a reference and positively selected sites were drawn onto the crystal structure using Phyre tool (http://www.sbg.bio.ic.ac.uk/phyre2/html). All the residues identified as under selection fall in the domain containing the ligand binding site. The sites which fall in the region identified as the ligand binding site and another cluster in a region immediately following the signal sequence. (b) Location of positively selected amino acid sites identified GDF9 gene. The crystal structure of human GDF9 as a reference and positively selected sites was drawn onto the crystal structure using Phyre tool (http://www.sbg.bio.ic.ac.uk/phyre2/html). All the residues identified as under selection fall in the domain containing the ligand binding site. The sites which fall in the region identified as the ligand binding site and another cluster in a region immediately following the signal sequence
Figure 2.

Selection pressures among goat BMP15 gene sequences using mechanistic empirical combination (MEC) model of selecton online tool. Yellow and brown highlights represent positive selection, gray and white highlights represent neutral selection, and purple highlight represents negative selection on codons
Figure 3.
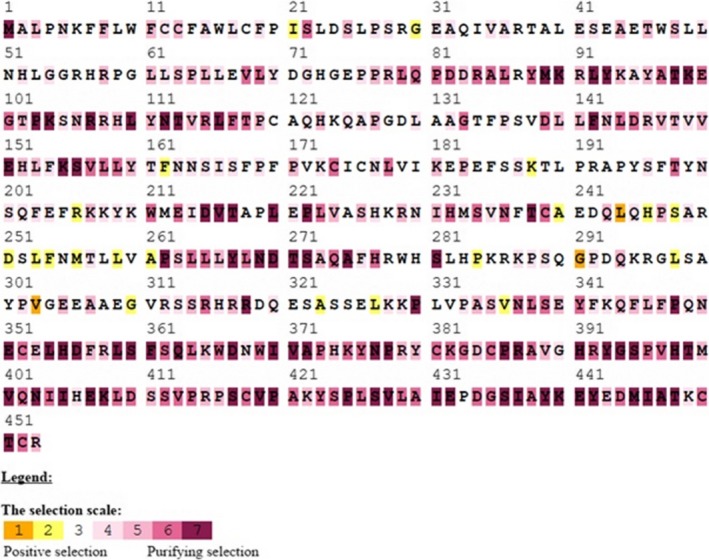
Selection pressures among goat GDF9 gene sequences using mechanistic empirical combination (MEC) model of selecton online tool. Yellow and brown highlights represent positive selection, gray and white highlights represent neutral selection, and purple highlight represents negative selection on codons
3.2. Protein–protein interaction network
By searching BMP15 and GDF9 encoded protein to the STRING databank, various PPI pairs were found. The PPI network had 21 nodes (denote BMP15 and GDF9 encoded proteins) and 102 edges (line networks between nodes) (Figure 4). In the PPI network, BMP15 and GDF9 are interacted with the other reproductive key genes which are co‐expressed. We found 19 genes: KITLG, KIT, TGFβ, TGFβR, SMAD2, SMAD3, SMAD4, SMAD7, BMP2, BMP4, BMP7, FSHβ, LHCGR, ZAR1, BRD2, BMPR2, and BMPR1β (Figure 4). Among these, TGFβ, TGFβR, SMAD2, SMAD3, SMAD4, SMAD7, BMP2, BMP4, BMP7, and FSHβ are vital genes involved in biological signaling pathways in reproduction because these are upregulated genes with BMP15 and GDF9 (Figure 4). The molecular pathways of all the interacted proteins are involved in various reproductive functions such as TGF beta binding receptor, cytokine receptor binding, SMAD binding, and others proteins are interconnected with each other in various biological molecular pathways that are involved in modulating the reproductive efficiency in mammals.
Figure 4.
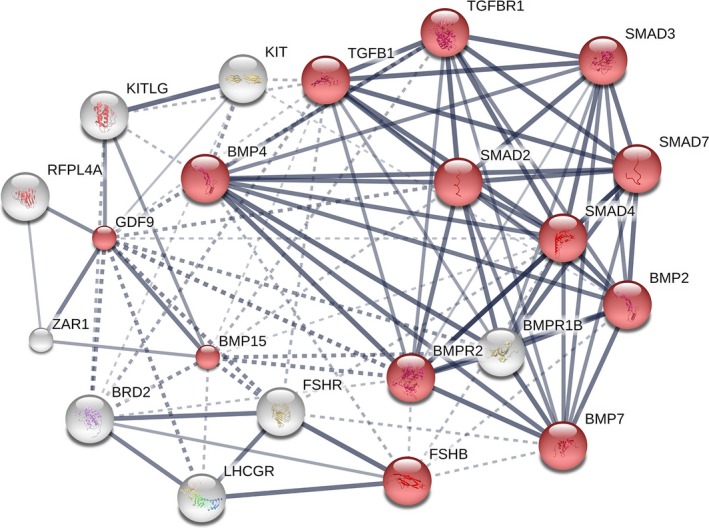
The protein–protein interaction (PPI) network built by STRING database for BMP15 and GDF genes. Gray and Red circles characterize downregulated and upregulated genes, respectively. Line thickness indicates the strength of the interaction. Dash and solid edge mean negative and positive correlation coefficient. Network nodes denote proteins' post‐transcriptional modifications or splice isoforms, and each node represents all the proteins produced by a single, protein‐coding gene locus
4. DISCUSSION
The BMP15 and GDF9 contribute in the development of primary follicle from primordial follicle and play an essential role in the subsequent phases of follicular growth and maturation, enhancing the expression of luteinizing hormone receptor (LHR), steroidogenic regulatory protein, and plasminogen activators. These factors are also involved in the communication among oocyte and cumulus cells, where they control the biosynthesis of cholesterol in cumulus cells, glycolysis, and absorption of amino acids. Although the mode of action has not been fully understood, in vitro studies revealed that the factors BMP15 and GDF9 stimulate the ovarian follicles growth and cumulus cells proliferation through the initiation of mitosis in cells and theca and granulosa expression of genes connected to follicular maturation (de Castro, Cruz, & Leal, 2016). Assumed that positive selection is generally linked with the branch length and rapid gene evolution and the genes related to reproduction evolve rapidly and often proved positive selection (Meslin et al., 2012). We accomplished positive selection analyses on BMP15 and GDF9. We used coding nucleotide sequences of 32 mammalian species which were evaluated by branch‐site models in PAML package (Yang, 2007), in order to investigate whether the diverse species in the phylogenetic kinship experienced selection pressure and to identify clues of native periodic positive selection. These evaluates were performed on the complete sequences, the mature, and the pro‐region form of BMP15 and GDF9. We studied all branches of the phylogenetic tree, and various codon sites were found under positive selection in mammalian clade (Figure 1a,b and Table 1). We identified fifteen positively selected sites with PAML, six sites with FEL and REL, and four sites with SLAC models for BMP15; eleven of these fifteen amino acids (Figure 2) were also recognized by the estimation of positive selection using the mechanistic empirical combination (MEC) model for measuring adaptive selection pressure at codons on the BMP15 sequence. We identified seven positively selected sites with PAML, eight with FEL, six with REL, and four with SLAC model for GDF9. Furthermore 22 amino acids were found positively selected for the hominidae clade, corresponding to human coding sequences (Figure 3) using the mechanistic empirical combination (MEC) model in Selecton server. It has been testified that GC influenced gene conversion might rise the dN/dS ratio, particularly in primates and yield false‐positive recognition of amino acids in branch‐site model of positive selection (Ratnakumar et al., 2010). It has been revealed that in hominidae clade, the third codon position of BMP15, the G‐C content (53%) is higher than the gene in these taxa (46%) (Romiguier, Ranwez, Douzery, & Galtier, 2010). However, a current study (Gharib & Robinson‐Rechavi, 2013) describes that the G‐C rate of a sequence did not considerably interfere the branch‐site model. A phylogenetic analysis was performed to study the evolving role of BMP15. A maximum‐likelihood phylogenetic kinship of mammalian BMP15 expressed by the ovary exhibited that BMP15 has a very robust divergence and a fast evolution compared to other genes. Furthermore, positive selection signals detected in the hominidae clade consistent to F146, L189 and Y235 codon sites in human BMP15 (Auclair et al., 2013). We performed selection analyses to investigate amino acid positions under the positive selection through Bayes Empirical Bayes and found various amino acid sites of BMP15 and GDF9 under positive selection. We found positive selection signals at 131D, 163Y, 191W, 147E, and 236P codon sites of BMP15 (Figure 2) and 162F, 188K, 206R, 240A, 244L, 246H, 248S, 251D, 253L, 254F, and other codon sites of GDF9 (Figure 3). The positively selected amino acid sites such as alanine, leucine, arginine, and lysine are important for signaling. Among 24 mammalian species, positive selection signals were detected signals in the human BMP15 (Auclair et al., 2013). Therefore, some amino acid sites under positive selection are essential for particular role of protein and consequently for female fertility (Persani, Rossetti, Di Pasquale, Cacciatore, & Fabre, 2014). Moreover, the transformed was more effective than wild type in deterring the progesterone production in granulosa cells of ovine cell culture. It is evidenced that BMP15 has evolved faster than other TGF family members and was acquiesced to positive selection in mammalian clade (Persani et al., 2014). The sequence alignment reveals that BMP15 belongs to the TGF family of cytokines due to the existence of “cystine‐knot” motif, together with GDF9 as the next homolog. Like other TGF members, these molecules are first decoded as signal peptide with a pre‐pro‐peptide at N‐terminal followed by pro‐domain and the C‐terminal mature sphere that delivers the biological action (Chang, Brown, & Matzuk, 2002). The particular functions of TGF superfamily member's pro‐domains are unidentified. The proteolytically treated pro‐region and mature regions of BMPs remain attached noncovalently, usually networking with the extracellular matrix (Sengle, Ono, Sasaki, & Sakai, 2011). Regarding the BMP15 pro‐region, it drives the dimerization and subsequent secretion of the mature dimers and may help to alleviate the mature region bioactivity (Pulkki et al., 2011). In our study, positive selection at BMP15 and GDF9 was found with ω > 1 (Table 1). This indicates that nonsynonymous (dN) sites evolved quicker than those of synonymous sites and positive Darwinian selection influence purifying/balancing selection favored new variants and raised allelic polymorphism (Bergström & Gyllensten, 1995) which in turn might introduce an alteration in protein structure validation, thus affecting the signaling pathways (Cui et al., 2009). The changing amino acid substitutions across species might be the result of discrete divergence from their common lineages, which agrees with former submissions. The orthologs differ from their most recent common forebear have different evolutionary routes which may direct the deviations in the selective constraints on homologous sites (Marini, Thomas, & Rine, 2010). Our analysis of bone morphogenetic protein genes involved in recent selection provides insights of some biological processes that have been objectives of selection in current and much longer evolutionary timescales (Voight, Kudaravalli, Wen, & Pritchard, 2006). Hence, understanding the story of selection in mammalian genome promises to be an interesting research area for years to come.
5. CONCLUSIONS
In present study, we investigated that BMP15 and GDF9 genes have evolved rapidly than other TGFß superfamily members and was allowed to selection pressure in mammalian clade. Some positively selected amino acid sites are of significant for the particular role of protein and consequently for female fertility. We presented comprehensive analyses in determination of genetic importance of BMP15 and GDF9. Selection analyses of bone morphogenetic proteins modulating reproduction could facilitate the development of unique strategies that may help for genetic improvement and select individuals with high breeding values for traits of interest as parentages to produce the next generation.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
HIA wrote original draft of manuscript. SGE, TW, BT, YY, LP, CL, YC, ZJY, and HJ help in data analysis. GL and XJ supervised the study.
Supporting information
ACKNOWLEDGMENTS
Author is thankful to anonymous reviewers for their valuable comments, suggestions, and critical reading of the manuscript. This work was funded by China National Meat‐type Sheep and Goat Industrial Technology System CARS‐39‐1A and Yichang Goat Breeding Program 2015.
Ahmad HI, Liu G, Jiang X, et al. Maximum‐likelihood approaches reveal signatures of positive selection in BMP15 and GDF9 genes modulating ovarian function in mammalian female fertility. Ecol Evol. 2017;7:8895–8902. https://doi.org/10.1002/ece3.3336
REFERENCES
- Ahmad, H. I. , Liu, G. , Jiang, X. , Liu, C. , Chong, Y. , & Huarong, H. (2017). Adaptive molecular evolution of MC1R gene reveals the evidence for positive diversifying selection in indigenous goat populations. Ecology and Evolution, 7, 5170–5180. https://doi.org/10.1002/ece3.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, H. I. , Liu, G. , Jiang, X. , Liu, C. , Fangzheng, X. , Chong, Y. , … Huarong, H. (2017). Adaptive selection at agouti gene inferred breed specific selection signature within the indigenous goat populations. Asian‐Australasian Journal of Animal Sciences, 00, 1–7. https://doi.org/10.5713/ajas.16.0994 [DOI] [PubMed] [Google Scholar]
- Asif, A. R. , Awais, M. , Qadri, S. , Ahmad, H. I. , & Du, X. (2017). Positive selection of IL‐33 in adaptive immunity of domestic Chinese goats. Ecology and Evolution, 7, 1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair, S. , Rossetti, R. , Meslin, C. , Monestier, O. , Di Pasquale, E. , Pascal, G. , … Fabre, S. (2013). Positive selection in bone morphogenetic protein 15 targets a natural mutation associated with primary ovarian insufficiency in human. PLoS ONE, 8, e78199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström, T. , & Gyllensten, U. (1995). Evolution of Mhc class II polymorphism: The rise and fall of class II gene function in primates. Immunological Reviews, 143, 13–31. [DOI] [PubMed] [Google Scholar]
- Bielawski, J. P. , & Yang, Z. (2003). Maximum likelihood methods for detecting adaptive evolution after gene duplication Journal of Structural and Functional Genomics; 3 201–212. [PubMed] [Google Scholar]
- de Castro, F. C. , Cruz, M. H. C. , & Leal, C. L. V. (2016). Role of growth differentiation factor 9 and bone morphogenetic protein 15 in ovarian function and their importance in mammalian female fertility—A review. Asian‐Australasian Journal of Animal Sciences, 29, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceko, M. J. , Hummitzsch, K. , Hatzirodos, N. , Bonner, W. M. , Aitken, J. B. , Russell, D. L. , … Harris, H. H. (2014). X‐Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics, 7, 71–82. [DOI] [PubMed] [Google Scholar]
- Chang, H. , Brown, C. W. , & Matzuk, M. M. (2002). Genetic analysis of the mammalian transforming growth factor‐β superfamily. Endocrine Reviews, 23, 787–823. [DOI] [PubMed] [Google Scholar]
- Cui, H. X. , Zhao, S. M. , Cheng, M. L. , Guo, L. , Ye, R. Q. , Liu, W. Q. , & Gao, S. Z. (2009). Cloning and expression levels of genes relating to the ovulation rate of the Yunling black goat. Biology of Reproduction, 80, 219–226. [DOI] [PubMed] [Google Scholar]
- Dias, F. C. F. , Khan, M. I. R. , Adams, G. P. , Sirard, M. A. , & Singh, J. (2014). Granulosa cell function and oocyte competence: Super‐follicles, super‐moms and super‐stimulation in cattle. Animal Reproduction Science, 149, 80–89. [DOI] [PubMed] [Google Scholar]
- Franceschini, A. , Szklarczyk, D. , Frankild, S. , Kuhn, M. , Simonovic, M. , Roth, A. , … Jensen, L. J. (2012). STRING v9. 1: Protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Research, 41, D808–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib, W. H. , & Robinson‐Rechavi, M. (2013). The branch‐site test of positive selection is surprisingly robust but lacks power under synonymous substitution saturation and variation in GC. Molecular Biology and Evolution, 30, 1675–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, F. , Pupko, T. , Paz, I. , Bell, R. E. , Bechor‐Shental, D. , Martz, E. , & Ben‐Tal, N. (2003). ConSurf: Identification of functional regions in proteins by surface‐mapping of phylogenetic information. Bioinformatics, 19, 163–164. [DOI] [PubMed] [Google Scholar]
- Hatzirodos, N. , Hummitzsch, K. , Irving‐Rodgers, H. F. , Harland, M. L. , Morris, S. E. , & Rodgers, R. J. (2014). Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genomics, 15, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Ma, X. , Liu, X. , Zhang, C. , & Li, J. (2010). Candidate genes polymorphism and its association to prolificacy in Chinese goats. Journal of Agricultural Science, 2, 88. [Google Scholar]
- Kelley, L. A. , & Sternberg, M. J. (2009). Protein structure prediction on the Web: A case study using the Phyre server. Nature Protocols, 4, 363–371. [DOI] [PubMed] [Google Scholar]
- Li, H. , Zhao, X. , Wang, J. , Zong, M. , & Yang, H. (2017). Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene, 596, 98–104. [DOI] [PubMed] [Google Scholar]
- Marini, N. J. , Thomas, P. D. , & Rine, J. (2010). The use of orthologous sequences to predict the impact of amino acid substitutions on protein function. PLoS Genetics, 6, e1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslin, C. , Mugnier, S. , Callebaut, I. , Laurin, M. , Pascal, G. , Poupon, A. , … Monget, P. (2012). Evolution of genes involved in gamete interaction: Evidence for positive selection, duplications and losses in vertebrates. PLoS ONE, 7, e44548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , Li, Q. , Wigglesworth, K. , Rangarajan, A. , Kattamuri, C. , Peterson, R. T. , … Matzuk, M. M. (2013). Growth differentiation factor 9: Bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proceedings of the National Academy of Sciences, 110, E776–E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persani, L. , Rossetti, R. , Di Pasquale, E. , Cacciatore, C. , & Fabre, S. (2014). The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Human Reproduction Update, 20, 869–883. [DOI] [PubMed] [Google Scholar]
- Poon, A. F. , Frost, S. D. , & Pond, S. L. K. (2009). Detecting signatures of selection from DNA sequences using Datamonkey. Bioinformatics for DNA Sequence Analysis, 537, 163–183. [DOI] [PubMed] [Google Scholar]
- Pulkki, M. M. , Myllymaa, S. , Pasternack, A. , Lun, S. , Ludlow, H. , Al‐Qahtani, A. , … Laitinen, M. (2011). The bioactivity of human bone morphogenetic protein‐15 is sensitive to C‐terminal modification: Characterization of the purified untagged processed mature region. Molecular and Cellular Endocrinology, 332, 106–115. [DOI] [PubMed] [Google Scholar]
- Ratnakumar, A. , Mousset, S. , Glémin, S. , Berglund, J. , Galtier, N. , Duret, L. , & Webster, M. T. (2010). Detecting positive selection within genomes: The problem of biased gene conversion. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 365, 2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romiguier, J. , Ranwez, V. , Douzery, E. J. , & Galtier, N. (2010). Contrasting GC‐content dynamics across 33 mammalian genomes: Relationship with life‐history traits and chromosome sizes. Genome Research, 20, 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, F. , & Smitz, J. (2012). Molecular control of oogenesis. Biochimica et Biophysica Acta (BBA)‐Molecular Basis of Disease, 1822, 1896–1912. [DOI] [PubMed] [Google Scholar]
- Sengle, G. , Ono, R. N. , Sasaki, T. , & Sakai, L. Y. (2011). Prodomains of transforming growth factor β (TGFβ) superfamily members specify different functions extracellular matrix interactions and growth factor bioavailability. Journal of Biological Chemistry, 286, 5087–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Stecher, G. , Peterson, D. , Filipski, A. , & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight, B. F. , Kudaravalli, S. , Wen, X. , & Pritchard, J. K. (2006). A map of recent positive selection in the human genome. PLoS Biology, 4, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth, K. , Lee, K. B. , O'Brien, M. J. , Peng, J. , Matzuk, M. M. , & Eppig, J. J. (2013). Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proceedings of the National Academy of Sciences, 110, E3723–E3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. (2007). PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24, 1586–1591. [DOI] [PubMed] [Google Scholar]
- Yang, J. R. , Liao, B. Y. , Zhuang, S. M. , & Zhang, J. (2012). Protein misinteraction avoidance causes highly expressed proteins to evolve slowly. Proceedings of the National Academy of Sciences, 109, E831–E840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


